
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol., 20 September 2022
Sec. Cancer Imaging and Image-directed Interventions
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1013801
This article is part of the Research TopicSite Specific Imaging Guidelines In Head & Neck, and Skull Base CancersView all 5 articles
 Florent Carsuzaa1,2*
Florent Carsuzaa1,2* Benjamin Verillaud3
Benjamin Verillaud3 Pierre-Yves Marcy4
Pierre-Yves Marcy4 Philippe Herman3
Philippe Herman3 Xavier Dufour1,2
Xavier Dufour1,2 Valentin Favier5
Valentin Favier5 Juliette Thariat6,7
Juliette Thariat6,7In sinonasal cancer surgery, a fundamental challenge is to understand the postoperative imaging changes after reconstruction. Misinterpretation of post-operative imaging may lead to a misdiagnosis of tumor recurrence. Because radiotherapy planning is based on imaging, there are many gaps in knowledge to be filled in the interpretation of postoperative imaging to properly define radiotherapy tumor volumes in the presence of flaps. On the other hand, radiotherapy may be responsible for tissue fibrosis or atrophy, the anatomy of the reconstructed region and the functional outcomes may change after radiotherapy compared to surgery alone. This narrative review illustrates the interdisciplinary aims and challenges of sinonasal reconstructive surgery using flaps or grafts. It is particularly relevant to radiologists and radiation oncologists, at a time when intensity modulated radiotherapy and proton therapy have the potential to further contribute to reduction of morbidity.
Sinonasal cancers represent 3-5% of head and neck cancers with a peak incidence in the 5th to 7th decades and with a male preponderance (1, 2). Histological types are more varied than in the other head and neck cancer locations where squamous cell carcinoma remains the most frequent, followed by adenocarcinoma, melanoma, olfactory neuroblastoma and adenoid cystic carcinoma (1, 3, 4). For a majority of histologic subtypes, surgery, when feasible, is the gold standard of treatment and radiotherapy is usually indicated as an adjuvant modality to optimize local control (5). Surgical techniques have evolved over time, with a clear switch from open to endoscopic approaches in most cases. However, the main surgical challenge remains the same: to preserve critical structures such as the brain, the orbit, the optic nerve and the internal carotid artery. Reconstruction techniques after removal of sinonasal cancers focus on preventing complications associated with the procedure, reducing short- and long-term morbidity (cerebrospinal fluid (CSF) leaks, vascular and functional damages) and preventing aesthetic and functional sequelae (enophthalmos, oronasal fistula). These reconstruction techniques have also significantly changed over the past 2 decades and the reconstructed anatomy is meant to be robust enough to withstand radiotherapy.
Understanding the postoperative imaging changes after head and neck cancer reconstructions is a challenge (6, 7). Sinonasal reconstructions are all the more difficult to analyze on imaging due to their variable anatomy and versatility in the immediate postoperative setting of early complications or routine long-term follow-up. Misinterpretation of post-operative imaging may lead to either a misdiagnosis of tumor recurrence, or on the contrary to delayed diagnosis if the recurrence is mistaken for a reconstruction-related change. Among the daily multidisciplinary challenges of cancer treatment, transfer of surgical advances into radiotherapy planning is little assessed.
Similarly, there are many gaps in knowledge to be filled in the interpretation of postoperative imaging to properly define radiotherapy tumor volumes in the presence of imaging variability imposed by reconstruction. On the other hand, radiotherapy may be responsible for tissue fibrosis or atrophy, the anatomy of the reconstructed region and the functional outcomes may change after radiotherapy compared to surgery alone. Together, these factors impose challenges for all physicians involved in follow-up
This narrative review illustrates the interdisciplinary aims and challenges of sinonasal reconstructive surgery using flaps or grafts. It is particularly relevant to radiologists and radiation oncologists, at a time when intensity modulated radiotherapy (IMRT) and proton therapy have the potential to further contribute to reduction of morbidity following on strategy initiated by surgeons.
Open surgery with transfacial and craniofacial resection has long been the only method for the surgical treatment of sinonasal tumors, historically. Since the emergence of endoscopic endonasal surgery, open surgical techniques were progressively being replaced unless involvement of the lateral or anterior wall of the frontal sinus or the anterior wall of the maxillary sinus, for example, are involved (8–10). Open surgery is also used in cases of bone invasion or subcutaneous soft tissue involvement, requiring wide excision.
Initially reserved for treatment of chronic rhinosinusitis and functional surgeries limited to the nasal cavities and paranasal sinuses, endoscopic endonasal surgery has evolved with the development of endoscopic skull base approaches and transnasal craniotomy techniques and has since been used for malignant tumors (11–13). Endoscopic approaches were first described for intestinal-type adenocarcinoma (ITAC), then for adenoid cystic carcinomas (14), melanomas (15) and are now implemented regardless of histology. For malignant tumors, the resection uses a centripetal technique. Tumor debulking is primarily performed in order to identify the tumor attachment base (often called pedicle, although this should not be confused for vascular pedicle). Then, clinically uninvolved sinuses preoperatively are opened to allow visualization of the medial orbits, nasofrontal recesses, and sphenoid sinuses. Bony landmarks (optic canals, carotid canals) are identified. Finally, centripetal tumor resection is performed as well as resection of a safety plane (additional margin) where tumor was present. Iterative frozen section biopsy is performed in macroscopically healthy tissue remote from the tumor throughout the procedure to ensure comprehensive resection (16). The operative report should accurately describe the gross tumor, its base and extensions as well as resection areas depending on whether margins are possibly involved (and to be confirmed with pathology report of similar granularity/accuracy).
Open or endoscopic endonasal techniques may use different reconstruction techniques. Local grafts or flaps are used in endoscopic endonasal reconstruction whereas larger defects may be repaired in open approaches. If reconstruction is performed, it should be described in terms of tissue components, insertions and vascularization. Peroperative and immediate postoperative complications should also be reported.
When the tumor involves the skull base, a post-operative CSF leak may occur in 0.5 to 5% of surgical series (8, 17, 18). This risk depends mostly on the location and the size of the defect (19). Several complications can occur as a result of CSF leak including meningitis, empyema, brain abscess, decreased cranial pressure, brain herniation, and death (20). Reconstructive surgery therefore aims at restoring the barrier between the subdural space and sinonasal cavities to avoid postoperative CSF leak. The development of duraplasty techniques (multilayered reconstruction with grafts or vascularized flaps) allowed a large reduction in morbidity and mortality. In recent series, the overall success of CSF leak repair with endonasal flaps is now about 95% (21). A raised intracranial pressure is the main factor associated with failure of duraplasty but other factors depending on the location and size of the defect, or the pathology itself (e.g.: craniopharyngiomas) must be taken into account (22). A special attention to the quality of CSF leak closure is of paramount importance in these situations. A history of radiotherapy (poor tissue healing) or prior surgery with compromise of local vascularized tissue reconstructive options make reconstruction techniques more difficult (23).
In case of intraconal tumor extension and invasion of the infrastructure of the maxilla, the oncologic resection requires open approach with orbital exenteration and open maxillectomy, respectively. In these situations, a reconstruction for functional and aesthetic purposes is mandatory. These reconstructions are mainly performed using free flaps providing bony components (24, 25). Bony reconstructions can further allow the installation of nasal or orbital epithesis, or the implementation of a good dental rehabilitation in maxillary reconstructions. In situations where bone or soft tissue are resected, free flap reconstruction often necessitates a bone flap to reconstruct the maxillary defect. The subcutaneous soft tissues and the cutaneous part are often reconstructed by chimeric flaps (associating independent components and each having their own vascularization) or composite flaps (associating independent components and having the same vascularization).
When critical anatomical structures such as the internal carotid artery, the optic nerve or the clivus are exposed to air or saliva postoperatively, reconstructive surgery aims to protect these structures from damages, ideally with a vascularized flap as it improves the effectiveness of the reconstruction in the long term (26, 27). This is particularly important when postoperative radiotherapy is considered (26–28).
Tissues used for reconstruction surgery include grafts and flaps (Table 1). Grafts are tissues harvested locally (nasal mucopericondrium/mucoperiostium) or from another site (abdominal fat, fascia lata). Grafts do not bring their own blood supply and may indeed not need vascularization as their main aim is to protect an anatomic area from by use of solid coverage. Graft living tissues may turn into fibrous inert tissues. They are usually harvested from the patient, however occasionally, the reconstruction will not use autologous tissues. Flaps are tissues harvested from a donor site and moved to the recipient site with their blood vessels. Flaps can be harvested locally (nasal fossae), regionally from an adjacent region (temporal fossa, face) or at distant sites (free flaps requiring microvascular anastomosis).
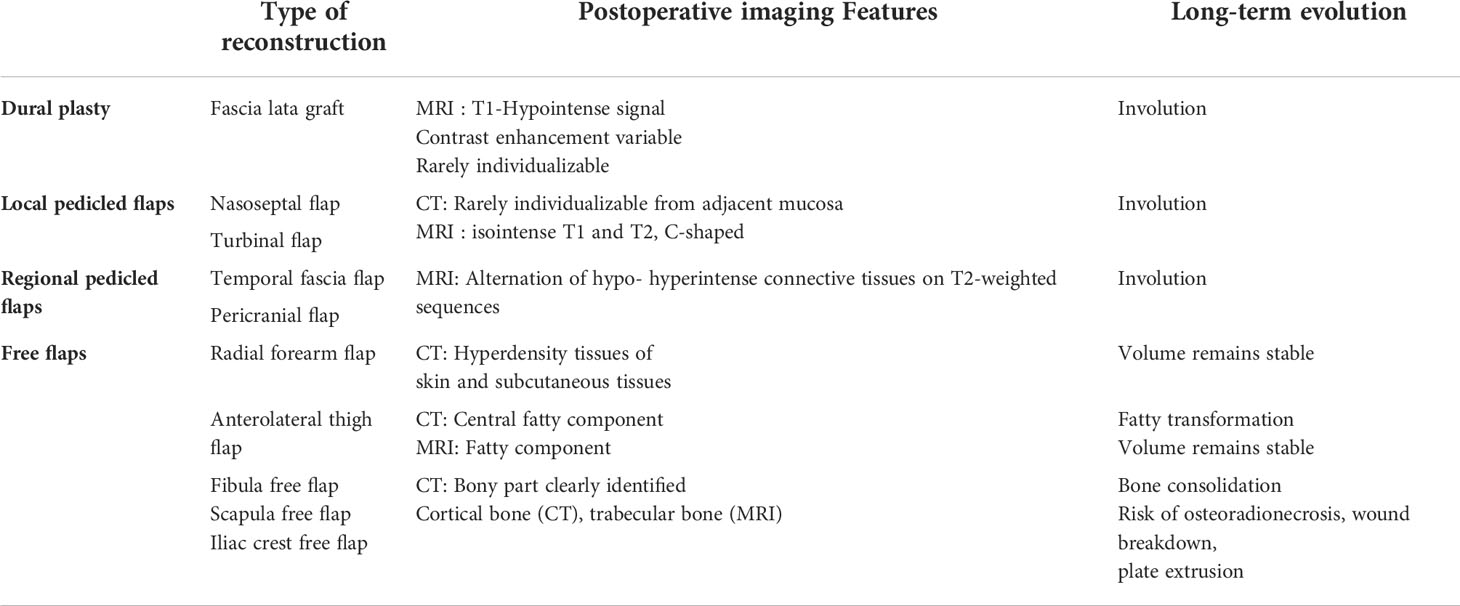
Table 1 Types of various surgical flaps used in sinonasal cancer surgery, postoperative & long-term follow-up imaging features.
Free grafts are frequently used for duraplasty. Free grafts can be used in combination with another free graft [e.g. fascia lata with a piece of cartilage (29)], or in combination with a local pedicled flap. In post-operative imaging (Table 1), the different components of the grafts may no longer be individualized and a hypointense signal without contrast enhancement is observed between the two parts of the bone defect.
The fascia lata graft is a non-vascularized connective tissue, very thin (1-2mm), harvested from the patient’s thigh and positioned to close the defect in a single or multilayer fashion depending on surgeon preferences (Figure 1). This technique is effective and safe with 3% donor site morbidity (30, 31). Using facia lata graft protect from the increased risk of CSF leak reconstruction after radiotherapy (31). In the long term, fibrosis of the various components used for reconstruction appears.
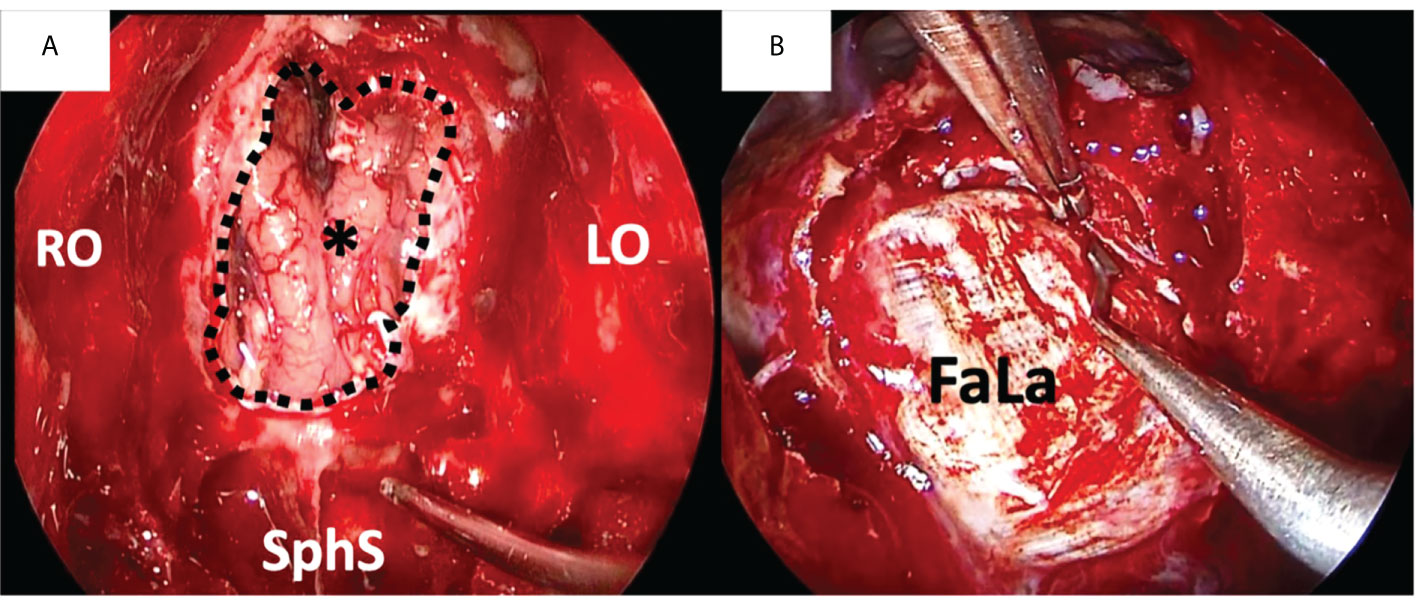
Figure 1 Duraplasty with fascia lata (FaLa) after endoscopic transnasal craniectomy for an intestinal type adenocarcinoma. (A) Endoscopic view of the surgical field after tumor removal: the right and left orbital walls (RO and LO, respectively), the sphenoid sinus (SphS) and the frontal lobe (asterisk) are exposed; the dotted line marks the limits of dural resection. (B) The first layer of fascia lata is placed intradurally to obtain watertight closure.
Adult abdominal fat, which is known to contain pluripotent stem cells, is frequently used to repair limited anterior skull base defects. Fat lobules are taken from the abdomen by a sub-umbilical incision or from the subcutis of the tight incision in case of simultaneous need of fascia lata. In large skull base defects, fat grafts can’t be left in a place in contact with air flow inside the nose because of the risk of liponecrosis, increased by radiotherapy. It is then recommended to cover fat graft with mucosal graft or a pedicle flap in order to avoid this complication. This technique is safe and has a low rate of complications. In particular, an increase in carcinological risk has never been described following stimulation by stem cells derived from adipocytes (32). In the long term, fat atrophy is observed (32). In early postoperative, fatty components can be visualized in hypersignal in T1-weighted and T2-weighted MRI sequences, however this examination is rarely performed in the very early postoperative period (Figure 2).
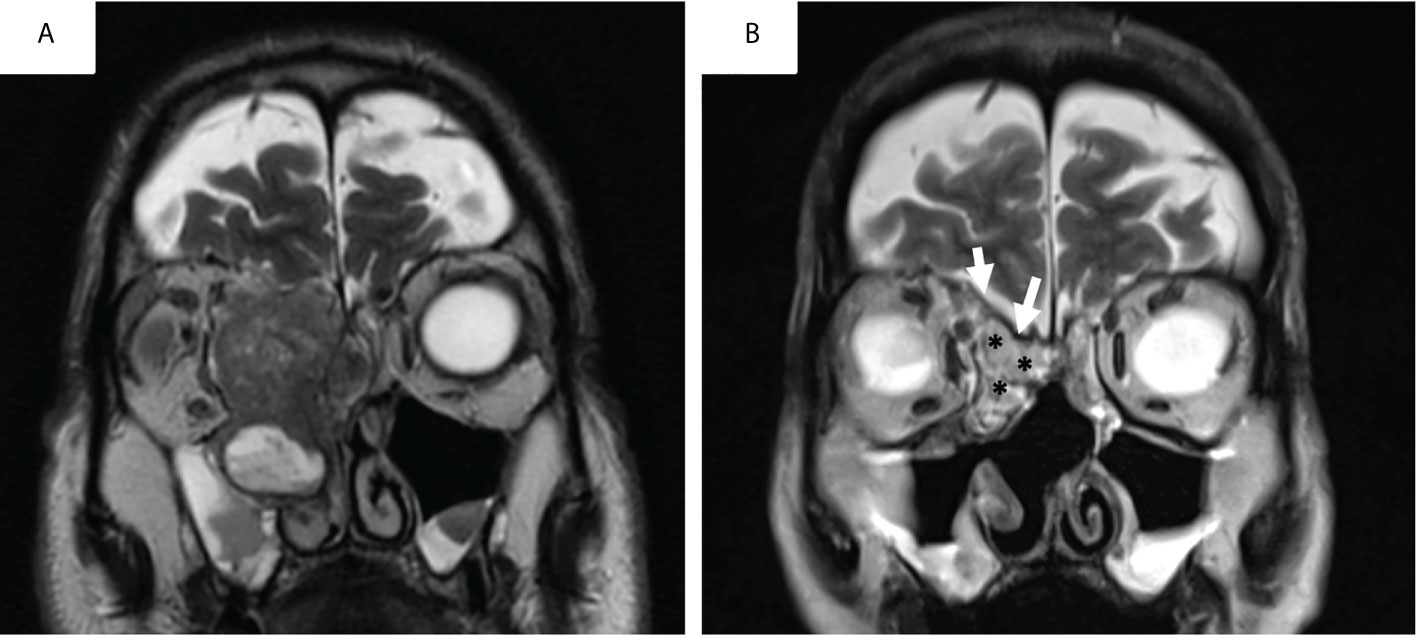
Figure 2 Pre-operative (A) and early post-operative aspect (postoperative day5) of a skull base reconstruction with fascia-lata and abdominal fat graft (B) for an intestinal type adenocarcinoma on T2-weighted MRI sequences.
Mucosal grafts are pieces of mucosa, harvested from the nasal cavity floor, middle or inferior turbinate or nasal septum in a quick and minimally morbid way (33) that can be used to reconstruct limited skull base defects in combination with other free grafts.
Cartilaginous grafts, harvested from the septal cartilage can be used as a first layer of closure and fashioned to the size of defect. Bone graft, harvested from the vomer, could also be used in this case. Although less effective than a fascia lata, this type of reconstruction can be useful for limited skull base defects and small orbital defects (34).
The use of free grafts makes it possible to cover a loss of substance. However, its lifespan is limited due to the absence of vascular blood supply. The use of local or regional pedicled flaps ensures vascularization and improves the effectiveness of the flap over the long term. In postoperative in CT-scan, local flaps are rarely individualizable from the adjacent mucosa. In MRI, local flaps have a C-shaped configuration within the operative defect, isointense on T1-weighted and T2-weighted images on both immediate and delayed MRI. After contrast enhancement, local flaps have an hyperintense aspect (Figure 3).
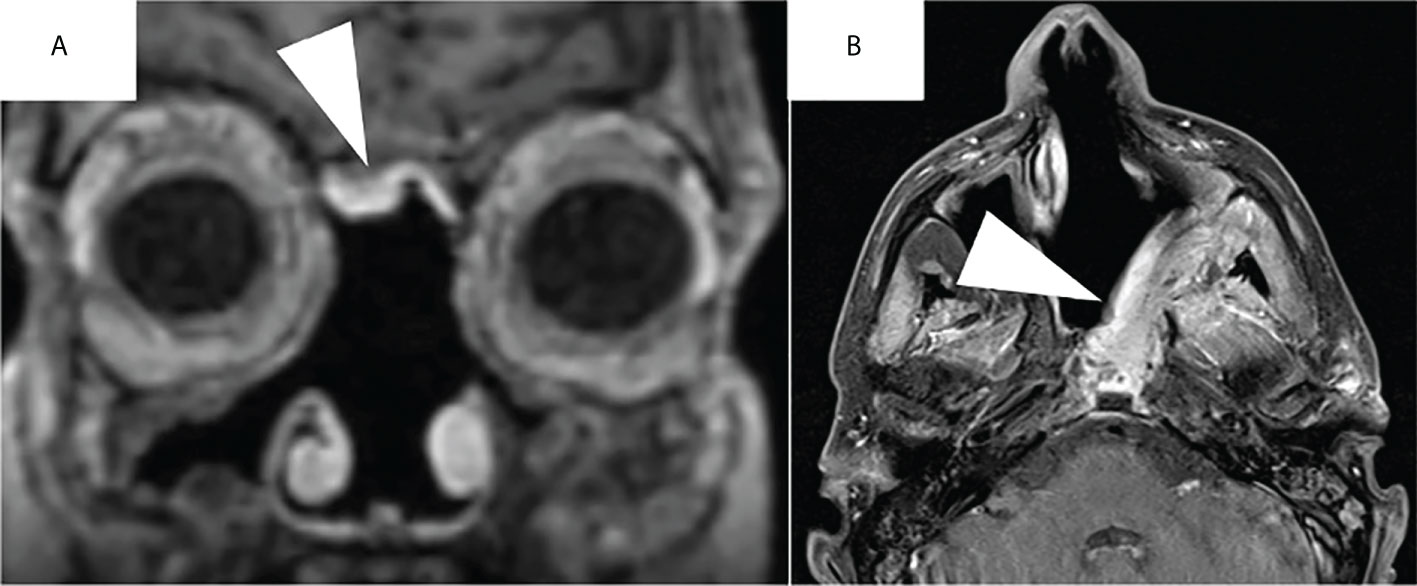
Figure 3 Post-operative aspect of a nasoseptal flap (A) and of a left superficial temporoparietal fascia flap (B) on contrast-enhanced T1-weighted MRI sequences. Note the hyperintense aspect of the flaps after contrast enhancement (white arrowheads).
The pedicled nasoseptal flap (NSF) is the first choice for most skull base reconstructions thanks to its large surface, ease of harvesting and versatile rotational angle of the pedicle. The use of this flap as described by Hadad et al. (35), has diminished the risk of postoperative CSF leaks and morbidity associated with extended endoscopic skull base approaches (36, 37). NSF is a mucoperiosteal flap pedicled on the posterior nasoseptal artery. It could be elevated to repair the skull base defect or to cover an exposed structure (e.g. carotid artey).
The turbinal flap is a mucoperiostal pedicled flap based on the middle and superior turbinates and pedicled on the ethmoidal artery system. Compared with other endonasal flaps, the turbinal flap has a double pedicle from the anterior and posterior ethmoidal arteries, which confers abundant blood supply. A vertical incision is performed at the middle turbinate anterior edge and a subperiostal dissection is carried out on the lateral side of the middle turbinate until the mucoperiostal flap is isolated from the bony framework and the flap elevated. This flap is adequate to cover defects along the entire ethmoid roof up to a size of 8cm2. It can also cover the medial orbital wall and medial portion of the roof of maxillary sinus (38).
The septal flip flap is a mucoperiostal pedicled flap harvested from the contralateral nasal septum based on the septal branches of the anterior and posterior ethmoidal arteries and can be rotated to resurface an anterior skull base defect (39). This flap provides vascularized mucosal coverage extending from the frontal recess back to the planum sphenoidalis (40).
The lateral nasal wall flap is a mucoperiostal pedicled flap harvested from the lateral nasal wall. It is based on a rich vascular supply based on an anterior pedicle comprising branches of the facial (angular and lateral nasal) and ethmoidal arteries. This flap is useful when an anterior pedicle is needed and when the septum is absent (41).
When a local pedicled flap cannot be used due to a too large defect, an unavailability of nasal mucosa in cases of salvage reconstructions or a prior irradiation which causes an unfavorable condition for local flap viability, a regional pedicled flap can be used. These flaps allow to cover large skull base defects and vascular exposures in the nasal cavities. In postoperative imaging, local flaps are rarely individualizable in CT-scan. Frequently composed of connective tissue, they can be distinguished in T2-weighted MRI sequences. Regional flaps have also a C-shaped configuration within the operative defect, isointense on T1-weighted and T2-weighted images and an hyperintense aspect after contrast enhancement.
The superficial temporoparietal fascia flap (TPFF) is a connective tissue flap vascularized by the superficial temporal artery, which has a larger diameter than the ethmoidal or sphenopalatine arteries and is usually spared by external radiations in case of prior radiotherapy (42, 43). Its properties, such as thinness, foldability and its long pedicle make it a versatile flap for reconstruction of various defects of the skull base, both in adult and children. It is a good reconstructive option for defects of the middle and posterior fossa (44). It is less used for anterior skull base repairs because of the orientation of its pedicle (45). Its transposition into the nasal cavity through a hemi-coronal approach is frequently performed through a temporal-infratemporal tunnel (42). Other way for TPFF transposition were more recently described like the supraorbital epidural corridor (46). It represents a safe and effective technique with morbidity limited to potential alopecia, facial nerve damage and scalp necrosis (Figure 4).
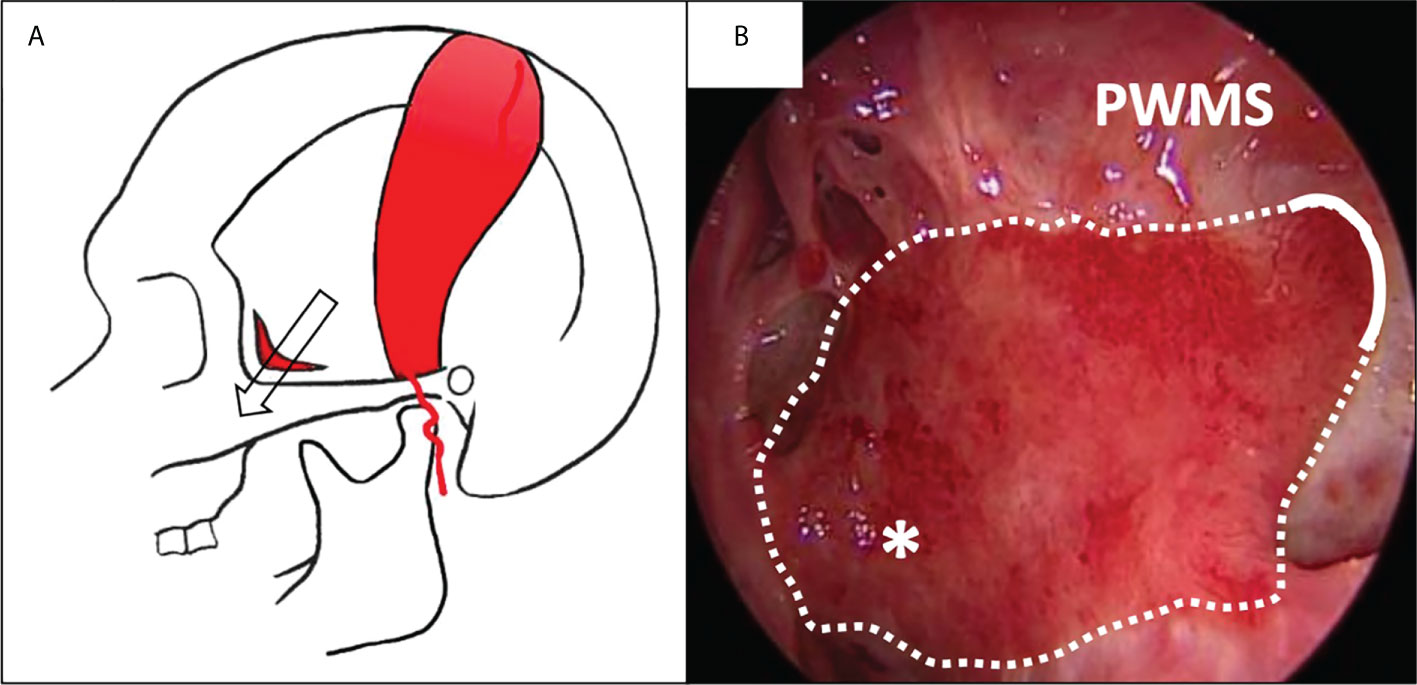
Figure 4 Covering of the lateral nasopharyngeal wall with a superficial temporoparietal fascia flap (sTPFF) after left endoscopic rhinopharyngectomy for recurrent nasopharyngeal carcinoma. (A) the sTPFF is pedicled on the superficial temporal artery; it is transposed in the nasal cavity through a temporal/infratemporal fossa tunnel (arrow). (B) Post-operative endoscopic view after 2 months: the flap (dotted line) has been introduced through an opening in the posterior wall of the left maxillary sinus (PWMS) and covers the lateral wall of the nasopharynx (asterisk), thus protecting the parapharyngeal internal carotid artery.
The pericranial flap is a versatile and robust pedicled flap used for skull base reconstruction and has been the workhorse of anterior skull base reconstruction for several decades. It is taken from the aponeurotic system between the frontalis and occipitalis muscles. It receives its blood supply from the supraorbital and supratrochlear vessels, terminal branches of the ophthalmic artery (47). Initially requiring a coronal incision, minimal invasive techniques for harvesting the pericranial flap were gradually developed with small scalp incision, endoscopic dissection and minimal cosmetic bone defect to gain access to the nasal aspect of the skull base (48, 49). It can cover from the anterior cranial fossa as far as the sella, without reaching posterior cranial base defects (44). The thickness of the flap remains relatively stable over time for most patients even following radiotherapy (50, 51). Although there is a risk of obstruction of the frontal sinuses, passage of the flap in the midline in conjunction with complete removal of the floor of the frontal sinuses maintains a lateral drainage pathway (52) (Figure 5).
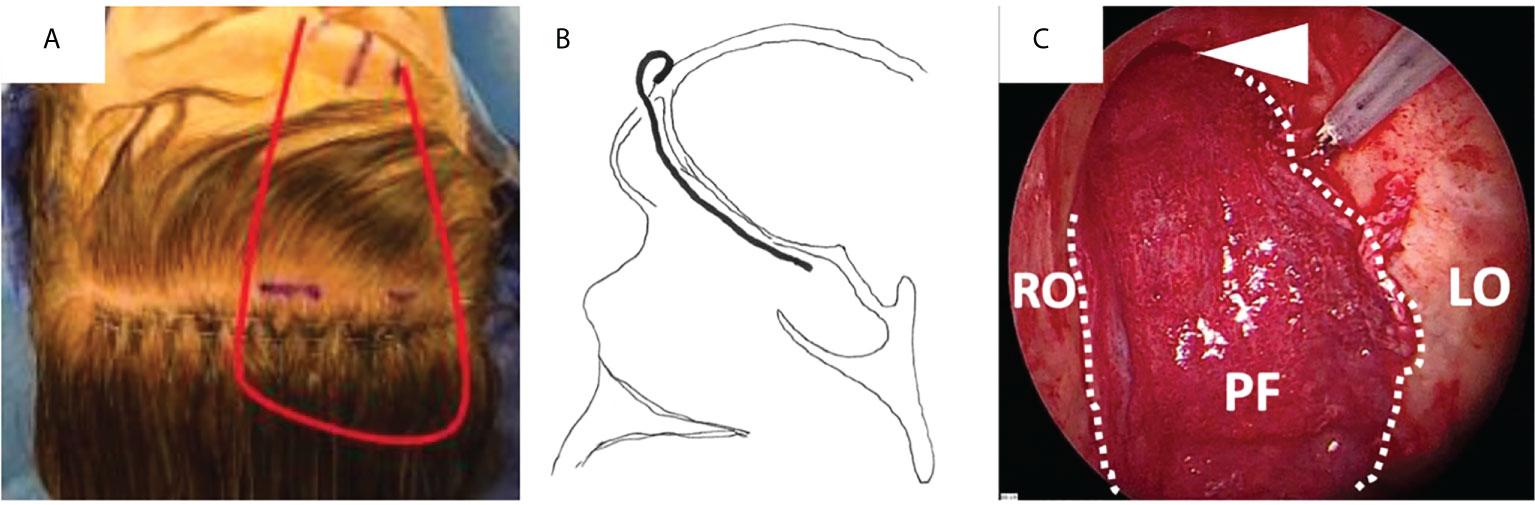
Figure 5 Covering of the anterior skull base with a pericranial flap (PF) after endoscopic transnasal craniectomy for an olfactory neuroblastoma in a previously irradiated patient. (A) Lining of the pericranial flap (red line) pedicled on the left supraorbital and supratrochlear pedicles. (B) The flap is placed against the whole anterior skull base through an opening in the superior aspect of the anterior wall of the frontal sinus. (C) Final endoscopic view, with the flap covering the whole anterior skull base, from the right to the left orbit (RO and LO, respectively) laterally and from the frontal sinus (white arrow head) to the sellar region posteriorly.
When the size and location of the skull base defect to be repaired exceeds the excursion limits of pedicled flaps, free flaps can be considered. They are mainly used during open sinus surgeries, leading to an important facial defect. Endoscopic assisted inset of the free flap in the anterior skull base is also feasible in selected cases (53).
The radial forearm flap has been shown to be a safe free flap when used for both head and neck as well as skull base reconstruction (54). This flap is composed with skin, fascia, fat (2-10mm layer) and muscle. Its skin paddle is taken from the distal part of the forearm, supplied by the radial artery and vein. This flap has a long pedicle which allows for usage of neck vessels as recipient vessels. It is exclusively composed of skin and fascia and is relatively thin. Due to its pliability, it is versatile for skull base defect reconstructions. It can also be folded back on itself to create more volume where needed (55). This makes it an ideal choice of flap when the defect is small, but reliable skull base or dural closure is required (56). However, reconstruction of the donor site requires a thin skin graft, often taken from the thigh, which can be a secondary source of pain and skin discoloration on healing (57). In postoperative imaging, the hyperdense tissues of the skin and subcutaneous tissues are thin and located on the mucosal side of the flap. Postoperative radiotherapy might reduce soft-tissue flap versatility due to fibrosis but in the lack of correlative studies, it remains controversial.
The anterolateral thigh flap is frequently used when a free flap with both muscle and adipose bulk is desired (58). This flap offers versatility in the components taken when harvesting the flap as well as creative inset options. It is mostly composed of fat and skin and can be harvested with or without muscle depending on the volume needed (59). The volume of transplanted fat vary according to the Body Mass Index of the patient (60). Its vascularization is supplied by a descending branch of lateral circumflex artery. Two veins accompany the artery and then merge into one at profundal femoral vein junction. It could be used for full-thickness nasal defect reconstructions, mostly orbital walls or total maxillectomy defects, with good functional results (61, 62). In postoperative imaging, it is easily differentiated from adjacent tissues, thanks to the hypodense central fatty component, which can reach 1 to 3cm. Although it is frequently observed a fatty atrophy, this flap provides relatively stable volume maintenance over time even after postoperative radiation and the impact of flap atrophy on functional deterioration or the need for surgical overcompensation are both controversial from surgical and radiotherapy standpoints (60).
Rectus abdominis free flap is a versatile flap that is well suited to a variety of reconstructive defects in the head and neck including maxillary or orbital defects. Vascularized by the deep inferior epigastric artery, this flap can be harvested with or without a skin paddle with a long pedicle. The versatility of this donor site is due to the ability to transfer large areas of skin with varying thicknesses and varying amounts of underlying muscle (63).
The latissimus dorsi free flap can be used for maxillary or orbital reconstructions. It is harvested from the thoracodorsal artery (terminal branch of the subscapular artery) as a simple muscle flap or a musculocutaneous flap. Due to the size of the muscle it can be used to cover large maxillary or orbital defects (64).
Restoring the facial contour based on the concept of facial buttress reconstruction improves aesthetic outcomes (65). Osteocutaneous free flaps should be used in case of significant orbital or maxillary defects (66).
The fibula free flap is the main composite bone flap used in head and neck cancer bone reconstruction. It can be used as an osteo-cutaneous flap or a bone flap without skin, and its fatty part is very thin. It provides a large amount of bone and a long pedicle. It is vascularized by interosseous and segmental perforators.
Scapula free flap reconstruction is also versatile in orbitomaxillary reconstruction (67). Scapula free flap can be harvested as a chimeric flap with bone, muscle and skin all harvested on separate branches from the subscapular system (circumflex scapular artery). The scapula free flap provides large flat bone and is surrounded by fat (hypodense) and muscle (homogenous moderate density compared to mucosa). One of the advantages of the scapula free flap over the fibula free flap is that the subscapular arterial system is usually protected from atherosclerotic disease as compared to the fibular vascular system (68).
Iliac crest free flap is useful for composite bone and soft tissue reconstructions. It is harvested on the deep circumflex iliac artery and offers a large quantity of high-quality bone, necessary for dental rehabilitation with osseointegrated implants. It has however a relatively short pedicle length (8-10 cm) and induces an important donor site morbidity (69).
Bony reconstructions require fixation with plates and screws and utilization of mirror image based virtual surgical planning and a 3D-printing guides improves aesthetics and cosmetics results (70, 71). Long-term complications occurred in about 15% of cases and concern principally wound breakdown, plate extrusion or osteoradionecrosis. Bone flaps are known to be at higher risk for osteoradionecrosis than the native bone (7). Dose backscatter from plates and screws might result in increased dose to the native and flap segments at their junction and increased risk of osteonecrosis, but lack of data prevents any estimate of the risk. There also might be an increased risk of osteoradionecrosis with 3D-printed piecemeal osteotomies. These bone fragments are deperiosted and the frail vascular suppliance might further contribute to the vascular-related risk factor for osteoradionecrosis. Prior radiotherapy predisposes patients to long-term complications (72).
Success rates of microvascular free flap reconstruction approach 95% and the main complication of these reconstruction is flap failure. It must be detected early and managed efficiently because of the short window of opportunity for flap salvage. Free flap failure may primarily be due to thrombosis. Arterial thrombosis occurs early in the immediate postoperative setting whereas venous thrombosis occurs later, frequently after 72h. Necrosis of free flap is rarely due to radiation-induced damage of vascular anastomosis or thrombosis but often occurs in the early postoperative period and could be caused by the vessel quality, comorbidities or technical procedures (7). Perforator flaps would seem to be more robust to radiotherapy. However, experience is currently mostly limited to skin cancer and data on perforator flaps in mucosal head and neck cancers are needed.
In the literature, there is no consensus concerning a reconstruction choice algorithm. Some parameters must be taken into account when making the decision: the size and the location of the defect, the materials and flaps available locally, the need for postoperative radiotherapy (or past history of radiotherapy) and the surgeon’s expertise and choices. The most widespread indications described in the literature are reported below (Figure 6).
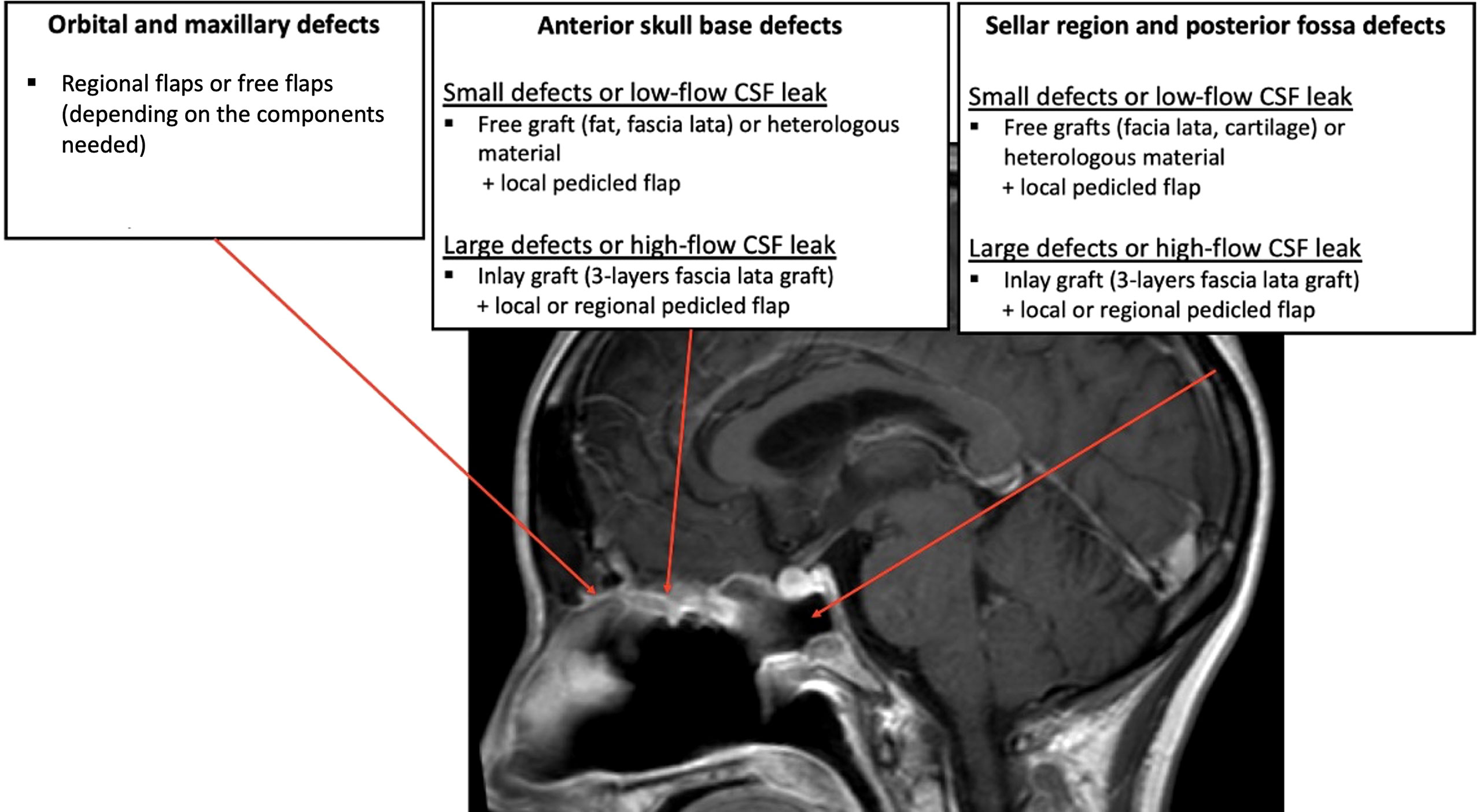
Figure 6 A proposition for reconstruction strategies according to the location and size of the defect after surgical removal of the tumor.
Anterior fossa defects, excepted limited defects (<1 cm), are best repaired with inlay grafts, for example fat or fascia lata, because the pressure from the brain helps maintaining the material in position and avoid its endonasal migration. A 3-layer reconstruction with the iliotibial tract of the fascia lata is usually necessary in case of large defect (>3 cm) after transnasal craniectomy (30). The skull base primary reconstruction can then be covered with a vascularized mucosal local flap to protect the graft and facilitate healing (30). Indeed, a flap does not replace the primary dural plasty and watertight closure must be obtained before placing the flap on the reconstruction. For very small defects, for example in the area of the cribriform plate, it is possible to use a free graft (or even heterologous material such as the Tachosil® sealant) in an onlay position, ideally covered by a local pedicled flap.
Defects of the sellar region or of the clivus are more difficult to close because of the high intracranial pressure associated with the proximity to the cisterns and ventricles. Small defects (<1 cm) may be repaired using multilayered free grafts, for example fascia lata and cartilage in the “gasket seal” technique with high success rates (29). Large dural defects (>3 cm) involving wide dural and arachnoid dissection and high-flow CSF leaks should preferably reconstructed with multilayer duraplasty covered by a vascularized flap (73, 74). To better guide the choice of the surgeon on the type of reconstruction and the type of flap to be used, surgical base defects could be predicted, with the help of computer tools, on preoperative CT-scans (75).
When a multilayer reconstruction is performed, the different components of the reconstruction are maintained in their final position by biological glue and/or packing. The flaps are very rarely maintained by sutures or staples.
In case of exposure or critical structures (internal carotid artery, optic nerve, clivus bone, or even intact dura), the best way to prevent post-operative complications is to cover the exposed structure with a vascularized flap. Depending on the location of the defect and on the available flaps, local pedicled flaps or regional pedicled flaps can be used in these situations.
When an open technique is performed with bony defects (infrastructure of the maxilla, orbit), the extent of injury needs to be assessed and reconstructed accordingly. Free flaps providing bone component should be considered and utilized whenever possible as they provide excellent functional and aesthetics results (76). For these reconstructions, flaps used are increasingly versatile by providing muscle, fat and bone components depending on the subunits to be reconstructed.
Skull base tumor recurrence after radiation therapy is difficult to resect and reconstruct. In this case, multilayer reconstruction with vascularized flap helps to optimize healing of the operative field.
Because of a frequency of 30 to 50% of local recurrence after surgery for malignant sinonasal tumors (3), it is necessary to detect early a possible recurrence. This detection is both clinical and paraclinical using imaging assessments. Postoperative imaging evaluation is therefore one of the cornerstones of monitoring patients undergoing endoscopic sinonasal surgery for malignant tumors. The detection of local recurrences is difficult in this context since it is necessary to integrate the post-operative changes related to the removal of the tumor but also the elements of reconstruction (grafts and flaps). Performing post-operative imaging is essential since it serves as a baseline and integrates post-operative changes that will be key to the interpretation of further local changes and to better detect subsequent recurrences. In this sense, MRI is the most appropriate imaging examination since it makes it possible to detect the various elements of reconstruction and allows most of the time to distinguish between a recurrence and post-therapeutic changes. PET/CT is often useful to visualize a possible recurrence after oncological surgery. However, the inflammatory environment of the posttreatment in sinonasal cavity leads to a high number of false positives. PET/CT has a worse specificity and positive predictive value in sinonasal malignancy than in head and neck malignancy overall, especially in the early posttreatment period (77). There is no optimal timeframe for performing the first postoperative imaging. We can however think that an MRI performed between 3 and 6 months post-operative is mandatory. In order to perform a proper assessment of the radiological findings, it is essential to know the radiological variations of sinonasal reconstructions during the healing process.
In free grafts and local or regional flaps reconstructions, the different components of the reconstruction (grafts and flaps) may no longer be individualized in CT-scan and a hypodense signal without contrast enhancement is observed between the two parts of the bone defect. In MRI, local and regional flaps have a C-shaped configuration within the operative defect, isointense on T1-weighted and T2-weighted images on both immediate and delayed MRI. After contrast enhancement, it is observed an hyperintense aspect (78). The presence of fatty components in the reconstruction technique can be confirmed in MRI with the fat suppression technique. Flaps composed of connective tissues can be distinguished in T2-weighted sequences, alternating hypointense-hyperintense appearance representing the layers (49). Thickening of the sinonasal mucosa is typical after surgical and radiation treatment. Inflammation manifests as ballooning of the mucosa with hyperintense T2 signal. After contrast enhancement, the epithelial lining enhances whereas the underlying edematous submucosa does not. Graft and flap reconstructive surgery and associated synthetic/metallic materials substantially impact surveillance. Better awareness of radiologists and detailed description of their procedure by surgeons as well as interdisciplinary discussions are critical to the detection of complications and recurrences during follow up.
After radiotherapy, such changes are seen immediately in early follow-up studies and may persist as long as 30 months. Chronic inflammation of the mucosa may also favor the formation of synechiae between bone or mucosal structures and the flap. In free flaps reconstructions, bone flaps can be easily distinguished from the native bone by the rupture of continuity of the cortical. Spontaneous bone resorption occurs over time by about 0.2 mm/year in the native mandible and can be applied by analogy to the maxillary. This resorption occurs in transplanted bone to a lesser degree (79). Fixation screws and plates at the flap-native bone interface can be responsible for metallic artifacts and backscatter radiation, which might increase their susceptibility to osteoradionecrosis (80). In these reconstructions, numerous clips are usually present at the vascular pedicle or at the anastomosis between the flap and the tumor bed. They are rarely used to indicate areas of dubious margins and thanks to their small size, they little degrade image quality (7).
Although success rates of microvascular free flap reconstruction approach 95% (81), the vascular supply may be compromised in the early postoperative period because of injury or compression. Free flaps for extended maxillofacial reconstruction are at risk of necrosis in connection with vascular phenomena (82). To evaluate the vitality of these flaps, dynamic contrast-enhanced CT-scan and MRI can be performed. The heterogenous contrast enhancement is difficult to correlate with flap failure because there is overlap in the imaging appearance of an enhancing flap and granulation tissue, particularly of the delayer scans. Immediate postoperative imaging are more helpful to evaluate the enhancement of the flap because there would be less time for granulation tissue to form (76).
As with other flaps of the head and neck, the fatty part within the reconstructions is likely to atrophy. The fatty part is visualized hypointense in postoperative CT scan and with the fat suppression technique in postoperative MRI. This atrophy is all the more important as postoperative radiotherapy is routinely performed for sinonasal carcinomas. Although flap fibrosis is a functional disadvantage in the context of extensive reconstruction, it is also the desired objective in the case of CSF leak repair.
Recent imaging techniques, as Dual-Energy Computed Tomography (DECT) provides high overall image quality for tumor delineation in head and neck imaging and reduces beam hardening artifacts. After sinonasal reconstruction, 3D volume rendering reconstruction allows virtual visualization of the flap and the feeding vessels to be spared (83).
Sinonasal flap reconstructive surgery does substantially modify the anatomy. During radiotherapy planning, ectopic fat or fascia/skin of graft can be seen as thin hypodense or spontaneously hyperdense layers at the surgical borders of the operative bed. Ectopic flap tissues can be identified by their components: ectopic fat well seen as hypodense central areas, “geometric” bone with metallic fixation devices (and often surrounding artefacts that render delineation even more complex), iso/slightly hyperdense muscle/skin/fascia components.
As reported in conventional head and neck carcinomas, radiotherapy planning is currently blind to surgical changes and flaps are not delineated per lack of knowledge on their aspect on imaging (7). In head and neck free flaps, the concept of flap sparing was however published by Bittermann yet neither implemented nor challenged in practice since (84). Flap changes may occur spontaneously or due to radiotherapy. However, several surgical series have reported that radiotherapy may deteriorate the results of reconstructive surgery in terms of functional outcomes. To assess dose-effect relationships of flaps and functional outcomes in comparison with surgery only, radiation oncologists will have to be able to delineate flaps on their planning CT or MRI as was done in conventional head and neck sites (85). Current practice for free flaps seems to include flaps in the target volumes with clinical target volumes centered on flap hypodense fatty portion (86). For that, a perfect knowledge of the tumor location (tumor implantation, and sites of involved margins), the type of surgery and reconstruction performed with comprehensive operatory reports are critical for the radiation oncologists (Figure 7).
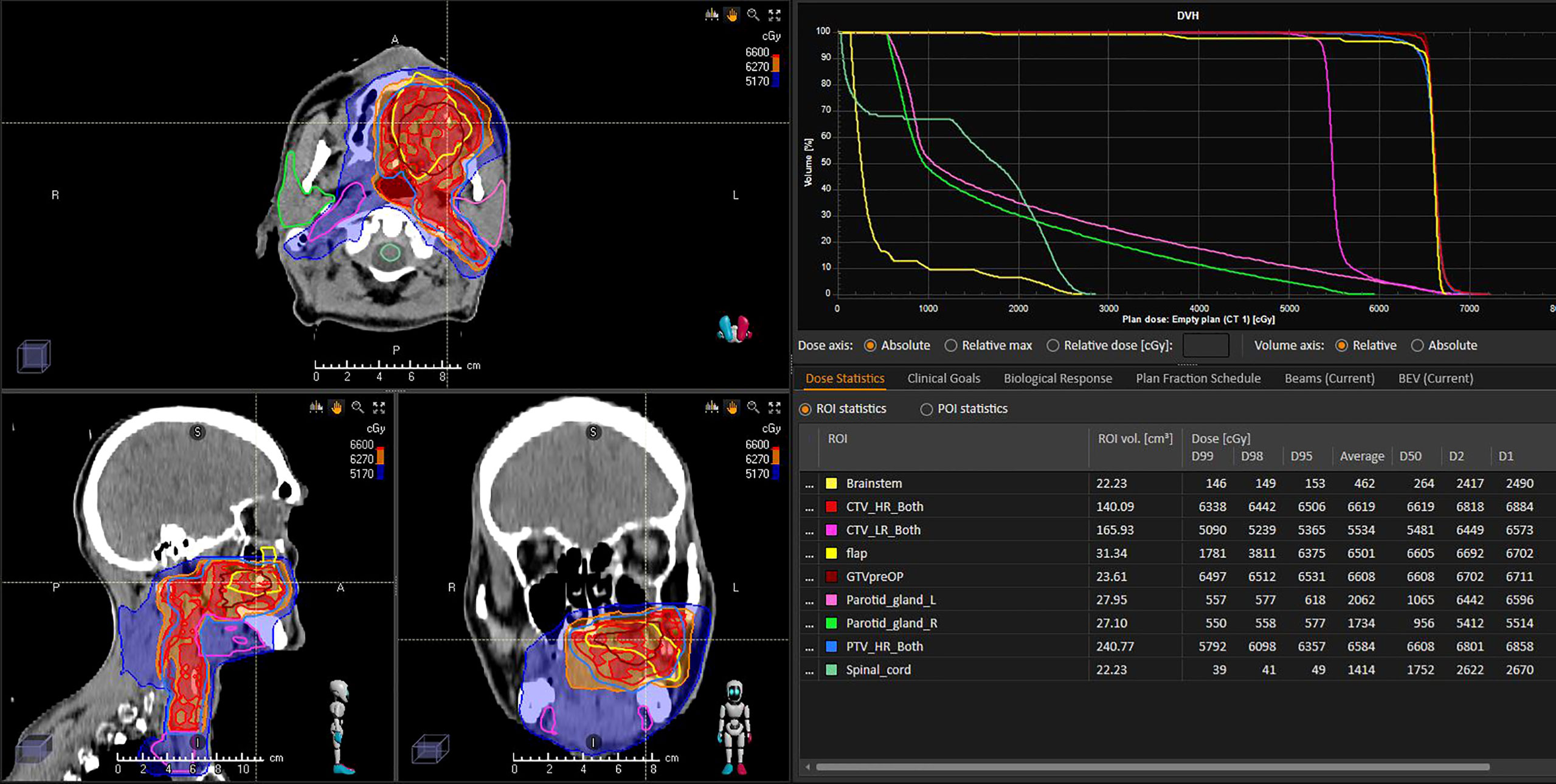
Figure 7 Postoperative radiotherapy planning of a left palatine squamous cell carcinoma following reconstructive surgery with a soft-tissue forearm flap. The flap (yellow line) was not delineated for RT planning and has been delineated a posteriori independently of referring radiation oncologist and blind to CTV delineation. Analysis of radiation dose distribution suggests that the flap was considered as a target volume and received the highest dose prescription level (66 Gy, within red 62.7Gy isodose).
Delineation of flaps is critical to better understanding of radiation effects, versus surgery alone. There are hardly any data on flap management during radiotherapy planning. Based on unpublished data, it seems that current practice trends towards including most of the flap volume into the high-risk high-dose radiotherapy volumes. Although one might consider avoiding irradiation of an ectopic, flap, tissue to limit the risk of toxicity and functional deterioration, data are critically lacking. Ambispective studies including flap reconstructive surgery of sinonasal tumors are ongoing.
The management of defects after sinonasal cancer surgery is a major challenge for patients. Many technical advances in techniques for reconstructing sinonasal defects after sinonasal cancer surgery have been described in recent years. The increasingly common use of vascular pedicled flaps and free flaps, both endonasal and extranasal, is contributing to the meaningful reduction in surgical complications. The impact of reconstruction techniques on postoperative imaging and radiotherapy planning should be taken into account more systematically to assess post-operative radiotherapy effects on reconstructed anatomy, flaps and grafts.
Manuscript conceptualization: FC and JT. Manuscript writing: all authors. Manuscript correction: all authors. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Dutta R, Dubal PM, Svider PF, Liu JK, Baredes S, Eloy JA. Sinonasal malignancies: A population-based analysis of site-specific incidence and survival: Sinonasal malignancies. Laryngoscope (2015) 125:2491–7. doi: 10.1002/lary.25465
2. Eggesbø HB. Imaging of sinonasal tumours. Cancer Imaging (2012) 12:136–52. doi: 10.1102/1470-7330.2012.0015
3. Turner JH, Reh DD. Incidence and survival in patients with sinonasal cancer: A historical analysis of population-based data. Head Neck (2012) 34:877–85. doi: 10.1002/hed.21830
4. Stelow EB, Bishop JA. Update from the 4th edition of the world health organization classification of head and neck tumours: Tumors of the nasal cavity, paranasal sinuses and skull base. Head Neck Pathol (2017) 11:3–15. doi: 10.1007/s12105-017-0791-4
5. Ferrari M, Orlandi E, Bossi P. Sinonasal cancers treatments: State of the art. Curr Opin Oncol (2021) 33:196–205. doi: 10.1097/CCO.0000000000000726
6. Beddok A, Guzene L, Coutte A, Thomson D, Yom S, Calugaru V, et al. International assessment of interobserver reproducibility of flap delineation in head and neck carcinoma. Acta Oncol (2022) 61(6):672–9. doi: 10.1080/0284186X.2022.2036367
7. Carsuzaa F, Lapeyre M, Gregoire V, Maingon P, Beddok A, Marcy P-Y, et al. Recommendations for postoperative radiotherapy in head & neck squamous cell carcinoma in the presence of flaps: A GORTEC internationally-reviewed HNCIG-endorsed consensus. Radiother Oncol (2021) 160:140–7. doi: 10.1016/j.radonc.2021.04.026
8. Hagemann J, Roesner J, Helling S, Jacobi C, Doescher J, Engelbarts M, et al. Long-term outcome for open and endoscopically resected sinonasal tumors. Otolaryngol Head Neck Surg (2019) 160:862–9. doi: 10.1177/0194599818815881
9. Awad AJ, Mohyeldin A, El-Sayed IH, Aghi MK. Sinonasal morbidity following endoscopic endonasal skull base surgery. Clin Neurol Neurosurg (2015) 130:162–7. doi: 10.1016/j.clineuro.2015.01.004
10. Hartl DM, Brasnu DF, Shah JP, Hinni ML, Takes RP, Olsen KD, et al. Is open surgery for head and neck cancers truly declining? Eur Arch Otorhinolaryngol (2013) 270:2793–802. doi: 10.1007/s00405-012-2322-y
11. Villaret AB, Yakirevitch A, Bizzoni A, Bosio R, Bignami M, Pistochini A, et al. Endoscopic transnasal craniectomy in the management of selected sinonasal malignancies. Am J Rhinol�Allergy (2010) 24:60–5. doi: 10.2500/ajra.2010.24.3397
12. Nicolai P, Battaglia P, Bignami M, Villaret AB, Delù G, Khrais T, et al. Endoscopic surgery for malignant tumors of the sinonasal tract and adjacent skull base: A 10-year experience. Am J Rhinol (2008) 22:308–16. doi: 10.2500/ajr.2008.22.3170
13. Castelnuovo P, Battaglia P, Locatelli D, Delù G, Sberze F, Bignami M. Endonasal micro-endoscopic treatment of malignant tumors of the paranasal sinuses and anterior skull base. Operative Techniques Otolaryngol-Head Neck Surg (2006) 17:152–67. doi: 10.1016/j.otot.2006.06.002
14. Volpi L, Bignami M, Lepera D, Karligkiotis A, Pistochini A, Ottini G, et al. Endoscopic endonasal resection of adenoid cystic carcinoma of the sinonasal tract and skull base. Laryngoscope (2019) 129:1071–7. doi: 10.1002/lary.27485
15. Lundberg M, Haapaniemi A, Hagstrom J, Juteau S, Hernberg M, Makitie AA, et al. Similar survival outcome after endoscopic and open approaches for sinonasal mucosal melanoma. Rhin (2019) 0:0–0. doi: 10.4193/Rhin18.123
16. Moya-Plana A, Bresson D, Temam S, Kolb F, Janot F, Herman P. Development of minimally invasive surgery for sinonasal malignancy. Eur Ann Otorhinolaryngo Head Neck Dis (2016) 133:405–11. doi: 10.1016/j.anorl.2016.06.001
17. Abdelmeguid AS, Raza SM, Su SY, Kupferman M, Roberts D, DeMonte F, et al. Endoscopic resection of sinonasal malignancies. Head Neck (2020) 42:645–52. doi: 10.1002/hed.26047
18. Consortium CRANIAL. CSF rhinorrhoea after endonasal intervention to the skull base (CRANIAL) - part 1: Multicenter pilot study. World Neurosurg (2021) 149:e1077–89. doi: 10.1016/j.wneu.2020.12.171
19. Bernal-Sprekelsen M, Rioja E, Enseñat J, Enriquez K, Viscovich L, Agredo-Lemos FE, et al. Management of anterior skull base defect depending on its size and location. BioMed Res Int (2014) 2014:346873. doi: 10.1155/2014/346873
20. Soni AJ, Modi G. Outcome of uncorrected CSF leak and consequent recurrent meningitis in a patient: A case presentation and literature review. Br J Neurosurg (2020) 34:492–4. doi: 10.1080/02688697.2018.1478063
21. Bubshait RF, Almomen AA. The endonasal endoscopic management of cerebrospinal fluid rhinorrhea. Cureus (2021) 13:e13457. doi: 10.7759/cureus.13457
22. Ivan ME, Iorgulescu JB, El-Sayed I, McDermott MW, Parsa AT, Pletcher SD, et al. Risk factors for postoperative cerebrospinal fluid leak and meningitis after expanded endoscopic endonasal surgery. J Clin Neurosci (2015) 22:48–54. doi: 10.1016/j.jocn.2014.08.009
23. Moore AG, Srinivasan A. Postoperative and postradiation head and neck: Role of magnetic resonance imaging. Top Magn Reson Imaging (2015) 24:3–13. doi: 10.1097/RMR.0000000000000042
24. Moya-Plana A, Veyrat M, Honart JF, de Fremicourt K, Alkhashnam H, Sarfati B, et al. Reconstruction of maxillectomy and midfacial defects using latissimus dorsi-scapular free flaps in a comprehensive cancer center. Oral Oncol (2019) 99:104468. doi: 10.1016/j.oraloncology.2019.104468
25. Vass G, Bella Z, Tóbiás Z, Nagy A, Iván L, Rovó L. Esthetically favorable surgical alternative for the removal of sinonasal malignant tumors–the modified facial degloving technique. J Oral Maxillofac Surg (2017) 75:2272. doi: 10.1016/j.joms.2017.06.036
26. Jang JW, Chan AW. Prevention and management of complications after radiotherapy for skull base tumors: A multidisciplinary approach. Adv Otorhinolaryngol (2013) 74:163–73. doi: 10.1159/000342293
27. Sharma MB, Jensen K, Urbak SF, Funding M, Johansen J, Bechtold D, et al. A multidimensional cohort study of late toxicity after intensity modulated radiotherapy for sinonasal cancer. Radiother Oncol (2020) 151:58–65. doi: 10.1016/j.radonc.2020.07.029
28. Mohamed Ali A, Mathis T, Bensadoun R-J, Thariat J. Radiation induced optic neuropathy: Does treatment modality influence the risk? Bull Cancer (2019) 106:1160–76. doi: 10.1016/j.bulcan.2019.09.008
29. Leng LZ, Brown S, Anand VK, Schwartz TH. “Gasket-seal” watertight closure in minimal-access endoscopic cranial base surgery. Neurosurgery (2008) 62:ONSE342-343. doi: 10.1227/01.neu.0000326017.84315.1f
30. Mattavelli D, Schreiber A, Ferrari M, Accorona R, Bolzoni Villaret A, Battaglia P, et al. Three-layer reconstruction with iliotibial tract after endoscopic resection of sinonasal tumors. World Neurosurg (2017) 101:486–92. doi: 10.1016/j.wneu.2017.02.066
31. Mattavelli D, Schreiber A, Villaret AB, Accorona R, Turri-Zanoni M, Lambertoni A, et al. Complications and donor site morbidity of 3-layer reconstruction with iliotibial tract of the anterior skull base: Retrospective analysis of 186 patients. Head Neck (2018) 40:63–9. doi: 10.1002/hed.24931
32. Fonmarty D, Bastier P-L, Lechot A, Gimbert E, de Gabory L. Assessment of abdominal fat graft to repair anterior skull base after malignant sinonasal tumor extirpation. Otolaryngol Head Neck Surg (2016) 154:540–6. doi: 10.1177/0194599815620781
33. Suh JD, Ramakrishnan VR, DeConde AS. Nasal floor free mucosal graft for skull base reconstruction and cerebrospinal fluid leak repair. Ann Otol Rhinol Laryngol (2012) 121:91–5. doi: 10.1177/000348941212100203
34. Chavan SS, Potdukhe KV, Kale V, Naik H, Thomas I. A comparitive study of endoscopic skull base reconstruction in CSF rhinorrea using nasoseptal flap with septal cartilage v/s fascia lata with fat. Indian J Otolaryngol Head Neck Surg (2021) 73:233–9. doi: 10.1007/s12070-021-02379-1
35. Hadad G, Bassagasteguy L, Carrau RL, Mataza JC, Kassam A, Snyderman CH, et al. A novel reconstructive technique after endoscopic expanded endonasal approaches: Vascular pedicle nasoseptal flap. The laryngoscope (2006) 116:1882–6. doi: 10.1097/01.mlg.0000234933.37779.e4
36. Zanation AM, Carrau RL, Snyderman CH, Germanwala AV, Gardner PA, Prevedello DM, et al. Nasoseptal flap reconstruction of high flow intraoperative cerebral spinal fluid leaks during endoscopic skull base surgery. Am J Rhinol�Allergy (2009) 23:518–21. doi: 10.2500/ajra.2009.23.3378
37. Zanation AM, Thorp BD, Parmar P, Harvey RJ. Reconstructive options for endoscopic skull base surgery. Otolaryngol Clinics North America (2011) 44:1201–22. doi: 10.1016/j.otc.2011.06.016
38. Schreiber A, Mattavelli D, Ferrari M, Rampinelli V, Lancini D, Belotti F, et al. The turbinal flap: An additional option for anterior skull base reconstruction. cadaveric feasibility study and case report: The turbinal flap. Int Forum Allergy Rhinol (2017) 7:199–204. doi: 10.1002/alr.21857
39. Battaglia P, Turri-Zanoni M, De Bernardi F, Dehgani Mobaraki P, Karligkiotis A, Leone F, et al. Septal flip flap for anterior skull base reconstruction after endoscopic resection of sinonasal cancers: Preliminary outcomes. Acta Otorhinolaryngol Ital (2016) 36:194–8. doi: 10.14639/0392-100X-748
40. Bozkurt G, Leone F, Arosio AD, Dehgani Mobaraki P, Elhassan HA, Seyhun N, et al. Septal flip flap for anterior skull base reconstruction after endoscopic transnasal craniectomy: Long-term outcomes. World Neurosurg (2019) 128:e409–16. doi: 10.1016/j.wneu.2019.04.166
41. Hadad G, Rivera-Serrano CM, Bassagaisteguy LH, Carrau RL, Fernandez-Miranda J, Prevedello DM, et al. Anterior pedicle lateral nasal wall flap: A novel technique for the reconstruction of anterior skull base defects. Laryngoscope (2011) 121:1606–10. doi: 10.1002/lary.21889
42. Fortes FSG, Carrau RL, Snyderman CH, Kassam A, Prevedello D, Vescan A, et al. Transpterygoid transposition of a temporoparietal fascia flap: A new method for skull base reconstruction after endoscopic expanded endonasal approaches. Laryngoscope (2007) 117:970–6. doi: 10.1097/MLG.0b013e3180471482
43. Arosio AD, Coden E, Karligkiotis A, Volpi L, Petruzzi G, Pellini R, et al. Temporoparietal fascia flap endonasal transposition in skull base reconstruction: Surgical technique. World Neurosurg (2021) 146:118. doi: 10.1016/j.wneu.2020.10.169
44. Reyes C, Mason E, Solares CA. Panorama of reconstruction of skull base defects: from traditional open to endonasal endoscopic approaches, from free grafts to microvascular flaps. Int Arch Otorhinolaryngol (2014) 18:S179–186. doi: 10.1055/s-0034-1395268
45. Gutierrez WR, Bennion DM, Walsh JE, Owen SR. Vascular pedicled flaps for skull base defect reconstruction. Laryngoscope Investig Otolaryngol (2020) 5:1029–38. doi: 10.1002/lio2.471
46. Ferrari M, Vural A, Schreiber A, Mattavelli D, Gualtieri T, Taboni S, et al. Side-door temporoparietal fascia flap: A novel strategy for anterior skull base reconstruction. World Neurosurg (2019) 126:e360–70. doi: 10.1016/j.wneu.2019.02.056
47. Patel MR, Shah RN, Snyderman CH, Carrau RL, Germanwala AV, Kassam AB, et al. Pericranial flap for endoscopic anterior skull-base reconstruction. Neurosurgery (2010) 66:506–12. doi: 10.1227/01.NEU.0000365620.59677.FF
48. Majer J, Herman P, Verillaud B. “Mailbox slot” pericranial flap for endoscopic skull base reconstruction. Laryngoscope (2016) 126:1736–8. doi: 10.1002/lary.25686
49. Santamaría A, Langdon C, López-Chacon M, Cordero A, Enseñat J, Carrau R, et al. Radio-anatomical analysis of the pericranial flap “money box approach” for ventral skull base reconstruction. Laryngoscope (2017) 127:2482–9. doi: 10.1002/lary.26574
50. Xu X, Lwin S, Ting E, Ong YK. Magnetic resonance imaging study of the pericranial flap and its local effects following endoscopic craniofacial resection. Laryngoscope (2021) 131(1):E90–7. doi: 10.1002/lary.28735
51. Yano T, Tanaka K, Kishimoto S, Iida H, Okazaki M. Reliability of and indications for pericranial flaps in anterior skull base reconstruction. J Craniofacial Surg (2011) 22:482–5. doi: 10.1097/SCS.0b013e318207b714
52. Zanation AM, Snyderman CH, Carrau RL, Kassam AB, Gardner PA, Prevedello DM. Minimally invasive endoscopic pericranial flap: A new method for endonasal skull base reconstruction. Laryngoscope (2009) 119:13–8. doi: 10.1002/lary.20022
53. Rodriguez-Lorenzo A, Driessen C, Mani M, Lidian A, Gudjonsson O, Stigare E. Endoscopic assisted insetting of free flaps in anterior skull base reconstruction: A preliminary report of five cases. Microsurgery (2020) 40:460–7. doi: 10.1002/micr.30542
54. Soutar DS, Scheker LR, Tanner NS, McGregor IA. The radial forearm flap: A versatile method for intra-oral reconstruction. Br J Plast Surg (1983) 36:1–8. doi: 10.1016/0007-1226(83)90002-4
55. Lin AC, Lin DT. Reconstruction of lateral skull base defects with radial forearm free flaps: The double-layer technique. J Neurol Surg B Skull Base (2015) 76:257–61. doi: 10.1055/s-0035-1548551
56. Wang W, Vincent A, Sokoya M, Kohlert S, Kadakia S, Ducic Y. Free-flap reconstruction of skull base and orbital defects. Semin Plast Surg (2019) 33:72–7. doi: 10.1055/s-0039-1677881
57. Bell EB, Cohen ER, Sargi Z, Leibowitz J. Free tissue reconstruction of the anterior skull base: A review. World J Otorhinolaryngol Head Neck Surg (2020) 6:132–6. doi: 10.1016/j.wjorl.2020.01.004
58. Kwon D, Iloreta A, Miles B, Inman J. Open anterior skull base reconstruction: A contemporary review. Semin Plast Surg (2017) 31:189–96. doi: 10.1055/s-0037-1607273
59. Ali RS, Bluebond-Langner R, Rodriguez ED, Cheng M-H. The versatility of the anterolateral thigh flap. Plast Reconstr Surg (2009) 124:e395–407. doi: 10.1097/PRS.0b013e3181bcf05c
60. Strohl MP, Junn JC, House AE, Heaton CM, Seth R, Park AM, et al. Long-term stability of vascularized adipofascial flaps in facial reconstruction. Facial Plast Surg Aesthet Med (2020) 22:262–7. doi: 10.1089/fpsam.2019.0015
61. Livaoğlu M, Karacal N, Bektaş D, Bahadir O. Reconstruction of full-thickness nasal defect by free anterolateral thigh flap. Acta Otolaryngol (2009) 129:541–4. doi: 10.1080/00016480802258810
62. Lin X, uan SY, Liu C. The anterolateral thigh flap for reconstruction of the defect after maxillectomy. J Craniofac Surg (2020) 31:e89–92. doi: 10.1097/SCS.0000000000005975
63. Urken M, Turk J, Weinberg H, Vickery C, Biller H. The rectus abdominis free flap in head and neck reconstruction. Arch Otolaryngol Head Neck Surg (1991) 117(8):857–66. doi: 10.1001/archotol.1991.01870210103021
64. Aviv J, Urken M, Vickery C, Weinberg H, Buchbinder D, Biller H. The combined latissimus dorsi-scapular free flap in head and neck reconstruction. Arch Otolaryngol Head Neck Surg (1991) 117(11):1242–50. doi: 10.1001/archotol.1991.01870230058008
65. Bluebond-Langner R, Rodriguez ED. Application of skeletal buttress analogy in composite facial reconstruction. Craniomaxillofac Trauma Reconstr (2009) 2:19–25. doi: 10.1055/s-0028-1098966
66. Heredero S, Solivera J, García B, Dean A. Osteomyocutaneous peroneal artery perforator flap for reconstruction of the skull base. Br J Oral Maxillofac Surg (2016) 54:99–101. doi: 10.1016/j.bjoms.2015.10.016
67. Bartholomew R, Zenga J, Lin D, Deschler D, Richmon J. Tip-on-Tip scapular (TOTS) flap for reconstruction of combined palatectomy and rhinectomy defects. Facial Plast Surg (2018) 34:389–93. doi: 10.1055/s-0038-1666784
68. Brown J, Bekiroglu F, Shaw R. Indications for the scapular flap in reconstructions of the head and neck. Br J Oral Maxillofac Surg (2010) 48:331–7. doi: 10.1016/j.bjoms.2009.09.013
69. Bianchi B, Ferri A, Ferrari S, Copelli C, Boni P, Sesenna E. Iliac crest free flap for maxillary reconstruction. J Oral Maxillofac Surg (2010) 68(11):2706–13. doi: 10.1016/j.joms.2010.01.008
70. Jang W-H, Lee JM, Jang S, Kim H-D, Ahn K-M, Lee J-H. Mirror image based three-dimensional virtual surgical planning and three-dimensional printing guide system for the reconstruction of wide maxilla defect using the deep circumflex iliac artery free flap. J Craniofac Surg (2019) 30:1829–32. doi: 10.1097/SCS.0000000000005577
71. Stranix JT, Stern CS, Rensberger M, Ganly I, Boyle JO, Allen RJ, et al. A virtual surgical planning algorithm for delayed maxillomandibular reconstruction. Plastic and Reconstructive Surgery (2019) 143:1197–206. doi: 10.1097/PRS.0000000000005452
72. Swendseid B, Kumar A, Sweeny L, Wax MK, Zhan T, Goldman RA, et al. Long-term complications of osteocutaneous free flaps in head and neck reconstruction. Otolaryngol Head Neck Surg (2020) 162:641–8. doi: 10.1177/0194599820912727
73. Snyderman CH, Wang EW, Zenonos GA, Gardner PA. Reconstruction after endoscopic surgery for skull base malignancies. J Neurooncol (2020) 150:463–8. doi: 10.1007/s11060-020-03465-0
74. Rotman LE, Kicielinski KP, Broadwater DR, Davis MC, Vaughan TB, Woodworth BA, et al. Predictors of nasoseptal flap use after endoscopic transsphenoidal pituitary mass resection. World Neurosurg (2018) S1878-8750(18):32920–6. doi: 10.1016/j.wneu.2018.12.097
75. MacArthur FJD, McGarry GW. The radioanatomy of endonasal flap coverage of skull base defects: A tool for preoperative planning. Laryngoscope (2018) 128:1287–93. doi: 10.1002/lary.26925
76. Dang RP, Roland LT, Sharon JD, Doering M, Chicoine MR, Pipkorn P. Pedicle corridors and vessel options for free flap reconstruction following endoscopic endonasal skull base surgery: A systematic review. J Neurol Surg B Skull Base (2021) 82:196–201. doi: 10.1055/s-0039-1695001
77. Workman A, Palmer J, Adappa N. Posttreatment surveillance for sinonasal malignancy. Curr Opin Otolaryngol Head Neck Surg (2017) 25(1):86–92. doi: 10.1097/moo.0000000000000330
78. Kang MD, Escott E, Thomas AJ, Carrau RL, Snyderman CH, Kassam AB, et al. The MR imaging appearance of the vascular pedicle nasoseptal flap. AJNR Am J Neuroradiol (2009) 30:781–6. doi: 10.3174/ajnr.A1453
79. Shokri T, Stahl LE, Kanekar SG, Goyal N. Osseous changes over time in free fibular flap reconstruction. Laryngoscope (2019) 129:1113–6. doi: 10.1002/lary.27337
80. Witjes MJH, Schepers RH, Kraeima J. Impact of 3D virtual planning on reconstruction of mandibular and maxillary surgical defects in head and neck oncology. Curr Opin Otolaryngol Head Neck Surg (2018) 26:108–14. doi: 10.1097/MOO.0000000000000437
81. Genden EM, Rinaldo A, Suárez C, Wei WI, Bradley PJ, Ferlito A. Complications of free flap transfers for head and neck reconstruction following cancer resection. Oral Oncol (2004) 40:979–84. doi: 10.1016/j.oraloncology.2004.01.012
82. Fujioka M. Factors predicting total free flap loss after microsurgical reconstruction following the radical ablation of head and neck cancers. ISRN Plast Surg (2013) 2013:1–5. doi: 10.5402/2013/952971
83. Diekhoff T, Scheel M, Kress W, Hamm B, Jahnke P. Dual-energy computed tomography of the neck–optimizing tube current settings and radiation dose using a 3D-printed patient phantom. Quant Imaging Med Surg (2021) 11:1144–55. doi: 10.21037/qims-20-854
84. Bittermann G, Voss P, Duttenhoefer F, Zimmerer R, Vach K, Metzger MC. The validity of surgical clips as radiographic markers for the tumour resection cavity in head and neck cancer treatment. J Craniomaxillofac Surg (2015) 43:758–62. doi: 10.1016/j.jcms.2015.04.005
85. Le Guevelou J, Bastit V, Marcy PY, Lasne-Cardon A, Guzene L, Gerard M, et al. Flap delineation guidelines in postoperative head and neck radiation therapy for head and neck cancers. Radiother Oncol (2020) 151:256–65. doi: 10.1016/j.radonc.2020.08.025
86. Gérard M, Le Guevelou J, Jacksic N, Lequesne J, Bastit V, Géry B, et al. Postoperative radiotherapy after flap reconstructive surgery in patients with head and neck cancer: A retrospective monocentric study with flap delineation to assess toxicity and relapse. Cancer Radiother (2020) 24:851–9. doi: 10.1016/j.canrad.2020.06.024
Keywords: sinonasal tumors, flap, reconstructive surgery, radiotherapy, imaging
Citation: Carsuzaa F, Verillaud B, Marcy P-Y, Herman P, Dufour X, Favier V and Thariat J (2022) Interdisciplinary challenges and aims of flap or graft reconstruction surgery of sinonasal cancers: What radiologists and radiation oncologists need to know. Front. Oncol. 12:1013801. doi: 10.3389/fonc.2022.1013801
Received: 07 August 2022; Accepted: 25 August 2022;
Published: 20 September 2022.
Edited by:
Abhishek Mahajan, The Clatterbridge Cancer Centre, United KingdomReviewed by:
Stefano Taboni, University of Brescia, ItalyCopyright © 2022 Carsuzaa, Verillaud, Marcy, Herman, Dufour, Favier and Thariat. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Florent Carsuzaa, ZmxvcmVudC5jYXJzdXphYUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.