- Department of Gastroenterology, Cancer Hospital, Chongqing University, Chongqing, China
Background: To date, there is no evidence that intensive follow-up provides survival benefit in gastric cancer patients undergoing curative gastrectomy. The aim of this study is to investigate the efficacy of detection of asymptomatic recurrence using intensive surveillance strategy in long-term survival after curative gastric cancer surgery.
Methods: A systematic review of electronic databases including PubMed, Embase, Web of Science, the Cochrane Library and China National Knowledge Infrastructure, Clinical Trials Registry and Google Scholar was performed up to April 2022. The primary outcomes were survival outcomes: overall survival, recurrence-free survival and post-recurrence survival. The secondary endpoints were clinicopathological features, recurrence patterns and treatment after recurrence. The registration number of this protocol is PROSPERO CRD42022327370.
Results: A total of 11 studies including 1898 participants were included. In the pooled analysis, the detection of asymptomatic recurrence was significantly associated with an improved overall survival compared to patients showing symptoms of recurrence (HR=0.67; 95%CI: 0.57-0.79; P<0.001), which was primarily driven by the prolongation of post-recurrence survival (HR=0.51; 95%CI: 0.42-0.61; P<0.001), since there was no significant difference observed in recurrence-free survival (HR=1.12; 95%CI: 0.81-1.55; P=0.48) between the two groups. Meanwhile, male sex and advanced T stage were more frequently observed in the symptomatic recurrence group. Furthermore, patients in the symptomatic recurrence group had a higher proportion of peritoneal relapse but lower proportion of distant lymph node metastasis. Additionally, patients in the symptomatic recurrence group were less likely to receive surgery treatment and post-recurrence chemotherapy.
Conclusion: The detection of asymptomatic recurrence using intensive follow-up was associated with an appreciable improvement in overall survival. However, more robust data from high-quality studies are still required to verify this issue.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=327370, identifier CRD42022327370.
Background
Gastric cancer (GC) is still one of the most common causes of cancer-related deaths worldwide, especially in East Asia (1, 2). Once the GC is detected, surgery with curative intent is the treatment choice for nonmetastatic disease (3, 4). However, despite curative resection was performed, 70%-80% GC recurrences occur within the first 2 years and about 90% of recurrences occur by 5 years, which makes the prognosis of GC patients poor (5, 6).
Currently, international guidelines from the National Cancer Comprehensive Network (NCCN) (7), the Japanese Gastric Cancer Association (JGCA) (8) and the European Society for Medical Oncology (ESMO) (8) consistently recommend postoperative intensive follow-up strategies for GC survivors, despite no consensus on the frequency and regimen of surveillance. Intensive follow-up is beneficial to manage treatment-related complications and collect data (9, 10). Most importantly, intensive follow-up is considered to enable early detection of recurrence disease, therefore instigating further treatment without delay and improving survival outcomes of these patients (11). Consequently, intensive follow-up is actively recommended by clinicians, aiming to detect recurrence as soon as possible, preferably before tumor-related symptoms develop (6).
In colorectal cancer, several meta-analyses have demonstrated improved survival in patients with asymptomatic recurrence (AR) using intensive follow-up strategies compared to these who present later with symptomatic recurrence (SR) (12, 13). The same is true for lung cancer (14) and pancreatic cancer (15). Nevertheless, in gastric cancer, the literature regarding the benefits of intensive follow-up on survival is scarce and extremely inconsistent. Therefore, the JGCA guidelines (16) and the ESMO guidelines (8) clearly state that there is no evidence that intensive follow-up provides survival benefit to GC patients undergoing curative resection. Recently, a newly published meta-analysis based on five studies also failed to demonstrate the benefit of planned surveillance strategies in improving detection of recurrence and post-recurrence survival (PRS) in GC patients (17).
Herein, we performed a systematic review and meta-analysis based on previously published studies to investigate whether the detection of AR using intensive follow-up could improve the survival outcomes, especially overall survival (OS), in GC patients who underwent curative resection.
Methods
The present study was conducted in line with the requirements from Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to identify studies that assess the association of the detection of AR and survival outcomes in GC patients who underwent curative resection. The meta-analysis was registered in PROSPERO (CRD42022327370).
Search strategy
Relevant studies from electronic datasets including PubMed, Embase, Web of Science, and the Cochrane Library were systematically examined up to April 1, 2022. In addition, with the same cutoff date, the Clinical Trials Registry and Google Scholar were used to search unpublished trials and grey literature, respectively. Language restrictions were not applied during the search process. The following combination of key words were used to identify potential studies: [“asymptomatic recurrence” OR “symptomatic recurrence” OR “intensive surveillance” OR “intensive follow-up”] AND [“gastric cancer” OR “gastric carcinoma” OR “gastric neoplasm” OR “stomach cancer” OR “stomach carcinoma” OR “stomach neoplasm”]. Additionally, the references of the included studies were searched for additional reports. The search was performed by two investigators independently (HY-P and MH-Y).
Inclusion and exclusion criteria
The inclusion criteria were determined according to the PICOS approach as follows. P: Patients were pathologically diagnosed as gastric cancer and underwent curative resection; I: AR, which was defined as recurrence discovered by a routine laboratory, imaging, or endoscopic test that was not prompted by any clinical concerns; C: SR, which was defined as the presence of patient-reported symptoms triggering further investigations; O: Time-to-event survival outcomes; S: Comparative studies including randomized controlled trials, cohort and case-controlled studies.
The exclusion criteria were studies 1) reported as case reports, reviews, letters and abstracts; 2) with overlapping data; 3) whose data were not adequate for meta-analysis or unavailable from the authors.
Data extraction and quality assessment
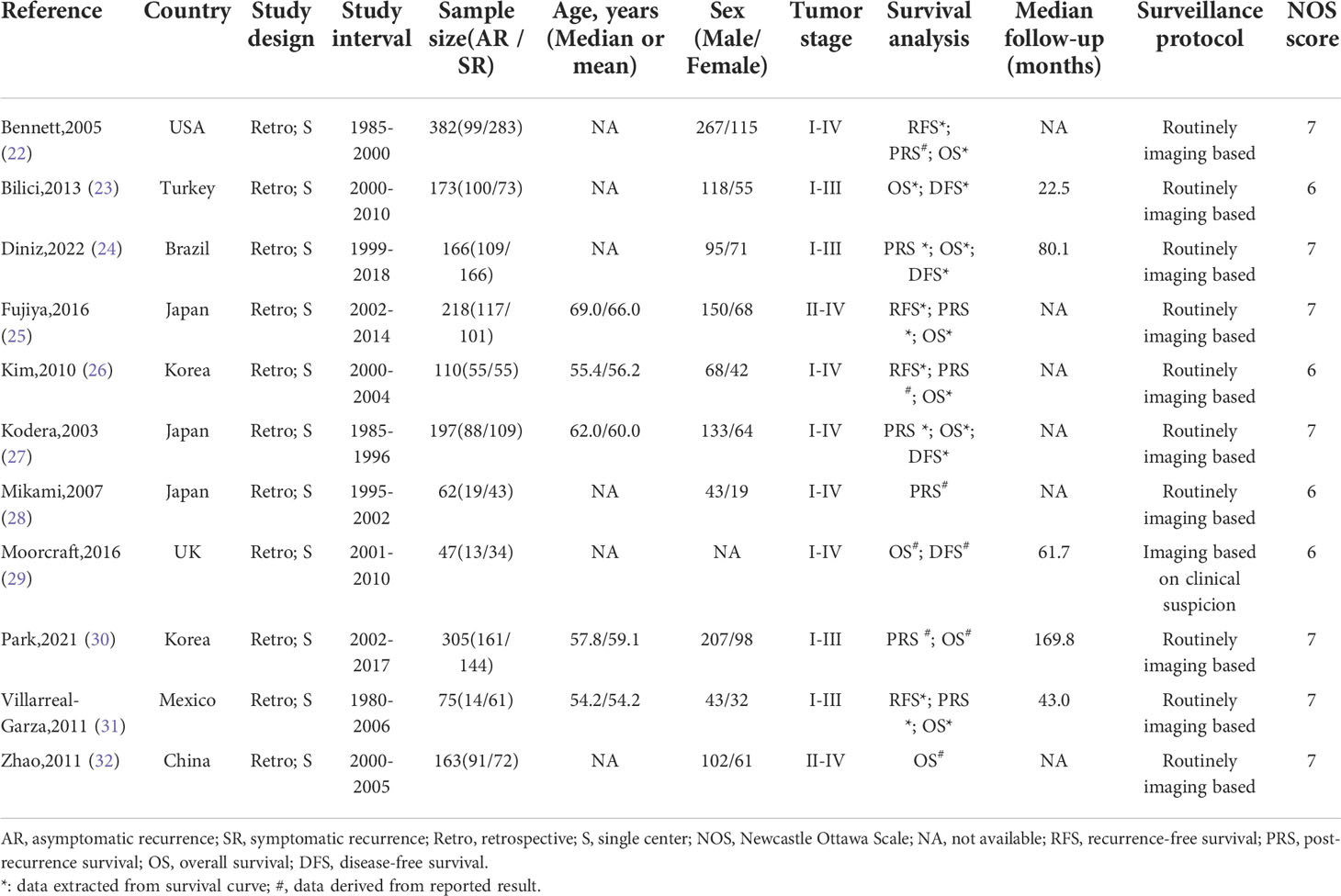
Two independent reviewers (HY-P and MH-Y) conducted the data extraction and cross-checked all the results. The extracted data included first author, year of publication, study interval, country, study design and sample size, clinicopathological features like age, sex, tumor location, histological differentiation, Lauren type, tumor stage, adjuvant chemotherapy, recurrence patterns, treatment after recurrence, follow-up time, and survival outcome measures.
The Newcastle Ottawa Scale (NOS) (18) was used to assess the quality of included observational studies and a study with NOS score >6 is regarded as of high quality.
Outcomes
In the present study, the primary outcomes were to investigate the impact of detection of AR on survival outcomes, including OS (from the date of surgery to the date of any cause of death), PRS (from the date of recurrence to the date of any cause of death) and recurrence-free survival (RFS: from the date of surgery to the date of recurrence). Secondary outcomes were aimed to compare the clinicopathological features, recurrence patterns and treatment after recurrence between the two groups.
Of note, since disease free survival (DFS: from the date of surgery to the date of recurrence or any cause of death) and RFS share the similar endpoints, they were analyzed together as one outcome, RFS (19, 20).
Statistical analysis
The hazard ratio (HR) and odds ratio (OR) with their 95% confidence intervals (CI) were used as the effect size for survival outcomes and dichotomous variables, respectively. For studies that HR with 95%CI was not reported, we then calculated them from the necessary data following the methods reported by Tierney et al. (21) Heterogeneity among studies was assessed using I (2) statistic. All pooled analyses were performed assuming the random-effects model, which accounts for variance across included studies. Subgroup analysis was performed to explore the source of heterogeneity. A leave-one-out sensitivity analysis was conducted to evaluate the robustness and credibility of the results. Publication bias was evaluated using Begg’s funnel plot. A P value <0.05 was considered statistically significant. All of these statistical analyses were performed by Review Manager Software, version 5.3 (Cochrane, London, UK) and Stata, version 12.0 (Statacorp, College Station, TX).
Results
Study characteristics
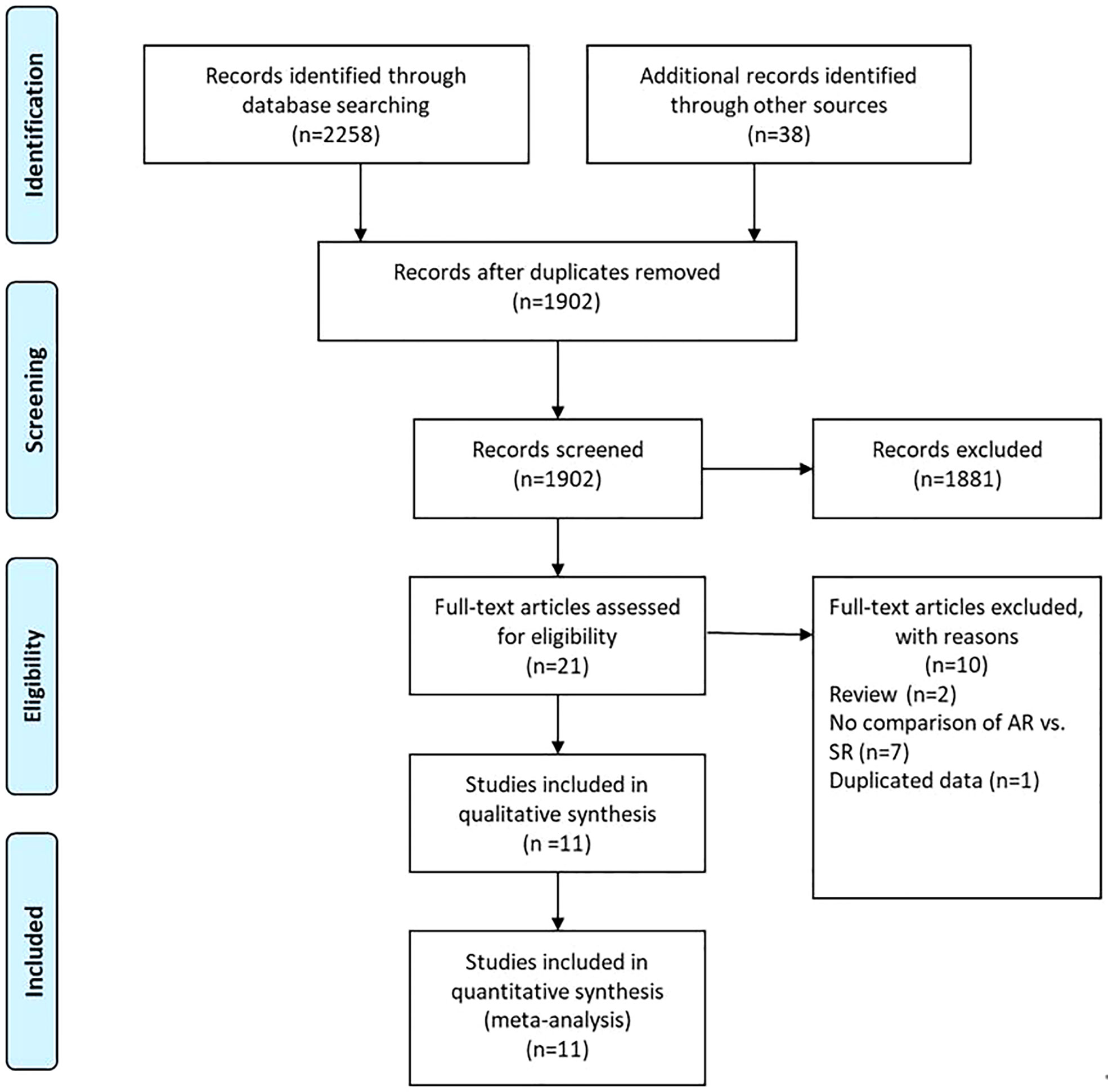
A flow chart of the selection process was shown in Figure 1. The search strategy yielded 2296 potential studies. After title, abstract assessment and full text assessment, 11 retrospective cohort studies (22–32) were finally included in the present study. The characteristics of the included studies were shown in Table 1. A total of 1898 GC patients from 8 countries who underwent curative (R0) resection were included in this study. These studies were published from 2003 to 2022 with a sample size ranging from 47 to 382. Among these studies, the median follow-up time ranged from 22.5 to 169.8 months. With respect to the survival outcomes, the OS was reported in all of these included studies, while RFS/DFS and PRS were reported in 8 and 8 studies, respectively. The surveillance protocols of most studies were routinely based on the combination of history, physical examination, blood tests and imaging-based evidence mainly from CT. However, Moorcraft et al. (29) did not routinely perform imaging-based examination during the follow-up unless the recurrence was clinically suspected. The details of quality assessment of the included studies were shown in the Supplementary File (Table S1), and the scores of these studies ranged from 6 to 7 after careful assessment with NOS.
Overall survival
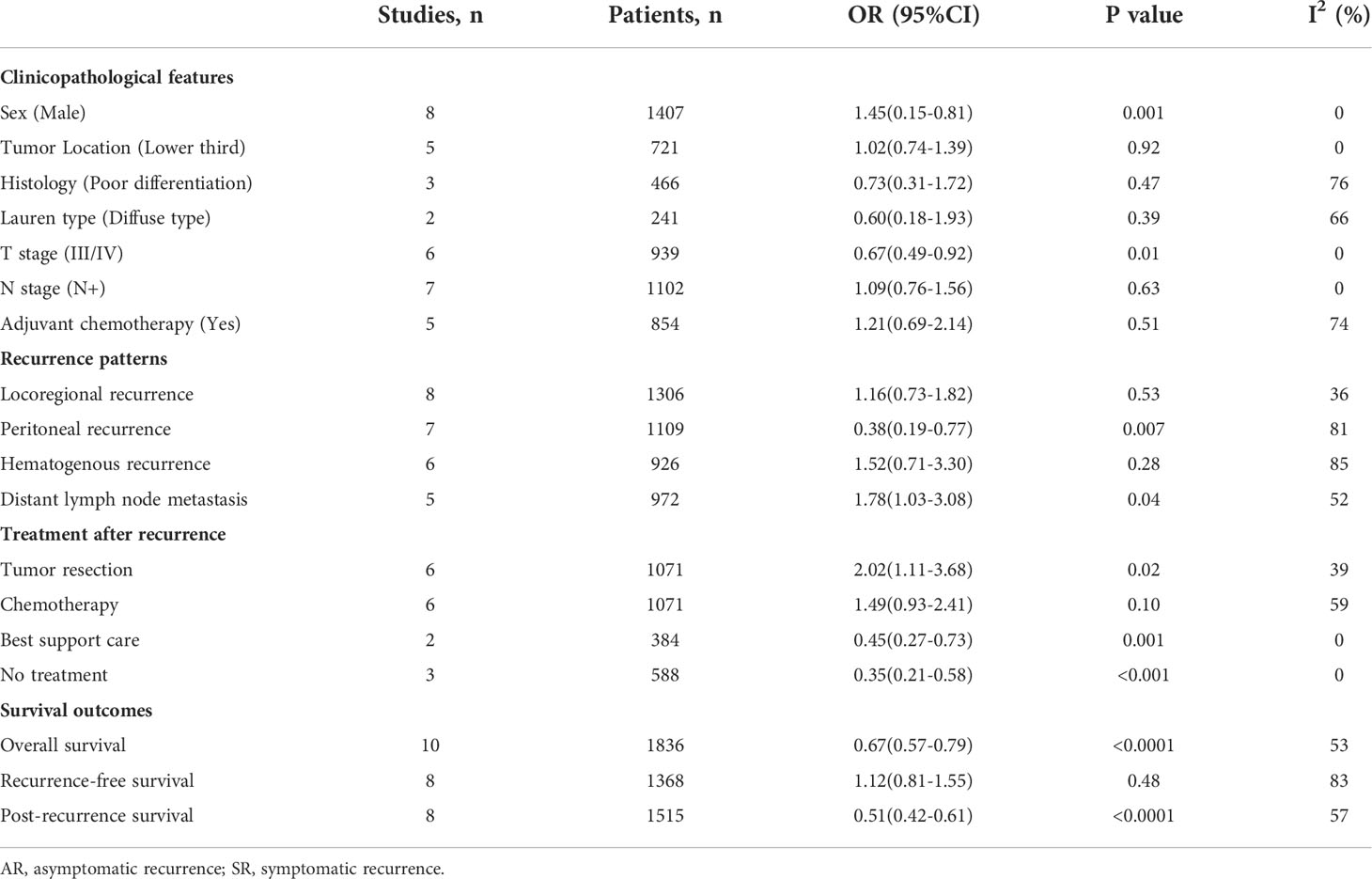
Ten studies (22–27, 29–32) involving 1836 patients (795 in the AR group and 1041 in the SR group) reported the OS outcome. Briefly, five studies (22, 23, 26, 30, 32) showed patients in the AR group had a superior OS than patients in the SR group, but another five studies (24, 25, 27, 29, 31) showed no differences between the two groups. After adding these results, the pooled HR was 0.67 (95%CI: 0.57-0.79; P<0.001), which indicated that the detection of AR could improve the OS in GC patients undergoing curative resection (Figure 2 and Table 2), although there was a moderate heterogeneity (I2 = 53%; P=0.02). Subgroup analyses based on country, sample size, TNM stage, survival analysis, NOS score were performed and shown in Table 3 and Figure S1. Despite heterogeneity in some subsets, the pooled results of almost all subgroup analyses revealed that patients in the AR group had a significantly better OS when compared with these in the SR group. Additionally, sensitivity analysis showed that the pooled outcome did not substantially change (Figure S3A).
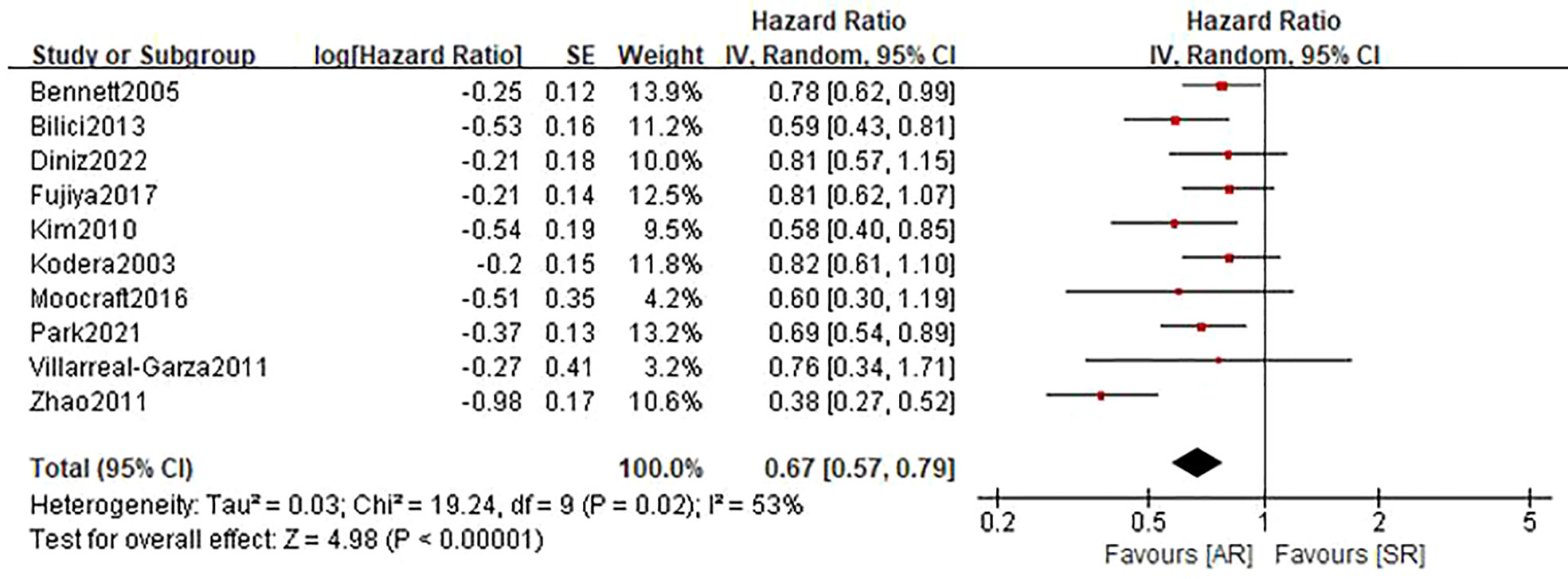
Figure 2 Forest plot assessing overall survival between the asymptomatic and symptomatic recurrence group.
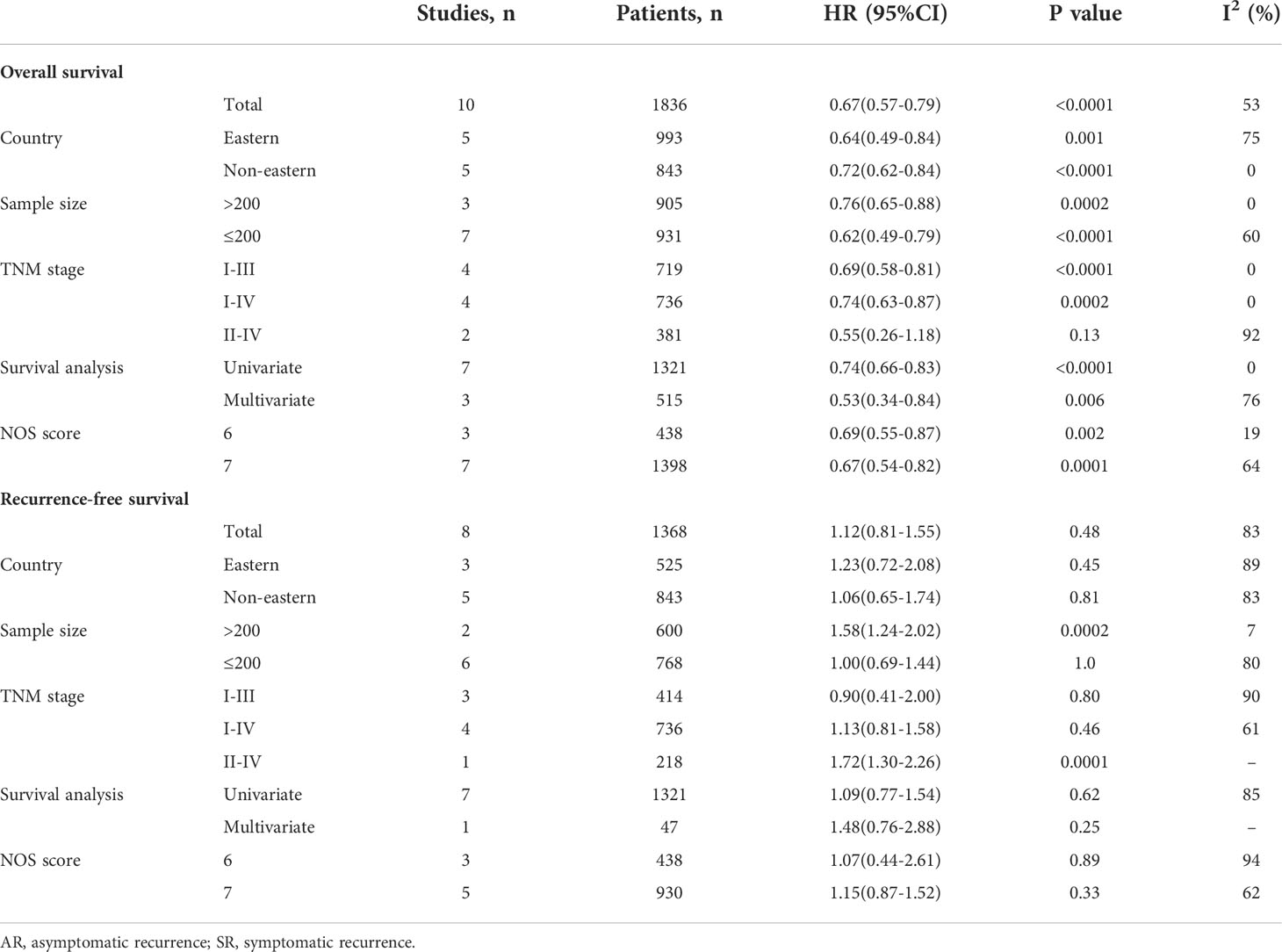
Table 3 Subgroup analysis for overall survival and recurrence-free survival of AR patients vs. SR patients.
Recurrence free survival
A total of 8 studies (22–27, 29, 31) involving 1368 patients (543 in the AR group and 825 in the SR group) reported on RFS. Among these studies, one study (23) reported a favorable RFS in the AR group, 2 studies (24, 25) showed a better RFS in SR group, and the remaining 5 studies (22, 26, 27, 29, 31) did not show differences in this outcome. The pooled HR was 1.12 (95%CI: 0.81-1.55; P=0.48; I2 = 83%), which suggested that there was no significant difference in the time to recurrence between the two groups (Figure 3 and Table 2). Further subgroup analyses based on the above-mentioned parameters indicated that there were no significant differences in terms of RFS in most subgroups, except for the subgroup which had a sample size of more than 200 patients and the subgroup which included patients with stage II-IV disease, showed a favorable RFS in the SR group (Table 3 and Figure S2). Sensitivity analysis indicated that the pooled outcome did not substantially change (Figure S3B).
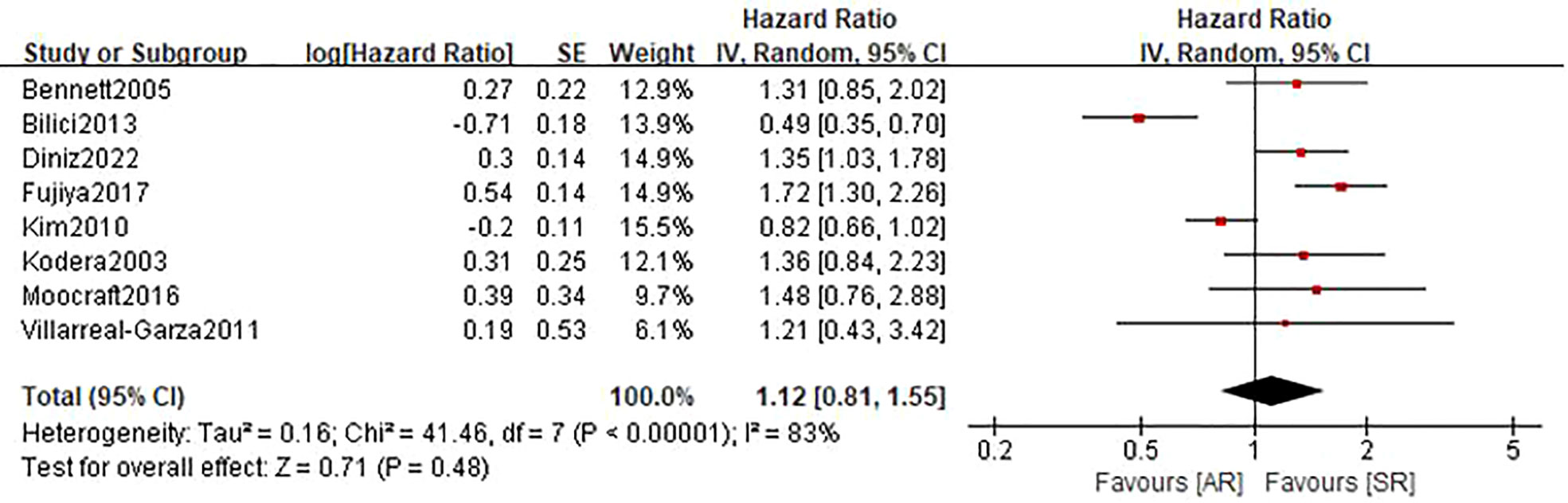
Figure 3 Forest plot assessing recurrence-free survival between the asymptomatic and symptomatic recurrence group.
Post-recurrence survival
Eight studies (22, 24–27, 30, 31) involving 1515 patients (610 in the AR group and 905 in the SR group) reported this outcome. The pooled HR was 0.51 (95%CI: 0.42-0.61; P<0.001), which suggested that the detection of AR was associated with an improved PRS (Figure 4). Even though there was a degree of heterogeneity (I2 = 57%), all of these studies consistently concluded that the detection of AR helps improve PRS.
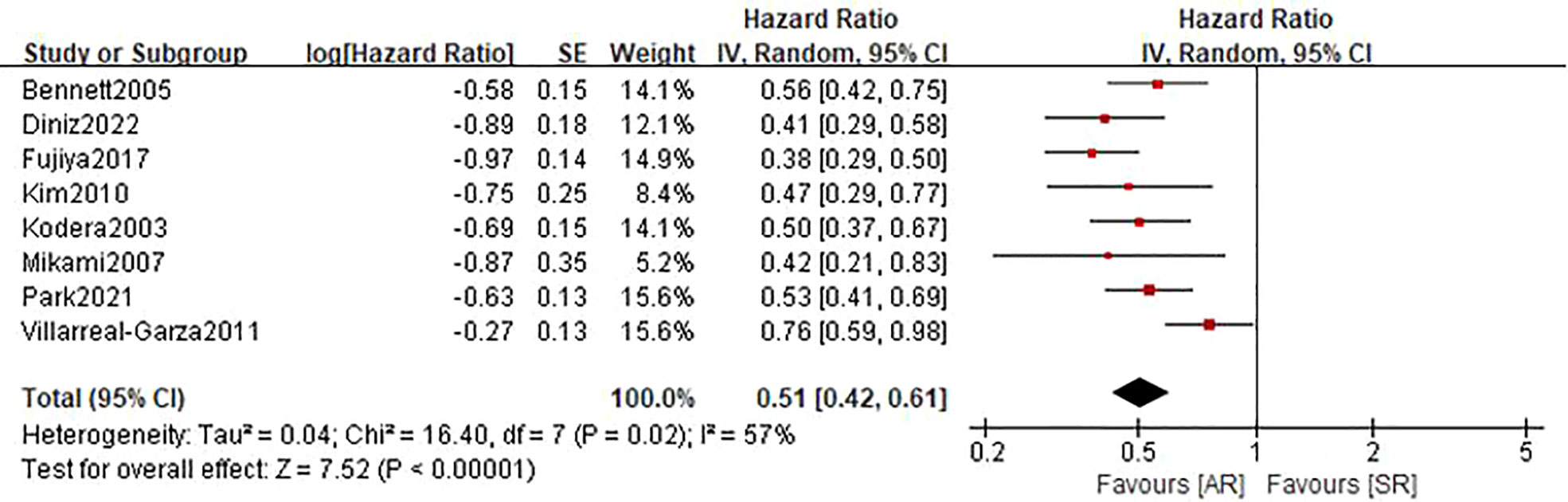
Figure 4 Forest plot assessing post-recurrence survival between the asymptomatic and symptomatic recurrence group.
Clinicopathological features
As shown in Table 2 and Figure S4, pooled results showed that asymptomatic recurrence was significantly associated with a higher proportion of male (OR=1.45, 95%CI: 1.15-1.81, P=0.001; I2 = 0%), and less commonly presented as T3/4 tumors (OR=0.67, 95%CI: 0.49-0.92, P=0.01; I2 = 0%). No association was found between symptomatic recurrence with tumor location, histological differentiation, Lauren type, nodal metastasis and adjuvant chemotherapy. Pooled analysis for age was not feasible due to the lack of available data.
Recurrence patterns
Figure 5 presents results of specific recurrence patterns between the AR and SR groups. Compared with the SR group, the pooled analyses identified that patients in the AR group were more likely to occur distant lymph node metastasis (OR=1.78, 95%CI: 1.03-3.08, P=0.04; I2 = 52%) but less likely to develop peritoneal relapse (OR=0.38, 95%CI: 0.19-0.77, P=0.007; I2 = 81%). With respect to locoregional recurrence and hematogenous recurrence, there were no obvious differences observed between the two groups.
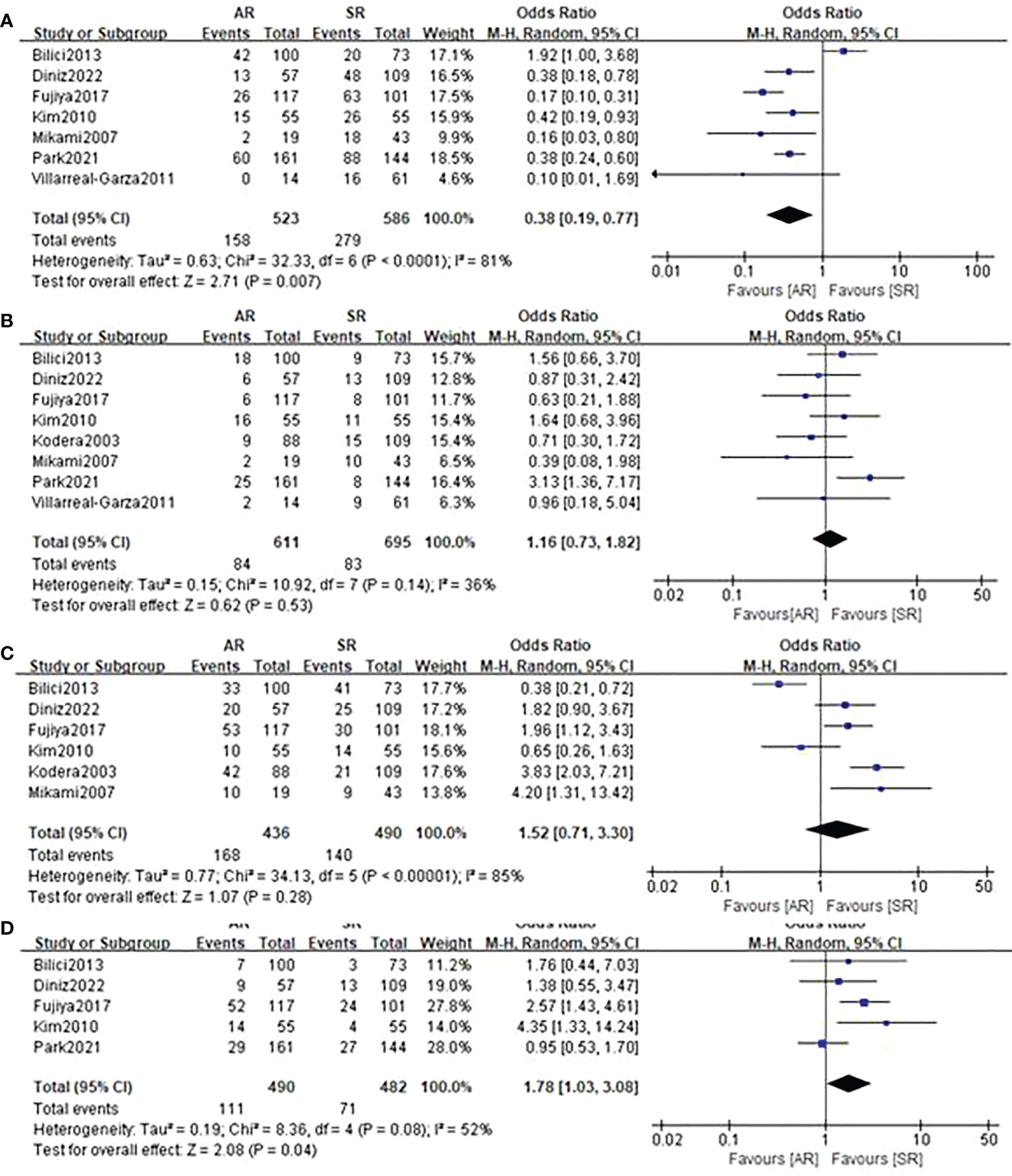
Figure 5 Forest plot assessing recurrence patterns including (A) peritoneal recurrence, (B) locoregional recurrence, (C) hematogenous recurrence, (D) distant lymph node metastasis between the asymptomatic and symptomatic recurrence group.
Treatment characteristics
In terms of treatment after recurrence (Figure 6), the incorporated analyses revealed that patients in the AR group had a higher rate of surgery for recurrent lesions (OR=2.02, 95%CI: 1.11-3.68, P=0.02; I2 = 39%) and a trend towards a higher rate of chemotherapy treatment (OR=1.49, 95%CI: 0.93-2.41, P=0.10; I2 = 59%). On the contrary, the detection of AR could significantly decrease the proportion of patients receiving basic support care (OR=0.45, 95%CI: 0.27-0.73, P=0.001; I2 = 0%) and no treatment (OR=0.35, 95%CI: 0.21-0.58, P<0.001; I2 = 0%).

Figure 6 Forest plot assessing treatment after recurrence including (A) tumor resection, (B) chemotherapy, (C) basic support care, (D) no treatment between the asymptomatic and symptomatic recurrence group.
Publication bias
The Begg’s funnel plot was performed to assess the potential publication bias of the primary outcomes. As shown in Figure S5, the funnel plots of OS, RFS and PRS were virtually symmetric, and the P values of Begg’s test were 0.371, 0.803 and 0.258, respectively, indicating that these pooled outcomes had a low risk of publication bias.
Discussion
This systematic review and meta-analysis demonstrated that asymptomatic GC patients undergoing intensive follow-up after curative gastrectomy had a significantly longer OS than patients showing symptoms of recurrence. The survival benefit was primarily driven by the prolongation of PRS, since there was no significant difference observed in RFS between the two groups. Meanwhile, male sex and advanced T stage were more frequently observed in the SR group than in the AR group. Patients in the SR group had a higher proportion of peritoneal relapse but a lower proportion of distant lymph node metastasis. Additionally, patients in the SR group were less likely to receive surgery treatment and post-recurrence chemotherapy.
In colorectal cancer, studies have suggested that intensive follow-up leads to improved OS compared with less intensive or no follow-up (33, 34). Meanwhile, as a good surrogate of intensive follow-up, accumulating studies have also found that detection of AR helps achieve a superior survival in colorectal patients undergoing curative resection (35). While in gastric cancer, there were only 2 directly comparative studies with inconsistent outcomes (36, 37), we therefore meta-analyzed studies which evaluated the survival of patients according to the presence of cancer-related symptoms or not to investigate the relationship between intensive follow-up and survival outcomes in GC patients undergoing curative resection.
In our meta-analysis, we demonstrated that the detection of AR indeed helps improve OS in GC patients undergoing curative resection. Although there was a degree of heterogeneity in this outcome, our detailed subgroup analyses and sensitivity analysis demonstrated the stability and reliability of this result. Therefore, it is convinced that intensive follow-up should be recommended for patients undergoing radical gastrectomy, owing to the substantial survival benefit.
Intuitively, intensive follow-up is more likely to lead to early detection of recurrence, which have been confirmed in colorectal cancer (13). However, in the present study, despite an OS benefit, the time to detection of recurrence was not different between the two groups through our overall meta-analysis. For this, some scholars explained that the detection of AR may not be the same with early detection of recurrence, and AR is just less biologically aggressive than SR (22, 23). However, considering the high heterogeneity among these included studies, we further performed sensitivity analysis and a series of subgroup analyses. And a subgroup involving studies with a sample size of more than 200 indicated that patients in the AR group had a shorter RFS compared with these in the SR group with I2 = 7%, which indicated that detection of AR may help detect recurrence earlier. However, given that only 2 studies were incorporated into this subgroup, this result should be interpreted with caution, and more prospective studies with a substantial sample size are needed to further validate this issue.
When PRS was analyzed, we identified that the AR group had a better survival compared to the SR group, which remained consistent in all available studies. Certainly, this finding should be mainly attributed to more effective treatment delivered to AR patients (24). In our pooled analyses, we revealed that patients in the AR group had a higher proportion of tumor resection and a trend towards a higher rate of chemotherapy treatment in the AR group. This was demonstrated in the study by Sisic et al. (36), which showed that GC patients who received surgery had an improved PRS compared with these who did not. Besides, Diniz et al. (24) indicated a longer PRS of the patients who received chemotherapy when compared with these receiving basic support care. Predictably, as more effective therapies including chemotherapy, targeted therapy, immunotherapy and surgical resection become available, we believe that the PRS will be further prolonged in patients with early recurrent gastric cancer.
In addition, through our pooled analyses, we found that patients in the SR group were more likely to occur peritoneal recurrence, which is closely associated with deeper tumor invasion (38). Consistently, our clinicopathological analysis also confirmed that the SR group had more advanced T staging. Meanwhile, Diniz et al. (24) demonstrated that patients with peritoneal recurrence had a worse OS when compared with these with other recurrence patterns, which may partially explain why patients in the SR group had an inferior long-term survival. However, in cases with peritoneal recurrence, Fujiya et al. (25) revealed that patients in the AR group still had an obviously improved OS than these in the SR group (median OS: 35.9 vs. 24.0 months; P=0.039). Therefore, intensive follow-up still should not be ignored, especially for patients with a high risk of peritoneal recurrence (e.g., advanced T stage).
A growing body of research focusing on different surveillance strategies has provided us with a more thorough understanding of how intensive follow-up facilitates the promotion of oncological outcomes in cancer patients (15, 17). A meta-analysis by Halle-Smith et al. (15) showed that in pancreatic ductal adenocarcinoma, imaging-based routine surveillance is more likely to detect recurrence at the asymptomatic stage than other routine surveillance methods, and that OS may be superior in patients whose recurrence is detected at the asymptomatic stage. Meanwhile, Chidambaram et al. (17) also demonstrated the crucial role of imaging-based planned surveillance post-esophagectomy in improving PRS. On the other hand, among our included studies, limited evidence from the study by Moorcraft et al. (29) that routinely performed surveillance with clinical assessment and tumor markers, along with endoscopy and CT scans as clinically suspected, failed to confirm the benefits of surveillance in early detection of recurrence and extended OS. Therefore, we infer that the survival benefit from follow-up may be largely attributable to imaging-based surveillance strategies.
The present study has some limitations that should be acknowledged. First, all of the included studies were retrospective in nature, which may increase the risk of selective bias. Thus, more prospective studies with a large sample size are expected to provide more credible evidence on this issue. Second, substantial heterogeneity across the included studies was found in the primary outcome measures. Even though the results of sensitivity analyses and most subgroup analyses remained unchanged, we failed to change the heterogeneity. Third, the median follow-up time among the included studies ranged from 22.5 to 169.8 months, or was not reported. The insufficiency of follow-up time may affect the occurrence of time-to-event outcomes, leading to survivor bias (39, 40). Fourth, due to the limited data comprising different surveillance strategies, we were unable to quantitatively analyze which types of follow-up protocols played a central role in the survival of GC patients.
Conclusions
This systematic review and meta-analysis offered encouraging evidence that detection of AR using intensive follow-up was associated with an appreciable improvement in overall survival. Therefore, it is convinced that intensive follow-up should be recommended to GC patients undergoing curative gastrectomy. However, taking into account the aforementioned limitations, more high-quality prospective studies are still required to verify the results of our study.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
HY-P wrote the manuscript. HY-P, MH-Y and LH-C performed the data search and data analysis. HY-P, MH-Y and LH-C prepared figures. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by Chongqing Technology innovation and Application Development Special general project (cstc2019jscx-msxmX0194).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1011683/full#supplementary-material
References
1. Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet (London England). (2020) 396(10251):635–48. doi: 10.1016/S0140-6736(20)31288-5
2. Pang H, Zhang W, Liang X, Zhang Z, Chen X, Zhao L, et al. Prognostic score system using preoperative inflammatory, nutritional and tumor markers to predict prognosis for gastric cancer: A two-center cohort study. Adv Ther (2021) 38(9):4917–34. doi: 10.1007/s12325-021-01870-z
3. Pang HY, Zhao LY, Wang H, Chen X, Liu K, Zhang W, et al. Impact of type of postoperative complications on long-term survival of gastric cancer patients: Results from a high-volume institution in China. Front Oncol (2021) 11:587309. doi: 10.3389/fonc.2021.587309
4. Yang H, Ji K, Ji J. Current status and perspectives of conversion therapy for advanced gastric cancer. Chin J Cancer Res = Chung-kuo yen cheng yen chiu. (2022) 34(2):109–14. doi: 10.21147/j.issn.1000-9604.2022.02.05
5. Roviello F, Marrelli D, de Manzoni G, Morgagni P, Leo AD, Saragoni L, et al. Prospective study of peritoneal recurrence after curative surgery for gastric cancer. Br J surg (2003) 90(9):1113–9. doi: 10.1002/bjs.4164
6. Cardoso R, Coburn NG, Seevaratnam R, Mahar A, Helyer L, Law C, et al. A systematic review of patient surveillance after curative gastrectomy for gastric cancer: a brief review. Gastric cancer: Off J Int Gastric Cancer Assoc Japanese Gastric Cancer Assoc (2012) 15 Suppl 1:S164–167. doi: 10.1007/s10120-012-0142-9
7. Ajani JA, D’Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, et al. Gastric cancer, version 2.2022. JNCCN J Natl Compr Cancer Netw (2022) 20(2):167–92. doi: 10.6004/jnccn.2022.0008
8. Smyth EC, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D. Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann oncol: Off J Eur Soc Med Oncol (2016) 27(suppl 5):v38–49. doi: 10.1093/annonc/mdw350
9. Bali C, Roukos DH, Ziogas DE. Follow-up for gastric cancer: how extensive and intensive should it be? Ann Surg Oncol (2010) 17(3):941. doi: 10.1245/s10434-009-0821-x
10. Zanotti D, Baiocchi GL, Coniglio A, Mohammadi B, Ministrini S, Mughal M, et al. Follow-up after surgery for gastric cancer: how to do it. Updates surg (2018) 70(2):293–9. doi: 10.1007/s13304-018-0524-6
11. Whiting J, Sano T, Saka M, Fukagawa T, Katai H, Sasako M. Follow-up of gastric cancer: a review. Gastric cancer: Off J Int Gastric Cancer Assoc Japanese Gastric Cancer Assoc (2006) 9(2):74–81. doi: 10.1007/s10120-006-0360-0
12. Bruinvels DJ, Stiggelbout AM, Kievit J, van Houwelingen HC, Habbema JD, van de Velde CJ. Follow-up of patients with colorectal cancer. A meta-analysis. Ann surg (1994) 219(2):174–82. doi: 10.1097/00000658-199402000-00009
13. Pita-Fernández S, Alhayek-Aí M, González-Martín C, López-Calviño B, Seoane-Pillado T, Pértega-Díaz S. Intensive follow-up strategies improve outcomes in nonmetastatic colorectal cancer patients after curative surgery: a systematic review and meta-analysis. Ann oncol: Off J Eur Soc Med Oncol (2015) 26(4):644–56. doi: 10.1093/annonc/mdu543
14. Calman L, Beaver K, Hind D, Lorigan P, Roberts C, Lloyd-Jones M. Survival benefits from follow-up of patients with lung cancer: a systematic review and meta-analysis. J Thorac oncol: Off Publ Int Assoc Study Lung Cancer (2011) 6(12):1993–2004. doi: 10.1097/JTO.0b013e31822b01a1
15. Halle-Smith JM, Hall L, Daamen LA, Hodson J, Pande R, Young A, et al. Clinical benefit of surveillance after resection of pancreatic ductal adenocarcinoma: A systematic review and meta-analysis. Eur J Surg oncol: J Eur Soc Surg Oncol Br Assoc Surg Oncol (2021) 47(9):2248–55. doi: 10.1016/j.ejso.2021.04.031
16. Japanese Gastric Cancer Association. Japanese Gastric cancer treatment guidelines 2018 (5th edition). Gastric cancer: Off J Int Gastric Cancer Assoc Japanese Gastric Cancer Assoc (2021) 24(1):1–21. doi: 10.1007/s10120-020-01042-y
17. Chidambaram S, Sounderajah V, Maynard N, Markar SR. Evaluation of post-operative surveillance strategies for esophageal and gastric cancers: a systematic review and meta-analysis. diseases of the esophagus. Off J Int Soc Dis Esophagus (2022) 00:1–13. doi: 10.1093/dote/doac034
18. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
19. Chen X, Li J, Zhang X, Liu Y, Wu J, Li Y, et al. Prognostic and clinicopathological significance of pretreatment mean platelet volume in cancer: a meta-analysis. BMJ Open (2020) 10(10):e037614. doi: 10.1136/bmjopen-2020-037614
20. Papakonstantinou A, Gonzalez NS, Pimentel I, Suñol A, Zamora E, Ortiz C, et al. Prognostic value of ctDNA detection in patients with early breast cancer undergoing neoadjuvant therapy: A systematic review and meta-analysis. Cancer Treat Rev (2022) 104:102362. doi: 10.1016/j.ctrv.2022.102362
21. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials (2007) 8:16. doi: 10.1186/1745-6215-8-16
22. Bennett JJ, Gonen M, D’Angelica M, Jaques DP, Brennan MF, Coit DG. Is detection of asymptomatic recurrence after curative resection associated with improved survival in patients with gastric cancer? J Am Coll Surgeons (2005) 201(4):503–10. doi: 10.1016/j.jamcollsurg.2005.05.033
23. Bilici A, Salman T, Oven Ustaalioglu BB, Unek T, Seker M, Aliustaoglu M, et al. The prognostic value of detecting symptomatic or asymptomatic recurrence in patients with gastric cancer after a curative gastrectomy. J Surg Res (2013) 180(1):e1–9. doi: 10.1016/j.jss.2012.03.035
24. Diniz TP, da Costa WL Jr., Gomes CC, de Jesus VHF, Felismino TC, Torres SM, et al. Symptomatic recurrence and survival outcomes after curative treatment of gastric cancer: Does intensive follow-up evaluation improve survival? Ann Surg Oncol (2022) 29(1):274–84. doi: 10.1245/s10434-021-10724-5
25. Fujiya K, Tokunaga M, Makuuchi R, Nishiwaki N, Omori H, Takagi W, et al. Early detection of nonperitoneal recurrence may contribute to survival benefit after curative gastrectomy for gastric cancer. Gastric cancer: Off J Int Gastric Cancer Assoc Japanese Gastric Cancer Assoc (2017) 20(Suppl 1):141–9. doi: 10.1007/s10120-016-0661-x
26. Kim JH, Jang YJ, Park SS, Park SH, Mok YJ. Benefit of post-operative surveillance for recurrence after curative resection for gastric cancer. J gastrointest surg: Off J Soc Surg Alimentary Tract. (2010) 14(6):969–76. doi: 10.1007/s11605-010-1200-4
27. Kodera Y, Ito S, Yamamura Y, Mochizuki Y, Fujiwara M, Hibi K, et al. Follow-up surveillance for recurrence after curative gastric cancer surgery lacks survival benefit. Ann Surg Oncol (2003) 10(8):898–902. doi: 10.1245/ASO.2003.12.009
28. Mikami K, Yamashita Y, Maekawa T, Shinohara T, Yamauchi Y, Hoshino S, et al. Surveillance program for recurrence after curative gastric cancer surgery. Chir Gastroenterol (2007) 23(4):392–8. doi: 10.1159/000109243
29. Moorcraft SY, Fontana E, Cunningham D, Peckitt C, Tom Waddell T, Smyth EC, et al. Characterising timing and pattern of relapse following surgery for localised oesophagogastric adenocarcinoma: a retrospective study. BMC cancer. (2016) 16:112. doi: 10.1186/s12885-016-2145-0
30. Park JS, Choe EA, Park S, Nam CM, Hyung WJ, Noh SH, et al. Detection of asymptomatic recurrence improves survival of gastric cancer patients. Cancer Med (2021) 10(10):3249–60. doi: 10.1002/cam4.3899
31. Villarreal-Garza C, Rojas-Flores M, Castro-Sánchez A, Villa AR, García-Aceituno L, León-Rodríguez E. Improved outcome in asymptomatic recurrence following curative surgery for gastric cancer. Med Oncol (Northwood London England). (2011) 28(4):973–80. doi: 10.1007/s12032-010-9576-2
32. Zhao JZ, Zhang RP, Wang G, Li F, Wang X, Xue Q, et al. Prognosis of patients with recurrence after curative resection of advanced gastric cancer. Zhonghua wei chang wai ke za zhi = Chin J gastrointest surg (2011) 14(2):107–10. doi: 10376/cma.j.issn.1671-0274.2011.02.010
33. Pietra N, Sarli L, Costi R, Ouchemi C, Grattarola M, Peracchia A. Role of follow-up in management of local recurrences of colorectal cancer: a prospective, randomized study. Dis colon rectum (1998) 41(9):1127–33. doi: 10.1007/BF02239434
34. Secco GB, Fardelli R, Gianquinto D, Bonfante P, Baldi E, Ravera G, et al. Efficacy and cost of risk-adapted follow-up in patients after colorectal cancer surgery: a prospective, randomized and controlled trial. Eur J Surg oncol: J Eur Soc Surg Oncol Br Assoc Surg Oncol (2002) 28(4):418–23. doi: 10.1053/ejso.2001.1250
35. Duineveld LA, van Asselt KM, Bemelman WA, Smits AB, Tanis PJ, van Weert HCPM, et al. Symptomatic and asymptomatic colon cancer recurrence: A multicenter cohort study. Ann Family Med (2016) 14(3):215–20. doi: 10.1370/afm.1919
36. Sisic L, Strowitzki MJ, Blank S, Nienhueser H, Dorr S, Haag GM, et al. Postoperative follow-up programs improve survival in curatively resected gastric and junctional cancer patients: a propensity score matched analysis. Gastric cancer: Off J Int Gastric Cancer Assoc Japanese Gastric Cancer Assoc (2018) 21(3):552–68. doi: 10.1007/s10120-017-0751-4
37. Tan IT, So BY. Value of intensive follow-up of patients after curative surgery for gastric carcinoma. J Surg Oncol (2007) 96(6):503–6. doi: 10.1002/jso.20823
38. Kus T, Kose F, Aktas G, Arslan UY, Sedef AM, Cinkiret HY, et al. Prediction of peritoneal recurrence in patients with gastric cancer: a multicenter study. J gastrointest cancer. (2021) 52(2):634–42. doi: 10.1007/s12029-020-00419-7
39. Miller DP, Gomberg-Maitland M, Humbert M. Survivor bias and risk assessment. Eur Respir J (2012) 40(3):530–2. doi: 10.1183/09031936.00094112
Keywords: gastri cancer, intensive follow-up, recurrence, survival outcomes, meta-analysis
Citation: Pang H-Y, Yan M-H, Chen L-H, Chen X-F, Chen Z-X, Zhang S-R and Sun H (2022) Detection of asymptomatic recurrence following curative surgery improves survival in patients with gastric cancer: A systematic review and meta-analysis. Front. Oncol. 12:1011683. doi: 10.3389/fonc.2022.1011683
Received: 04 August 2022; Accepted: 10 October 2022;
Published: 26 October 2022.
Edited by:
Jinqiu Jacky Yuan, Sun Yat-sen University, ChinaCopyright © 2022 Pang, Yan, Chen, Chen, Chen, Zhang and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hao Sun, c3VuaGFvNjhAc2luYS5jb20=
†These authors share first authorship
 Hua-Yang Pang†
Hua-Yang Pang† Meng-Hua Yan
Meng-Hua Yan Hao Sun
Hao Sun