- 1Department of Urology, The Third Medical Center, Chinese PLA General Hospital, Beijing, China
- 2Department of Urology, No.971 Hospital of PLA Navy, Tsingtao, Shandong, China
- 3Department of Postgraduate, Hebei North University, Zhangjiakou, Hebei, China
- 4Department of Urology, Shanxi Medical University, Taiyuan, Shanxi, China
- 5Affiliated Hospital of Weifang Medical University, School of Clinical Medicine, Weifang Medical University, Weifang, China
Purpose: Posttransplant skin cancer is the most common malignancy after patients have undergone renal transplantation. Through comprehensive observation with a large sample size nationwide, understanding the risk factors and outcome of posttransplant skin cancer will help to develop appropriate patient surveillance and disease prevention strategies.
Materials and methods: This retrospective population-based cohort study was based on Organ Procurement and Transplantation Network data released in March 2021. Characteristics and outcomes, including patient survival and graft survival of recipients, were compared. Risk factors for posttransplant skin cancer, cancer onset momentum, and mortality were determined.
Results: A total of 199,564 renal transplant recipients were included. After renal transplantation, 7,334 (3.68%), 6,093 (3.05%), and 936 (0.47%) were diagnosed with squamous cell carcinoma, basal cell carcinoma, and melanoma, respectively. Skin cancer was the major cause of death (squamous cell carcinoma: 23.8%, basal cell carcinoma: 18%, and melanoma: 41.6%). Five-year survival rates ranked from best to worst were as follows: basal cell carcinoma (96.7 [95% confidence interval: 96.3–97.2]%), squamous cell carcinoma (94.1 [93.5–94.6]%), melanoma (89.7 [87.7–91.6]%), and cancer-free (87.4 [87.2–87.5]%) (p < 0.001 for all except melanoma vs. cancer-free, p = 0.534). Regarding graft survival, death-censored graft survival, posttransplant skin cancer, and melanoma were significantly better than the cancer-free group (p < 0.001). Independent risk factors for developing posttransplant skin cancer included older age, male sex, Caucasian race, pretransplant malignancy, polycystic kidney disease-induced end-stage renal disease (ESRD), retransplantation, private health insurance, T-cell depletion induction, and tacrolimus/mycophenolic acid use. Caucasian race and pretransplant malignancy were independent risk factors for posttransplant skin cancer onset momentum. Male sex, Caucasian race, pretransplant malignancy, hypertension- or diabetes-induced ESRD, retransplantation, diabetes history, deceased donor, cyclosporin, and mTOR inhibitor use were independent risk factors for posttransplant skin cancer mortality.
Conclusion: Although posttransplant skin cancer is a major cause of recipient death, information regarding its impact on patient and graft survival is limited. Given the differences regarding risk factors for posttransplant skin cancer incidence, onset momentum, and mortality, personalized approaches to screening may be appropriate to address the complex issues encountered by kidney transplant recipients.
Introduction
Renal transplantation (RT) is the most effective treatment for end-stage renal disease (ESRD). However, posttransplant malignancy is the leading cause of death and allograft loss, reducing the recipient’s lifespan and requiring heightened surveillance (1–3). Skin cancer, commonly categorized as non-melanoma skin cancer (NMSC) and melanoma according to tumor incidence, recurrence, metastasis, and aggressiveness (4, 5), represents a major threat to RT recipients. NMSC, primarily squamous cell carcinoma (SCC) and basal cell carcinoma (BCC), is the most frequent posttransplant malignancy, with a total incidence of 7.5%, which is up to 100-fold greater than that in the general population (6–11). Melanoma has a posttransplant incidence 1.5–2.5 times greater than that during dialysis (12–15) and is associated with high mortality and metastasis, comprising only 4% of cutaneous malignancies, but accounting for 80% of skin cancer deaths in the general population (16, 17).
Several studies have been conducted on posttransplant skin cancer (PTSC). Ponticelli et al. reviewed PTSC regarding its histopathological classification, characteristics, and treatment (18). In the general population, SCC is the second most common cancer in Caucasians, outnumbered by BCC at a 4:1 ratio (19). However, in transplant recipients, this ratio is reversed; the incidence of SCC is higher, followed by BCC (16, 17). This may reflect different pathogenic mechanisms underlying the development of these tumors in kidney transplant recipients. Ascha et al. highlighted the risk factors for melanoma and reported a greater risk of melanoma in American RT recipients than in the general population (20). Immunosuppressive therapy, skin color, and viral infection are widely recognized risk factors for skin cancer (17). Immunosuppressive agents are known to accelerate melanoma development in transplant recipients (21), whereas NSMC is closely related to sun exposure [especially ultraviolet (UV) radiation] (6, 22). Genetic factor as a fundamental cause of oncogenic activity should not be ignored. Laing et al. revealed that methylene tetrahydrofolate reductase (MTHFR) polymorphism plays a critical role in the elevated rate of SCC in renal transplant recipients (23, 24). Nevertheless, research in this field has mainly focused on the prevalence and risk factors for skin cancer after RT, but has not explored further aspects. Other studies were limited to one kind of skin cancer, NMSC or melanoma, and lack comprehensive comparison of different kinds of skin cancer.
Hence, we conducted a retrospective population-based cohort study using data from the Organ Procurement and Transplantation Network (OPTN) that includes three pathological types of PTSC. The study aimed to compare the outcomes of recipients and grafts and to identify risk factors for PTSC. We explore the impact of risk factors on PTSC development and outcomes. Our findings will improve our understanding of PTSC and could aid in the development of strategies to prevent or treat skin cancers in RT recipients.
Methods
Data source, study design, and participants
We analyzed data from the OPTN Standard Transplant Analysis and Research file released in March 2021. This is a retrospective population-based cohort study that included all adult kidney transplant recipients who underwent transplantation between 2000 and 2014 in the United States. Recipients who were <18 years old; who were ABO incompatible; who received multiorgan transplants, primary non-functional grafts, and other confirmed post-transplant malignancies; and who underwent en bloc kidney transplantation were excluded from the analysis (Figure 1). This paper is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does the mention of trade names, commercial, or organizations imply endorsement by the U.S. government.
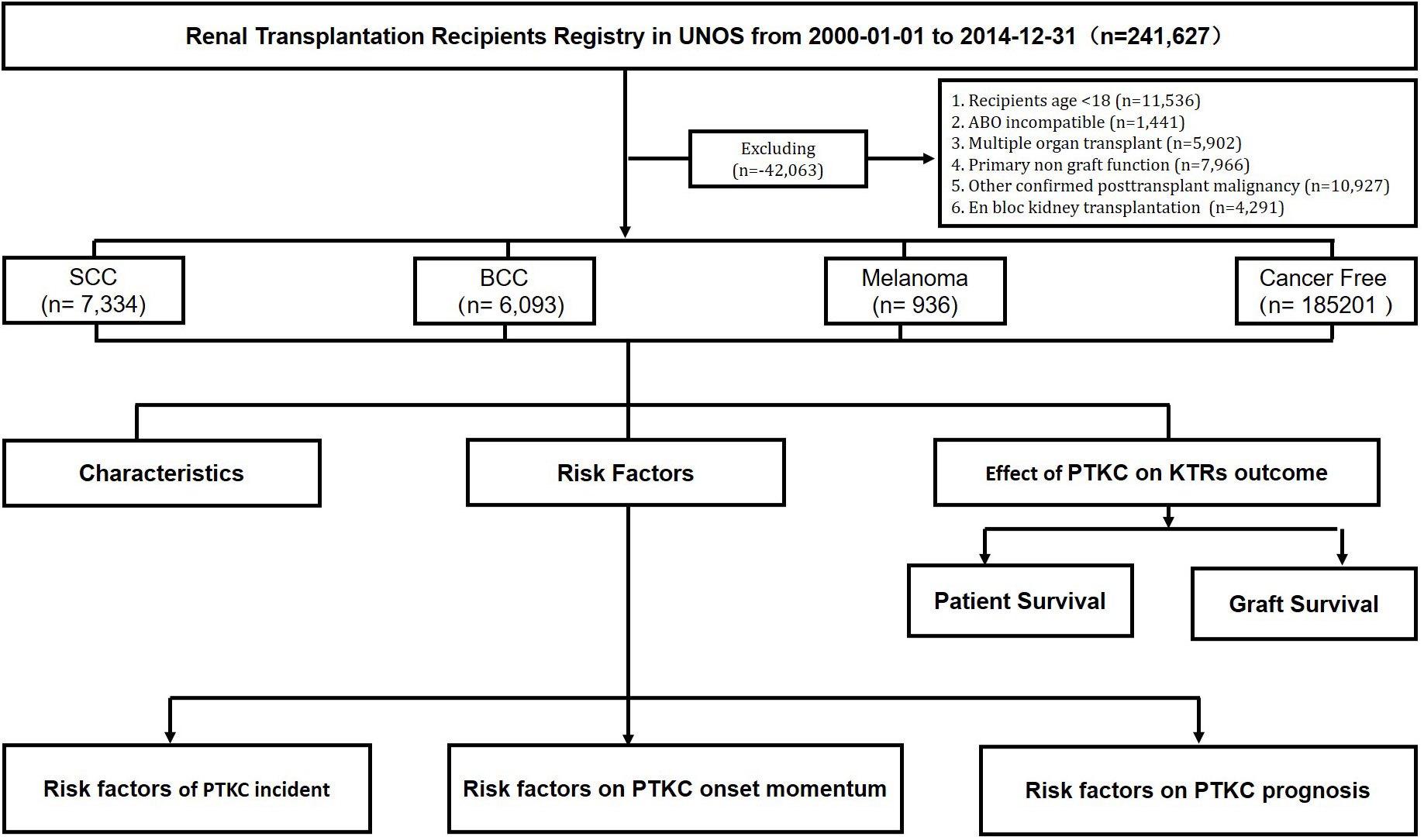
Figure 1 Flowchart of study cohort identification. SCC, squamous cell carcinoma; BCC, basal cell carcinoma. All kidney transplant recipients from 1 January 2000 to 31 December 2014 were followed from the date of transplantation to the date of outcome, censored for loss to follow-up, or end of the study period.
Exposure and outcomes classification and assessment
Time to outcome was defined as the date from transplantation until the date of the specified outcome (patient death or graft failure), censored for loss to follow-up, or the end of the study period. Outcomes included patient survival (PS), graft survival (GS), and death-censored GS (DCGS). The patients were divided into SCC, BCC, melanoma, and cancer-free (control) groups (Figure 1).
Statistical analysis
Patient demographics and clinical characteristics were compared using the chi-square test for categorical variables and Student’s t-test for continuous variables; the distributions of the variables approximated normality, matched by the probability of skin cancer exposure based on a multivariable logistic regression model with odds ratios. The survival analysis is presented as Kaplan–Meier curves and compared using log-rank tests. The impact of potential skin cancer risk factors on cancer onset momentum was accounted for in the multivariable linear regression model. Cox proportional hazards models were fitted to estimate hazard ratios for skin cancer patient outcomes after adjusting for most potential confounders. Lasso regression was used to further estimate the optimal value for penalization coefficient lambda 22 confounders: (AGE+GENDER+ETHCAT_FACTOR+BMI_CALC_FACTOR+MALIG_HISTORY_FACTOR+DIAG_KI_FACTOR+ABO_FACTOR+DIALYSIS_DURATION+NUM_PREV_TX_FACTOR+DIAB_FACTOR+PRI_PAYMENT_TRR_KI_FACTOR+AGE_DON+GENDER_DON+ETHCAT_DON_FACTOR+BMI_DON_CALC_FACTOR+DON_TY_FACTOR+INDUCTION_THERAPY_AT_DISCHARGE_IL2RA+INDUCTION_THERAPY_AT_DISCHARGE_TCELL+MAINTENANCE_THERAPY_AT_DISCHARGE_CSA+MAINTENANCE_THERAPY_AT_DISCHARGE_TAC+MAINTENANCE_THERAPY_AT_DISCHARGE_MPA+MAINTENANCE_THERAPY_AT_DISCHARGE_MTOR) was selected for the prediction model. All analyses were performed using RStudio software version 1.1.456 (R. RStudio, Inc., Boston, MA, USA). Statistical significance was set at p < 0.05, and all confidence intervals were set at a 95% threshold. Descriptive statistics were used to summarize and present the data.
Results
From January 2000 to December 2014, 241,627 patients received renal transplant registration in the United Network for Organ Sharing (UNOS). A total of 199,564 RT recipients met the inclusion criteria, of whom 7,334 (3.68%), 6,093 (3.05%), and 936 (0.47%) were diagnosed with SCC, BCC, and melanoma after RT, respectively.
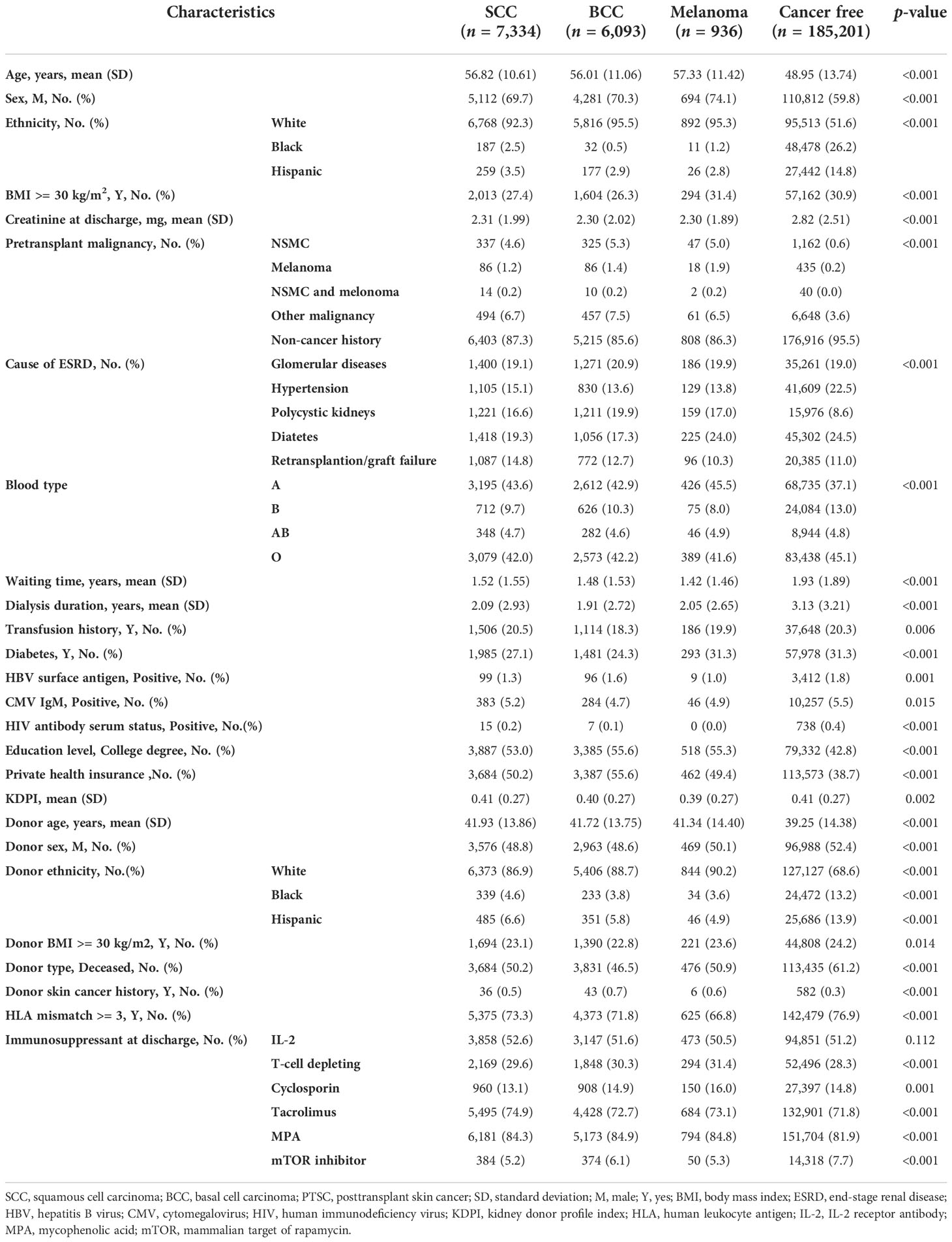
Compared with the cancer-free group, cancer groups were older (56.01–57.33 vs. 48.95 years), were more male (69.7%–74.1% vs. 59.8%), had a higher proportion of Caucasians (92.3%–95.5% vs. 51.6%), had lower creatinine at discharge (2.30 vs. 2.82 mg/dl), had more pretransplant malignancy history (12.7%–13.7% vs. 4.5%), and had more polycystic kidney disease (PKD)-induced ESRD (16.6%–19.9% vs. 8.6%), but fewer had diabetes (17.3%–24% vs. 24.5%)- or hypertension (13.6%–15.1% vs. 22.5%)-induced ESRD, had more blood type A (42.9%–45.5% vs. 37.1%) and type B (8%–10.3% vs. 13%) but less type O (41.6%–42.2% vs. 45.1%), had a shorter waiting time (1.42–1.52 vs. 1.93 years) and dialysis duration (1.91–2.09 vs. 3.13 years), had less diabetes history (24.3%–31.3% vs. 31.3%), had less viral infection history including hepatitis B virus (HBV; 1%–1.6% vs. 1.8%), cytomegalovirus (CMV; 4.7%–5.2% vs. 5.5%), and human immunodeficiency virus (HIV; 0%–0.2% vs. 0.4%), had a higher proportion of college degrees (53%–55.6% vs. 42.8%), and had more private insurance (49.4%–55.6% vs. 38.7%). Regarding donors, cancer groups exhibited lower Kidney Donor Profile Index scores (KDPI; 0.39–0.41 vs. 0.41), were older (41.72–41.93 vs. 39.25, years), were not male (48.8%–50.1% vs. 52.4%), had a significantly higher proportion of Caucasians (86.9%–90.2% vs. 68.6%), had less obesity (22.8%–23.6% vs. 24.2%), had less deceased donors (46.5%–50.9% vs. 61.2%), had more donor skin cancer history (0.5%–0.7% vs. 0.3%), and had less human leukocyte antigen (HLA) mismatch (66.8%–73.3% vs. 76.9%). With regard to immunosuppressant therapy at discharge, cancer groups used less T-cell-depleting antibodies (29.6%–31.4% vs. 28.3%) and mTOR inhibitors (5.2%–6.1% vs. 7.7%), but more tacrolimus (72.7%–74.9% vs. 71.8%) and mycophenolic acid (MPA; 84.3%–84.9% vs.81.9%) (Table 1).
Melanoma patients were the oldest (57.33 [11.42] years), were mostly male (74.1%), were the most obese (31.4%), had the most pretransplant malignancy (13.7%) and skin cancer (6.9%) histories, had a history of diabetes (31.3%), had the lowest KDPI (0.39 [0.27]), had the lowest HLA mismatch (66.8%), and had the lowest acute rejection incidence rate (0.7%). Among NSMC, there were no significant differences between baseline SCC and BCC rates (Table 1).
The skin color of the patients was associated with the incidence of skin cancer. The proportion of Caucasian patients with skin cancer was 40% higher than that in cancer-free patients. Interestingly, in the donor cohort, the proportion of Caucasians in the skin cancer group was 20% higher than that in the cancer-free group (Table 1). To clarify the significant proportion difference in donor ethnicity, we analyzed the proportion of recipient ethnicity who received Caucasians’ donor kidney. Compared to the cancer-free group, there is an obviously higher proportion of Caucasians who received kidney from a Caucasian donor (95.8%–97.1% vs. 65.7%), while a lower proportion of Blacks received kidney from a Caucasian donor in the skin cancer group (0.6%–1.5% vs. 20.6%). Furthermore, in both living donors and deceased donors, Caucasian recipients in skin cancer groups are more likely to receive a Caucasian donor kidney than those in the cancer-free group (living donor, 51.4%–57.2% vs. 34.5%; deceased donor, 40.0%–45.3% vs. 31.1%) (Supplementary Table 1). Regarding proportion of cancer type in ethnic groups, the most common type of PTSC in Blacks is SCC (81.3%). Pretransplant malignancy was also a risk factor for skin cancer incidence after transplantation. The incidence rate of skin cancer history in the cancer group was 5% higher than that in the cancer-free group and nearly 10% higher in terms of total malignancy history (Table 1).
Effect of de novo kidney cancer on kidney transplant outcome
At the most recently reported follow-up, compared with the cancer-free group, the PTSC group had the highest skin cancer mortality (23.8%, 18%, and 41.6%) and exhibited a significantly lower incidence of delayed graft function and acute rejection, a longer follow-up period, and a higher death rate. SCC had the slowest cancer onset speed (6.0 [3.5, 9.2] years). BCC showed the longest follow-up period (IQR, median, 10.7 [7.7, 14.0] years) and lowest death rate (25.3%), even lower than those of cancer-free patients. Melanoma presented the worst outcome, including the shortest follow-up period (9.4 [6.8, 12.9] years), the fastest speed of cancer onset (4.9 [2.8, 8.2] years), and the highest death rate (42.6%). Melanoma displayed the lowest rate of graft function, while cancer-free patients exhibited the highest rate of graft failure (Table 2).
Kaplan–Meier curves depict that BCC and SCC had the best outcomes for both patients and grafts of the four groups, whereas cancer-free patients had the worst outcomes (Figure 2). The PS between SCC, BCC, melanoma, and cancer-free patients showed statistically significant differences. p-values of pairwise comparisons between the four groups also confirmed significant differences in crude survival (p < 0.001 for each pair), except cancer-free and melanoma (p = 0.534). The 5-year survival rates of the four groups ranked from best to worst were as follows: BCC (96.7 [95% confidence interval: 96.3–97.2]%), SCC (94.1 [93.5–94.6]%), melanoma (89.7 [87.7–91.6]%), and cancer-free (87.4 [87.2–87.5]%) (Figure 2A). Regarding GS, BCC (95.6 [95.1–96.2]%), SCC (92.8 [92.2–93.4]%), melanoma (88.4 [86.3–90.4]%), and cancer-free (78.9 [78.7–79.1]%) also displayed significant differences (p < 0.001 for each pairwise comparison). BCC and SCC still ranked as the first two best GSs, but melanoma exceeded the cancer-free group (Figure 2B). DCGS ranking of the four groups was parallel to GS; however, the p-values for pairwise comparisons between each cancer group increased. The differences between the three cancer groups were smaller (p = 0.052, p = 0.668, and p = 0.017), whereas the differences between the cancer groups and cancer-free groups were greater (p < 0.001) (Figure 2C).
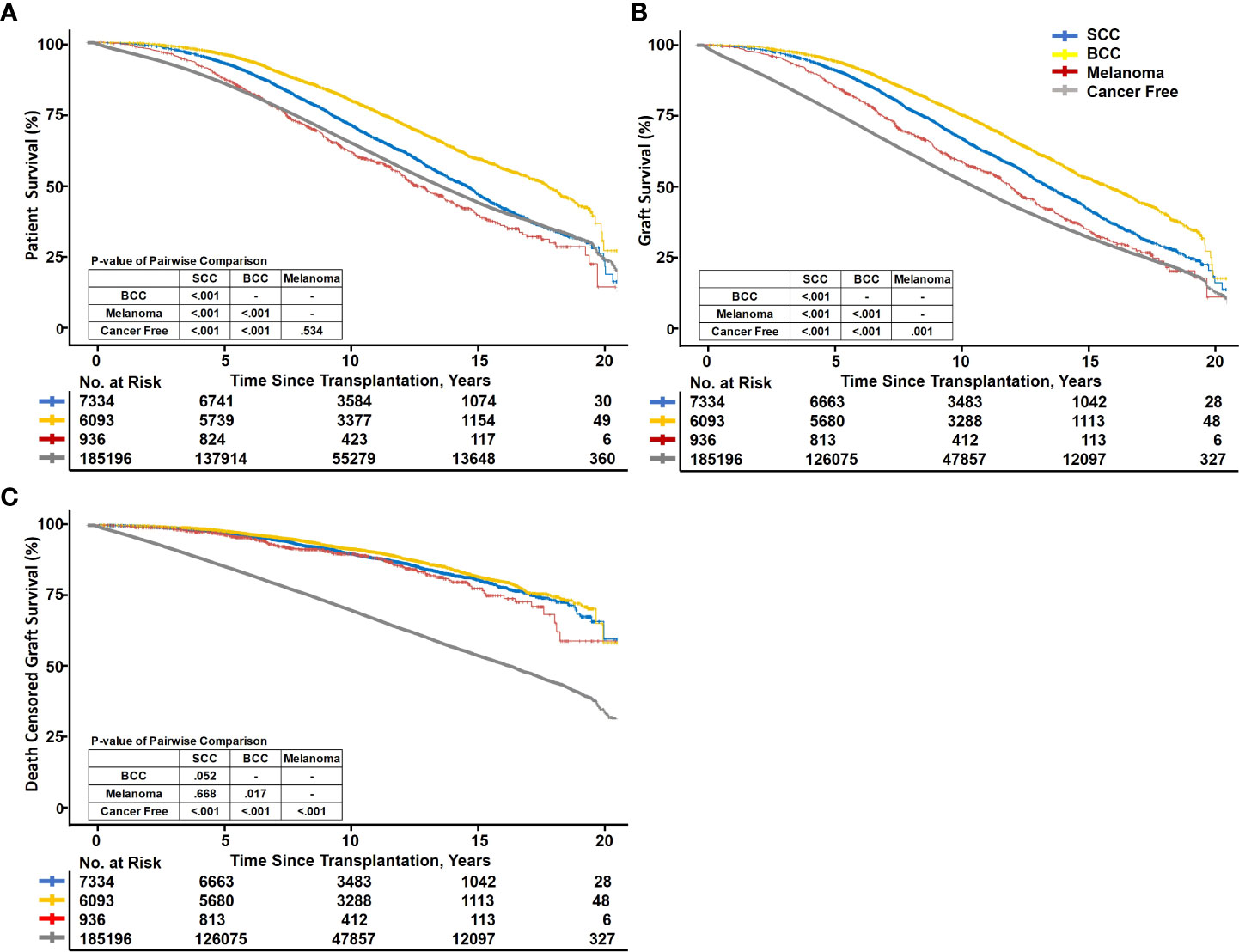
Figure 2 Kaplan–Meier survival curve fit of recipient and graft survival. SCC, squamous cell carcinoma; BCC, basal cell carcinoma. Kaplan–Meier curves showing patient survival (A), graft survival (B), and death-censored graft survival (C) between PTSC and cancer-free kidney transplant recipients.
Among the three types of skin cancer, the cumulative incidence of SCC and BCC was higher and the incidence rate increased with follow-up duration, while melanoma increased gradually with a lower incidence after RT (5- and 10-year cumulative incidence:1.8%, 4.7%; 1.5%, 4.9%; and 0.3%, 0.6%, respectively) (Figure 3).
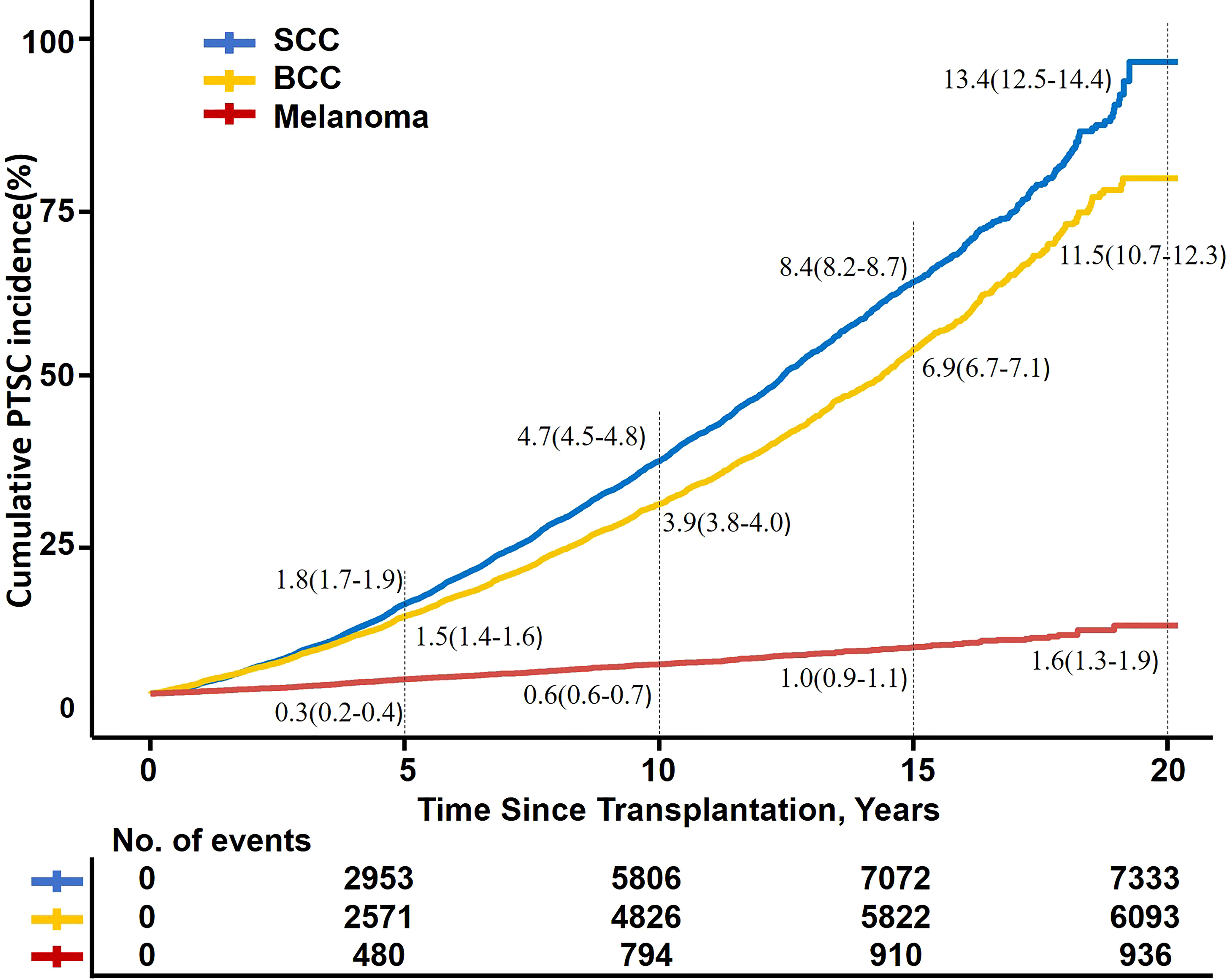
Figure 3 Kaplan–Meier fit of PTSC incidence by time after transplantation. SCC, squamous cell carcinoma; BCC, basal cell carcinoma; PTSC, posttransplant skin cancer.
Risk factors of PTSC incidence
To identify unmodifiable factors at the time of transplantation that conferred a greater risk for subsequent malignancy within the transplant population, initial unadjusted analyses suggested that most variables were associated with a significantly increased risk of PTSC. After allowing for the effects of adjusted multivariate analysis, logistic regression model suggested that recipient characteristics such as older age (aOR: 1.05, 1.05, and 1.05, respectively), male (adjusted odds ratio: 1.56, 1.58, and 1.75, respectively), Caucasian (aOR: 9.95, 38.78, and 2.04, respectively), pretransplant malignancy (aOR: 1.63, 1.94, and 1.66, respectively), PKD-induced ESRD (aOR: 1.22, 1.30, and 1.21, respectively), retransplantation (aOR: 1.64, 1,29, and 1.13, respectively), private health insurance (aOR: 1.30, 1.48, and 1.33, respectively), Caucasian donor (aOR: 1.16, 1.30, and 1.61, respectively), T-cell depletion (aOR: 1.15, 1.20, and 1.08, respectively), tacrolimus (aOR: 1.21, 1.05, and 1.27, respectively), and MPA (aOR: 1.05, 1.22, and 1.10, respectively) were the independent risk factors for PTSC development. Obesity (aOR: 0.87, 0.83, NA, respectively), group B blood (aOR: 0.93, 1.00, 0.81, respectively), hypertension (aOR: 0.75, 0.70, 0.75, respectively), diabetes (aOR, 0.75, 0.72, 0.90, respectively)-induced ESRD, diabetes history (aOR: 0.85, 0.80, 0.81, respectively), deceased donor (aOR: 0.77, 0.76, 0.89, respectively), and mTOR inhibitor (aOR: 0.74, 0.87, 0.77, respectively) could decrease the risk of PTSC incidence. Moreover, other cofounders like IL-2 and longer dialysis duration exhibited differences that were not significant and would therefore not be discussed further (Table 3).
Risk factors for PTSC onset momentum
The linear regression model suggested that Caucasian (SCC: [0.28], BCC: [0.25]) and pretransplant malignancies (SCC: [0.13], BCC: [0.20]) were associated with a longer duration of NMSC diagnosis.
Risk factors for PTSC mortality
Male (adjusted hazard ratio [aHR]: 1.15, 1.20, and 1.08, respectively), Caucasian (aHR: 1.16, 1.16, and 1.18, respectively), pretransplant malignancy (aHR: 1.11, 1.09, and 1.02, respectively), hypertension (aHR: 1.25, 1.28, and 1.24, respectively)- or diabetes (aHR: 1.40, 1.41, and 1.38, respectively)-induced ESRD, retransplantation (aHR: 1.11, 1.12, and 1.14, respectively), diabetes history (aHR: 1.63, 1.65, and 1.64, respectively), deceased donor (aHR: 1.38, 1.38, and 1.37, respectively), and cyclosporin (aHR: 1.22, 1.22, and 1.22, respectively) and mTOR inhibitor (aHR: 1.20, 1.20, and 1.21, respectively) use significantly increased the mortality of patients with PTSC, whereas Hispanic (aHR: 0.77, 1.76, and 1.76, respectively), PKD-induced ESRD (aHR: 0.78, 0.76, and 0.77, respectively), private insurance (aHR: 0.76, 0.75, and 0.75, respectively), Hispanic donor (aHR: 0.89, 0.88, and 0.88, respectively), and T-cell depletion (aHR: 0.9, 0.9, and 0.9, respectively) were associated with decreased mortality of patients with PTSC (Table 3).
Discussion
It is important to acknowledge the PTSC from three disease dimensions of incidence, onset momentum, and prognosis (mortality). We found not only that the risk factors for three dimensions of PTSC were different, but also that the effects of some cofounders on PTSC incidence, onset momentum, and mortality were opposite. Patients with hypertension, diabetes-induced ESRD, deceased donor, or T-cell-depleting agents have a significantly lower incidence risk of PTSC but a poorer prognosis if diagnosed with PTSC. On the contrary, PKD-induced ESRD or mTOR inhibitor use will raise the patient’s PTSC risk but exhibit relatively better outcome. Regarding outcomes, PTSC, particularly BCC, exhibited better outcomes, while patients with melanoma showed comparable PS and better GS compared with cancer-free cancer RT recipients.
Skin carcinogenesis following RT is associated with several factors, including immunosuppressive therapy, UV exposure, light skin color, genetic and epigenetic factors, viral infection, and psychogenic factors (20, 25–27). Laing et al. depicted that SCC was featured by aberrant methylation of DNA, which appears related to polymorphisms of MTHFR (28). An international survey indicated that the incidence rates of skin cancer were observed to increase with age in all of the studied countries including the USA (29). Skin cancer is more common in light-skinned Caucasians than in persons with skin of color and is often associated with greater morbidity and mortality, but is related to a slower onset momentum because skin color would minimize the likelihood of early detection of these tumors (30). Patients with pretransplant malignancies are prone to PTSC, which was also reported in another study using UNOS data. Patients with a history of malignancies had a hazard ratio of 1.77 to develop first posttransplant malignancy and 1.23-fold risk of mortality (31). A population-based study assessed the incidence of NMSC in American men, and it was twice that in women. Clearly, sex-related hormonal differences may play key roles in UV-induced skin inflammation and cancer development (32, 33). Considering that graft kidneys are unlikely to affect the patient’s skin and Caucasian candidates are more likely to be paired with Caucasian donors in living donor donations, whether Caucasian donors are a risk factor need to be determined by further studies. Generally, lower socioeconomic status is associated with decreased sun protection practices (34–36), but recipients with private insurance in our study, which usually means greater socioeconomic status, displayed an elevated risk of PTSC, which may be explained by the fact that most private insurance owners are Caucasian. Meanwhile, Caucasians or private insurance owners have a relatively lower risk of mortality, resulting from timely therapy with a shorter delay between diagnosis and definitive surgery (37, 38). Previous studies showed that PKD is an independent risk factor for NMSC development after RT, but the mechanisms linking PKD and NMSC are unclear. T-cell-depleting agents increasing the risk of cancers, such as melanoma, have already been proven (39, 40). The maintenance regimen often includes a calcineurin inhibitor (tacrolimus or cyclosporin), an antiproliferative agent (MPA or azathioprine), and steroids, all which are known to increase the risk of cutaneous malignancies. Our analysis corroborates results from current literature (41). Ascha et al. reported that sirolimus was a risk factor for melanoma after RT; however, another prospective clinical study suggested that mTOR inhibitors could have a preventive effect on PTSC genesis (42). We found that patients who used mTOR inhibitors at discharge, an immunosuppressant well known for its antioncogenic effects (43, 44), had a significantly decreased risk of PTSC. Interestingly, obesity is associated with a decreased risk of developing non-melanoma skin cancers. On one hand, obese individuals may likely spend less time outdoors with less chronic sun exposure (45). On the other hand, the “obesity paradox” has revealed that obesity may potentially attenuate the magnitude of inflammation (46). The protective effect of metformin, an antidiabetic drug associated with decreased cancer risk, on skin cancer in patients with diabetes has been proven in a cohort study in Taiwan (47, 48). Nevertheless, diabetes negatively impacts the recipients’ health and results in a worse prognosis. Several plausible hypotheses have been proposed for the observed association between ABO blood groups and skin cancer risk. Celi´c et al. demonstrated that the AB blood group was significantly associated with the occurrence of NMSCs (49), whereas Xie et al. revealed an association between the non-O blood group and a decreased risk of each type of skin cancer (50). One study found that patients with deceased donor had a significantly lower risk of PTSC incidence compared with those who did not. In our study, recipients of kidney from a deceased donor had a 1.38 greater hazard of mortality compared with living donor recipients. This is partially discordant with prior studies, with patients from deceased donors experiencing greater incidence of cancer and mortality (3, 51).
Skin cancer is the most commonly diagnosed cancer in the United States, in both RT recipients and the general population (4, 52, 53), threatens RT recipients’ lifespan and quality (11, 16, 22), and is regarded as a contraindication to transplantation (54). Partially congruent with previous studies, we observed higher morbidity but moderate skin cancer mortality in RT recipients. Compared with cancer-free patients after transplantation, posttransplant NMSC patients exhibited better survival, even melanoma, which usually results in early metastasis and is highly aggressive, also presented comparable survival in this study. This may be explained by the following reasons: (1) Tumor patients may have an inactive (immune escape) immune system to favor tumor progression but a better immune tolerance, which would be beneficial in terms of reducing transplant kidney rejection (55). It could also explain the fact that PTSC patients show lower proportion of HLA mismatch, lower creatinine at discharge, lower rate of delay graft function, and acute rejection. (2) RT patients are more proactive and regular in the early stages after RT, resulting in early treatment. Considering that skin cancer rarely impacts the kidney or allograft, and recipients with PTSC were more likely to undergo closer medical follow-up and thorough surveillance strategies, these recipients exhibited better GS features than cancer-free patients. (3) Given the time of skin cancer diagnosis in our study was more than 5 years after transplantation, most PTSC patients experience immunosuppression, and reducing it would not significantly impair their immune balance. However, skin cancer, especially melanoma, remains a common cause of death in patients with PTSC. Several studies found that melanoma and NMSC after transplantation are common causes of substantial mortality (26, 56). Combined with evidence from our study, the mean time to developing PTSC after RT was approximately 10 years and PTSC incidence increased gradually. The epidemiological features and tendency of PTSC provide important perspectives on prevention and evaluation of PTSC over the long-term and allow clinicians to take subsequent active steps to achieve the expected benefit during the early follow-up period.
Notably, this study may provide a basis for PTSC screening and treatment, especially for risk stratification and the development of individualized strategies. Considering that PTSC does not obviously impact PS and GS, which was demonstrated in our study, routine screening for PTSC may not be necessary for all recipients, but individualized screening among high-risk individuals particularly in the early follow-up period, especially cancer history, may be a more suitable and cost-effective approach.
Our research is a comprehensive observation that enrolled a large sample size spanning 15 years of registry and 7–10 years of follow-up period nationwide. However, several limitations exist in this study. First, despite the relatively large sample size, we lack information regarding cancer risk after transplant failure and are limited to avoiding loss to follow-up, which might result in underestimation of PTSC mortality in RT recipients. Secondly, information regarding sun exposure, such as occupational or recreational pastimes and sun protective habits, was unavailable. Thirdly, granular patient information of the data, particularly as it relates to immunosuppressive scheme during follow-up, and skin cancer details such as grade, stage, and therapies are insufficiently detailed in the UNOS registry. Finally, we could not assess certain risk factors for melanoma because they were not captured in the UNOS database.
Older age, male, Caucasian recipients, having pretransplant malignancy, PKD-induced ESRD, retransplantation, private health insurance, use of T-cell depletion, tacrolimus, and MPA are risk factors of PTSC incidence. Obesity, B blood group, hypertension- or diabetes-induced ESRD, diabetes history, deceased donor, and mTOR inhibitor use decreased the risk of PTSC incidence. Despite PTSC being a major cause of recipient death, its impact on both PS and GS remains limited. Given the differences in individual risks for PTSC and overall prognosis, a personalized approach to screening may be an appropriate strategy to address the complex issues encountered by RT recipients.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://optn.transplant.hrsa.gov/data/request-data/.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
XH, WL, JD, and QY designed the study. XH and WL conducted the statistical analysis and wrote the manuscript. All authors contributed to the critical revision of the manuscript for intellectual content and revised the manuscript. All authors read and approved the final manuscript.
Acknowledgments
We gratefully acknowledge research support of the OPTN/UNOS data and Dr. Nahel Elias of the Center for Transplantation Sciences and Division of Transplant Surgery, Department of Surgery, Massachusetts General Hospital, Boston, MA, USA, for providing guidance in data analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1017498/full#supplementary-material
Supplementary Table 1 | Recipient ethnicity and donor type in patient received caucasian donor kidney.
Abbreviations
BCC, basal cell carcinoma; BMI, body mass index; DCGS, death-censored graft survival; ESRD, end-stage renal disease; OPTN, Organ Procurement and Transplantation Network; PKD, polycystic kidney disease; PTSC, post-transplant skin cancer; RT, renal transplantation; SCC, squamous cell carcinoma; UNOS, United Network for Organ Sharing.
References
1. Francis A, Johnson DW, Melk A, Foster BJ, Blazek K, Craig JC, et al. Survival after kidney transplantation during childhood and adolescence. Clin J Am Soc Nephrol (2020) 15:392–400. doi: 10.2215/cjn.07070619
2. Lim WH, Badve S V, Wong G. Long-term allograft and patient outcomes of kidney transplant recipients with and without incident cancer - a population cohort study. Oncotarget (2017) 8:77771–82. doi: 10.18632/oncotarget.20781
3. Farrugia D, Mahboob S, Cheshire J, Begaj I, Khosla S, Ray D, et al. Malignancy-related mortality following kidney transplantation is common. Kidney Int (2014) 85:1395–403. doi: 10.1038/ki.2013.458
4. Linares MA, Zakaria A, Nizran P. Skin cancer. Prim Care (2015) 42:645–59. doi: 10.1016/j.pop.2015.07.006
5. Gordon R. Skin cancer: An overview of epidemiology and risk factors. Semin Oncol Nurs (2013) 29:160–9. doi: 10.1016/j.soncn.2013.06.002
6. O'Reilly Zwald F, Brown M. Skin cancer in solid organ transplant recipients: advances in therapy and management:Part i. epidemiology of skin cancer in solid organ transplant recipients. J Am Acad Dermatol (2011) 65:253–61. doi: 10.1016/j.jaad.2010.11.062
7. Kalinova L, Majek O, Stehlik D, Krejci K, Bachleda P. Skin cancer incidence in renal transplant recipients - a single center study. Biomed Pap Med Faculty Univ Palacky Olomouc Czechoslovakia (2010) 154:257–60. doi: 10.5507/bp.2010.039
8. Engels EA, Pfeiffer RM, Fraumeni JF, Kasiske BL, Israni AK, Snyder JJ, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA (2011) 306:1891. doi: 10.1001/jama.2011.1592
9. Collett D, Mumford L, Banner NR, Neuberger J, Watson C. Comparison of the incidence of malignancy in recipients of different types of organ: a UK registry audit. Am J Transplant (2010) 10:1889–96. doi: 10.1111/j.1600-6143.2010.03181.x
10. Tessari G, Naldi L, Boschiero L, Minetti E, Sandrini S, Nacchia F, et al. Incidence of primary and second cancers in renal transplant recipients: A multicenter cohort study. Am J Transplant (2013) 13:214–21. doi: 10.1111/j.1600-6143.2012.04294.x
11. Au E, Wong G, Chapman JR. Cancer in kidney transplant recipients. Nat Rev Nephrol (2018) 14:508–20. doi: 10.1038/s41581-018-0022-6
12. Hollenbeak CS, Todd MM, Billingsley EM, Harper G, Dyer A M, Lengerich EJ. Increased incidence of melanoma in renal transplantation recipients. Cancer (2005) 104:1962–7. doi: 10.1002/cncr.21404
13. Vajdic CM, McDonald SP, McCredie MRE, Van Leeuwen MT, Stewart JH, Law M, et al. Cancer incidence before and after kidney transplantation. JAMA (2006) 296:2823. doi: 10.1001/jama.296.23.2823
14. Villeneuve PJ, Schaubel DE, Fenton SS, Shepherd FA, Jiang Y, Mao Y. Cancer incidence among Canadian kidney transplant recipients. Am J Transplant (2007) 7:941–8. doi: 10.1111/j.1600-6143.2007.01736.x
15. Kasiske BL, Snyder JJ, Gilbertson D T, Wang C. Cancer after kidney transplantation in the united states. Am J Transplant (2004) 4:905–13. doi: 10.1111/j.1600-6143.2004.00450.x
16. Kovach B T, Stasko T. Skin cancer after transplantation. Transplant Rev (Orlando) (2009) 23:178–89. doi: 10.1016/j.trre.2009.02.004
17. Stoff B, Salisbury C, Parker D, O'Reilly Zwald F. Dermatopathology of skin cancer in solid organ transplant recipients. Transplant Rev (Orlando Fla) (2010) 24:172–89. doi: 10.1016/j.trre.2010.05.002
18. Ponticelli C, Cucchiari D, Bencini P. Skin cancer in kidney transplant recipients. J Nephrol (2014) 27:385–94. doi: 10.1007/s40620-014-0098-4
19. Alam M, Ratner D. Cutaneous squamous-cell carcinoma. N Engl J Med (2001) 344:975–83. doi: 10.1056/NEJM200103293441306
20. Ascha M, Ascha MS, Tanenbaum J, Bordeaux JS. Risk factors for melanoma in renal transplant recipients. JAMA Dermatol (2017) 153:1130–6. doi: 10.1001/jamadermatol.2017.2291
21. Stewart JH, Vajdic CM, van Leeuwen MT, Amin J, Webster AC, Chapman JR, et al. The pattern of excess cancer in dialysis and transplantation. Nephrol Dial Transplant (2009) 24:3225–31. doi: 10.1093/ndt/gfp331
22. Ulrich C, Kanitakis J, Stockfleth E, Euvrard S. Skin cancer in organ transplant recipients–where do we stand today? Am J Transplant (2008) 8:2192–8. doi: 10.1111/j.1600-6143.2008.02386.x
23. Griffin L, Ho L, Akhurst RJ, Arron ST, Boggs JME, Conlon P, et al. Genetic polymorphism in methylenetetrahydrofolate reductase chloride transport protein 6 (MTHFR CLCN6) gene is associated with keratinocyte skin cancer in a cohort of renal transplant recipients. Skin Health Dis (2022) 2:e95. doi: 10.1002/ski2.95
24. Laing ME, Dicker P, Moloney FJ, Ho WL, Murphy GM, Conlon P, et al. Association of methylenetetrahydrofolate reductase polymorphism and the risk of squamous cell carcinoma in renal transplant patients. Transplantation (2007) 84:113–6. doi: 10.1097/01.tp.0000266069.41882.28
25. Krásová M, Sečníková Z, Göpfertová D, Hercogová J, Viklický O, Jůzlová K, et al. Immunosuppressive therapy in the posttransplant period and skin cancer. Dermatol Ther (2016) 29:433–6. doi: 10.1111/dth.12379
26. Garrett GL, Blanc PD, Boscardin J, Lloyd AA, Ahmed RL, Anthony T, et al. Incidence of and risk factors for skin cancer in organ transplant recipients in the united states. JAMA Dermatol (2017) 153:296–303. doi: 10.1001/jamadermatol.2016.4920
27. Comeau S, Jensen L, Cockfield SM, Sapijaszko M, Gourishankar S. Non-melanoma skin cancer incidence and risk factors after kidney transplantation: A Canadian experience. Transplantation (2008) 86:535–41. doi: 10.1097/TP.0b013e318180482d
28. Laing ME, Cummins R, O'Grady A, O'Kelly P, Kay E W, Murphy GM. Aberrant DNA methylation associated with MTHFR C677T genetic polymorphism in cutaneous squamous cell carcinoma in renal transplant patients. Br J Dermatol (2010) 163:345–52. doi: 10.1111/j.1365-2133.2010.09774.x
29. Niino M, Matsuda T. Age-specific skin cancer incidence rate in the world. Jpn J Clin Oncol (2021) 51:848–9. doi: 10.1093/jjco/hyab057
30. Gloster H M, Neal K. Skin cancer in skin of color. J Am Acad Dermatol (2006), 55:741–64. doi: 10.1016/j.jaad.2005.08.063
31. Livingston-Rosanoff D, Foley DP, Leverson G, Wilke LG. Impact of pre-transplant malignancy on outcomes after kidney transplantation: United network for organ sharing database analysis. J Am Coll Surg (2019) 229:568–79. doi: 10.1016/j.jamcollsurg.2019.06.001
32. Oberyszyn TM. Non-melanoma skin cancer: importance of gender, immunosuppressive status and vitamin d. Cancer Lett (2008) 261:127–36. doi: 10.1016/j.canlet.2008.01.009
33. Zhong Q-Y, Lin B, Chen Y-T, Huang Y-P, Feng W-P, Wu Y, et al. Gender differences in UV-induced skin inflammation, skin carcinogenesis and systemic damage. Environ Toxicol Pharmacol (2021) 81. doi: 10.1016/j.etap.2020.103512
34. Abudu B, Cook KA, Gershenwald JE, Cohen P R, Geller AC. Quantitative associations between health insurance and stage of melanoma at diagnosis among nonelderly adults in the united states. Cancer (2019) 126:775–81. doi: 10.1002/cncr.32587
35. Engel TN, Nguyen M, Pan A, Sivamani RK. Health insurance relationship to sun protection practices and beliefs. Dermatol Online J (2021) 27. doi: 10.5070/D327955135
36. Schafer I, Reusch M, Siebert J, Hilbring C, Augustin M. Association of health insurance and socio-economic factors with health care for malignant melanoma. Gesundheitswesen (2017) 79:21–7. doi: 10.1055/s-0035-1564164
37. Tripathi R, Archibald LK, Mazmudar RS, Conic RRZ, Rothermel LD, Scott JF, et al. Racial differences in time to treatment for melanoma. J Am Acad Dermatol (2020) 83:854–9. doi: 10.1016/j.jaad.2020.03.094
38. Adamson AS, Zhou L, Baggett CD, Thomas N E, Meyer AM. Association of delays in surgery for melanoma with insurance type. JAMA Dermatol (2017) 153:1106–13. doi: 10.1001/jamadermatol.2017.3338
39. Al-Adra DP, Hammel L, Roberts J, Woodle ES, Levine D, Mandelbrot D, et al. Preexisting melanoma and hematological malignancies, prognosis, and timing to solid organ transplantation: A consensus expert opinion statement. Am J Transplant (2021) 21:475–83. doi: 10.1111/ajt.16324
40. Hall EC, Engels EA, Pfeiffer R M, Segev DL. Association of antibody induction immunosuppression with cancer after kidney transplantation. Transplantation (2015) 99:1051–7. doi: 10.1097/TP.0000000000000449
41. Collins L, Quinn A, Stasko T. Skin cancer and immunosuppression. Dermatol Clin (2019) 37:83–94. doi: 10.1016/j.det.2018.07.009
42. Campistol JM, Eris J, Oberbauer R, Friend P, Hutchison B, Morales JM, et al. Sirolimus therapy after early cyclosporine withdrawal reduces the risk for cancer in adult renal transplantation. J Am Soc Nephrol (2006) 17:581–9. doi: 10.1681/ASN.2005090993
43. Monaco AP. The role of mTOR inhibitors in the management of posttransplant malignancy. Transplantation (2009) 87:157–63. doi: 10.1097/TP.0b013e318193886e
44. de Fijter JW. Cancer and mTOR inhibitors in transplant recipients. Transplantation (2017) 101:45–55. doi: 10.1097/TP.0000000000001447
45. Pothiawala S, Qureshi AA, Li Y, Han J. Obesity and the incidence of skin cancer in US caucasians. Cancer Causes Control (2012) 23:717–26. doi: 10.1007/s10552-012-9941-x
46. Park J, Ahmadi SF, Streja E, Molnar MZ, Flegal KM, Gillen D, et al. Obesity paradox in end-stage kidney disease patients. Prog Cardiovasc Dis (2014) 56:415–25. doi: 10.1016/j.pcad.2013.10.005
47. Tseng H-W, Shiue Y-L, Tsai K-W, Huang W-C, Tang P-L, Lam H-C. Risk of skin cancer in patients with diabetes mellitus: A nationwide retrospective cohort study in Taiwan. Medicine (2016) 95:e4070. doi: 10.1097/MD.0000000000004070
48. Tseng C-H. Metformin is associated with decreased skin cancer risk in Taiwanese patients with type 2 diabetes. J Am Acad Dermatol (2018) 78:694–700. doi: 10.1016/j.jaad.2017.12.016
49. Celic D, Lipozencic J, Kolaric B, Ferencak G, Rajkovic J K, Borlinic T. Association between blood group and nonmelanoma skin cancers (Basal cell carcinoma and squamous cell carcinoma). Int J Environ Res Public Health (2019) 16. doi: 10.3390/ijerph16132267
50. Xie J, Qureshi AA, Li Y, Han J. ABO blood group and incidence of skin cancer. PloS One (2010) 5:e11972. doi: 10.1371/journal.pone.0011972
51. Nafar M, Einollahi B, Hemati K, Gholi F P, Firouzan A. Development of malignancy following living donor kidney transplantation. Transplant Proc (2005) 37:3065–7. doi: 10.1016/j.transproceed.2005.08.011
52. Skin cancer in the USA. Lancet (London England) (2011) 378:1528. doi: 10.1016/S0140-6736(11)61660-7
53. Madan V, Lear J T, Szeimies R-M. Non-melanoma skin cancer. Lancet (London England) (2010) 375:673–85. doi: 10.1016/S0140-6736(09)61196-X
54. Otley CC, Hirose R, Salasche SJ. Skin cancer as a contraindication to organ transplantation. Am J Transplant (2005) 5:2079–84. doi: 10.1111/j.1600-6143.2005.01036.x
55. Bedke J, Stenzl A. Immunologic mechanisms in RCC and allogeneic renal transplant rejection. Nat Rev Urol (2010) 7:339–47. doi: 10.1038/nrurol.2010.59
Keywords: skin cancer, renal transplantation (RT), end stage renal disease (ESRD), UNOS/OPTN, risk factors
Citation: Hao X, Lai W, Xia X, Xu J, Wu Y, Lv C, Meng Q, Lv K, Huang S, Luo Z, Dong J and Yuan Q (2022) Skin cancer outcomes and risk factors in renal transplant recipients: Analysis of organ procurement and transplantation network data from 2000 to 2021. Front. Oncol. 12:1017498. doi: 10.3389/fonc.2022.1017498
Received: 12 August 2022; Accepted: 03 November 2022;
Published: 24 November 2022.
Edited by:
Mahesh R. Desai, Muljibhai Patel Urological Hospital, IndiaReviewed by:
Mary Laing, University of Galway, IrelandJane Tomimori, Universidade Federal de São Paulo, Brazil
Copyright © 2022 Hao, Lai, Xia, Xu, Wu, Lv, Meng, Lv, Huang, Luo, Dong and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Dong, SnVuZG9uZ0B2aXAuMTI2LmNvbQ==; Qing Yuan, cXl1YW5tZEBvdXRsb29rLmNvbQ==
†These authors share first authorship
 Xiaowei Hao1,2†
Xiaowei Hao1,2† Junnan Xu
Junnan Xu Kaikai Lv
Kaikai Lv Jun Dong
Jun Dong Qing Yuan
Qing Yuan