
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 29 September 2022
Sec. Genitourinary Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1013941
Objectives: We aimed to systematically assess the inter-reader agreement of the Prostate Imaging Reporting and Data System Version (PI-RADS) v2.1 for the detection of prostate cancer (PCa).
Methods: We included studies reporting inter-reader agreement of different radiologists that applied PI-RADS v2.1 for the detection of PCa. Quality assessment of the included studies was performed with the Guidelines for Reporting Reliability and Agreement Studies. The summary estimates of the inter-reader agreement were pooled with the random-effect model and categorized (from slight to almost perfect) according to the kappa (κ) value. Multiple subgroup analyses and meta-regression were performed to explore various clinical settings.
Results: A total of 12 studies comprising 2475 patients were included. The pooled inter-reader agreement for whole gland was κ=0.65 (95% CI 0.56-0.73), and for transitional zone (TZ) lesions was κ=0.62 (95% CI 0.51-0.72). There was substantial heterogeneity presented throughout the studies (I2= 95.6%), and meta-regression analyses revealed that only readers’ experience (<5 years vs. ≥5 years) was the significant factor associated with heterogeneity (P<0.01). In studies providing head-to-head comparison, there was no significant difference in inter-reader agreement between PI-RADS v2.1 and v2.0 for both the whole gland (0.64 vs. 0.57, p=0.37), and TZ (0.61 vs. 0.59, p=0.81).
Conclusions: PI-RADS v2.1 demonstrated substantial inter-reader agreement among radiologists for whole gland and TZ lesions. However, the difference in agreement between PI-RADS v2.0 and v2.1 was not significant for the whole gland or the TZ.
Prostate cancer is the second most common cancer in men worldwide, and it is estimated that one in nine will be affected by this disease at some point during their lifetime (1, 2). Multiparametric magnetic resonance imaging (mpMRI) has been established as an effective noninvasive method for the detection, staging, and guiding management of PCa (3–5). Compared with conventional methods that depend on digital rectal examination (DRE) or transrectal ultrasonography (TRUS)–guided biopsy alone, mpMRI demonstrated higher accuracy and thus could substantially reduce unnecessary biopsies (6–8). In recent years, various novel models and nomograms were developed to improve the diagnostic performance of PCa, which utilizes one or more of following tools or biomarkers: serum prostate-specific antigen density (PSA), PSAD, age, history of prior prostate biopsy, DRE, radiomics, genomics, and molecular imaging (9, 10)
To standardize performing, interpreting, and reporting the PCa with mpMRI, the European Society of Urogenital Radiology (ESUR) introduced the first version of the Prostate Imaging Reporting and Data System in 2012, which was generated based on expert consensus and provides a detailed scoring system using mpMRI (11). The PI-RADS has been validated as an effective scoring system in clinical practice, and an early meta-analysis reported that the pooled sensitivity and specificity for the first version were 0.78 and 0.79 (12). To address some shortcomings in the PI-RADS v1, the American College of Radiology (ACR) and the ESUR released PI-RADS v2, which was widely applied for risk stratification and determination of biopsy pathway, showing good diagnostic performance (13, 14). However, inter-reader agreement among radiologists for PI-RADS v2 varied widely, especially for TZ lesions (15, 16). Therefore, a revision termed PI-RADS v2.1 was released in 2019, which primarily aimed to improve the inter-reader agreement on TZ (17). The major revisions are as follows: 1) introducing the concept of atypical nodules to score 2 in TZ, which are defined as a mostly encapsulated or homogeneous circumscribed nodules; 2) employing the DWI features to upgrade atypical nodules to score 3; and 3) downgrading these completely encapsulated nodules to score 1. Several studies evaluating the reproducibility of PI-RADS v2.1 especially for TZ have been published; however, the reported inter-reader agreements varied widely. Therefore, the purpose of our study was to systematically assess the reproducibility of PI-RADS v2.1 among various radiologists. Besides, we aimed to compare the inter-reader agreement of PI-RADS v2.1 with PI-RADS v2 in studies providing head-to-head comparison.
This meta-analysis and systematic review is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement, and performed with a predefined review and data extraction protocol (18). The primary outcome of our study was the pooled inter-reader agreement of using the PI-RADS v2.1 for the prediction of PCa. As the primary revision in PI-RADS v2.1 was the improvement of reproducibility for TZ lesions, the secondary outcome of our study was the inter-reader agreement regarding TZ. In addition, we would compare the PI-RADS v2.1 and v2.0 in studies providing head-to-head comparison.
A computerized literature search of PubMed, EMBASE, Cochrane Library, Web of Science, and Google Scholar was performed to identify potential eligible articles published between September 1, 2019 and March 31, 2022. We included studies reporting the inter-reader agreement on PI-RADS v2.1, with no language restriction applied. The terms combined synonyms used for searching were as follows: ([PI-RADS] OR [Prostate Imaging Reporting and Data System]) AND ([prostate cancer] OR [prostate carcinoma] OR [PCa]). An additional search was performed by manually screening the bibliographies of the included articles and reviews. Studies identified by the literature search were assessed by two independent reviewers (W.J. with 6 years and J.Y.G. with 8 years of experience in performing systematic reviews and meta-analyses), and disagreements were resolved by consensus via discussion with a third reviewer (Q.Y.).
We included studies that satisfied all of the following criteria: 1) reported the inter-reader agreement of PI-RADS v2.1 for detection of PCa; 2) provided κvalues and 95% confidence intervals (95% CI), or other measurements allowing assessment of inter-reader agreement; and 3) reported TRUS-guided biopsy, MRI-US fusion biopsy, or radical prostatectomy (RP) pathological results as the reference standard. We excluded studies that met any of the following criteria: 1) studies with a small sample size that involved less than 20 participants; 2) studies that did not provide detailed data to evaluate the inter-reader agreement; and 3) reviews, letters, guidelines, conference abstracts, or editorials.
We used a predefined standardized form to extract relevant information as follows: 1) demographic characteristics such as the number of patients and lesions, patient age, PSA level, and zonal anatomy (peripheral zone [PZ] and/or TZ); 2) study characteristics such as first author, publication year, location and institution, period of the study conducted, number of readers and their experience, whether blinded to final results, reference standard, inter-reader agreement; and 3) technical characteristics such as magnetic field strength, coil type, and MRI sequences used. The quality assessment of included studies was performed according to the Quality Appraisal of Diagnostic Reliability (QAREL) Checklist (19). For individual studies, these categories were scored as high quality if it was described in sufficient detail in the article with no potential bias.
The summary estimates of inter-reader agreement values were calculated with the random-effects model (Sidik-Jonkman method) (20, 21), and then categorized as follows: a κ value of <0.20 indicates slight agreement; a κ value between 0.21 and 0.40, fair agreement; a κ value between 0.41 and 0.60, moderate agreement; a κ value between 0.61 and 0.80, substantial agreement; and a κ value of between 0.81 and 1.00, almost perfect agreement (22).
Aside from the grouping of all PI-RADS category 1-5 lesions, we performed multiple subgroup analyses regarding the following variables: 1) for PI-RADS category ≥3; 2) for PI-RADS category ≥4; 3) for lesions of TZ; 4) for lesions of the whole gland; 5) for readers with experience at least 5 years (experienced); 6) for readers with experience less than 4 years or not dedicated in this area (inexperienced). Considering that several studies provide head-to-head comparison between PI-RADS v2.1 and v2.0, we thus compared these two versions in available studies. The meta-regression analysis was performed to explore the causes of heterogeneity by adding the following covariates: 1) type of analysis (per patient vs. per lesion); 2) PI-RADS score (for lesions with score ≥3 vs. all lesions; 3) zonal anatomy (whole gland vs. TZ); and 4) readers’ level of experience (experienced vs. varied experience).
Heterogeneity throughout studies was determined with the Q statistics and the inconsistency index (I2) as follows: for value between 0% and 40%, unimportant; between 30% and 60%, moderate; between 50% and 90%, substantial; and between 75% and 100%, considerable (23). Funnel plots and the rank test were used for the assessment of any possible publication bias. All analyses were performed with STATA 16.0 (StataCorp, Texas, USA), with P values <0.05 considered statistically significant. Two reviewers (W.J. and J.Y.G.) independently conducted the data extraction and quality assessment, disagreements were resolved through discussion and arbitrated by the third one (Q.Y.).
Figure 1 shows the flow chart of the publication selection process. Our literature search identified 901 references initially, of which 294 were excluded due to duplicates. After inspecting the titles and abstracts, a total of 497 articles were excluded. The full-text review was conducted among the remaining 110 potential articles, of them 98 articles were excluded for insufficient data and not in the field of interest. Finally, a total of 12 articles comprising 2475 patients were included in the current meta-analysis (24–35).
The detailed demographic and study characteristics are summarized in Tables 1, 2. The patient sample ranged from 58 to 355, with the lesion number of 58-638. The mean patient age was 63.1-73 years and with a PSA level of 4.9-13.7 ng/ml. Regarding zonal anatomy, 5 studies reported the inter-reader agreement only for TZ (27, 30, 31, 33, 34), 2 studies reported the inter-reader agreement both for TZ and PZ (24, 32), 4 studies reported the reproducibility for the whole gland and did not differentiate location (25, 26, 28, 35), only 1 study reported the inter-reader agreement merely on PZ lesions (29). In 4 studies the MRI images were interpreted by 2 radiologists (28, 29, 31, 34), whereas in the remaining 8 studies the MRI images were interpreted by at least 3 radiologists. Regarding readers’ experience, 5 studies reported that the MRI images were interpreted by experienced or dedicated radiologists (25, 27, 31, 34, 35), whereas in the remaining 7 studies the images were interpreted by radiologists with varied experience (1-20 years). With regard to magnetic field strength, in 10 studies the MRI examinations were performed with 3.0 T, while the remaining 2 studies used 1.5 T MRI (25, 26). Endorectal coils were used in only 2 studies (25, 26), and all other studies used the phased-array coil (PAC). The majority of studies used mpMRI sequences of diffusion-weighted imaging (DWI), dynamic contrast-enhanced (DCE), and T2; however, 1 study used biparametric MRI (bpMRI) that only included DWI and T2 sequences (33).
In general, the quality assessment for included studies was high. In 10 of 12 studies the readers were completely blinded to the final results, for the remaining 2 studies the radiologists were aware of part of the patient’s information (age and/or PSA level) that may influence the inter-reader agreement (26, 31). 5 studies did not explicitly report that the images were interpreted by readers independently, thus were categorized as high risk (24, 26, 32, 33, 35). Further details on the study quality are provided in Supplementary Table 1.
The pooled summary estimates of inter-reader agreement of the PI-RADS v2.1 are summarized in Figure 2. For individual studies, the κ values ranged from 0.42 to 0.89, and the pooled summary estimates of the κ value was 0.65 (95% CI 0.56-0.73) for all PI-RADS 1-5 lesions. We performed comparison between PI-RADS v2.0 and v2.1 using the available head-to-head comparison studies. For TZ lesions, the pooled κ value of 0.59 (95% CI 0.48-0.69) vs. 0.61 (95% CI 0.47-0.75) from 6 head-to-head comparison studies, suggesting the inter-reader agreement was comparable between the two PI-RADS versions (P=0.81, Figure 3) (24, 27, 31–34). Concerning the inter-reader agreement of all PI-RADS 1-5 lesions, the pooled κ values from 7 head-to-head comparison studies demonstrated that PI-RADS v2.1 had a non-significant difference compared with v2.0 with values of 0.64 (95% CI, 0.55-0.72) vs. 0.57 (95% CI, 0.45-0.69), respectively (p=0.37). We did not observe significant publication bias among included studies (Figure 4). Concerning the diagnostic accuracy for clinically significant PCa, the pooled sensitivity and specificity were 0.89 (95% CI 0.84-0.93) and 0.76 (95% CI 0.64, 0.85), with area under receiver operating characteristic (ROC) curve of 0.91 (95% CI 0.88-0.93).
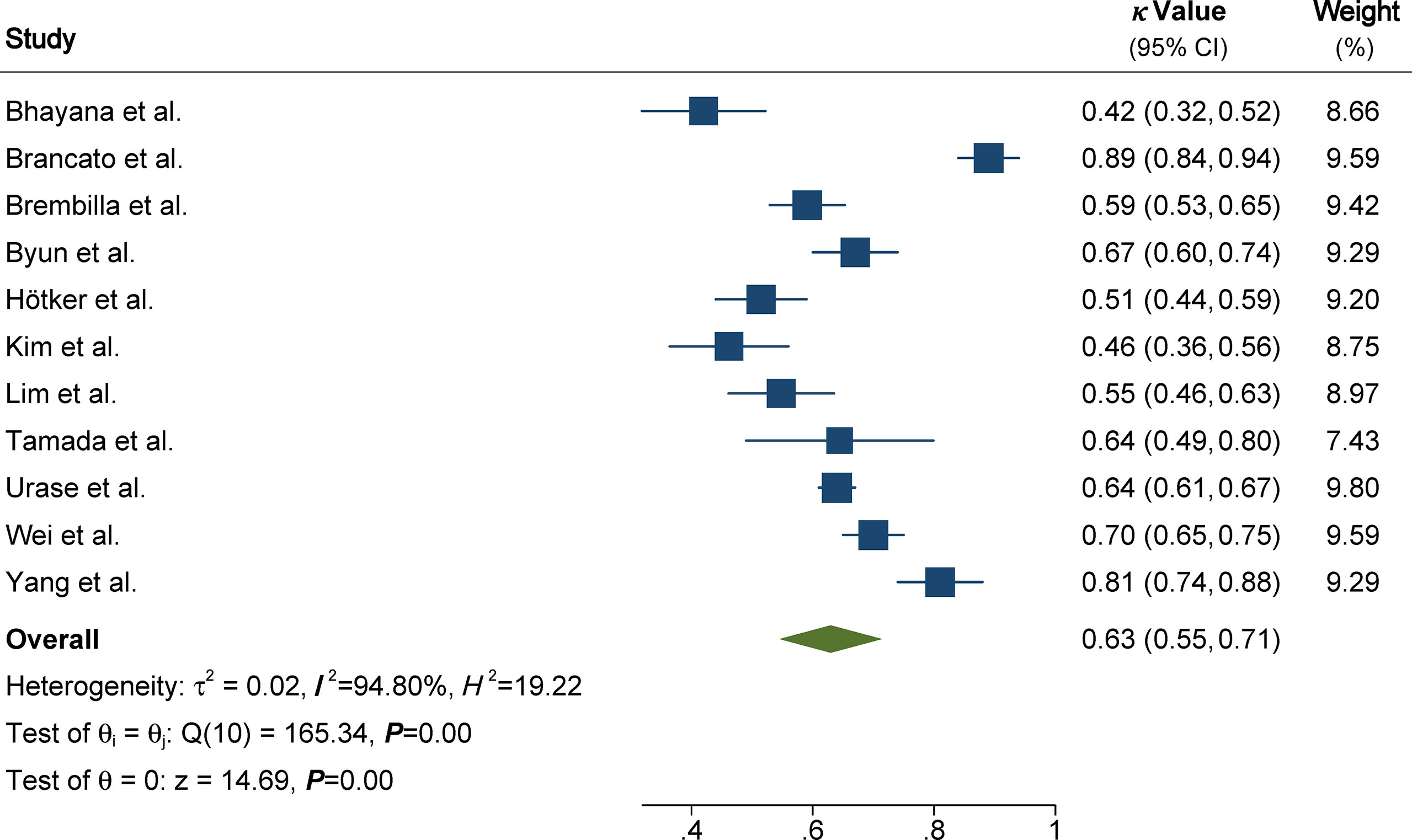
Figure 2 Coupled forest plot of pooled inter-reader agreement of all Prostate Imaging Reporting and Data System Version v2.1 lesions. CI, confidence interval.
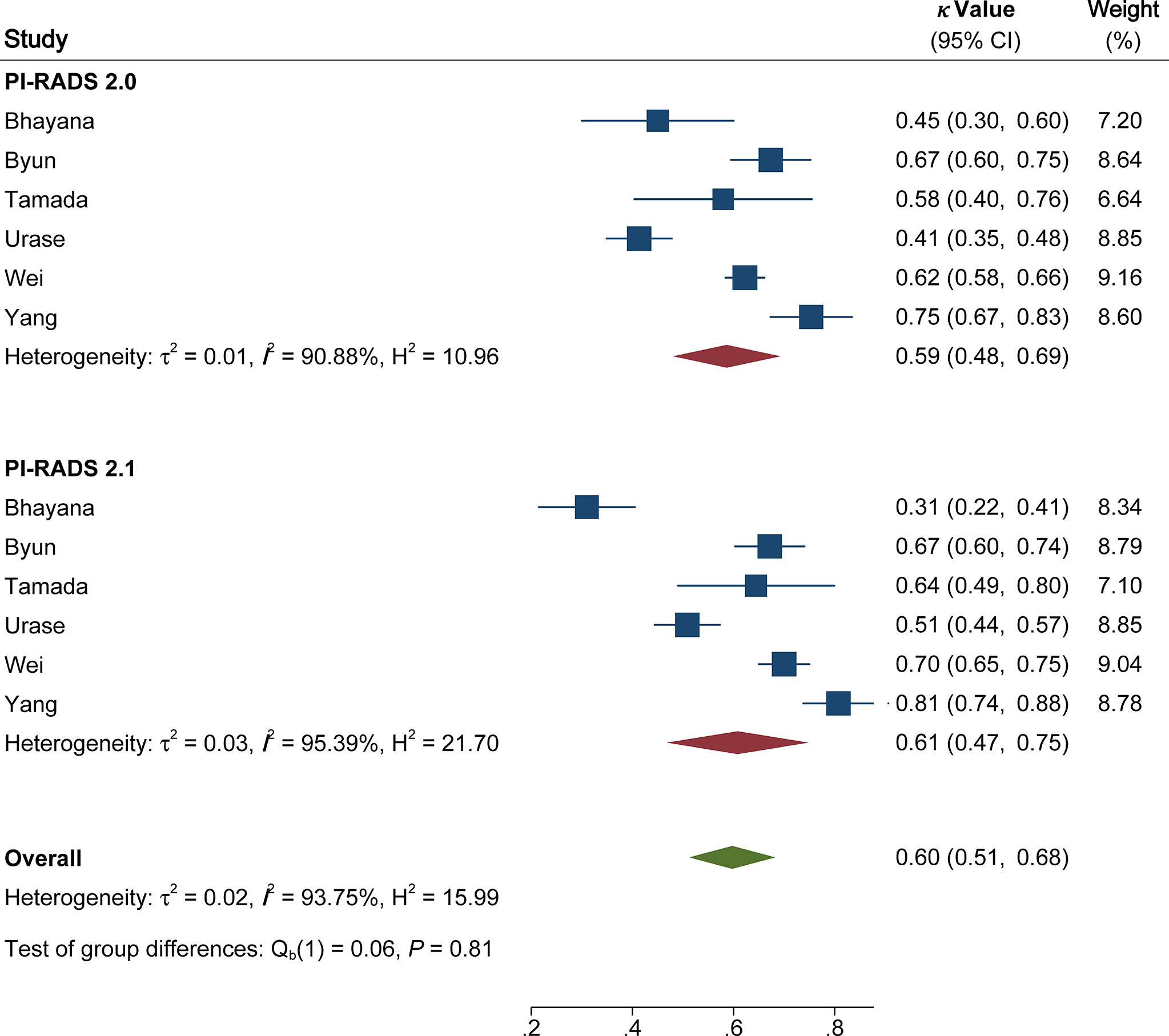
Figure 3 Coupled forest plot of pooled inter-reader agreement of Prostate Imaging Reporting and Data System Version v2.1 vs. 2.0 for transitional zone. CI, confidence interval.

Figure 4 The funnel plot. A P value of 0.17 suggests that the likelihood of publication bias is low.
Concerning specific PI-RADS cutoff values, the pooled κ value was moderate (0.58, 95% CI 0.52-0.64) for PI-RADS score ≥3. Whereas for the threshold of PI-RADS score ≥4, the inter-reader agreement was substantial, with pooled κ value of 0.70 (95% CI 0.63-0.77). For the subgroup of any PCa, the pooled κ value was 0.67 (95% CI 0.55-0.79), which was comparable with the inter-reader agreement for clinically significant PCa (0.65, 95% CI 0.53-0.77). In terms of TZ lesions, the pooled inter-reader agreement from 8 studies was 0.62 (95% CI 0.51-0.72). By comparison, the inter-reader agreement for PZ lesions was slightly higher, with pooled κ value of 0.65 (95% CI 0.51-0.80). Regarding subgroup analysis according to experience, the pooled κ value for experienced readers (≥5 years) was not significantly different from inexperienced readers, with values of 0.72 (95%CI 0.66-0.78) and 0.64 (95% CI 0.60-0.68), respectively. For subgroup analysis of reference standard, the pooled κ value for TRUS was 0.62 (95% CI 0.49-0.75) for 4 studies, which was lower than 6 studies using MRI-TRUS as the reference standard (0.66, 95% CI 0.51-0.81).
As substantial heterogeneity was observed between included studies, we performed meta-regression analysis to investigate the sources. Among the various potential factors, we found that only the experience of radiologists (0.76 vs. 0.56, P<0.01) was significantly associated with heterogeneity. Other covariates such as type of analysis (per patient vs. per lesion), PI-RADS score (lesions with score ≥3 vs. all lesions), and zonal anatomy (whole gland vs. TZ) were not substantial factors, with P values ranging from 0.14 to 0.43 (Supplementary Table 2).
The inter-reader agreement is critical for the standardized scoring system, as it relates to reducing the variability of interpretation between radiologists and improving the categorization of the level of suspicion for PCa. In this study, we systematically assessed the inter-reader agreement of PI-RADS v2.1 for the detection of PCa based on 12 studies. The pooled κ value of 0.65 (95% CI 0.56-0.73) suggested that the PI-RADS v2.1 exhibited substantial reproducibility among radiologists. Such reliability is critical for a standardized scoring system, as it reduces variable interpretation among radiologists and improves the association of categorization with likelihood of clinically significant PCa. As the revision of PI-RADS v2.1 served a main purpose of improving the inter-reader agreement for TZ lesions, the majority of included studies assessed the reproducibility for lesions located in TZ. Nevertheless, the pooled κ values suggested that there was no significant improvement as compared to PI-RADS v2.0 in several head-to-head comparison studies. Furthermore, half of the included studies reported no improvement of inter-reader agreement or even a decrease, though findings may have been at least partially affected by experience of readers (24, 25, 27).
In this meta-analysis substantial heterogeneity was observed between included studies, hence meta-regression analysis was performed to investigate the sources. Among the various potential factors, we found that only the level of readers’ experience was significantly associated with the degree of heterogeneity (P<0.01). In some studies, the κ value was generated from readers of widely varied experience, which is usually lower than those from experienced readers (24, 26, 28–30). Another possible but less studied influence on reader agreement is that of image quality (e.g. high b value images of DWI), as clear, quantifiable quality standards are not established (15). The meta-regression is considered exploratory and there may have been too few head-to-head studies to detect a difference in heterogeneity according to PI-RADS version. Our analysis revealed that the inter-reader agreement was lower in PI-RADS score ≥3 lesions relative to all lesions (0.58 vs. 0.65), one possible explanation is that more detailed diagnostic criteria of PI-RADS 1-2 score introduced in v2.1. On the other side, the inter-reader agreement for PI-RADS ≥4 was substantial, with a pooled κ value of 0.7. Therefore, the classification of score 3 lesions still a challenge in the new PI-RADS version, which may more depend on readers’ experience and personal patterns, and future studies may focus on more quantitative means of scoring. Although studies demonstrated that bpMRI has comparable diagnostic accuracy as multiparametric MRI in the detection of PCa (36), the majority of studies included the DCE sequence. In 3 studies that provided the inter-reader agreement from bpMRI, the reported pooled κ value of 0.7-0.86 suggested substantial to good agreement among readers. Because of too small sample, the κ values were unfeasible to pool and need validation in large prospective multi-center studies in the future.
In an earlier meta-analysis conducted by Park et al., the pooled κ value for PI-RADS v2 was 0.61 (95% CI 0.55-0.67) based on 30 studies, which is slightly lower than our results (15). In a more recent study, the reported κ values for PI-RADS v2.0 and v2.1 were 0.42-0.70 and 0.48-0.69, respectively, which also demonstrated comparable inter-reader agreement (37). Although many modifications in PI-RADS v2.1 were related to TZ lesions, some important revisions were also introduced to the interpretation of PZ lesions. On the basis of 5 studies reporting the inter-reader agreement on PZ lesions (24–26, 29, 32), the pooled κ value was comparable to a previous study (0.65 vs. 0.64) (15). In general, our study indicated that there was no substantial improvement in reproducibility between readers based on current evidence. Recently, radiomics has been widely studied in PCa, with investigations between quantitative image features and single gene expression, which demonstrated promising diagnostic results. In addition, artificial intelligence and machine learning also have been thoroughly studies in management and treatment of PCa such as biopsy, surgery, histopathology, and active surveillance (38, 39).
There are some limitations to our study. First, all studies included were retrospective in the study design, leading to a high risk of bias for the patient selection domain. Nevertheless, because of insufficient data it is unfeasible to pool the inter-reader agreement from prospective studies. Second, substantial heterogeneity was presented among included studies, which affected the general applicability of this systematic review. We performed multiple subgroup analyses and meta-regression to explore the sources, and the results indicated that level of experience was the significant factor that contributed to the heterogeneity. However, these analyses only explained part of the heterogeneity, and these analyses were based on only a few studies, thus the results should be interpreted cautiously. Lastly, most included studies used MRI-TRUS fusion targeted biopsy as the reference standard, while compared to RP which may miss potential lesions with a negative MRI but positive pathology.
PI-RADS v2.1 demonstrated substantial inter-reader agreement between radiologists for whole gland and TZ lesions. Nevertheless, no statistically significant improvement in reproducibility was observed in PI-RADS v2.1 of as compared to v2.0.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1013941/full#supplementary-material
1. Ferlay J, Colombet M, Soerjomataram I, Dyba T, Randi G, Bettio M, et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer (2018) 103:356–87. doi: 10.1016/j.ejca.2018.07.005
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA: A Cancer J Clin (2020) 70(1):7–30. doi: 10.3322/caac.21590
3. Tamada T, Sone T, Higashi H, Jo Y, Yamamoto A, Kanki A, et al. Prostate cancer detection in patients with total serum prostate-specific antigen levels of 4-10 ng/mL: Diagnostic efficacy of diffusion-weighted imaging, dynamic contrast-enhanced MRI, and T2-weighted imaging. AJR Am J Roentgenol (2011) 197:664–70. doi: 10.2214/AJR.10.5923
4. Vilanova JC, Barceló-Vidal C, Comet J, Boada M, Barceló J, Ferrer J, et al. Usefulness of prebiopsy multifunctional and morphologic MRI combined with free-to-Total prostate-specific antigen ratio in the detection of prostate cancer. Am J Roentgenol (2011) 196(6):W715–22. doi: 10.2214/AJR.10.5700
5. Delongchamps NB, Rouanne M, Flam T, Beuvon F, Liberatore M, Zerbib M, et al. Multiparametric magnetic resonance imaging for the detection and localization of prostate cancer: Combination of T2-weighted, dynamic contrast-enhanced and diffusion-weighted imaging. Bju Int (2011) 107(9):1411–8. doi: 10.1111/j.1464-410X.2010.09808.x
6. Kasivisvanathan V, Stabile A, Neves JB, Giganti F, Valerio M, Shanmugabavan Y, et al. Magnetic resonance imaging-targeted biopsy versus systematic biopsy in the detection of prostate cancer: A systematic review and meta-analysis. Eur Urol (2019) 76:284–303. doi: 10.1016/j.eururo.2019.04.043
7. Ahmed HU, Bosaily AES, Brown LC, Gabe R, Kaplan R, Parmar MK, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet (2017) 389(10071):815–22. doi: 10.1016/S0140-6736(16)32401-1
8. Rouvière O, Puech P, Renard-Penna R, Claudon M, Roy C, Mège-Lechevallier F, et al. Use of prostate systematic and targeted biopsy on the basis of multiparametric MRI in biopsy-naive patients (MRI-FIRST): a prospective, multicentre, paired diagnostic study. Lancet Oncol (2019) 20(1):100–9. doi: 10.1016/S1470-2045(18)30569-2
9. Wen J, Tang T, Ji Y, Zhang Y. PI-RADS v2.1 combined with prostate-specific antigen density for detection of prostate cancer in peripheral zone. Front Oncol (2022) 12:861928. doi: 10.3389/fonc.2022.861928
10. de la Calle CM, Fasulo V, Cowan JE, Lonergan PE, Maggi M, Gadzinski AJ, et al. Clinical utility of 4Kscore®, ExosomeDxTM and magnetic resonance imaging for the early detection of high grade prostate cancer. J Urol (2021) 205(2):452–60. doi: 10.1097/JU.0000000000001361
11. Barentsz JO, Richenberg J, Clements R, Choyke P, Verma S, Villeirs G, et al. ESUR prostate MR guidelines 2012. Eur Radiol (2012) 22(4):746–57. doi: 10.1007/s00330-011-2377-y
12. Hamoen EHJ, de Rooij M, Witjes JA, Barentsz JO, Rovers MM. Use of the prostate imaging reporting and data system (PI-RADS) for prostate cancer detection with multiparametric magnetic resonance imaging: A diagnostic meta-analysis. Eur Urology (2015) 67(6):1112–21. doi: 10.1016/j.eururo.2014.10.033
13. Weinreb JC, Barentsz JO, Choyke PL, Cornud F, Haider MA, Macura KJ, et al. PI-RADS prostate imaging – reporting and data system: 2015, version 2. Eur Urology (2016) 69(1):16–40. doi: 10.1016/j.eururo.2015.08.052
14. Woo S, Suh CH, Kim SY, Cho JY, Kim SH. Diagnostic performance of prostate imaging reporting and data system version 2 for detection of prostate cancer: A systematic review and diagnostic meta-analysis. Eur Urology (2017) 72(2):177–88. doi: 10.1016/j.eururo.2017.01.042
15. Park KJ, Choi SH, Lee JS, Kim JK, Kim MH. Interreader agreement with prostate imaging reporting and data system version 2 for prostate cancer detection: A systematic review and meta-analysis. J Urol (2020) 204(4):661–70. doi: 10.1097/JU.0000000000001200
16. Rosenkrantz AB, Ginocchio LA, Cornfeld D, Froemming AT, Gupta RT, Turkbey B, et al. Interobserver reproducibility of the PI-RADS version 2 lexicon: A multicenter study of six experienced prostate radiologists. Radiology (2016) 280(3):793–804. doi: 10.1148/radiol.2016152542
17. Turkbey B, Rosenkrantz AB, Haider MA, Padhani AR, Villeirs G, Macura KJ, et al. Prostate imaging reporting and data system version 2.1: 2019 update of prostate imaging reporting and data system version 2. Eur Urol (2019) 76(3):340–51. doi: 10.1016/j.eururo.2019.02.033
18. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. Epidemiol Biostat Public Health (2009) 6(4):e1–34. doi: 10.1136/bmj.b2700
19. Del Giudice F, Pecoraro M, Vargas HA, Cipollari S, De Berardinis E, Bicchetti M, et al. Systematic review and meta-analysis of vesical imaging-reporting and data system (VI-RADS) inter-observer reliability: An added value for muscle invasive bladder cancer detection. Cancers (Basel) (2020) 12(10):E2994. doi: 10.3390/cancers12102994
20. Sidik K, Jonkman JN. A simple confidence interval for meta-analysis. Stat Med (2002) 21(21):3153–9. doi: 10.1002/sim.1262
21. Sidik K, Jonkman JN. On constructing confidence intervals for a standardized mean difference in meta-analysis. Commun Stat - Simul Comput (2003) 32(4):1191–203. doi: 10.1081/SAC-120023885
22. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics (1977) 33(1):159–74. doi: 10.2307/2529310
23. Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ Br Med J (2011) 343(7829):889–93. doi: 10.1136/bmj.d5928
24. Bhayana R, O’Shea A, Anderson MA, Bradley WR, Gottumukkala RV, Mojtahed A, et al. PI-RADS versions 2 and 2.1: Interobserver agreement and diagnostic performance in peripheral and transition zone lesions among six radiologists. AJR Am J Roentgenol (2021) 217(1):141–51. doi: 10.2214/AJR.20.24199
25. Brancato V, Di Costanzo G, Basso L, Tramontano L, Puglia M, Ragozzino A, et al. Assessment of DCE utility for PCa diagnosis using PI-RADS v2.1: Effects on diagnostic accuracy and reproducibility. Diagnostics (2020) 10(3):164. doi: 10.3390/diagnostics10030164
26. Brembilla G, Dell’Oglio P, Stabile A, Damascelli A, Brunetti L, Ravelli S, et al. Interreader variability in prostate MRI reporting using prostate imaging reporting and data system version 2.1. Eur Radiol (2020) 30(6):3383–92. doi: 10.1007/s00330-019-06654-2
27. Byun J, Park KJ, Kim MH, Kim JK. Direct comparison of PI-RADS version 2 and 2.1 in transition zone lesions for detection of prostate cancer: Preliminary experience. J Magn Reson Imaging (2020) 52(2):577–86. doi: 10.1002/jmri.27080
28. Hötker AM, Blüthgen C, Rupp NJ, Schneider AF, Eberli D, Donati OF. Comparison of the PI-RADS 2.1 scoring system to PI-RADS 2.0: Impact on diagnostic accuracy and inter-reader agreement. PloS One (2020) 15(10):e0239975. doi: 10.1371/journal.pone.0239975
29. Kim HS, Kwon GY, Kim MJ, Park SY. Prostate imaging-reporting and data system: Comparison of the diagnostic performance between version 2.0 and 2.1 for prostatic peripheral zone. Korean J Radiol (2021) 22(7):1100–9. doi: 10.3348/kjr.2020.0837
30. Lim CS, Abreu-Gomez J, Carrion I, Schieda N. Prevalence of prostate cancer in PI-RADS version 2.1 transition zone atypical nodules upgraded by abnormal DWI: Correlation with MRI-directed TRUS-guided targeted biopsy. Am J Roentgenol (2021) 216(3):683–90. doi: 10.2214/AJR.20.23932
31. Tamada T, Kido A, Takeuchi M, Yamamoto A, Miyaji Y, Kanomata N, et al. Comparison of PI-RADS version 2 and PI-RADS version 2.1 for the detection of transition zone prostate cancer. Eur J Radiol (2019) 121:108704. doi: 10.1016/j.ejrad.2019.108704
32. Urase Y, Ueno Y, Tamada T, Sofue K, Takahashi S, Hinata N, et al. Comparison of prostate imaging reporting and data system v2.1 and 2 in transition and peripheral zones: Evaluation of interreader agreement and diagnostic performance in detecting clinically significant prostate cancer. Br J Radiol (2022) 95:20201434. doi: 10.1259/bjr.20201434
33. Wei CG, Zhang YY, Pan P, Chen T, Yu HC, Dai GC, et al. Diagnostic accuracy and inter-observer agreement of PI-RADS version 2 and version 2.1 for the detection of transition zone prostate cancers. AJR Am J Roentgenol (2021) 216:1247–56. doi: 10.2214/AJR.20.23883
34. Yang S, Zhang CY, Zhang YY, Tan SX, Wei CG, Shen XH, et al. The diagnostic value of version 2.1 prostate imaging reporting and data system for prostate transitional zone lesions. Zhonghua Yi Xue Za Zhi (2020) 100(45):3609–13. doi: 10.3760/cma.j.cn112137-20200506-01442
35. Bao J, Zhi R, Hou Y, Zhang J, Wu CJ, Wang XM, et al. Optimized MRI assessment for clinically significant prostate cancer: A STARD-compliant two-center study. J Magn Reson Imaging (2021) 53(4):1210–9. doi: 10.1002/jmri.27394
36. Niu X, Chen Xh, Chen Zf, Chen L, Li J, Peng T. Diagnostic performance of biparametric MRI for detection of prostate cancer: A systematic review and meta-analysis. Am J Roentgenol (2018) 211(2):369–78. doi: 10.2214/AJR.17.18946
37. Lee CH, Vellayappan B, Tan CH. Comparison of diagnostic performance and inter-reader agreement between PI-RADS v2.1 and PI-RADS v2: systematic review and meta-analysis. Br J Radiol (2022) 95:20210509. doi: 10.1259/bjr.20210509
38. Ferro M, de Cobelli O, Vartolomei MD, Lucarelli G, Crocetto F, Barone B, et al. Prostate cancer radiogenomics-from imaging to molecular characterization. Int J Mol Sci (2021) 22(18):9971. doi: 10.3390/ijms22189971
Keywords: prostate cancer, magnetic resonance imaging, inter-reader agreement, meta-analysis, PI-RADS
Citation: Wen J, Ji Y, Han J, Shen X and Qiu Y (2022) Inter-reader agreement of the prostate imaging reporting and data system version v2.1 for detection of prostate cancer: A systematic review and meta-analysis. Front. Oncol. 12:1013941. doi: 10.3389/fonc.2022.1013941
Received: 08 August 2022; Accepted: 13 September 2022;
Published: 29 September 2022.
Edited by:
Stefano Salciccia, Sapienza University of Rome, ItalyReviewed by:
Martina Maggi, Sapienza University of Rome, ItalyCopyright © 2022 Wen, Ji, Han, Shen and Qiu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Qiu, Y29udHJpYnV0ZV9zY2lAMTI2LmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.