
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 30 November 2022
Sec. Genitourinary Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1013806
Background: Previous studies have explored the role of PD-L1 in the survival outcomes of penile cancer patients with controversies existed. Thus, the meta-analysis was conducted to report and review the association between PD-L1 and survival in penile cancer patients.
Methods: PubMed, Cochrane Library, EMBASE, and Web of Science were all searched, screened, and reviewed by June 1, 2022. Hazard ratio (HR) was used to evaluate the relationship between PD-L1 and survival outcome, and odds ratio (OR) was for tumor features.
Results: Nine retrospective studies (1,003 patients) were incorporated. The prevalence of PD-L1 in patients with penile cancer was 51.4% (95% CI = 42.1%-60.8%, I2 = 88.5%). Higher PD-L1 on tumor cells was related to shorter cancer-specific survival (CSS) in patients (HR = 1.578, 95% CI = 1.227-2.029, I2 = 23.3%), but had no associations with overall survival (OS) (HR = 1.123, 95% CI = 0.511-2.465, I2 = 0.0%). Subgroup analysis indicated that higher PD-L1 was related to shorter CSS in Caucasus (HR = 1.827, 95% CI = 1.355-2.465, I2 = 0.0%) only. Furthermore, PD-L1 had associations with tumor stage (pT1 vs. pT2-4, OR = 0.480, 95% CI = 0.346-0.667, P = 0.001) and tumor grade (Well and moderate vs. Poor, OR = 0.377, 95% CI = 0.264-0.538, P < 0.001). PD-L1 positivity was also related to lymph node (LN) status (pN0/NX vs. pN1–3, OR = 0.541, 95% CI = 0.385-0.759, P = 0.001) and HPV status (Positive vs. Negative, OR = 0.510, 95% CI = 0.322-0.810, P = 0.003). A trend toward statistical significance between PD-L1 and histological types was also observed (Usual SCC vs. Others, OR = 1.754, 95% CI = 0.984-3.124, P = 0.070).
Conclusions: PD-L1 over-expression was related to worse survival outcomes and several clinicopathological features of penile cancer. PD-L1 expression can be applied to select appropriate treatment strategies for penile malignancies.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=343041, identifier CRD42022343041.
Penile cancer, with substantial differences in prevalence, is a rare carcinoma in industrialized countries (1). Penile squamous cell carcinoma (SqCC) accounts for 95% of all penile malignancies (2). Therapeutic options for locally advanced metastatic penile SqCC are limited and most of the patients die within one year (3). Surgery combined with subsequent platinum-based chemotherapy usually had a moderate response, while 63.3% of patients still suffer tumor recurrence or progression after first-line chemotherapy (paclitaxel, ifosfamide, and cisplatin) and the median survival is only 5.6 months (4). Recently, immunotherapy has yielded dramatically improved long-term survival benefits in several SqCC, especially among patients with high programmed death receptor ligand-1 (PD-L1)/programmed death receptor-1 (PD-1) expression (5, 6). Given the high expression of PD-L1 in the penile SqCC tumor microenvironment, immunotherapy may be an efficient strategy (7).
The immune checkpoint had been regarded as the milestone event in tumor research for decades. The mechanisms underlying tumor development and progression in the background of immunotherapy have also been widely discussed (8, 9). Recently, the favorable efficacy of immunotherapy has been found and validated in many malignancies (10–12). PD-L1, on the tumor cells, could bind to PD-1, suppressing immune cell proliferation and release of immune molecules. Tumor cells can evade immune surveillance through immune checkpoints. Finally, tumor recurrence or metastasis happened (13, 14). In the field of penile cancer, increasing studies have demonstrated the role of PD-L1 expression in survival outcomes and they got controversial findings. Some evidence indicated that higher PD-L1 was related to poor survival for penile cancer (15), while some reported opposing findings (16, 17). Therefore, we formulated clinical questions under the guidance of the PICOS strategy and firstly assessed the role of PD-L1 expression (high or low) in survival outcomes and the clinicopathological features in penile cancer patients through a meta-analysis.
Detailed inclusion criteria were raised according to the established reporting guidelines (18, 19). Three authors independently reviewed all available literature in PubMed, Cochrane Library, EMBASE, and Web of Science in June 2022. No eligible randomized-controlled trials (RCTs) with interventions were found and observational studies reporting the effect of PD-L1 on tumor behaviors or survival outcomes in penile cancer patients were all included. The references and citations were also searched and checked carefully. The keywords for the search were “PD-L1” and “penile cancer”. Supplementary Table 1 showed the detailed search strategy. Notably, this study is a conventional trial-level meta-analysis, thus no individual patient-level data were available. The protocol of the study was registered in PROSPERO (CRD42022343041).
Inclusion criteria: (a) Population: penile cancer patients without non-surgical treatments. (b) Interventions: Expression of PD-L1 (≥ cut-off value) on tumor cells. (c) Comparators: Expression of PD-L1 (< cut-off value) on tumor cells. (d) Outcomes: Survival outcomes or clinicopathological characteristics of penile carcinoma cases. (e) Study design: No restriction. (f) Article types: Original article or study with standard reporting and sufficient data. (g) Information on survival outcomes: Hazard ratio (HR) and 95% confidence interval (95%CI) could be obtained directly or indirectly. (i) Studies with a sample size of more than 20. We excluded studies that can’t meet the inclusion criteria or those with low quality for reporting.
Three authors screened the retrieved records independently. Items including the first author, study year, study design, study region, demographic information, cut-off value, median follow-up duration, and survival outcomes were extracted. We obtained missing or unclear information through contact with the article authors. Information will be considered as not mentioned or not available if there was no reply. By using the validated tool (20), HRs and their 95%CIs were digitized from studies that only had Kaplan-Meier curves.
A modified Newcastle–Ottawa scale (NOS) was used for RoB analysis (21). An agreement was reached through consensus among the 3 authors and communication with the article authors.
PD-L1 expression and its association with tumor behaviors were presented by pooled odds ratio (ORs) and HR was used to demonstrate the relationship between PD-L1 and survival outcomes. If significant heterogeneity was found or I2 > 50%, we utilized random-effect models, otherwise we chose fixed-effect models (22). Publication bias was evaluated through Begg’s test and Egger’s test, and displayed by funnel plots. Sensitivity analyses were done by excluding a study at one time and a cumulative meta-analysis was also done. STATA 12.0 (Stata-Corp.) was used for statistical analyses. A two-tailed P < 0.05 was considered statistically significant.
Two hundred and eighty-five non-repeated records were identified. We excluded records for the following reasons: not original articles or irrelevant topics (n=239), studies with limited sample size (≤ 20) (n=20), or insufficient data (n=17). Finally, we included 9 retrospective cohort studies (1,003 individuals) in the study (shown in Figure 1) (15–17, 22–27).
All 9 studies were published in recent six years (Table 1) and they were conducted across 6 countries, with 3 studies in China, 2 in the USA, 1 in Brazil, 1 in Sweden, 1 in the Netherlands, and 1 in Germany. Immunohistochemistry (IHC) was adopted to analyze the expression of PD-L1 in tumor tissues in all the studies. All studies had NOS grades ≥ 7 (Supplementary Table 2).
The prevalence ranges from 48.0 to 67.4% in studies (Table 1). The pooled prevalence of PD-L1 in penile cancer was 51.4% (random effect, 95%CI=42.1-60.8%, I2 = 88.5%) (Figure 2).
Seven studies, with 903 individuals, reported CSS (summarized in Table 2). The pooled results demonstrated that higher PD-L1 level was associated with shorter CSS (HR = 1.578, 95% CI = 1.227-2.029, I2 = 23.3%) (Figure 3). Three studies, with 153 individuals, reported OS. We found that PD-L1 had no significant association with OS in patients with penile carcinoma (HR = 1.123, 95% CI = 0.511-2.465, I2 = 0.0%) (Figure 4).
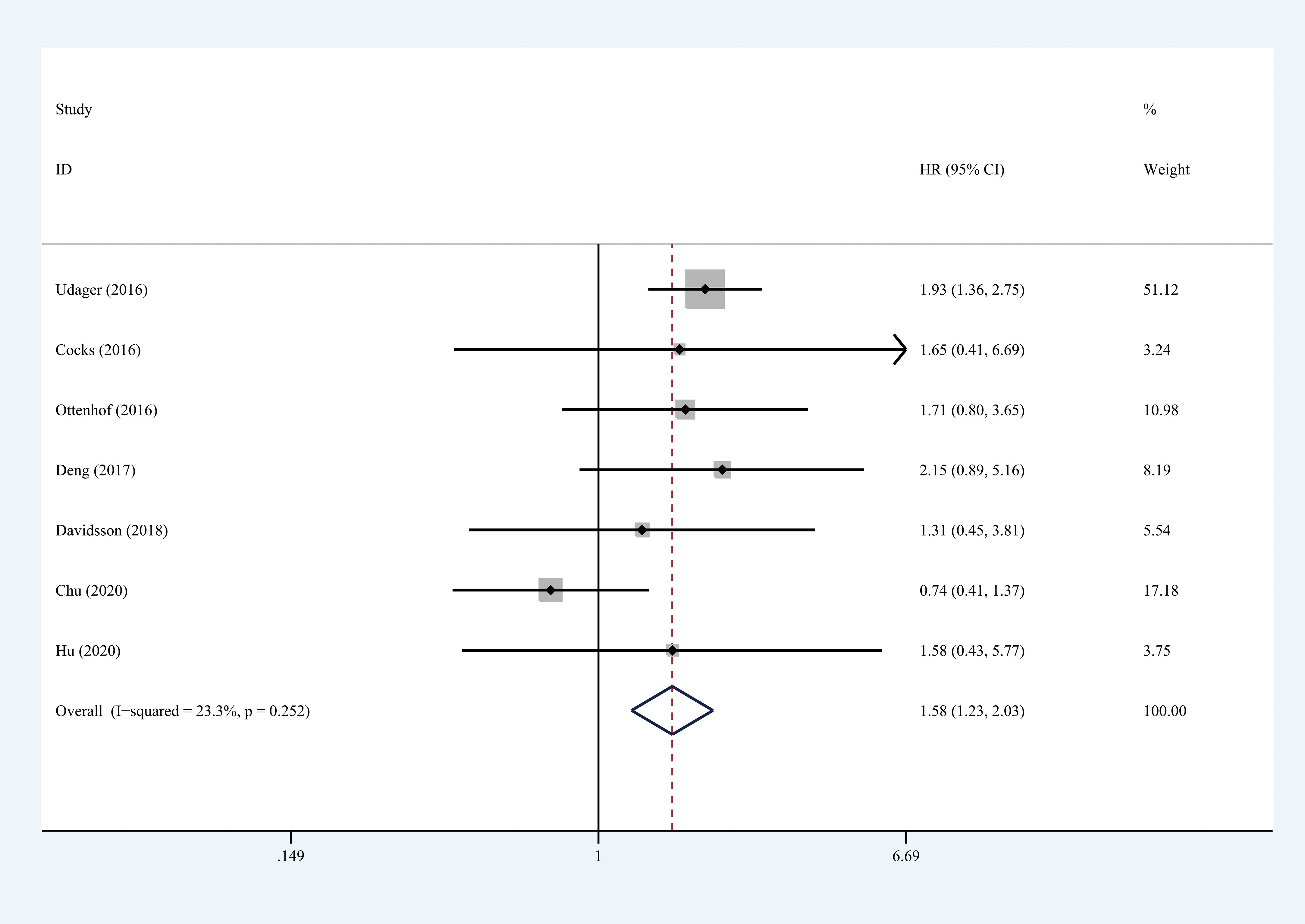
Figure 3 Prognostic value of PD-L1 for CSS. CSS, cancer specific survival; HR, hazard ratio; CI, confidence interval.
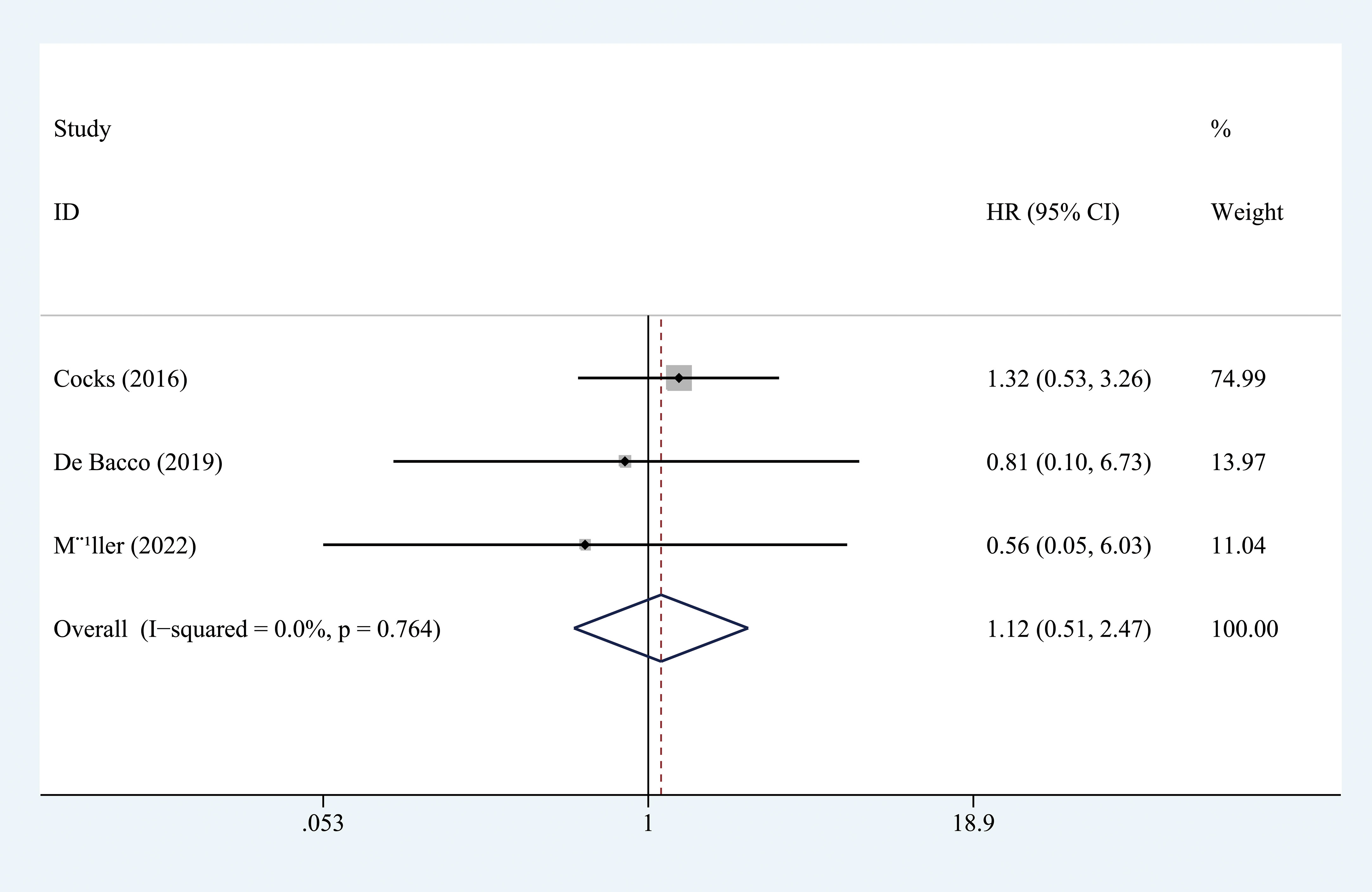
Figure 4 Prognostic value of PD-L1 for OS. OS, overall survival; HR, hazard ratio; CI, confidence interval.
Results of meta-regression and subgroup analyses were presented in Table 2. No significant results were determined among the subgroups in terms of OS. The subgroup analysis by race indicated that higher PD-L1 (higher than cut-off values) was associated with shorter CSS in Caucasians (HR = 1.827, 95% CI = 1.355-2.465, I2 = 0.0%) but not in Asians (HR = 0.852, 95% CI = 0.491-1.475, I2 = 5.8%). Moreover, results indicated that higher PD-L1 levels were associated with shorter CSS in the studies conducted in the USA (HR = 1.915, 95% CI = 1.362-2.693, I2 = 0.0%), but not in other countries. No significant difference was determined in any subgroup (Pinteraction > 0.05 for all).
Results on this were recorded in Table 3. We observed that higher PD-L1 in penile cancer had relationships with higher tumor stage (pT1 vs. pT2-4, OR = 0.480, 95% CI = 0.346-0.667, P = 0.001) and more advanced tumor grade (Well and moderate vs. Poor, OR = 0.377, 95% CI = 0.264-0.538, P < 0.001). Significant associations were also found between PD-L1 positivity and lymph node (LN) positivity (pN0/NX vs. pN1–3, OR = 0.541, 95% CI = 0.385-0.759, P = 0.001) and HPV negativity (Positive vs. Negative, OR = 0.510, 95% CI = 0.322-0.810, P = 0.003). Moreover, there was a trend toward statistical significance between PD-L1 positivity and usual histological types (Usual SCC vs. Others, OR = 1.754, 95% CI = 0.984-3.124, P = 0.07). However, PD-L1 levels had no significant associations with penile cancer in terms of lympho-vascular invasion (LVI) (presence vs. absence, OR = 1.005, 95% CI = 0.561-1.800, P = 0.087). Local recurrence, distant progression, and p16 were only reported in a single article, thus pooled results were not achievable (15).
No significant publication bias was found (CSS: Begg’s test, P = 0.652; Egger’s test, P = 0.764 (Figure 5).
We extracted each study subsequently in each analysis, finding that no study could affect the pooled result significantly, thus the results were reliable (Figure 6). The cumulative meta-analysis was performed in the order of publication year (Figure 7). It revealed that higher PD-L1 levels were related to shorter CSS, but not the OS. Furthermore, we also found that the 95%CIs narrowed and the pooled results gradually moved near the null.
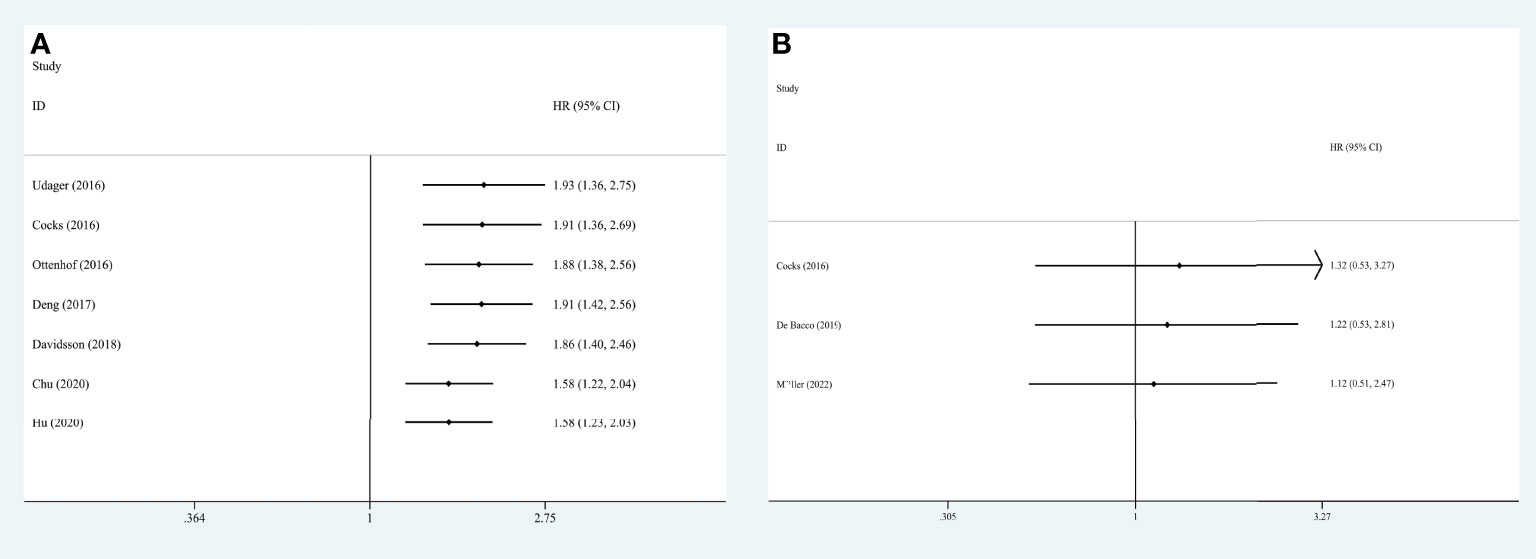
Figure 7 Cumulative meta-analysis for OS (A) and CSS (B), based on year of publication. OS, overall survival; CSS, cancer specific survival; HR, hazard ratio; CI, confidence interval.
To our knowledge, we firstly assessed the role of PD-L1 in penile cancer patients by conducting a meta-analysis. The pooled prevalence of PD-L1 in patients with penile carcinoma was 51.4% (42.1% to 60.8%) and results indicated that higher PD-L1 was not related to OS but was associated with shorter CSS. Notably, higher PD-L1 had associations with worse CSS in Caucasian penile cancer patients, especially patients from the USA. When it comes to tumor behavior, higher PD-L1 had associations with higher tumor stage and grade of penile carcinoma, LN positivity, and HPV negativity. Moreover, a trend toward statistical significance was also observed between positive PD-L1 and usual SqCC.
In the meta-analysis, seven studies reported a high expression rate (>40%) of PD-L1 (15–17, 23, 24, 26, 27), which provided rationality for immunotherapy application in such cancer. However, different cut-off values, small sample sizes, and diverse primary antibody species may influence the prevalence.
Due to the rarity of penile carcinoma, it had been largely neglected by urologic academic associations (28). Up till now, studies reporting the role of PD-L1/PD-1 in the prognosis of urological tumors had been focused mainly on urothelial carcinoma of the bladder (UCB), prostate cancer, and RCC (29). Only the well-designed studies included in the meta-analysis investigated the role of PD-L1 in penile carcinoma, but these studies were often designed differently. The meta-analysis only pooled the results from 9 studies reporting PD-L1 levels from tumor cells. Previous large clinical trials firstly explored the role of immunotherapy in advanced penile cancer or metastatic penile cancer (NCT03333616 and NCT03774901) (30, 31). They showed that these refractory conditions can’t achieve prognostic improvement after first-line modality treatment, while Avelumab or the combination of Nivolumab and Ipilimumab can enhance the prognosis. However, they did not compare the efficacy difference between subgroups of PD-L1 high and low, as researchers did in other types of tumors. Under the light of clinical trials, a few case series and case reports were published, promoting the application of immunotherapy in penile carcinoma and indicating the crucial role of PD-L1 expression in immunotherapy (32–35). Unfortunately, there were few studies in this area, and more well-designed studies are warranted.
Currently, checkpoint inhibitors have been widely rationalized in several cancers for their great efficacy and accepted adverse effects compared with conventional therapies (36). If PD-L1 levels in tumor cells were linked to clinical and pathological features, the PD-L1 inhibitors would inhibit the tumor biology, such as invasion, recurrence, and metastasis, etc. In the study, PD-L1 was related to penile cancer in T stage, tumor grade, and LN status, similar findings have been indicated in both penile cancer and other types of tumors (37). Results indicated that PD-L1 could not promote the lymphovascular invasion (LVI) of penile cancer, while only three studies mentioned related data and some heterogeneity existed in these studies (17, 24, 26). The results may provide evidence for immunotherapy and rationalize it as a promising perioperative therapy for penile cancer.
Apart from PD-L1, some newly developed molecules related to genetic, epigenetic, and immune responses in patients with penile cancer should also be mentioned. Marchi et al. utilized data from a public database identifying STAT1, which is potentially associated with dysfunction of the immune system, as prognostic factor for penile cancer (38). Later, it was found that the knockdown of CCL20, CXCL13, or CXCL5 gave the same results shown by significant suppression of STAT1 level and inhibition of MMP2 and MMP9 expression, leading to the diminished proliferation, migration, and invasion of penile cancer cells (39, 40). SHCBP1 is a gene that has physiological associations with T cell proliferation and signaling. Researchers have concluded that SHCBP1 may be used as a prognostic biomarker. In vitro and in vivo experiments indicated that SHCBP1 knockdown results in the decrease of the proliferation, migration, and invasion of penile cancer cells, while forced activation of STAT3 reverses this process (41). Together, SHCBP1 has the potential to be used not only as a biomarker but also as a target for future treatment strategies. Furthermore, more and more promising molecules and pathways, including Pten, Smad4/Apc, PPARG, JAK–STAT, and MMP1 were found, providing potential therapeutic targets for the uncommon cancer (42, 43).
Our findings have some research and clinical implications. Firstly, the expression of PD-L1 may be a meaningful marker for prognosis anticipation in penile cancer cases. Patients with positive PD-L1 may tend to show more advanced tumor features and have a potentially worse cancer-specific prognosis. Secondly, PD-L1 expression maybe not the only biomarker associated with prognosis in the population, which should be identified in future laboratory studies. Thirdly, PD-1/PD-L1 blockades could be an effective treatment selection for penile cancer patients with positive PD-L1. We have also conducted a similar study in RCC (44). We found that the expression of PD-L1 in RCC is 27%, thus the expression of PD-L1 is more frequent in penile caner. Now that in the post-surgical treatment of advanced or metastatic renal cell carcinoma (RCC), immunotherapy has become the first-line drug (45). We believed PD-L1-related immunotherapy should have much more potential in the treatment of penile cancer. While the responsiveness of penile cancer to immunotherapy, just like other cancers, depends on several indicators, such as tumor mutation burden, immune infiltration patterns, macrophage infiltration patterns, and HPV status, etc. Future findings from clinical trials will hopefully elucidate the tangible conclusion regarding the role of immunotherapy in penile cancer. Given the rarity of the cancer, accruing patients to trials is difficult, suggesting the importance of multidisciplinary and global collaboration.
The study has some strengths. (A) The reliability of the results was repeatedly validated in the methodology. (B) IHC is an easy-to-use and widely-used method to evaluate the expression of protein. Therefore, the findings are clinically performable. The study also has some limitations. First and foremost, heterogeneity originating from the different cut-off values and sometimes the differences in primary antibody species, makes it difficult to reach a solid conclusion. Secondly, PD-L1 or PD-1 expressed by other cells or tissues were not evaluated and reviewed. Thirdly, four studies did not mention the staging standard (15, 22, 25, 26). Among other studies, those published after 2018 used different staging systems from those published before 2018. For studies after 2018, they even use different staging standards. This makes subgroup analysis unavailable. Thus, the differences in staging may cause bias, making the pooled results on stage unconvincing. Fourthly, we only roughly compared the usual SCC with all other unusual SCC. This was conducted and displayed because four of the included studies made this comparison and the pooled result was statistically calculatable. However, the clinical significance of the result is limited because unusual penile SCC consists of various subtypes and each subtype has its own pathological and prognostic features.
In conclusion, the positive rate of PD-L1 was 51.4% (95%CI: 42.1% to 60.8%) in penile cancer. Higher PD-L1 levels in tumor cells were related to shorter CSS in penile cancer patients and higher T stage, higher tumor grade, positive LN status, HPV negativity, and usual SqCC of penile cancer. Incorporating PD-L1 into prognostic tools for adjuvant treatment selection might help improve the survival of penile cancer. More studies comparing the efficacy difference and prognosis between patients with high PD-L1 and those with low PD-L1 are expected in the promising field.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
(I) Conception: YL and HJL. (II) Administrative support: HJL. (III) Collection: YL, HS, and YTW. (IV) Data analysis and interpretation: HS and YTW. (V) Manuscript writing, revision and approval: All authors. All authors contributed to the article and approved the submitted version.
This work is supported by the grant from National Population Health Science Data Sharing Service Platform Clinical Medical Science Data Center (NCMI-ABD02-201906) to HJL.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1013806/full#supplementary-material
1. Kayes O, Ahmed HU, Arya M, Minhas S. Molecular and genetic pathways in penile cancer. Lancet Oncol (2007) 8(5):420–9. doi: 10.1016/S1470-2045(07)70137-7
2. Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO classification of tumours of the urinary system and Male genital organs-part a: Renal, penile, and testicular tumours. Eur Urol (2016) 70(1):93–105. doi: 10.1016/j.eururo.2016.02.029
3. Pettaway CA, Pagliaro L, Theodore C, Haas G. Treatment of visceral, unresectable, or bulky/unresectable regional metastases of penile cancer. Urology (2010) 76(2 Suppl 1):S58–65. doi: 10.1016/j.urology.2010.03.082
4. Wang J, Pettaway CA, Pagliaro LC. Treatment for metastatic penile cancer after first-line chemotherapy failure: Analysis of response and survival outcomes. Urology (2015) 85(5):1104–10. doi: 10.1016/j.urology.2014.12.049
5. Garassino MC, Gadgeel S, Esteban E, Felip E, Speranza G, Domine M, et al. "Patient-reported outcomes following pembrolizumab or placebo plus pemetrexed and platinum in patients with previously untreated, metastatic, non-squamous non-small-cell lung cancer (KEYNOTE-189): A multicentre, double-blind, randomised, placebo-controlled, phase 3 trial." the lancet. Oncology (2020) 21(3):387–97. doi: 10.1016/S1470-2045(19)30801-0
6. Mansfield AS, Każarnowicz A, Karaseva N, Sánchez A, De Boer R, Andric Z, et al. Safety and patient-reported outcomes of atezolizumab, carboplatin, and etoposide in extensive-stage small-cell lung cancer (IMpower133): A randomized phase I/III trial. Ann Oncol (2020) 31(2):310–7. doi: 10.1016/j.annonc.2019.10.021
7. de Vries H-M, Ottenhof SR, Horenblas S, van der Heijden MS, Jordanova ES. Defining the tumor microenvironment of penile cancer by means of the cancer immunogram. Eur Urol Focus (2019) 5(5):718–21. doi: 10.1016/j.euf.2019.02.019
8. Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med (2018) 24(5):541–50. doi: 10.1038/s41591-018-0014-x
9. Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer (2019) 19(3):133–50. doi: 10.1038/s41568-019-0116-x
10. Siefker-Radtke A, Curti B. Immunotherapy in metastatic urothelial carcinoma: focus on immune checkpoint inhibition. Nat Rev Urol (2018) 15(2):112–24. doi: 10.1038/nrurol.2017.190
11. Siefker-Radtke AO, Apolo AB, Bivalacqua TJ, Spiess PE, Black PC. Immunotherapy with checkpoint blockade in the treatment of urothelial carcinoma. J Urol (2018) 199(5):1129–42. doi: 10.1016/j.juro.2017.10.041
12. Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, et al. Cancer treatment and survivorship statistic. CA Cancer J Clin (2019) 69(5):363–85. doi: 10.3322/caac.21565
13. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer (2012) 12(4):252–64. doi: 10.1038/nrc3239
14. Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol (2015) 33(17):1974–82. doi: 10.1200/JCO.2014.59.4358
15. Udager AM, Liu TY, Skala SL, Magers MJ, McDaniel AS, Spratt DE, et al. Frequent PD-L1 expression in primary and metastatic penile squamous cell carcinoma: Potential opportunities for immunotherapeutic approaches. Ann Oncol (2016) 27(9):1706–12. doi: 10.1093/annonc/mdw216
16. Chu C, Yao K, Lu J, Zhang Y, Chen K, Lu J, et al. Immunophenotypes based on the tumor immune microenvironment allow for unsupervised penile cancer patient stratification. Cancers (Basel) (2020) 12(7). doi: 10.3390/cancers12071796
17. Hu J, Li H, He T, Deng H, Gong G, Cui Y, et al. A nomogram incorporating PD-L1, NLR, and clinicopathologic features to predict inguinal lymph node metastasis in penile squamous cell carcinoma. Urol Oncol (2020) 38(7):641.e619–641.e629. doi: 10.1016/j.urolonc.2020.04.015
18. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. meta-analysis of observational studies in epidemiology (MOOSE) group. Jama (2000) 283(15):2008–12. doi: 10.1001/jama.283.15.2008
19. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Bmj (2009) 339:b2535. doi: 10.1136/bmj.b2535
20. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials (2007) 8:16. doi: 10.1186/1745-6215-8-16
21. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
22. Cocks M, Taheri D, Ball MW, Bezerra SM, Del Carmen Rodriguez M, Ricardo BFP, et al. Immune-checkpoint status in penile squamous cell carcinoma: A north American cohort. Hum Pathol (2016) 59:55–61. doi: 10.1016/j.humpath.2016.09.003
23. Deng C, Li Z, Guo S, Chen P, Chen X, Zhou Q, et al. Tumor PD-L1 expression is correlated with increased TILs and poor prognosis in penile squamous cell carcinoma. Oncoimmunology (2017) 6(2):e1269047. doi: 10.1080/2162402X.2016.1269047
24. Ottenhof SR, Djajadiningrat RS, de Jong J, Thygesen HH, Horenblas S, Jordanova ES. Expression of programmed death ligand 1 in penile cancer is of prognostic value and associated with HPV status. J Urol (2016) 197(3 Pt 1):690–7. doi: 10.1016/j.juro.2016.09.088
25. Davidsson S, Carlsson J, Giunchi F, Harlow A, Kirrander P, Rider J, et al. PD-L1 expression in men with penile cancer and its association with clinical outcomes. Eur Urol Oncol (2018) 2(2):214–21. doi: 10.1016/j.euo.2018.07.010
26. De Bacco MW, Carvalhal GF, MacGregor B, Marçal JMB, Wagner MB, Sonpavde GP, et al. PD-L1 and p16 expression in penile squamous cell carcinoma from an endemic region. Clin Genitourin Cancer (2019) 18(3):e254–9. doi: 10.1016/j.clgc.2019.10.014
27. Müller T, Demes M, Lehn A, Köllermann J, Vallo S, Wild PJ, et al. The peri- and intratumoral immune cell infiltrate and PD-L1 status in invasive squamous cell carcinomas of the penis. Clin Transl Oncol (2022) 24(2):331–41. doi: 10.1007/s12094-021-02694-7
28. Bandini M, Ahmed M, Basile G, Watkin N, Master V, Zhu Y, et al. A global approach to improving penile cancer care. Nat Rev Urol (2022) 19(4):231–9. doi: 10.1038/s41585-021-00557-y
29. Xiong W, Gao Y, Wei W, Zhang J. Extracellular and nuclear PD-L1 in modulating cancer immunotherapy. Trends Cancer (2021) 7(9):837–46. doi: 10.1016/j.trecan.2021.03.003
30. Gassian N, Frontczak A, Mouillet G, Vernerey D, Manseur O, Goujon M, et al. Activity and tolerability of maintenance avelumab immunotherapy after first line polychemotherapy including platinum in patients with locally advanced or metastatic squamous cell penile carcinoma: PULSE. Bull Cancer (2020) 107(5s):eS16–21. doi: 10.1016/S0007-4551(20)30282-4
31. McGregor BA, Campbell MT, Xie W, Farah S, Bilen MA, Schmidt AL, et al. Results of a multicenter, phase 2 study of nivolumab and ipilimumab for patients with advanced rare genitourinary malignancies. Cancer (2021) 127(6):840–9. doi: 10.1002/cncr.33328
32. Chahoud J, t. Skelton WP, Spiess PE, Walko C, Dhillon J, Gage KL, et al. Case report: Two cases of chemotherapy refractory metastatic penile squamous cell carcinoma with extreme durable response to pembrolizumab. Front Oncol (2020) 10:615298. doi: 10.3389/fonc.2020.615298
33. Su X, Zhang J, Fu C, Xiao M, Wang C. Recurrent metastatic penile cancer patient with positive PD-L1 expression obtained significant benefit from immunotherapy: A case report and literature review. Onco Targets Ther (2020) 13:3319–24. doi: 10.2147/OTT.S231258
34. Hahn AW, Chahoud J, Campbell MT, Karp DD, Wang J, Stephen B, et al. Pembrolizumab for advanced penile cancer: a case series from a phase II basket trial. Invest New Drugs (2021) 39(5):1405–10. doi: 10.1007/s10637-021-01100-x
35. Hu L, Shan X, Han D, Guo Z, Wang H, Xiao Z. Multimodal treatment combining salvage surgery-assisted chemotherapy and checkpoints blockade immunotherapy achieves complete remission on a recurrent penile cancer patient: A case report. Onco Targets Ther (2021) 14:4891–6. doi: 10.2147/OTT.S319932
36. Kubli SP, Berger T, Araujo DV, Siu LL, Mak TW. Beyond immune checkpoint blockade: emerging immunological strategies. Nat Rev Drug Discov (2021) 20(12):899–919. doi: 10.1038/s41573-021-00155-y
37. Nishino M, Ramaiya NH, Hatabu H, Hodi FS. Monitoring immune-checkpoint blockade: response evaluation and biomarker development. Nat Rev Clin Oncol (2017) 14(11):655–68. doi: 10.1038/nrclinonc.2017.88
38. Marchi FA, Martins DC, Barros-Filho MC, Kuasne H, Busso Lopes AF, Brentani H, et al. Multidimensional integrative analysis uncovers driver candidates and biomarkers in penile carcinoma. Sci Rep (2017) 7(1). doi: 10.1038/s41598-017-06659-1
39. Mo M, Tong S, Huang W, Cai Y, Zu X, Hu X. High serum CCL20 is associated with tumor progression in penile cancer. J Cancer (2020) 11(23):6812–22. doi: 10.7150/jca.48939
40. Mo M, Li Y, Hu X. Serum CXCL5 level is associated with tumor progression in penile cancer. Biosci Rep (2021) 41(1). doi: 10.1042/BSR20202133
41. Chen R, Li H, Li Y, Fazli L, Gleave M, Nappi L, et al. Loss of nuclear functions of HOXA10 is associated with testicular cancer proliferation. Front Oncol (2018) 8:594. doi: 10.3389/fonc.2018.00594
42. Aydin AM, Chahoud J, Adashek JJ, Azizi M, Magliocco A, Ross JS, et al. Understanding genomics and the immune environment of penile cancer to improve therapy. Nat Rev Urol (2020) 17(10):555–70. doi: 10.1038/s41585-020-0359-z
43. Huang T, Cheng X, Chahoud J, Sarhan A, Tamboli P, Rao P, et al. Effective combinatorial immunotherapy for penile squamous cell carcinoma. Nat Commun (2020) 11(1). doi: 10.1038/s41467-020-15980-9
44. Lu Y, Song Y, Xu Y, Ou N, Liang Z, Hu R, et al. The prevalence and prognostic and clinicopathological value of PD-L1 and PD-L2 in renal cell carcinoma patients: A systematic review and meta-analysis involving 3,389 patients. Transl Androl Urol (2020) 9(2):367–81. doi: 10.21037/tau.2020.01.21
Keywords: PD-L1, immune checkpoint, prognostic biomarker, meta-analysis, penile carcinoma
Citation: Lu Y, Wang Y, Su H and Li H (2022) PD-L1 is associated with the prognosis of penile cancer: A systematic review and meta-analysis. Front. Oncol. 12:1013806. doi: 10.3389/fonc.2022.1013806
Received: 07 August 2022; Accepted: 03 November 2022;
Published: 30 November 2022.
Edited by:
Marco Borghesi, University of Genoa, ItalyReviewed by:
Guru P. Sonpavde, Dana–Farber Cancer Institute, United StatesCopyright © 2022 Lu, Wang, Su and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongjun Li, eXBsbWFpbGJveEAxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.