
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 06 October 2022
Sec. Breast Cancer
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1019925
This article is part of the Research TopicNeoadjuvant Therapy in Breast Cancer: Biomarkers and Early Response PredictionView all 16 articles
 Xiaoyan Qian1,2
Xiaoyan Qian1,2 Meng Xiu1
Meng Xiu1 Qing Li1
Qing Li1 Jiayu Wang1
Jiayu Wang1 Ying Fan1
Ying Fan1 Yang Luo1
Yang Luo1 Ruigang Cai1
Ruigang Cai1 Qiao Li1
Qiao Li1 Shanshan Chen1
Shanshan Chen1 Peng Yuan3
Peng Yuan3 Fei Ma1
Fei Ma1 Binghe Xu1
Binghe Xu1 Pin Zhang1*
Pin Zhang1*Background: Although achieving pathological complete response (pCR) and near-pathological complete response (near-pCR) after neoadjuvant chemotherapy (NAC) in breast cancer predicts a better outcome, some patients still experience recurrence. The aim of our study was to investigate the predictive factors of recurrence in the pCR and near-pCR population.
Methods: We reviewed 1,209 breast cancer patients treated with NAC between January 2010 and April 2021 in the Cancer Hospital, Chinese Academy of Medical Sciences (CHCAMS). A total of 292 patients achieving pCR and near-pCR were included in our analysis. pCR was defined as ypT0N0/ypTisN0. Near-pCR was defined as ypT1mi/1a/1bN0 or ypT0/isN1mi. Clinical features and follow-up information were collected. Survival and predictive factors of recurrence were analyzed.
Results: Of the 292 patients, 173 were pCR and 119 were near-pCR. The median age was 46 years (range, 23–75 years). The predominant tumor subtypes were human epidermal growth factor receptor type 2 (HER2)-positive breast cancer (49.0%) and triple-negative breast cancer (TNBC) (30.8%). The median duration of follow-up was 53 months (range, 9–138 months). A total of 25 (8.6%) patients developed recurrence, with 9 (5.2%) in the pCR group and 16 (13.4%) in the near-pCR group. The vast majority of recurrence occurred within 36 months from onset of NAC. The 5-year recurrence-free survival (RFS) rate of patients achieving pCR was significantly higher than that of patients achieving near-pCR (94.6% vs. 85.6%, p = 0.008). However, the 5-year overall survival (OS) rate between the two cohorts had no statistical difference (94.3% vs. 89.6%, p = 0.304). Clinical N3 (cN3) before NAC was an independent risk factor of recurrence in patients who achieved pCR (p = 0.003) and near-pCR (p = 0.036). Tumor size before NAC, subtypes of breast cancer, and chemotherapy regimens showed no significant association with RFS both for patients who achieved pCR and for those who achieved near-pCR (p > 0.05).
Conclusions: cN3 before NAC was an independent risk factor of recurrence in patients who achieved pCR and near-pCR. It is worthwhile to closely monitor patients with cN3, especially in the first 3 years.
Neoadjuvant chemotherapy (NAC) was widely used in patients with human epidermal growth receptor 2 (HER2)-positive breast cancer and triple-negative breast cancer (TNBC) (1–3). HER2-positive breast cancer and TNBC are relatively sensitive to NAC, and pathological reaction to NAC can provide prognostic information and guide the selection of postoperative treatment (4–9). Due to the rapid development of antineoplastic drugs in recent decades, the rate of pathological complete response (pCR) after NAC has significantly increased (10). Studies have demonstrated that patients achieving pCR had significantly better disease-free survival (DFS) and overall survival (OS) than patients with residual disease (11, 12). The assessment of obtaining a real pCR is of great importance and has been gradually standardized nowadays. The generally accepted definition of pCR is that there is no residual invasive carcinoma in the breast and in all sampled lymph nodes (ypT0/isN0) (13–15). More recently, the concept of near-pCR was gradually being proposed and has attracted more and more attention. Substantial research elucidated that patients who achieved near-pCR also had outstanding DFS and OS (13, 14). A variety of definitions of near-pCR have been used in neoadjuvant clinical trials in breast cancer. The most common consensus was that the residual disease ≤1 cm (9, 16).
In spite of the outstanding outcomes of patients achieving pCR and near-pCR, some of them may still experience recurrence. In order to identify clinical and pathological predictive factors of cancer recurrence, we performed this retrospective analysis among breast cancer patients who achieved pCR and near-pCR in the Cancer Hospital, Chinese Academy of Medical Sciences (CHCAMS). In this study, we aimed to explore the predictive factors associated with recurrence for the patients achieving pCR and near-pCR, and investigate whether the risk for recurrence and death of patients achieving near-pCR was comparable with those achieving pCR.
We reviewed 1,209 breast cancer patients that were treated with NAC between January 2010 and April 2021 in CHCAMS. The inclusion criteria in this study were as follows: (1) patients who were pathologically diagnosed with invasive breast cancer based on WHO criteria; (2) patients who have early-stage or locally advanced breast cancer (4, 13); (3) patients receiving surgery after NAC; (4) patients with complete clinical information; and (5) patients with follow-up data. Exclusion criteria were as follows: (1) patients with distant metastasis before or during NAC; (2) patients without detailed pathology after surgery; and (3) patients who withdraw active follow-up data. A final cohort of 292 patients who achieved pCR and near-pCR was incorporated in this study. Clinical and pathological data of these patients were collected: age, menstruation, tumor size, regional lymph node, estrogen receptor (ER), progesterone receptor (PR), HER2, Ki67 index, chemotherapy, radiation, endocrine, and surgery regimens.
pCR was defined as no residual invasive carcinoma in the breast and negative axillary lymph nodes, including ypT0N0 and ypTisN0 (13–15). Near-pCR was defined as the residual tumor size ≤1 cm in the breast and negative axillary lymph nodes, or no residual invasive carcinoma in the breast yet existing micrometastasis in lymph node, including ypT1mi/a/bN0 and ypT0/isN1mi (9, 16). Pathologically, T and N were defined according to the AJCC Staging System of Breast Cancer, 8th edition (17).
The Miller–Payne grade system was used to evaluate breast cancer pathological responses to NAC (18). Grade 1: no significant reduction in tumor cells; Grade 2: a minor reduction in tumor cells (≤30%); Grade 3: reduction in tumor cells between 30% and 90%; Grade 4: disappearance of tumor cells > 90%; Grade 5: no invasive tumor cells identifiable, and DCIS may be present.
ER and PR status was assessed by immunohistochemistry (IHC) and categorized as positive when more than 1% of cancer cells were stained (19). HER2 positive was defined as 3+ by IHC or positive by fluorescence in situ hybridization (FISH) (20). Ki67 index was defined as the mean tumor cells with marker expression by IHC: low (<20%), intermediate (20%–49%), and high (≥50%) (21–23).
The molecular subtype classification was on the basis of IHC of ER, PR, HER2, and Ki67 (24). Luminal A: ER and PR positive (PR ≥ 20%), HER2 negative, and Ki67 low expression; Luminal B HER2-negative: ER and/or PR positive, HER2 negative; Luminal B HER2-positive: ER and/or PR positive, HER2 positive; HER2-positive (non-luminal): ER and PR negative, HER2 positive; Triple-negative: ER and PR negative, HER2 negative.
Recurrence-free survival (RFS) was calculated as the time from the onset of NAC to local or distant recurrence, or death due to any cause, whichever came first. OS was calculated as the time from the onset of NAC to death due to any cause.
All statistical analyses were conducted using SPSS 25.0 and R (version 3.5.1). The Kaplan–Meier method with the log-rank test was used for recurrence and survival analysis. The factors significant at the 20% level in the univariate analysis were considered for inclusion in the multivariate model. The Cox proportional hazards regression model was used to assess the association of clinical and pathological predictive factors with RFS. C-statistics was conducted to evaluate the predictive value of the factors. All tests were two tailed and a p-value less than 0.05 was considered to indicate a statistically significant difference.
A total of 292 patients with pCR and near-pCR were included in this study. Their clinical and pathological characteristics are described in Table 1. The median age of patients was 46 years (range, 23–75 years); 62.3% were premenopausal. The median duration of follow-up for these patients was 53 months (range, 9–138 months). There were 173 patients achieving pCR and 119 achieving near-pCR. The predominant tumor subtypes were HER2 positive (49.0%) (including luminal B HER2+ and non-luminal HER2+) and TNBC (30.8%). Among the patients with HER2 positive, 63.6% received trastuzumab, while 24.4% received trastuzumab and pertuzumab. The majority of the tumors were T2+ (91.4%) and N+ (76.0%). Overall, 77.0% of the patients underwent mastectomy and 83.2% of the patients had axillary lymph node dissection.
As shown in Table 2, a total of 25 (8.6%) patients developed recurrence. Twenty-one (84.0%) recurrences occurred within 36 months. Among patients achieving pCR, 9 (5.2%) patients developed cancer recurrence, with 2 patients presenting with both local recurrence and distant metastasis, while 7 patients presented with distant metastasis. The median time to recurrence was 14 months (range, 8–62 months) from the onset of NAC. Four (44.4%) patients presented liver metastasis and 2 (22.2%) patients presented brain metastasis as the first event.
With regard to patients achieving near-pCR, 16 (13.4%) patients developed cancer recurrence, with 4 patients presenting with local recurrence only, 4 patients with both local recurrence and distant metastasis, while 8 patients presenting with distant metastasis only. The median time to recurrence was 18 months (range, 4–69 months). Three (18.8%) patients experienced lung metastasis and 6 (37.5%) patients presented bone metastasis as the first event.
The 3-year RFS rates of patients achieving pCR and near-pCR were 95.6% and 85.6%, respectively. The 5-year RFS rates of patients achieving pCR and near-pCR were 94.6% and 85.6%, respectively. The risk of cancer recurrence was significantly higher in patients achieving near-pCR than that in patients achieving pCR (HR = 3.01, 95% CI: 1.34–7.01, p = 0.008, Figure 1A). A total of 15 (5.1%) patients died. The 3-year OS rates of the pCR group and the near-pCR group were 96.6% and 96.3%, respectively. The 5-year OS rates of the pCR and near-pCR groups were 94.3% and 89.6%, respectively. There was no statistical difference in OS between the two cohorts (HR = 1.69, 95% CI: 0.61–4.67, p = 0.304, Figure 1B).
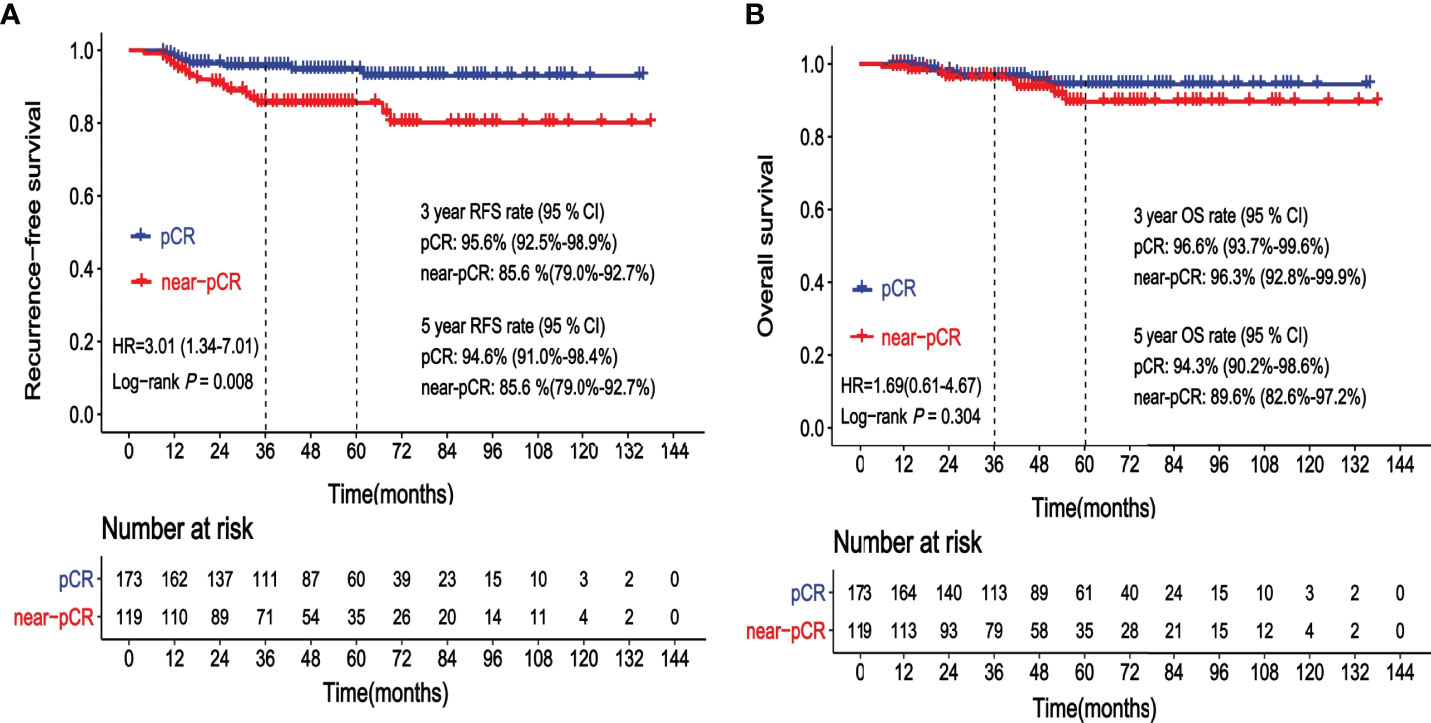
Figure 1 (A) Kaplan–Meier curve showing recurrence-free survival (RFS) according to the status after neoadjuvant chemotherapy (NAC): pathological complete response (pCR) vs. near-pathological complete response (near-pCR). (B) Kaplan–Meier curve showing overall survival (OS) according to the status after neoadjuvant chemotherapy (NAC): pathological complete response (pCR) vs. near-pathological complete response (near-pCR).
Table 3 shows the results of the analyses for factors associated with RFS of patients achieving pCR. Clinical lymph node status (cN) before NAC was a significant covariate in the univariate analysis for RFS in patients achieving pCR (p < 0.001). The 5-year RFS rates for cN0–2 and cN3 patients who achieved pCR were 98.0% and 82.7%, respectively. cN3 was an independent factor of higher risk for recurrence on the multivariate analysis (Figure 2, HR = 9.8, 95% CI: 2.1–44.5, p = 0.003). The C-statistics was 0.77 (95% CI: 0.63–0.91) of cN3 for RFS prediction. Age at diagnosis, tumor size at diagnosis, subtypes of breast cancer, and other factors showed no significant association with RFS of patients who achieved pCR (p > 0.05).
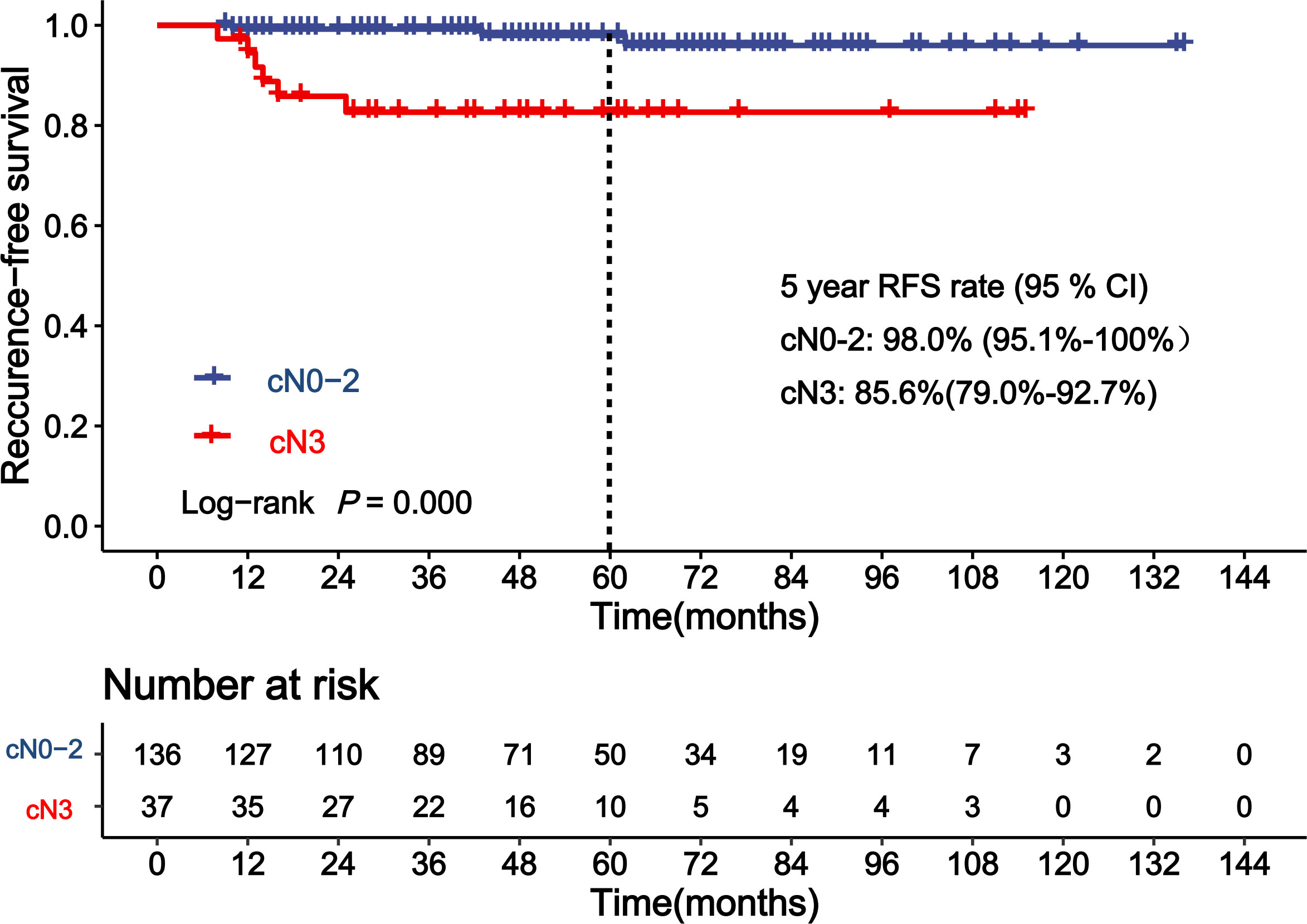
Figure 2 Kaplan–Meier curve showing recurrence-free survival (RFS) of patients achieving pathological complete response (pCR) according to clinical lymph node status (cN).
Table 4 shows the results of the analyses for the factors associated with RFS of patients achieving near-pCR. cN before NAC was a significant covariate in the univariate analysis for RFS in patients achieving near-pCR (Figure 3, p = 0.036). The 5-year RFS rates for cN0–2 and cN3 patients who achieved near-pCR were 88.5% and 71.1%, respectively. The C-statistics was 0.63 (95% CI: 0.52–0.74) of cN3 for RFS prediction. There was no difference between ypT1miN0, ypT1aN0, and ypT1bN0 for RFS (p = 0.942). The Miller–Payne grade after NAC also showed no significant association with the RFS of patients who achieved near-pCR (p > 0.05). There were no other factors significant at the 20% level in the univariate analyses of RFS for patients achieving near-pCR; thus, we did not conduct multivariate analyses further.
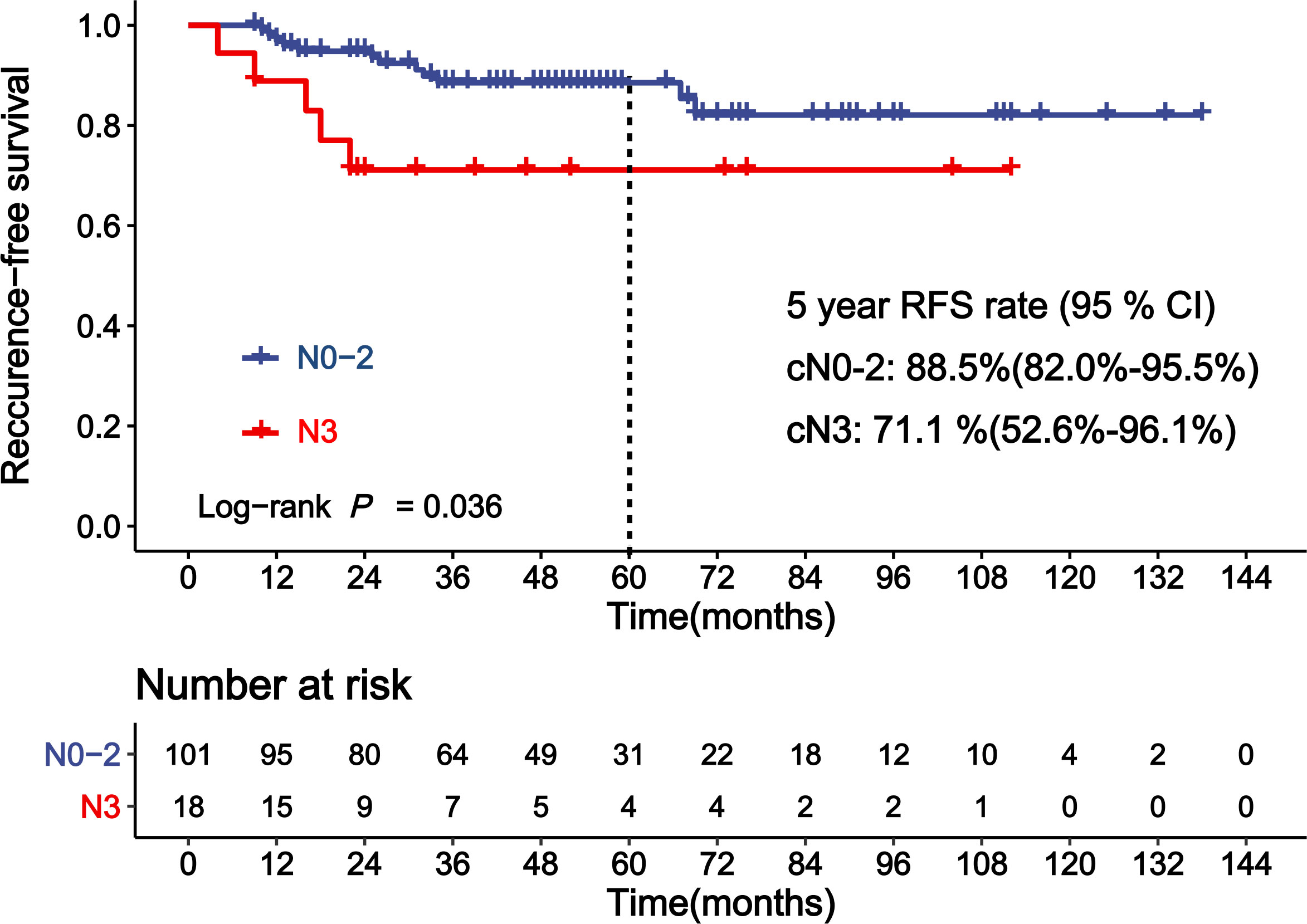
Figure 3 Kaplan–Meier curve showing recurrence-free survival (RFS) of patients achieving near-pCR according to clinical lymph node status (cN).
In this retrospective study of 292 patients achieving pCR and near-pCR after NAC, the recurrence pattern of patients was described, and the vast majority of recurrence occurred within 36 months from onset of NAC. This study found that the risk for recurrence of patients achieving near-pCR after NAC was higher than those achieving pCR. Moreover, cN3 before NAC was identified as a robust predictive factor of RFS for patients achieving pCR and near-pCR.
The 5-year RFS rate of pCR was 94.6% in our study, which was consistent with previous studies (25–27). The sub-study of EORTC 10994/BIG 1-00 phase III trial including 283 patients found that clinical tumor size was the only predictor for distant recurrence-free interval (DRFI) after pCR (27). In the research from the Anderson group, the authors identified that clinical stage IIIB–C and inflammatory breast cancer, premenopausal status, and resection of fewer than 10 lymph nodes were associated with an increased risk of developing distant metastasis for patients achieving pCR (28). Since cN contributes to the clinical stage, our study was partly consistent with the Anderson research. The predictive value of cN3 was also confirmed by C-statistics (0.77, 95% CI: 0.63–0.91) and Cox proportional hazards regression (cN3 vs. cN0–2, HR = 9.8, 95% CI: 2.1–44.5, p = 0.003). Asaoka and colleagues’ research also found that lymph node metastasis before NAC was the only predictor of cancer recurrence on multivariate analyses for patients achieving pCR (29).
The 5-year RFS rate of near-pCR was 85.6%, which was 9% lower than that of patients achieving pCR, but the OS of the two cohorts had no significant difference. The Spring et al. meta-analysis (30), which included 27,895 patients from 52 publications, showed that patients with residual disease after NAC had a 5-year DFS rate of 67%, which was much lower compared with the near-pCR population (85.6%) in our study. This illustrated the fact that it was necessary to distinguish the near-pCR population from the residual disease. There has been controversy regarding the definition of near-pCR in the past few years (31). In Cheng’s study, near-pCR was defined as residual tumor volume <1 cm3 (16). While residual tumor size ≤ 1 cm was excluded in KATHERINE, a clinical trial focused on intensive postoperative treatment (9). However, Lee and colleagues defined near-pCR as tumor size ≤ 0.5 cm (32). In our study, near-pCR was defined as the residual tumor size ≤1 cm in the breast (ypT1mi/1a/1bN0), or no residual invasive carcinoma in the breast yet existing micrometastasis in lymph node (ypT0/isN1mi).
To our best knowledge, this study is the first one to report the potential predictive factors of RFS for patients achieving near-pCR. We found that cN3 was an independent factor of higher risk for recurrence in the near-pCR subgroup, which was consistent with the pCR subgroup. The 5-year RFS rates for cN0–2 and cN3 patients who achieved near-pCR were 88.5% and 71.1%, respectively (p = 0.036). According to AJCC 8th edition staging system of breast cancer, cN3 is defined as metastasis to ipsilateral infraclavicular/supraclavicular lymph node(s), or metastasis to ipsilateral internal mammary lymph node(s) and axillary lymph node(s). There is controversy regarding the treatment of the local supraclavicular and internal mammary lymph node(s). It is difficult to remove the supraclavicular lymph node(s) and internal mammary lymph node(s) during the surgery. Radiation therapy is usually applied to deal with the supraclavicular and internal mammary lymph node(s) involvement. However, it is difficult to evaluate whether the status of no evidence of disease (NED) is reached. In recent years, growing interest was focused on post-NAC treatment, and some trials noted that reinforcing the adjuvant treatment could improve prognosis for patients with residual disease. In the subset of CREATE-X, TNBC patients with residual invasive disease who received capecitabine had a 5-year DFS rate of 69.8%, 13.7% higher than the control group (HR = 0.58, 95% CI: 0.39–0.87) (8). In the KATHERINE clinical trial, the invasive DFS at 3 years of HER2-positive breast cancer patients with residual invasive disease who received T-DM1 was 88.3%, higher than patients receiving trastuzumab (HR = 0.5, p < 0.001) (9). However, numerous post-NAC clinical trials incorporated patients with a residual disease of at least 1.0 cm or node positive disease, excluding patients who achieved near-pCR. Our study showed that patients with near-pCR still had a certain risk of recurrence. Adjuvant therapy to minimize the risk of recurrence for patients with near-pCR is needed to be illuminated in further prospective research.
Our study also has several limitations. First, it was a retrospective study; therefore, selection bias was inevitable. Second, because the number of death events was small, we did not conduct analysis on the predictive factors of OS in patients achieving pCR and near-pCR.
Patients achieving pCR had excellent outcomes. The recurrence risk of patients achieving near-pCR after NAC was higher than that of patients achieving pCR. The vast majority of recurrence occurred within 3 years from onset of NAC. Patients with cN3 before NAC had a higher risk of developing local and distant metastasis, even achieving pCR or near-pCR after NAC. It is worthwhile to closely monitor patients with cN3, especially in the first 3 years.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
PZ contributed to the study concept, design, and patient management, and revised the manuscript. XQ contributed to data collection and data analysis, and drafted the manuscript. MX revised the manuscript. QinL, JW, YF, YL, RC, QiaL, SC, PY, FM, BX participated in patient management. All authors approved the final version of the manuscript.
This project was funded by Cancer Prevention and Research Fund of China Medical Foundation and the fund supported the follow-up and publication costs.
The authors wish to thank all the study participants and research staff who participated in this work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Loibl S, Poortmans P, Morrow M, Denkert C, Curigliano G. Breast cancer. Lancet (2021) 397(10286):1750–69. doi: 10.1016/S0140-6736(20)32381-3
2. Loibl S, Gianni L. HER2-positive breast cancer. Lancet (2017) 389(10087):2415–29. doi: 10.1016/S0140-6736(16)32417-5
3. Harbeck N, Gluz O. Neoadjuvant therapy for triple negative and HER2-positive early breast cancer. Breast (2017) 34 Suppl 1:S99–S103. doi: 10.1016/j.breast.2017.06.038
4. Korde LA, Somerfield MR, Carey LA, Crews JR, Denduluri N, Hwang ES, et al. Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline. J Clin Oncol (2021) 39(13):1485–505. doi: 10.1200/JCO.20.03399
5. Zardavas D, Piccart M. Neoadjuvant therapy for breast cancer. Annu Rev Med (2015) 66:31–48. doi: 10.1146/annurev-med-051413-024741
6. Killelea BK, Yang VQ, Mougalian S, Horowitz NR, Pusztai L, Chagpar AB, et al. Neoadjuvant chemotherapy for breast cancer increases the rate of breast conservation: results from the national cancer database. J Am Coll Surg (2015) 220(6):1063–9. doi: 10.1016/j.jamcollsurg.2015.02.011
7. Volders JH, Negenborn VL, Spronk PE, Krekel NMA, Schoonmade LJ, Meijer S, et al. Breast-conserving surgery following neoadjuvant therapy-a systematic review on surgical outcomes. Breast Cancer Res Treat (2018) 168(1):1–12. doi: 10.1007/s10549-017-4632-7
8. Masuda N, Lee SJ, Ohtani S, Im YH, Lee ES, Yokota I, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med (2017) 376(22):2147–59. doi: 10.1056/NEJMoa1612645
9. von Minckwitz G, Huang CS, Mano MS, Loibl S, Mamounas EP, Untch M, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med (2019) 380(7):617–28. doi: 10.1056/NEJMoa1814017
10. Funt SA, Chapman PB. The role of neoadjuvant trials in drug development for solid tumors. Clin Cancer Res (2016) 22(10):2323–8. doi: 10.1158/1078-0432.CCR-15-1961
11. Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet (2014) 384(9938):164–72. doi: 10.1016/S0140-6736(13)62422-8
12. Berruti A, Amoroso V, Gallo F, Bertaglia V, Simoncini E, Pedersini R, et al. Pathologic complete response as a potential surrogate for the clinical outcome in patients with breast cancer after neoadjuvant therapy: a meta-regression of 29 randomized prospective studies. J Clin Oncol (2014) 32(34):3883–91. doi: 10.1200/JCO.2014.55.2836
13. Guerini-Rocco E, Botti G, Foschini MP, Marchio C, Mastropasqua MG, Perrone G, et al. Role and evaluation of pathologic response in early breast cancer specimens after neoadjuvant therapy: consensus statement. Tumori (2022) 108:196–203. doi: 10.1177/03008916211062642
14. Bossuyt V, Provenzano E, Symmans WF, Boughey JC, Coles C, Curigliano G, et al. And c. breast international group-north American breast cancer group, recommendations for standardized pathological characterization of residual disease for neoadjuvant clinical trials of breast cancer by the BIG-NABCG collaboration. Ann Oncol (2015) 26:1280–91. doi: 10.1093/annonc/mdv161
15. Bossuyt V, Symmans WF. Standardizing of pathology in patients receiving neoadjuvant chemotherapy. Ann Surg Oncol (2016) 23:3153–61. doi: 10.1245/s10434-016-5317-x
16. Cheng Q, Huang J, Liang J, Ma M, Ye K, Shi C, et al. The diagnostic performance of DCE-MRI in evaluating the pathological response to neoadjuvant chemotherapy in breast cancer: A meta-analysis. Front Oncol (2020) 10:93. doi: 10.3389/fonc.2020.00093
17. Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, et al. AJCC cancer staging manual[M]. 8th ed. New York: Springer (2017). Available at: https://link.springer.com/book/10.1007/978-1-4757-3656-4
18. Ogston KN, Miller ID, Payne S, Hutcheon AW, Sarkar TK, Smith I, et al. A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast (2003) 12:320–7. doi: 10.1016/s0960-9776(03)00106-1
19. Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of clinical Oncology/College of American pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol (2010) 28:2784–95. doi: 10.1200/JCO.2009.25.6529
20. Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical Oncology/College of American pathologists clinical practice guideline focused update. J Clin Oncol (2018) 36:2105–22. doi: 10.1200/JCO.2018.77.8738
21. Li XR, Liu M, Zhang YJ, Wang JD, Zheng YQ, Li J, et al. Evaluation of ER, PgR, HER-2, ki-67, cyclin D1, and nm23-H1 as predictors of pathological complete response to neoadjuvant chemotherapy for locally advanced breast cancer. Med Oncol (Northwood London England) (2011) 28 Suppl 1:S31–8. doi: 10.1007/s12032-010-9676-z
22. Alba E, Lluch A, Ribelles N, Anton-Torres A, Sanchez-Rovira P, Albanell J, et al. High proliferation predicts pathological complete response to neoadjuvant chemotherapy in early breast cancer. oncologist (2016) 21:778. doi: 10.1634/theoncologist.2015-0312erratum
23. Chen X, He C, Han D, Zhou M, Wang Q, Tian J, et al. The predictive value of ki-67 before neoadjuvant chemotherapy for breast cancer: a systematic review and meta-analysis. Future Oncol (2017) 13:843–57. doi: 10.2217/fon-2016-0420
24. Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ. And m. panel, strategies for subtypes–dealing with the diversity of breast cancer: highlights of the st. gallen international expert consensus on the primary therapy of early breast cancer 2011. Ann Oncol (2011) 22:1736–47. doi: 10.1093/annonc/mdr304
25. Mittendorf EA, Vila J, Tucker SL, Chavez-MacGregor M, Smith BD, Symmans WF, et al. The neo-bioscore update for staging breast cancer treated with neoadjuvant chemotherapy: Incorporation of prognostic biologic factors into staging after treatment. JAMA Oncol (2016) 2(7):929–36. doi: 10.1001/jamaoncol.2015.6478
26. Symmans WF, Wei C, Gould R, Yu X, Zhang Y, Liu M, et al. Long-term prognostic risk after neoadjuvant chemotherapy associated with residual cancer burden and breast cancer subtype. J Clin Oncol (2017) 35(10):1049–60. doi: 10.1200/JCO.2015.63.1010
27. Fei F, Messina C, Slaets L, Chakiba C, Cameron D, Bogaerts J, et al. Tumor size is the only predictive factor of distant recurrence after pathological complete response to neoadjuvant chemotherapy in patients with large operable or locally advanced breast cancers: a sub-study of EORTC 10994/BIG 1-00 phase III trial. Eur J Cancer (2015) 51(3):301–9. doi: 10.1016/j.ejca.2014.11.023
28. Gonzalez-Angulo AM, McGuire SE, Buchholz TA, Tucker SL, Kuerer HM, Rouzier R, et al. Factors predictive of distant metastases in patients with breast cancer who have a pathologic complete response after neoadjuvant chemotherapy. J Clin Oncol (2005) 23(28):7098–104. doi: 10.1200/JCO.2005.11.124
29. Asaoka M, Narui K, Suganuma N, Chishima T, Yamada A, Sugae S, et al. Clinical and pathological predictors of recurrence in breast cancer patients achieving pathological complete response to neoadjuvant chemotherapy. Eur J Surg Oncol (2019) 45(12):2289–94. doi: 10.1016/j.ejso.2019.08.001
30. Spring LM, Fell G, Arfe A, Sharma C, Greenup R, Reynolds KL, et al. Pathologic complete response after neoadjuvant chemotherapy and impact on breast cancer recurrence and survival: A comprehensive meta-analysis. Clin Cancer Res (2020) 26(12):2838–48. doi: 10.1158/1078-0432.CCR-19-3492
31. Cortazar P, Geyer CE Jr. Pathological complete response in neoadjuvant treatment of breast cancer. Ann Surg Oncol (2015) 22(5):1441–6. doi: 10.1245/s10434-015-4404-8
32. Lee HB, Han W, Kim SY, Cho N, Kim KE, Park JH, et al. Prediction of pathologic complete response using image-guided biopsy after neoadjuvant chemotherapy in breast cancer patients selected based on MRI findings: a prospective feasibility trial. Breast Cancer Res Treat (2020) 182(1):97–105. doi: 10.1007/s10549-020-05678-3
Keywords: breast cancer, pathological complete response, near-pathological complete response, survival, predictive factors
Citation: Qian X, Xiu M, Li Q, Wang J, Fan Y, Luo Y, Cai R, Li Q, Chen S, Yuan P, Ma F, Xu B and Zhang P (2022) Clinical N3 is an independent risk factor of recurrence for breast cancer patients achieving pathological complete response and near-pathological complete response after neoadjuvant chemotherapy. Front. Oncol. 12:1019925. doi: 10.3389/fonc.2022.1019925
Received: 15 August 2022; Accepted: 20 September 2022;
Published: 06 October 2022.
Edited by:
Raffaella Massafra, Bari John Paul II Cancer Institute (IRCCS), ItalyReviewed by:
Shengchun Liu, First Affiliated Hospital of Chongqing Medical University, ChinaCopyright © 2022 Qian, Xiu, Li, Wang, Fan, Luo, Cai, Li, Chen, Yuan, Ma, Xu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pin Zhang, enBwdW1jQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.