- 1Unit for Psycho-Oncology and Health Psychology, Department of Psychology and Behavioural Sciences, Aarhus University, Aarhus, Denmark
- 2Sleep and Circadian Psychology Research Group, Department of Psychology and Behavioural Sciences, Aarhus University, Aarhus, Denmark
- 3Aarhus Institute of Advanced Studies, Aarhus University, Aarhus, Denmark
- 4Department of Medical Social Sciences, Northwestern University Feinberg School of Medicine, Chicago, IL, United States
Cancer patients experience a number of co-occurring side- and late-effects due to cancer and its treatment including fatigue, sleep difficulties, depressive symptoms, and cognitive impairment. These symptoms can impair quality of life and may persist long after treatment completion. Furthermore, they may exacerbate each other’s intensity and development over time. The co-occurrence and interdependent nature of these symptoms suggests a possible shared underlying mechanism. Thus far, hypothesized mechanisms that have been purported to underlie these symptoms include disruptions to the immune and endocrine systems. Recently circadian rhythm disruption has emerged as a related pathophysiological mechanism underlying cancer- and cancer-treatment related symptoms. Circadian rhythms are endogenous biobehavioral cycles lasting approximately 24 hours in humans and generated by the circadian master clock – the hypothalamic suprachiasmatic nucleus. The suprachiasmatic nucleus orchestrates rhythmicity in a wide range of bodily functions including hormone levels, body temperature, immune response, and rest-activity behaviors. In this review, we describe four common approaches to the measurement of circadian rhythms, highlight key research findings on the presence of circadian disruption in cancer patients, and provide a review of the literature on associations between circadian rhythm disruption and cancer- and treatment-related symptoms. Implications for future research and interventions will be discussed.
1 Introduction
Cancer patients suffer from a range of co-occurring side- and late-effects associated with cancer and/or its treatment including fatigue (1), sleep difficulties (2), depressive symptoms (3, 4) and cognitive impairment (5). These cancer- and treatment-related symptoms (CTRS), sometimes described as the “cancer symptom cluster” (6), have a range of negative implications for patients such as delaying cancer treatments, impacting treatment adherence, and detrimental effects on quality of life (7) and daily life functioning (8). Symptoms can be present prior to treatment (9–13), may often worsen during treatment (9, 14–16), and for a large subset, may persist well beyond treatment completion (4, 7, 17–21). Furthermore, CTRS may exacerbate each other’s intensity and development over time (22). The co-occurrence and interdependent nature of these symptoms suggests a possible shared underlying mechanism (23, 24), and while the importance of investigating these symptoms together has been emphasized (25), most research has had a single-symptom focus. Hence, mechanisms underlying CTRS remain unclear.
To date, the predominant hypothesis of a shared underlying mechanism for CTRS has been based on an immune system response (24, 26, 27) as presented in the “sickness behavior model” (6, 28). Sickness behaviors are physiological and behavioral changes, such as fatigue, disturbed sleep and mood, and impaired cognition (6, 27–29) that occur in reaction to an immune response and the release of proinflammatory cytokines such as tumor necrosis factor–α (TNF-α), interleukin (IL)-6 and IL-1β (30). It is generally accepted that inflammation plays an important role in tumorigenesis and that tumor development leads to an intrinsic inflammatory immune response (31). Evidence also suggests that cancer is associated with both immunostimulation and immunosuppression with increased concentrations of various cytokines including TNF-α and IL-6 (32). During the course of treatment, a strong additional inflammatory response may be triggered by both local and systemic therapies such as surgery, radiotherapy and chemotherapies (31, 33, 34). Cancer and treatment-induced immune responses and the release of peripheral proinflammatory cytokines may induce central inflammation mediated by microglial activation within the brain, which can lead to behavioral and cognitive deficits (35). While a meta-analysis supports the sickness behavior model, the strength of association between markers of inflammatory responses and the CTRS varies (30). Furthermore, this model does not generally account for why and how CTRS may persist well beyond the disease and treatment completion, nor does it readily translate into targeted interventions.
Another proposed mechanism of CTRS relates to disruption of the endocrine system and most notably that of the hypothalamic-pituitary-adrenal (HPA) axis. Heightened and chronic stress associated with the cancer disease and its treatment may impact the HPA axis resulting in altered cortisol secretion patterns, which have been associated with CTRS (12, 36, 37). In particular, studies have shown diurnal variations to be altered with evidence of associations between flatter diurnal cortisol slope and more severe CTRS (36, 38, 39). While these lines of evidence underscore the importance of HPA dysregulation as an underlying mechanism of CTRS, these findings may also be closely linked with dysfunction of another fundamental system – the circadian system. Diurnal variations in cortisol reciprocally interact with circadian mechanisms within the brain (40), and thus, disrupted diurnal variations in cortisol may reflect underlying disruptions to this biological timing system.
2 Circadian disruption in cancer survivors
Recently, circadian rhythm disruption has emerged as an important and related pathophysiological mechanism underlying CTRS (41–44). Circadian rhythms are endogenous biobehavioral cycles lasting slightly longer than 24 hours in humans and generated by the circadian master clock (45) – the hypothalamic suprachiasmatic nucleus (SCN) (46). The SCN orchestrates rhythmicity in a wide range of bodily functions including rest-activity behaviors, body temperature, immune response, and hormone levels (46, 47). The unique role of circadian rhythms in CTRS is perhaps best demonstrated in animal models in which disturbance of the master clock has resulted in sleep disturbance (48–50), altered mood-related behaviors (51–53) and cognitive impairment (54, 55). In cancer patients, several lines of evidence also support the possible role of circadian disruption in the development of CTRS as will be highlighted in further detail below.
A major appeal of a circadian disruption hypothesis of CTRS is that the expression and regulation of the previously proposed mechanisms of CTRS are reciprocally related to the circadian system. For example, research points to a bidirectional link between circadian rhythms and inflammatory processes (56, 57). On the one hand, the inflammatory immune response may be caused by disrupted circadian rhythms (58). Higher circulating levels of proinflammatory cytokines have been observed in cancer patients with disrupted activity rhythms (59). On the other hand, circadian disruption may occur due to the impact of cytokines on the SCN. Animal studies have shown that proinflammatory cytokines can produce phase shifts in activity rhythms (60), and that TNF-α has a suppressing effect on clock genes with detrimental effects on the circadian system (61). More than a decade ago, there was a call for studies to examine inflammatory responses and circadian rhythms in relation to CTRS to clarify associations and identify points of therapeutic intervention (27).
A bidirectional link between the endocrine and the circadian system is also supported by research. Various endocrine factors are shown to be under direct circadian control (62), including hormones produced by the HPA axis (40, 63), and there’s accumulating evidence to show that chronic disruption of the circadian system may lead to disorders of metabolic, reproductive and mood systems (64). Emerging evidence also suggests that endocrine feedback may play a role in the entrainment of the circadian system. In this regard, altered endocrine functioning has been implicated in the disruption of circadian rhythms likely mediated by altered glucocorticoids and metabolic hormones (65).
Behavioral and psychological alterations following cancer diagnosis and treatment may also independently impact the circadian system either directly through behavioral changes, such as reduced exposure to light (66) or indirectly through the aforementioned pathophysiological mechanisms. There are also well-known bidirectional links between sleep and the immune system (67) with evidence suggesting that both disrupted sleep and long sleep duration is associated with increased systemic inflammation (68). Other psychosocial factors, including stress, anxiety, and depression, are also known to have bidirectional associations with the immune system (69, 70).
Taken together, a circadian disruption hypothesis of CTRS is not only compatible with other predominant pathophysiological models but adds to them by highlighting the potentially key modulatory role of the circadian system in the manifestation of CTRS (see Figure 1). Furthermore, the appeal of the circadian system as an underlying mechanism lies in its modifiability, as it can be targeted in both pharmacological (e.g., melatonin administration) (71) and non-pharmacological interventions (e.g. light therapy) (72, 73) with the potential to stabilize multiple biobehavioral systems and ultimately lead to symptom reduction and improved quality of life.
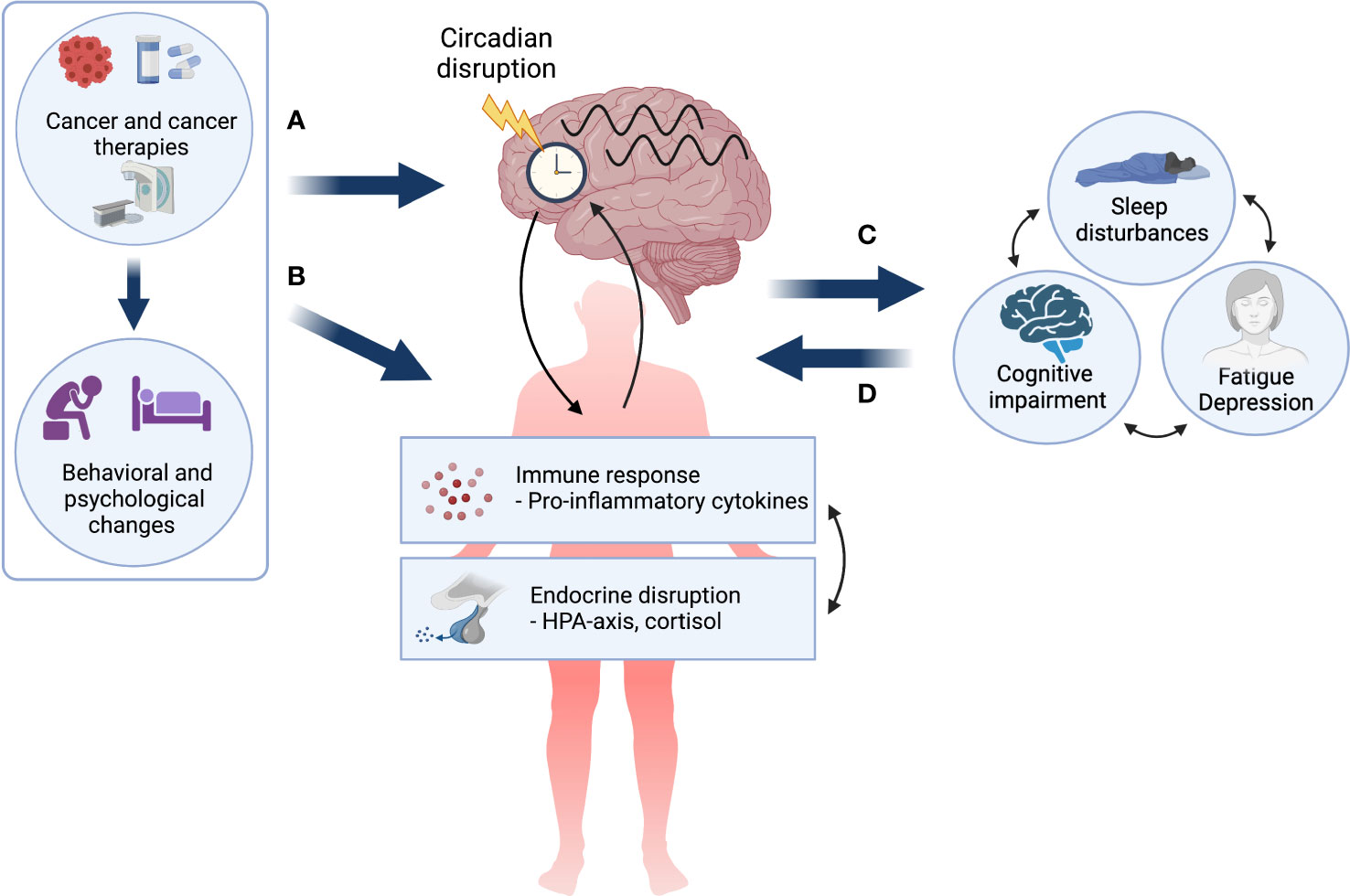
Figure 1 The circadian disruption hypothesis of cancer- and cancer treatment-related symptoms. Cancer and its treatment, as well as associated behavioral and psychological changes may (A) directly impact the circadian system resulting in circadian disruption in both biological and behavioral rhythms, and (B) lead to a dysregulated immune response and endocrine disruption, which are themselves bidirectionally linked and may both impact the circadian system. Circadian disruption may result in cancer- and treatment-related symptoms (CTRS) or exacerbate pre-existing symptoms (C). Finally, it is important to note that once manifested, chronic CTRS burden may further alter both behavioral and pathophysiological factors creating a self-perpetuating negative loop (D). Created with BioRender.com.
In the present review, we aim to highlight key research findings of the presence of circadian disruption in cancer patients and provide a detailed review of associations between circadian rhythm disruption and CTRS. Methods of assessment related to CTRS, including patient-reported outcome measures, as well as behavioral and performance-based approaches will be briefly described below. Furthermore, assessment of circadian rhythms through measurement of secretion patterns of melatonin and cortisol, rest-wake activity, and 24-hour body temperature, will be described. Finally, implications for future research and potential interventions to strengthen the circadian system will be discussed.
3 Cancer- and treatment-related symptoms
In this section, each of the CTRS will be described and the main methodologies discussed.
3.1 Fatigue
Fatigue is among the most prevalent symptoms of cancer and cancer treatment and refers to a “distressing, persistent, subjective sense of physical, emotional, and/or cognitive tiredness related to cancer treatment that is not proportional to recent activity and interferes with usual functioning” (74). It is estimated that between 70 – 90% of cancer patients undergoing radio- or chemotherapy will experience fatigue, and although the number decreases over time, long-term fatigue is prevalent in approximately 30% (75).
The vast majority of studies measuring cancer-related fatigue use patient-reported outcome measures. Although research has identified several biomarkers of fatigue including immune, metabolic, and neuroendocrine markers (76), fatigue is inherently subjective and, thus, most appropriately captured by self-reported measures. Measures of fatigue can be either one- or multi-dimensional. An example of a one-dimensional measure is the widely used Functional Assessment of Chronic Illness Therapy – Fatigue (FACIT-F) (77). An example of an often-used multi-dimensional measure of fatigue is the Multidimensional Fatigue Symptom Inventory (MFSI) (78), which distinguishes between general, emotional, physical, and mental fatigue, as well as vigor.
3.2 Sleep problems
Sleep problems are also highly prevalent both during and years after cancer treatments with estimates ranging from 30-50% (79). A variety of methods exist for the assessment of sleep outcomes spanning from patient-reported to actigraphy-based to EEG-defined sleep with polysomnography (PSG). Although the latter method is considered the gold standard to measure objective sleep, PSG is both costly and time-consuming, and therefore less frequently applied in CTRS research.
Because insomnia is subjectively defined, patient-reported measures of sleep quality and insomnia severity have been extensively used in the literature with established cut-offs for determining clinical levels of sleep disturbances. The most widely used measures of patient-reported insomnia severity and sleep quality are the Insomnia Severity index (ISI) and the Pittsburgh Sleep Quality Index (PSQI), which have both been shown to be valid and reliable measures in cancer populations (80, 81).
Another patient-reported measure of sleep behavior can be collected through sleep diaries that require patients to fill out details about the timing and duration of various sleep-related behaviors such as time spent trying to fall asleep, early- and night-time awakenings, and overall time spent in bed. Diaries allow for the extraction of common sleep metrics including sleep onset latency (SOL), wake after sleep onset (WASO), early awakenings (EA), time in bed (TIB), total sleep time (TST), and sleep efficiency (SE).
Actigraphy is yet another measure of sleep behavior often used in cancer populations as it is relatively cost-effective and easy to use, allowing for continuous measurement across longer time periods (82, 83). While actigraphy does not allow for the direct measurement of sleep, rest-activity patterns are good indicators of the timing and duration of sleep (84) and allow for the calculation of common sleep metrics such as SE, WASO and TST. Sleep diaries are often concomitantly collected with actigraphy to edit the rest-activity data.
3.3 Depression symptoms
Both during and after cancer treatment, many patients suffer from high psychological distress including symptoms of depression, which may last for years (85). Depending on the method of assessment, prevalence rates across cancer types have been reported to range between 8 – 24% (86). While individual clinical interviews are considered the gold standard for diagnosing depression, due to time- and resource limits, symptoms of depression are most commonly assessed by using validated and reliable self-report scales. Examples of these include the Hospital Anxiety and Depression Scale (HADS) (87) and the Center for Epidemiologic Studies Depression Scale (CES-D) (88), but many more exist (89).
3.4 Cognitive impairment
Cognitive impairment refers to changes in mental functions and abilities such as memory decline, and impaired attention and executive functioning. Impairments to cognition are highly prevalent and distressing, and often associated with treatments such as chemotherapy and antihormonal treatment (90), as well as with the cancer disease itself (91), although the underlying mechanisms are still poorly understood.
A neuropsychological test battery is considered the “gold standard” measure of domain-specific cognitive functions. The test battery consists of a range of different standardized and performance-based cognitive tests to assess a patient’s strengths and cognitive weaknesses. Guidelines have been published by the International Cancer & Cognition Task Force with recommended tests to be used in the field of cancer (92).
Although neuropsychological tests are considered to be robust measures of cognitive function, their use is often limited as they are time-consuming and their proper administration requires specialized training. Therefore, self-report measures of cognitive functions are widely used in the research literature using various instruments. A review from 2018 reported considerable diversity in cognitive measures used and found that the two items from the European Organisation for Research and Treatment of Cancer QLQ-C30 (EORTC QLQ-C30) were the most often used items (93). Other common measures included the Functional Assessment of Cancer Therapy-Cognitive Function (FACT-Cog) (94) and the Cognitive Failures Questionnaire (CFQ) (95).
One major limitation of self-report measures of cognitive function is that they are often poor correlates of performance-based neuropsychological tests (96) and instead tend to be more indicative of psychological distress (93). In order to strengthen the scientific rigor of the use of self-report measures of cognitive function, recent recommendations of their use have also been published (97).
4 Assessment of circadian rhythms in cancer patients
Circadian rhythm research in cancer patients has typically focused on the measurement of four key markers of circadian rhythms: melatonin, cortisol, activity, and body temperature. Their measurement is described in detail below.
4.1 Measurement of melatonin rhythms
Melatonin (5-methoxy-N-acetyltryptamine) is a circadian hormone synthesized in the corpus pineale and regulated by the SCN in response to light information received directly through the retinohypothalamic tract (98, 99). As a result of direct anatomical connections between the SCN and the pineal gland, the circadian rhythm of melatonin is considered the best peripheral estimator of the timing of the internal circadian pacemaker (100). In normally entrained individuals, melatonin secretion has a clear circadian rhythm characterized by low levels secreted during the day and a peak in the early morning. Levels typically rise between 8 p.m. and 11 p.m. reaching acrophase between 2 a.m. and 4 a.m. and returning to baseline levels between 8 a.m. and 10 a.m. (101).
The measurement of melatonin concentrations can be undertaken in plasma, serum, urine and/or saliva. For the assessment of circadian phase, plasma is considered the method of choice due to higher values compared with saliva (101). In order to accurately capture the circadian rhythm, it is important to collect samples at regular intervals (e.g., every hour) during the 24-hour day. High frequency blood sampling, thus, requires indwelling canulla in a hospital or laboratory setting. Saliva sampling, on the other hand, is non-invasive and can be undertaken at home, but the drawback is that patients need to be awake during normal sleeping hours for night samples. Alternatively, routine urine sampling in 2 to 8 hour intervals can be used for the measurement of the major metabolite of melatonin, 6-sulphatoxymelatonine. However, given the longer sampling intervals, this method is less accurate when measuring the circadian phase of melatonin secretion (101, 102). The dim light melatonin onset (DLMO) protocol is widely used to assess the melatonin phase. DLMO requires repeated melatonin assessment usually from saliva samples taken every 30 to 60 minutes during evening hours to capture the phase of the evening rise. Although melatonin levels in saliva are generally stable, enabling individuals to store samples at home until delivery to a laboratory, rather strict conditions for collection of samples need to be adhered to that can affect sample quality. For example, while research suggests that 1 hour sampling may be as accurate as 30 minute sampling schemes (103), it is important to initiate sampling several hours before the expected rise. In addition, saliva collection typically needs to occur under dim light conditions or wearing blue light blocking glasses in order to avoid photic melatonin suppression. Individuals also need to avoid food and water 10-15 minutes before sampling times (102, 104, 105), and certain foods, products and drugs ideally ought to be avoided during, at minimum, the sampling period, due to interactions with melatonin levels (including caffeine, alcohol, bananas, chocolate, toothpaste, beta-blockers and non-steroidal anti-inflammatory drugs) (106–111).
4.2 Measurement of cortisol rhythms
Cortisol is a glucocorticoid circadian hormone regulated by the HPA axis (112). Cortisol rhythms tend to be diurnal with levels rising early in the morning, then decreasing over the course of the day (113).
The measurement of circadian rhythms in cortisol can be obtained by frequent 24 hour blood serum and plasma sampling (114, 115). However, given the invasive nature of this sampling method, salivary cortisol is the most common method of measuring the amount of unbound, biologically active cortisol in the blood. Most studies use repeated daytime measurements to assess diurnal cortisol rhythms, and thus, possible HPA dysregulation (38). Depending on the variable of interest, different sampling schemes have been recommended. Most commonly used variables include the cortisol awakening response (CAR) (116), diurnal slope (117) and area under the curve (AUC) (118). Irrespective of the variable of interest, it is recommended to collect daily samples on two consecutive days at each time point to increase reliability. For the measurement of CAR specifically, a minimum of three morning samples has been recommended with the first sample being collected at personal awakening time and then 30 and 45 minutes later (119). For diurnal cortisol rhythms, there are unfortunately, as yet, no published consensus guidelines, but the literature recommends the collection of three to six samples across the day for diurnal variables including AUC (117).
4.3 Measurement of activity rhythms
In cancer patients, circadian rhythms have mainly been investigated through examination of rest-wake activity rhythms (120). The analysis of inactivity/activity is translated into rest/wake and is based on the observation that there is less movement during rest (or sleep) periods and more movement during wake periods. The rhythm of locomotor activity across the 24 hour day has been described as the circadian activity rhythm (121).
Rest-wake activity is typically measured using an actigraph, a device similar in size to a watch and worn on the wrist. It provides a convenient way to approximate rest versus wake states continuously for 24-hours a day for days, weeks, or even longer (82). A number of circadian parameters can be derived from rest-wake spans including mesor, amplitude, acrophase, rhythm quotient, circadian quotient, peak activity, R-squared, F-statistics, circadian quotient, interdaily stability, intradaily variability, 24-h autocorrelation (r24), and a dichotomy index (I<O, which is the percentage of activity in-bed that is less than the median activity out-of-bed) (44, 120, 122). See section 4.5 for further details.
4.4 Measurement of body temperature rhythms
Core body temperature is another robust marker of the circadian system (123). Core body temperature in homeothermic organisms is regulated around a narrow temperature range with its own distinct rhythm and with an amplitude plateauing between 2 p.m. and 8 p.m. and a minimum temperature in the early morning (124, 125). While the core body temperature rhythm is tightly controlled by the SCN and plays an important role in the coordination of peripheral clocks, the SCN itself has been shown to be resistant to temperature entrainment (126). Research has also shown that the sleep-wake cycle is closely associated with circadian body temperature rhythms (127). In healthy individuals, the sleep period usually occurs when the core temperature curve is decreasing and ends with the rising phase of the curve.
It has been argued that there is no gold standard for the measurement of core body temperature (128). Nevertheless, core body temperature has traditionally been measured in a variety of different sites such as the rectum, the mouth, and the tympanic membrane (128). Continuous measurement of temperature in these sites requires patients to be awake, making it less optimal for 24-hour rhythm assessments. Recently, the development of wireless data loggers has facilitated noninvasive and continuous assessment of both proximal and distal skin temperature without the active involvement of participants (129). While proximal skin temperature (e.g. forehead, thigh, stomach) is positively correlated with core body temperature, distal skin temperature (e.g. hands, feet) is inversely associated with core body temperature (130). It is also known that distal skin temperature is phase advanced with respect to core body temperature (129), suggesting that heat loss from extremities may drive the circadian rhythm of core body temperature.
4.5 Methodological considerations for the analysis of circadian markers
Depending on the methods of assessment and sampling rate frequencies, various methods and statistical approaches exist for analyzing rhythmic data to determine important rhythm parameters and circadian rhythm disruption. While it is beyond the scope of the present paper to review all approaches, a few key approaches will be highlighted here.
Both parametric and non-parametric approaches have been developed to analyze circadian rhythm markers (131). An example of the former is cosinor analyses, which use the method of least squares to fit a cosine curve to periodic 24 hour data. Common metrics derived from this method to analyze markers of circadian rhythms (e.g. melatonin, cortisol, rest-activity, and temperature) include the mesor, the rhythm-adjusted mean; the amplitude, the difference between the peak and the wave mean; the period, the duration of one cycle; and the acrophase, the time of day of peak activity. Another variable sometimes reported and that represents overall circadian rhythm robustness is the pseudo F-statistic, which is based on the residuals from cosine fitting models (132, 133).
A limitation of the above methods to assess circadian rhythms, however, is that there are no established cut-offs or thresholds to readily determine circadian disruption. Thus, circadian disruption is often operationalized by employing general linear models to assess between-group differences or changes over time in these measures (134, 135). Furthermore, the application of cosinor-based methods may be better suited to some circadian markers than others. Although commonly used with actigraphy-based rest-activity assessments, motor activity patterns, for example, do not typically resemble a sinusoid, and thus, other approaches have been warranted (131, 136).
To overcome some of these challenges, non-parametric approaches to circadian activity rhythms have been developed with the aim to assess intra-daily variability as a marker of sleep-wake cycle disturbances, and inter-daily stability as a marker of circadian entrainment (131). One promising approach in cancer populations has been the use of the dichotomy index (I<O). The I<O is a measure of the relative amount of activity in-bed below the median of activity out-of-bed (137). Lower I<O is considered to reflect weaker rest-activity rhythmicity (138) and studies have shown that lower I<O is associated with poorer outcomes in cancer patients (136, 139). A strength of the I<O is the reporting of general cut-off values. An I<O value close to 100% is indicative of non-disrupted rest-activity rhythms as seen in healthy subjects, whereas a median value of 97.5% has been reported in cancer patients and considered the threshold for circadian rest-activity disruption (140). Finally, more sophisticated non-parametric approaches have also been applied to rest activity data, such as Hidden Markov Modelling that can i) threshold activity into different states in a probabilistic way and in a time dependent manner, ii) capture square wave forms observed in activity data alongside heterogeneous ultradian variances in human activity, and iii) can generate circadian rhythm parameter estimates based on probabilities of transitions between rest and activity (141).
Finally, given that circadian markers are often measured continuously across time, dynamical modelling that describe the state of the rhythm as a function of time capturing the ongoing fluctuations or change in the rhythms may also be applied, although in practice these approaches are less widely used (142).
5 Circadian rhythm disruption in cancer patients: Key research findings
In the following section, key research findings related to the assessment of each of the circadian markers in cancer populations will be presented and associations with CTRS will be reviewed.
5.1 Melatonin levels in cancer patients
Disrupted melatonin rhythms have been observed in a wide variety of diseases (143–146). Unfortunately, research regarding the effects of cancer and cancer treatments on circadian melatonin rhythms have been sparse, possibly due to the aforementioned methodological challenges associated with assessing melatonin rhythms. However, there are notable and relatively consistent patterns of findings from the few, small studies that exist. A recent study that compared salivary melatonin levels in newly diagnosed prostate cancer patients with controls found that the cancer patients had lower melatonin levels compared with the controls (147). Breast cancer patients have also been found to excrete lower levels of melatonin from 24-hour urine samples (148) and have exhibited suppressed nocturnal peak, mesor, and amplitude of serum melatonin when compared with benign patient groups (149). Melatonin rhythms and secretion levels have also been examined over the course of cancer treatment. Among early-stage breast and ovarian cancer patients receiving chemotherapy, studies have found significant reductions in the level of night-time melatonin over the course of chemotherapy (150, 151). Melatonin has also been examined in other cancer types including cervical cancer (152), lung cancer (153, 154), and colorectal cancer (155). Typically, these studies have found lower melatonin concentrations than patient or healthy control groups, though two studies found differences from healthy controls in circadian melatonin profiles as well, including a flatter slope (152). Using a DLMO protocol, a small recent study found indications for earlier melatonin secretion in gastrointestinal cancer patients with disrupted activity rhythms (140). However, it ought to be mentioned that inter-subject variability was markedly larger for cancer patients than controls, and such variability highlights a potential weakness of the DLMO protocol.
5.1.1 Melatonin and CTRS
Few studies have specifically investigated the association between circadian melatonin rhythms and CTRS (see Table 1). Chang and colleagues (154) investigated diurnal variation in salivary melatonin in newly-diagnosed lung cancer patients prior to treatment compared with matched healthy controls. Although lung cancer patients evidenced lower melatonin levels and flatter diurnal slopes than controls, there were no significant associations observed between melatonin slope or melatonin levels and sleep quality, symptoms of depression, or fatigue. In another study (156), serum melatonin levels were investigated in a group of newly diagnosed breast cancer patients. Pre-surgical levels were negatively associated with self-reported symptoms of depression, while melatonin levels post-surgery were negatively associated with daytime sleepiness. Clearly, more research is needed with the aim of prospectively investigating associations between the development of CTRS and melatonin rhythms. Although there are evident methodological challenges in capturing circadian melatonin rhythms, the DLMO protocol may be useful for capturing the slope of dim-light melatonin secretion and phase shifts in cancer-patients throughout the cancer treatment trajectory (102).
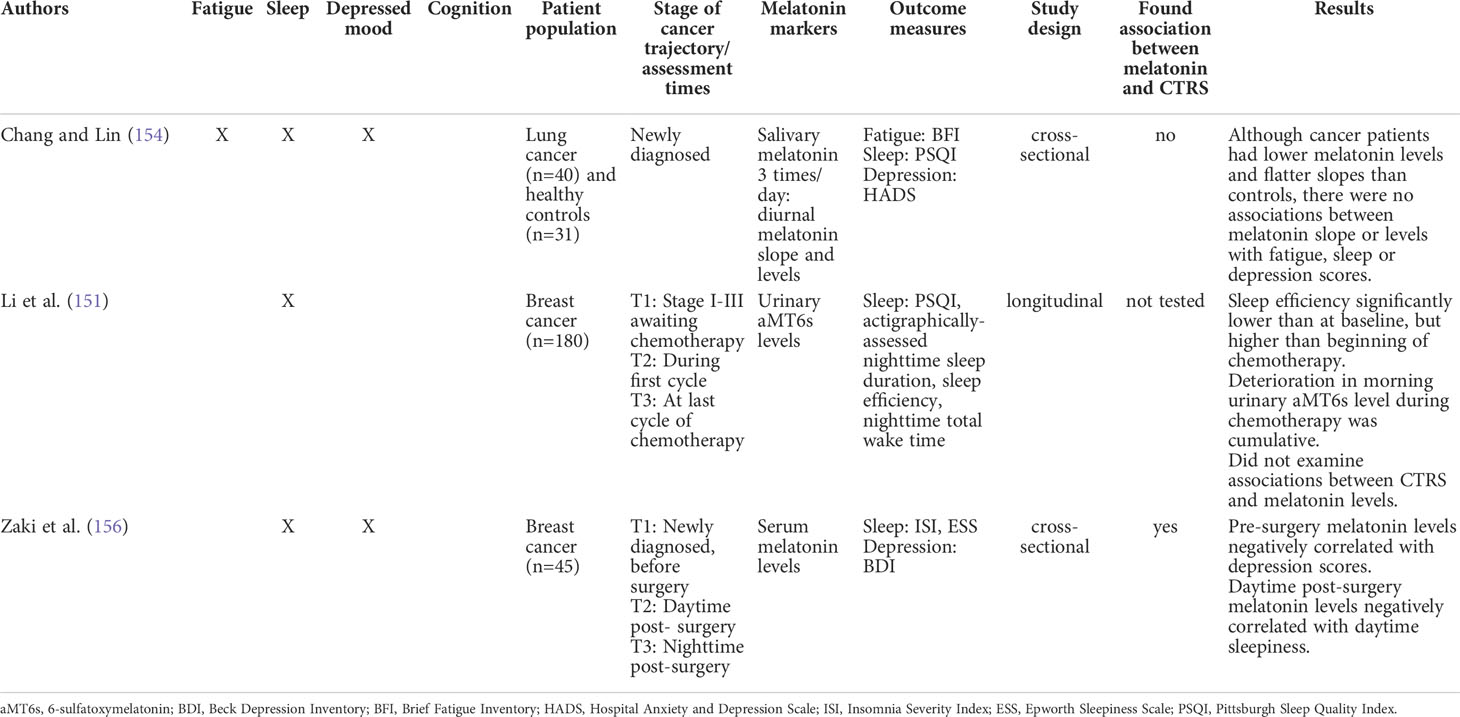
Table 1 Summary of studies that examined melatonin and cancer- and treatment-related symptoms (CTRS).
5.2 Cortisol levels in cancer patients
In a broad array of studies focused predominantly on breast cancer and ovarian cancer patients, increased disruption to cortisol rhythms or secretion levels has been found based on comparisons with control groups or patients at an earlier stage of disease. The predominant finding is that compared with comparison groups, the primary cancer groups tend to experience elevations in mean or nocturnal cortisol levels (38, 157–159) and flatter diurnal cortisol rhythms (38, 157, 159). A study that followed ovarian cancer patients prior to primary treatment to 1 year post-treatment, found that patients showed significant reductions in nocturnal salivary cortisol secretion and plasma IL-6 and a more normalized diurnal cortisol rhythm at 6 months with changes maintained at 1 year (160). In studies of lung cancer patients, similar findings of loss of circadian rhythmicity have been found when compared with healthy controls (161, 162).
5.2.1 Cortisol and CTRS
Research focused on cortisol and CTRS has primarily focused on salivary cortisol (as opposed to urinary, serum or plasma cortisol) and examined diurnal cortisol slope, cortisol awakening response or cortisol levels at a particular point in time (e.g., morning or nocturnal levels) (see Table 2). Numerous studies have examined associations between markers of cortisol rhythms and depressed mood in cancer patients at different stages of the cancer trajectory, primarily among breast cancer patients, but also among lung, colorectal, gynecologic, and prostate cancer patients (38, 59, 154, 160, 164–168, 170–172, 175, 177, 179, 180). The findings have been equivocal with many studies finding no association, including among newly diagnosed lung, endometrial and breast cancer patients (154, 175, 179), advanced breast cancer patients (115), and breast cancer survivors (164, 166, 180). Others have found associations, including associations between evening cortisol levels in ovarian cancer patients and depressive symptoms both before and after primary treatment (38, 160, 172), higher morning cortisol levels in women with metastatic breast cancer (177), and reduced diurnal variation in cortisol levels among depressed advanced metastatic cancer inpatients compared with those who were non-depressed (170). The cortisol awakening response has also been found to be blunted in depressed metastatic breast cancer patients compared with those who were non-depressed (165). In contrast, a study by Kuhlman (171) found the opposite; the cortisol awakening response positively predicted changes in depressed mood over time in early stage breast cancer patients. Sephton also found, contrary to expectations, that accentuated diurnal cortisol rhythms were associated with greater depressed mood (177).
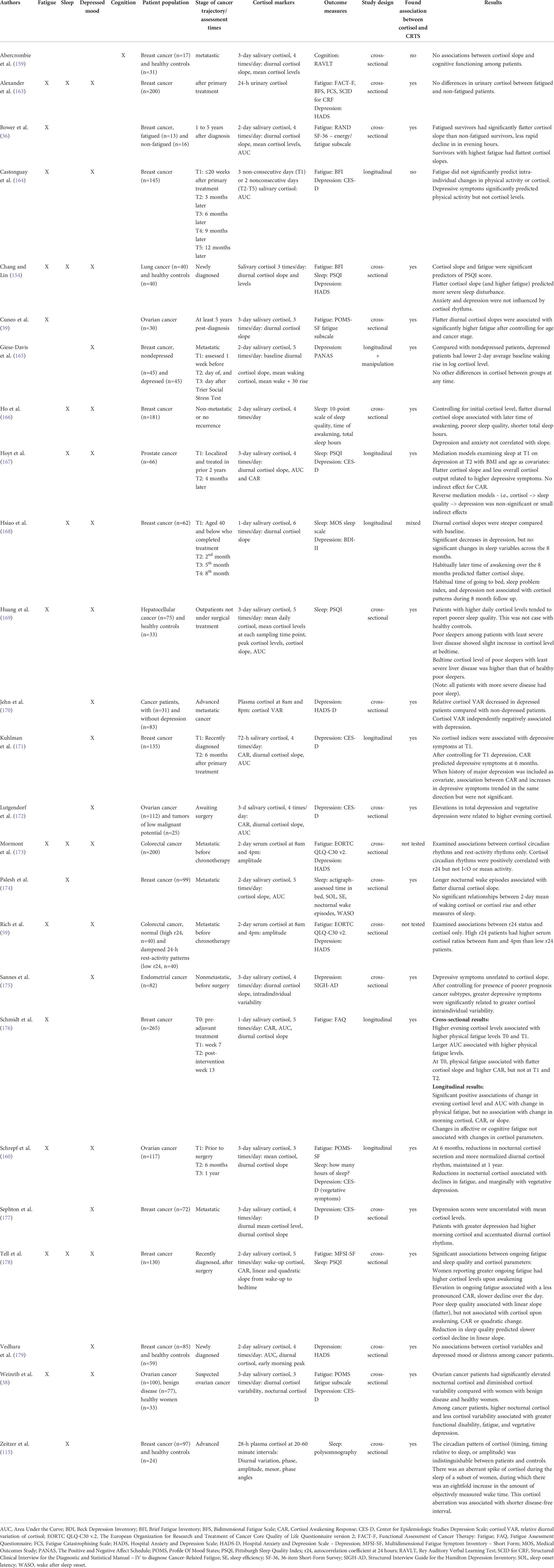
Table 2 Summary of studies that examined cortisol and cancer- and treatment-related symptoms (CTRS).
More consistent associations between markers of cortisol rhythms and fatigue and sleep quality have been found (36, 38, 39, 115, 154, 160, 166, 174, 176, 178). Flatter diurnal cortisol slopes have been associated with greater fatigue in breast cancer patients post-surgery (178), pre-adjuvant treatment (176), and 1 to 5 years after diagnosis (36) and in ovarian cancer survivors (39), as well as poorer sleep quality in breast cancer survivors (166, 174, 178) and among newly diagnosed lung cancer patients (154). Higher cortisol upon awakening has also been associated with fatigue in breast cancer patients evaluated post-surgery (178) and higher daily cortisol levels with poorer sleep quality among hepatocellular cancer patients (169). In a large, longitudinal study of 265 breast cancer patients undergoing adjuvant therapies (176), higher evening cortisol levels were associated with higher physical fatigue both pre-adjuvant therapy and 7 weeks later. Importantly, this study evaluated changes in cortisol levels over time and found associations between changes in evening cortisol levels and AUC with changes in physical fatigue from pre-adjuvant therapy to 13 weeks later, though neither morning cortisol, the cortisol awakening response, nor slope were associated with fatigue.
Highlighting the interrelationships between different CTRS, Hoyt (167) found that lower cortisol output and a flatter diurnal slope accounted for 45-57% of the effect of sleep quality at study entry upon depressed mood 4 months later in prostate cancer survivors.
Not all studies have found associations between cortisol rhythms and CTRS. For example, a large study (n=200) of breast cancer patients after primary therapy that measured 24-hour urinary cortisol instead of diurnal salivary cortisol, found no differences between fatigued and non-fatigued patients (163). Abercrombie etal. (159) investigated metastatic breast cancer patients and found no association between cortisol slope and cognition.
5.3 Activity rhythms in cancer patients
Circadian activity rhythm disruption has been detected across the cancer trajectory. Soon after diagnosis, many cancer patients undergo surgery. In one study of 60 endometrial cancer patients, significant rest-activity disruption (as measured by lower mesor and weaker amplitude) 1 week and 1 month post-surgery was found, with significant recovery on all parameters by 4 months post-surgery (181). Furthermore, the cancer group had more impaired rhythms than a reference group at 1-week post-surgery suggesting that surgery may also be associated with circadian disruption. A large majority of research in this area has focused on circadian activity rhythm disruption associated with chemotherapy, particularly in breast cancer patients. In one such longitudinal study, circadian impairments were examined in breast cancer patients before and during chemotherapy (182). Ninety-five women scheduled to receive neoadjuvant or adjuvant anthracycline based chemotherapy for stage I-III breast cancer wore wrist actigraphs for 72 consecutive hours pre-chemotherapy, and during weeks 1, 2 and 3 of cycles 1 and 4 of chemotherapy. Compared to baseline, amplitude, mesor, up-mesor, down-mesor, and rhythmicity were all significantly impaired during the first week of both chemotherapy cycles with some recovery during weeks 2 and 3. However, most variables remained significantly more impaired than baseline during weeks 2 and 3 of cycle 4. These findings were corroborated by another longitudinal study that included a cancer-free control group (14). One hundred and forty-eight women with stage I-III breast cancer scheduled to receive at least 4 cycles of chemotherapy and matched cancer-free controls participated. Circadian activity rhythm data was collected via 72 consecutive hour actigraphy before the start of chemotherapy, at the end of cycle 4 of chemotherapy, and 1 year after the start of chemotherapy. R-squared was the circadian outcome of interest indicating rhythm robustness. At baseline, breast cancer patients had more disrupted rhythms than the controls. At cycle 4, the cancer patients had more disrupted rhythms compared to their own baseline levels and to controls. At 1 year, cancer patients’ circadian activity rhythms did not differ from non-cancer controls. The number of chemotherapy cycles also appear to be important. One study examined rest-activity in newly diagnosed breast cancer patients during chemotherapy cycles (183). Average scores of all rhythm parameters (i.e., mesor, amplitude, acrophase, rhythm quotient, circadian quotient, peak activity, dichotomy index, and autocorrelation coefficient) significantly decreased with an increasing number of chemotherapy cycles. In addition, activity rhythm disruptions during chemotherapy are likely to peak at the start of the cycles and decrease during the periods between cycles (120).
Other studies have found circadian activity rhythm disruptions in other cancer populations or associated with other cancer treatments (including mixed cancer patients undergoing chemotherapy and/or radiation therapy, colorectal cancer patients undergoing chemotherapy, gynecologic cancer patients undergoing chemotherapy, and breast cancer patients undergoing endocrine therapy). Such studies have generally shown disruptions to circadian parameters when compared with pre-treatment, the beginning of treatment, with cancer controls, or with healthy controls (184–189). Studies have also investigated activity rhythms in lung cancer populations at different stages of the cancer trajectory (190–192). In one longitudinal study of 82 newly diagnosed lung cancer patients undergoing cancer treatment (193), sleep-wake rhythms were assessed at baseline prior to treatment and at four subsequent time points at weeks 6, 12, 24, and 48. While poorer sleep-wake rhythms were observed at baseline, significant improvements were observed at week 48.
Even years after cancer treatment, circadian activity rhythm alterations have been detected. One small scale study of breast cancer survivors found circadian activity rhythm alterations 5 years after primary diagnosis when compared with a healthy control group (194).
Overall, numerous studies suggest that circadian activity rhythms may be disrupted prior to, during and after cancer treatment. In addition, a recent scoping review of actigraphy-based circadian activity rhythms revealed that up to 55% of patients with advanced cancer had disrupted activity rhythms (195).
5.3.1 Activity rhythms and CTRS
Numerous studies have elucidated potential associations between important circadian rhythm markers and various CTRS, typically through the use of actigraphy over 24 to 72 hours of continuous measurement (see Table 3). Studies on the associations between circadian activity rhythms and CTRS have been undertaken in cancer populations across the cancer trajectory.
Several studies have revealed associations between circadian activity disruption and CTRS prior to treatment onset (115, 196, 199, 200, 204–206). More disrupted circadian activity rhythms have been found to be associated with greater depressed mood prior to treatment among head and neck cancer patients and lung cancer patients (200, 201). A study of metastatic colorectal cancer patients prior to chronotherapy also found that patients with a high r24 coefficient (i.e., greater regularity) had fewer fatigue symptoms than those with a low r24 coefficient (59). In a study of breast cancer patients prior to chemotherapy, lower mesor (i.e, mean level of activity) was associated with worse sleep quality and higher sleep onset latency (199). A study of a mixed group of cancer patients before treatment, also found a limited number of significant correlations between circadian activity rhythm markers and sleep quality (204). However, another study of breast cancer patients scheduled for chemotherapy did not find associations between circadian activity rhythms and CTRS of fatigue, sleep quality or depression (196).
Many studies have examined circadian activity rhythms and CTRS during cancer treatment (14, 122, 183, 185, 188, 190, 193, 198, 201, 208). An early study by Roscoe and colleagues (207) directly examined and found significant temporal associations between increases in circadian activity disruption across cycles of chemotherapy and increases in depression and fatigue among breast cancer patients undergoing chemotherapy. Another study focused on depression, this time in lung cancer patients, found associations between disrupted sleep-activity rhythms and worse depression among outpatients prior to chemotherapy, but not among inpatients during chemotherapy (201). A subsequent study by Liu and colleagues of 148 Stage I-III breast cancer patients undergoing chemotherapy, also found that more disrupted circadian activity rhythms were significantly associated with increases in fatigue (122). Other cross-sectional studies have had similar findings (188, 191).
Disturbances to circadian rhythms have also been associated with CTRS post-treatment. For example, in a cross-sectional study by Chen and colleagues (192) of 106 lung cancer patients, poorer circadian function, including a lower dichotomy index, was associated with poorer objective sleep quality. A recent study examined circadian activity rhythms and cognition in breast cancer patients during and after treatment. There was a significant group-by-time effect in self-reported, but not objective cognition when compared with matched controls. Changes in objective cognitive functioning were positively associated with changes in circadian rhythmicity (i.e., a decrease in cognitive functioning at follow-up was predicted by reduced circadian activity rhythm robustness, worsening sleep quality, and increases in nap time compared to baseline (197).
Finally, among cohorts of advanced cancer patients, significant associations have been detected between disrupted circadian activity rhythms and fatigue (202, 205), depressed mood (205), and poorer subjective sleep/sleep quality (203, 206).
5.4 Temperature rhythms in cancer patients
Thus far, research on circadian temperature rhythms in cancer patients has been sparse. In one small observation study of 9 breast cancer survivors (209), circadian core body temperature was measured using an ingested radio telemetry pill. Results were suggestive of circadian disruption of skin temperature in all participants. However, due to the lack of a comparison group, larger controlled studies are indicated. Another small study involving 10 breast cancer patients receiving chemotherapy used wireless skin surface temperature patches on the front thorax (210). Half of the patients exhibited disrupted circadian skin surface temperature rhythms following chemotherapy. In a recent study, significantly deteriorated chest surface temperature rhythms were observed in gastrointestinal cancer patients (N = 25) with disrupted activity rhythms as indicated by a low dichotomy score (< 97.5%) compared with patients without such disruptions (140).
5.4.1 Temperature rhythms and CTRS
To the best of our knowledge, no studies have specifically examined the relationship between circadian temperature rhythms and CTRS.
6 Discussion
This review describes key findings of studies that have examined circadian rhythms in cancer patients and associations with CTRS. The majority of studies focused on circadian activity rhythm disruptions in cancer patients and many found associations between activity rhythm disruptions and fatigue, sleep and depressed mood. A number of studies also examined cortisol and CTRS in cancer, particularly by examining diurnal variation or cortisol levels. The findings were more mixed, especially with respect to associations with depressed mood. However, apart from a couple of exceptions, more consistent associations were found between indicators of cortisol disruption (including flatter diurnal cortisol slopes and higher cortisol levels at different times of the day) and fatigue and sleep outcomes. Few studies examined melatonin levels in cancer patients across time, and even fewer examined associations with CTRS, which is surprising given the current interest in exogenous melatonin as a potential antiproliferative agent for some cancers (211). Cognition was rarely examined in any of the reviewed studies, with only one finding associations between circadian activity rhythm disruption and cognitive impairment.
For the most part, the reviewed studies have focused on one or maybe two approaches to the assessment of circadian rhythms. Studies in this area would likely benefit from a multi-modal approach to the assessment of circadian rhythms, e.g., through the use of advanced actigraphy that includes measurement of multiple markers, such as activity and skin temperature rhythms. In addition, longitudinal studies assessing multiple circadian rhythms and associations with CTRS over time would provide richer data regarding the nature and strength of these associations. Furthermore, the inclusion of health or non-cancer control groups would provide the field with a clearer picture of circadian rhythm changes, and associations with side effects and symptoms that are unique to the cancer patient experience. The field would also benefit from further work to develop an operationalized standard for what a normative healthy circadian rhythm ought to be, so that there are clearer cut-offs for determining clinically significant circadian rhythm disruption. In conclusion, given the potential modifiability of the circadian system through enhancement of both photic and non-photic zeitgebers, targeting the circadian system in the treatment of CTRS is a fertile area for future research.
Overall, we have highlighted the important role that the circadian system may play in the manifestation of CTRS. A limitation of this review is that we did not review the potential role of circadian disruption on mortality. Indeed, there have been numerous seminal studies that have found associations between circadian markers and mortality in cancer patients, and that deserve mention due to their obvious relevance to this topic (e.g., 136, 173, 212, 213). Pioneering work by Mormont and colleagues (173) examined circadian rest-activity rhythms in 192 metastatic colorectal cancer patients receiving chronomodulated chemotherapy after failure of a first treatment protocol. Survival at two years was five times higher in patients with stronger activity rhythms (I<O in upper quartile) than those with weaker activity rhythms (I<O in lower quartile). A later study reinforced these findings in 192 previously untreated metastatic colorectal cancer patients undergoing chronomodulated chemotherapy (139). A pooled study of 436 patients that included the aforementioned cohorts plus an additional cohort of colorectal cancer patients, the majority of whom had failed prior chemotherapy for metastatic disease, confirmed that I<O was a robust predictor of overall survival, particularly among those with an I<O above 97.5% (136). Important studies focusing on cortisol markers in cancer patients have also found associations with survival. In studies by Sephton and colleagues (213, 214) that examined 104 metastatic breast cancer patients and 62 lung cancer patients, diurnal cortisol slope positively predicted survival after seven and across three years respectively. However, these findings need to be considered in light of poor correlation between cortisol concentrations in the serum and in saliva, particularly in the case of metastatic colorectal cancer (212). Thus, future studies would benefit from further examination of associations between rigorous markers of circadian rhythms and survival, in addition to CTRS.
A further limitation of this review is that it focused on circadian rhythm disruption at the physiological and behavioral levels. We did not examine disruption at molecular and/or cellular levels. For example, there is research showing that clock gene variations, particularly to NPAS2, CLOCK, RORA, RORB, and PER3, may contribute to small but statistically significantly elevated cancer risk (215). In addition, disrupted cellular signaling pathways in cancer patients (e.g., of the mechanistic target of rapamycin [mTOR]) may be controlled by the circadian clock (216), and thus may also underlie CTRS.
6.1 Future directions and conclusion
Overall, this review suggests that circadian rhythms may be disrupted in cancer patients, and that such disruptions may contribute to the development and persistence of CTRS. In this regard, the circadian system offers a potential modifiable target for a variety of pharmacological and non-pharmacological interventions that aim to normalize circadian rhythms and, thus, ameliorate CTRS. Importantly, synchronization of circadian rhythms to the external environment occurs through entrainment via exposure to environmental “zeitgebers” or time-givers. Such zeitgebers include bright light, which potently drives the SCN rhythm, and non-photic zeitgebers (e.g., physical activity, timing of eating), which may drive rhythms of peripheral systems (217–219). Under healthy conditions, the central SCN rhythm directly coordinates peripheral rhythms through endocrine and autonomic nervous system signals and regulation of core body temperature, and indirectly through feedback from activity and feeding rhythms (219). Misalignment occurs if the central rhythm is misaligned to the light/dark cycle or if central and peripheral rhythms are not aligned with each other (220), which can impair the homeostasis of the body (219) and potentially contribute to CTRS. Importantly, the receptivity of circadian rhythms to zeitgebers illustrates how the circadian system is inherently modifiable, making it an attractive intervention target. Thus, the enhancement of central and peripheral zeitgebers may be a pathway to improving circadian health in cancer patients and, in turn, CTRS. In this regard, the optimization of the timing of multiple zeitgebers in cancer patients through what we term “Chrono-Behavioral Therapy” may be an approach worth investigating in future research (as conceptualized in Figure 2 below).
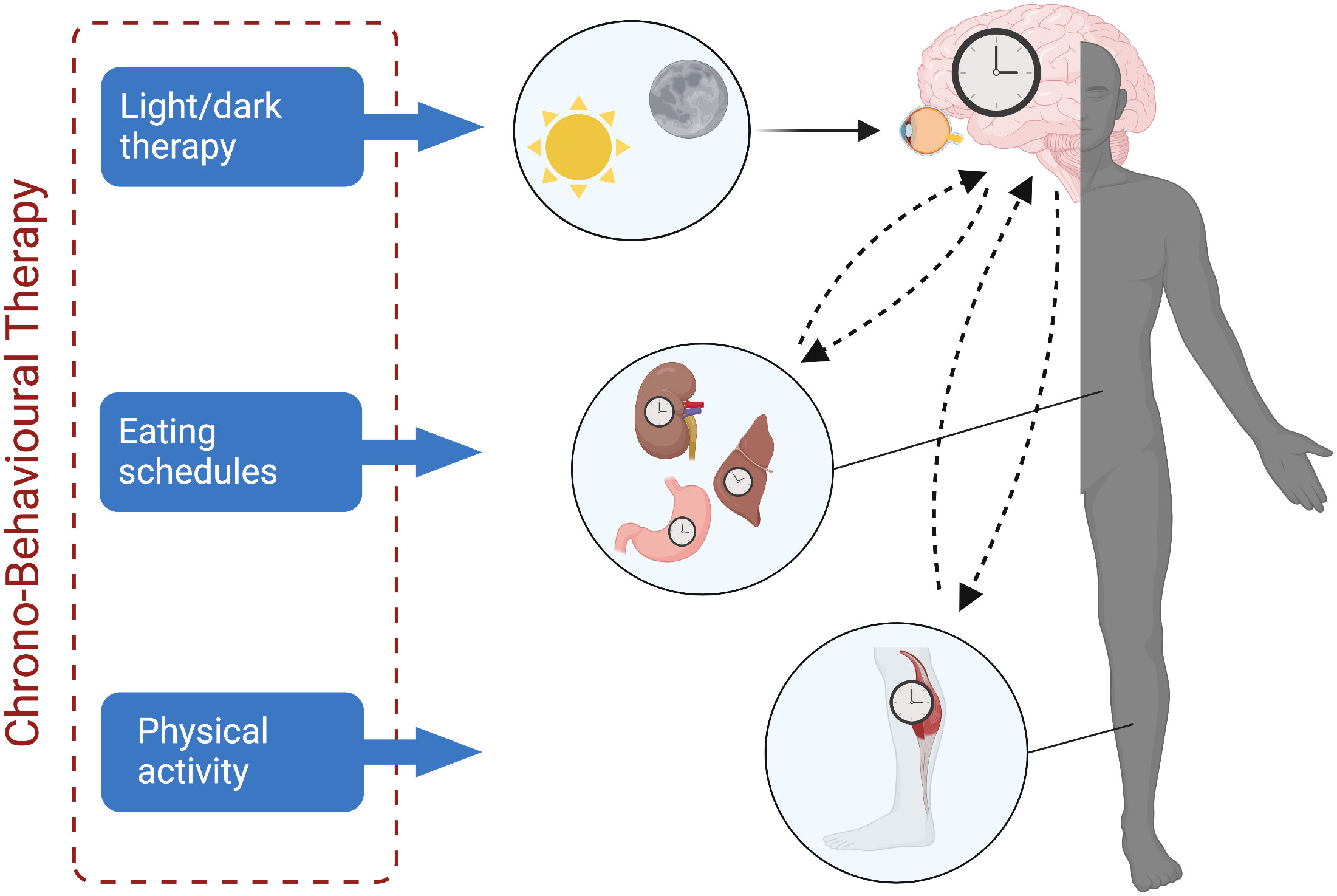
Figure 2 Entrainment of central and peripheral clocks through targeted interventions. Strengthening of the circadian system through direct entrainment of the central clock (i.e., the suprachiasmatic nucleus) may occur through implementation of light/dark therapy; entrainment of peripheral clocks may occur through interventions that target the timing of eating and physical activity. Created with BioRender.com.
Light (both natural and artificial) is the strongest, direct zeitgeber of the SCN (i.e., the central clock of the circadian system), and has been used as a therapeutic tool to treat other disorders, including seasonal affective and other mood disorders for decades already (221). Thus, it is not a surprise that there has been a particular focus on light and its association with CTRS. A study by Liu and colleagues (66) assessed circadian activity rhythms with actigraphy in breast cancer patients who were undergoing chemotherapy. Increased fatigue was significantly associated with decreased light exposure, possibly due to patients spending less time outdoors in bright light. This work triggered a range of intervention studies that tested the use of light exposure to treat CTRS (72, 73, 222–225). In general, protocols instruct cancer patients to use a light box or glasses emitting circadian stimulating light each morning upon waking for 30-45 minutes for 4 weeks or during treatment in order to improve the robustness of the circadian system. Results have shown that light therapy can prevent fatigue and depression in cancer patients undergoing treatment (222, 224), and ameliorate fatigue and improve sleep in cancer survivors after primary treatment (72, 73, 223, 226). Unfortunately, these studies have generally not been sufficiently powered to determine if circadian rhythms mediate light therapy’s effect on CTRS, but one study did determine that bright light therapy protected breast cancer patients from experiencing circadian activity rhythm deterioration during chemotherapy (227).
Another potential area of work focuses on enhancement of peripheral zeitgebers including the timing of physical activity and the timing of eating. Physical activity is a strong non-photic zeitgeber for the mammalian circadian clock (228) likely in part due to effects on central clock genes in skeletal muscles that regulate biological processes (229). Non-photic zeitgebers may support the circadian system through associative learning processes that engage circadian time as a conditioned stimulus (217, 230). In addition, non-photic behavioral zeitgebers tend to be salient to the individual and can serve as a “gatekeeper” to photic zeitgebers (i.e., light/dark exposure) (217). Indeed, there is evidence that physical activity, particularly at night, can phase delay circadian rhythms (i.e., shift the circadian rhythm to later) (231–234). A recent systematic review also confirmed exercise’s phase-shifting properties across studies (235). The timing of eating is another potential peripheral zeitgeber of the circadian system (219, 236) via homeostatic effects on core body temperature (237). Importantly, metabolic dysfunction is a comorbidity of many types of cancers and implicated in peripheral fatigue (238). Furthermore, circadian misalignment can occur if food intake occurs during the dark phase, resulting in systemic metabolic dysregulation (219, 239, 240). Both animal and human research indicates that later timing of food intake may result in negative health outcomes (241–243). Indeed, a recent study found that night eating during the COVID-19 pandemic was associated with greater swings in fatigue (244).
In the field of psychiatry, attempts have already been made to harness the power of peripheral zeitgebers through a therapeutic approach called “interpersonal social rhythm therapy,” originally developed to treat patients with bipolar disorder (245). The therapy is based on the hypothesis that bipolar disorder arises due to dysregulated neurotransmitter systems and perturbations in the circadian system, and therefore focuses on behavioral techniques to improve the regularity of a person’s daily routines. Thus far, interpersonal social rhythm therapy has been found to be feasible and satisfactory in patients with bipolar disorder, but has not yet been proven to be efficacious as more rigorous randomized controlled studies are yet to be undertaken (246). It has not yet been evaluated in cancer patients.
A final point to consider is the potential of telemonitoring for the assessment of circadian rhythms and CTRS in the future. For the most part, the measurement approaches described in the studies reviewed in this paper are not used in routine clinical practice, likely due to the difficulties and expense of collecting and tracking patient data in real-time. However, in recent years, the rapid evolution of wearable sensor technology, E-Health applications, and cloud-based computing have made the implementation of new IT-based health care management methods possible (247, 248). Indeed, a number of recent studies have demonstrated the feasibility of telemonitoring of circadian markers (including rest-wake and biological) and patient-reported outcomes of cancer patients in their own homes (140, 249–252). Thus, the effect of an increased interest in circadian rhythms and health combined with the wave of popularity of new health monitoring technology, has provided the research and health care community with optimal conditions for telemonitoring research to grow. Furthermore, such work would likely form a solid basis for a precision health approach to cancer patient care into the future.
Thus far, the medical field has already attempted to harness the circadian system in cancer treatment itself through chronotherapy approaches that time drug delivery to the appropriate phase of the circadian rhythm with varying degrees of success (215). Our review adds to that important work by summarizing the increasing body of work linking circadian disruption with CTRS, and thus, it highlights the potential of the circadian system as an important target for clinical monitoring and interventions in the future with the ultimate goal of improving cancer patients’ quality of life.
Author contributions
AA and LW were responsible for conceptualization of this review and contributed to the scientific analysis of existing research. Both authors contributed equally to manuscript preparation, and read and approved the final submitted version.
Funding
AA’s effort was supported by grants from the Danish Cancer Society (R174-A11447-17-S52) and Independent Research Fund Denmark (5053-00220B). LW’s effort was supported by the European Union’s Horizon 2020 Research and Innovation Programme under the Marie Sklodowska-Curie grant agreement no. 754513 and the Aarhus University Research Foundation, as well as by the American Cancer Society award number 131642-RSG-18-053-01-PCSM.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR. Cancer-related fatigue: The scale of the problem. Oncologist (2007) 12 Suppl 1:4–10. doi: 10.1634/theoncologist.12-S1-4
2. Lowery-Allison AE, Passik SD, Cribbet MR, Reinsel RA, O’Sullivan B, Norton L, et al. Sleep problems in breast cancer survivors 1–10 years posttreatment. Palliat. Support. Care (2018) 16, 325–34. doi: 10.1017/S1478951517000311
3. Christensen S, Zachariae R, Jensen AB, Vaeth M, Møller S, Ravnsbaek J, et al. Prevalence and risk of depressive symptoms 3-4 months post-surgery in a nationwide cohort study of Danish women treated for early stage breast-cancer. Breast Cancer Res Treat (2009) 113:339–55. doi: 10.1007/s10549-008-9920-9
4. Maass SWMC, Roorda C, Berendsen AJ, Verhaak PFM, de Bock GH. The prevalence of long-term symptoms of depression and anxiety after breast cancer treatment: A systematic review. Maturitas (2015) 82:100–8. doi: 10.1016/j.maturitas.2015.04.010
5. Janelsins MC, Kesler SR, Ahles TA, Morrow GR. Prevalence, mechanisms, and management of cancer-related cognitive impairment. Int Rev Psychiatry (2014) 26:102–13. doi: 10.3109/09540261.2013.864260
6. Cleeland CS, Bennett GJ, Dantzer R, Dougherty PM, Dunn AJ, Meyers CA, et al. Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? a cytokine-immunologic model of cancer symptoms. Cancer (2003) 97:2919–25. doi: 10.1002/cncr.11382
7. Schmidt ME, Chang-Claude J, Vrieling A, Heinz J, Flesch-Janys D, Steindorf K. Fatigue and quality of life in breast cancer survivors: Temporal courses and long-term pattern. J Cancer Surviv. (2012) 6:11–9. doi: 10.1007/s11764-011-0197-3
8. Calvio L, Peugeot M, Bruns GL, Todd BL, Feuerstein M. Measures of cognitive function and work in occupationally active breast cancer survivors. J Occup. Environ Med (2010) 52:219–27. doi: 10.1097/JOM.0b013e3181d0bef7
9. Liu L, Fiorentino L, Natarajan L, Parker BA, Mills PJ, Sadler GR, et al. Pre-treatment symptom cluster in breast cancer patients is associated with worse sleep, fatigue and depression during chemotherapy. Psychooncology. (2009) 18:187–94. doi: 10.1002/pon.1412
10. Van Onselen C, Aouizerat BE, Dunn LB, Paul SM, West C, Hamolsky D, et al. Differences in sleep disturbance, fatigue and energy levels between women with and without breast pain prior to breast cancer surgery. Breast (2013) 22:273–6. doi: 10.1016/j.breast.2012.07.007
11. Lange M, Giffard B, Noal S, Rigal O, Kurtz J-E, Heutte N, et al. Baseline cognitive functions among elderly patients with localised breast cancer. Eur J Cancer (2014) 50:2181–9. doi: 10.1016/j.ejca.2014.05.026
12. Amidi A, Wu LM, Agerbæk M, Larsen PL, Pedersen AD, Mehlsen M, et al. Cognitive impairment and potential biological and psychological correlates of neuropsychological performance in recently orchiectomized testicular cancer patients. Psychooncology. (2015) 24:1174–80. doi: 10.1002/pon.3804
13. Doong S-H, Dhruva A, Dunn LB, West C, Paul SM, Cooper BA, et al. Associations between cytokine genes and a symptom cluster of pain, fatigue, sleep disturbance, and depression in patients prior to breast cancer surgery. Biol Res Nurs. (2015) 17:237–47. doi: 10.1177/1099800414550394
14. Ancoli-Israel S, Liu L, Rissling M, Natarajan L, Neikrug AB, Palmer BW, et al. Sleep, fatigue, depression, and circadian activity rhythms in women with breast cancer before and after treatment: A 1-year longitudinal study. Support. Care Cancer (2014) 22:2535–45. doi: 10.1007/s00520-014-2204-5
15. Sanford SD, Beaumont JL, Butt Z, Sweet JJ, Cella D, Wagner LI. Prospective longitudinal evaluation of a symptom cluster in breast cancer. J Pain Symptom Manage (2014) 47:721–30. doi: 10.1016/j.jpainsymman.2013.05.010
16. Hsu H-T, Lin K-C, Wu L-M, Juan C-H, Hou M-F, Hwang S-L, et al. Symptom cluster trajectories during chemotherapy in breast cancer outpatients. J Pain Symptom Manage (2017) 53:1017–25. doi: 10.1016/j.jpainsymman.2016.12.354
17. Colagiuri B, Christensen S, Jensen AB, Price MA, Butow PN, Zachariae R. Prevalence and predictors of sleep difficulty in a national cohort of women with primary breast cancer three to four months postsurgery. J Pain Symptom Manage (2011) 42:710–20. doi: 10.1016/j.jpainsymman.2011.02.012
18. Koppelmans V, Breteler MMB, Boogerd W, Seynaeve C, Gundy C, Schagen SB. Neuropsychological performance in survivors of breast cancer more than 20 years after adjuvant chemotherapy. J Clin Oncol (2012) 30:1080–6. doi: 10.1200/JCO.2011.37.0189
19. Suppli NP, Johansen C, Christensen J, Kessing LV, Kroman N, Dalton SO. Increased risk for depression after breast cancer: A nationwide population-based cohort study of associated factors in denmark 1998-2011. J Clin Oncol (2014) 32:3831–9. doi: 10.1200/JCO.2013.54.0419
20. Amidi A, Wu LM, Pedersen AD, Mehlsen M, Pedersen CG, Rossen P, et al. Cognitive impairment in testicular cancer survivors 2 to 7 years after treatment. Support. Care Cancer (2015) 23:2973–9. doi: 10.1007/s00520-015-2663-3
21. Wu LM, Tanenbaum ML, Dijkers MPJM, Amidi A, Hall SJ, Penedo FJ, et al. Cognitive and neurobehavioral symptoms in patients with non-metastatic prostate cancer treated with androgen deprivation therapy or observation: A mixed methods study. Soc Sci Med (2016) 156:80–9. doi: 10.1016/j.socscimed.2016.03.016
22. Nguyen J, Cramarossa G, Bruner D, Chen E, Khan L, Leung A, et al. A literature review of symptom clusters in patients with breast cancer. Expert Rev Pharmacoecon. Outcomes Res (2011) 11:533–9. doi: 10.1586/erp.11.55
23. Fan G, Filipczak L, Chow E. Symptom clusters in cancer patients: a review of the literature. Curr Oncol (2007) 14:173–9. doi: 10.3747/co.2007.145
24. Kim H-J, Barsevick AM, Fang CY, Miaskowski C. Common biological pathways underlying the psychoneurological symptom cluster in cancer patients. Cancer Nurs. (2012) 35:E1–E20. doi: 10.1097/NCC.0b013e318233a811
25. Miaskowski C, Dodd M, Lee K. Symptom clusters: The new frontier in symptom management research. J Natl Cancer Inst Monogr (2004) 2004:17–21. doi: 10.1093/jncimonographs/lgh023
26. Bower JE, Ganz PA, Irwin MR, Kwan L, Breen EC, Cole SW. Inflammation and behavioral symptoms after breast cancer treatment: Do fatigue, depression, and sleep disturbance share a common underlying mechanism? J Clin Oncol (2011) 29:3517–22. doi: 10.1200/JCO.2011.36.1154
27. Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol (2008) 26:971–82. doi: 10.1200/JCO.2007.10.7805
28. Dantzer R. Cytokine, sickness behavior, and depression. Immunol Allergy Clin North Am (2009) 29:247–64. doi: 10.1016/j.iac.2009.02.002
29. Rich TA. Analyzing the symptoms in cancer patients. IEEE Eng. Med Biol Mag. (2008) 27:25–8. doi: 10.1109/MEMB.2007.907364
30. Shattuck EC, Muehlenbein MP. Towards an integrative picture of human sickness behavior. Brain. Behav Immun (2016) 57:255–62. doi: 10.1016/j.bbi.2016.05.002
31. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell (2010) 140:883–99. doi: 10.1016/j.cell.2010.01.025
32. Lippitz BE. Cytokine patterns in patients with cancer: A systematic review. Lancet Oncol (2013) 14:e218–28. doi: 10.1016/S1470-2045(12)70582-X
33. McKelvey KJ, Hudson AL, Back M, Eade T, Diakos CI. Radiation, inflammation and the immune response in cancer. Mamm. Genome (2018) 29:843–65. doi: 10.1007/s00335-018-9777-0
34. Edwardson DW, Parissenti AM, Kovala AT. “Chemotherapy and inflammatory cytokine signalling in cancer cells and the tumour microenvironment,”. In: Advances in experimental medicine and biology. Cham: Springer (2019). p. 173–215. doi: 10.1007/978-3-030-20301-6_9
35. Réus GZ, Fries GR, Stertz L, Badawy M, Passos IC, Barichello T, et al. The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. Neuroscience (2015) 300:141–54. doi: 10.1016/j.neuroscience.2015.05.018
36. Bower JE, Ganz PA, Dickerson SS, Petersen L, Aziz N, Fahey JL. Diurnal cortisol rhythm and fatigue in breast cancer survivors. Psychoneuroendocrinology (2005) 30:92–100. doi: 10.1016/j.psyneuen.2004.06.003
37. Oh I-J, Kim K-S, Kim Y-C, Park J-Y, Yoo K-Y, Do S-H, et al. Altered hypothalamus-Pituitary-Adrenal axis function: A potential underlying biological pathway for multiple concurrent symptoms in patients with advanced lung cancer. Psychosom. Med (2019) 81:41–50. doi: 10.1097/PSY.0000000000000648
38. Weinrib AZ, Sephton SE, Degeest K, Penedo F, Bender D, Zimmerman B, et al. Diurnal cortisol dysregulation, functional disability, and depression in women with ovarian cancer. Cancer (2010) 116:4410–9. doi: 10.1002/cncr.25299
39. Cuneo MG, Schrepf A, Slavich GM, Thaker PH, Goodheart M, Bender D, et al. Diurnal cortisol rhythms, fatigue and psychosocial factors in five-year survivors of ovarian cancer. Psychoneuroendocrinology (2017) 84:139–42. doi: 10.1016/j.psyneuen.2017.06.019
40. Russell G, Lightman S. The human stress response. Nat Rev Endocrinol (2019) 15:525–34. doi: 10.1038/s41574-019-0228-0
41. Rich TA. Symptom clusters in cancer patients and their relation to EGFR ligand modulation of the circadian axis. J Support. Oncol (2007) 5:167–74.
42. Payne JK. Altered circadian rhythms and cancer-related fatigue outcomes. Integr Cancer Ther (2011) 10:221–33. doi: 10.1177/1534735410392581
43. Porter LS. Circadian disruption–a new direction for psycho-oncology research? Ann Behav Med (2012) 44:1–2. doi: 10.1007/s12160-012-9376-3. A Comment on Dedert et al.
44. Innominato PF, Roche VP, Palesh OG, Ulusakarya A, Spiegel D, Lévi FA. The circadian timing system in clinical oncology. Ann Med (2014) 46:191–207. doi: 10.3109/07853890.2014.916990
45. Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: Organization and coordination of central and peripheral clocks. Annu Rev Physiol (2010) 72:517–49. doi: 10.1146/annurev-physiol-021909-135821
46. Videnovic A, Lazar AS, Barker RA, Overeem S. The clocks that time us’-circadian rhythms in neurodegenerative disorders. Nat Rev Neurol (2014) 10:683–93. doi: 10.1038/nrneurol.2014.206
47. Scheiermann C, Kunisaki Y, Frenette PS. Circadian control of the immune system. Nat Rev Immunol (2013) 13:190–8. doi: 10.1038/nri3386
48. Franken P, Dijk D-J. Circadian clock genes and sleep homeostasis. Eur J Neurosci (2009) 29:1820–9. doi: 10.1111/j.1460-9568.2009.06723.x
49. Yu X, Zecharia A, Zhang Z, Yang Q, Yustos R, Jager P, et al. Circadian factor BMAL1 in histaminergic neurons regulates sleep architecture. Curr Biol (2014) 24:2838–44. doi: 10.1016/j.cub.2014.10.019
50. Mang GM, La Spada F, Emmenegger Y, Chappuis S, Ripperger JA, Albrecht U, et al. Altered sleep homeostasis in rev-erbα knockout mice. Sleep (2016) 39:589–601. doi: 10.5665/sleep.5534
52. Kronfeld-Schor N, Einat H. Circadian rhythms and depression: Human psychopathology and animal models. Neuropharmacology (2012) 62:101–14. doi: 10.1016/j.neuropharm.2011.08.020
53. Schnell A, Albrecht U, Sandrelli F. Rhythm and mood: Relationships between the circadian clock and mood-related behavior. Behav Neurosci (2014) 128:326–43. doi: 10.1037/a0035883
54. Kondratova AA, Dubrovsky YV, Antoch MP, Kondratov RV. Circadian clock proteins control adaptation to novel environment and memory formation. Aging (Albany. NY). (2010) 2:285–97. doi: 10.18632/aging.100142
55. De Bundel D, Gangarossa G, Biever A, Bonnefont X, Valjent E. Cognitive dysfunction, elevated anxiety, and reduced cocaine response in circadian clock-deficient cryptochrome knockout mice. Front Behav Neurosci (2013) 7:152. doi: 10.3389/fnbeh.2013.00152
56. Habbal OA, Al-Jabri AA. Circadian rhythm and the immune response: A review. Int Rev Immunol (2009) 28:93–108. doi: 10.1080/08830180802645050
57. Cermakian N, Lange T, Golombek D, Sarkar D, Nakao A, Shibata S, et al. Crosstalk between the circadian clock circuitry and the immune system. Chronobiol. Int (2013) 30:870–88. doi: 10.3109/07420528.2013.782315
58. Narasimamurthy R, Hatori M, Nayak SK, Liu F, Panda S, Verma IM. Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proc Natl Acad Sci (2012) 109:12662–7. doi: 10.1073/pnas.1209965109
59. Rich T, Innominato PF, Boerner J, Mormont MC, Iacobelli S, Baron B, et al. Elevated serum cytokines correlated with altered behavior, serum cortisol rhythm, and dampened 24-hour rest-activity patterns in patients with metastatic colorectal cancer. Clin Cancer Res (2005) 11:1757–64. doi: 10.1158/1078-0432.CCR-04-2000
60. Leone MJ, Marpegan L, Duhart JM, Golombek DA. Role of proinflammatory cytokines on lipopolysaccharide-induced phase shifts in locomotor activity circadian rhythm. Chronobiol. Int (2012) 29:715–23. doi: 10.3109/07420528.2012.682681
61. Cavadini G, Petrzilka S, Kohler P, Jud C, Tobler I, Birchler T, et al. TNF- suppresses the expression of clock genes by interfering with e-box-mediated transcription. Proc Natl Acad Sci (2007) 104:12843–8. doi: 10.1073/pnas.0701466104
62. Gamble KL, Berry R, Frank SJ, Young ME. Circadian clock control of endocrine factors. Nat Rev Endocrinol (2014) 10:466–75. doi: 10.1038/nrendo.2014.78
63. Ikegami K, Refetoff S, Van Cauter E, Yoshimura T. Interconnection between circadian clocks and thyroid function. Nat Rev Endocrinol (2019) 15:590–600. doi: 10.1038/S41574-019-0237-Z
64. Bedrosian TA, Fonken LK, Nelson RJ. Endocrine effects of circadian disruption. Annu Rev Physiol (2016) 78:109–31. doi: 10.1146/annurev-physiol-021115-105102
65. Tsang AH, Astiz M, Friedrichs M, Oster H. Endocrine regulation of circadian physiology. J Endocrinol (2016) 230:R1–R11. doi: 10.1530/JOE-16-0051
66. Liu L, Marler MR, Parker BA, Jones V, Johnson S, Cohen-Zion M, et al. The relationship between fatigue and light exposure during chemotherapy. Support. Care Cancer (2005) 13:1010–7. doi: 10.1007/s00520-005-0824-5
67. Irwin MR. Sleep and inflammation: Partners in sickness and in health. Nat Rev Immunol (2019) 19:702–15. doi: 10.1038/s41577-019-0190-z
68. Irwin MR, Olmstead R, Carroll JE. Sleep disturbance, sleep duration, and inflammation: A systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry (2016) 80:40–52. doi: 10.1016/j.biopsych.2015.05.014
69. Godbout J, Godbout JP, Glaser R. Stress-induced immune dysregulation: Implications for wound healing, infectious disease and cancer. Artic. J Neuroimmune Pharmacol (2007) 1:421–7. doi: 10.1007/s11481-006-9036-0
70. Aldea M, Craciun L, Tomuleasa C, Crivii C. The role of depression and neuroimmune axis in the prognosis of cancer patients. J B.U.ON. (2014) 19:5–14. doi: 10.1016/j.mce.2020.111093
71. Cajochen C, Kräuchi K, Wirz-Justice A. Role of melatonin in the regulation of human circadian rhythms and sleep. J Neuroendocrinol. (2003) 15:432–7. doi: 10.1046/j.1365-2826.2003.00989.x
72. Redd WH, Valdimarsdottir H, Wu LM, Winkel G, Byrne EE, Beltre MA, et al. Systematic light exposure in the treatment of cancer-related fatigue: A preliminary study. Psychooncology. (2014) 23:1431–4. doi: 10.1002/pon.3553
73. Wu LM, Amidi A, Valdimarsdottir H, Ancoli-Israel S, Liu L, Winke G, et al. The effect of systematic light exposure on sleep in a mixed group of fatigued cancer survivors. J Clin Sleep Med (2018) 14:31–9. doi: 10.5664/jcsm.6874
74. Denlinger CS, Ligibel JA, Are M, Baker KS, Demark-Wahnefried W, Friedman DL, et al. Survivorship: Fatigue, version 1.2014. J Natl Compr Canc Netw (2014) 12:876–87. doi: 10.6004/jnccn.2014.0082
75. Bower JE. Cancer-related fatigue–mechanisms, risk factors, and treatments. Nat Rev Clin Oncol (2014) 11:597–609. doi: 10.1038/nrclinonc.2014.127
76. Saligan LN, Olson K, Filler K, Larkin D, Cramp F, Sriram Y, et al. The biology of cancer-related fatigue: A review of the literature. Support. Care Cancer (2015) 23:2461–78. doi: 10.1007/S00520-015-2763-0/FIGURES/2
77. Webster K, Cella D, Yost K. The functional assessment of chronic illness therapy (FACIT) measurement system: Properties, applications, and interpretation. Health Qual. Life Outcomes (2003) 1:79. doi: 10.1186/1477-7525-1-79
78. Stein KD, Jacobsen PB, Blanchard CM, Thors C. Further validation of the multidimensional fatigue symptom inventory-short form. J Pain Symptom Manage (2004) 27:14–23. doi: 10.1016/j.jpainsymman.2003.06.003
79. Savard J, Morin CM. Insomnia in the context of cancer: A review of a neglected problem. J Clin Oncol (2001) 19:895–908. doi: 10.1200/JCO.2001.19.3.895
80. Beck SL, Schwartz AL, Towsley G, Dudley W, Barsevick A. Psychometric evaluation of the Pittsburgh sleep quality index in cancer patients. J Pain Symptom Manage (2004) 27:140–8. doi: 10.1016/j.jpainsymman.2003.12.002
81. Savard MH, Savard J, Simard S, Ivers H. Empirical validation of the insomnia severity index in cancer patients. Psychooncology. (2005) 14:429–41. doi: 10.1002/PON.860
82. Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep (2003) 26:342–92. doi: 10.1093/sleep/26.3.342
83. Madsen MT, Huang C, Gögenur I. Actigraphy for measurements of sleep in relation to oncological treatment of patients with cancer: A systematic review. Sleep Med Rev (2015) 20:73–83. doi: 10.1016/J.SMRV.2014.07.002
84. Marino M, Li Y, Rueschman MN, Winkelman JW, Ellenbogen JM, Solet JM, et al. Measuring sleep: accuracy, sensitivity, and specificity of wrist actigraphy compared to polysomnography. Sleep (2013) 36:1747–55. doi: 10.5665/sleep.3142
85. Götze H, Friedrich M, Taubenheim S, Dietz A, Lordick F, Mehnert A. Depression and anxiety in long-term survivors 5 and 10 years after cancer diagnosis. Support. Care Cancer (2020) 28:211–20. doi: 10.1007/S00520-019-04805-1
86. Krebber AMH, Buffart LM, Kleijn G, Riepma IC, De Bree R, Leemans CR, et al. Prevalence of depression in cancer patients: A meta-analysis of diagnostic interviews and self-report instruments. Psychooncology. (2014) 23:121–30. doi: 10.1002/PON.3409
87. Johnston M, Pollard B, Hennessey P. Construct validation of the hospital anxiety and depression scale with clinical populations. J Psychosom. Res (2000) 48:579–84. doi: 10.1016/S0022-3999(00)00102-1
88. Radloff LS. The CES-d scale: A self-report depression scale for research in the general population. Appl Psychol Meas. (1977) 1:385–401. doi: 10.1177/014662167700100306
89. Vodermaier A, Linden W, Siu C. Screening for emotional distress in cancer patients: A systematic review of assessment instruments. J Natl Cancer Inst (2009) 101:1464–88. doi: 10.1093/JNCI/DJP336
90. Lange M, Joly F, Vardy J, Ahles T, Dubois M, Tron L, et al. Cancer-related cognitive impairment: An update on state of the art, detection, and management strategies in cancer survivors. Ann Oncol Off J Eur Soc Med Oncol (2019) 30:1925–40. doi: 10.1093/ANNONC/MDZ410
91. Lange M, Hardy-Leger I, Licaj I, Pistilli B, Rigal O, Le Fel J, et al. Cognitive impairment in patients with breast cancer before surgery: Results from a CANTO cohort subgroup. Cancer Epidemiol. Biomarkers Prev (2020) 29:1759–66. doi: 10.1158/1055-9965.EPI-20-0346
92. Wefel JS, Vardy J, Ahles T, Schagen SB. International cognition and cancer task force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol (2011) 12:703–8. doi: 10.1016/S1470-2045(10)70294-1
93. Bray VJ, Dhillon HM, Vardy JL. Systematic review of self-reported cognitive function in cancer patients following chemotherapy treatment. J Cancer Surviv. (2018) 12:537–59. doi: 10.1007/s11764-018-0692-x
94. Jacobs SR, Jacobsen PB, Booth-Jones M, Wagner LI, Anasetti C. Evaluation of the functional assessment of cancer therapy cognitive scale with hematopoietic stem cell transplant patients. J Pain Symptom Manage (2007) 33:13–23. doi: 10.1016/j.jpainsymman.2006.06.011
95. Broadbent DE, Cooper PF, FitzGerald P, Parkes KR. The cognitive failures questionnaire (CFQ) and its correlates. Br J Clin Psychol (1982) 21:1–16. doi: 10.1111/J.2044-8260.1982.TB01421.X
96. Hutchinson AD, Hosking JR, Kichenadasse G, Mattiske JK, Wilson C. Objective and subjective cognitive impairment following chemotherapy for cancer: A systematic review. Cancer Treat Rev (2012) 38:926–34. doi: 10.1016/j.ctrv.2012.05.002
97. Henneghan AM, Van Dyk K, Kaufmann T, Harrison R, Gibbons C, Heijnen C, et al. Measuring self-reported cancer-related cognitive impairment: Recommendations from the cancer neuroscience initiative working group. J Natl Cancer Inst (2021) 113:1625–33. doi: 10.1093/JNCI/DJAB027
98. Claustrat B, Brun J, Chazot G. The basic physiology and pathophysiology of melatonin. Sleep Med Rev (2005) 9:11–24. doi: 10.1016/j.smrv.2004.08.001
99. Couto-Moraes R, Palermo-Neto J, Markus RP. The immune-pineal axis: stress as a modulator of pineal gland function. Ann N Y. Acad Sci (2009) 1153:193–202. doi: 10.1111/j.1749-6632.2008.03978.x
100. Arendt J. Melatonin: Characteristics, concerns, and prospects. J Biol Rhythms (2005) 20:291–303. doi: 10.1177/0748730405277492
101. Middleton B. “Measurement of melatonin and 6-sulphatoxymelatonin,” in. Methods Mol Biol (2013) 1065, 171–99. doi: 10.1007/978-1-62703-616-0_11
102. Benloucif S, Burgess HJ, Klerman EB, Lewy AJ, Middleton B, Murphy PJ, et al. Measuring melatonin in humans. J Clin Sleep Med (2008) 4:66–9. doi: 10.5664/jcsm.27083
103. Crowley SJ, Suh C, Molina TA, Fogg LF, Sharkey KM, Carskadon MA. Estimating the dim light melatonin onset of adolescents within a 6-h sampling window: the impact of sampling rate and threshold method. Sleep Med (2016) 20:59–66. doi: 10.1016/J.SLEEP.2015.11.019
104. Pandi-Perumal SR, Smits M, Spence W, Srinivasan V, Cardinali DP, Lowe AD, et al. Dim light melatonin onset (DLMO): A tool for the analysis of circadian phase in human sleep and chronobiological disorders. Prog Neuropsychopharmacol Biol Psychiatry (2007) 31:1–11. doi: 10.1016/j.pnpbp.2006.06.020
105. Reid KJ. Assessment of circadian rhythms. Neurol Clin (2019) 37:505–26. doi: 10.1016/j.ncl.2019.05.001
106. Surrall K, Smith JA, Bird H, Okala B, Othman H, Padwick DJ. Effect of ibuprofen and indomethacin on human plasma melatonin. J Pharm Pharmacol (1987) 39:840–3. doi: 10.1111/J.2042-7158.1987.TB05129.X
107. Voultsios A, Kennaway DJ, Dawson D. Salivary melatonin as a circadian phase marker: validation and comparison to plasma melatonin. J Biol Rhythms (1997) 12:457–66. doi: 10.1177/074873049701200507
108. Stoschitzky K, Sakotnik A, Lercher P, Zweiker R, Maier R, Liebmann P, et al. Influence of beta-blockers on melatonin release. Eur J Clin Pharmacol (1999) 55:111–5. doi: 10.1007/S002280050604
109. Härtter S, Korhonen T, Lundgren S, Rane A, Tolonen A, Turpeinen M, et al. Effect of caffeine intake 12 or 24 hours prior to melatonin intake and CYP1A2*1F polymorphism on CYP1A2 phenotyping by melatonin. Basic Clin Pharmacol Toxicol (2006) 99:300–4. doi: 10.1111/J.1742-7843.2006.PTO_491.X
110. Burgess HJ, Wyatt JK, Park M, Fogg LF. Home circadian phase assessments with measures of compliance yield accurate dim light melatonin onsets. Sleep (2015) 38:889–97. doi: 10.5665/SLEEP.4734
111. Colletti A, Cicero AFG. Nutraceutical approach to chronic osteoarthritis: From molecular research to clinical evidence. Int J Mol Sci (2021) 22:12920. doi: 10.3390/IJMS222312920
112. Wu H-S, Davis JE, Natavio T. Fatigue and disrupted sleep-wake patterns in patients with cancer: A shared mechanism. Clin J Oncol Nurs. (2012) 16:E56–68. doi: 10.1188/12.CJON.E56-E68
113. Kirschbaum C, Hellhammer DH. Salivary cortisol in psychobiological research: an overview. Neuropsychobiology (1989) 22:150–69. doi: 10.1159/000118611
114. Selmaoui B, Touitou Y. Reproducibility of the circadian rhythms of serum cortisol and melatonin in healthy subjects: A study of three different 24-h cycles over six weeks. Life Sci (2003) 73:3339–49. doi: 10.1016/J.LFS.2003.05.007
115. Zeitzer JM, Nouriani B, Rissling MB, Sledge GW, Kaplan KA, Aasly L, et al. Aberrant nocturnal cortisol and disease progression in women with breast cancer. Breast Cancer Res Treat (2016) 158:43–50. doi: 10.1007/s10549-016-3864-2
116. Ryan R, Booth S, Spathis A, Mollart S, Clow A. Use of salivary diurnal cortisol as an outcome measure in randomised controlled trials: A systematic review. Ann Behav Med (2016) 50:210–36. doi: 10.1007/S12160-015-9753-9
117. Adam EK, Quinn ME, Tavernier R, McQuillan MT, Dahlke KA, Gilbert KE. Diurnal cortisol slopes and mental and physical health outcomes: A systematic review and meta-analysis. Psychoneuroendocrinology (2017) 83:25–41. doi: 10.1016/J.PSYNEUEN.2017.05.018
118. Fekedulegn DB, Andrew ME, Burchfiel CM, Violanti JM, Hartley TA, Charles LE, et al. Area under the curve and other summary indicators of repeated waking cortisol measurements. Psychosom. Med (2007) 69:651–9. doi: 10.1097/PSY.0B013E31814C405C
119. Stalder T, Kirschbaum C, Kudielka BM, Adam EK, Pruessner JC, Wüst S, et al. Assessment of the cortisol awakening response: Expert consensus guidelines. Psychoneuroendocrinology (2016) 63:414–32. doi: 10.1016/J.PSYNEUEN.2015.10.010
120. Huang C, Madsen MT, Gögenur I. Circadian rhythms measured by actigraphy during oncological treatments: A systematic review. Biol Rhythm Res (2015) 46:329–48. doi: 10.1080/09291016.2015.1004840
121. Rogers VE, Zhu S, Ancoli-Israel S, Hinds PS. Impairment in circadian activity rhythms occurs during dexamethasone therapy in children with leukemia. Pediatr Blood Cancer (2014) 61:1986–91. doi: 10.1002/pbc.25147
122. Liu L, Rissling M, Neikrug A, Fiorentino L, Natarajan L, Faierman M, et al. Fatigue and circadian activity rhythms in breast cancer patients before and after chemotherapy: A controlled study. Fatigue Biomed Heal Behav (2013) 1:12–26. doi: 10.1080/21641846.2012.741782
123. Aschoff J. Circadian control of body temperature. J Therm. Biol (1983) 8:143–7. doi: 10.1016/0306-4565(83)90094-3
124. Weinert D, Waterhouse J. The circadian rhythm of core temperature: Effects of physical activity and aging. Physiol Behav (2007) 90:246–56. doi: 10.1016/j.physbeh.2006.09.003
125. Weinert D. Circadian temperature variation and ageing. Ageing Res Rev (2010) 9:51–60. doi: 10.1016/j.arr.2009.07.003
126. Buhr ED, Yoo S-H, Takahashi JS. Temperature as a universal resetting cue for mammalian circadian oscillators. Sci. (2010) 330:379–85. doi: 10.1126/science.1195262
127. Kräuchi K. The thermophysiological cascade leading to sleep initiation in relation to phase of entrainment. Sleep Med Rev (2007) 11:439–51. doi: 10.1016/j.smrv.2007.07.001
128. Taylor NAS, Tipton MJ, Kenny GP. Considerations for the measurement of core, skin and mean body temperatures. J Therm. Biol (2014) 46:72–101. doi: 10.1016/j.jtherbio.2014.10.006
129. Sarabia JA, Rol MA, Mendiola P, Madrid JA. Circadian rhythm of wrist temperature in normal-living subjects: A candidate of new index of the circadian system. Physiol Behav (2008) 95:570–80. doi: 10.1016/j.physbeh.2008.08.005
130. Kräuchi K, Wirz-Justice A. Circadian rhythm of heat production, heart rate, and skin and core temperature under unmasking conditions in men. Am J Physiol (1994) 267:R819–29. doi: 10.1152/ajpregu.1994.267.3.R819
131. Gonçalves BSB, Cavalcanti PRA, Tavares GR, Campos TF, Araujo JF. Nonparametric methods in actigraphy: An update. Sleep Sci (2014) 7:158–64. doi: 10.1016/J.SLSCI.2014.09.013
132. Marler MR, Gehrman P, Martin JL, Ancoli-Israel S. The sigmoidally transformed cosine curve: A mathematical model for circadian rhythms with symmetric non-sinusoidal shapes. Stat Med (2006) 25:3893–904. doi: 10.1002/SIM.2466
133. Paudel ML, Taylor BC, Ancoli-Israel S, Stone KL, Tranah G, Redline S, et al. Rest/activity rhythms and cardiovascular disease in older men. Chronobiol. Int (2011) 28:258–66. doi: 10.3109/07420528.2011.553016
134. Cornelissen G. Cosinor-based rhythmometry. Theor Biol Med Model (2014) 11:16. doi: 10.1186/1742-4682-11-16
135. Doyle MM, Murphy TE, Miner B, Pisani MA, Lusczek ER, Knauert MP. Enhancing cosinor analysis of circadian phase markers using the gamma distribution. Sleep Med (2022) 92:1–3. doi: 10.1016/J.SLEEP.2022.01.015
136. Lévi F, Dugué P-A, Innominato P, Karaboué A, Dispersyn G, Parganiha A, et al. Wrist actimetry circadian rhythm as a robust predictor of colorectal cancer patients survival. Chronobiol. Int (2014) 31:891–900. doi: 10.3109/07420528.2014.924523
137. Minors D, Akerstedt T, Atkinson G, Dahlitz M, Folkard S, Levi F, et al. The difference between activity when in bed and out of bed. i. healthy subjects and selected patients. Chronobiol. Int (1996) 13:27–34. doi: 10.3109/07420529609040839
138. Natale V, Innominato PF, Boreggiani M, Tonetti L, Filardi M, Parganiha A, et al. The difference between in bed and out of bed activity as a behavioral marker of cancer patients: A comparative actigraphic study. Chronobiol Int (2015) 32:925–33. doi: 10.3109/07420528.2015.1053909
139. Innominato PF, Focan C, Gorlia T, Moreau T, Garufi C, Waterhouse J, et al. Circadian rhythm in rest and activity: A biological correlate of quality of life and a predictor of survival in patients with metastatic colorectal cancer. Cancer Res (2009) 69:4700–7. doi: 10.1158/0008-5472.CAN-08-4747
140. Lévi F, Komarzynski S, Huang Q, Young T, Ang Y, Fuller C, et al. Tele-monitoring of cancer patients’ rhythms during daily life identifies actionable determinants of circadian and sleep disruption. Cancers (Basel). (2020) 12:1–21. doi: 10.3390/cancers12071938
141. Huang Q, Cohen D, Komarzynski S, Li XM, Innominato P, Lévi F, et al. Hidden Markov models for monitoring circadian rhythmicity in telemetric activity data. J R Soc Interface (2018) 15:20170885. doi: 10.1098/RSIF.2017.0885
142. Asgari-Targhi A, Klerman EB. Mathematical modeling of circadian rhythms. Wiley Interdiscip. Rev Syst Biol Med (2019) 11:e1439. doi: 10.1002/WSBM.1439
143. Slats D, Claassen JAHR, Verbeek MM, Overeem S. Reciprocal interactions between sleep, circadian rhythms and alzheimer’s disease: Focus on the role of hypocretin and melatonin. Ageing Res Rev (2013) 12:188–200. doi: 10.1016/J.ARR.2012.04.003
144. Kalliolia E, Silajdžić E, Nambron R, Hill NR, Doshi A, Frost C, et al. Plasma melatonin is reduced in huntington’s disease. Mov. Disord (2014) 29:1511–5. doi: 10.1002/mds.26003
145. Videnovic A, Noble C, Reid KJ, Peng J, Turek FW, Marconi A, et al. Circadian melatonin rhythm and excessive daytime sleepiness in Parkinson disease. JAMA Neurol (2014) 71:463. doi: 10.1001/jamaneurol.2013.6239
146. Bradley AJ, Webb-Mitchell R, Hazu A, Slater N, Middleton B, Gallagher P, et al. Sleep and circadian rhythm disturbance in bipolar disorder. Psychol Med (2017) 47:1678–89. doi: 10.1017/S0033291717000186
147. Lozano-Lorca M, Olmedo-Requena R, Rodríguez-Barranco M, Redondo-Sánchez D, Jiménez-Pacheco A, Vázquez-Alonso F, et al. Salivary melatonin rhythm and prostate cancer: CAPLIFE study. J Urol. (2021). 207:565–72 doi: 10.1097/JU.0000000000002294
148. Bartsch C, Bartsch H, Jain AK, Laumas KR, Wetterberg L. Urinary melatonin levels in human breast cancer patients. J Neural Transm (1981) 52:281–94. doi: 10.1007/BF01256753
149. Bartsch C, Bartsch H, Fuchs U, Lippert TH, Bellmann O, Gupta D. Stage-dependent depression of melatonin in patients with primary breast cancer. Correlation with prolactin, thyroid stimulating hormone, and steroid receptors. Cancer (1989) 64:426–33. doi: 10.1002/1097-0142(19890715)64:2<426::AID-CNCR2820640215>3.0.CO;2-O
150. Payne JK. The trajectory of fatigue in adult patients with breast and ovarian cancer receiving chemotherapy. Oncol Nurs. Forum (2002) 29:1334–40. doi: 10.1188/02.ONF.1334-1340
151. Li W, Kwok CCH, Chan DCW, Ho AWY, Ho CS, Zhang J, et al. Disruption of sleep, sleep-wake activity rhythm, and nocturnal melatonin production in breast cancer patients undergoing adjuvant chemotherapy: Prospective cohort study. Sleep Med (2019) 55:14–21. doi: 10.1016/j.sleep.2018.11.022
152. Karasek M, Kowalski AJ, Suzin J, Zylinska K, Swietoslawski J. Serum melatonin circadian profiles in women suffering from cervical cancer. J Pineal Res (2005) 39:73–6. doi: 10.1111/j.1600-079X.2005.00221.x
153. Hu S, Shen G, Yin S, Xu W, Hu B. Melatonin and tryptophan circadian profiles in patients with advanced non-small cell lung cancer. Adv Ther (2009) 26:886–92. doi: 10.1007/s12325-009-0068-8
154. Chang WP, Lin CC. Relationships of salivary cortisol and melatonin rhythms to sleep quality, emotion, and fatigue levels in patients with newly diagnosed lung cancer. Eur J Oncol Nurs. (2017) 29:79–84. doi: 10.1016/j.ejon.2017.05.008
155. Khoory R, Stemme D. Plasma melatonin levels in patients suffering from colorectal carcinoma. J Pineal Res (1988) 5:251–8. doi: 10.1111/j.1600-079X.1988.tb00651.x
156. Zaki NFW, Sabri YM, Farouk O, Abdelfatah A, Spence DW, Bahammam AS, et al. Depressive symptoms, sleep profiles and serum melatonin levels in a sample of breast cancer patients. Nat Sci Sleep (2020) 12:135–49. doi: 10.2147/NSS.S206768
157. Touitou Y, Bogdan A, Levi F, Benavides M, Auzeby A. Disruption of the circadian patterns of serum cortisol in breast and ovarian cancer patients: Relationships with tumour marker antigens. Br J Cancer (1996) 74:1248–52. doi: 10.1038/bjc.1996.524
158. van der Pompe G, Antoni MH, Heijnen CJ. Elevated basal cortisol levels and attenuated ACTH and cortisol responses to a behavioral challenge in women with metastatic breast cancer. Psychoneuroendocrinology (1996) 21:361–74. doi: 10.1016/0306-4530(96)00009-1
159. Abercrombie HC, Giese-Davis J, Sephton S, Epel ES, Turner-Cobb JM, Spiegel D. Flattened cortisol rhythms in metastatic breast cancer patients. Psychoneuroendocrinology (2004) 29:1082–92. doi: 10.1016/j.psyneuen.2003.11.003
160. Schrepf A, Clevenger L, Christensen D, DeGeest K, Bender D, Ahmed A, et al. Cortisol and inflammatory processes in ovarian cancer patients following primary treatment: relationships with depression, fatigue, and disability. Brain. Behav Immun (2013) 30 Suppl:S126–34. doi: 10.1016/j.bbi.2012.07.022
161. Mazzoccoli G, Vendemiale G, De Cata A, Carughi S, Tarquini R. Altered time structure of neuro-endocrine-immune system function in lung cancer patients. BMC Cancer (2010) 10:314. doi: 10.1186/1471-2407-10-314
162. Kim KS, Kim YC, Oh IJ, Kim SS, Choi JY, Ahn RS. Association of worse prognosis with an aberrant diurnal cortisol rhythm in patients with advanced lung cancer. Chronobiol. Int (2012) 29:1109–20. doi: 10.3109/07420528.2012.706767
163. Alexander S, Minton O, Andrews P, Stone P. A comparison of the characteristics of disease-free breast cancer survivors with or without cancer-related fatigue syndrome. Eur J Cancer (2009) 45:384–92. doi: 10.1016/J.EJCA.2008.09.010
164. Castonguay AL, Wrosch C, Sabiston CM. The roles of negative affect and goal adjustment capacities in breast cancer survivors: Associations with physical activity and diurnal cortisol secretion. Health Psychol (2017) 36:320–31. doi: 10.1037/HEA0000477
165. Giese-Davis J, Wilhelm FH, Conrad A, Abercrombie HC, Sephton S, Yutsis M, et al. Depression and stress reactivity in metastatic breast cancer. Psychosom. Med (2006) 68:675–83. doi: 10.1097/01.PSY.0000238216.88515.E5
166. Ho RTH, Fong TCT, Chan CKP, Chan CLW. The associations between diurnal cortisol patterns, self-perceived social support, and sleep behavior in Chinese breast cancer patients. Psychoneuroendocrinology (2013) 38:2337–42. doi: 10.1016/J.PSYNEUEN.2013.05.004
167. Hoyt MA, Bower JE, Irwin MR, Weierich MR, Stanton AL. Sleep quality and depressive symptoms after prostate cancer: The mechanistic role of cortisol. Behav Neurosci (2016) 130:351–6. doi: 10.1037/BNE0000107
168. Hsiao FH, Kuo WH, Jow GM, Chang KJ, Yang PS, Lam HB, et al. Habitual sleep-wake behaviors and lifestyle as predictors of diurnal cortisol patterns in young breast cancer survivors: a longitudinal study. Psychoneuroendocrinology (2015) 53:60–8. doi: 10.1016/J.PSYNEUEN.2014.12.014
169. Huang TW, Cheung DST, Xu X, Loh EW, Lai JH, Su WW, et al. Relationship between diurnal cortisol profile and sleep quality in patients with hepatocellular carcinoma. Biol Res Nurs. (2020) 22:139–47. doi: 10.1177/1099800419881195
170. Jehn CF, Kühnhardt D, Bartholomae A, Pfeiffer S, Schmid P, Possinger K, et al. Association of IL-6, hypothalamus-pituitary-adrenal axis function, and depression in patients with cancer. Integr Cancer Ther (2010) 9:270–5. doi: 10.1177/1534735410370036
171. Kuhlman KR, Irwin MR, Ganz PA, Crespi CM, Petersen L, Asher A, et al. Cortisol awakening response as a prospective risk factor for depressive symptoms in women after treatment for breast cancer. Psychosom. Med (2017) 79:763–9. doi: 10.1097/PSY.0000000000000499
172. Lutgendorf SK, Weinrib AZ, Penedo F, Russell D, DeGeest K, Costanzo ES, et al. Interleukin-6, cortisol, and depressive symptoms in ovarian cancer patients. J Clin Oncol (2008) 26:4820–7. doi: 10.1200/JCO.2007.14.1978
173. Mormont MC, Waterhouse J, Bleuzen P, Giacchetti S, Jami A, Bogdan A, et al. Marked 24-h rest/activity rhythms are associated with better quality of life, better response, and longer survival in patients with metastatic colorectal cancer and good performance status. Clin Cancer Res (2000) 6:3038–45.
174. Palesh O, Zeitzer JM, Conrad A, Giese-Davis J, Mustian KM, Popek V, et al. Vagal regulation, cortisol, and sleep disruption in women with metastatic breast cancer. J Clin Sleep Med (2008) 4:441–9. doi: 10.5664/jcsm.27280
175. Sannes TS, Jensen SE, Dodd SM, Kneipp SM, Garey Smith S, Patidar SM, et al. Depressive symptoms and cortisol variability prior to surgery for suspected endometrial cancer. Psychoneuroendocrinology (2013) 38:241–9. doi: 10.1016/J.PSYNEUEN.2012.06.001
176. Schmidt ME, Semik J, Habermann N, Wiskemann J, Ulrich CM, Steindorf K. Cancer-related fatigue shows a stable association with diurnal cortisol dysregulation in breast cancer patients. Brain. Behav Immun (2016) 52:98–105. doi: 10.1016/J.BBI.2015.10.005
177. Sephton SE, Dhabhar FS, Keuroghlian AS, Giese-Davis J, McEwen BS, Ionan AC, et al. Depression, cortisol, and suppressed cell-mediated immunity in metastatic breast cancer. Brain. Behav Immun (2009) 23:1148–55. doi: 10.1016/J.BBI.2009.07.007
178. Tell D, Mathews HL, Janusek LW. Day-to-Day dynamics of associations between sleep, napping, fatigue, and the cortisol diurnal rhythm in women diagnosed as having breast cancer. Psychosom. Med (2014) 76:519–28. doi: 10.1097/PSY.0000000000000097
179. Vedhara K, Stra JT, Miles JNV, Sanderman R, Ranchor AV. Psychosocial factors associated with indices of cortisol production in women with breast cancer and controls. Psychoneuroendocrinology (2006) 31:299–311. doi: 10.1016/J.PSYNEUEN.2005.08.006
180. Hsiao FH, Jow GM, Kuo WH, Wang MY, Chang KJ, Lai YM, et al. A longitudinal study of diurnal cortisol patterns and associated factors in breast cancer patients from the transition stage of the end of active cancer treatment to post-treatment survivorship. Breast (2017) 36:96–101. doi: 10.1016/J.BREAST.2017.06.016
181. Rumble ME, Rose SL, White KH, Moore AH, Gehrman P, Benca RM, et al. Circadian actigraphic rest-activity rhythms following surgery for endometrial cancer: A prospective, longitudinal study. Gynecol. Oncol (2015) 137:448–55. doi: 10.1016/j.ygyno.2015.04.001
182. Savard J, Liu L, Natarajan L, Rissling MB, Neikrug AB, He F, et al. Breast cancer patients have progressively impaired sleep-wake activity rhythms during chemotherapy. Sleep (2009) 32:1155–60. doi: 10.1093/sleep/32.9.1155
183. Sultan A, Choudhary V, Parganiha A. Worsening of rest-activity circadian rhythm and quality of life in female breast cancer patients along progression of chemotherapy cycles. Chronobiol. Int (2017) 34:609–23. doi: 10.1080/07420528.2017.1286501
184. Pati AK, Parganiha A, Kar A, Soni R, Roy S, Choudhary V. Alterations of the characteristics of the circadian rest-activity rhythm of cancer in-patients. Chronobiol. Int (2007) 24:1179–97. doi: 10.1080/07420520701800868
185. Berger AM, Grem JL, Visovsky C, Marunda HA, Yurkovich JM. Fatigue and other variables during adjuvant chemotherapy for colon and rectal cancer. Oncol Nurs. Forum (2010) 37:E359–69. doi: 10.1188/10.ONF.E359-E369
186. Jim HSL, Small B, Faul LA, Franzen J, Apte S, Jacobsen PB. Fatigue, depression, sleep, and activity during chemotherapy: Daily and intraday variation and relationships among symptom changes. Ann Behav Med (2011) 42:321–33. doi: 10.1007/s12160-011-9294-9
187. Innominato PF, Giacchetti S, Bjarnason GA, Focan C, Garufi C, Coudert B, et al. Prediction of overall survival through circadian rest-activity monitoring during chemotherapy for metastatic colorectal cancer. Int J Cancer (2012) 131:2684–92. doi: 10.1002/ijc.27574
188. Ortiz-Tudela E, Iurisci I, Beau J, Karaboue A, Moreau T, Rol MA, et al. The circadian rest-activity rhythm, a potential safety pharmacology endpoint of cancer chemotherapy. Int J Cancer (2014) 134:2717–25. doi: 10.1002/ijc.28587
189. Martin T, Duivon M, Bessot N, Grellard J-M, Emile G, Polvent S, et al. Rest activity rhythms characteristics of breast cancer women following endocrine therapy. Sleep (2021) 45:1–11. doi: 10.1093/SLEEP/ZSAB248
190. Levin RD, Daehler MA, Grutsch JF, Quiton J, Lis CG, Peterson C, et al. Circadian function in patients with advanced non-small-cell lung cancer. Br J Cancer (2005) 93:1202–8. doi: 10.1038/sj.bjc.6602859
191. Grutsch JF, Ferrans C, Wood PA, Du-Quiton J, Quiton DFT, Reynolds JL, et al. The association of quality of life with potentially remediable disruptions of circadian sleep/activity rhythms in patients with advanced lung cancer. BMC Cancer (2011) 11:193. doi: 10.1186/1471-2407-11-193
192. Chen HM, Wu YC, Tsai CM, Tzeng JI, Lin CC. Relationships of circadian rhythms and physical activity with objective sleep parameters in lung cancer patients. Cancer Nurs. (2015) 38:215–23. doi: 10.1097/NCC.0000000000000163
193. Chang WP, Lin CC. Changes in the sleep–wake rhythm, sleep quality, mood, and quality of life of patients receiving treatment for lung cancer: A longitudinal study. Chronobiol. Int (2017) 34:451–61. doi: 10.1080/07420528.2017.1293678
194. Roveda E, Bruno E, Galasso L, Mulè A, Castelli L, Villarini A, et al. Rest-activity circadian rhythm in breast cancer survivors at 5 years after the primary diagnosis. Chronobiol. Int (2019) 36:1156–65. doi: 10.1080/07420528.2019.1621330
195. Milanti A, Chan DNS, Li C, So WKW. Actigraphy-measured rest-activity circadian rhythm disruption in patients with advanced cancer: A scoping review. Support. Care Cancer (2021) 1:1–25. doi: 10.1007/s00520-021-06317-3
196. Ancoli-Israel S, Liu L, Marler MR, Parker BA, Jones V, Sadler GR, et al. Fatigue, sleep, and circadian rhythms prior to chemotherapy for breast cancer. Support. Care Cancer (2006) 14:201–9. doi: 10.1007/s00520-005-0861-0
197. Ancoli-Israel S, Liu L, Natarajan L, Rissling M, Neikrug AB, Youngstedt SD, et al. Reductions in sleep quality and circadian activity rhythmicity predict longitudinal changes in objective and subjective cognitive functioning in women treated for breast cancer. Support. Care Cancer (2022) 30:3187–200. doi: 10.1007/s00520-021-06743-3
198. Berger AM, Wielgus K, Hertzog M, Fischer P, Farr L. Patterns of circadian activity rhythms and their relationships with fatigue and anxiety/depression in women treated with breast cancer adjuvant chemotherapy. Support. Care Cancer (2010) 18:105–14. doi: 10.1007/s00520-009-0636-0
199. Berger AM, Farr LA, Kuhn BR, Fischer P, Agrawal S. Values of Sleep/Wake, Activity/Rest, circadian rhythms, and fatigue prior to adjuvant breast cancer chemotherapy. J Pain Symptom Manage (2007) 33:398–409. doi: 10.1016/j.jpainsymman.2006.09.022
200. Cash E, Duck CR, Brinkman C, Rebholz W, Albert C, Worthen M, et al. Depressive symptoms and actigraphy-measured circadian disruption predict head and neck cancer survival. Psychooncology. (2018) 27:2500–7. doi: 10.1002/pon.4862
201. Du-Quiton J, Wood PA, Burch JB, Grutsch JF, Gupta D, Tyer K, et al. Actigraphic assessment of daily sleep-activity pattern abnormalities reflects self-assessed depression and anxiety in outpatients with advanced non-small cell lung cancer. Psychooncology. (2010) 19:180–9. doi: 10.1002/pon.1539
202. Innominato PF, Komarzynski S, Palesh OG, Dallmann R, Bjarnason GA, Giacchetti S, et al. Circadian rest-activity rhythm as an objective biomarker of patient-reported outcomes in patients with advanced cancer. Cancer Med (2018) 7:4396–405. doi: 10.1002/cam4.1711
203. Ma CL, Chang WP, Lin CC. Rest/activity rhythm is related to the coexistence of pain and sleep disturbance among advanced cancer patients with pain. Support. Care Cancer (2014) 22:87–94. doi: 10.1007/S00520-013-1918-0
204. Miaskowski C, Lee K, Dunn L, Dodd M, Aouizerat BE, West C, et al. Sleep-wake circadian activity rhythm parameters and fatigue in oncology patients before the initiation of radiation therapy. Cancer Nurs. (2011) 34:255–68. doi: 10.1097/NCC.0b013e3181f65d9b
205. Mormont MC, Waterhouse J. Contribution of the rest-activity circadian rhythm to quality of life in cancer patients. Chronobiol. Int (2002) 19:313–23. doi: 10.1081/CBI-120002606
206. Palesh O, Haitz K, Lévi F, Bjarnason GA, Deguzman C, Alizeh I, et al. Relationship between subjective and actigraphy-measured sleep in 237 patients with metastatic colorectal cancer. Qual. Life Res (2017) 26:2783–91. doi: 10.1007/s11136-017-1617-2
207. Roscoe JA, Morrow GR, Hickok JT, Bushunow P, Matteson S, Rakita D, et al. Temporal interrelationships among fatigue, circadian rhythm and depression in breast cancer patients undergoing chemotherapy treatment. Support. Care Cancer (2002) 10:329–36. doi: 10.1007/s00520-001-0317-0
208. Dhruva A, Lee K, Paul SM, West C, Dunn L, Dodd M, et al. Sleep-wake circadian activity rhythms and fatigue in family caregivers of oncology patients. Cancer Nurs. (2012) 35:70–81. doi: 10.1097/NCC.0b013e3182194a25
209. Carpenter JS, Gilchrist JM, Chen K, Gautam S, Freedman RR. Hot flashes, core body temperature, and metabolic parameters in breast cancer survivors. Menopause (2004) 11:375–81. doi: 10.1097/01.GME.0000113848.74835.1A
210. Roche VP, Mohamad-Djafari A, Innominato PF, Karaboué A, Gorbach A, Lévi FA. Thoracic surface temperature rhythms as circadian biomarkers for cancer chronotherapy. Chronobiol. Int (2014) 31:409–20. doi: 10.3109/07420528.2013.864301
211. Jung-Hynes B, Huang W, Reiter RJ, Ahmad N. Melatonin resynchronizes dysregulated circadian rhythm circuitry in human prostate cancer cells. J Pineal Res (2010) 49:60. doi: 10.1111/J.1600-079X.2010.00767.X
212. Mormont MC, Bogdan A, Cormont S, Touitou Y, Lévi F. Cortisol diurnal variation in blood and saliva of patients with metastatic colorectal cancer: Relevance for clinical outcome. Anticancer Res (2002) 22:1243–9.
213. Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer Inst (2000) 92:994–1000. doi: 10.1093/jnci/92.12.994
214. Sephton SE, Lush E, Dedert EA, Floyd AR, Rebholz WN, Dhabhar FS, et al. Diurnal cortisol rhythm as a predictor of lung cancer survival. Brain. Behav Immun (2013) 30: S163–70. doi: 10.1016/j.bbi.2012.07.019
215. Sancar A, Van Gelder RN. Clocks, cancer, and chronochemotherapy. Sci . (2021) 371:eabb0738. doi: 10.1126/science.abb0738
216. Patel SA, Kondratov RV. Clock at the core of cancer development. Biol (Basel). (2021) 10:1–16. doi: 10.3390/BIOLOGY10020150
218. Roenneberg T, Kantermann T, Juda M, Vetter C, Allebrandt KV. Light and the human circadian clock. Handb. Exp Pharmacol (2013) 217:311–31. doi: 10.1007/978-3-642-25950-0_13
219. Pickel L, Sung HK. Feeding rhythms and the circadian regulation of metabolism. Front Nutr (2020) 7:39. doi: 10.3389/fnut.2020.00039
220. Baron KG, Reid KJ. Circadian misalignment and health. Int Rev Psychiatry (2014) 26:139–54. doi: 10.3109/09540261.2014.911149
221. Golden RN, Gaynes BN, Ekstrom RD, Hamer RM, Jacobsen FM, Suppes T, et al. The efficacy of light therapy in the treatment of mood disorders: A review and meta-analysis of the evidence. Am J Psychiatry (2005) 162:656–62. doi: 10.1176/APPI.AJP.162.4.656
222. Ancoli-Israel S, Rissling M, Neikrug A, Trofimenko V, Natarajan L, Parker BA, et al. Light treatment prevents fatigue in women undergoing chemotherapy for breast cancer. Support. Care Cancer (2012) 20:1211–9. doi: 10.1007/s00520-011-1203-z
223. Johnson JA, Garland SN, Carlson LE, Savard J, Simpson JSA, Ancoli-Israel S, et al. Bright light therapy improves cancer-related fatigue in cancer survivors: a randomized controlled trial. J Cancer Surviv. (2018) 12:206–15. doi: 10.1007/s11764-017-0659-3
224. Valdimarsdottir HB, Figueiro MG, Holden W, Lutgendorf S, Wu LM, Ancoli-Israel S, et al. Programmed environmental illumination during autologous stem cell transplantation hospitalization for the treatment of multiple myeloma reduces severity of depression: A preliminary randomized controlled trial. Cancer Med (2018) 7:4345–53. doi: 10.1002/cam4.1690
225. Starreveld DEJ, Daniels LA, Kieffer JM, Valdimarsdottir HB, de Geus J, Lanfermeijer M, et al. Light therapy for cancer-related fatigue in (Non-)Hodgkin lymphoma survivors: Results of a randomized controlled trial. Cancers 2021 (2021) 13:4948. doi: 10.3390/CANCERS13194948
226. Fox RS, Baik SH, McGinty H, Garcia SF, Reid KJ, Bovbjerg K, et al. Feasibility and preliminary efficacy of a bright light intervention in ovarian and endometrial cancer survivors. Int J Behav Med (2021) 28:83–95. doi: 10.1007/s12529-020-09861-0
227. Neikrug AB, Rissling M, Trofimenko V, Liu L, Natarajan L, Lawton S, et al. Bright light therapy protects women from circadian rhythm desynchronization during chemotherapy for breast cancer. Behav Sleep Med (2012) 10:202–16. doi: 10.1080/15402002.2011.634940
228. Tahara Y, Aoyama S, Shibata S. The mammalian circadian clock and its entrainment by stress and exercise. J Physiol Sci (2017) 67:1–10. doi: 10.1007/s12576-016-0450-7
229. Aoyama S, Shibata S. The role of circadian rhythms in muscular and osseous physiology and their regulation by nutrition and exercise. Front Neurosci (2017) 11:63. doi: 10.3389/fnins.2017.00063
230. Mistlberger RE, Skene DJ. Social influences on mammalian circadian rhythms: animal and human studies. Biol Rev (2004) 79:533–56. doi: 10.1017/S1464793103006353
231. Baehr EK, Eastman CI, Revelle W, Olson SHL, Wolfe LF, Zee PC. Circadian phase-shifting effects of nocturnal exercise in older compared with young adults. Am J Physiol - Regul Integr Comp Physiol (2003) 284:R1542–50. doi: 10.1152/ajpregu.00761.2002
232. Barger LK, Wright KP, Hughes RJ, Czeisler CA. Daily exercise facilitates phase delays of circadian melatonin rhythm in very dim light. Am J Physiol - Regul Integr Comp Physiol (2004) 286:R1077–84. doi: 10.1152/ajpregu.00397.2003
233. Okamoto A, Yamamoto T, Matsumura R, Node K, Akashi M. An out-of-lab trial: A case example for the effect of intensive exercise on rhythms of human clock gene expression. J Circadian Rhythms (2013) 11:10. doi: 10.1186/1740-3391-11-10
234. Youngstedt SD, Kline CE, Elliott JA, Zielinski MR, Devlin TM, Moore TA. Circadian phase-shifting effects of bright light, exercise, and bright light + exercise. J Circadian Rhythms (2016) 14:1–8. doi: 10.5334/jcr.137
235. Lewis P, Korf HW, Kuffer L, Groß JV, Erren TC. Exercise time cues (zeitgebers) for human circadian systems can foster health and improve performance: A systematic review. BMJ Open Sport Exerc. Med (2018) 4:443. doi: 10.1136/bmjsem-2018-000443
236. Lewis P, Oster H, Korf HW, Foster RG, Erren TC. Food as a circadian time cue — evidence from human studies. Nat Rev Endocrinol (2020) 16:213–23. doi: 10.1038/s41574-020-0318-z
237. Kräuchi K, Cajochen C, Werth E, Wirz-Justice A. Alteration of internal circadian phase relationships after morning versus evening carbohydrate-rich meals in humans. J Biol Rhythms (2002) 17:364–76. doi: 10.1177/074873040201700409
238. Ryan JL, Carroll JK, Ryan EP, Mustian KM, Fiscella K, Morrow GR. Mechanisms of cancer-related fatigue. Oncologist (2007) 12:22–34. doi: 10.1634/theoncologist.12-S1-22
239. Challet E. “Circadian clocks, food intake, and metabolism,”. In: Progress in molecular biology and translational science. (Oxford: Elsevier B.V) (2013). p. 105–35. doi: 10.1016/B978-0-12-396971-2.00005-1
240. Yoshizaki T, Tada Y, Hida A, Sunami A, Yokoyama Y, Togo F, et al. Influence of dietary behavior on the circadian rhythm of the autonomic nervous system as assessed by heart rate variability. Physiol Behav (2013) 118:122–8. doi: 10.1016/j.physbeh.2013.05.010
241. Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obesity (2009) 17:2100–2. doi: 10.1038/oby.2009.264
242. Reid KJ, Baron KG, Zee PC. Meal timing influences daily caloric intake in healthy adults. Nutr Res (2014) 34:930–5. doi: 10.1016/j.nutres.2014.09.010
243. McHill AW, Phillips AJK, Czeisler CA, Keating L, Yee K, Barger LK, et al. Later circadian timing of food intake is associated with increased body fat. Am J Clin Nutr (2017) 106:1213–9. doi: 10.3945/ajcn.117.161588
244. Turgut M, Soylu Y, Metin SN. Physical activity, night eating, and mood state profiles of athletes during the COVID-19 pandemic. Prog Nutr (2020) 22:2020019. doi: 10.23751/pn.v22i2-S.10567
245. Frank E, Swartz HA, Kupfer DJ. Interpersonal and social rhythm therapy: Managing the chaos of bipolar disorder. Biol Psychiatry (2000) 48:593–604. doi: 10.1016/S0006-3223(00)00969-0
246. Haynes PL, Gengler D, Kelly M. Social rhythm therapies for mood disorders: an update. Curr Psychiatry Rep (2016) 18:75. doi: 10.1007/S11920-016-0712-3
247. Haghi M, Thurow K, Stoll R. Wearable devices in medical Internet of things: Scientific research and commercially available devices. Healthc. Inform. Res (2017) 23:4. doi: 10.4258/HIR.2017.23.1.4
248. Usak M, Kubiatko M, Shabbir MS, Dudnik OV, Jermsittiparsert K, Rajabion L. Health care service delivery based on the Internet of things: A systematic and comprehensive study. Int J Commun Syst (2020) 33:e4179. doi: 10.1002/DAC.4179
249. Innominato PF, Komarzynski S, Mohammad-Djafari A, Arbaud A, Ulusakarya A, Bouchahda M, et al. Clinical relevance of the first domomedicine platform securing multidrug chronotherapy delivery in metastatic cancer patients at home: The inCASA European project. J Med Internet Res (2016) 18:e6303. doi: 10.2196/JMIR.6303
250. Innominato P, Komarzynski S, Karaboué A, Ulusakarya A, Bouchahda M, Haydar M, et al. Home-based e-health platform for multidimensional telemonitoring of symptoms, body weight, sleep, and circadian activity: Relevance for chronomodulated administration of irinotecan, fluorouracil-leucovorin, and oxaliplatin at home–results from a pilot study. JCO Clin Cancer Inf (2018) 2, 1–15. doi: 10.1200/cci.17.00125
251. Komarzynski S, Huang Q, Innominato PF, Maurice M, Arbaud A, Beau J, et al. Relevance of a mobile Internet platform for capturing inter- and intrasubject variabilities in circadian coordination during daily routine: Pilot study. J Med Internet Res (2018) 20:e204. doi: 10.2196/JMIR.9779
252. Bouchahda M, Ulusakarya A, Thirot-Bidault A, Colle E, Attari A, Bossevot R, et al. A multicenter telemonitoring-telecare study with automatic assessment of physiological parameters and patient-reported outcomes in remote pancreatic cancer patients on mFOLFIRINOX: Interim technology report. J Clin Oncol (2022) 40:e13617–7. doi: 10.1200/JCO.2022.40.16_SUPPL.E13617
Keywords: circadian rhythms, cancer, sleep, fatigue, cognitive impairment, depressed mood
Citation: Amidi A and Wu LM (2022) Circadian disruption and cancer- and treatment-related symptoms. Front. Oncol. 12:1009064. doi: 10.3389/fonc.2022.1009064
Received: 01 August 2022; Accepted: 28 September 2022;
Published: 28 October 2022.
Edited by:
Alfredo Berruti, University of Brescia, ItalyReviewed by:
Francis Albert Lévi, University of Warwick, United KingdomRuifeng Ray Cao, University of Minnesota Twin Cities, United States
Copyright © 2022 Amidi and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ali Amidi, YWxpQHBzeS5hdS5kaw==
†These authors have contributed equally to this work and share first authorship
 Ali Amidi
Ali Amidi Lisa M. Wu
Lisa M. Wu