- 1Department of General Surgery, Nanjing Drum Tower Hospital, The Affiliated Hospital of Nanjing University Medical School, Nanjing, China
- 2Department of Pathology, Nanjing Drum Tower Hospital, The Affiliated Hospital of Nanjing University Medical School, Nanjing, China
- 3Institute of Thoracic Oncology, West China Hospital, Sichuan University, Chengdu, China
Objective: To evaluate the prognostic impact of folate receptor (FR)-positive circulating tumor cells (FR+ CTCs) for patients with pancreatic cancer (PC).
Background: Risk stratification before surgery for PC patients remains challenging as there are no reliable prognostic markers currently. FR+ CTCs, detected by ligand-targeted polymerase chain reaction (LT-PCR), have shown excellent diagnostic value for PC in our previous study and prognostic value in a variety of cancer types.
Methods: Peripheral blood samples from 44 consecutive patients diagnosed with PC were analyzed for FR+ CTCs. 25 patients underwent tumor resection and were assigned to the surgical group. 19 patients failed to undergo radical resection because of local advance or distant metastasis and were assigned to the non-surgical group. The impact of CTCs on relapse and survival were explored.
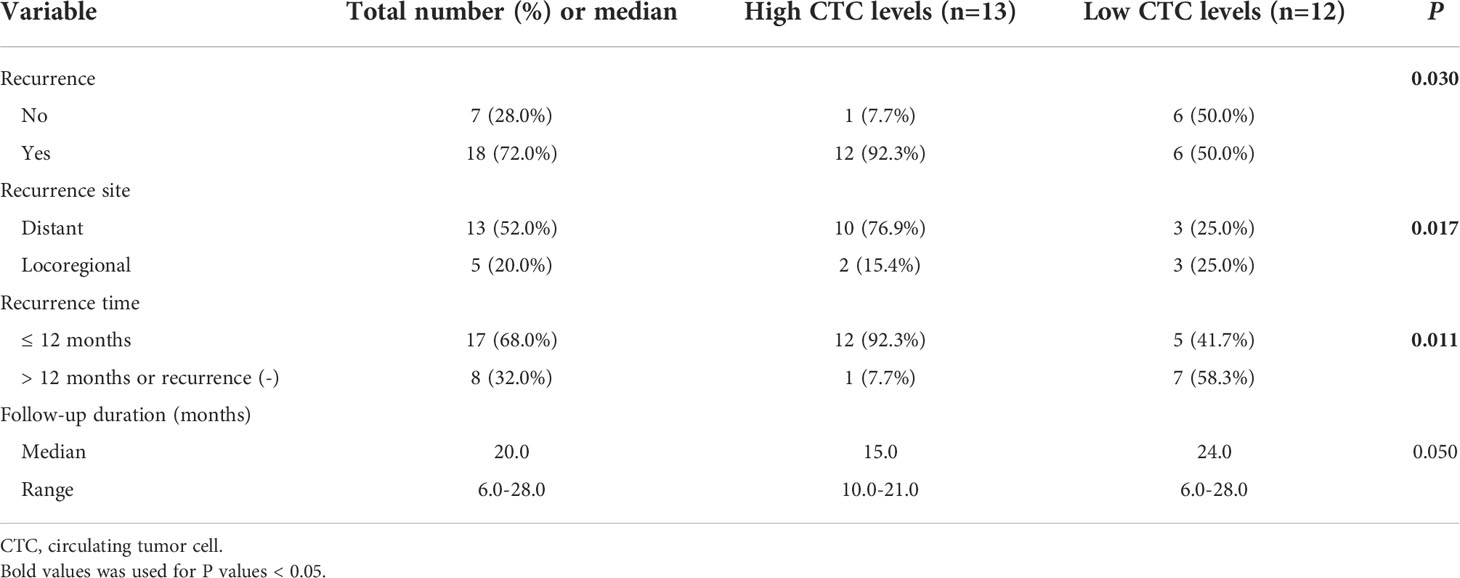
Results: For the prognostic stratification, the optimal cut-off value of CTCs analyzed by receiver operating characteristic (ROC) curve was 14.49 folate units (FU)/3 ml. High CTC levels (> 14.49 FU/3 ml) were detected in 52.0% (13/25) of the patients in the surgical group and 63.2% (12/19) in the non-surgical group. In the surgical group, median disease-free survival (DFS) for patients with high CTC levels versus low CTC levels (< 14.49 FU/3 ml) was 8.0 versus 26.0 months (P = 0.008). In multivariable analysis, CTCs were an independent risk factor for DFS (HR: 4.589, P = 0.012). Concerning the recurrence patterns, patients with high CTC levels showed a significantly frequent rate of distant and early recurrence (P = 0.017 and P = 0.011). CTC levels remained an independent predictor for both distant (OR: 8.375, P = 0.014) and early recurrence (OR: 8.412, P = 0.013) confirmed by multivariable logistic regression. However, CTCs did not predict survival in the non-surgical group (P = 0.220).
Conclusion: FR+ CTCs in resected PC patients could predict impaired survival and recurrence patterns after surgery. Preoperative CTC levels detected by LT-PCR may help guide treatment strategies and further studies in a larger cohort are warranted.
Introduction
Pancreatic cancer (PC) is the fourth leading cause of cancer mortality in the United States, with an estimated 62,210 new cases diagnosed in 2022 (1). It is currently predicted to become the second leading cause by 2030 (2). Radical resection, in combination with systemic therapy, remains the only hope of cure or meaningful long-time survival with overall 5-year survival rates as high as 30% for patients with PC (3). Due to a combination of late presentation and early metastasis, 80% of the patients are diagnosed in the advanced stage and only 10%-20% of PC patients can get a curative resection at the time of diagnosis. However, due to a substantial rate of under-staging, around 20% of patients already recure within the first 6 months after surgery, resulting the median disease-free survival (DFS) is just over 12 months (4, 5). Therefore for many of these patients, the survival advantage from curative resection is questionable. The main cause of early relapse is likely occult systemic disease below the detection limit of cross-sectional imaging, although there may be other contributing factors such as postoperative morbidity (6, 7). Neoadjuvant therapy is recently recommended to address the issue of occult systemic disease (8). Currently, selection of potentially resectable patients for surgery remains challenging as there are no methods to stratify a patient’s risk for metastasis to help guide neoadjuvant and adjuvant therapies (9). One possible strategy is to identify effective prognostic biomarkers to distinguish patients at high risk of early systemic progression who may benefit from systemic treatment first and patients with favourable prognosis who are more likely to benefit from upfront resection (10).
Circulating tumor cells (CTCs), functioning as the “seeds” of metastasis, are tumor cells that originate from primary tumors, survive in circulating and disseminate to colonize distant sites through invading adjacent vasculature (11, 12). Involvement of CTCs in the metastatic process has been identified in the majority of solid tumors (13–15). Accumulating evidence has demonstrated that, as a non-invasive assessment of tumor biology, CTCs are a readily available biomarker for predicting survival in colorectal, breast, and prostate cancers (16–18). In patients with potentially resectable PC, the lack of reliable biomarkers to guide surgical or neoadjuvant treatment decisions also motivated the present studies to investigate the prognostic impact of preoperative CTCs analysis (19–22). Thus far, the CellSearch System remains the first and only CTC detection platform approved by Food and Drug Administration (FDA), although detection, enumeration, and isolation of CTCs have been facilitated by recent technological advancements in a sensitive and reproducible manner for clinical and research applications. It utilizes immunomagnetic separation for isolation of CTCs, and then quantitative evaluation of CTCs is analyzed by capturing epithelial cell adhesion molecule (EpCAM), an epithelial cell marker (23). CellSearch is also the most commonly used method to examine CTCs as a prognostic marker in PC patients in a number of studies, however, across various stages of PC, it has performed with relatively poor detection rates of 7–48% (24–26). In addition, in a study evaluating the efficiency of the CellSearch System, CTC detection rate and counts have been found to be lowest in PC among different types of metastatic cancers (27). Therefore, diverse techniques and technologies have been developed for enrichment, isolation, and identification of CTCs from peripheral blood samples in PC patients in recent years.
Folate receptors (FRs), cysteine-rich cell-surface glycoproteins, are highly expressed in a variety of cancers, including PC (28–31). Our previous study has shown promising clinical value of detecting FR-positive (FR+) CTCs by a novel ligand-targeted polymerase chain reaction (LT-PCR) method in patients with PC (32). Although FR+ CTCs have been demonstrated to be useful in the diagnosis of PC, their application in predicting prognosis requires further clarification. Therefore, the purpose of this study was to investigate the prognostic impact of pretreatment FR+ CTCs analysis in patients with PC.
Patients and methods
Patients, study design, and clinical data collection
Between September 2018 to December 2019, 50 consecutive patients with suspected PC treated at our hospital were enrolled into this observational study. The treatment strategies for all patients were discussed and determined by the multidisciplinary team of pancreatic disease, independent of the results of CTCs analysis. Exclusion criteria were (1): a history of any other malignancy or any anticancer therapies in the last 10 years (2); failure to adhere to standard surgical procedures in the surgical group (3); other than PC confirmed by final histopathologic observations. Diagnosis was confirmed from the specimens obtained by resection or biopsy. A database of demographic, laboratory, and relevant clinicopathologic variables, including preoperative carbohydrate antigen 19-9 (CA19-9), age, gender, location of primary tumor, tumor differentiation, tumor size, tumor stages et al, was prospectively maintained. Disease stages - tumor, node, and metastasis (TNM) staging, were based on the eighth edition of the American Joint Committee on Cancer (AJCC) manual. A distance from the tumor to the resection margin≥1 mm was defined as R0.
Standard pancreaticoduodenectomy (PD), distal pancreatectomy (DP) or other procedures were performed in patients who were selected for radical surgery in accordance with the tumor location and extension. In addition, patients were followed with a standard postoperative protocol, with routine postoperative clinical status and CA19-9 assessment every 3 months and contrast-enhanced computerized tomography (CT) or magnetic resonance imaging (MRI) scan every 6 months. If necessary, positron emission tomography (PET) was conducted to evaluate recurrence. Early recurrence was defined as within 12 months of surgery, as described in previous studies (5, 33). Locoregional recurrence was defined as recurrent disease along the superior mesenteric artery (SMA)/superior mesenteric vein (SMV), celiac axis, or porta hepatis and in the pancreatic bed, retroperitoneum, or remnant pancreas through radiographic or pathological evidence. Distant recurrence was tumor spread outside of the locoregional area (liver, lungs, peritoneum and extra-regional lymph nodes) (34). All procedures were in accordance with the ethical standards of the Helsinki Declaration. The study was approved by the Ethics Committee of Nanjing Drum Tower Hospital (No. 2020-079-01), and informed written consent was obtained from all subjects before the study.
CTC detection
Before commencing treatment, peripheral venous blood samples (3 ml) from every patient were collected in vacuum tubes containing the anticoagulant ethylenediaminetetraacetic acid for CTCs analysis. For patients with neoadjuvant treatment followed by curative-intent pancreatectomy, CTC detection was performed before surgery. A commercially available CTC detection kit, CytoploRare Kit, invented by Geno Biotech and approved by the China FDA, was used for isolation, enrichment and enumeration of FR+ CTCs, as described and detailed previously (32). All blood specimens were stored at 4°C and processed within 24 hours of blood withdrawal. The experimental procedure was performed strictly according to the manufacturer’s protocol. Briefly, the isolation and enrichment of CTCs was initially achieved by lysing erythrocytes, followed by immunomagnetic depletion of leukocytes from the whole blood. Then, LT-PCR was used for quantitative analysis of the FR+ CTCs in each blood sample. Finally, the level of FR+ CTCs in each sample was calculated on the basis of a calibration curve generated with the standard reference materials provided in the kit. FR+ CTC levels were measured in folate units (FU)/3 ml of blood.
Statistical analysis
Continuous variables were presented as medians (ranges) and categorical variables were summarized as number (percentage). Statistical analysis for these two types of variable was examined using Student’s t test (or Mann-Whitney U test) and Fisher’s exact test, respectively. Optimal cut-off value of CTCs for recurrence prediction was analyzed by receiver operating characteristic (ROC) curve and calculated with Youden Index. Survival analyses were carried out with the Kaplan-Meier method, using Log-rank test for difference of curve pairs. Overall survival (OS) was defined as the time from date of diagnosis or resection to either death by any cause or censored at last follow-up. DFS was defined as the time interval between date of operation and date of tumor relapse showed by radiological or clinical evidence. To assess the independent influence of CTCs and other covariates on tumor recurrence, univariable and multivariable Cox Proportional Hazard regression model analyses were performed. Multivariable analyses for distant and early recurrence were calculated with a logistic regression model. Variables with a P value < 0.2 in univariable analyses and clinically relevant variables were included in multivariable analyses. Data were analyzed using IBM SPSS, v.25 (IBM Corp., Armonk, NY) and graphs were prepared using PRISM 8 (GraphPad Software, Inc, La Jolla, CA). A P value < 0.05 was considered statistically significant.
Results
Patient characteristics
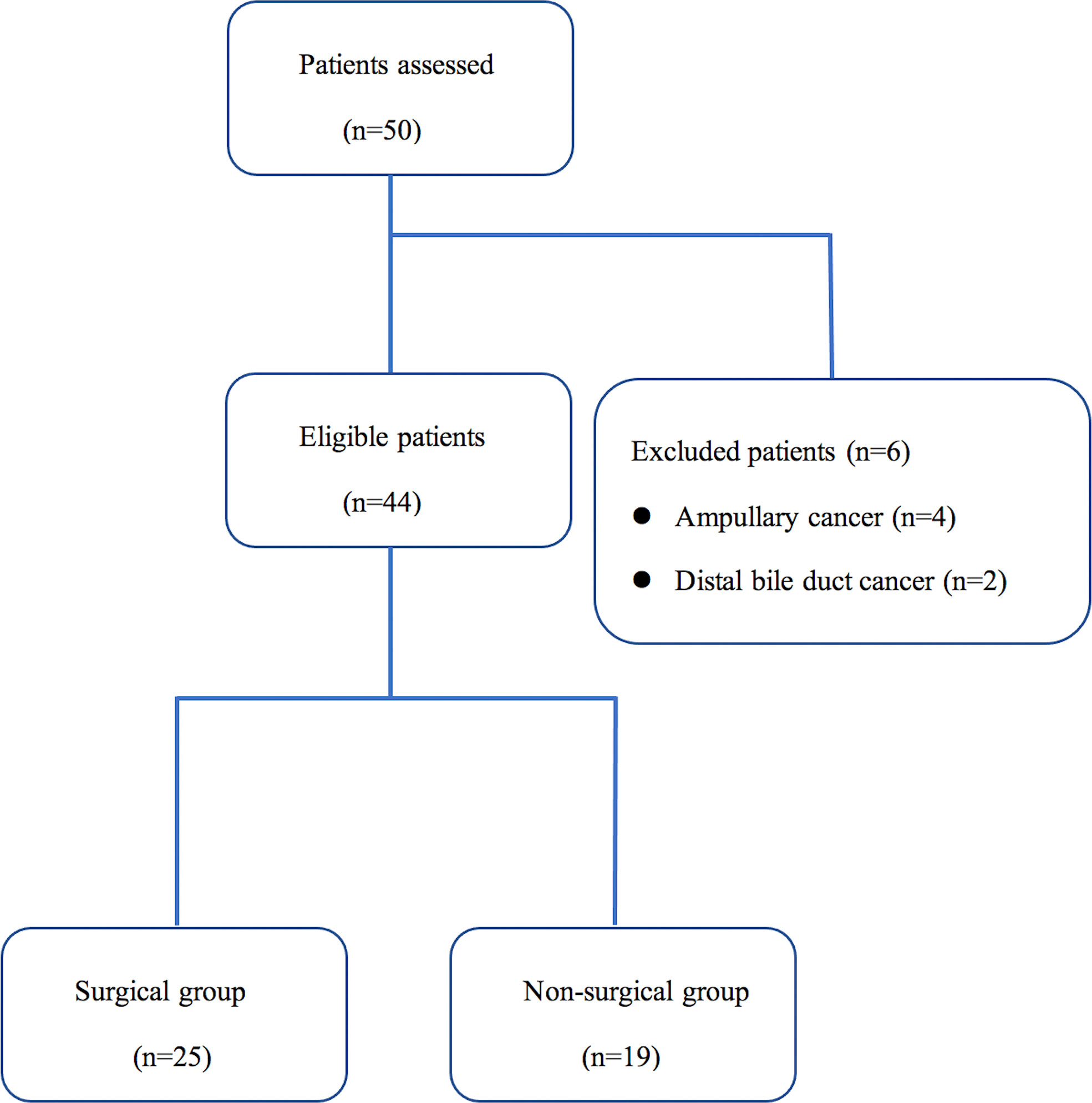
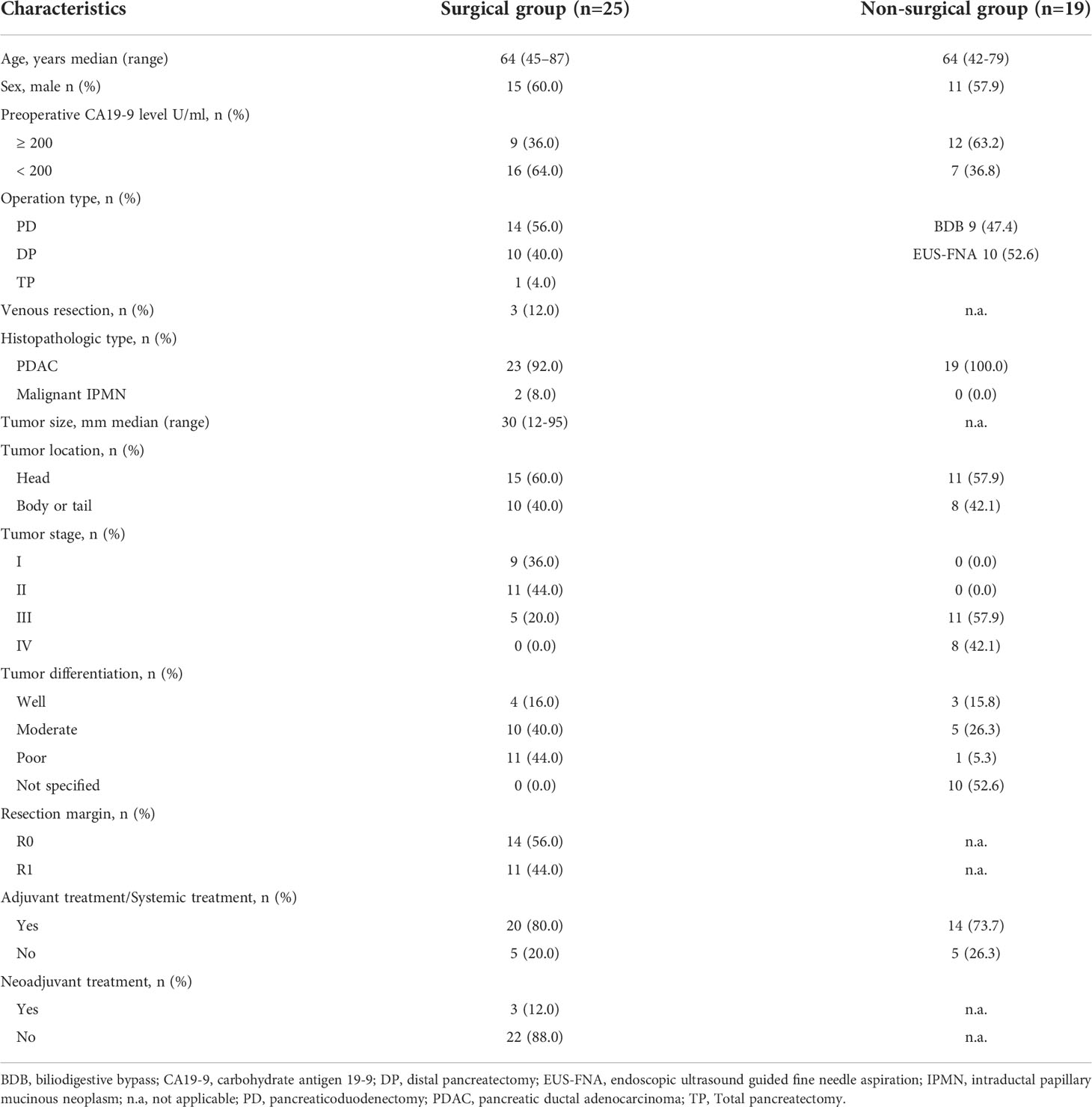
Of the 50 patients assessed during the study period, 6 patients (12.0%) did not meet the inclusion criteria. The Consort diagram showing the stratification of the 44 eligible cases is presented in Figure 1. Tumor resection with curative intent was performed in 56.8% of the patients (25/44) (surgical group), while 43.2% (19/44) were not resected due to local advance or distant metastasis (non-surgical group) (Table 1). The median age of these enrolled patients was both 64 years in the two groups and 60.0% (15/25) were men in the surgical group, while 57.9% (11/19) in the non-surgical group. In the surgical group, 60.0% of the tumors were located in the head, and according to the histopathologic type, most were pancreatic ductal adenocarcinoma (PDAC), and only two were malignant intraductal papillary mucinous neoplasm (IPMN). R0 resection was achieved in 56.0% (14/25) of the patients and 80.0% (20/25) received adjuvant treatment. 3 patients received neoadjuvant chemotherapy preoperatively according to the decision taken on pancreatic multidisciplinary and the regimen was gemcitabine with albumin-bound paclitaxel for all patients. In the non-surgical group, 73.7% (14/19) of the patients received systemic treatment.
CTC levels in the patient cohort
For the prognostic stratification, ROC curve analysis showed that the area under the ROC curve (AUROC) was 0.883 (P = 0.003), with 14.49 FU/3 ml as the optimal cut-off value of CTCs and the Youden Index was 0.727 (Figure 2). The CTC levels of patients in the surgical group (median 14.92 FU/3 ml, range 5.61 to 26.98 FU/3 ml) were slightly lower than those of patients in the non-surgical group (median 16.76 FU/3 ml, range 5.49 to 41.22 FU/3 ml), although the difference was not significant (P = 0.147). In the surgical group, high CTC levels (> 14.49 FU/3 ml) were detected in 52.0% (13/25) of the patients and in the non-surgical group, 12 patients had high CTC levels. We also compared the clinicopathological characteristics of patients with high and low CTC levels, and the results are shown in Table 2. In the surgical group and non-surgical group, there were both no significant difference of clinicopathological characteristics between the two subgroups.
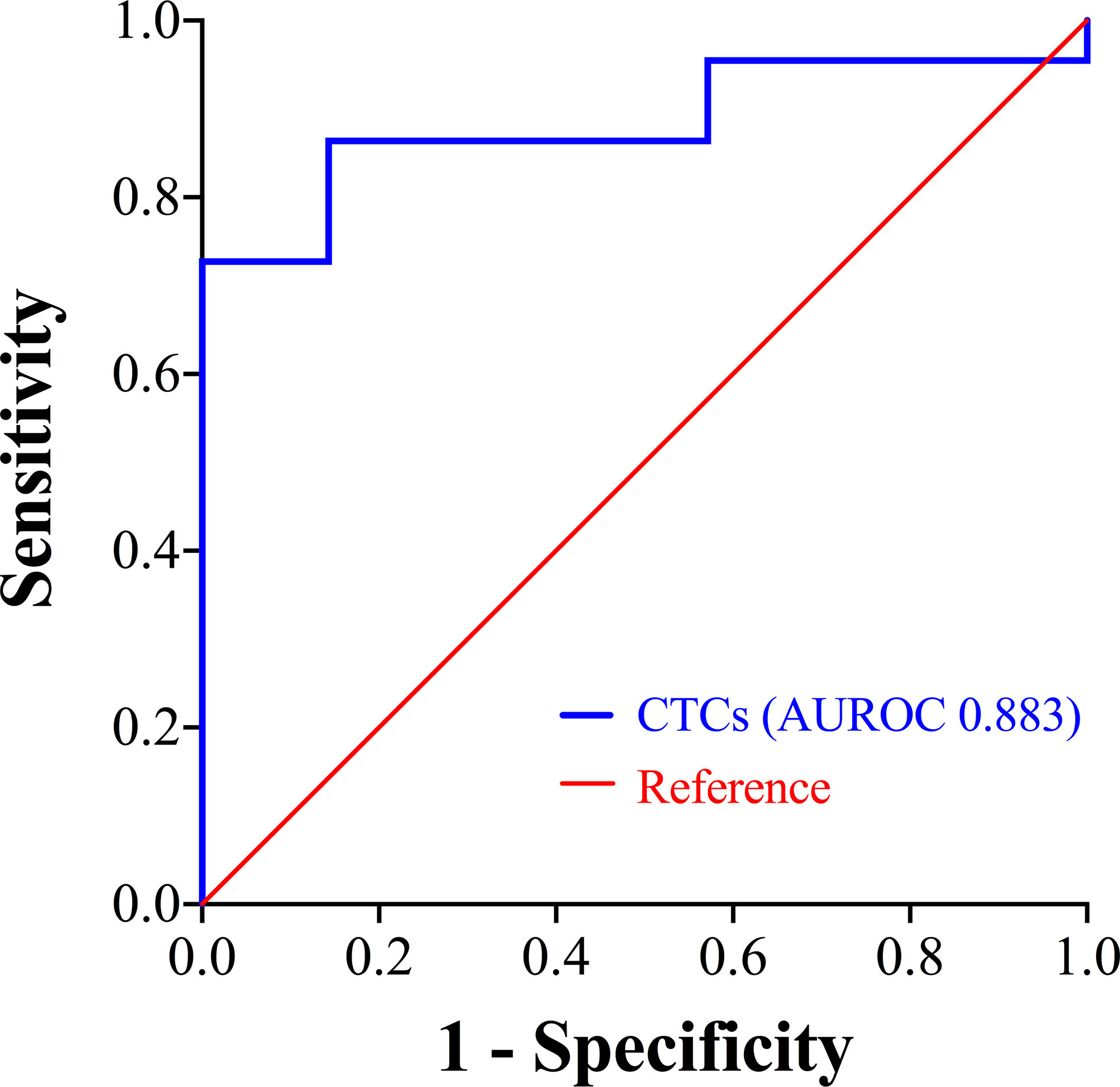
Figure 2 ROC curve showing the prognostic stratification performance of CTCs. CTCs, circulating tumor cells; ROC, receiver operating characteristic; AUROC, area under the ROC curve.
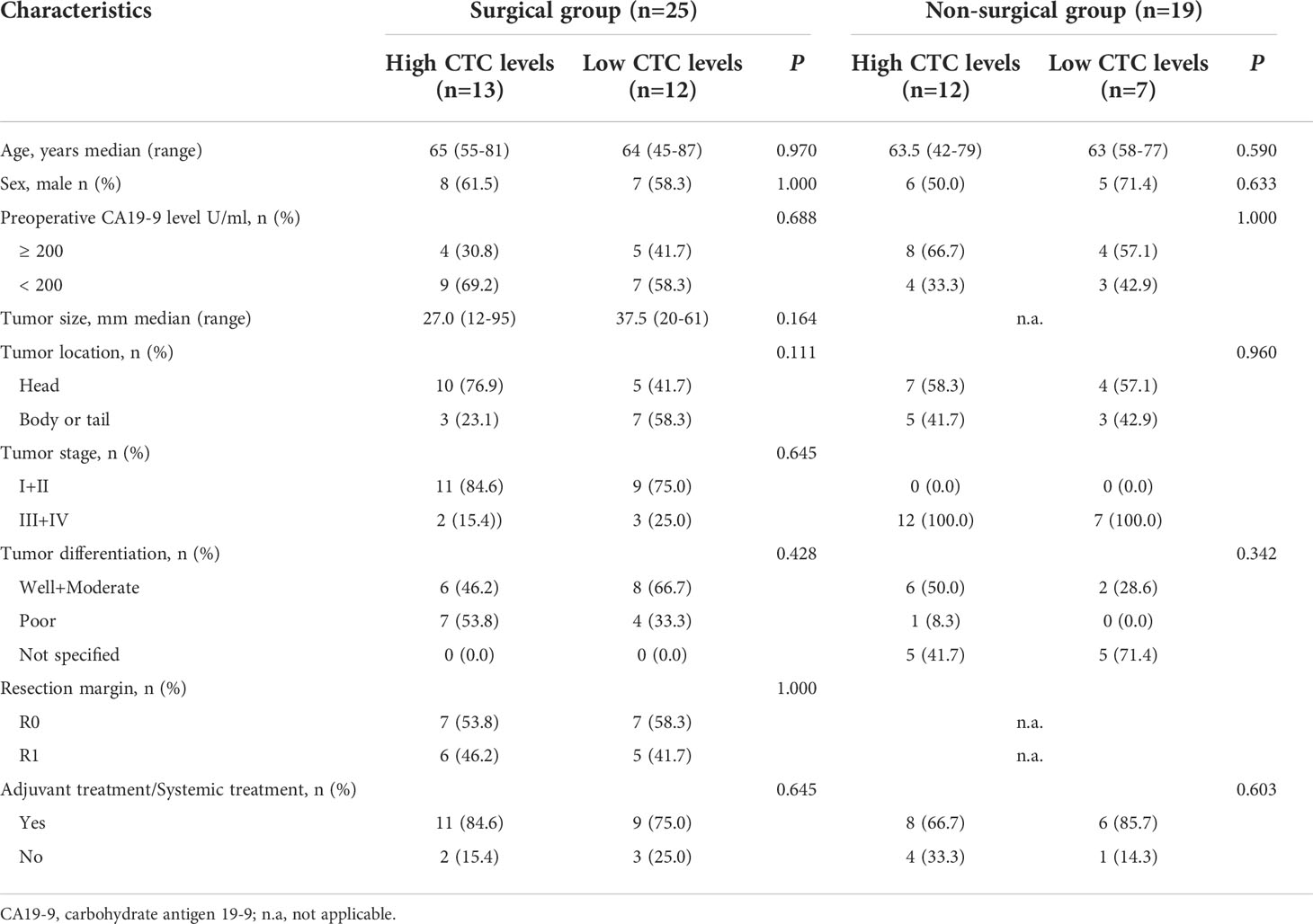
Table 2 Comparison of the clinicopathological characteristics of patients with high and low CTC levels in different groups.
CTC detection and survival
In the surgical group, the median observation time was 20.0 months (range 6.0 to 28.0). Median DFS was 10.0 months for all patients (Figure 3A). Patients with high CTC levels had a significantly shorter DFS (median 8.0 vs. 26.0 months, P = 0.008; Figure 3B) compared with patients with low CTC levels. The univariable survival analyses of DFS for CTC levels (HR 3.735; 95% CI: 1.263–11.049; P = 0.017) and other risk factors are presented in Table 3. Of those, age also affected the prognosis. After inclusion of these confounding factors as well as other known risk factors (R, tumor stage) in multivariable analysis, CTC levels remained a significant prognostic factor (HR 4.589; 95% CI: 1.404–14.997; P = 0.012; Table 3).
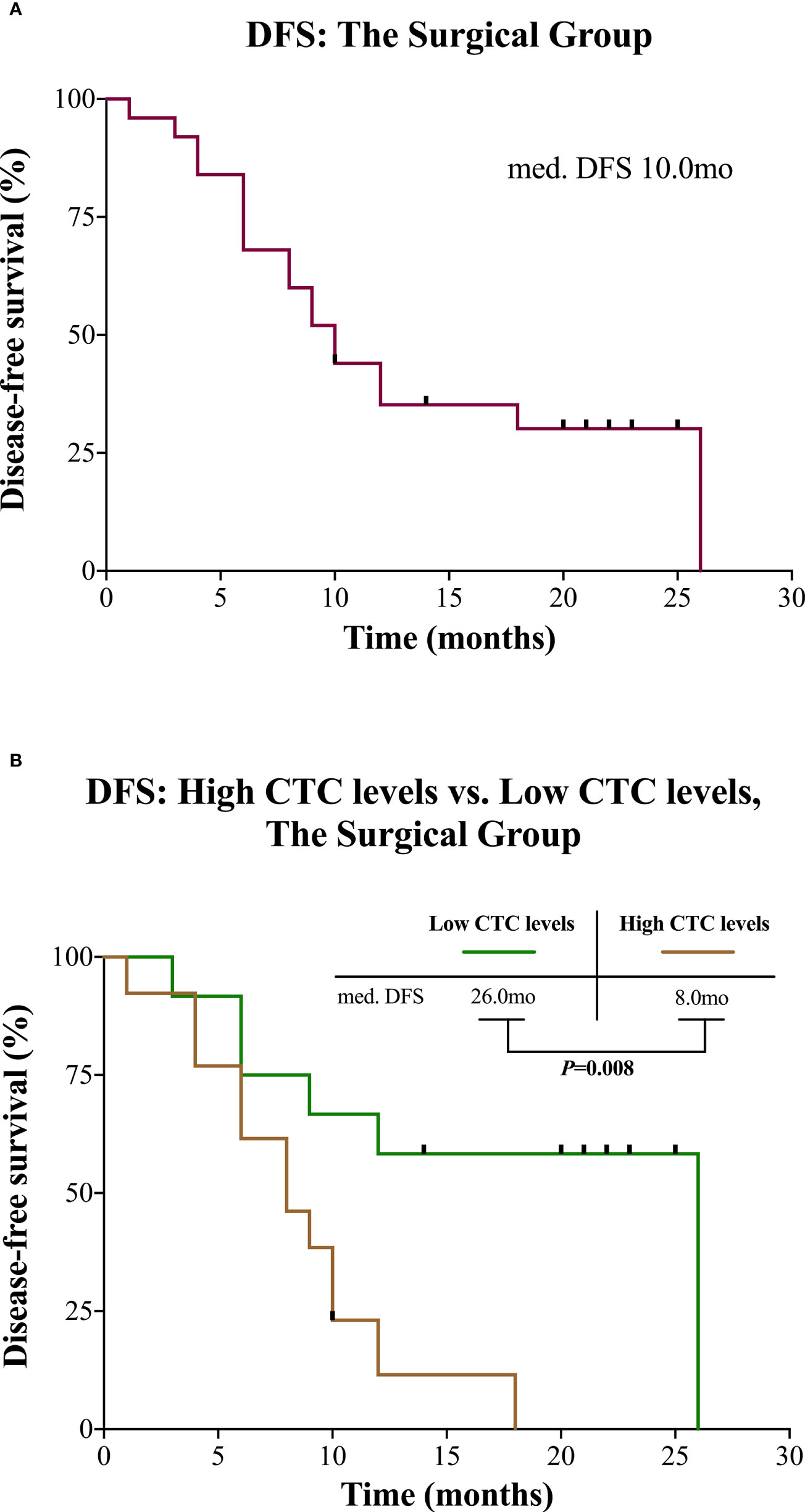
Figure 3 Kaplan-Meier curves for patients in the surgical group. (A) DFS for the entire group. (B) DFS of the surgical group divided into high and low CTC levels. CTC, circulating tumor cell; DFS, disease-free survival; mo, months; med., median.
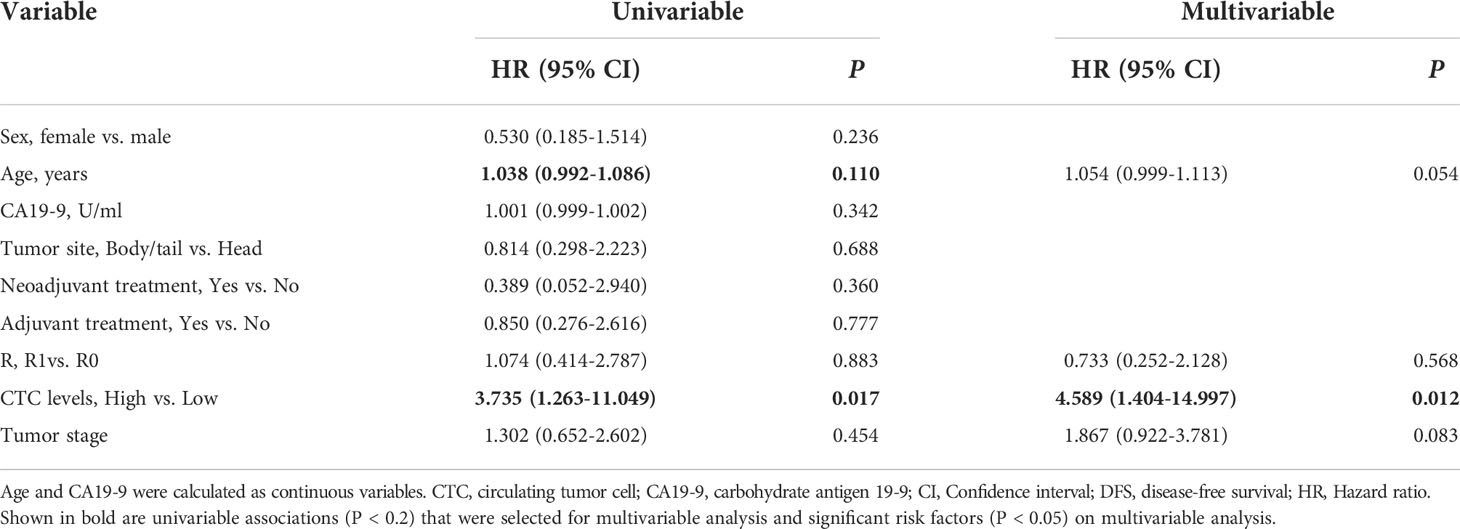
Table 3 Uni- and multivariable analyses of prognostic factors of DFS for patients in the surgical group.
In the non-surgical group, the median observation time was 13.0 months (range 2.0 to 21.0). Median OS was 11.0 months for all patients (Figure 4A). Median OS for patients with high CTC levels was 8.5 months and 17.0 months for patients with low CTC levels, but the difference was not statistically significant (P = 0.220; Figure 4B).
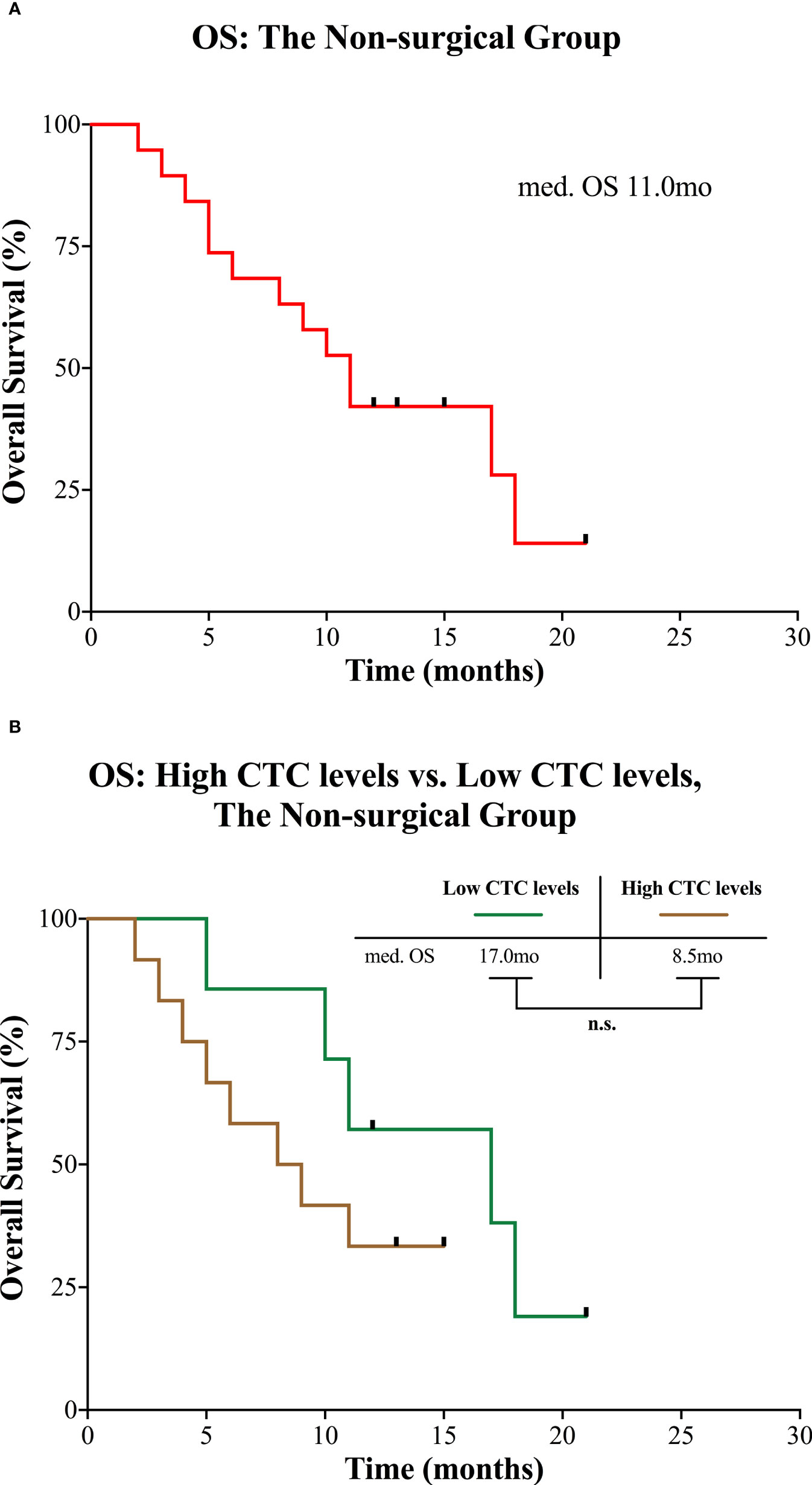
Figure 4 Kaplan-Meier curves for patients in the non-surgical group. (A) OS for the entire group. (B) OS of the non-surgical group divided into high and low CTC levels. CTC, circulating tumor cell; mo, months; med., median; n.s., not significant; OS, overall survival.
Patterns of recurrence according to the CTC levels in the surgical group
We compared the presence or absence of recurrence in accordance with CTC levels, and we found that recurrence was significantly higher in the patients with high CTC levels (12/13, 92.3%) compared with patients with low CTC levels (6/12, 50.0%) (P = 0.030). Then the recurrence site and time of emergence were further analyzed (Table 4 and Figure 5). Distant recurrence was significantly higher in the patients with high CTC levels as compared with patients with low CTC levels (76.9% vs. 25.0%, P = 0.017). In the patients with low CTC levels, 5 out of 12 (41.7%) instances of recurrence were within 12 months, but nearly all recurrences (12/13, 92.3%) in the patients with high CTC levels occurred within 12 months (P = 0.011). Finally, we confirmed using multivariable logistic regression analysis that CTCs are an independent risk factor for both distant (OR 8.375; 95% CI 1.915–28.222; P = 0.014) and early recurrence (OR 8.412; 95% CI 2.342–27.234; P = 0.013) (Table 5).
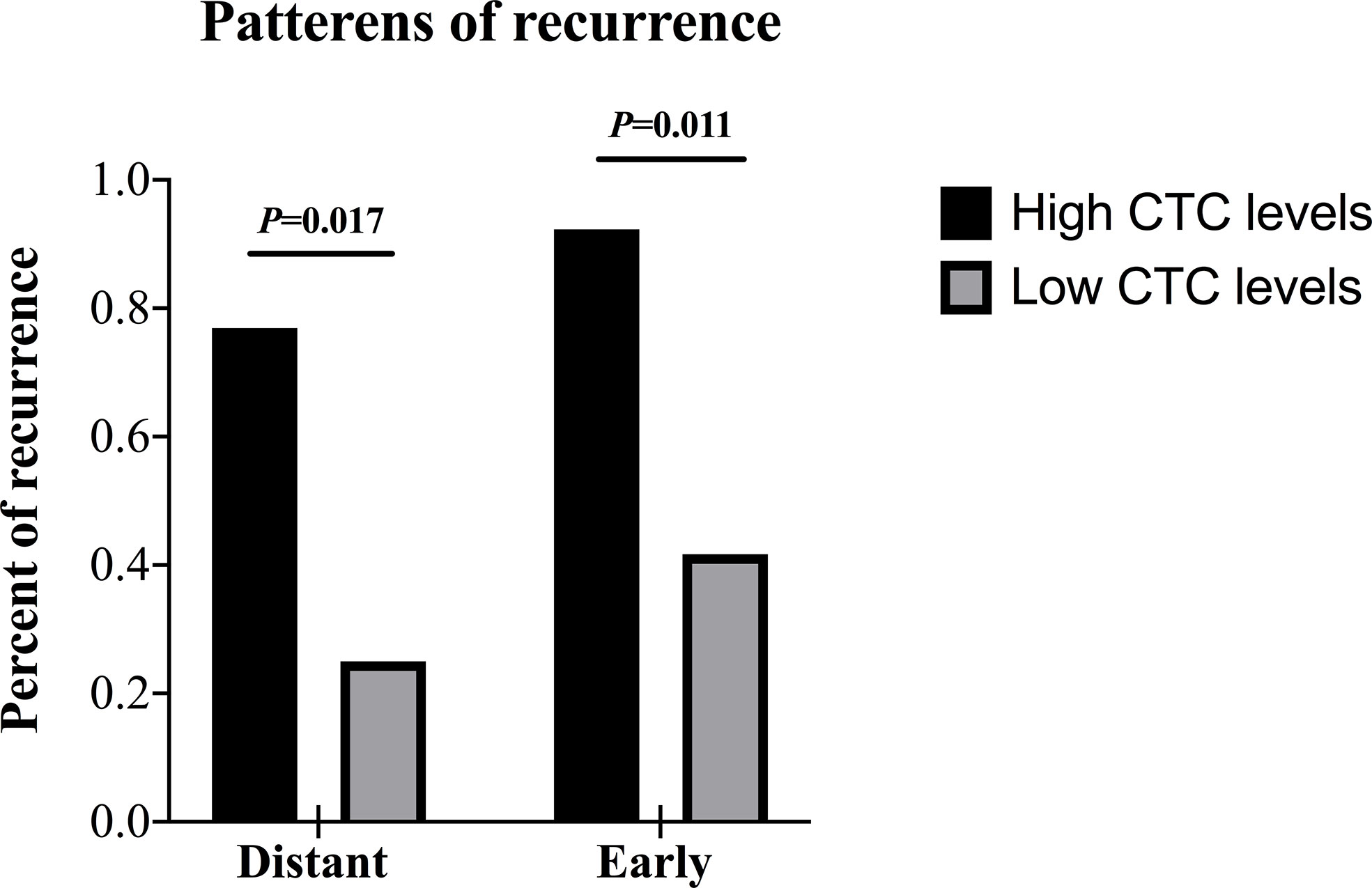
Figure 5 Patterns of recurrence of patients in the surgical group according to the CTC levels. CTC, circulating tumor cell.
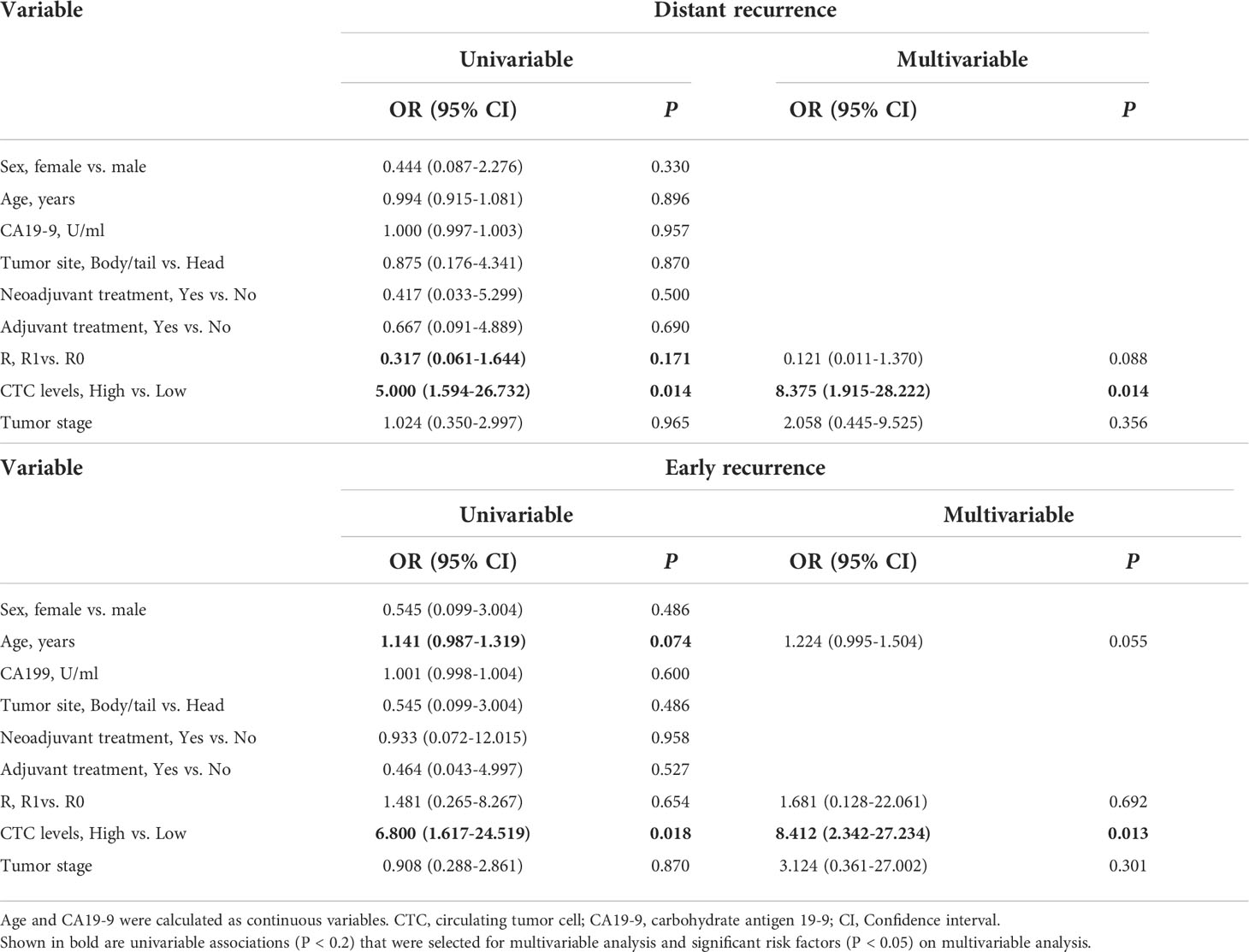
Table 5 Uni- and multivariable logistic regression analysis for risk factors in distant and early recurrence.
Based on the results, we summarized the relationship between preoperative CTC levels and postoperative patterns of recurrence. If the CTC levels were high (> 14.49 FU/3 ml) before surgery, it tended to show patterns of early and distant recurrence.
Discussion
This is the first report to demonstrate the clinical significance of FR+ CTCs detected by LT-PCR for predicting the survival in patients with PC. The effect of high CTC levels in the present cohort was detrimental. In patients with resected PC, we demonstrated a strong association between the CTC levels and reduced DFS and in the patterns of recurrence, CTCs were also associated with early and distant recurrence.
As a ‘‘liquid biopsy’’, the utilization of CTCs to assess the tumor biology and guide treatment decisions has developed into an emerging field of study (35). Especially in PC, the current lack of individualized up-front treatment stratification by preoperative risk assessment is a major hindrance for improved treatment results of patients with presumed resectable PC and CTCs have been considered as a very useful biomarker to establish the appropriate therapeutic protocol (22). However, there is a broad heterogeneity in the CTC detection platforms to date and as the “gold standard” technique, the detection rate of the CellSearch system is too low to limit its clinical application (36). In recent years, although the presence of sensitive and reproducible platforms for the isolation, enrichment and detection of CTCs from peripheral blood, methodological standardization for such technologies is still required to have a significant value on clinical care (37). As FRs are also highly expressed in PC, our previous study has shown that FR+ CTCs have potential as a biomarker for the diagnosis of PC and LT-PCR is feasible and reliable for detecting FR+ CTCs in patients with PC (32). In addition, FR+ CTCs have been shown to serve as prognostic markers in several cancer types, including gastric, breast and lung cancers (38–40). In a prospective cohort study including 132 gastric cancer patients, combined model including FR+ CTC level and other biomarkers (CA19-9, prealbumin and peripheral lymphocyte count) presented high sensitivity (100%) and moderate specificity (59.3%) in predicting peritoneal metastasis, the preoperative FR+ CTC level could also predict short-term recurrence after surgery (38). In another prospective study to investigate the prognostic and predictive significance of FR+ CTC in non-small cell lung cancer patients who underwent surgery, patients with lower preoperative CTC level had longer relapse-free survival (RFS) and OS, CTC level (HR = 4.10) and pathological stage (HR = 3.16) were independent prognostic factors of RFS (40). Accordingly, in the present analysis, we gave insight into the association between pretreatment CTC levels and patient survival.
The frequency of CTC-detection varies due to the detection methods and the disease status of the patients (19–22). However, in our current study, with 14.49 FU/3 ml as the optimal cut-off value of CTCs, the detection rate of high CTC levels was 52.0% in the patients with resectable PC and 63.2% in the patients with advanced stage cancer. Compared to previous studies, especially the studies in which Cellsearch was used as the detection method, our study showed a higher detection rate in both early and advanced stage cancer. Consequently, these results revealed again that LT-PCR is feasible and reliable for detecting FR+ CTCs in PC patients.
In our cohort, 43.2% of the patients were assigned to the non-surgical group due to local advance or distant metastasis of tumor. In this group, median OS was 11.0 months and there was no significant difference in OS between the patients with high and low CTC levels. These results are consistent with other reports utilizing the different methods (41, 42). However, in our study, we have secured meaningful results for the difference between the two groups (8.5 months vs. 17.0 months) and larger-scale studies are warranted in the future.
In the current study, 56.8% of the patients were assigned to the surgical group and tumor resection with curative intent was performed. Median DFS was 10.0 months for all patients, which was similar to other studies with larger number of patients (5, 34). More importantly, high CTC levels predicted impaired DFS following potentially curative surgery and patients with low CTC levels could have a DFS as long as 26.0 months, which was an amazing result. However, this may be related to the limitations of our study with small number of cases and short duration for follow-up, and only 50.0% of the patients with low CTC levels during the observation time recured. Moreover, multivariable analysis indicated that CTCs are an independent risk factor for DFS. Concerning OS in the surgical group, also due to the short follow-up, we did not analyze the OS.
Although there are abundant studies on the association between CTCs and survival, we are aware of only few reports of an association between CTCs from peripheral blood and recurrence rate or pattern (22, 43). Therefore, in the present analysis, we particularly analyzed whether CTC levels could be used as an indicator of early recurrence and recurrence patterns among these patients. In this cohort, the recurrence was significantly higher in the patients with high CTC levels compared with patients with low CTC levels. Meanwhile, in patients with high CTC levels, early recurrence (i.e. within 12 months post-operatively) and distant recurrence were significantly frequent. Moreover, multivariable logistic regression analysis indicated that CTCs are a risk factor for both early and distant recurrence.
Noteworthy, in the present study, the CTC levels were the only independent predictor of DFS and recurrence patterns although other clinically relevant factors such as preoperative CA19-9 level, R status, neoadjuvant and adjuvant therapy, tumor stage were included in the analyses. However, in some previous studies, including large-scale and multicenter clinical trials, CA19-9 level, R status, neoadjuvant and adjuvant therapy were all significantly associated with survival and recurrence (44–47). The discrepancy may be mainly explained by the small sample size and the selection bias of patients in our study. Another alternate explanation may be that we did not analyze the surgical margins separately as different surgical margins could have different prognostic roles (48).
Our study showed that with the currently available techniques for CTC-detection and treatment modalities in PC, to support surgical treatment decisions probably would be a promising use of CTC-analysis. Similar to CTCs, circulating tumor DNA (ctDNA) also has the potential to be a preoperative prognostic tool for the stratification of patients with resectable PC (49–51). Although ctDNA was more abundant and easier to detect than CTCs in comparative studies, the prognostic impact of different mutational signatures in ctDNA was not fully resolved (52–54). In a recent meta-analysis, ctDNA was detected in 8.3–68.6% of patients with resectable PDAC preoperatively and was associated with lower RFS and OS (49). Most probably, future improvements in systemic treatment are dependent upon identification of the core molecular characteristics or driver mutations of the cancer cells.
We acknowledge that our study has several important limitations. First, the number of patients is relatively small and the follow-up time is relatively short, which limit the generalization of the results reported. In addition, FR+ CTCs can not predict survival in the non-surgical group, indicating that this prognostic biomarker has less utility in patients with advanced disease, which might mainly due to the universally poor prognosis of this patient population. Moreover, the present study design did not include the CTC detection at multiple time points to observe how CTC dynamics predict outcome with treatment, however, our study does provided evidence on the utility of FR+ CTCs as a biomarker at a time when key treatment decisions are made. Finally, the recurrences of some patients were identified based on radiological evidence, without pathological verification, which may include potential for some provider variability regarding postoperative imaging.
In conclusion, this small-scale, exploratory clinical trial revealed that FR+ CTCs detected by LT-PCR predict shorter DFS and are associated with early and distant recurrence in patients with resectable PC. Our results indicate that FR+ CTCs could be a promising tool to individualize treatment planning and to improve outcomes in PC. Further studies to investigate the prognostic value of CTCs detected by LT-PCR in a larger cohort are warranted.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of Nanjing Drum Tower Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
HC, JY, and XF: study design, data acquisition, data analysis, data interpretation; HC: drafting of the manuscript; LM, XC, CL, and GL: data acquisition; YQ and WH: study concept, study design and study supervision; YQ: critical revision of the manuscript for important intellectual content; WH: contribution of CTC detection method and reagents. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by National Natural Science Foundation of China (NSFC) (No. 31971518).
Acknowledgments
The authors thank all members of the multidisciplinary team treating pancreatic tumors at Nanjing Drum Tower Hospital for their guidance in this study. We also appreciate the technical support of Yidu Cloud (Beijing) Technology Co. Ltd., China, in extracting data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA: Cancer J Clin (2022) 72:7–33. doi: 10.3322/caac.21708
2. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the united states. Cancer Res (2014) 74:2913–21. doi: 10.1158/0008-5472.Can-14-0155
3. Strobel O, Neoptolemos J, Jäger D, Büchler MW. Optimizing the outcomes of pancreatic cancer surgery. Nat Rev Clin Oncol (2019) 16:11–26. doi: 10.1038/s41571-018-0112-1
4. Hugenschmidt H, Labori KJ, Brunborg C, Verbeke CS, Seeberg LT, Schirmer CB, et al. Circulating tumor cells are an independent predictor of shorter survival in patients undergoing resection for pancreatic and periampullary adenocarcinoma. Ann Surg (2020) 271:549–58. doi: 10.1097/sla.0000000000003035
5. Groot VP, Gemenetzis G, Blair AB, Rivero-Soto RJ, Yu J, Javed AA, et al. Defining and predicting early recurrence in 957 patients with resected pancreatic ductal adenocarcinoma. Ann Surg (2019) 269:1154–62. doi: 10.1097/sla.0000000000002734
6. Court CM, Ankeny JS, Sho S, Winograd P, Hou S, Song M, et al. Circulating tumor cells predict occult metastatic disease and prognosis in pancreatic cancer. Ann Surg Oncol (2018) 25:1000–8. doi: 10.1245/s10434-017-6290-8
7. Labori KJ, Katz MH, Tzeng CW, Bjørnbeth BA, Cvancarova M, Edwin B, et al. Impact of early disease progression and surgical complications on adjuvant chemotherapy completion rates and survival in patients undergoing the surgery first approach for resectable pancreatic ductal adenocarcinoma - a population-based cohort study. Acta Oncol (2016) 55:265–77. doi: 10.3109/0284186x.2015.1068445
8. Versteijne E, Vogel JA, Besselink MG, Busch ORC, Wilmink JW, Daams JG, et al. Meta-analysis comparing upfront surgery with neoadjuvant treatment in patients with resectable or borderline resectable pancreatic cancer. Br J Surg (2018) 105:946–58. doi: 10.1002/bjs.10870
9. Poruk KE, Valero V, Saunders T, Blackford AL, Griffin JF, Poling J, et al. Circulating tumor cell phenotype predicts recurrence and survival in pancreatic adenocarcinoma. Ann Surg (2016) 264:1073–81. doi: 10.1097/sla.0000000000001600
10. Hank T, Hinz U, Reiner T, Malleo G, Konig AK, Maggino L, et al. A pretreatment prognostic score to stratify survival in pancreatic cancer. Ann Surg (2021). doi: 10.1097/SLA.0000000000004845
11. Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science (2011) 331:1559–64. doi: 10.1126/science.1203543
12. Massagué J, Obenauf AC. Metastatic colonization by circulating tumour cells. Nature (2016) 529:298–306. doi: 10.1038/nature17038
13. Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science (2013) 339:580–4. doi: 10.1126/science.1228522
14. Connor AA, McNamara K, Al-Sukhni E, Diskin J, Chan D, Ash C, et al. Central, but not peripheral, circulating tumor cells are prognostic in patients undergoing resection of colorectal cancer liver metastases. Ann Surg Oncol (2016) 23:2168–75. doi: 10.1245/s10434-015-5038-6
15. Carter L, Rothwell DG, Mesquita B, Smowton C, Leong HS, Fernandez-Gutierrez F, et al. Molecular analysis of circulating tumor cells identifies distinct copy-number profiles in patients with chemosensitive and chemorefractory small-cell lung cancer. Nat Med (2017) 23:114–9. doi: 10.1038/nm.4239
16. Bidard FC, Peeters DJ, Fehm T, Nolé F, Gisbert-Criado R, Mavroudis D, et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol (2014) 15:406–14. doi: 10.1016/s1470-2045(14)70069-5
17. Salvianti F, Gelmini S, Mancini I, Pazzagli M, Pillozzi S, Giommoni E, et al. Circulating tumour cells and cell-free DNA as a prognostic factor in metastatic colorectal cancer: the OMITERC prospective study. Br J Cancer (2021) 125:94–100. doi: 10.1038/s41416-021-01399-6
18. Antonarakis ES, Lu C, Luber B, Wang H, Chen Y, Zhu Y, et al. Clinical significance of androgen receptor splice variant-7 mRNA detection in circulating tumor cells of men with metastatic castration-resistant prostate cancer treated with first- and second-line abiraterone and enzalutamide. J Clin Oncol (2017) 35:2149–56. doi: 10.1200/jco.2016.70.1961
19. Poruk KE, Blackford AL, Weiss MJ, Cameron JL, He J, Goggins M, et al. Circulating tumor cells expressing markers of tumor-initiating cells predict poor survival and cancer recurrence in patients with pancreatic ductal adenocarcinoma. Clin Cancer Res (2017) 23:2681–90. doi: 10.1158/1078-0432.Ccr-16-1467
20. Effenberger KE, Schroeder C, Hanssen A, Wolter S, Eulenburg C, Tachezy M, et al. Improved risk stratification by circulating tumor cell counts in pancreatic cancer. Clin Cancer Res (2018) 24:2844–50. doi: 10.1158/1078-0432.Ccr-18-0120
21. Song BG, Kwon W, Kim H, Lee EM, Han YM, Kim H, et al. Detection of circulating tumor cells in resectable pancreatic ductal adenocarcinoma: A prospective evaluation as a prognostic marker. Front Oncol (2020) 10:616440. doi: 10.3389/fonc.2020.616440
22. Hugenschmidt H, Labori KJ, Borgen E, Brunborg C, Schirmer CB, Seeberg LT, et al. Preoperative CTC-detection by CellSearch is associated with early distant metastasis and impaired survival in resected pancreatic cancer. Cancers (2021) 13:485. doi: 10.3390/cancers13030485
23. Yeo D, Bastian A, Strauss H, Saxena P, Grimison P, Rasko JEJ. Exploring the clinical utility of pancreatic cancer circulating tumor cells. Int J Mol Sci (2022) 23:1671. doi: 10.3390/ijms23031671
24. Catenacci DV, Chapman CG, Xu P, Koons A, Konda VJ, Siddiqui UD, et al. Acquisition of portal venous circulating tumor cells from patients with pancreaticobiliary cancers by endoscopic ultrasound. Gastroenterology (2015) 149:1794–803.e4. doi: 10.1053/j.gastro.2015.08.050
25. Dotan E, Alpaugh RK, Ruth K, Negin BP, Denlinger CS, Hall MJ, et al. Prognostic significance of MUC-1 in circulating tumor cells in patients with metastatic pancreatic adenocarcinoma. Pancreas (2016) 45:1131–5. doi: 10.1097/mpa.0000000000000619
26. Buscail E, Alix-Panabières C, Quincy P, Cauvin T, Chauvet A, Degrandi O, et al. High clinical value of liquid biopsy to detect circulating tumor cells and tumor exosomes in pancreatic ductal adenocarcinoma patients eligible for up-front surgery. Cancers (2019) 11:1656. doi: 10.3390/cancers11111656
27. Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res (2004) 10:6897–904. doi: 10.1158/1078-0432.Ccr-04-0378
28. Chen C, Ke J, Zhou XE, Yi W, Brunzelle JS, Li J, et al. Structural basis for molecular recognition of folic acid by folate receptors. Nature (2013) 500:486–9. doi: 10.1038/nature12327
29. Nunez MI, Behrens C, Woods DM, Lin H, Suraokar M, Kadara H, et al. High expression of folate receptor alpha in lung cancer correlates with adenocarcinoma histology and EGFR [corrected] mutation. J Thorac Oncol (2012) 7:833–40. doi: 10.1097/JTO.0b013e31824de09c
30. Norton N, Youssef B, Hillman DW, Nassar A, Geiger XJ, Necela BM, et al. Folate receptor alpha expression associates with improved disease-free survival in triple negative breast cancer patients. NPJ Breast Cancer (2020) 6:4. doi: 10.1038/s41523-020-0147-1
31. Parker N, Turk MJ, Westrick E, Lewis JD, Low PS, Leamon CP. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal Biochem (2005) 338:284–93. doi: 10.1016/j.ab.2004.12.026
32. Cheng H, He W, Yang J, Ye Q, Cheng L, Pan Y, et al. Ligand-targeted polymerase chain reaction for the detection of folate receptor-positive circulating tumour cells as a potential diagnostic biomarker for pancreatic cancer. Cell Prolif (2020) 53:e12880. doi: 10.1111/cpr.12880
33. Gemenetzis G, Groot VP, Yu J, Ding D, Teinor JA, Javed AA, et al. Circulating tumor cells dynamics in pancreatic adenocarcinoma correlate with disease status: Results of the prospective CLUSTER study. Ann Surg (2018) 268:408–20. doi: 10.1097/sla.0000000000002925
34. Honselmann KC, Pergolini I, Castillo CF, Deshpande V, Ting D, Taylor MS, et al. Timing but not patterns of recurrence is different between node-negative and node-positive resected pancreatic cancer. Ann Surg (2020) 272:357–65. doi: 10.1097/SLA.0000000000003123
35. Wan L, Pantel K, Kang Y. Tumor metastasis: moving new biological insights into the clinic. Nat Med (2013) 19:1450–64. doi: 10.1038/nm.3391
36. Reimers N, Pantel K. Liquid biopsy: novel technologies and clinical applications. Clin Chem Lab Med (2019) 57:312–6. doi: 10.1515/cclm-2018-0610
37. Martini V, Timme-Bronsert S, Fichtner-Feigl S, Hoeppner J, Kulemann B. Circulating tumor cells in pancreatic cancer: Current perspectives. Cancers (2019) 11:1659. doi: 10.3390/cancers11111659
38. Zeng CDD, Jin CC, Gao C, Xiao AT, Tong YX, Zhang S. Preoperative folate receptor-positive circulating tumor cells are associated with occult peritoneal metastasis and early recurrence in gastric cancer patients: A prospective cohort study. Front Oncol (2022) 12:769203. doi: 10.3389/fonc.2022.769203
39. Wu Q, Zheng H, Gu J, Cheng Y, Qiao B, Wang J, et al. Detection of folate receptor-positive circulating tumor cells as a biomarker for diagnosis, prognostication, and therapeutic monitoring in breast cancer. J Clin Lab Anal (2022) 36:e24180. doi: 10.1002/jcla.24180
40. Li H, Li B, Pan Y, Zhang Y, Xiang J, Zhang Y, et al. Preoperative folate receptor-positive circulating tumor cell level is a prognostic factor of long term outcome in non-small cell lung cancer patients. Front Oncol (2020) 10:621435. doi: 10.3389/fonc.2020.621435
41. Khoja L, Backen A, Sloane R, Menasce L, Ryder D, Krebs M, et al. A pilot study to explore circulating tumour cells in pancreatic cancer as a novel biomarker. Br J Cancer (2012) 106:508–16. doi: 10.1038/bjc.2011.545
42. Sergeant G, Roskams T, van Pelt J, Houtmeyers F, Aerts R, Topal B. Perioperative cancer cell dissemination detected with a real-time RT-PCR assay for EpCAM is not associated with worse prognosis in pancreatic ductal adenocarcinoma. BMC Cancer (2011) 11:47. doi: 10.1186/1471-2407-11-47
43. Park Y, Jun HR, Choi HW, Hwang DW, Lee JH, Song KB, et al. Circulating tumour cells as an indicator of early and systemic recurrence after surgical resection in pancreatic ductal adenocarcinoma. Sci Rep (2021) 11:1644. doi: 10.1038/s41598-020-80383-1
44. Aziz MH, Sideras K, Aziz NA, Mauff K, Haen R, Roos D, et al. The systemic-immune-inflammation index independently predicts survival and recurrence in resectable pancreatic cancer and its prognostic value depends on bilirubin levels: A retrospective multicenter cohort study. Ann Surg (2019) 270:139–46. doi: 10.1097/sla.0000000000002660
45. Ghaneh P, Kleeff J, Halloran CM, Raraty M, Jackson R, Melling J, et al. The impact of positive resection margins on survival and recurrence following resection and adjuvant chemotherapy for pancreatic ductal adenocarcinoma. Ann Surg (2019) 269:520–9. doi: 10.1097/sla.0000000000002557
46. Macedo FI, Ryon E, Maithel SK, Lee RM, Kooby DA, Fields RC, et al. Survival outcomes associated with clinical and pathological response following neoadjuvant FOLFIRINOX or Gemcitabine/Nab-paclitaxel chemotherapy in resected pancreatic cancer. Ann Surg (2019) 270:400–13. doi: 10.1097/sla.0000000000003468
47. Moaven O, Clark CJ, Russell GB, Votanopoulos KI, Howerton R, Levine EA, et al. Optimal adjuvant treatment approach after upfront resection of pancreatic cancer: Revisiting the role of radiation based on pathologic features. Ann Surg (2021) 274:1058–66. doi: 10.1097/sla.0000000000003770
48. Crippa S, Giannone F, Schiavo Lena M, Belfiori G, Partelli S, Tamburrino D, et al. R status is a relevant prognostic factor for recurrence and survival after pancreatic head resection for ductal adenocarcinoma. Ann Surg Oncol (2021) 28:4602–12. doi: 10.1245/s10434-020-09467-6
49. Guven DC, Sahin TK, Yildirim HC, Aktepe OH, Dizdar O, Yalcin S. A systematic review and meta-analysis of the association between circulating tumor DNA (ctDNA) and prognosis in pancreatic cancer. Crit Rev Oncol Hematol (2021) 168:103528. doi: 10.1016/j.critrevonc.2021.103528
50. Woo SM, Kim MK, Park B, Cho EH, Lee TR, Ki CS, et al. Genomic instability of circulating tumor DNA as a prognostic marker for pancreatic cancer survival: A prospective cohort study. Cancers (2021) 13:5466. doi: 10.3390/cancers13215466
51. Affolter KE, Hellwig S, Nix DA, Bronner MP, Thomas A, Fuertes CL, et al. Detection of circulating tumor DNA without a tumor-informed search using next-generation sequencing is a prognostic biomarker in pancreatic ductal adenocarcinoma. Neoplasia (2021) 23:859–69. doi: 10.1016/j.neo.2021.06.005
52. Earl J, Garcia-Nieto S, Martinez-Avila JC, Montans J, Sanjuanbenito A, Rodríguez-Garrote M, et al. Circulating tumor cells (Ctc) and kras mutant circulating free dna (cfdna) detection in peripheral blood as biomarkers in patients diagnosed with exocrine pancreatic cancer. BMC Cancer (2015) 15:797. doi: 10.1186/s12885-015-1779-7
53. Zhu Y, Zhang H, Chen N, Hao J, Jin H, Ma X. Diagnostic value of various liquid biopsy methods for pancreatic cancer: A systematic review and meta-analysis. Medicine (2020) 99:e18581. doi: 10.1097/md.0000000000018581
Keywords: pancreatic cancer, folate receptor, circulating tumor cells, surgical resection, prognosis, recurrence
Citation: Cheng H, Yang J, Fu X, Mao L, Chu X, Lu C, Li G, Qiu Y and He W (2022) Folate receptor-positive circulating tumor cells predict survival and recurrence patterns in patients undergoing resection for pancreatic cancer. Front. Oncol. 12:1012609. doi: 10.3389/fonc.2022.1012609
Received: 05 August 2022; Accepted: 26 September 2022;
Published: 13 October 2022.
Edited by:
Xiaodong Tian, First Hospital, Peking University, ChinaReviewed by:
Jishu Wei, Nanjing Medical University, ChinaYinmo Yang, First Hospital, Peking University, China
Copyright © 2022 Cheng, Yang, Fu, Mao, Chu, Lu, Li, Qiu and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yudong Qiu, WXVkb25ncWl1TkpAMTYzLmNvbQ==; Wei He, aGV3ZWlAd2Noc2N1LmNu
†These authors have contributed equally to this work
 Hao Cheng
Hao Cheng Jun Yang2†
Jun Yang2† Chenglin Lu
Chenglin Lu Yudong Qiu
Yudong Qiu