- 1Department of Urology, Peking Union Medical College Hospital, Peking Union Medical College, Chinese Academy of Medical Sciences, Beijing, China
- 2Department of Pathology, Peking Union Medical College Hospital, Peking Union Medical College, Chinese Academy of Medical Sciences, Beijing, China
Background: Papillary Renal Neoplasm (PRN) with polarity inversion is a less common subtype of kidney cancer with an apparently recognizable morphology, distinct immunohistochemical profiles, and frequent KRAS mutations. It has been estimated to account 4% of previously diagnosed PRN.
Case presentation: This is a retrospective case report of two patients diagnosed with PRNRP. Two males were found to have kidney mass accidentally through imaging examination in clinic. Both of the patients had no obvious discomfort and abnormal test indicators. Subsequently, they underwent partial nephrectomy in our center by the same surgeon and followed up closely with an impressive clinical outcome. The pathology reports indicated that their pathological features were consistent with PRNRP. The HE staining showed a monolayer of papillary or tubular structures, with small nuclei away from the cytoplasmic top of the basement membrane. The immunohistochemical results were GATA3 (+), vimentin (-).
Conclusion: Our case reports and literature review suggested that PRNRP should be separated from traditional PRN and partial nephrectomy is a robust modality for PRNRP. The morphological, immunohistochemical, and genetic information of the cases we presented would provide important material for PRNRP to become a distinct category with benign clinical outcome.
Introduction
Papillary renal neoplasm (PRN) is the second most common subtype of kidney cancer, which takes up 15%-20% of renal cell carcinoma (RCC) (1, 2). PRN with reverse polarity (PRNRP) is a rare and newly defined subtype of PRN, accounting for 4-8.6% of the total (3–5). In 2019, Al-Obaidy et al. named the diagnosis for the first time as PRNRP and demonstrated that it was different from common PRN in histomorphological, immunohistochemical, and chromosomal features (3). This tumor type has been described as being characterized by branching papillae, or rarely, predominant tubules covered by bland oncocytic cells with apical low- International Society of Urological Pathology (ISUP)-grade nuclei. This article would report two cases PRNRP and review the current literature reports. We believe our results would offer new insights into PRNRP, thus aiding future efforts for the diagnosis and treatment of this disease. Both patients have fulfilled the written informed consent.
Case presentation
Case 1
The patient is a 54-year-old male, who was admitted to the hospital due to the discovery of a left kidney tumor by ultrasound. The patient had no complains of clinical symptoms, no fever, low back pain, or hematuria. He was a heavy smoker (30 pack/year). Urine culture and urine cytology of that patents demonstrated normal results. The results of blood routine, liver and kidney function, coagulation test indexes also showed no obvious abnormality. Ultrasonography of the urinary system demonstrated mixed echoes in the lower pole of the left kidney, which measured 4×3 cm in size, with a clear boundary, thick septa. A 1.1×1.0 cm regular shape, medium-high echo was detected on the wall. Enhanced CT showed a slightly high-density nodule at the lower pole of the left kidney, with a diameter of about 2.5 cm and a CT value of 57 HU on plain scan with no obvious strengthening (Figures 1A, B). Based on Bosniak criteria (6), the lesion was sorted as category IV. Laparoscopic left partial nephrectomy was then performed. The tumor showed a complete capsule, and the section composed of cystic and solid components.

Figure 1 (A, B) Computed tomography revealed a slightly high-density nodule at the lower pole of the left kidney for case one, with a diameter of about 2.5 cm and a CT value of 57 HU on plain scan with no obvious strengthening. (C, D) CT demonstrated a 1.6×1.3 cm round high-density nodule in the right kidney of case 2 with a CT value of about 80HU on plain scan. No enhancement after intravenous administration of contrast was noted.
The postoperative pathology revealed PRNRP (grade 2 PRN, stage pT1a). The tumor incision is grayish red with medium texture and fine papillary shape. Tumor was confined to the kidney, with no extension into the perinephric adipose tissue, renal sinus, renal pelvis, or Gerota fascia. Immunohistochemical phenotypes were as follow: GATA3 (+), Keratin 34βe12 (localized +), AE1/AE3 (+), Keratin 7 (+), EMA (+), vimentin (-), PAX-8(-), CD10(-), CD117(-), CA9(-), TFE3(-), RCC(-), P504(-). After one year of follow-up, the patient recovered well without disease progression or recurrence.
Case two
The patient was a 35-year-old male, who admitted to the hospital due to physical examination of a right kidney mass for 1 week. The patient had no clinical manifestations, no fever, low back pain, or hematuria. The findings of laboratory tests, including complete blood count, liver and renal function tests, and urine analysis, were within normal range. The patient was previously healthy with no family history of tumor.
Ultrasound of the urinary system demonstrated an anechoic, regular mass at the middle and lower part of the right kidney measuring 1.9×1.6×1.3 cm in size, with clear boundary. Enhanced CT showed a 1.6×1.3 cm round high-density nodule in the right kidney with a CT value of about 80HU on plain scan. No enhancement after intravenous administration of contrast was noted. The mass was classified as Bosniak type III (Figures 1C, D). Chest CT showed a 4mm nodules at upper lobes.
Laparoscopic right partial nephrectomy was then performed, and the postoperative specimen showed a complete capsule, with solid and cystic changes in cut section. The pathological study confirmed the diagnosis of PRNRP polarity, grade 2 with 20% of necrosis, without vascular embolus or peri-renal infiltration, pT1aNx. No renal capsule invasion, no renal pelvis and renal sinus fat involvement was detected. Immunohistochemical phenotypes: GATA3(+), AE1/AE3(+), Keratin 7(+), EMA(+), vimentin(-), PAX-8(-), CD10(-), CD117(-), CA9(-), TFE3(-), RCC(-), P504(-). The patient did not report any specific discomfort and disease progression at his one year follow-up.
Both the pathological manifestations of the tumors from the aforementioned two patients were consistent with typical PRNRP. The HE staining showed papillary or tubular structures, the surface was covered with a monolayer of eosinophils, the cytoplasm was granular, with small nuclei away from the cytoplasmic top of the basement membrane. The immunohistochemical phenotype was GATA3 (+), vimentin (-) (Figure 2).
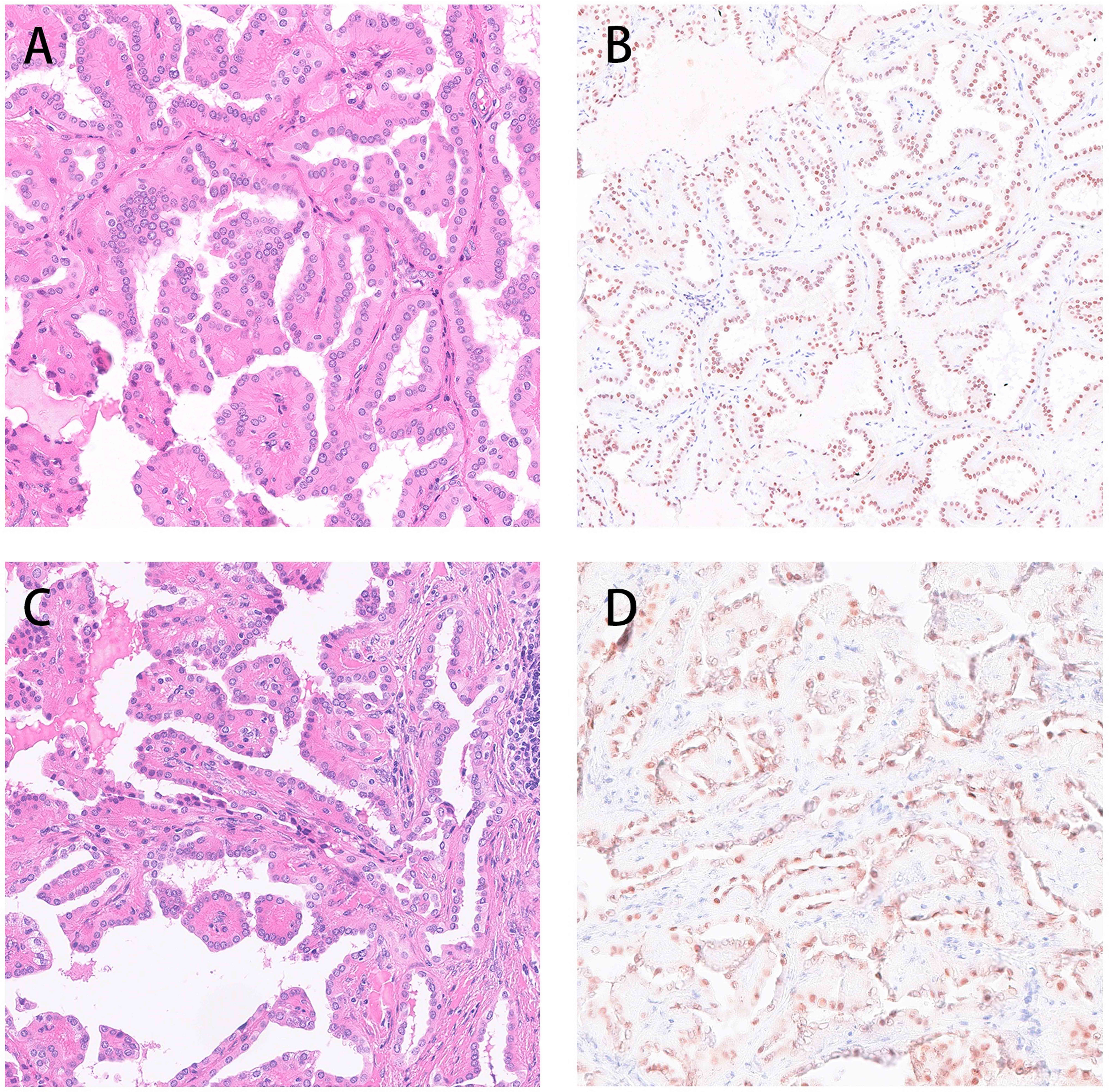
Figure 2 Both the pathological manifestations of the tumors from the aforementioned two patients were consistent with typical PRNRP. The HE staining showed papillary or tubular structures, the surface was covered with a monolayer of eosinophils, the cytoplasm was granular, with small nuclei away from the cytoplasmic top of the basement membrane. The immunohistochemical phenotype was GATA3 (+), vimentin (-) (A, B: Case 1 C, D Case 2).
Discussion
With the development of molecular biology and genetics, PRN including three molecular subtypes with different prognostic propensities based on whether it is combined with CDKN2A gene silencing, SETD2 gene mutation, TFE3 gene fusion, or increased expression of NRF2–antioxidant response element signaling pathway (7). PRNRP is pathologically characterized as papillary or tubular structures covered with a monolayer of eosinophilic cells with granular cytoplasm and small, inconspicuous nuclei located at the cytoplasm away from the basement membrane (8). Immunohistochemistry showed that GATA3 and L1CAM were positive, but vimentin was negative (9). Fluorescence in situ hybridization (FISH) analysis demonstrated a low level of chromosomal abnormalities (10). Trisomy 7/17 was found in 20% of tumors, and chromosome Y deletion was found in 14% of male patients. All the aforementioned pathology characteristics are significantly different from typical PRN (9).
Genetically, 80-100% of PRNRP would activate mutations in KRAS (4, 8, 11), which is relatively rare in other types of RCC. According to TCGA database (The Cancer Genome Atlas database), only 0.4% (2/488) of clear cell RCC have KRAS gene mutations; about 1.8% (5/274) of PRN have KRAS gene mutations; no KRAS gene mutation was detected in chromophobe RCC (7, 11, 12). PRNRP is the only pathological subtype of RCC with a high frequency of KRAS mutations. KRAS gene belongs to RAS gene family, which can encode p21 protein with GTPase activity. It forms a classic tyrosine kinase signal pathway with downstream raf/mek/erk and MAPK, and plays an important role in regulating cell proliferation, differentiation, migration and apoptosis (13, 14). KRAS gene is an important proto-oncogene, and its abnormal activation is associated with a variety of malignant tumors, such as lung cancer, colon cancer and pancreatic cancer (15, 16). Different tumor types have different KRAS mutation profiles. For example, p.Gly12Cys and p.Gly12Val missense mutations are common in non-small cell lung cancer, whereas p.Gly12Asp missense mutations are more likely to occur in colorectal cancer (17, 18). Different KRAS gene mutations can produce different downstream effects, thus affecting the prognosis of the disease. In non-small cell lung cancer, patients with p.Gly12Cys and p.Gly12Val mutations would promote RAS-related protein signaling (v-ral simian leukemia viral oncogene homolog ras related, Ral signaling) and reduce the activation of growth factor-dependent AKT (growth factor-dependent Akt) thus leads to a worse prognosis than patients with other mutations; whereas p. Gly12Asp mutation plays critical a role by increasing the activation of PI3K (phosphatidylinosi-tol 3-kinase) and MEK (mitogen-activated protein/extracellular signal-regulated kinase kinase). In PRNRP, the common KRAS gene mutation sites are p.Gly12Val, p. Gly12Asp and p. Gly12Arg, accounting for 61.5%, 34.6% and 3.9%, respectively (11). Nevertheless, the effect of different genotypes on clinical prognosis remains to be unclear.
The mean onset age of PRNRP was around 60 years, with no significant gender differences. Like common RCC, PRNRP may not generally demonstrate any symptoms in the early phase and may be found accidentally through physical examination. A few patients may have symptoms of waist discomfort or hematuria. In present study, The CT imaging features of the two cases were both high-density cysts without obvious enhancement. Tong et al. demonstrated that 5 of 12 of their cases were cystic (19). Chang et al. also demonstrated that 6 of their 10 tumors were cystic (4). The cystic features were also proved by the loose papillae floating in the large spaces shown by the images in the published cases (20). Thus we can infer that CT images features of PRNRP were small-sized, well circumscribed neoplasm, often encapsulated and cystic, with no obvious enhancement. According to previous study, the majority of the patients had tumors less than 3 cm in diameter, TNM staging was mainly T1aN0M0, and the mainstay of treatment is partial nephrectomy. The biological behavior of PRNRP is generally indolent, with promising prognosis. Up to now, the longest follow-up time in the published literature is nearly 20 years, and no disease progression or recurrence has been reported in all patients (3–5, 11, 19, 21, 22). Nevertheless, previous study also highlighted that more studies are needed to further evaluate the clinical characteristics and prognosis of partial nephrectomy. In our report, two patients presented in our case report were young and middle-aged males, both of whom were found to have renal masses accidentally during physical examination, without any obvious clinical manifestations. In addition, the ultrasound results of them showed a solid cystic mass with clear borders. CT demonstrated the possibility of high-density cysts without obvious enhancement. As of yet, no disease progression or recurrence was observed in the one year follow-up. We firmly believe the morphological, immunohistochemical, and genetic information of the cases we presented would provide important material for PRNRP to become a distinct category with benign clinical outcome.
Conclusion
PRNRP is a newly defined papillary renal cell carcinoma with characteristic pathomorphology, immunohistochemical phenotype and genetic phenotype, with promising prognosis. PRNRP should be separated from PRN and treated by partial nephrectomy instead of radical nephrectomy.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LZ: substantial contributions to acquisition of data and involved in drafting. ZY, YW, WX, PB, and XY: equal contributions to acquisition of data. XY: substantial contributions to data acquisition, involved in drafting. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Prendeville S, Richard PO, Jewett MAS, Kachura JR, Sweet JM, van der Kwast TH, et al. Accuracy of renal tumour biopsy for the diagnosis and subtyping of papillary renal cell carcinoma: Analysis of paired biopsy and nephrectomy specimens with focus on discordant cases. J Clin Pathol (2019) 72(5):363–7. doi: 10.1136/jclinpath-2018-205655
2. Capitanio U, Bensalah K, Bex A, Boorjian SA, Bray F, Coleman J, et al. Epidemiology of renal cell carcinoma. Eur Urol (2019) 75(1):74–84. doi: 10.1016/j.eururo.2018.08.036
3. Al-Obaidy KI, Eble JN, Cheng L, Williamson SR, Sakr WA, Gupta N, et al. Papillary renal neoplasm with reverse polarity: A morphologic, immunohistochemical, and molecular study. Am J Surg Pathol (2019) 43(8):1099–111. doi: 10.1097/PAS.0000000000001288
4. Chang HY, Hang JF. Clinicopathological and molecular characterisation of papillary renal neoplasm with reverse polarity and its renal papillary adenoma analogue. Histopathology (2021) 78(7):1019–31. doi: 10.1111/his.14320
5. Kiyozawa D, Kohashi K, Takamatsu D, Yamamoto T, Eto M, Iwasaki T, et al. Morphological, immunohistochemical, and genomic analyses of papillary renal neoplasm with reverse polarity. Hum Pathol (2021) 112:48–58. doi: 10.1016/j.humpath.2021.03.009
6. Silverman SG, Pedrosa I. Bosniak classification of cystic renal masses, version 2019: An update proposal and needs assessment. Radiology (2019) 292(2):475–88. doi: 10.1148/radiol.2019182646
7. Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov (2012) 2(5):401–4. doi: 10.1158/2159-8290.CD-12-0095
8. Al-Obaidy KI, Eble JN, Nassiri M, Cheng L, Eldomery MK, Williamson SR. Recurrent KRAS mutations in papillary renal neoplasm with reverse polarity. Cancer Discov (2020) 33(6):1157–64. doi: 10.1038/s41379-019-0362-1
9. Wei S, Kutikov A, Patchefsky AS, Flieder DB, Talarchek JN, Al-Saleem T, et al. Papillary renal neoplasm with reverse polarity is often cystic: Report of 7 cases and review of 93 cases in the literature. Am J Surg Pathol (2022) 46(3):336–43. doi: 10.1097/PAS.0000000000001773
10. Yang T, Kang E, Zhang L, Zhuang J, Li Y, Jiang Y, et al. Papillary renal neoplasm with reverse polarity may be a novel renal cell tumor entity with low malignant potential. Diagn Pathol (2022) 17(1):66. doi: 10.1186/s13000-022-01235-2
11. Kim SS, Cho YM. Recurrent KRAS mutations identified in papillary renal neoplasm with reverse polarity-a comparative study with papillary renal cell carcinoma. Mod Pathol (2020) 33(4):690–9. doi: 10.1038/s41379-019-0420-8
12. Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal (2013) 6(269):pl1. doi: 10.1126/scisignal.2004088
13. Peyssonnaux C, Eychène A. The Raf/MEK/ERK pathway: New concepts of activation. Biol Cell (2001) 93(1-2):53–62. doi: 10.1016/S0248-4900(01)01125-X
14. Kranenburg O. The KRAS oncogene: past, present, and future. biochimica et biophysica acta. Biochim Biophys Acta (2005) 1756(2):81–2. doi: 10.1016/j.bbcan.2005.10.001
15. Eberhard DA, Johnson BE, Amler LC, Goddard AD, Heldens SL, Herbst RS, et al. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol (2005) 23(25):5900–9. doi: 10.1200/JCO.2005.02.857
16. Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol (2008) 26(10):1626–34. doi: 10.1200/JCO.2007.14.7116
17. Ostrow SL, Simon E, Prinz E, Bick T, Shentzer T, Nagawkar SS, et al. Variation in KRAS driver substitution distributions between tumor types is determined by both mutation and natural selection. Sci Rep (2016) 6:21927. doi: 10.1038/srep21927
18. Ihle NT, Byers LA, Kim ES, Saintigny P, Lee JJ, Blumenschein GR, et al. Effect of KRAS oncogene substitutions on protein behavior: Implications for signaling and clinical outcome. J Natl Cancer Inst (2012) 104(3):228–39. doi: 10.1093/jnci/djr523
19. Tong K, Zhu W, Fu H, Cao F, Wang S, Zhou W, et al. Frequent KRAS mutations in oncocytic papillary renal neoplasm with inverted nuclei. Histopathology (2020) 76(7):1070–83. doi: 10.1111/his.14084
20. Nova-Camacho LM, Martin-Arruti M, Ruiz Díaz I, Panizo-Santos Á. Papillary renal neoplasm with reverse polarity. Arch Pathol Lab Med (2022). doi: 10.5858/arpa.2022-0156-OA
21. Zhou L, Xu J, Wang S, Yang X, Li C, Zhou J, et al. Papillary renal neoplasm with reverse polarity: A clinicopathologic study of 7 cases. Int J Surg Pathol (2020) 28(7):728–34. doi: 10.1177/1066896920918289
Keywords: renal cell carcinoma, reverse polarity, papillary renal cell carcinoma, pathology, partial nephrectomy
Citation: Yuzhi Z, Zhen L, Yu X, Boju P, Weigang Y and Xingcheng W (2022) Papillary renal neoplasm with reverse polarity with a favorable prognosis: Two cases report and literature review. Front. Oncol. 12:1011422. doi: 10.3389/fonc.2022.1011422
Received: 04 August 2022; Accepted: 14 October 2022;
Published: 31 October 2022.
Edited by:
Haoran Liu, Stanford University, United StatesReviewed by:
Xiang-hui Ning, First Affiliated Hospital of Zhengzhou University, ChinaSean R. Williamson, Cleveland Clinic, United States
Copyright © 2022 Yuzhi, Zhen, Yu, Boju, Weigang and Xingcheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Weigang, cHVtY2h5d2dAc2luYS5jb20=; Wu Xingcheng, cWlhbnh1bjgwQHNpbmEuY29t
†These authors have contributed equally to this work
 Zuo Yuzhi
Zuo Yuzhi Liang Zhen
Liang Zhen Xiao Yu
Xiao Yu Pan Boju2
Pan Boju2 Yan Weigang
Yan Weigang