
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 01 December 2022
Sec. Hematologic Malignancies
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1013781
Objective: We evaluated the correlation between cerebrospinal fluid (CSF) cytokine levels and central nervous system (CNS) involvement in adult acute myeloid leukemia (AML).
Methods: The study sample consisted of 90 patients diagnosed with AML and 20 with unrelated CNS involvement. The AML group was divided into two sub-groups: those with (CNS+, n=30) and without CNS involvement (CNS-, n=60). We used a cytometric bead assay to measure CSF interleukin (IL)-2, IL-4, IL-6, and IL-10, tumor necrosis factor-α, interferon-γ, and IL-17A. We used receiver operating characteristic curves to evaluate the ability of CSF cytokine levels to identify CNS involvement in adult AML.
Results: CSF IL-6 levels were significantly higher in CNS+adult AML patients and positively correlated with the lactate dehydrogenase levels (r=0.738, p<0.001) and white blood cell (WBC) count (r=0.455, p=0.012) in the blood, and the protein (r=0.686, p<0.001) as well as WBC count in the CSF (r=0.427, p=0.019). Using a CSF IL-6 cut-off value of 8.27 pg/ml yielded a diagnostic sensitivity and specificity was 80.00% and 88.46%, respectively (AUC, 0.8923; 95% CI, 0.8168–0.9678). After treating a subset of tested patients, their CSF IL-6 levels decreased. Consequently, the elevated CSF IL-6 levels remaining in CNS+ adult AML patients post-treatment were associated with disease progression.
Conclusion: CSF IL-6 is a promising marker for the diagnosis of adult AML with CNS involvement and a crucial dynamic indicator for therapeutic response.
Central nervous system (CNS) involvement is a well-known and serious extramedullary disease in adults with acute myeloid leukemia (AML). However, it is less common than acute lymphoblastic leukemia (ALL) (1–3). The therapies available for ALL and AML have significantly improved, and viable CNS disease treatments, such as intrathecal chemotherapy and more refined radiation techniques, have been developed. Despite this progress, CNS relapses remain life-threatening clinical and therapeutic challenges (4, 5). Most studies have focused on CNS involvement in pediatric AML patients; additionally, routine diagnostic lumbar punctures (LPs) are not performed in adult AML patients without obvious CNS signs or symptoms. Consequently, little is known about the incidence and clinical course of in adult AMLwith CNS involvement. This lack of information often results in unfavorable outcomes (5–7).
The current diagnosis of CNS diseases includes the clinical evaluation of neurologic symptoms and cerebrospinal fluid (CSF) by LPs, as well as radiographic imaging, which may be used independently or in combination (4). The LPs and conventional cytological examination of CSF is the standard diagnostic method for CNS leukemia (8). Recent studies have shown that flow cytometry (FCM) has superior specificity and sensitivity in detecting CNS leukemia compared to conventional cytology (CC) (9–11). However, both ways yield a high percentage (>40%) of false-negative reports owing to the low cellularity in the CSF. This percentage of false-negative reports was obtained from patients who were eventually diagnosed with CNS involvement by neuroimaging or autopsy (12). In addition, clinical evaluation of CNS leukemia may overlap with other neurological conditions, and radiographic imaging is not routinely performed during AML and ALL diagnosis, especially when there are no clinical symptoms (4, 13). Thus, developing effective diagnostic tools to detect CNS involvement in acute leukemia is imperative.
CSF biomolecules can be used as biomarkers to promote the diagnosis of CNS disease (14–16). Epstein-Barr viral DNA and the germ cell markers, α-fetoprotein and/or human chorionic gonadotropin, are applied in the diagnosis of AIDS-related CNS lymphoma and childhood CNS germinoma, respectively, and the differential expression of microRNAs in the CSF are potential noninvasive biomarkers for the diagnosis of CNS lymphoma (17–19). In primary central nervous system lymphoma (PCNSL), CSF levels of interleukin (IL)-10 have been reported as potential diagnostic and prognostic biomarkers (20, 21). Meanwhile, in large B cell PCNSL, increased IL-10/IL-6 ratios have been reported to be reliable diagnostic biomarkers (22). Some cytokines and chemokines, such as C-X-C motif chemokine ligand 10 (CXCL10), C-C motif chemokine ligand 4 (CCL4), CCL17, and IL-8, are also highly expressed in the CSF of metastatic tumors (23). In addition, a recent study reported that CSF miR-181a quantification might provide novel tools for monitoring CNS involvement in pediatric ALL (24). However, CSF biomarkers for the diagnosis of CNS involvement in adult AML patients have rarely been reported.
Thus, in this study, we retrospectively analyzed cytokine expression in the CSF of adult AML patients at CNS relapse. We explored the correlation between CSF cytokine levels and clinical characteristics of CNS involvement in these patients. We evaluated the diagnostic significance of CSF IL-6 and examined the cytokine levels after treatment to evaluate the dynamics of treatment-induced alteration of the IL-6 expression. Collectively, we aimed to identify a new and effective diagnostic tool for CNS involvement in adult patients with AML.
Ninety AML patients admitted to the First Affiliated Hospital, College of Medicine, Zhejiang University, were enrolled in the study from January 2021 to February 2022. Clinical and laboratory data were collected from the hospital electronic databases. All patients underwent peripheral blood microscopic evaluations, bone marrow and flow cytometric analyses to diagnose AML and classify its morphology. None of the patients had the presence of other diseases that may affect the change of central immune status, such as other tumor, infection, acute trauma to the brain, cerebrovascular disease and a history of immunodeficiency etc. Twenty patients diagnosed with other CNS diseases, such as autoimmune encephalitis (n=6), metastatic brain tumor of lung cancer (n=3), neurosyphilis (n=2), HIV encephalopathy (n=2), viral meningitis (n=3), and headache of unknown cause (n=4), were included for comparison. This study protocol was approved by the ethics committee of the First Affiliated Hospital, College of Medicine, Zhejiang University.
All AML patients underwent diagnostic and follow-up LPs to identify CNS involvement at different time points after their first prophylactic intrathecal chemotherapy therapy with cytarabine (50 mg), dexamethasone (5 mg), and methotrexate 10 mg. To diagnose CNS involvement, we used a cell counting chamber to confirm the presence of >5 white blood cells (WBCs) of CSF. To this end, we also confirmed the presence of leukemic blast cells in the CSF with CC or FCM analysis after cytocentrifugation. Intrathecal therapy for AML in CNS+ patients consisted of intrathecal injection twice a week until CSF blast clearance and minimal residual disease (MRD) FCM were negative.
CSF samples (90 CSF samples were obtained at different time points after the first chemotherapy, and 16 CSF samples were obtained after completion of intrathecal prophylactic therapy) were obtained from 90 AML patients and 20 subjects with unrelated CNS involvement. The levels of seven cytokines (IL-2, IL-4, IL-6, and IL-10, tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), and IL-17A were quantitatively determined immediately with the use of a cytometric bead array kit (Human Th1/Th2/TH17 Cytokine Kit; JiangXi Cellgene, NanChang, China). The minimum and maximum limits of detection for all 6 cytokines were 0.10 and 5000 pg/ml, respectively. Samples were analyzed using a BD FACS Canto II flow cytometer (Becton Dickinson, San Jose, CA, USA). The data were generated in graphical and tabular format using FCAP Array software (BD Biosciences, San Jose, CA, USA).
Statistical analyses were performed using SPSS version 23.0 (SPSS, Chicago, IL, USA). The Mann–Whitney U test was used to the compare the differences of non-normal distribution data between CNS involvement group and non-CNS involvement group, categorical values were analysed by χ2 test or Fisher’s exact test. We evaluated the correlation of clinical features and CSF cytokines with Spearman’s correlation analysis (GraphPad Prism 5.0). The Kolmogorov–Smirnov test on these cytokines revealed a non-normal distribution, thus the Mann–Whitney U test was used to compare the concentrations of seven cytokines among the three groups. The sensitivity, specificity, cut-off value, the area under the curve (AUC), and 95% confidence interval (CI) of CSF cytokine levels to identify CNS involvement was assessed using receiver operating characteristic (ROC) curves (GraphPad Prism 5.0). All tests were two-tailed, and statistical significance was set at p<0.05.
Ninety adult AML patients and 20 individuals diagnosed with other CNS disease were included in this study (N=110). Patients with AML were divided into those with CNS involvement (CNS+, n=30) and those without CNS involvement (CNS-, n=60). Patients with CNS involvement were significantly older than those without CNS involvement and had a higher proportion of CNS symptoms/signs and adverse cytogenetic risk. Based on the French-American British (FAB) morphology classification scheme, M5 morphologywas associated with CNS involvement. In addition, among the 30 AML patients with CNS+ in this study, 21 cases of FCM of CSF and 18 cases of CC of CSF were positive. Significant differences were observed in white blood cell WBC count, lactate dehydrogenase (LDH) level, CSF protein level, and CSF WBC count, but not in sex, bone marrow status and chemotherapy regimens between the two groups (Table 1).
As shown in Figure 1, the CSF IL-6 levels was significantly higher in CNS+ adult AML patients than in the CNS− group and other CNS disease patients (p<0.001). In contrast, no statistically significant differences in the IL-2, IL-4, IL-10, TNF-α, IFN-γ, and IL-17A levels were observed among these groups. At the same time, serum IL-6 results were obtained in 16 CNS+AML patients and 43 CNS-AML patients, but there was no statistically difference between the two groups.(See Supplementary Figure 1)
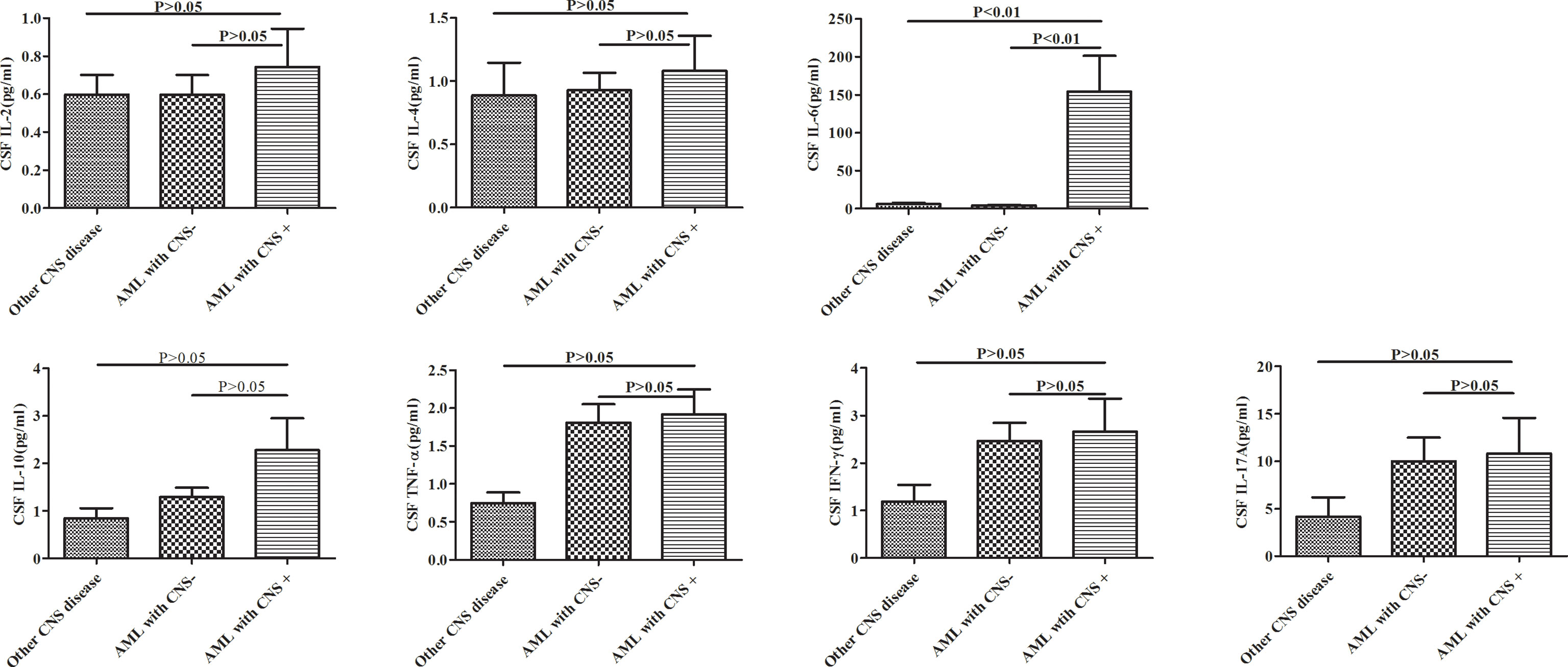
Figure 1 Cytokine profiles in adult AML patients with and without CNS involvement (AML with CNS+ and CNS-) and other CNS diseases. AML, acute myeloid leukemia; CNS, central nervous system.
To evaluate the potential correlation between CSF IL-6 levels and clinical features, pairwise Spearman’s rank coefficients were calculated. The data in Figure 2 suggests that CSF IL-6 levels positively correlated with LDH levels (r=0.738, p<0.001) and blood WBC count (r=0.455, p=0.012), and CSF protein level (r=0.686, p<0.001) and WBC count (r=0.427, p=0.019).
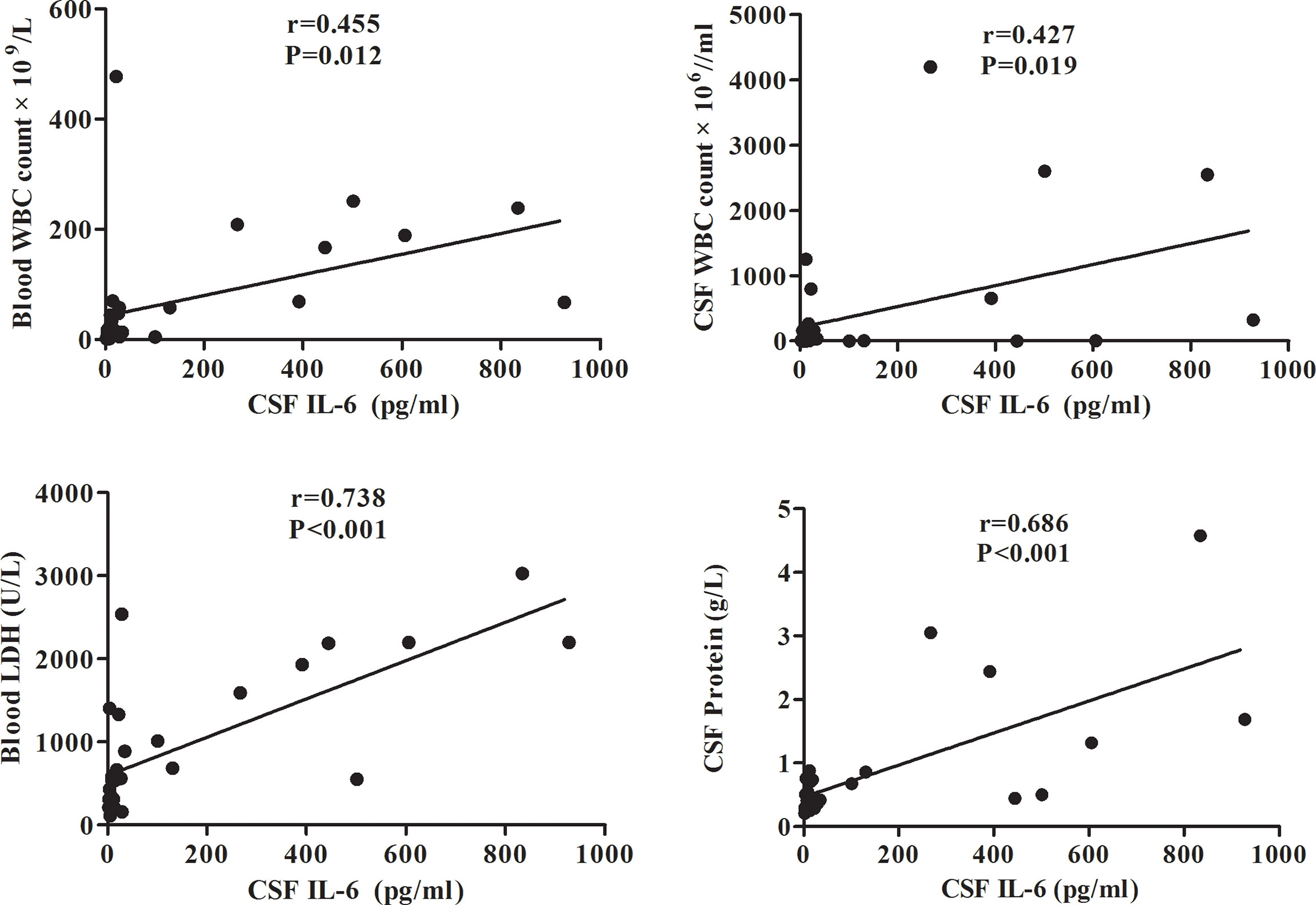
Figure 2 Correlation between the CSF IL-6 level and blood WBC count/CSF WBC count/blood LDH/CSF protein levels in adult AML patients. CSF, cerebrospinal fluid; IL, interleukin; LDH, lactate dehydrogenase; AML, acute myeloid leukemia.
The diagnostic efficacy of CSF IL-6 was assessed using the ROC curve. With the CSF IL-6 cut-off at 8.27 pg/ml, the diagnostic sensitivity and specificity for AML with CNS involvement were 80.00% and 88.46%, respectively (AUC, 0.8923; 95% CI, 0.8168–0.9678) (Figure 3). Because the patients with CNS involvement were selected based on positive CC or FCM, the CSF WBC had 100% specificity and discrimination for each procedure. However, the sensitivity of the CC and FCM was only 60.00% and 76.67%, respectively, which was lower than that of CSF IL-6-based diagnosis (Supplementary Table 1).
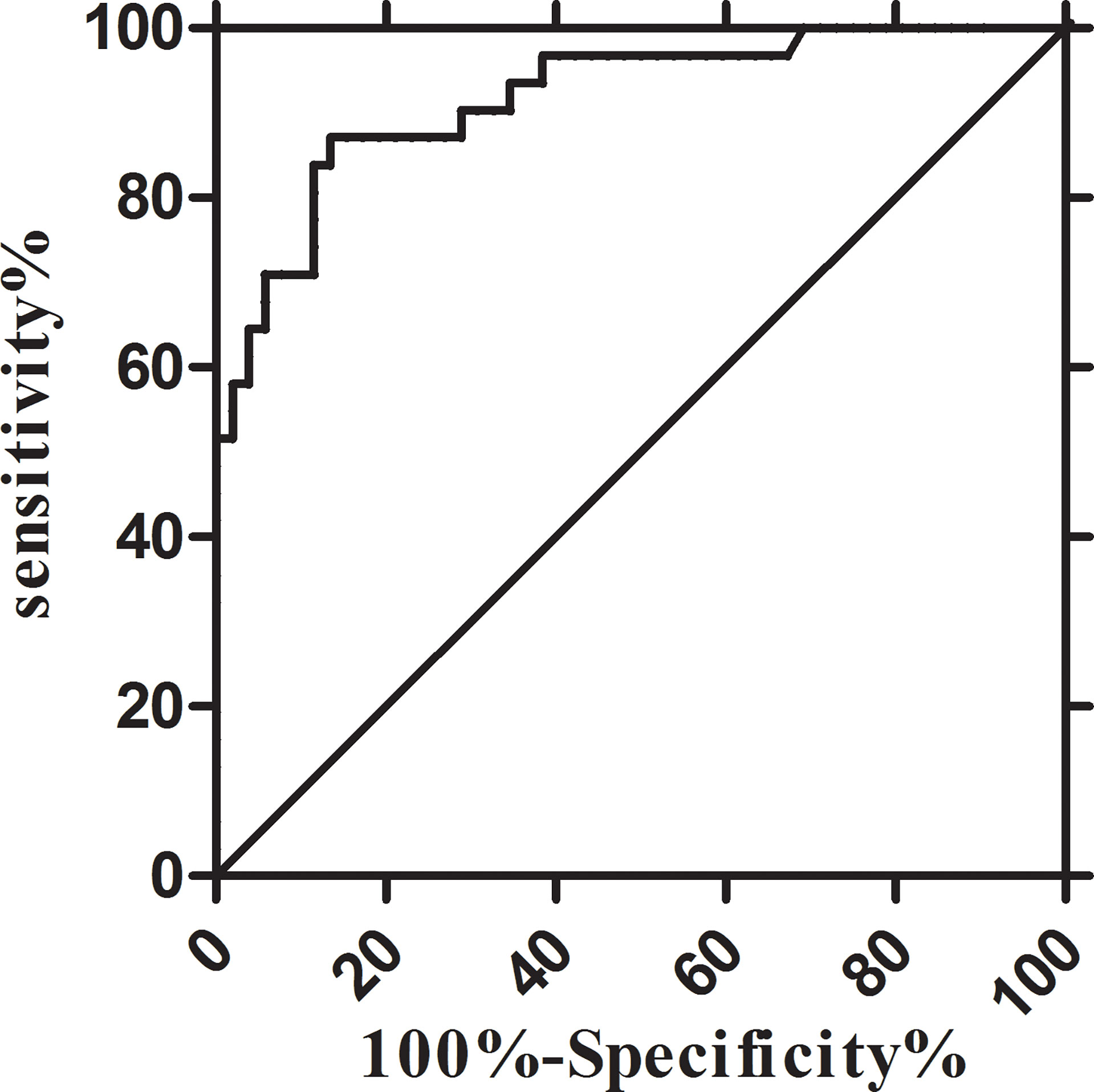
Figure 3 Receiver-operator characteristic (ROC) curves of the CSF IL-6. IL-6: sensitivity 80.00% and specificity 88.46% at 8.27 pg/ml (AUC, 0.8923; 95% CI, 0.8168–0.9678). AUC, area under the curve; CI, confidence interval.
Additionally, we explored the relationship between CSF IL-6 levels and disease status. CSF IL-6 concentrations were available before and after intrathecal treatment in 16 AML patients with CNS involvement. The cytokine levels after chemotherapy treatment decreased in 12 patients, four of whom were in partial remission (PR), whereas the rest were in complete remission (CR). The other four had CSF-IL-6 levels elevation, despite having completed intrathecal chemotherapy treatment; Notably, two of them experienced early death (<12 months), and two reached “progressive disease” status during follow-up visits (Figure 4).
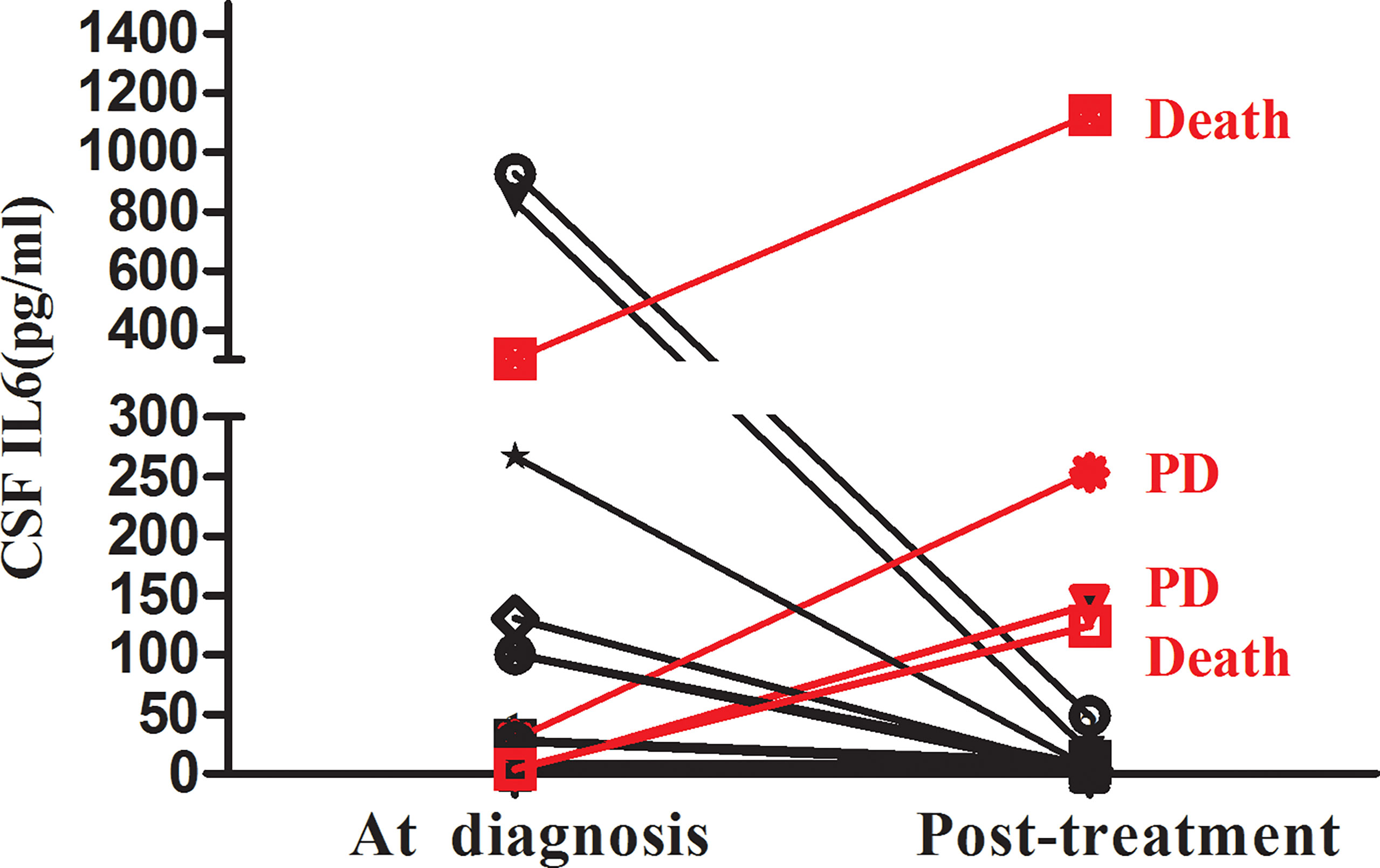
Figure 4 Changes in the CSF IL-6 level between AML with CNS involvement during diagnosis and post-treatment (n=16).
CNS involvement in AML remains a clinical challenge related to therapeutic obstacles and confers a poor prognosis (25). In adult AML, occult CNS infiltrations are undetected (3). Moreover, sensitive methods for diagnosing CNS leukemia are still lacking. Cytokines/chemokines involved in tumor metastasis, including leukemia, reportedly play a role in early diagnostics and assessment of metastatic tumor development (26–28). In this retrospective study, we identified a potential biomarker of CNS status in AML. We explored the cytokine concentrations in the CSF of adult patients with AML at CNS relapse. Our results revealed unexpectedly high IL-6 expression levels in CNS+AML patients diagnosed with CC/FCM, whereas those in CNS−AML were similar to those in the control group. We then demonstrated a correlation between CSF IL-6 levels and several clinical features of CNS involvement in adult AML. This is the first demonstration that elevated CSF IL-6 levels have high sensitivity and specificity in diagnosing CNS involvement in adults with AML. Additionally, using serial sample collection in a subset of patients, we examined IL-6 dynamics during treatment and confirmed the significant relationship between the CSF IL-6 levels and the therapeutic response in CNS involvement in adults with AML.
Cytokines are involved in various malignancies. IL-6, a pleiotropic cytokine, plays a role in various cancers, including hematological malignancy (29–31). IL-6 plays a vital role in the cytokines network, which is involved in the regulation of hematopoiesis and leukemic blast formation (32, 33). In patients with AML, serum IL-6 levels have been found to be highly expressed. However, its role as a predictive biomarker or therapeutic target is currently unclear, owing to its contradictory effects (34, 35). Recently, IL-6 has received increasing attention as a vital cytokine involved in tumor invasion (36). In leukemia CNS metastasis, cancerous cells migrate through the vasculature, from the bone marrow to the vertebrae and brain, by crossing the blood-brain-barrier(BBB)via endothelial disruption or trans-endothelial migration, multistep processes (37–41), in which cytokines (such as TNF, IL-1β, or IL-15) and chemokines play critical roles. Moreover, the expression of chemokines and cytokines is positively related to the development of CNS leukemia (28). Here, we revealed that CSF IL-6 concentration was elevated in CNS involvement in adult AML, and the CSF IL-6 levels positively correlated with its clinical features, including elevated blood WBC and LDH levels during diagnosis, as well as CSF protein and WBC, suggesting that IL-6 might play a part in CNS involvement in AML. Furthermore, this novel marker yielded a diagnostic sensitivity of 80.00% and specificity of 88.46%, which provided approximately 20.00% and 10.00% sensitivity benefits, compared to the CC and FCM methods, respectively. As previously reported, CC has a high specificity (>95%), while the sensitivity is relatively low (<50%) (4, 9). In addition, it is difficult to define the precise immunophenotype of neoplastic cells, owing to their paucity (42). In addition, research indicates that IL levels are stable, a significant advantage in contrast to identifying cells within the CSF (20). Thus, our results suggest that CSF IL-6 measurement could be a novel and efficient approach to aid the clinical diagnosis of CNS involvement in adult AML.
However, the cellular origin of CSF IL-6 in this study is unclear. In this study, IL-6 expression in peripheral blood (PB) samples did not differ between AML patients wtih CNS involvement and without CNS involvement, which suggesting that the high expression of CSF IL-6 might not be derived from the peripheral circulation. IL-6 is produced by macrophages (30) as well as neurons, glial and endothelial cells, and fibroblasts, which could play a role in the CNS (43). Thus, we speculated that CSF IL-6 in this study might be produced by non-leukemic cells in the CNS microenvironment. In contrast, a previous study reported that lymphoblasts release multiple cytokines and exosomes and regulate the brain microenvironment to breach the BBB for metastatic invasion (44). Concordantly, emerging evidence demonstrates that cancer cells release cytokines, extracellular vesicles, and exosomes, which have a potential role in altering the microenvironment at extramedullary sites, suggesting that CSF IL-6 is probably to be secreted by leukemia cells (45–47).
Furthermore, we examined the CSF IL-6 levels after intrathecal therapy to evaluate the treatment-induced alteration dynamics of IL-6 expression over time. Our results demonstrated that the CSF IL-6 concentration after treatment was significantly connected with the therapeutic response in adult AML with CNS involvement. Moreover, four patients after intrathecal therapy who were negative for CSF CC/FCM but with an elevated CSF IL-6 concentration exhibited a progressive form of the disease in the subsequent follow-up. Thus, our findings suggest that measurement of IL-6 levels in the CSF could be combined with cytologic/FCM analysis to better assess the therapeutic response as well as to help clinicians determine further treatment. However, whether post-treatment IL-6 levels in the CSF of patients with CR or PR affect PFS requires further investigation.
The present study still had several limitations. First, since CNS involvement is uncommon in adult AML, the sample size was small. Second, as a retrospective study, we lack CSF samples of CNS involvement in adult AML at initial diagnosis; thus, future studies are needed to investigate whether the CSF IL-6 levels in adult AML at initial diagnosis could predict CNS status. Finally, owing to a lack of exploration of the relationship between CSF IL-6 and survival analysis, an accumulation of prospective studies is necessary to provide definitive conclusions on the influence of CSF IL-6 on the prognosis of adult AML with CNS involvement.
In conclusion, our study provides the first description of CSF IL-6 as a promising diagnostic biomarker for CNS involvement in adult AML patients. We also revealed the correlation between CSF IL-6 level and clinical features of CNS involvement in adult with AML. In addition, we demonstrated that CSF IL-6 level, after intrathecal treatment, correlated with the therapeutic response. Further studies are required to validate CSF IL-6 level as a highly sensitive and specific diagnostic marker for adult AML with CNS involvement in. The molecular mechanisms underlying the cytokine network in the CNS involvement in adult AML require further investigation.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethics committee of the First Affiliated Hospital, College of Medicine, Zhejiang University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
JG and XH was involved in the conception design, analysis and interpretation of the data, and drafting the manuscript. YZ, CB and ZZ collected and analyzed the data and assisted in manuscript drafting. JJ and HT developed the conception design, analyzed and interpreted the data, and revised the manuscript critically for intellectual content. All authors approved the final of the version of the manuscript to be published.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1013781/full#supplementary-material
1. Abbott BL, Rubnitz JE, Tong X, Srivastava DK, Pui CH, Ribeiro RC, et al. Clinical significance of central nervous system involvement at diagnosis of pediatric acute myeloid leukemia: a single institution's experience. Leukemia (2003) 17:2090–6. doi: 10.1038/sj.leu.2403131
2. Bisschop MM, Révész T, Bierings M, van Weerden JF, van Wering ER, Hählen K, et al. Extramedullary infiltrates at diagnosis have no prognostic significance in children with acute myeloid leukaemia. Leukemia (2001) 15:46–9. doi: 10.1038/sj.leu.2401971
3. Alakel N, Stölzel F, Mohr B, Kramer M, Oelschlägel U, Röllig C, et al. Symptomatic central nervous system involvement in adult patients with acute myeloid leukemia. Cancer Manag Res (2017) 9:97–102. doi: 10.2147/cmar.s125259
4. Paul S, Short NJ. Central nervous system involvement in adults with acute leukemia: Diagnosis, prevention, and management. Curr Oncol Rep (2022) 24:427–36. doi: 10.1007/s11912-022-01220-4
5. Pui CH, Howard SC. Current management and challenges of malignant disease in the CNS in paediatric leukaemia. Lancet Oncol (2008) 9:257–68. doi: 10.1016/s1470-2045(08)70070-6
6. Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood (2017) 129:424–47. doi: 10.1182/blood-2016-08-733196
7. Kobayashi R, Tawa A, Hanada R, Horibe K, Tsuchida M, Tsukimoto I. Extramedullary infiltration at diagnosis and prognosis in children with acute myelogenous leukemia. Pediatr Blood Cancer (2007) 48:393–8. doi: 10.1002/pbc.20824
8. Ranta S, Nilsson F, Harila-Saari A, Saft L, Tani E, Söderhäll S, et al. Detection of central nervous system involvement in childhood acute lymphoblastic leukemia by cytomorphology and flow cytometry of the cerebrospinal fluid. Pediatr Blood Cancer (2015) 62:951–6. doi: 10.1002/pbc.25363
9. Bromberg JE, Breems DA, Kraan J, Bikker G, van der Holt B, Smitt PS, et al. CSF flow cytometry greatly improves diagnostic accuracy in CNS hematologic malignancies. Neurol (2007) 68:1674–9. doi: 10.1212/01.wnl.0000261909.28915.83
10. Craig FE, Foon KA. Flow cytometric immunophenotyping for hematologic neoplasms. Blood (2008) 111:3941–67. doi: 10.1182/blood-2007-11-120535
11. Del Principe MI, Buzzatti E, Piciocchi A, Forghieri F, Bonifacio M, Lessi F, et al. Clinical significance of occult central nervous system disease in adult acute lymphoblastic leukemia. a multicenter report from the campus ALL network. Haematologica (2021) 106:39–45. doi: 10.3324/haematol.2019.231704
12. Crespo-Solis E, López-Karpovitch X, Higuera J, Vega-Ramos B. Diagnosis of acute leukemia in cerebrospinal fluid (CSF-acute leukemia). Curr Oncol Rep (2012) 14:369–78. doi: 10.1007/s11912-012-0248-6
13. Shen H, Zhao Y, Shi Y, Sun J, Zhou D, Li L, et al. The diagnostic and prognostic value of MRI in central nervous system involvement of acute myeloid leukemia: a retrospective cohort of 84 patients. Hematology (2020) 25:258–63. doi: 10.1080/16078454.2020.1781500
14. Rubenstein JL, Wong VS, Kadoch C, Gao HX, Barajas R, Chen L, et al. CXCL13 plus interleukin 10 is highly specific for the diagnosis of CNS lymphoma. Blood (2013) 121:4740–8. doi: 10.1182/blood-2013-01-476333
15. Lewczuk P, Zimmermann R, Wiltfang J, Kornhuber J. Neurochemical dementia diagnostics: a simple algorithm for interpretation of the CSF biomarkers. J Neural Transm (Vienna) (2009) 116:1163–7. doi: 10.1007/s00702-009-0277-y
16. Frankfort SV, Tulner LR, van Campen JP, Verbeek MM, Jansen RW, Beijnen JH. Amyloid beta protein and tau in cerebrospinal fluid and plasma as biomarkers for dementia: a review of recent literature. Curr Clin Pharmacol (2008) 3:123–31. doi: 10.2174/157488408784293723
17. Antinori A, De Rossi G, Ammassari A, Cingolani A, Murri R, Di Giuda D, et al. Value of combined approach with thallium-201 single-photon emission computed tomography and Epstein-Barr virus DNA polymerase chain reaction in CSF for the diagnosis of AIDS-related primary CNS lymphoma. J Clin Oncol (1999) 17:554–60. doi: 10.1200/jco.1999.17.2.554
18. Diez B, Balmaceda C, Matsutani M, Weiner HL. Germ cell tumors of the CNS in children: recent advances in therapy. Childs Nerv Syst (1999) 15:578–85. doi: 10.1007/s003810050546
19. Baraniskin A, Kuhnhenn J, Schlegel U, Chan A, Deckert M, Gold R, et al. Identification of microRNAs in the cerebrospinal fluid as marker for primary diffuse large b-cell lymphoma of the central nervous system. Blood (2011) 117:3140–6. doi: 10.1182/blood-2010-09-308684
20. Nguyen-Them L, Costopoulos M, Tanguy ML, Houillier C, Choquet S, Benanni H, et al. The CSF IL-10 concentration is an effective diagnostic marker in immunocompetent primary CNS lymphoma and a potential prognostic biomarker in treatment-responsive patients. Eur J Cancer (2016) 61:69–76. doi: 10.1016/j.ejca.2016.03.080
21. Sasayama T, Nakamizo S, Nishihara M, Kawamura A, Tanaka H, Mizukawa K, et al. Cerebrospinal fluid interleukin-10 is a potentially useful biomarker in immunocompetent primary central nervous system lymphoma (PCNSL). Neuro Oncol (2012) 14:368–80. doi: 10.1093/neuonc/nor203
22. Song Y, Zhang W, Zhang L, Wu W, Zhang Y, Han X, et al. Cerebrospinal fluid IL-10 and IL-10/IL-6 as accurate diagnostic biomarkers for primary central nervous system Large b-cell lymphoma. Sci Rep (2016) 6:38671. doi: 10.1038/srep38671
23. Lok E, Chung AS, Swanson KD, Wong ET. Melanoma brain metastasis globally reconfigures chemokine and cytokine profiles in patient cerebrospinal fluid. Melanoma Res (2014) 24:120–30. doi: 10.1097/cmr.0000000000000045
24. Egyed B, Kutszegi N, Sági JC, Gézsi A, Rzepiel A, Visnovitz T, et al. MicroRNA-181a as novel liquid biopsy marker of central nervous system involvement in pediatric acute lymphoblastic leukemia. J Transl Med (2020) 18:250. doi: 10.1186/s12967-020-02415-8
25. Cheng CL, Li CC, Hou HA, Fang WQ, Chang CH, Lin CT, et al. Risk factors and clinical outcomes of acute myeloid leukaemia with central nervous system involvement in adults. BMC Cancer (2015) 15:344. doi: 10.1186/s12885-015-1376-9
26. Van Etten RA. Aberrant cytokine signaling in leukemia. Oncogene (2007) 26:6738–49. doi: 10.1038/sj.onc.1210758
27. Kornblau SM, McCue D, Singh N, Chen W, Estrov Z, Coombes KR. Recurrent expression signatures of cytokines and chemokines are present and are independently prognostic in acute myelogenous leukemia and myelodysplasia. Blood (2010) 116:4251–61. doi: 10.1182/blood-2010-01-262071
28. Si MY, Fan ZC, Li YZ, Chang XL, Xie QD, Jiao XY. The prognostic significance of serum and cerebrospinal fluid MMP-9, CCL2 and sVCAM-1 in leukemia CNS metastasis. J Neurooncol (2015) 122:229–44. doi: 10.1007/s11060-014-1707-8
29. Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol (2014) 6:a016295. doi: 10.1101/cshperspect.a016295
30. Burger R. Impact of interleukin-6 in hematological malignancies. Transfus Med Hemother (2013) 40:336–43. doi: 10.1159/000354194
31. Gu J, Huang X, Zhang Y, Bao C, Zhou Z, Jin J. Cytokine profiles in patients with newly diagnosed multiple myeloma: Survival is associated with IL-6 and IL-17A levels. Cytokine (2021) 138:155358. doi: 10.1016/j.cyto.2020.155358
32. Suzuki T, Morio T, Tohda S, Nagata K, Yamashita Y, Imai Y, et al. Effects of interleukin-6 and granulocyte colony-stimulating factor on the proliferation of leukemic blast progenitors from acute myeloblastic leukemia patients. Jpn J Cancer Res (1990) 81:979–86. doi: 10.1111/j.1349-7006.1990.tb03335.x
33. Ikebuchi K, Wong GG, Clark SC, Ihle JN, Hirai Y, Ogawa M. Interleukin 6 enhancement of interleukin 3-dependent proliferation of multipotential hemopoietic progenitors. Proc Natl Acad Sci U S A (1987) 84:9035–9. doi: 10.1073/pnas.84.24.9035
34. Horacek JM, Kupsa T, Vasatova M, Jebavy L, Zak P. Biochip array technology and evaluation of serum levels of multiple cytokines and adhesion molecules in patients with newly diagnosed acute myeloid leukemia. Exp Oncol (2014) 36:50–1.
35. Binder S, Luciano M, Horejs-Hoeck J. The cytokine network in acute myeloid leukemia (AML): A focus on pro- and anti-inflammatory mediators. Cytokine Growth Factor Rev (2018) 43:8–15. doi: 10.1016/j.cytogfr.2018.08.004
36. Cao F, Zhang Q, Chen W, Han C, He Y, Ran Q, et al. IL-6 increases SDCBP expression, cell proliferation, and cell invasion by activating JAK2/STAT3 in human glioma cells. Am J Transl Res (2017) 9:4617–26.
37. Basu SK, Remick SC, Monga M, Gibson LF. Breaking and entering into the CNS: clues from solid tumor and nonmalignant models with relevance to hematopoietic malignancies. Clin Exp Metastasis (2014) 31:257–67. doi: 10.1007/s10585-013-9623-4
38. Argaw AT, Zhang Y, Snyder BJ, Zhao ML, Kopp N, Lee SC, et al. IL-1beta regulates blood-brain barrier permeability via reactivation of the hypoxia-angiogenesis program. J Immunol (2006) 177:5574–84. doi: 10.4049/jimmunol.177.8.5574
39. Connell JJ, Chatain G, Cornelissen B, Vallis KA, Hamilton A, Seymour L, et al. Selective permeabilization of the blood-brain barrier at sites of metastasis. J Natl Cancer Inst (2013) 105:1634–43. doi: 10.1093/jnci/djt276
40. Williams MT, Yousafzai Y, Cox C, Blair A, Carmody R, Sai S, et al. Interleukin-15 enhances cellular proliferation and upregulates CNS homing molecules in pre-b acute lymphoblastic leukemia. Blood (2014) 123:3116–27. doi: 10.1182/blood-2013-05-499970
41. Si M, Jiao X, Li Y, Chen H, He P, Jiang F. The role of cytokines and chemokines in the microenvironment of the blood-brain barrier in leukemia central nervous system metastasis. Cancer Manag Res (2018) 10:305–13. doi: 10.2147/cmar.s152419
42. Moriarty AT, Wiersema L, Snyder W, Kotylo PK, McCloskey DW. Immunophenotyping of cytologic specimens by flow cytometry. Diagn Cytopathol (1993) 9:252–8. doi: 10.1002/dc.2840090303
43. Erta M, Quintana A, Hidalgo J. Interleukin-6, a major cytokine in the central nervous system. Int J Biol Sci (2012) 8:1254–66. doi: 10.7150/ijbs.4679
44. Kinjyo I, Bragin D, Grattan R, Winter SS, Wilson BS. Leukemia-derived exosomes and cytokines pave the way for entry into the brain. J Leukoc Biol (2019) 105:741–53. doi: 10.1002/jlb.3a0218-054r
45. Buonamici S, Trimarchi T, Ruocco MG, Reavie L, Cathelin S, Mar BG, et al. CCR7 signalling as an essential regulator of CNS infiltration in T-cell leukaemia. Nat (2009) 459:1000–4. doi: 10.1038/nature08020
46. Steinbichler TB, Dudás J, Riechelmann H, Skvortsova II. The role of exosomes in cancer metastasis. Semin Cancer Biol (2017) 44:170–81. doi: 10.1016/j.semcancer.2017.02.006
Keywords: cerebrospinal fluid, interleukin-6, central nervous system, acute myeloid leukemia, biomarker
Citation: Gu J, Huang X, Zhang Y, Bao C, Zhou Z, Tong H and Jin J (2022) Cerebrospinal fluid interleukin-6 is a potential diagnostic biomarker for central nervous system involvement in adult acute myeloid leukemia. Front. Oncol. 12:1013781. doi: 10.3389/fonc.2022.1013781
Received: 07 August 2022; Accepted: 14 November 2022;
Published: 01 December 2022.
Edited by:
Michele Redell, Baylor College of Medicine, United StatesReviewed by:
Sheng-Li Xue, The First Affiliated Hospital of Soochow University, ChinaCopyright © 2022 Gu, Huang, Zhang, Bao, Zhou, Tong and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Jin, amllajA1MDNAemp1LmVkdS5jbg==; Hongyan Tong, dG9uZ2hvbmd5YW5Aemp1LmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.