
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 16 September 2022
Sec. Thoracic Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1009298
This article is part of the Research Topic Recent Advances in Surgical Management of NSCLC View all 13 articles
Introduction: Although robot-assisted thoracoscopic surgery (RATS) has been widely applied in treating non-small cell lung cancer (NSCLC), its advantages remain unclear for very old patients. The present study compared the perioperative outcomes and survival profiles among RATS, video-assisted thoracoscopic surgery (VATS), and open lobectomy (OL), aiming to access the superiority of RATS for NSCLC patients aged ≥75 years.
Methods: Pathological IA-IIIB NSCLC patients aged ≥75 years who underwent RATS, VATS, or OL between June 2015 and June 2021 in Shanghai Chest Hospital were included. Propensity score matching (PSM, 1:1:1 RATS versus VATS versus OL) was based on 10 key prognostic factors. The primary endpoints were perioperative outcomes, and the secondary endpoints were disease-free (DFS), overall (OS), and cancer-specific survival (CS).
Results: A total of 504 cases (126 RATS, 200 VATS, and 178 OL) were enrolled, and PSM led to 97 cases in each group. The results showed that RATS led to: 1) the best surgical-related outcomes including the shortest operation duration (p <0.001) and the least blood loss (p <0.001); 2) the fastest postoperative recoveries including the shortest ICU stay (p = 0.004), chest tube drainage duration (p <0.001), and postoperative stay (p <0.001), and the most overall costs (p <0.001); 3) the lowest incidence of postoperative complications (p = 0.002), especially pneumonia (p <0.001). There was no difference in the resection margins, reoperation rates, intraoperative blood transfusion, and volume of chest tube drainage among the three groups. Moreover, RATS assessed more N1 (p = 0.009) and total (p = 0.007) lymph nodes (LNs) than VATS, while the three surgical approaches dissected similar numbers of N1, N2, and total LN stations and led to a comparable incidence of postoperative nodal upstaging. Finally, the three groups possessed comparable DFS, OS, and CS rates. Further subgroup analysis found no difference in DFS or OS among the three groups, and multivariable analysis showed that the surgical approach was not independently correlated with survival profiles.
Conclusion: RATS possessed the superiority in achieving better perioperative outcomes over VATS and OL in very old NSCLC patients, though the three surgical approaches achieved comparable survival outcomes.
Lung cancer is one of the most prevalent and deadly malignancies worldwide, and non-small cell lung cancer (NSCLC) occupies 80-85% of total lung cancer morbidities (1). Optimal surgical treatment is critical for patients with resectable NSCLC to achieve good long-term outcomes and is becoming increasingly important given the implementation of lung cancer screening approaches has contributed to the earlier diagnosis of the malignancy (2). However, with the average age at diagnosis of approximately 70 years, most NSCLC patients are elderly and are frequently associated with comorbidities and poor cardiopulmonary functions, which have created great challenges for surgical treatments (3). More importantly, NSCLC patients aged ≥75 years who represent up to 40% of total NSCLC cases are associated with less surgical frequencies, more preoperative comorbidities, increased postoperative complications, and worse long-term outcomes compared with those aged 65-74 years (4, 5). Therefore, great attention should be attached to identifying well-tolerated and oncological effective surgical approaches for these very old populations.
Although open lobectomy (OL) is still the standard surgical approach for resectable NSCLC, it is associated with considerable postoperative complications and even surgery-related mortalities, especially in elderly patients (6). Thus, minimally invasive surgeries (MISs) which could reduce postoperative complications and shorten postoperative hospital stay, such as video-assisted thoracoscopic surgery (VATS), have been widely adopted (7). Numerous studies have suggested that VATS achieved better perioperative outcomes and similar long-term survival compared to OL for older NSCLC patients (8–10). Nowadays, robotic-assisted thoracoscopic surgery (RATS), an innovative MIS with high-quality visualization and great maneuverability which allows surgeons to perform complex operations with great convenience and precision, has been increasingly applied in treating NSCLC (11). Currently, a few studies evaluated the safety and effectiveness of RATS in NSCLC patients aged 65 years or older, suggesting that RATS reduced postoperative complications and noncancer-specific mortalities than OL, and assessed increased lymph nodes (LNs) than VATS (3, 12, 13). However, merely a few patients aged ≥75 years were included in these studies, and the advantages of RATS specified for this important group of populations remain unknown.
The present study retrospectively investigated the perioperative outcomes and survival profiles of RATS, VATS, and OL in NSCLC patients aged ≥75 years, aiming to assess the superiority of RATS for very old NSCLC cases. Propensity score-matched (PSM) analysis was applied to mitigate the patient selection bias.
This study was a single-center retrospective cohort study focusing on NSCLC patients aged ≥75 years who underwent lobectomy at the Department of Thoracic Surgical Oncology, Shanghai Chest Hospital. The Institutional Review Board of Shanghai Lung Tumor Clinical Medical Center, Shanghai Chest Hospital, Shanghai Jiao Tong University approved this study (No. KS1735). All procedures conducted on human participants were following the Declaration of Helsinki (as revised in 2013).
We retrospectively identified NSCLC patients aged ≥75 years receiving lobectomy from June 2015 to June 2021. Preoperative exams including pulmonary function testing, electrocardiogram, and echocardiography were conducted to ensure the operation tolerance of patients. Distant metastasis was evaluated by using positron emission tomography/CT (PET/CT), bone scintigraphy, and cranial enhanced magnetic resonance imaging (MRI). Contrast-enhanced chest CT imaging was conventionally used to assess the mediastinal and pulmonary lymph nodal involvement, and PET-CT, endobronchial ultrasound trans-bronchial needle aspiration (EBUS-TBNA), and/or mediastinoscopy were further applied when CT scan indicated a short-axis >1 cm of lymph nodes for suitable patients. For a few patients who could not tolerate or rejected the invasive assessments, CT scan and/or PET-CT were applied for the preoperative lymph nodal evaluation. The inclusion criteria included: aged ≥75 years, underwent RATS, VATS, or OL combining with systemic LNs dissection, and pathologically diagnosed NSCLC. The exclusion criteria included: malignancy other than NSCLC, surgical methods other than lobectomy, neoadjuvant therapy, and preoperative distant metastasis. A total of 504 cases were finally included and divided into the RATS, VATS, and OL groups. Following data were recorded: clinicopathological characteristics including age, gender, smoking status, body mass index (BMI), preoperative comorbidities, pulmonary functions [% of predicted forced expiratory volume in 1 s (FEV1%) and % of predicted diffusing capacity for carbon monoxide (DLCO%)], anatomic location, tumor size, histological type, visceral pleural invasion, and pathological T (pT), N (pN), and TNM (pTNM) stage; perioperative outcomes including resection margins, operation duration, conversion rates, blood loss, intraoperative blood transfusion, ICU stay, duration and volume of chest tube drainage, length of postoperative stay, overall costs, and postoperative complications; LNs assessment including the number of total dissected lymph nodes (LNs) and LN stations, number of harvested N1 and N2 LNs and LN stations and postoperative nodal upstaging; survival profiles including 1-, 3-, and 5-year disease-free (DFS), overall (OS), and cancer-specific survival (CS). Among 504 NSCLC patients identified in our database, 418 cases were staged by the 8th edition of the tumor-node-metastasis (TNM) staging system of the International Association for the Study of Lung Cancer. However, the other 86 enrolled cases were staged according to the 7th TNM version in the database, and therefore these patients were all restaged by the 8th TNM version based on their postoperative paraffin pathology reports before the analyses and propensity score matching.
RATS, VATS, and OL were conducted according to the procedures described previously (6, 14, 15). Briefly, patients received general anesthesia with double-lumen tracheal intubation and contralateral single-lung ventilation and underwent radical pulmonary lobectomy combined with systemic pulmonary and mediastinal LNs dissection. RATS was performed using the da Vinci Surgical System (Intuitive Surgical, Sunnyvale, CA, USA). For RATS and VATS, 4 incisions were created without rib-spreading. For OL, patients received a conventional rib-spreading thoracotomy through an incision of about 15 cm.
After surgery, patients were discharged from the hospital 1-2 days after removing drainage tubes unless there were comorbidities requiring intervention. Follow-up assessments included thoracic CT and brain MRI scans and were conducted every 3-6 months after the surgery during the first 2-year period and once a year afterward. For patients who did not come to the outpatient clinic regularly, telephone follow-up was performed every 1 year until death or June 2022. Patients lost to follow-up were evaluated based on the latest electronic medical records.
We performed the statistical analysis according to the methods published previously (6, 16–18). Variables were expressed using appropriate descriptive statistics, including frequencies and percentages for categorical variables, and mean ± standard deviation (SD) or median and interquartile range (IQR) for continuous variables. Pearson’s chi-square tests or Fisher’s exact tests with Bonferroni post-hoc tests were applied to compare categorical variables. For continuous variables, the normality of distribution and homogeneity of variance was analyzed by Kolmogorov-Smirnov tests, and analysis of variance (ANOVA) was performed if a normal distribution and homogeneity of variance were assumed. If not, the Kruskal-Wallis rank sum tests were performed to compare the three groups, followed by Dunn’s multiple comparisons tests to correct for multiple comparisons. Wilcoxon rank sum tests were applied to compare the conversion rates of RATS and VATS. Survival profiles were analyzed by Kaplan-Meier curves log-rank (Mantel-Cox) tests. Factors relevant to DFS and OS were further analyzed using multivariable Cox’s regression model analysis. Statistical analysis was conducted using SPSS version 26.0 (IBM Corporation, Armonk, NY, USA), and survival profiles were analyzed using GraphPad Prism 9 (GraphPad Software Inc., San Diego, CA, USA). The p value of less than 0.05 was considered to be statistically significant.
To mitigate potential selection bias, propensity score matching (PSM) was applied to balance baseline confounding features of patients among the three groups using the nearest matching method with a 1:1:1 RATS versus VATS versus OL group ratio. Enrolled patients were matched by the following variables: age, gender, history of smoke, BMI, FEV1%, DLCO%, tumor size, anatomic location, histological type, pT, and pN stage. PSM was conducted using R version 4.1.3 (The R Foundation for Statistical Computing, Vienna, Austria).
The baseline clinicopathological characteristics of patients were expressed in Table 1. Among the three groups, the OL group had the highest proportion males (OL 64.05% vs RATS 53.97% vs VATS 51.50%, p = 0.039), the lowest FEV1% (OL 85.40 ± 16.52 vs RATS 90.23 ± 18.42 vs VATS 89.63 ± 15.94, p = 0.022) and DLCO% (OL 86.11 ± 19.98 vs RATS 89.14 ± 18.71 vs VATS 93.19 ± 18.70, p <0.001), and the largest tumor size (OL 4.05 ± 2.15 vs RATS 2.58 ± 1.16 vs VATS 2.67 ± 1.29 cm, p <0.001). The three groups also differed in the tumor location (p = 0.034), histology type (p <0.001), pT (p <0.001), pN (p = 0.009), and pTNM (p <0.001) stage. Therefore, PSM was used to balance the baseline characteristics of patients among the three groups. Finally, a total of 291 cases were included. As summarized in Table 2, three groups were well balanced with a similar distribution of all included characteristics following the application of PSM.
The perioperative outcomes of enrolled patients were shown in Table 3. Patients who underwent RATS were associated with the shortest operation duration (RATS 100.85 ± 29.06 vs VATS 113.75 ± 33.40 vs OL 112.76 ± 22.85 mins, p <0.001) and the least blood loss (p <0.001). RATS also led to the shortest ICU stay (RATS 0[0-1] vs VATS 1[0-1] vs OL 1[0-1] days, p = 0.004), chest tube drainage duration (RATS 4[3-6] vs VATS 5[4-6] vs OL 5[5-7] days, p <0.001) and postoperative stay (RATS 5[4-6] vs VATS 5[4-7] vs OL 6[5-8] days, p <0.001) among three surgical approaches, and had a similar conversion rate compared with VATS (p = 0.184). However, the overall cost in the RATS group was $14838.26 ± 2841.65, which was significantly higher than that in the VATS ($13190.51 ± 2120.18, p <0.001) and OL ($13429.58 ± 2582.36, p <0.001) group. There was no significant difference in terms of the resection margins (p = 0.608), reoperation rates (p = 0.543), intraoperative blood transfusion (p = 0.377), and volume of chest tube drainage (p = 0.061) among the three groups. Moreover, patients in the RATS group had the lowest incidence of postoperative complications (RATS 30.93% vs VATS 41.24% vs OL 55.67%, p = 0.002). More importantly, patients who received RATS were associated with a significantly lower incidence of pneumonia than those who received VATS (p <0.050) or OL (p <0.050). Finally, there was no in-hospital or 30-day mortality in all three groups.
As expressed in Table 4, OL harvested the highest number of N1 (OL 5.79 ± 3.62 vs RATS 5.10 ± 2.40 vs VATS 4.18 ± 2.78, p <0.001), N2 (OL 7.74 ± 4.29 vs RATS 6.91 ± 4.50 vs VATS 5.63 ± 3.53, p <0.001), and total (OL 13.54 ± 6.05 vs RATS 12.01 ± 5.55 vs VATS 9.81 ± 4.55, p <0.001) LNs. Nevertheless, RATS dissected comparable N1 (p = 0.730), N2 (p = 0.289), and total (p = 0.075) LNs than OL. When comparing the two MISs, RATS assessed a higher number of N1 (p = 0.009) and total (p = 0.007) LNs than VATS, while having no superiority over VATS in assessing N2 LNs (p = 0.056). Finally, three surgical approaches dissected similar numbers of N1 (p = 0.415), N2 (p = 0.298), and total (p = 0.124) LN stations, and also led to a comparable incidence of postoperative nodal upstaging (p = 0.356).
The median follow-up of the RATS, VATS, and OL groups was 43[7-80], 44[3-80], and 53[1-81] months, respectively. In the RATS group, 1-, 3-, and 5-year DFS rates were 96.78%, 80.00%, and 67.72%, respectively, and 1-, 3-, and 5-year OS rates were 95.81%, 87.19%, and 59.26%, respectively (Figure 1). Besides, patients receiving VATS had the 1-, 3- and 5-year DFS rates of 89.69%, 79.33%, and 61.85%, respectively, and possessed the 1-, 3- and 5-year OS rates of 94.85%, 80.90%, and 55.07%, respectively. Moreover, OL led to the 1-, 3- and 5-year DFS rates of 88.51%, 81.58% and 67.46% respectively, and 1-, 3- and 5- OS rates of 95.88%, 77.61% and 59.87%, respectively. The three groups possessed comparable DFS (p = 0.574) and OS (p = 0.704). Moreover, the three surgical approaches also achieved similar CS rates (p = 0.470, Supplementary Figure S1). Further subgroup analyses also suggested no survival profile difference among the three groups in terms of pTNM or pN stage (Supplementary Figure S2). Furthermore, we found that the surgical type was not independently correlated with DFS [hazard ratio = 1.190, p = 0.478; Table 5) or OS (hazard ratio = 1.162, p = 0.480) through multivariable Cox regression analysis. Nevertheless, the LNs metastasis was independently correlated with shortened DFS (HR = 3.785, p <0.001) and OS (HR = 1.857, p <0.001).
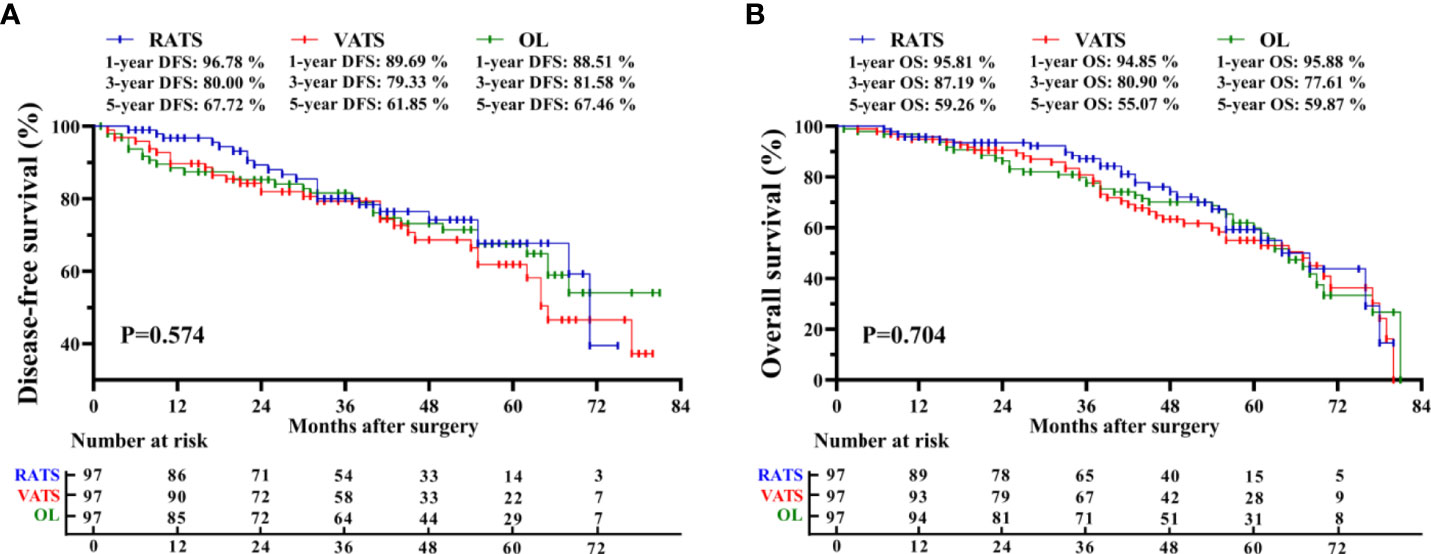
Figure 1 Kaplan-Meier survival curve of matched patients. Comparison of disease-free survival (A) and overall survival (B) among the RATS, VATS, and OL groups. RATS, robotic-assisted thoracoscopic surgery; VATS, video-assisted thoracoscopic surgery; OL, open lobectomy.
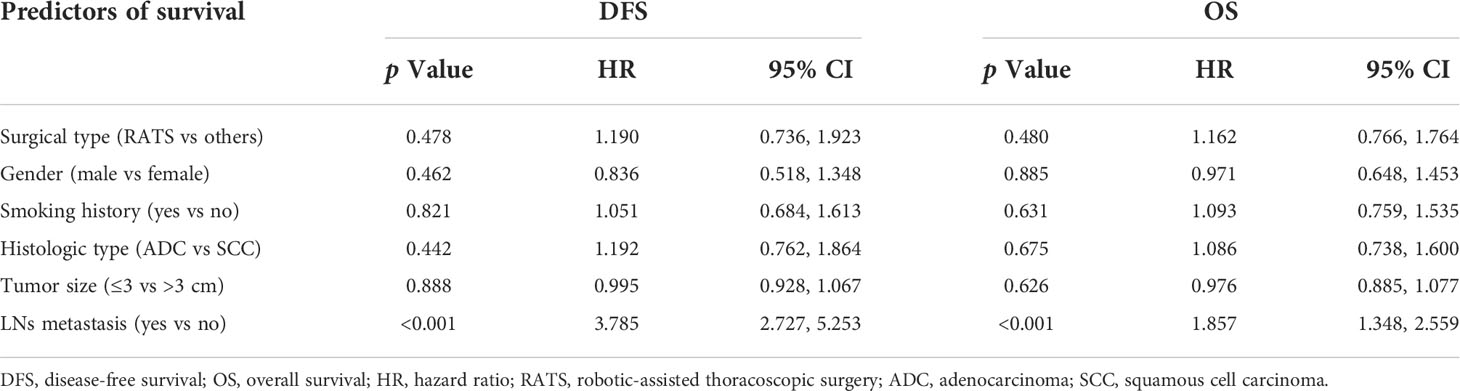
Table 5 Cox’s proportional hazards regression model analysis for survival profiles of matched populations.
The robot-assisted surgical system provides surgeons with wide visibilities through high-definition three-dimensional views, improved dexterity by wide-range motioned mechanical wrists, and better maneuverability by delicate instruments, allowing operators to perform complex operations with great convenience and precision (19, 20). Previous studies have shown that RATS led to better perioperative outcomes than OL and harvested more lymph nodes than VATS, and was also associated with the best cost-effective among the three surgical approaches (3, 21–23). Nowadays, the continuing aged population and increased prevalence of NSCLC have contributed to the rapid growth in the number of older people diagnosed with NSCLC (24). Given the increased incidence of preoperative comorbidities and worsening cardiopulmonary functions when individuals grow older, very old patients more frequently experience postoperative complications, slow recoveries, and poor outcomes than younger individuals, which has promoted critical challenges to surgical resections (15, 25). Although the feasibility and oncological efficacy of RATS in younger NSCLC patients have been widely investigated and well established, the research on RATS for very old NSCLC patients is still limited. Our study compared the perioperative outcomes and survival profiles of RATS, VATS, and OL for NSCLC patients aged 75 years or older, suggesting that RATS led to the best surgical-related outcomes, the fastest postoperative recoveries, and the least postoperative complications, especially postoperative pneumonia, among the three surgical approaches, and also assessed more lymph nodes than VATS. Taken together, our results showed for the first time that RATS possesses the superiority in achieving better perioperative outcomes over VATS and OL in very old NSCLC patients.
The most interesting finding of our study was that RATS led to the lowest incidence of postoperative pneumonia, a prevalent postoperative complication that may be a marker of increased long-term mortality in NSCLC patients undergoing surgery, among the three surgical approaches in NSCLC patients aged ≥75 years (26). Such superiority might be partly attributed to the high-definition visualization and improved dexterity and maneuverability provided by the robotic-assisted surgical system which allowed surgeons to perform surgeries more precisely to avoid causing unnecessary damage (19, 20). Besides, RATS also led to shorter surgical duration and fewer blood loss than VATS, which may mitigate the impact of mechanical ventilation and anesthesia and altered internal environments for patients. More importantly, to the best of our knowledge, it was the first time to find that RATS reduced postoperative pneumonia in old NSCLC patients compared with VATS, which might be attributed to the high incidence of this postoperative complication in the very old patients we enrolled which makes this superiority of RATS more apparent.
When considering surgical-related outcomes, RATS reduced intraoperative blood loss compared with VATS and OL. However, VATS had a similar conversion rate to thoracotomy compared with RATS, and all three groups achieved excellent bleeding control with low incidences of intraoperative blood transfusion. For these reasons, all three surgical approaches appear to be safe and effective with regard to bleeding control for elderly NSCLC patients. Moreover, according to previous studies reported by other surgical teams, the operative time is prevalently longer in robot-assisted surgery than that in VATS or OL due to the additional docking time and the impact of a learning curve (27–29). However, our study indicated that RATS was associated with shortened surgical duration than VATS and OL, which might be attributed to the well-organized surgical team and the experienced operators from a high-volume medical center.
The dissection of LNs is of key importance in the surgical resection of NSCLC. Similar to the results reported by previous studies that enrolled younger patients, the total number of LNs harvested by RATS was 12.01 ± 5.55 in our study, suggesting that LNs dissection using robot-assisted surgical systems may not be significantly affected by growth in ages (11, 15, 30, 31). Nowadays, numerous studies have compared RATS with VATS and/or OL in terms of LNs dissection, but have drawn conflicting conclusions. Jin et al. and Haruki et al. independently reported that RATS harvested more N1 and total LNs than VATS, while other studies indicated that RATS was comparable to VATS with regard to LNs dissection (11, 30). Moreover, by comparing RATS, VATS, and OL, Toker et al. found that RATS dissected more N1 and total LNs than VATS and OL (21). However, Kneuertz et al. reported that RATS, VATS, and OL dissected a similar number of LNs (32). Our study showed that LNs assessed by RATS were comparable to that dissected by OL and more than that harvested by VATS, suggesting that RATS was an effective surgical technic and even superior to VATS regarding LNs assessment in very old NSCLC patients. Although OL harvested the most LNs, the rate of nodal upstaging of the three groups was comparable. This might be explained by most of the enrolled cases had the early-stage disease without nodal involvement and three surgical approaches assessed a similar number of LN stations. The relevance between LNs assessment and long-term survival remains controversial. Dezube et al. and Hennon et al. independently reported that additional LNs dissection conferred no survival benefit for lobectomy, while other studies suggested that increased LNs assessment was associated with better long-term outcomes (33–36). In our study, increased LNs sampling was not correlated with prolonged DFS and OS in very old NSCLC patients undergoing lobectomy, and further follow-up is still necessary to confirm this result.
Although various kinds of preoperative LNs assessment approaches have been prompted, there is still a 3%-15% of rate occult N2 disease identified at the pathological stage (11, 32, 37–40). However, the occult N2 disease could lead to a poor prognosis and therefore influence the survival profiles in our study. In our hospital, mediastinal LNs were systemically assessed by using thoracic CT and PET-CT, and invasive approaches including EBUS-TBNA and mediastinoscopy were further applied when necessary to minimize the incidence of occult N2 disease. Moreover, in our study, the incidence of occult N2 disease in RATS, VATS, and OL groups was 4.12%, 4.12%, and 8.25%, respectively, which was consistent with many previous studies (11, 32, 37–40). Our results also showed that the three groups had comparable incidences of postoperative lymph nodal upstaging. Therefore, the occult N2 disease may not change our survival outcomes.
When considering the oncological effectiveness, our study showed that RATS achieved comparable DFS, OS, and CS as VATS and OL, and further subgroup analysis also indicated similar survival profiles in terms of pTNM and pN stages among three surgical approaches, suggesting that RATS might be an effective surgical method for both early- and advanced-stage resectable NSCLC patients aged ≥75 years. However, our recruitment ended in June 2021 and only a few patients had long-term follow-up data. In our hospital, RATS was performed for the first time in 2009 by our surgical team (which was also the first RAT in China mainland) and widely applied since 2015. The poor surgical tolerances and high surgical risks of very old NSCLC patients have created great challenges for our surgeons, requiring the operators to be experienced and highly skilled, therefore many enrolled patients underwent RATS in recent years. In order to avoid potential bias due to the surgical dates, we included patients who received RATS, VATS, or OL in a similar period. Consequently, long-term follow-up data were available for a few patients. Nevertheless, the median follow-up of the RATS, VATS, and OL groups was 43[7-80], 44[3-80], and 53[1-81] months, respectively, and follow-up of the particular patient was less than 1 year due to his/her death. Therefore, 3-year survival data were available for most patients, and our results suggested that the three groups possessed comparable 1- and 3-year DFS, OS, and CS. More importantly, the primary endpoints of our study were perioperative outcomes and the results showed that RATS possessed the superiority in achieving better perioperative outcomes over VATS and OL in very old NSCLC patients. The DFS, OS, and CS were the secondary endpoints and we are continuing the follow-up and also enrolling more eligible cases currently, aiming to further compare the long-term survival outcomes of RATS, VATS, and OL based on a larger cohort and the longer follow-up data, and the results will be reported afterward. We also noticed that for pathological I stage NSCLC, all three surgical approaches achieved lower 5-year OS than DFS. This was attributed to the fact that a high proportion of elderly patients with early-stage NSCLC died from non-tumor-specific factors, such as cardiovascular diseases, cerebrovascular accidents, and dysfunction of critical organs. Nevertheless, the relapse and metastasis of malignancy was still the major reason contributing to mortalities of II-III stage NSCLC patients in our cohorts.
There are still some limitations of this study. Despite PSM being used, enrolled patients were not randomized before the surgery and the retrospective nature of this study might lead to undiscovered selection bias. Thus, further randomized, controlled trials are necessary to validate the results of our study. Moreover, this study was performed in a single high-volume center, which largely limited the representativeness of participants, thus further multi-center researches are essential to confirm whether the present study could represent real-world practices. Finally, for patients with relapsed disease, the recurrence patterns (locally or distant) and the relevance to surgical approaches were not described, and further studies are needed.
In summary, we retrospectively compared the perioperative outcomes and survival profiles of RATS, VATS, and OL in treating NSCLC patients aged 75 years or older. The results suggested that RATS possessed the superiority in achieving better perioperative outcomes over VATS and OL in very old NSCLC patients, though the three surgical approaches achieved comparable survival outcomes.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Institutional Review Board of Shanghai Chest Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
JH and QL contributed to the study design. HP, ZG, and YT were responsible for interpreting the results. LJ, HZ, and JN contributed to the statistical analysis. JH and QL wrote the manuscript. All authors contributed to data collection and analysis. All authors contributed to the article and approved the submitted version.
This work was supported by the National Nature Science Foundation of China (Grant No. 81972176). The funders were not involved in the study design, collection, analysis, or interpretation of data, the writing of this article, or the decision to submit it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1009298/full#supplementary-material
1. Thai AA, Solomon BJ, Sequist LV, Gainor JF, Heist RS. Lung cancer. Lancet (2021) 398(10299):535–54. doi: 10.1016/S0140-6736(21)00312-3
2. Balata H, Fong KM, Hendriks LE, Lam S, Ostroff JS, Peled N, et al. Prevention and early detection for NSCLC: Advances in thoracic oncology 2018. J Thorac Oncol (2019) 14(9):1513–27. doi: 10.1016/j.jtho.2019.06.011
3. Veluswamy RR, Brown SAW, Mhango G, Nicastri DG, Sigel K, Smith CB, et al. Comparative effectiveness of robotic-assisted surgery for resectable lung cancer in older patients. Chest (2020) 157(5):1313–21. doi: 10.1016/j.chest.2019.09.017
4. Driessen E, Detillon D, Bootsma G, Ruysscher DD, Veen E, Aarts M, et al. Population-based patterns of treatment and survival for patients with stage I and II non-small cell lung cancer aged 65–74 years and 75 years or older. J Geriatr Oncol (2019) 10(4):547–54. doi: 10.1016/j.jgo.2019.03.001
5. Qiang GL, Liang CY, Guo YQ, Shi B, Tian YC, Song ZY, et al. Video-assisted thoracoscopic lobectomy for elderly non-small cell lung cancer: Short-term and long-term outcomes. J Cancer Res Ther (2015) 11(4):793–7. doi: 10.4103/0973-1482.140930
6. Huang J, Tian Y, Li CW, Shen YF, Li HC, Lv FZ, et al. Robotic-assisted thoracic surgery reduces perioperative complications and achieves a similar long-term survival profile as posterolateral thoracotomy in clinical N2 stage non-small cell lung cancer patients: A multicenter, randomized, controlled trial. Transl Lung Cancer Res (2021) 10(11):4281–92. doi: 10.21037/tlcr-21-898
7. Xu JM, Ni H, Wu YH, Cao JL, Han XP, Liu LX, et al. Perioperative comparison of video-assisted thoracic surgery and open lobectomy for pT1-stage non-small cell lung cancer patients in China: A multi-center propensity score-matched analysis. Transl Lung Cancer Res (2021) 10(1):402–14. doi: 10.21037/tlcr-20-1132
8. Mao Y, Gao Z, Yin Y. Complete video-assisted thoracoscopic surgery and traditional open surgery for elderly patients with NSCLC. Front Surg (2022) 9:863273. doi: 10.3389/fsurg.2022.863273
9. Ezer N, Kale M, Sigel K, Lakha S, Mhango G, Goodman E, et al. Outcomes after video-assisted thoracoscopic lobectomy versus open lobectomy for early-stage lung cancer in older adults. Ann Am Thorac Soc (2018) 15(1):76–82. doi: 10.1513/AnnalsATS.201612-980OC
10. Pei G, Zhou S, Han Y, Liu Z, Xu S. Risk factors for postoperative complications after lung resection for non-small cell lung cancer in elderly patients at a single institution in China. J Thorac Dis (2014) 6(9):1230–8. doi: 10.3978/j.issn.2072-1439.2014.07.23
11. Jin RS, Zheng YY, Yuan Y, Han DP, Cao YQ, Zhang YJ, et al. Robotic-assisted versus video-assisted thoracoscopic lobectomy: Short-term results of a randomized clinical trial (RVlob trial). Ann Surg (2022) 275(2):295–302. doi: 10.1097/SLA.0000000000004922
12. Hristov B, Eguchi T, Bains S, Dycoco J, Tan KS, Isbell JM, et al. Minimally invasive lobectomy is associated with lower noncancer-specific mortality in elderly patients: A propensity score matched competing risks analysis. Ann Surg (2019) 270(6):1161–69. doi: 10.1097/SLA.0000000000002772
13. Chen DL, Kang PM, Tao SL, Wu LC, Li QY, Tan QY. Comparative short-term outcomes of robotic-assisted surgery for older patients with non-small cell lung cancer: A propensity matched study. Updates Surg (2021) 74(3):1087–96. doi: 10.1007/s13304-021-00992-x
14. Huang J, Li JT, Li HY, Lin H, Lu PJ, Luo QQ. Continuous 389 cases of da Vinci robot-assisted thoracoscopic lobectomy in treatment of non-small cell lung cancer: Experience in shanghai chest hospital. J Thorac Dis (2018) 10(6):3776–82. doi: 10.21037/jtd.2018.06.80
15. Huang J, Tian Y, Zhou QJ, Ning JW, Gu ZN, Lu PJ, et al. Comparison of perioperative outcomes of robotic-assisted versus video-assisted thoracoscopic right upper lobectomy in non-small cell lung cancer. Transl Lung Cancer Res (2021) 10(12):4549–57. doi: 10.21037/tlcr-21-960
16. Komotar RJ, Starke RM, Raper DM, Anand VK, Schwartz TH. Endoscopic endonasal compared with microscopic transsphenoidal and open transcranial resection of craniopharyngiomas. World Neurosurg (2012) 77(2):329–41. doi: 10.1016/j.wneu.2011.07.011
17. Qian LQ, Chen XK, Huang J, Lin H, Mao F, Zhao XJ, et al. A comparison of three approaches for the treatment of early-stage thymomas: Robot-assisted thoracic surgery, video-assisted thoracic surgery, and median sternotomy. J Thorac Dis (2017) 9(7):1997–2005. doi: 10.21037/jtd.2017.06.09
18. Casazza GC, Thomas AJ, Gurgel RK, Shelton C, Meier JD. Variation in tympanoplasty cost in a multihospital network. Otol Neurotol (2018) 39(10):1047–53. doi: 10.1097/MAO.0000000000001997
19. Kanzaki M. Current status of robot-assisted thoracoscopic surgery for lung cancer. Surg Today (2019) 49(10):795–802. doi: 10.1007/s00595-019-01793-x
20. Mangiameli G, Cioffi U, Testori A. Lung cancer treatment: From tradition to innovation. Front Oncol (2022) 12:858242. doi: 10.3389/fonc.2022.858242
21. Toker A, Ozyurtkan MO, Demirhan O, Ayalp K, Kaba E, Uyumaz E. Lymph node dissection in surgery for lung cancer: Comparison of open vs. video-assisted vs. robotic-assisted approaches. Ann Thorac Cardiovasc Surg (2016) 22(5):284–90. doi: 10.5761/atcs.oa.16-00087
22. Kent MS, Hartwig MG, Vallieres E, Abbas AE, Cerfolio RJ, Dylewski MR, et al. Pulmonary open, robotic and thoracoscopic lobectomy (PORTaL) study: An analysis of 5,721 cases. Ann Surg (2021). doi: 10.1097/SLA.0000000000005115
23. Chen DL, Kang PM, Tao SL, Li QY, Wang RW, Tan QY. Cost-effectiveness evaluation of robotic-assisted thoracoscopic surgery versus open thoracotomy and video-assisted thoracoscopic surgery for operable non-small cell lung cancer. Lung Cancer (2021) 153:99–107. doi: 10.1016/j.lungcan.2020.12.033
24. Takigawa N, Ochi N, Nakagawa N, Nagasaki Y, Taoka M, Ichiyama N, et al. Do elderly lung cancer patients aged ≥75 years benefit from immune checkpoint inhibitors? Cancers (Basel) (2020) 12(7):1995. doi: 10.3390/cancers12071995
25. Tantraworasin A, Siwachat S, Tanatip N, Lertprasertsuke N, Kongkarnka S, Euathrongchit J, et al. Outcomes of pulmonary resection in non-small cell lung cancer patients older than 70 years old. Asian J Surg (2020) 43(1):154–65. doi: 10.1016/j.asjsur.2019.03.006
26. Lugg ST, Agostini PJ, Tikka T, Kerr A, Adams K, Bishay E, et al. Long-term impact of developing a postoperative pulmonary complication after lung surgery. Thorax (2016) 71(2):171–6. doi: 10.1136/thoraxjnl-2015-207697
27. Van der Ploeg APT, Ayez N, Akkersdijk GP, van Rossem CC, de Rooij PD. Postoperative pain after lobectomy: Robot-assisted, video-assisted and open thoracic surgery. J Robot Surg (2020) 14(1):131–6. doi: 10.1007/s11701-019-00953-y
28. O’Sullivan KE, Kreaden US, Hebert AE, Eaton D, Redmond KC. A systematic review and meta-analysis of robotic versus open and video-assisted thoracoscopic surgery approaches for lobectomy. Interact Cardiovasc Thorac Surg (2019) 28(4):526–34. doi: 10.1093/icvts/ivy315
29. Reddy RM, Gorrepati ML, Oh DS, Mehendale S, Reed MF. Robotic-assisted versus thoracoscopic lobectomy outcomes from high-volume thoracic surgeons. Ann Thorac Surg (2018) 106(3):902–8. doi: 10.1016/j.athoracsur.2018.03.048
30. Haruki T, Takagi Y, Kubouchi Y, Kidokoro Y, Nakanishi A, Nozaka Y, et al. Comparison between robot-assisted thoracoscopic surgery and video-assisted thoracoscopic surgery for mediastinal and hilar lymph node dissection in lung cancer surgery. Interact Cardiovasc Thorac Surg (2021) 33(3):409–17. doi: 10.1093/icvts/ivab112
31. Zhang JY, Feng QB, Huang YR, Ouyang LW, Luo FM. Updated evaluation of robotic- and video-assisted thoracoscopic lobectomy or segmentectomy for lung cancer: A systematic review and meta-analysis. Front Oncol (2022) 12:853530. doi: 10.3389/fonc.2022.853530
32. Kneuertz PJ, Cheufou DH, D’Souza DM, Mardanzai K, Abdel-Rasoul M, Theegarten D, et al. Propensity-score adjusted comparison of pathologic nodal upstaging by robotic, video-assisted thoracoscopic, and open lobectomy for non–small cell lung cancer. J Thorac Cardiovasc Surg (2019) 158(5):1457–66. doi: 10.1016/j.jtcvs.2019.06.113
33. Dezube AR, Mazzola E, Bravo-Iñiguez CE, De León LE, Rochefort MM, Bueno R, et al. Analysis of lymph node sampling minimums in early-stage non-small-cell lung cancer. Semin Thorac Cardiovasc Surg (2021) 33(3):834–45. doi: 10.1053/j.semtcvs.2020.11.007
34. Hennon MW, DeGraaff LH, Groman A, Demmy TL, Yendamuriet S. The association of nodal upstaging with surgical approach and its impact on long-term survival after resection of non-small-cell lung cancer. Eur J Cardiothorac Surg (2020) 57(5):888–95. doi: 10.1093/ejcts/ezz320
35. Liang WH, He JX, Shen YX, Shen JF, He QH, Zhang JR, et al. Impact of examined lymph node count on precise staging and long-term survival of resected non-Small-Cell lung cancer: A population study of the US SEER database and a Chinese multi-institutional registry. J Clin Oncol (2017) 35(11):1162–70. doi: 10.1200/JCO.2016.67.5140
36. Chen DL, Mao YM, Wen JM, Shu J, Ye F, She YL, et al. Numbers and stations: Impact of examined lymph node on precise staging and survival of radiologically pure-solid NSCLC: A multi-institutional study. JTO Clin Res Rep (2020) 1(3):100035. doi: 10.1016/j.jtocrr.2020.100035
37. Bille A, Woo KM, Ahmad U, Rizk NP, Jones DR. Incidence of occult pN2 disease following resection and mediastinal lymph node dissection in clinical stage I lung cancer patients. Eur J Cardiothorac Surg (2017) 51(4):674–9. doi: 10.1093/ejcts/ezw400
38. Gallina FT, Melis E, Forcella D, Mercadante E, Marinelli D, Ceddia S, et al. Nodal upstaging evaluation after robotic-assisted lobectomy for early-stage non-small cell lung cancer compared to video-assisted thoracic surgery and thoracotomy: A retrospective single center analysis. Front Surg (2021) 8:666158. doi: 10.3389/fsurg.2021.666158
39. Toosi K, Velez-Cubian FO, Glover J, Ng EP, Moodie CC, Garrett JR, et al. Upstaging and survival after robotic-assisted thoracoscopic lobectomy for non-small cell lung cancer. Surgery (2016) 160(5):1211–8. doi: 10.1016/j.surg.2016.08.003
Keywords: non-small cell lung cancer, robot-assisted thoracoscopic surgery, video-assisted thoracoscopic surgery, open lobectomy, elderly patients, propensity score-matched study
Citation: Pan H, Gu Z, Tian Y, Jiang L, Zhu H, Ning J, Huang J and Luo Q (2022) Propensity score-matched comparison of robotic- and video-assisted thoracoscopic surgery, and open lobectomy for non-small cell lung cancer patients aged 75 years or older. Front. Oncol. 12:1009298. doi: 10.3389/fonc.2022.1009298
Received: 01 August 2022; Accepted: 31 August 2022;
Published: 16 September 2022.
Edited by:
Ugo Cioffi, University of Milan, ItalyCopyright © 2022 Pan, Gu, Tian, Jiang, Zhu, Ning, Huang and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jia Huang, aHVhbmdqaWFkcmFnb25AMTI2LmNvbQ==; Qingquan Luo, bHVvcWluZ3F1YW5AaG90bWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.