- 1Department of Oncology, Affiliated Hospital of Guilin Medical University, Guilin, China
- 2College of International Education of Guilin Medical University, Guilin, China
- 3Department of Radiotherapy, Affiliated Hospital of Guilin Medical University, Guilin, China
Novel anaplastic lymphoma kinase (ALK) fusions are still being discovered in non-small cell lung cancer (NSCLC). Most patients with ALK+ NSCLC respond favorably to ALK tyrosine kinase inhibitors. In this case report, we identified a novel nonreciprocal ALK fusion, namely, junctional sarcoplasmic reticulum protein 1 (JSRP1) intergenic region–ALK fusion (Jintergenic: A20) via next-generation sequencing in a female patient initially diagnosed with stage IV B lung adenocarcinoma. Further examination of biopsy specimen and analysis of clinical samples by a multidisciplinary team confirmed the diagnosis of ALK+ NSCLC. At the 2- and 4-months follow-up after receiving alectinib, the patient responded rapidly, implying that alectinib had a remarkable therapeutic effect. We identified a novel JSRP1 intergenic region–ALK fusion as a carcinogenic mutation that responds to alectinib, thereby expanding the spectrum of ALK fusion partners in ALK + NSCLC. This study may help clinicians detect oncogenic mutations and provide timely treatment to patients with ALK+ NSCLC.
Introduction
Lung cancer remains the leading cause of cancer-related deaths globally, accounting for 18% of all cancer-related deaths (1). In addition, 4–8% of patients with non-small cell lung cancer (NSCLC) harbor anaplastic lymphoma kinase (ALK) gene fusions, which are associated with diagnosis at a young age (2, 3). ALK tyrosine kinase inhibitors (TKIs) have shown remarkable effects on patients with ALK-rearranged NSCLC (4, 5). More than 90 distinct fusion partners have been found. Furthermore, 28 potential fusion partners due to intergenic ALK rearrangements have been discovered (6). ALK fusion is a heterogeneous biomarker that may vary in expression depending on its type (7, 8).
Case description
In December 2021, a 40-year-old Chinese woman, without a family history of cancer as well as no smoking history, presented in a community hospital with upper abdominal pain for 3 months. Computed tomography (CT) scans of her abdomen revealed multiple lesions of the liver, pancreas, and bilateral adrenal gland. Chest X-ray examination revealed a lung mass in the right lower lobe. A chest CT scan revealed multiple nodules, with the largest diameter measuring 27 mm and multiple lymph node involvement around the right hilum (Figure 1A). The patient visited our hospital for further examination on December 22, 2021. The Eastern Cooperative Oncology Group performance status was one, and physical examination revealed no abnormal signs. Enhanced magnetic resonance imaging of the brain showed no abnormalities. Emission CT revealed abnormal radioactive concentrations in the bone, which were then diagnosed as multiple bone metastases (Figure 1B). At the patient’s request, ultrasound-guided liver tumor biopsy was performed on December 23, 2021. A cytologic examination of the biopsy specimen showed adenocarcinoma cells (Figure 2A), which tested positive for cytokeratin 7 and thyroid transcription factor-1 and negative for Hepa in immunohistochemistry (IHC) (Figure 2B–D). Lung adenocarcinoma with stage IV (T1bN2M1c) was confirmed by a multidisciplinary team composed of a pathologist, a thoracic radiologist, and an oncologist.
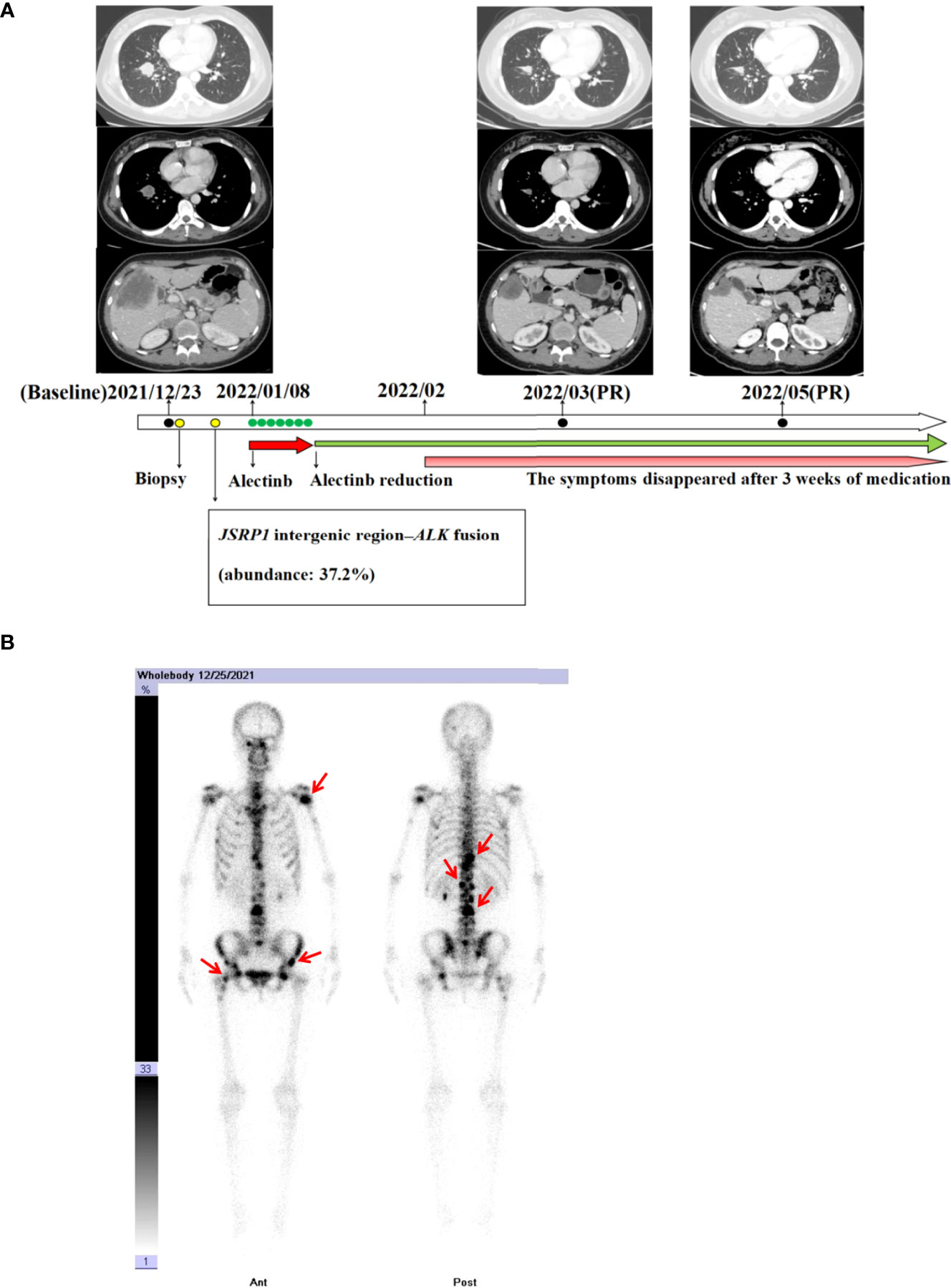
Figure 1 (A) Chest and abdomen computed tomography scans revealing the clinical response to alectinib. (B) Bone scan with technetium 99m-methyl diphosphonate prior to alectinib treatment revealing radioactive accumulation in the bones (red arrow).
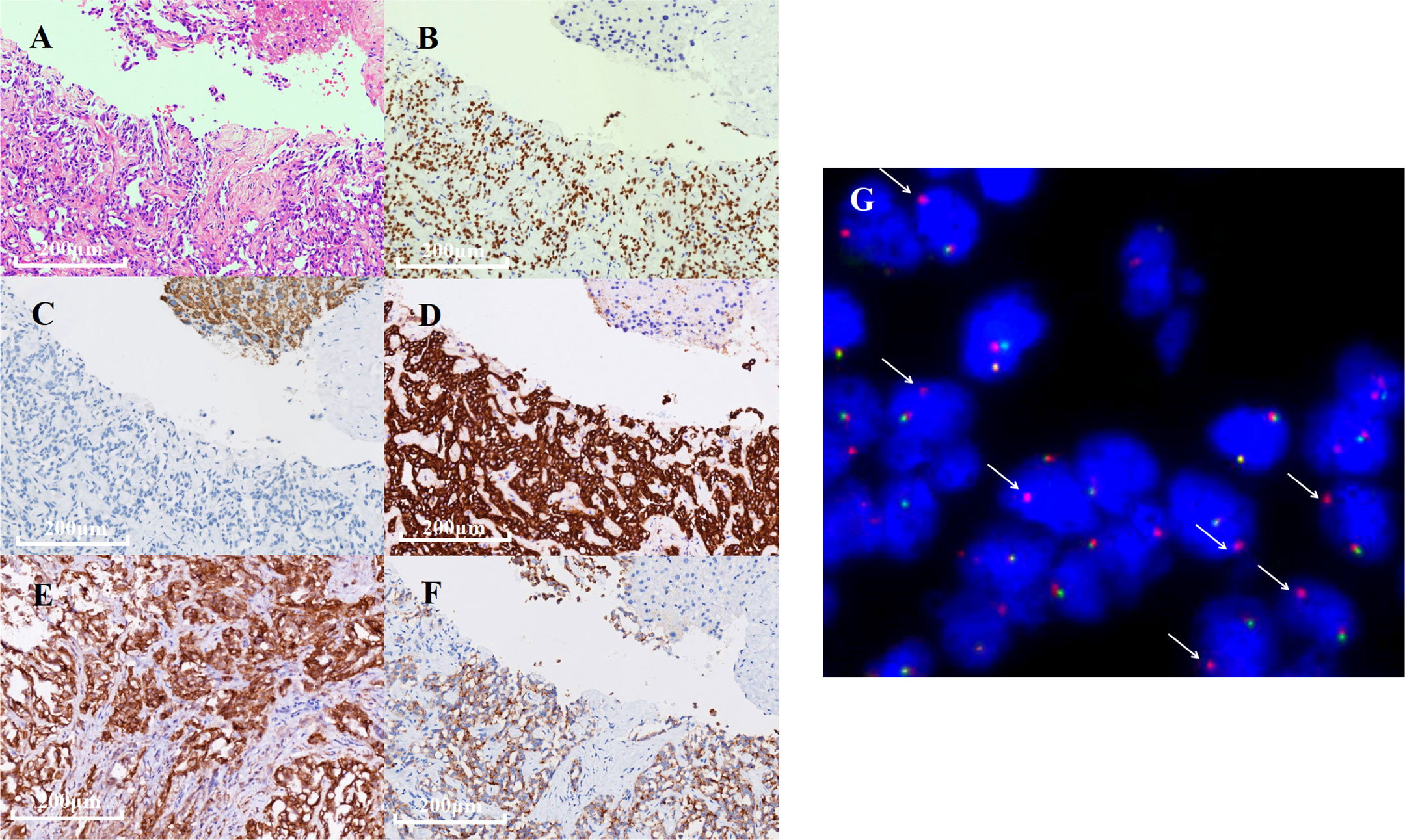
Figure 2 Immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH) results of the biopsy specimen. (A) Hematoxylin and eosin staining revealing an adenocarcinoma. (B–F) IHC results of TTF-1, Hepa, CK-7, PD-L1, and ALK expression detected by D5F3 IHC assay. (G) FISH showed ALK gene rearrangement positive signal (one red and one yellow, indicated by white arrows).
Next-generation sequencing (NGS) analysis of liver biopsy tissue was assessed by performing capture-based targeted deep sequencing using the Lung Core Panel (NextSeq 500 system, Illumina, Inc), covering the exons of 16 lung cancer-related genes (EGFR, ALK, RET, ROS1, MET, ERBB2, KRAS, BRAF, NTRK1, NTRK2, NTRK3, CDK4, CDK6, NRAS, PIK3CA, and TP53) (Guangxi Medical University REXPLO Medical Laboratory, Nanning, China). NGS revealed a novel ALK fusion, namely, junctional sarcoplasmic reticulum protein 1 (JSRP1) intergenic region–ALK fusion (Jintergenic: A20) (abundance: 37.2%). The microsatellite status was microsatellite instability-stable, and the tumor proportion score of PD-L1 (antibody: 28-8) was greater than 50% (Figure 2E). IHC (Ventana D5F3) and fluorescence in situ hybridization (FISH) of the biopsy specimen were performed, which confirmed a marked ALK fusion at the protein and DNA levels (Figures 2F, G).
Most ALK rearrangements are sensitive to ALK-TKI (6). The patient was orally administered 600 mg of alectinib twice daily in January 2022. The patient complained of significant fatigue and nausea 3 days after receiving alectinib. A week after initiation, alectinib administration was reduced to 450 mg orally twice daily. The symptoms disappeared after 3 weeks of medication. At the 2- and 4-months follow-up, a CT scan revealed a remarkable reduction of the tumors in the chest, liver, pancreas, adrenal gland, and mediastinal lymph node (Figure 1A). Partial response was achieved in accordance with the Response Evaluation Criteria in Solid Tumors version 1.1.
Written informed consent for submitting this case report was obtained from the patient.
Discussion
ALK rearrangements are common driver genes in NSCLC (9). With the increasing adoption of NGS for molecular profiling of NSCLC samples, more novel fusions and their corresponding efficacy on ALK-TKI have been reported (6). In this report, we identified a novel ALK gene fusion, JSRP1–ALK (Jintergenic: A20), via NGS and confirmed its efficacy using IHC and FISH assay. Subsequently, patient harboring this novel fusion was treated with alectinib, which exerted a remarkable effect in a short period.
JSRP1 is one of the proteins constituting the junctional face membrane with an apparent molecular mass of 45 kDa. It is critical in the process of functional expression of voltage-dependent Ca2+ channels (10, 11). In this report, JSRP1 intergenic region rearranged to exons 20–29 of ALK, retaining its intact domain (Figure 3A). Then, exons 1–19 of ALK rearranged to exon 2 of LINC00211 (A19: LINC00211) (Figure 3B). Based on the structure of the fusion products (Figure 3C), we speculated that the 3ʹ-ALK fusion product possesses kinase activity. Ying et al. found that uncommon ALK fusions detected using DNA NGS are an unreliable predictor of matched targeted therapy efficacy (12). In order to exclude the discordance of NGS, FISH, and IHC results caused by the complex biological mechanisms involved in transcriptional or post-transcriptional processes, we conducted FISH and IHC to confirm the positive ALK gene break and the marked ALK fusion protein expression in the tumor specimen. As per a previous study (13), we hypothesized that the JSRP1 intergenic region possibly generates an N-terminal-derived product that leads to the constitutive expression of ALK. According to the consistency of DNA NGS, FISH, and IHC results, and the good response of the patient to alectinb, we speculated that the JSRP1–ALK (Jintergenic: A20) fusion is a carcinogenic mutation in this case. Further studies should be conducted to confirm the biological function of the JSRP1–ALK (Jintergenic: A20) fusion in tumorigenesis. However, the biological function of 5ʹ-ALK fusion translocation products remains unclear.
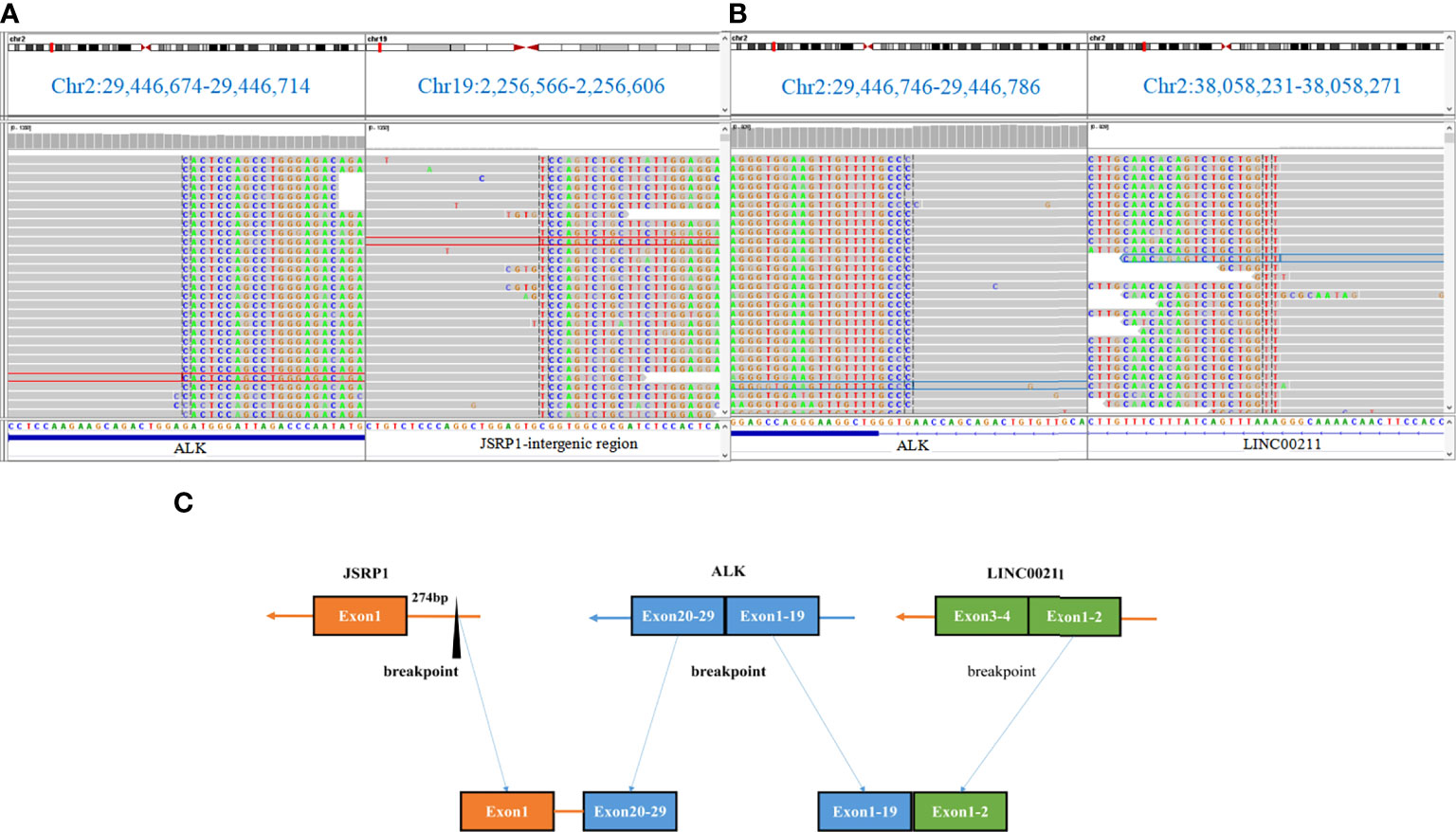
Figure 3 DNA-based next-generation sequencing findings for paraffin-embedded specimen of the patient. (A, B) The novel non-reciprocal JSRP1-ALK (Jintergenic:A20) rearrangement visualized using the Integrative Genomics Viewer.(C) Diagram depicting the non-reciprocal ALK fusion.
There are many studies and case reports on the treatment of NSCLC due to ALK rearrangements with crizotinib as it is the first ALK-TKI approved by the Food and Drug Administration for ALK-positive NSCLC patients. Zhang et al. reported that harboring non-reciprocal translocation is a poor predictive marker in patients with ALK-rearranged NSCLC treated with first-line crizotinib. Further, patients with non-reciprocal/reciprocal ALK translocation were reported to have a higher incidence of brain metastasis at baseline than those with classic 3ʹ-ALK fusion (7). Conversely, the biopsy sample had a high level of PD-L1 expression (PD-L1 TPS of > 50%). Yang et al. revealed that positive PD-L1 expression was associated with unfavorable clinical outcomes in patients with ALK positive lung adenocarcinoma receiving crizotinib (14). Studies have shown that different fusion modalities may have inconsistent responses to crizotinib (7, 8). Therefore, there may have not been a long survival benefit had crizotinib been given to this patient.
Compared with those receiving crizotinib, patients receiving alectinib, ceritinib, brigatinib, or lorlatinib showed better response and had considerably longer progression-free survival (PFS) in advanced ALK-positive NSCLC (15–18). The case we presented here had PFS for more than five months till date. Whether alectinib is more effective than crizotinb in rare ALK rearrangements requires more research. In addition, the resistance mechanism of rare ALK fusion to ALK-TKI is rarely reported. Further clinical observation is required to further improve the efficacy of ALK-TKI in patients with novel ALK rearrangements.
Conclusion
We identified a novel JSRP1 intergenic region–ALK fusion, expanding the spectrum of ALK fusion partners in patients with ALK + NSCLC. In addition, we found that alectinib exerted a remarkable and rapid therapeutic effect on the patient harboring this particular ALK fusion.
Data availability statement
The datasets presented in this article are not readily available because of ethical/privacy restrictions. Requests to access the datasets should be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by ethics committee of Affiliated Hospital of Guilin Medical University. The patient provided her written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
FX: Conceptualization and writing - original draft. LZ: Supervision and data curation. SX and CJ: Validation and editing of the figures. MK and MU: Writing - review and editing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (Grant no. 82160471) and Beijing Xisike Clinical Oncology Research Foundation (Y-QL202101-0214).
Acknowledgments
We would like to thank the patient and her family members. We are also grateful for Jun Lu from Shanghai Yichuang Yunkang Biotechnology for their kind assistance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Sacher AG, Dahlberg SE, Heng J, Mach S, Jänne PA, Oxnard GR. Association between younger age and targetable genomic alterations and prognosis in non-Small-Cell lung cancer. JAMA Oncol (2016) 2(3):313–20. doi: 10.1001/jamaoncol.2015.4482
3. Shimizu J, Masago K, Saito H, Nishino K, Kurata T, Itoh Y, et al. Biomarker testing for personalized, first-line therapy in advanced nonsquamous non-small cell lung cancer patients in the real world setting in Japan: a retrospective, multicenter, observational study (the BRAVE study). Ther Adv Med Oncol (2020) 12:1758835920904522. doi: 10.1177/1758835920904522
4. Wu YL, Lu S, Lu Y, Zhou J, Shi YK, Sriuranpong V, et al. Results of PROFILE 1029, a phase III comparison of first-line crizotinib versus chemotherapy in East Asian patients with ALK-positive advanced non-small cell lung cancer. J Thorac Oncol (2018) 13(10):1539–48. doi: 10.1016/j.jtho.2018.06.012
5. Camidge DR, Kim HR, Ahn MJ, Yang JCH, Han JY, Hochmair MJ, et al. Brigatinib versus crizotinib in ALK inhibitor-naive advanced ALK-positive NSCLC: Final results of phase 3 ALTA-1L trial. J Thorac Oncol (2021) 16(12):2091–108. doi: 10.1016/j.jtho.2021.07.035
6. Ou SI, Zhu VW, Nagasaka M. Catalog of 5' fusion partners in ALK-positive NSCLC circa 2020. JTO Clin Res Rep (2020) 1:100015. doi: 10.1016/j.jtocrr.2020.100015
7. Zhang Y, Zeng L, Zhou C, Li Y, Wu L, Xia C, et al. Detection of Nonreciprocal/Reciprocal ALK translocation as poor predictive marker in patients with first-line crizotinib-treated ALK-rearranged NSCLC. J Thorac Oncol (2020) 15:1027–36. doi: 10.1016/j.jtho.2020.02.007
8. Sun K, Nie L, Nong L, Cheng Y. Primary resistance to alectinib in a patient with STRN-ALK-positive non-small cell lung cancer: A case report. Thorac Cancer (2021) 12:1927–30. doi: 10.1111/1759-7714.13983
9. Solomon B, Varella-Garcia M, Camidge DR. ALK gene rearrangements: A new therapeutic target in a molecularly defined subset of non-small cell lung cancer. J Thorac Oncol (2009) 4:1450–4. doi: 10.1097/JTO.0b013e3181c4dedb
10. Zorzato F, Anderson AA, Ohlendieck K, Froemming G, Guerrini R, Treves S. Identification of a novel 45 kDa protein (JP-45) from rabbit sarcoplasmic-reticulum junctional-face membrane. Biochem J (2000) 351 Pt 2:537–43. doi: 10.1042/bj3510537
11. Anderson AA, Altafaj X, Zheng Z, Wang ZM, Delbono O, Ronjat M, et al. The junctional SR protein JP-45 affects the functional expression of the voltage-dependent Ca2+ channel Cav1.1. J Cell Sci (2006) 119:2145–55. doi: 10.1242/jcs.02935
12. Li W, Guo L, Liu Y, Dong L, Yang L, Chen L, et al. Potential unreliability of uncommon ALK, ROS1, and RET genomic breakpoints in predicting the efficacy of targeted therapy in NSCLC. J Thorac Oncol (2021) 16:404–18. doi: 10.1016/j.jtho.2020.10.156
13. Katayama R, Lovly CM, Shaw AT. Therapeutic targeting of anaplastic lymphoma kinase in lung cancer: a paradigm for precision cancer medicine. Clin Cancer Res (2015) 21:2227–35. doi: 10.1158/1078-0432.CCR-14-2791
14. Yang CY, Liao WY, Ho CC, Chen KY, Tsai TH, Hsu CL, et al. Association of programmed death-ligand 1 expression with fusion variants and clinical outcomes in patients with anaplastic lymphoma kinase-positive lung adenocarcinoma receiving crizotinib. Oncologist (2020) 25:702–11. doi: 10.1634/theoncologist.2020-0088
15. Fontana D, Ceccon M, Gambacorti-Passerini C, Mologni L. Activity of second-generation ALK inhibitors against crizotinib-resistant mutants in an NPM-ALK model compared to EML4-ALK. Cancer Med (2015) 4:953–65. doi: 10.1002/cam4.413
16. Soria JC, Tan D, Chiari R, Wu YL, Paz-Ares L, Wolf J, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): A randomised, open-label, phase 3 study. Lancet (2017) 389:917–29. doi: 10.1016/S0140-6736(17)30123-X
17. Camidge DR, Kim HR, Ahn MJ, Yang JCH, Han JY, Hochmair MJ, et al. Brigatinib versus crizotinib in advanced ALK inhibitor-naive ALK-positive non-small cell lung cancer: Second interim analysis of the phase III ALTA-1L trial. J Clin Oncol (2020) 38:3592–603. doi: 10.1200/JCO.20.00505
Keywords: intergenic region-ALK fusion, non-small cell lung cancer, alectinib, targeted therapy, case report
Citation: Xue F, Xu S, Jiang C, Kang M, Usman M and Zhu L (2022) Case report: Novel junctional sarcoplasmic reticulum protein 1 intergenic region–anaplastic lymphoma kinase fusion in a patient with lung adenocarcinoma responds to alectinib. Front. Oncol. 12:1019624. doi: 10.3389/fonc.2022.1019624
Received: 17 August 2022; Accepted: 20 September 2022;
Published: 04 October 2022.
Edited by:
Kohei Fujita, National Hospital Organization Kyoto Medical Center, JapanReviewed by:
Junichi Shimizu, Aichi Cancer Center, JapanChiao-En Wu, Linkou Chang Gung Memorial Hospital, Taiwan
Copyright © 2022 Xue, Xu, Jiang, Kang, Usman and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lin Zhu, ZG9jdG9yX3psMDMwMUAxNjMuY29t
 Feng Xue1
Feng Xue1 Muhammad Usman
Muhammad Usman Lin Zhu
Lin Zhu