
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol. , 27 October 2022
Sec. Thoracic Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1013299
This article is part of the Research Topic Molecular Genetic Testing and Emerging Targeted Therapies for Non-Small Cell Lung Cancer View all 7 articles
Background: Dysregulation of the mesenchymal epithelial transition (MET) pathway contributes to poor clinical outcomes in patients with non-small cell lung cancer (NSCLC). Numerous clinical trials are currently investigating several therapies based on modulation of the MET pathway.
Objectives: This study aimed to systematically evaluate the activity and safety of MET inhibitors in patients with NSCLC.
Methods: We searched PubMed, Embase, and the Cochrane Library from inception to June 02, 2022. The objective response rate (ORR) and disease control rate (DCR) were extracted as the main outcomes and pooled using the weighted mean proportion with fixed- or random-effects models in cases of significant heterogeneity (I2>50%). Safety analysis was performed based on adverse events reported in all studies.
Results: Eleven studies (882 patients) were included in the meta-analysis. The pooled ORR was 28.1% (95% confidence interval [CI], 0.223–0.354), while the pooled DCR was 69.1% (95% CI, 0.631–0.756). ORRs were higher for tepotinib (44.7% [95% CI, 0.365–0.530]) and savolitinib (42.9% [95% CI, 0.311–0.553]) than for other types of MET inhibitors. Patients with NSCLC with exon 14 skipping exhibited higher ORRs (39.3% (95% CI, 0.296–0.522)) and DCRs (77.8% (95% CI, 0.714–0.847)) than those with MET protein overexpression or amplification. Intracranial response rate and intracranial disease control rates were 40.1% (95% CI, 0.289–0.556) and 95.4% (95% CI, 0.892–0.100), respectively. Adverse events were mild (grade 1 to 2) in 87.2% of patients. Common adverse events above grade 3 included lower extremity edema (3.5% [95% CI, 0.027–0.044]), alanine aminotransferase (ALT) elevation (2.4% [95% CI, 0.014–0.033]), and lipase elevation (2.2% [95% CI, 0.016–0.031]).
Conclusion: MET inhibitors, which exhibited a satisfactory safety profile in the current study, may become a new standard of care for addressing MET dysregulation in patients with advanced or metastatic NSCLC, and even in those with brain metastases, particularly tepotinib, savolitinib and capmatinib. Further randomized trials are required to establish standard predictive biomarkers for MET therapies and to compare the effects of different MET inhibitors in NSCLC with MET dysregulation.
Lung cancer is the leading cause of cancer-related death worldwide, resulting in an estimated 1.6 million deaths each year (1, 2). Non-small-cell lung cancer (NSCLC) is a predominant lung cancer subtype that accounts for nearly 85% of lung cancer cases, with an annual global incidence that continues to increase (3, 4). The five-year overall survival rate for NSCLC is poor, decreasing from 68% in patients with stage IB disease to 0–10% in those with stage IVA–IVB disease (5). Understanding the pathophysiology of NSCLC and detecting relevant mutations is crucial for developing effective therapeutic strategies. However, NSCLC is molecularly heterogeneous (6), and its development and progression have been associated with various oncogenic drivers (7–9). Current diagnostic standards for NSCLC are based on the detection of epidermal growth factor receptor (EGFR), v-raf murine sarcoma viral oncogene homolog B1 (BRAF), and mesenchymal epithelial transition (MET) mutations, as well as analyses of anaplastic lymphoma kinase (ALK), ROS proto-oncogene receptor tyrosine kinase 1 (ROS1), and neurotrophic receptor tyrosine kinase (NTRK) translocations. MET dysregulation is notable in that extreme increases in MET activity can induce tumorigenesis and lead to invasion, proliferation, angiogenesis, metastatic spread of tumors, and resistance to cancer treatments (10, 11).
Dysregulation of the MET pathway in NSCLC is thought to occur via various mechanisms, including protein overexpression, gene mutation, amplification, and rearrangement (12). The reported prevalence of MET amplification in patients with NSCLC ranges from 1–5% (13–15), while that of MET protein overexpression ranges from 25–75% (16–18). In patients with non-squamous NSCLC, the rate of MET mutations ranges from 2–4% (15, 19, 20), although such mutations are clearly overrepresented among older adults, in whom the prevalence is comparable to that observed in patients with ALK-rearranged lung cancer (21). Studies have demonstrated that both MET overexpression and amplification are related to poor prognosis in patients with NSCLC (22–25), and MET amplification appears to be an independent marker of poor outcome following surgically resection of NSCLC (26–28). In a series of 687 Asian patients with resected NSCLC, MET alterations were poor prognostic factors for overall survival (OS) (27). A review by Pilotto et al. highlighted MET exon 14 skipping alterations as potential oncogenic targets in lung cancer given their ability to drive the activity of MET inhibitors in molecularly selected patients (29). Based on this evidence, the MET pathway has been explored as a potential therapeutic target NSCLC drug development. Within the last decade, several MET inhibitors have been developed and are undergoing investigation in clinical trials (30–34), including tyrosine kinase inhibitors (TKIs), monoclonal antibodies (mAb), and antibody-drug conjugates (ADCs). TKIs that target the MET pathway are generally divided into two types (I and II). Type I inhibitors are adenosine triphosphate (ATP) competitors that bind to the ATP-binding pocket of the active form (DFG-in), whereas type II TKIs are ATP competitors that bind to the inactive state (DFG-out), resulting in a configuration that may benefit those with acquired resistance to type I TKIs (35). Some of the prototypical drugs include crizotinib (type I), capmatinib (type I), tepotinib (type I), savolitinib (type I), cabozantinib (type II), glesatinib (type II), and merestinib (type II). Monoclonal antibodies inhibiting the MET pathway target the extracellular domain, leading to signaling inhibition (36), and include drugs such as onartuzumab and emibetuzumab. Mechanistically, ADCs such as telisotuzumab vedotin and amivantamab exert effects following antibody binding via more targeted, direct delivery of a cytotoxic payload to the tumor cells, limiting any resistance mechanisms that may be related to intracellular signaling, such as MET amplification in EGFR TKI resistance (37).
A 2015 study reported dramatic and durable partial responses (PRs) to crizotinib in patients with NSCLC harboring MET alterations (38). Durable PRs to capmatinib have also been reported in patients with advanced NSCLC exhibiting MET dysregulation (15). Subsequent case reports have confirmed these findings using different MET inhibitors across NSCLC histologies (23, 39–41). Given the promising responses in patients treated with MET inhibitors in some clinical trials, the Japanese Ministry of Health, US FDA, and China National Medical Products Administration approved tepotinib, capmatinib, and savolitinib for the treatment of NSCLC with MET dysregulation in 2020. This decision not has not only bridged the gap in the treatment landscape for NSCLS with MET dysregulation but has also ushered in a new era for MET inhibitors. However, despite the promise of MET inhibitors for NSCLC with MET dysregulation in most clinical trials, several studies have reported low treatment response rates, and the safety and precise mechanisms underlying the effects of MET inhibitors in the treatment of NSCLC remain unclear. Therefore, to promote optimal clinical treatment, we conducted a meta-analysis of studies related to the activity and safety of MET inhibitors in patients with NSCLC.
This study was registered at PROSPERO under registration number CRD42022341285 and aligned with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines during all stages of design, implementation, and reporting (42).
An exhaustive literature search involving computer-assisted and manual methods was conducted. Two independent investigators (LX and FW) conducted a systematic literature search of PubMed, Embase and the Cochrane library using the key words “c-Met inhibitor” and “non-small-cell lung cancer”. The last date of the search was June 02, 2022. The detailed search strategy is presented in Supplementary Table S1.
Studies with relevant information on patient characteristics, treatment interventions, and outcomes were included. Eligibility was limited to articles reporting the result of clinical trials and published in English. The inclusion criteria were as follows: 1) histologically confirmed diagnosis of locally advanced or metastatic NSCLC with dysregulation of the MET pathway; 2) single-agent treatment with a MET inhibitor; 3) assessments of objective response rate (ORR, defined as the proportion of patients with a complete or partial response) and/or disease control rate (DCR, defined as the proportion of patients with a complete response, a partial response, or stable disease) based on the guidelines provided by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 (43); and 4) adverse events recorded and graded by the investigators according to the National Cancer Institute Common Terminology Criteria for Adverse Events (AEs). All reported adverse events associated with drug treatment were included in the safety assessment. Articles for which only abstracts could be located were excluded.
Two reviewers independently performed the search, study selection, and data extraction steps. In case of discrepancies, consensus was reached via discussion. Two reviewers independently screened titles and abstracts from the data sources based on the eligibility criteria mentioned above. The full texts of the potentially relevant articles were then reviewed thoroughly to guarantee eligibility criteria. We recorded the following information from the original literature: (1) first author; (2) year of publication; (3) study design; (4) baseline patient data, including the number of patients who met the inclusion criteria, type of MET dysregulation, sex, and age; (5) type, dose, and schedule of MET inhibitor treatment; (6) Eastern Cooperative Oncology Group performance status (ECOG PS); (7) response/outcome; and (8) AEs.
We used the Methodological Index for Non-randomized Studies (MINORS) (44) to assess the quality of non-randomized studies.
Baseline patient characteristics, treatment responses, and AEs were analyzed for all enrolled studies. The ORR and DCR of NSCLC with MET-dysregulation were expressed as mean rates with 95% confidence intervals (95% CI). A subgroup analysis was performed based on different types of MET inhibitors, different types of MET dysregulation, and therapy types (monotherapy or combination therapy). The chi-square test (Q statistic) and I (2) statistics were used to assess heterogeneity (45). If P ≥ 0.10 and/or I (2) ≤ 50%, heterogeneity was considered low, and a fixed-effects model was selected. In other cases, random-effects models were used. Sensitivity analysis was performed by individually excluding each study with high heterogeneity from the pooled results. The risk of publication bias was determined using funnel plots and Egger’s test (46). Statistical significance was set at P < 0.05. AEs were extracted from all studies for the pooled safety analysis. We used logit transformation to perform a meta-analysis of raw proportions with a continuity correction of 0.5 in studies with zero cell frequencies. All analyses were performed using R programming language (package meta, version 3.6.1).
A flow chart of the screening process is shown in Figure 1. A total of 2,291 articles were identified via the database search. Among the selected studies, 1,355 duplicated articles were excluded using Endnote software. After exclusion based on titles and abstracts, 96 full-text articles were reviewed. After a full-text screening, 11 publications (882 patients) were included in this systematic review. The classifications and features of the included studies are presented in Table 1. A total of 3 (31, 34, 47) studies were phase I trials, while 8 (48–55) studies were phase II trials. All patients were diagnosed with advanced or metastatic NSCLC. Types of MET dysregulation included MET amplification, exon 14 skipping, and MET protein overexpression. One study included patients with MET amplification (53); three included patients with exon 14 skipping (34, 54, 55), four included patients with MET amplification and exon 14 skipping (49–52), one include patients with MET amplification and MET protein overexpression (31), and two included patients with all three types of MET dysregulation (47, 48). Patients were treated with capmatinib in three studies (48–50), crizotinib in four studies (34, 51–53), SAR125844 in one study (31), savolitinib in one studies (54), telisotuzumab vedotina in one studies (47), and tepotinib in one studies (55). Overall, most Eastern Cooperative Oncology Group performance status (ECOG PS) scores were 0 or 1. The characteristics of the included studies are described in detail in Table 1.
All non-randomized studies assessed using the MINORS index had scores ranging from 13 to 15 points, which were considered acceptable for the present meta-analysis (Table 2).
The pooled ORR was evaluated in 11 studies involving 882 patients with NSCLC. When all studies were considered, objective responses were reported in 270 patients (270/882, 30.6%) after MET inhibitor treatment. The ORR ranged from 10.0% to 44.7%, and the random-effects model was selected given the heterogeneity among the studies (I (2) = 71%, P < 0.01). The pooled rate was 28.1% (95% CI, 0.223–0.354) (Figure 2A).
The forest plots for the subgroup analyses of studies involving different MET inhibitors including SAR125844, crizotinib, capmatinib, savolitinib, telisotuzumab vedotin, and tepotinib are shown in Figure 3A. SAR125844, crizotinib, capmatinib, savolitinib, telisotuzumab vedotin, and tepotinib were associated with ORRs of 18.2% (95% CI, 0.052–0.403), 26.0% (95% CI, 0.187–0.362), 23.5% (95% CI, 0.154–0.360),42.9% (95% CI, 0.311–0.553), 22.5% (95% CI, 0.108–0.385), and 44.7% (95% CI, 0.365–0.530) without heterogeneity, respectively.
The forest plots for subgroup analyses of studies involving different types of MET dysregulation, including exon 14 skipping, MET protein overexpression, and amplification, are shown in Figure 4A. Among patients with NSCLC, MET amplification and MET protein overexpression were associated with ORRs of 24.5% (95% CI, 0.187–0.322) and 21.8% (95% CI, 0.150–0.317) without heterogeneity, respectively. In patients with exon 14 skipping, the ORR was 39.3% (95% CI, 0.296–0.522, I2 =60%).
All studies were included in the analysis of DCR (Figure 2B), which ranged from 45.3% to 81.4%. The pooled DCR was 69.1% (95% CI, 0.631–0.756), with high heterogeneity among studies (I (2) = 63%, P < 0.01).
Forest plots for subgroup analyses are shown in Figure 3B. SAR125844, crizotinib, capmatinib, savolitinib, telisotuzumab vedotin, and tepotinib were associated with DCRs of 77.3% (95% CI, 0.546–0.922), 62.6% (95% CI, 0.499–0.785), 66.3% (95% CI, 0.527–0.835), 81.4% (95% CI, 0.703–0.897), 70.0% (95% CI, 0.535–0.834), and 70.4% (95% CI, 0.625–0.775), respectively.
The forest plots for subgroup analyses of studies involving different types of MET dysregulation, including exon 14 skipping, MET protein overexpression, and amplification, are shown in Figure 4B. The DCRs for MET amplification, MET protein overexpression, and exon 14 skipping were 67.5% (95% CI, 0.568–0.802, I2 =75%), 70.8% (95% CI, 0.499–1.000, I2 =79%), and 77.8% (95% CI, 0.714–0.847, I2 =62%), respectively, with high heterogeneity among studies.
Figure 5 shows the forest plot for intracranial response and intracranial disease control rates. The pooled intracranial response rate and intracranial disease control rate were 40.1% (95% CI, 0.289–0.556; I2 =28%) and 95.4% (95% CI, 0.892–0.100; I2 =3%) without heterogeneity, respectively.
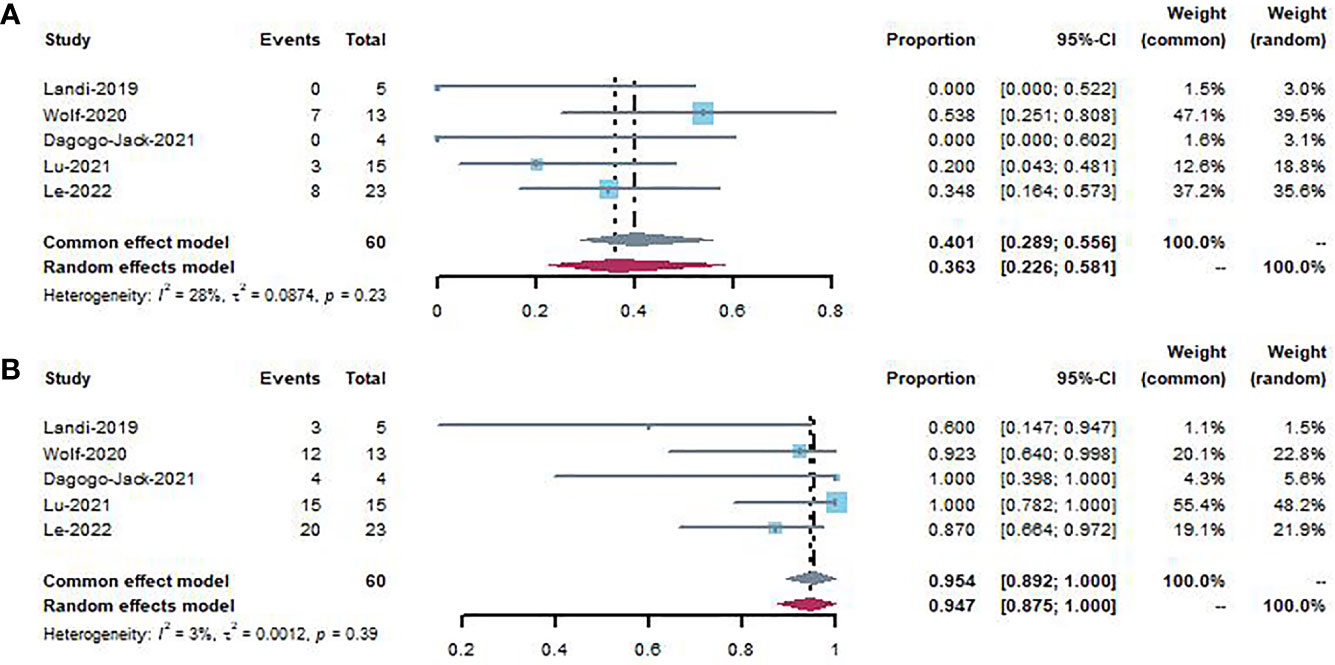
Figure 5 Forest plot showing intracranial response (A) and intracranial disease control (B) to patients using MET inhibitors.
All patients treated with MET inhibitors were evaluated for AEs, some of which were not included in the pooled analysis of response outcomes because the treatment response data were unavailable. There were 10 (31, 34, 47–51, 53–55) studies (829 patients) reporting 2,103 AEs associated with MET inhibitor treatment, most of which were mild. Five studies (34, 49, 50, 54, 55) reported 7 deaths due to MET inhibitor treatment. One study (52) did not provide relevant information on AEs. We evaluated AEs related to MET inhibitor treatment according to the National Cancer Institute Common Terminology Criteria for Adverse Events, which are shown in Table 3. A total of 829 patients from ten studies were included in the safety evaluation. In these patients, 87.2% AEs were mild to moderate (grade 1–2), the most common of which included lower extremity edema, nausea, fatigue, vomiting, diarrhea, anorexia, alanine aminotransferase (ALT) elevation, and creatinine elevation. A total of 270 grade 3 or 4 AEs were observed, the most common of which included lower extremity edema, ALT elevation, lipase elevation, fatigue, AST elevation, amylase elevation, vomiting, and nausea. Seven deaths were associated with MET inhibitor treatment in 829 patients: three due to pneumonitis, two in patients with interstitial lung disease, one in a patient with dyspnea, and one in a patient with tumor lysis syndrome. The pooled rates of ≥grade 3 common AEs were 3.5% (95% CI, 0.027-0.044) for lower extremity edema, 2.4% (95% CI, 0.014–0.033) for ALT elevation, and 2.2% (95% CI, 0.016–0.031) for lipase elevation (Table 4).
Sensitivity analysis was performed by removing individual studies one by one from the pooled results with high heterogeneity. The pooled analysis of ORR and DCR did not change significantly when studies were omitted, indicating that our combined results are reliable (Figure 6).
We used the Egger’s test and funnel plots to evaluate the publication bias in studies included. The results of the Egger’s test showed no evidence of publication bias in the studies on ORR (P =0.13) (Figure 7A) and DCR (P =0.29) (Figure 7B). This was consistent with the shape of funnel plots which had a good symmetry.
In this meta-analysis, we examined the activity and safety of MET inhibitors in patients with NSCLC exhibiting MET dysregulation based on the results of clinical trials. In our study including 882 patients with NSCLC, the estimated ORR was 28.1%, while the DCR was 69.1%. These results highlight the promise of MET inhibitors in patients with advanced/metastatic NSCLC with MET dysregulation, especially tepotinib and savolitinib. Moreover, patients with exon 14 skipping responded best to MET inhibitors, with an ORR of 39.3% and a DCR of 77.8%. Among 829 cases, 2,103 AEs associated with MET-inhibitor-treatment were reported, 87.2% of which were mild (grade 1–2), indicating that MET inhibitors are tolerated by most patients. The most common adverse events above grade 3 included lower extremity edema (3.5%), ALT elevation (2.4%), and lipase elevation (2.2%).
Although the overall ORR was not very high in patients treated with MET inhibitors, satisfactory overall DCRs were observed. In general, therapeutic responses vary widely for different MET inhibitors in different trials, from 10.0% to 44.7% for ORR and 45.3% to 81.4% for DCR. This may be explained by the different biological value of each individual MET alteration (MET overexpression, amplification, and MET exon 14 skipping) and the definitions/methods used to detect MET alterations in different trials. It remains highly debated whether patients with MET overexpression or amplification can benefit from MET inhibitors; however, MET exon 14 skipping is now an established biomarker, and patients with such alterations have been shown to benefit from MET-targeted therapies (56). MET inhibitors against MET exon 14 skipping offer hope to affected patients, as previous studies have demonstrated a low response to immune-oncology drugs in this population (57). Our pooled treatment response results for the MET dysregulation subgroup indicated that patients with exon 14 skipping had a higher ORR (39.3%) and DCR (77.8%) than those with MET protein overexpression or amplification, which is consistent with current views regarding the therapeutic tractability of different MET alterations. Immunohistochemistry (IHC) results for MET protein overexpression and fluorescence in situ hybridization (FISH) results for MET amplification are continuous variables, meaning that the selection of cut-off points is crucial. Only patients with a very high level of MET protein overexpression or amplification may benefit from these treatments, and patients with an MET status below a given threshold may exhibited diminished responses at the individual level (58). The results of initial trials evaluating MET inhibitor efficiency focused on unselected NSCLC have been negative (59), which may also be explained by wide variations in responses. MET amplification leads to overexpression or constitutive kinase activation via multiplication of the MET gene and synthesis of MET protein in excess; thus, the level of gene amplification may act as an oncogenic driver in patients with NSCLC (12). Previous studies involving patients with MET-amplified advanced NSCLC have reported greater efficacy in tumors with a high gene copy number than in those with a low gene copy number, and clinical trials of capmatinib have revealed that a gene copy number (GCN) ≥ 6 is associated with satisfactory anti-tumor activity (48, 49). Unlike MET amplification, MET overexpression can reflect both genomic and non-genomic processes; thus, the lack of correlation between protein expression and genomic alterations indicates that MET protein overexpression may not be a reliable patient selection criterion for MET-targeted therapies (60). The use of targeted therapies in this population has produced disappointing results in the context of advanced NSCLC (61, 62). Our pooled ORR was 23.5% in patients with MET overexpression, which is similar to the rate observed in patients with MET amplification, mainly because these patients had concurrent MET amplification and MET exon 14 skipping. Thus, MET overexpression alone may not be a reliable biomarker for predicting the activity of MET inhibitors. Next-generation sequencing (NGS) for MET exon 14 skipping mutations and/or amplification, FISH for MET amplification, and IHC for MET overexpression are widely used to detect MET dysregulation. Establishing standard predictive biomarkers for MET therapies remains an urgently requirement, as it is important for clinicians to distinguish between the various mechanisms of MET dysregulation to ensure appropriate testing and prompt treatment with optimal methods.
Current MET-signaling-targeted therapeutic strategies include inhibiting kinase activity, preventing phosphotransferase activity, and blocking MET signaling (58, 63, 64). This study evaluated the benefits of MET inhibitors, including anti-MET antibodies (SAR125844) (31), TKIs (crizotinib, savolitinib, tepotinib, capmatinib) (34, 48–55), and ADC (telisotuzumab vedotin) (47). Notably, the efficiency of tepotinib and savolitinib in clinical trials has been promising, with ORRs reaching nearly 50%. Both drugs are selective c-MET TKIs that have been approved for the treatment of NSCLC with MET dysregulation in Japan and China. One possible reason for the positive results is the proper selection of patients. In the included trials, patients treated with savolitinib monotherapy (54) and tepotinib monotherapy (55) all had MET exon 14 skipping mutations, which appears to be the most promising subset with sensitivity to MET inhibitors. In a clinical trial of capmatinib monotherapy, Wolf et al. reported similar ORRs to these two drugs in patients with NSCLC harboring MET exon 14 skipping mutations, and the overall ORR in patients with both MET exon 14 skipping mutations and MET amplification was 29.3%. Capmatinib has been approved for the treatment of NSCLC with MET dysregulation in the US. The inclusion of patients with different types of MET dysregulation (mutations, amplification, and overexpression) may have influenced the overall treatment effect in previous studies. Capmatinib has exhibited satisfactory antitumor activity in patients with MET exon 14 skipping mutations or high-GCN MET amplification, particularly in those who had not received previous treatment (48, 49); Tepotinib and savolitinib have exhibited satisfactory clinical activity in patients with MET exon 14 skipping mutations, irrespective of previous systemic treatment, and in those with brain metastases (54, 55). Another study reported satisfactory antitumor activity following crizotinib treatment for NSCLC with high levels of MET amplification (MET-to-CEP7 ratio ≥4, ORR: 38.1%) and for NSCLC with exon 14 skipping mutations (53). In addition, SAR125844 has shown modest antitumor activity (ORR: 28.6%) in NSCLC with MET amplification (31). In addition to monotherapy, combination therapies have been investigated in several clinical trials. MET dysregulation is known to confer primary or secondary resistance against EGFR TKIs, and research has demonstrated that inhibiting MET expression through shRNA can restore sensitivity to EGFR-TKIs (65). Based on this rationale, patients with MET amplification or MET protein overexpression plus MET-driven EGFR TKI resistance (32, 33, 66) may benefit from a combination of savolitinib and tepotinib (savolitinib plus osimertinib, savolitinib plus gefitinib, and tepotinib plus gefitinib), even those with disease progression following prior treatment with an EGFR inhibitor. These results highlight the potential of savolitinib and tepotinib to become the new standard of care for NSCLC, especially in patients with MET exon 14 skipping mutations. The combination of MET TKIs and EGFR TKIs (osimertinib plus savolitinib, savolitinib plus gefitinib, tepotinib plus gefitinib) may also be promising for MET-driven EGFR TKI resistance. Other combination therapies have been associated with minimal activity (ORRs lower than 20%), including capmatinib plus gefitinib, capmatinib plus erlotinib, telisotuzumab vedotina plus nivolumab, onartuzumab plus erlotinib) (30, 47, 61, 67, 68). However, these results may have been affected by weak preclinical rationale or inadequate patient selection.
Brain metastases may occur in up to 20 to 40% of patients with stage IV NSCLC (69). In the pooled analysis of MET activity in the CNS within a small patient group (N = 60), an intracranial response was observed in 40.1% of patients, while intracranial disease control was observed in 95.4% of patients. Given the importance of CNS control in maintaining the best disease response and quality of life, confirmation of these preliminary findings in a larger population is crucial.
The current findings indicate that MET inhibitors are well-tolerated and safe in patients with NSCLC. AEs mainly included lower-extremity edema, nausea, fatigue, diarrhea, vomiting, anorexia, ALT elevation, and creatinine elevation, most of which were mild and persisted for a short time only. Peripheral edema and nausea are the most frequent AEs in TKI trials, while anemia and fatigue are the most frequent events in trials of mAb and ADC. In our analysis, only seven deaths were reported to be associated with MET-inhibitor treatment. Although antibodies have excellent target specificity and predictable pharmacological properties, their toxicity is similar to that of molecular inhibitors.
There was high heterogeneity (I (2) = 71%, P < 0.01) among ORRs and DCRs (I (2) = 63%, P < 0.01). Subgroup analyses of each drug indicated that ORRs were higher for savolitinib (ORR, 42.9%) and tepotinib (ORR, 44.7%) than for other MET inhibitors, while subgroup analyses based on MET dysregulation type indicated that both ORRs and DCRs were higher for patients with exon 14 skipping (ORR, 39.3%; DCR, 77.8%) than for those with MET protein overexpression or amplification. These findings support additional evidence regarding the heterogeneity of NSCLC.
This meta-analysis had several limitations. First, the number of patients included was small, and no RCTs were included in this review. The small sample size may have influenced the strength of our study. Second, all studies failed to compare the activity of different types of MET inhibitors, meaning that we were unable to provide unbiased head-to-head comparisons of treatment effects. Third, despite a careful electronic search of the literature databases, some publications may have been missed. Fourth, most of this evidence is based on phase I and II studies; therefore, phase III studies are warranted to confirm the activity and safety of MET inhibitors.
The results of the current systematic review and meta-analysis suggest that MET inhibitors, especially savolitinib and tepotinib, are promising treatment options for NSCLC. Based on our analysis, most patients exhibit good tolerance to MET inhibitors. However, considering the limitations of previous studies, prospective randomized trials are required to assess the activity of different types of MET inhibitors in patients with NSCLC exhibiting MET dysregulation. Future studies should also aim to identify unique biomarkers and accurate diagnostic platforms for MET-targeted therapeutic strategies to avoid disparities in the evaluation of clinical outcomes.
Conception and design: FL and LX. Acquisition of data: LX and FW. Critical revision of the manuscript for important intellectual content: FL, LX, and FW. Statistical analysis: LX and FW. Obtain funding: FW. All authors contributed to the article and approved the submitted version.
This work was supported by Sichuan Science and Technology Program (No.2021YFQ0030); Tibet Science and Technology Program (XZ202201ZY0002G); 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYJC18021) and Post-Doctor Research Project, West China Hospital (2021HXBH074).
The authors thank Lichun Zhong (Laboratory of Pulmonary Immunology and Inflammation, Frontiers Science Center for Disease-related Molecular Network, West China Hospital, Sichuan University) for advising on the data collection.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1013299/full#supplementary-material
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA: Cancer J Clin (2015) 65(2):87–108. doi: 10.3322/caac.21262
3. Imyanitov EN, Iyevleva AG, Levchenko EV. Molecular testing and targeted therapy for non-small cell lung cancer: Current status and perspectives. Crit Rev oncology/hematology. (2021) 157:103194. doi: 10.1016/j.critrevonc.2020.103194
4. Duma N, Santana-Davila R, Molina JR. Non-small cell lung cancer: Epidemiology, screening, diagnosis, and treatment. Mayo Clinic Proc (2019) 94(8):1623–40. doi: 10.1016/j.mayocp.2019.01.013
5. Goldstraw P, Chansky K, Crowley J, Giroux DJ, Groome PA, Rami-Porta R, et al. The IASLC lung cancer staging project: Proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. (2016) 11(1):39–51. doi: 10.1016/j.jtho.2015.09.009
6. Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J cancer. (2001) 94(2):153–6. doi: 10.1002/ijc.1440
7. Cardarella S, Ortiz TM, Joshi VA, Butaney M, Jackman DM, Kwiatkowski DJ, et al. The introduction of systematic genomic testing for patients with non-small-cell lung cancer. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. (2012) 7(12):1767–74. doi: 10.1097/JTO.0b013e3182745bcb
8. Pao W, Girard N. New driver mutations in non-small-cell lung cancer. Lancet Oncol (2011) 12(2):175–80. doi: 10.1016/S1470-2045(10)70087-5
9. Shen C, Yang C, Xia B, You M. Long non-coding RNAs: Emerging regulators for chemo/immunotherapy resistance in cancer stem cells. Cancer letters. (2021) 500:244–52. doi: 10.1016/j.canlet.2020.11.010
10. Jeffers M, Rong S, Vande Woude GF. Hepatocyte growth factor/scatter factor-met signaling in tumorigenicity and invasion/metastasis. J Mol Med (Berlin Germany). (1996) 74(9):505–13. doi: 10.1007/BF00204976
11. Jeffers M, Schmidt L, Nakaigawa N, Webb CP, Weirich G, Kishida T, et al. Activating mutations for the met tyrosine kinase receptor in human cancer. Proc Natl Acad Sci United States America. (1997) 94(21):11445–50. doi: 10.1073/pnas.94.21.11445
12. Drilon A, Cappuzzo F, Ou SI, Camidge DR. Targeting MET in lung cancer: Will expectations finally be MET? J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. (2017) 12(1):15–26. doi: 10.1016/j.jtho.2016.10.014
13. Onozato R, Kosaka T, Kuwano H, Sekido Y, Yatabe Y, Mitsudomi T. Activation of MET by gene amplification or by splice mutations deleting the juxtamembrane domain in primary resected lung cancers. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. (2009) 4(1):5–11. doi: 10.1097/JTO.0b013e3181913e0e
14. Cappuzzo F, Marchetti A, Skokan M, Rossi E, Gajapathy S, Felicioni L, et al. Increased MET gene copy number negatively affects survival of surgically resected non-small-cell lung cancer patients. J Clin Oncol Off J Am Soc Clin Oncol (2009) 27(10):1667–74. doi: 10.1200/JCO.2008.19.1635
15. Frampton GM, Ali SM, Rosenzweig M, Chmielecki J, Lu X, Bauer TM, et al. Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer discovery. (2015) 5(8):850–9. doi: 10.1158/2159-8290.CD-15-0285
16. Ma PC, Jagadeeswaran R, Jagadeesh S, Tretiakova MS, Nallasura V, Fox EA, et al. Functional expression and mutations of c-met and its therapeutic inhibition with SU11274 and small interfering RNA in non-small cell lung cancer. Cancer Res (2005) 65(4):1479–88. doi: 10.1158/0008-5472.CAN-04-2650
17. Ma PC, Tretiakova MS, MacKinnon AC, Ramnath N, Johnson C, Dietrich S, et al. Expression and mutational analysis of MET in human solid cancers. Genes Chromosomes cancer. (2008) 47(12):1025–37. doi: 10.1002/gcc.20604
18. Nakamura Y, Niki T, Goto A, Morikawa T, Miyazawa K, Nakajima J, et al. C-met activation in lung adenocarcinoma tissues: an immunohistochemical analysis. Cancer science. (2007) 98(7):1006–13. doi: 10.1111/j.1349-7006.2007.00493.x
19. Mitiushkina NV, Kholmatov MM, Tiurin VI, Romankoet AA, Yatsuk OS, Sokolova TN, et al. Comparative analysis of expression of mutant and wild-type alleles is essential for reliable PCR-based detection of MET exon 14 skipping. Biochimie. (2019) 165:267–74. doi: 10.1016/j.biochi.2019.08.014
20. Jouneau S, Kerjouan M, Briens E, Lenormand JP, Meunier C, Letheulle J, et al. [Pulmonary alveolar proteinosis]. Rev Des maladies respiratoires. (2014) 31(10):975–91. doi: 10.1016/j.rmr.2014.08.009
21. Shaw AT, Engelman JA. ALK in lung cancer: Past, present, and future. J Clin Oncol Off J Am Soc Clin Oncol (2013) 31(8):1105–11. doi: 10.1200/JCO.2012.44.5353
22. Peters S, Adjei AA. MET: A promising anticancer therapeutic target. Nat Rev Clin Oncol (2012) 9(6):314–26. doi: 10.1038/nrclinonc.2012.71
23. Awad MM, Oxnard GR, Jackman DM, Savukoski DO, Hall D, Shivdasani P, et al. MET exon 14 mutations in non-Small-Cell lung cancer are associated with advanced age and stage-dependent MET genomic amplification and c-met overexpression. J Clin Oncol Off J Am Soc Clin Oncol (2016) 34(7):721–30. doi: 10.1200/JCO.2015.63.4600
24. Engelman JA, Jänne PA. Mechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Clin Cancer Res an Off J Am Assoc Cancer Res (2008) 14(10):2895–9. doi: 10.1158/1078-0432.CCR-07-2248
25. Turke AB, Zejnullahu K, Wu YL, Song Y, Dias-Santagata D, Lifshits E, et al. Preexistence and clonal selection of MET amplification in EGFR mutant NSCLC. Cancer Cell (2010) 17(1):77–88. doi: 10.1016/j.ccr.2009.11.022
26. Feng Y, Minca EC, Lanigan C, Liu A, Zhang W, Yin L, et al. High MET receptor expression but not gene amplification in ALK 2p23 rearrangement positive non-small-cell lung cancer. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. (2014) 9(5):646–53. doi: 10.1097/JTO.0000000000000145
27. Tong JH, Yeung SF, Chan AW, Chung LY, Chau SL, Lung RWM, et al. MET amplification and exon 14 splice site mutation define unique molecular subgroups of non-small cell lung carcinoma with poor prognosis. Clin Cancer Res an Off J Am Assoc Cancer Res (2016) 22(12):3048–56. doi: 10.1158/1078-0432.CCR-15-2061
28. Go H, Jeon YK, Park HJ, Sung SW, Seo JW, Chung DH. High MET gene copy number leads to shorter survival in patients with non-small cell lung cancer. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. (2010) 5(3):305–13. doi: 10.1097/JTO.0b013e3181ce3d1d
29. Pilotto S, Carbognin L, Karachaliou N, Ma PC, Rosell R, Tortora G, et al. Tracking MET de-addiction in lung cancer: A road towards the oncogenic target. Cancer Treat Rev (2017) 60:1–11. doi: 10.1016/j.ctrv.2017.08.002
30. Spigel DR, Ervin TJ, Ramlau RA, Daniel DB, Goldschmidt JH Jr, Blumenschein GR Jr, et al. Randomized phase II trial of onartuzumab in combination with erlotinib in patients with advanced non-small-cell lung cancer. J Clin Oncol Off J Am Soc Clin Oncol (2013) 31(32):4105–14. doi: 10.1200/JCO.2012.47.4189
31. Angevin E, Spitaleri G, Rodon J, Dotti K, Isambert N, Salvagni S, et al. A first-in-human phase I study of SAR125844, a selective MET tyrosine kinase inhibitor, in patients with advanced solid tumours with MET amplification. Eur J Cancer (Oxford Engl 1990). (2017) 87:131–9. doi: 10.1016/j.ejca.2017.10.016
32. Yang JJ, Fang J, Shu YQ, Chang JH, Chen GY, He JX, et al. A phase ib study of the highly selective MET-TKI savolitinib plus gefitinib in patients with EGFR-mutated, MET-amplified advanced non-small-cell lung cancer. Investigational New Drugs (2021) 39(2):477–87. doi: 10.1007/s10637-020-01010-4
33. Wu YL, Cheng Y, Zhou J, Lu S, Zhang Y, Zhao J, et al. Tepotinib plus gefitinib in patients with EGFR-mutant non-small-cell lung cancer with MET overexpression or MET amplification and acquired resistance to previous EGFR inhibitor (INSIGHT study): an open-label, phase 1b/2, multicentre, randomised trial. Lancet Respir Med (2020) 8(11):1132–43. doi: 10.1016/S2213-2600(20)30154-5
34. Drilon A, Clark JW, Weiss J, Ou SI, Camidge DR, Solomon BJ, et al. Antitumor activity of crizotinib in lung cancers harboring a MET exon 14 alteration. Nat Med (2020) 26(1):47–51. doi: 10.1038/s41591-019-0716-8
35. Fujino T, Kobayashi Y, Suda K, Koga T, Nishino M, Ohara S, et al. Sensitivity and resistance of MET exon 14 mutations in lung cancer to eight MET tyrosine kinase inhibitors in vitro. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. (2019) 14(10):1753–65. doi: 10.1016/j.jtho.2019.06.023
36. Mo HN, Liu P. Targeting MET in cancer therapy. Chronic Dis Trans Med (2017) 3(3):148–53. doi: 10.1016/j.cdtm.2017.06.002
37. Strickler JH, Weekes CD, Nemunaitis J, Ramanathan RK, Heist RS, Morgensztern D, First-in-Human Phase I. Dose-escalation and -expansion study of telisotuzumab vedotin, an antibody-drug conjugate targeting c-met, in patients with advanced solid tumors. J Clin Oncol Off J Am Soc Clin Oncol (2018) 36(33):3298–306. doi: 10.1200/JCO.2018.78.7697
38. Paik PK, Drilon A, Fan PD, Yu H, Rekhtman N, Ginsberg MS, et al. Response to MET inhibitors in patients with stage IV lung adenocarcinomas harboring MET mutations causing exon 14 skipping. Cancer discovery. (2015) 5(8):842–9. doi: 10.1158/2159-8290.CD-14-1467
39. Jenkins RW, Oxnard GR, Elkin S, Sullivan EK, Carter JL, Barbie DA. Response to crizotinib in a patient with lung adenocarcinoma harboring a MET splice site mutation. Clin Lung cancer. (2015) 16(5):e101–104. doi: 10.1016/j.cllc.2015.01.009
40. Lee C, Usenko D, Frampton GM, McMahon C, Ali SM, Weiss J. MET 14 deletion in sarcomatoid non-Small-Cell lung cancer detected by next-generation sequencing and successfully treated with a MET inhibitor. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. (2015) 10(12):e113–114. doi: 10.1097/JTO.0000000000000645
41. Mahjoubi L, Gazzah A, Besse B, Lacroix L. Soria JC. A never-smoker Lung adenocarcinoma patient MET exon 14 Mutat (D1028N) Rapid partial response after crizotinib. Investigational New Drugs (2016) 34(3):397–8. doi: 10.1007/s10637-016-0332-2
42. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ (Clinical Res ed.). (2021) 372:n71. doi: 10.1136/bmj.n71
43. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer (Oxford Engl 1990). (2009) 45(2):228–47. doi: 10.1016/j.ejca.2008.10.026
44. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): Development and validation of a new instrument. ANZ J surgery. (2003) 73(9):712–6. doi: 10.1046/j.1445-2197.2003.02748.x
45. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical Res ed.). (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
46. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical Res ed.). (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629
47. Camidge DR, Morgensztern D, Heist RS, Barve M, Vokes E, Goldman JW, et al. Phase I study of 2- or 3-week dosing of telisotuzumab vedotin, an antibody-drug conjugate targeting c-met, monotherapy in patients with advanced non-small cell lung carcinoma. Clin Cancer Res an Off J Am Assoc Cancer Res (2021) 27(21):5781–92. doi: 10.1158/1078-0432.CCR-21-0765
48. Schuler M, Berardi R, Lim WT, Jonge M, Bauer TM, Azaro A, et al. Molecular correlates of response to capmatinib in advanced non-small-cell lung cancer: clinical and biomarker results from a phase I trial. Ann Oncol Off J Eur Soc Med Oncol (2020) 31(6):789–97. doi: 10.1016/j.annonc.2020.03.293
49. Wolf J, Seto T, Han JY, Reguart N, Garon EB, Groen HJM, et al. Capmatinib in MET exon 14-mutated or MET-amplified non-Small-Cell lung cancer. New Engl J Med (2020) 383(10):944–57. doi: 10.1056/NEJMoa2002787
50. Dagogo-Jack I, Moonsamy P, Gainor JF, Lennerz JK, Piotrowska Z, Lin JJ, et al. A phase 2 study of capmatinib in patients with MET-altered lung cancer previously treated with a MET inhibitor. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. (2021) 16(5):850–9. doi: 10.1016/j.jtho.2021.01.1605
51. Landi L, Chiari R, Tiseo M, D'Incà F, Dazzi C, Chella A, et al. Crizotinib in MET-deregulated or ROS1-rearranged pretreated non-small cell lung cancer (METROS): A phase II, prospective, multicenter, two-arms trial. Clin Cancer Res an Off J Am Assoc Cancer Res (2019) 25(24):7312–9. doi: 10.1158/1078-0432.CCR-19-0994
52. Moro-Sibilot D, Cozic N, Pérol M, Mazières J, Otto J, Souquet PJ, et al. Crizotinib in c-MET- or ROS1-positive NSCLC: Results of the AcSé phase II trial. Ann Oncol Off J Eur Soc Med Oncol (2019) 30(12):1985–91. doi: 10.1093/annonc/mdz407
53. Camidge DR, Otterson GA, Clark JW, Ignatius Ou SH, Weiss J, Ades S, et al. Crizotinib in patients with MET-amplified NSCLC. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. (2021) 16(6):1017–29. doi: 10.1016/j.jtho.2021.02.010
54. Lu S, Fang J, Li X, Cao L, Zhou J, Guo Q, et al. Once-daily savolitinib in Chinese patients with pulmonary sarcomatoid carcinomas and other non-small-cell lung cancers harbouring MET exon 14 skipping alterations: a multicentre, single-arm, open-label, phase 2 study. Lancet Respir Med (2021) 9(10):1154–64. doi: 10.1016/S2213-2600(21)00084-9
55. Le X, Sakai H, Felip E, Veillon R, Garassino MC, Raskin J, et al. Tepotinib efficacy and safety in patients with MET exon 14 skipping NSCLC: Outcomes in patient subgroups from the VISION study with relevance for clinical practice. Clin Cancer Res an Off J Am Assoc Cancer Res (2022) 28(6):1117–26. doi: 10.1158/1078-0432.CCR-21-2733
56. Lai GGY, Guo R, Drilon A, Shao Weng Tan D. Refining patient selection of MET-activated non-small cell lung cancer through biomarker precision. Cancer Treat Rev (2022) 110:102444. doi: 10.1016/j.ctrv.2022.102444
57. Bittoni M, Yang JC, Shih JY, Peled N, Smit EF, Camidge DS, et al. Real-world insights into patients with advanced NSCLC and MET alterations. Lung Cancer (Amsterdam Netherlands). (2021) 159:96–106. doi: 10.1016/j.lungcan.2021.06.015
58. Comoglio PM, Trusolino L, Boccaccio C. Known and novel roles of the MET oncogene in cancer: a coherent approach to targeted therapy. Nat Rev Cancer. (2018) 18(6):341–58. doi: 10.1038/s41568-018-0002-y
59. Ye S, Li J, Hao K, Yan J, Zhou H. The efficacy and risk profile of c-met inhibitors in non-small cell lung cancer: A meta-analysis. Sci Rep (2016) 6:35770. doi: 10.1038/srep35770
60. Guo R, Luo J, Chang J, Rekhtman N, Arcila M, Drilon A. MET-dependent solid tumours - molecular diagnosis and targeted therapy. Nat Rev Clin Oncol (2020) 17(9):569–87. doi: 10.1038/s41571-020-0377-z
61. Spigel DR, Edelman MJ, O'Byrne K, Paz-Ares L, Mocci S, Phan S, et al. Results from the phase III randomized trial of onartuzumab plus erlotinib versus erlotinib in previously treated stage IIIB or IV non-Small-Cell lung cancer: METLung. J Clin Oncol Off J Am Soc Clin Oncol (2017) 35(4):412–20. doi: 10.1200/JCO.2016.69.2160
62. Yoshioka H, Azuma K, Yamamoto N, Takahashi T, Nishio M, Katakami N, et al. A randomized, double-blind, placebo-controlled, phase III trial of erlotinib with or without a c-met inhibitor tivantinib (ARQ 197) in Asian patients with previously treated stage IIIB/IV nonsquamous nonsmall-cell lung cancer harboring wild-type epidermal growth factor receptor (ATTENTION study). Ann Oncol Off J Eur Soc Med Oncol (2015) 26(10):2066–72. doi: 10.1093/annonc/mdv288
63. Koch JP, Aebersold DM, Zimmer Y, Medová M. MET targeting: time for a rematch. Oncogene. (2020) 39(14):2845–62. doi: 10.1038/s41388-020-1193-8
64. Recondo G, Che J, Jänne PA, Awad MM. Targeting MET dysregulation in cancer. Cancer discovery. (2020) 10(7):922–34. doi: 10.1158/2159-8290.CD-19-1446
65. Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Sci (New York N.Y.). (2007) 316(5827):1039–43. doi: 10.1126/science.1141478
66. Sequist LV, Han JY, Ahn MJ, et al. Osimertinib plus savolitinib in patients with EGFR mutation-positive, MET-amplified, non-small-cell lung cancer after progression on EGFR tyrosine kinase inhibitors: interim results from a multicentre, open-label, phase 1b study. Lancet Oncol (2020) 21(3):373–86. doi: 10.1016/S1470-2045(19)30785-5
67. McCoach CE, Yu A, Gandara DR, Riess JW, Vang DP, Li T, et al. Phase I/II study of capmatinib plus erlotinib in patients with MET-positive non-Small-Cell lung cancer. JCO Precis Oncol (2021) 5, 117–87. doi: 10.1200/PO.20.00279
68. Wu YL, Zhang L, Kim DW, Liu X, Lee DH, Yang JC, et al. Phase Ib/II study of capmatinib (INC280) plus gefitinib after failure of epidermal growth factor receptor (EGFR) inhibitor therapy in patients with EGFR-mutated, MET factor-dysregulated non-Small-Cell lung cancer. J Clin Oncol Off J Am Soc Clin Oncol (2018) 36(31):3101–9. doi: 10.1200/JCO.2018.77.7326
Keywords: non-small cell lung cancer, MET inhibitors, activity, safety, meta-analysis
Citation: Xu L, Wang F and Luo F (2022) MET-targeted therapies for the treatment of non-small-cell lung cancer: A systematic review and meta-analysis. Front. Oncol. 12:1013299. doi: 10.3389/fonc.2022.1013299
Received: 06 August 2022; Accepted: 17 October 2022;
Published: 27 October 2022.
Edited by:
Santiago Viteri, Clínica Mi Tres Torres, SpainReviewed by:
Carlos Cabrera-Gálvez, Clinica Mi Tres Torres, SpainCopyright © 2022 Xu, Wang and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fengming Luo, ZmVuZ21pbmdsdW9Ab3V0bG9vay5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.