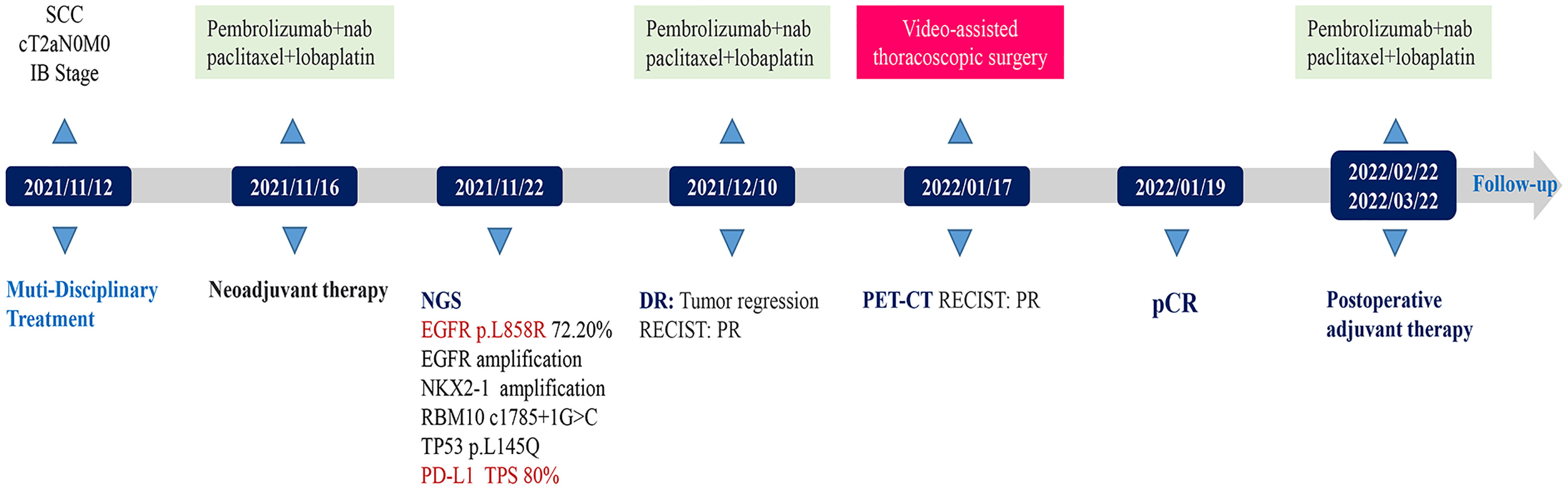
- 1Department of Thoracic Surgery, Chongqing General Hospital, Chongqing, China
- 2The Medical Department, 3D Medicines Inc., Shanghai, China
The treatment of lung cancer has fully entered the era of immunotherapy, which has significantly elevated the survival rate of patients with advanced non-small cell lung cancer (NSCLC), thus shedding light on resectable NSCLC. Previous clinical trial data suggested that neoadjuvant immuno-chemotherapy obtained a significant objective response rate (ORR) and disease control rate (DCR). Here, a case that achieved an excellent outcome following neoadjuvant immuno-chemotherapy was reported. The patient admitted to our hospital was 58 years old, female, with a rare case of stage IB lung squamous cell carcinoma (LUSC) harboring both epidermal growth factor receptor (EGFR) p.L858R mutations and high expression of programmed death ligand-1 (PD-L1) (tumor proportion score (TPS)=80%). Her tumor substantially shrunk following two cycles of neoadjuvant immuno-chemotherapy. The patient successively received single-port right upper thoracoscopic lobectomy + mediastinal lymph node dissection, which attained pathologic complete response (pCR). Additionally, the patient had grade 2 myelosuppression during the two cycles, which was treated with polyethylene glycol recombinant human granulocyte colony-stimulating factor (rhG-CSF). The patient was discharged uneventfully without any procedure-related complications. Two courses of adjuvant immuno-chemotherapy were administered postoperatively, leaving the patient in good physical condition at the 5-month follow-up visit. This case provided evidence for the feasibility and effectiveness of neoadjuvant immuno-chemotherapy in treating early-stage LUSC with EGFR mutations and high expression of PD-L1. However, randomized and multi-center controlled trials are required to validate the findings.
Introduction
As the molecular biology of lung cancer advances, immunotherapy and targeted therapy have become standard treatment protocols for advanced lung cancer in clinical practices with their application in early-stage lung cancer explored as well. Several clinical trials have proved the safety and efficacy of programmed death-1 (PD-1)/programmed death ligand-1 (PD-L1) inhibitors of neoadjuvant therapy in resectable non-small cell lung cancer (NSCLC) (1). The Checkmate 816 trial (2) was the first phase III randomized clinical trial to substantiate that neoadjuvant immuno-chemotherapy can significantly raise the percentage of pathologic complete response (pCR) in patients with resectable NSCLC, which was also the first and only preoperative neoadjuvant therapy protocol for lung cancer approved by the Food and Drug Administration (FDA). Moreover, identical to NEOSTAR (3) and NADIM (4) studies, patients with epidermal growth factor receptor (EGFR)+/anaplastic lymphoma kinase (ALK)+ were excluded and protein expression of PD-LI in baseline tumors was assessed in the Checkmate 816 trial, which revealed the association between up-regulated expression of PD-L1 and patient’s response to neoadjuvant immunotherapy. The targeted therapy exhibited prominent efficacy in treating NSCLC with EGFR mutations, especially the third generation of EGFR-tyrosine kinase inhibitors (EGFR-TKIs), which played essential roles in first/later line treatment and postoperative adjuvant therapy. However, only a few studies concerning neoadjuvant targeted therapy in lung cancer are available. NeoADAURA (5) and CTONG1103 (6) trials confirmed the efficacy and safety of neoadjuvant targeted therapy in patients with EGFR mutations, presenting superior clinical outcomes than neoadjuvant chemotherapy, while the effectiveness and safety of EGFR-TKIs of the neoadjuvant therapy for NSCLC remain undetermined. Hence, the optimal neoadjuvant therapy protocol is still undefined for NSCLC patients harboring both EGFR-sensitive mutations and PD-L1 positive. Here, we reported a case of stage IB LUSC with EGFR mutation, PD-L1 TPS=80%, and poor preoperative immune microenvironment who achieved pCR following neoadjuvant immuno-chemotherapy.
Case description
The 58-year-old patient was female and visited our hospital for one-month hemoptysis with no history of chronic diseases, smoking, and alcohol use (Figure 1). The X-ray and enhanced computed tomography (CT) demonstrated a mass in the upper lobe of the right lung (about 3.7 cm × 3.4 cm × 3.5 cm) with lobulated border and burrs, partially-occluded bronchial branches, and there were no obvious enlargement of mediastinal and bilateral hilar lymph nodes, and partial calcification. Central lung cancer was considered (Figures 2A–D). Carcinoembryonic antigen (CEA) was 86.98 ng/ml and the cytokeratin 19 fragment (CY21-1) was 4.06 ng/ml. Fiberoptic bronchoscopy indicated neoplasms in the posterior segment of the right upper lung, endoscopic biopsy suggested NSCLC (Figures 2G, H), and immunohistochemistry showed P40(+) and TTF-1 (–), suggestive of squamous cell carcinoma (Figures 3A, B). A few dyskaryotic cells were detected in bronchoalveolar lavage fluid (BALF). No significant metastasis was identified via upper abdominal enhanced CT scan, contrast-enhanced magnetic resonance imaging (MRI) of the head, and whole-body bone emission CT (ECT) scan. Taken together, the patient was classified as cT2aN0M0, stage IB. The patient’s tumor was closely related to the right upper pulmonary artery branch (Figure 2C), and the possibility of angioplasty during direct surgery was high, increasing the risk of surgery. There is also the possibility of conversion to thoracotomy, which would be more traumatic for the patient. Considering that the patient was a squamous carcinoma with a low possibility of having driver mutations, the multidisciplinary consultation recommended that neoadjuvant immune combination chemotherapy treatment should be performed first followed by surgery while waiting for NGS test and immunohistochemistry results. After signing the informed consent, the patient received anti-PD-1 therapy plus platinum-based neoadjuvant therapy (intravenous injection of 200 mg Pembrolizumab, 30 mg/m2 lobaplatin + 260 mg/m2 nab-paclitaxel, D1, q3w). The patient developed grade 2 myelosuppression during therapy, which was treated with an injection of polyethylene glycol recombinant human granulocyte colony-stimulating factor (rhG-CSF) with no grade 3-5 adverse events (AEs) observed. In the meantime, next-generation sequencing (NGS) and immunohistochemical(IHC) detected EGFR p.L858R mutations (abundance 72.2%), PD-L1 expression (TPS=80%, CPS(Combined Positive Score)=80), and tumor mutational burden (TMB, 5.03Mut/Mb), accompanied by EGFR copy-number amplification as well as multiple gene mutations, such as NKX2-1 and TP53. Considering the occurrence of EGFR mutation, the followed therapy was dilemma, which dependent on the outcome of the first treatment session as informed before. After one course of neoadjuvant immunochemotherpy, CEA and CY21-1 were 23.17 ng/ml and 2.43 ng/ml, respectively, significantly lower than the pre-treatment levels. In the meantime, chest digital radiography (DR) showed a reduction of the right upper lung occupancy (Figures 2E, F), implying partial radiographical response. So, the second cycle immunochemotherapy was perfomed as plan. Three weeks later, subsequent to the application of the second course of neoadjuvant therapy, a chest X-ray and enhanced CT scan were performed, indicating a marked reduction of the lesion in the right upper lung(about 1.9 cm × 1.4 cm) with partially bronchial stricture. At this point, CEA and CY21-1 were 3.06 ng/ml and 1.81 ng/ml respectively. Besides, positron emission tomography-computed tomography (PET-CT) illustrated an irregular patchy hyperdense shadow (2.76 cm × 2.18 cm × 1.59 cm) near the hilum of the right lung upper lobe and a mild increase in radioactivity uptake (maximum SUV = 2.57, mean SUV = 2.37), indicating that the tumor activity was remarkably suppressed (Figures 2I-L). Partial response (PR) was assessed according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. In the fifth week after the neoadjuvant therapy, the single-port right upper thoracoscopic lobectomy + mediastinal lymph node dissection was successfully performed, during which the blood loss was approximately 50 ml. Postoperative pathology (Figures 3C, D)showed that the mass was 2.5 cm × 2 cm × 1.5 cm and that the fibrous tissue of the tumor bed exhibited hyperplasia with degeneration. Lymphocytes infiltrated the areas containing multinucleated giant cells and foam cells without residual disease; the bronchial mucosa manifested chronic inflammation without residualdisease. Efficacy assessment of neoadjuvant therapy showed that pCR was achieved. The lymph nodes of 2R (0/2), 3A (0/2), 4R (0/1), group 7 (0/1), group 10 (0/1), group 11 (0/7), and group 12 (0/1) were disease-free. The bronchial stump of the right upper lung was also free of cancer cells. The patient recovered well and was discharged without any operation-related complications. Biopsy tissues and surgical samples were examined using multiplex immunohistochemistry (mIHC) to reveal the alterations of the tumor microenvironment (TME) after the application of neoadjuvant therapy, especially the inflammatory and immune cells. mIHC of biopsy tissues (Figures 3E, F) illustrated intratumoral presence of CD8+ T cells (111/mm2), and high infiltration of FoxP3 (244/mm2) and CD68 + CD163 + M2 macrophages (139/mm2). In the surgical samples (Figures 3G, H), large amounts of CD8+ T cells (511/mm2) and tertiary lymphoid structures were observed, while the number of CD68 + CD163 + M2 macrophages (32/mm2) and FoxP3+ (60/mm2) were substantially decreased. No genetic mutation was detected in the ctDNA of peripheral blood at the fourth postoperative week. The patient started receiving two cycles of adjuvant immuno-chemotherapy (intravenous injection of 200 mg Pembrolizumab, 30 mg/m2 lobaplatin + 260 mg/m2 nab-paclitaxel, D1, q3w) at the fifth week after surgery. The patient was in good physical condition at the 5-month follow-up visit and refused to accept the one-year single-agent immunotherapy due to financial factors.
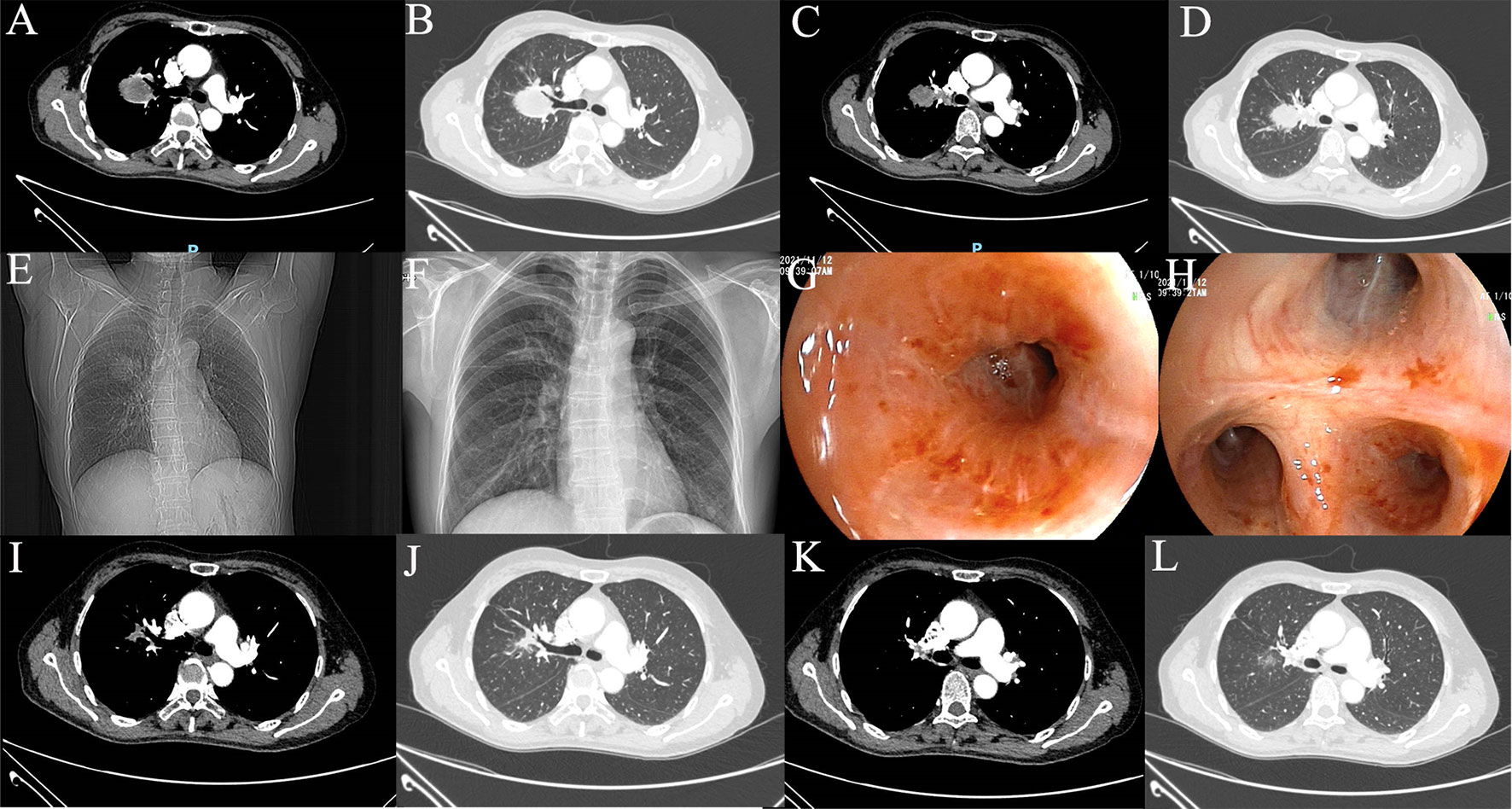
Figure 2 Imaging examination. (A–D) Preoperative CT scan; (E) Preoperative DR image; (F) DR image after one cycle of neoadjuvant therapy; (G, H) Preoperative fiberoptic bronchoscopy image; (I–L) CT image after 2 cycles of neoadjuvant therapy.
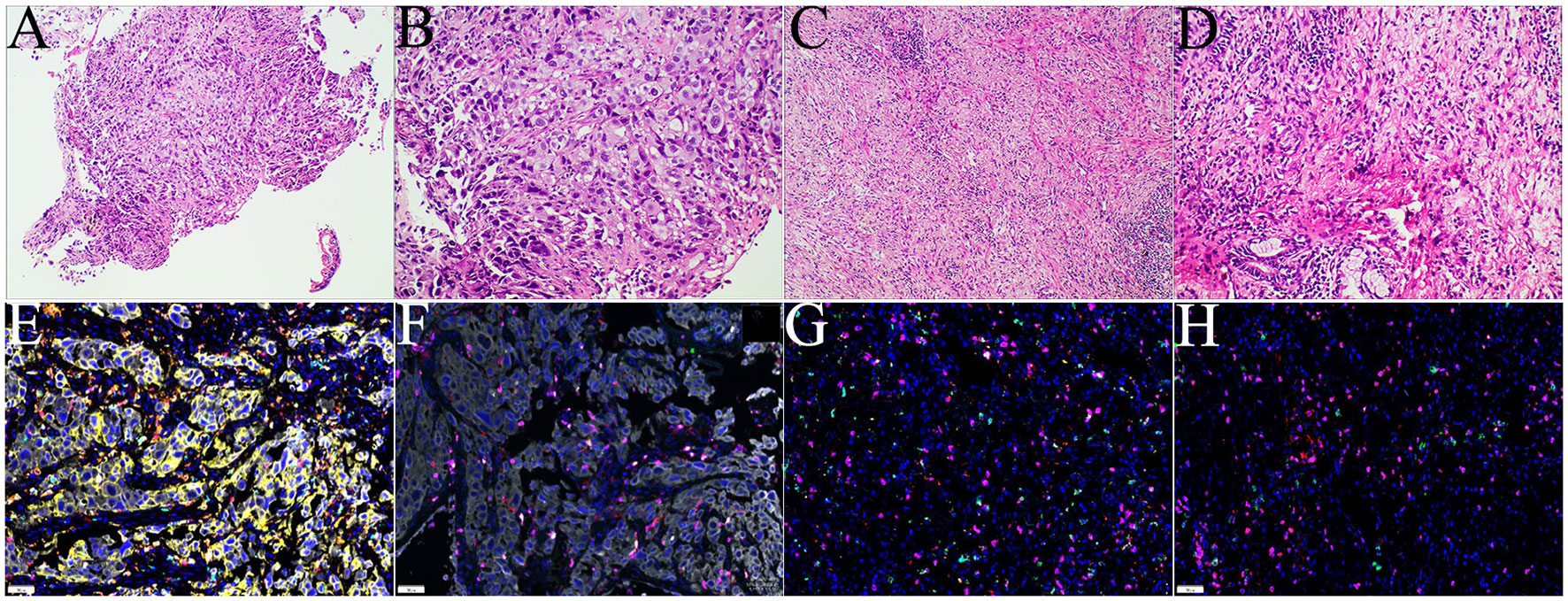
Figure 3 Examinations of pathology and mIHC. (A, B). Histology of biopsies samples (CT guided biopsy);(C, D) histology of surgical tissue sample; (E, F) mIHC staining of tumor microenvironment of puncture tissue; (G, H) mIHC staining of tumor microenvironment after operation.
Discussion
Multiple clinical studies (7) proved that neoadjuvant immune monotherapy or combined treatment played a crucial part in the treatment of early-stage NSCLC. The major pathologic response (MPR) rate of neoadjuvant immune monotherapy was 17% - 45% and the pCR was unsatisfactory (0%-16%), while the immuno-chemotherapy resulted in higher MPR (36.9%-83%) and pCR (24%-63%) with manageable AEs and no delay of the surgical schedule. Nevertheless, most of these studies excluded patients with EGFR+/ALK+. For advanced NSCLC patients with PD-L1 TPS >50%, immune checkpoint inhibitors(ICIs) prolonged the overall survival(OS) of patients with greater safety, as compared to the platinum-based doublet chemotherapy (8). Under neoadjuvant therapy, patients with high PD-L1 expression also tended to exhibit a higher response rate to immunotherapy. In the Checkmate 816 trial (2), neoadjuvant immuno-chemotherapy was confirmed to be effective in treating stage IB lung cancer, in which the pCR reached 40% and the event-free survival (EFS) risk was lowered by 76% in patients with PD-L1>50%.
EGFR or ALK gene alterations occur in patients with LUSC, especially non-smoking females, in which TKI treatments present clinical benefits in disease control with a considerably shorter drug-resistant duration in LUSC patients than in lung adenocarcinoma (LUAD) patients (9). A previous study (10) reported that the first-line pembrolizumab treatment was superior to conventional chemotherapy for lung cancer patients with high PD-L1 expression, and the subgroup analysis showed that pembrolizumab was more effective in treating LUSC than non-LUSC. Besides, the subgroup analysis conducted in the Checkmate 816 trial demonstrated the efficacy of preoperative neoadjuvant immono-chemotherapy in both LUAD and LUSC, while the efficacy was more significant in LUAD (HR: 0.5 vs HR: 0.77); this therapy also reduced the risk of recurrence or mortality by 23% (HR = 0.77) among LUSC patients versus chemotherapy alone, exhibiting therapeutic advantage (2). A cell experiment performed by Zhang et al. (11) revealed that higher PD-L1 expression was associated with lower sensitivity to EGFR-TKI of EGFR-mutant NSCLC cell lines, which leads to epithelial-to-mesenchymal transition (EMT) induced by the upregulation of Smad3 phosphorylation, potentially contributing to primary drug resistance.
Previous studies have reported that the efficacy of ICIs among NSCLC patients with EGFR mutations was minimal, and even EGFR alteration was considered to be related to the hyperprogression induced by ICIs, especially-monotherapy. In a single-center retrospective study (12), the objective response rate (ORR) yielded by immunotherapy of driver mutation-positive lung cancer was only 3.8%; while in another phase 2 clinical trial (NCT02879994), the ORR yielded by first-line pembrolizumab monotherapy was 0% among seven advanced NSCLC patients with EGFR mutations and strong PD-L1 expression (13). However, in the trials of IMPOWER 150, KEYNOTE-789, and CheckMate-722 (14–16), the advanced NSCLC patients with high PD-L1 expression and EGFR mutations responded well to immunotherapy combined with chemotherapy or anti-vascular endothelial growth factor (VEGF)agents. Collectively, ICIs should not be completely excluded in the treatment of EGFR-mutated NSCLC.
Several clinical trials also investigated the safety and efficacy of ICIs plus EGFR-TKIs. Jänne et al. (17) revealed that in advanced EGFR-mutated NSCLC patients administered with first-line osimertinib plus durvalumab, ORR was 82% (48–98), the median duration of response (DOR) was 7.1 months and median progression-free survival (PFS) was 9.0 months. However, the enrollment in this patient cohort was terminated due to the potential risk of interstitial lung disease (ILD)-related AEs. Considering the increased risk of treatment-related AEs as well as limited efficacy and benefits, the treatment strategy of ICIs plus EGFR-TKIs is possibly unacceptable.
A preclinical study (18) discovered that the activation of the EGFR pathway up-regulated the expression of PD-1, PD-L1, cytotoxic T lymphocyte antigen-4 (CTLA-4), and proinflammatory cytokines, rendering the feature of immunosuppression. This suggested that oncogenic EGFR signaling triggered immune escape by remodeling the TME. In the present case, the patient’s postoperative TME showed a significant increase of CD8 + T cells and an evident decrease of FoxP3 and CD68+CD163+ M2 macrophages(Supplementary Figure), together with the occurrence of tertiary lymphoid structure. T cells suppressed the anti-tumor functions of immune effector cells and were involved in tumor immune escape (19); M2 macrophages secreted anti-inflammatory cytokines and modulated wound healing to exert the immunosuppressive effects (20), and the tertiary lymphoid structure was associated with a promising prognosis immunotherapy response (21). Immuno-chemotherapy can directly kill cancer cells and regulate immune response to improve the tumor-immune microenvironment (TIME), thereby achieving better efficacy (22).
The ORR of neoadjuvant EGFR- TKI in the EGFR-sensitive mutation population is 48% and early stage NSCLC may reduce the ORR of neoadjuvant EGFR-TKIs (23), and the MPR of neoadjuvant Osimertinib was 15% (5). However the evidence for neoadjuvant targeted therapy especially in these subgroups of EGFR mutation status, PD-L1 expression is still not sufficient. In our case, ICIs were administered prior to EGFR-TKIs, given the low occurrence of driver gene mutation in LUSC, and the limited benefits of EGFR-TKIs. Besides, ICIs were more effective for LUSC, and their combination with chemotherapy might exhibit better efficacy with unknown expression of PD-L1. Due to the imaging remission, the treatment protocol was kept unchanged when the NGS- and IHC suggested high PD-L1 expression and EGFR p.L858R mutations accompanied by variation in multiple genes such as EGFR amplification. Finally, the patient underwent complete tumor resection and achieved pCR without grade 3-5 AEs during the treatment.
It was reported (24) that after receiving the first-line immuno-monotherapy, an elderly patient with metastatic squamous cell lung cancer harboring both EGFR mutation and high PD-L1 expression presented with continuously aggravated conditions and died 6 months later. Additionally, Oguri T et al. (25) reported that atezolizumab monotherapy led to immunotherapy-related hyperprogression in a patient with EGFR p.L858R-mutated pulmonary pleomorphic carcinoma. Similar to the above two cases, our case also involved high PD-L1 expression and EGFR p.L858R mutations with FOXP3 and M2 macrophages in the preoperative immune microenvironment, which was associated with the development of hyper progressive diseases (HPDs). Yet the tumor was obviously diminished without signs of HPD following the neoadjuvant immuno-chemotherapy, which may be attributed to the effects of immuno-chemotherapy on TIME and the good prognosis the therapy yielded. Still, HPD requires further exploration. Furthermore, Li et al. (26) reported an NSCLC patient with high PD-L1 expression, EGFR mutations, and cold tumors who experienced disease progression 3 weeks after neoadjuvant immuno-chemotherapy with LUAD different from LUSC in our case. Huang et al. (27) revealed that the squamous cell carcinoma components of lung adenosquamous adenocarcinoma were likely transformed from adenocarcinoma components and that EGFR-TKIs were effective for advanced EGFR-mutant lung adenosquamous carcinoma. Nevertheless, further researches on the immune microenvironment and immunotherapy of lung adenosquamous carcinoma need to be carried out.
Some limitations are present in this case. On the one hand, only one individual case was reported, thus it is tricky to promote this treatment protocol. On the other hand, due to the heterogeneity of tumor tissues, initial findings in pathologic biopsy were potentially inconsistent with the final ones, which means that adenosquamous carcinoma or inconsistent gene expression were probable, posing a potential risk for the selection of optimal neoadjuvant therapy modality.
Taken together, we reported a patient with stage IB NSCLC and LUSC harboring high PD-L1 expression and EGFR mutations who achieved pCR after two courses of neoadjuvant therapy of platinum-based chemotherapy plus pembrolizumab. This case illustrated the feasibility and effectiveness of neoadjuvant immuno-chemotherapy in treating early LUSC with EGFR mutations and high PD-L1 expression. Nonetheless, the conclusion requires validation in more patient samples. Moreover, therapeutic genes and biomarkers need to be further explored to select the optimal treatment protocol for clinical scenarios with co-existing PD-L1 upregulation and EGFR sensitivity mutation.
Data availability statement
The datasets presented in this article are not readily available because of ethical/privacy restrictions. Requests to access the datasets should be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Chongqing General Hospital of ethics committee. The patients/participants provided their written informed consent to participate in this study.
Author contributions
ZM was responsible for the organization and coordination of the case. XX was the attending physician. XX, ZS, and DH contributed to data collection. XX and DF were responsible for original draft preparation. All authors contributed to the article and approved the submitted version.
Conflict of interest
DF and DH were employed by the 3D Medicines Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1008932/full#supplementary-material
References
1. Provencio M, Calvo V, Romero A, Spicer JD, Cruz-Bermudez A. Treatment sequencing in resectable lung cancer: The good and the bad of adjuvant versus neoadjuvant therapy. Am Soc Clin Oncol Educ Book (2022) 42:1–18. doi: 10.1200/EDBK_358995
2. Forde PM, Spicer J, Lu S, Provencio M, Mitsudomi T, Awad MM, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med (2022) 386(21):1973–85. doi: 10.1056/NEJMoa2202170
3. Cascone T, William WN Jr., Weissferdt A, Leung CH, Lin HY, Pataer A, et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: the phase 2 randomized NEOSTAR trial. Nat Med (2021) 27(3):504–14. doi: 10.1038/s41591-020-01224-2
4. Provencio M, Nadal E, Insa A, García-Campelo MR, Casal-Rubio J, Dómine M, et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol (2020) 21(11):1413–22. doi: 10.1016/S1470-2045(20)30453-8
5. Tsuboi M, Weder W, Escriu C, Blakely C, He J, Dacic S, et al. Neoadjuvant osimertinib with/without chemotherapy versus chemotherapy alone for EGFR-mutated resectable non-small-cell lung cancer: NeoADAURA. Future Oncol (2021) 17(31):4045–55. doi: 10.2217/fon-2021-0549
6. Zhong WZ, Chen KN, Chen C, Gu CD, Wang J, Yang XN, et al. Erlotinib versus gemcitabine plus cisplatin as neoadjuvant treatment of stage IIIA-N2 EGFR-mutant non-Small-Cell lung cancer (EMERGING-CTONG 1103): A randomized phase II study. J Clin Oncol (2019) 37(25):2235–45. doi: 10.1200/JCO.19.00075
7. Chen S, Zhao Z, Long H. Application of neoadjuvant immuno-chemotherapy in NSCLC. Zhongguo Fei Ai Za Zhi (2021) 24(4):284–92. doi: 10.3779/j.issn.1009-3419.2021.102.10
8. Mok TSK, Wu Y-L, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet (2019) 393(10183):1819–30. doi: 10.1016/S0140-6736(18)32409-7
9. Lewis WE, Hong L, Mott FE, Simon G, Wu CC, Rinsurongkawong W, et al. Efficacy of targeted inhibitors in metastatic lung squamous cell carcinoma with EGFR or ALK alterations. JTO Clin Res Rep (2021) 2(11):100237. doi: 10.1016/j.jtocrr.2021.100237
10. Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Pembrolizumab versus chemotherapy for PD-L1-Positive non-Small-Cell lung cancer. N Engl J Med (2016) 375(19):1823–33. doi: 10.1056/NEJMoa1606774
11. Zhang Y, Zeng Y, Liu T, Du W, Zhu J, Liu Z, et al. The canonical TGF-beta/Smad signalling pathway is involved in PD-L1-induced primary resistance to EGFR-TKIs in EGFR-mutant non-small-cell lung cancer. Respir Res (2019) 20(1):164. doi: 10.1186/s12931-019-1137-4
12. Gainor JF, Shaw AT, Sequist LV, Fu X, Azzoli CG, Piotrowska Z, et al. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: A retrospective analysis. Clin Cancer Res (2016) 22(18):4585–93. doi: 10.1158/1078-0432.CCR-15-3101
13. Lisberg A, Cummings A, Goldman JW, Bornazyan K, Reese N, Wang T, et al. A phase II study of pembrolizumab in EGFR-mutant, PD-L1+, tyrosine kinase inhibitor naive patients with advanced NSCLC. J Thorac Oncol (2018) 13(8):1138–45. doi: 10.1016/j.jtho.2018.03.035
14. Reck M, Mok TSK, Nishio M, Jotte RM, Cappuzzo F, Orlandi F, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med (2019) 7(5):387–401. doi: 10.1016/S2213-2600(19)30084-0
15. Riely G, Hui R, Carbone D, Park K, Carrigan M, Xu X, et al. P1.01-81 phase 3 study of pemetrexed-platinum with or without pembrolizumab for TKI-Resistant/EGFR-Mutated advanced NSCLC: KEYNOTE-789. J Thorac Oncol (2018) 13(10):S494. doi: 10.1016/j.jtho.2018.08.637
16. Park K, Yang JC-H, Girard N, Mok T, Gainor J, Nakagawa K, et al. Nivolumab + chemotherapy vs chemotherapy in EGFR-mutated NSCLC after 1L or 2L EGFR-TKIs (CheckMate 722). Ann Oncol (2019) 30:vi126. doi: 10.1093/annonc/mdz343.039
17. Ahn MJ, Cho BC, Ou X, Walding A, Dymond AW, Ren S, et al. Osimertinib plus durvalumab in patients with EGFR-mutated, advanced NSCLC: A phase 1b, open-label, multicenter trial. J Thorac Oncol (2022) 17(5):718–23. doi: 10.1016/j.jtho.2022.01.012
18. Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, Christensen CL, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov (2013) 3(12):1355–63. doi: 10.1158/2159-8290.CD-13-0310
19. Toker A, Ohashi PS. Expression of costimulatory and inhibitory receptors in FoxP3(+) regulatory T cells within the tumor microenvironment: Implications for combination immunotherapy approaches. Adv Cancer Res (2019) 144:193–261. doi: 10.1016/bs.acr.2019.05.001
20. Fang P, Li X, Dai J, Cole L, Camacho JA, Zhang Y, et al. Immune cell subset differentiation and tissue inflammation. J Hematol Oncol (2018) 11(1):97. doi: 10.1186/s13045-018-0637-x
21. Lauss M, Donia M, Svane IM, Jönsson G. B cells and tertiary lymphoid structures: Friends or foes in cancer immunotherapy? Clin Cancer Res (2022) 28(9):1751–8. doi: 10.1158/1078-0432.CCR-21-1130
22. Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-Small-Cell lung cancer. N Engl J Med (2018) 378(22):2078–92. doi: 10.1056/NEJMoa1801005
23. Shi X, Dong X, Zhai J, Liu X, Lu D, Ni Z, et al. Current evidence of the efficacy and safety of neoadjuvant EGFR-TKIs for patients with non-small cell lung cancer. Front Oncol (2021) 11:608608. doi: 10.3389/fonc.2021.608608
24. Hamai K, Tanahashi H, Ueno S, Konishi H, Matsumura M, Nomura A, et al. First-line immune checkpoint therapy in metastatic squamous cell lung cancer harboring both EGFR mutation and high expression of PD-L1: A case report. Thorac Cancer (2020) 11(6):1716–9. doi: 10.1111/1759-7714.13436
25. Oguri T, Sasada S, Seki S, Murata S, Tsuchiya Y, Ishioka K, et al. A case of hyperprogressive disease following atezolizumab therapy for pulmonary pleomorphic carcinoma with epidermal growth factor receptor mutation. Respir Med Case Rep (2021) 33:101405. doi: 10.1016/j.rmcr.2021.101405
26. Zhao Q, Zhang X, Ma Q, Luo N, Liu Z, Wang R, et al. Case report: An “Immune-cold” EGFR mutant NSCLC with strong PD-L1 expression shows resistance to chemo-immunotherapy. Front Oncol (2022) 12:765997. doi: 10.3389/fonc.2022.765997
Keywords: neoadjuvant immunotherapy, lung squamous cell carcinoma, epidermal growth factor receptor (EGFR), mutations, pembrolizumab, programmed death ligand-1 (PD-L1)
Citation: Xu X, Shi Z, Fu D, Huang D and Ma Z (2022) EGFR mutations and high PD-L1 expression of lung squamous cell carcinoma patients achieving pCR following neoadjuvant immuno-chemotherapy: Case report. Front. Oncol. 12:1008932. doi: 10.3389/fonc.2022.1008932
Received: 03 August 2022; Accepted: 27 September 2022;
Published: 19 October 2022.
Edited by:
Kohei Fujita, National Hospital Organization Kyoto Medical Center, JapanReviewed by:
Jin Kang, Guangdong Provincial People’s Hospital, ChinaSong Xu, Tianjin Medical University General Hospital, China
Copyright © 2022 Xu, Shi, Fu, Huang and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zheng Ma, ZHJtYXIyMDIxQDE2My5jb20=
 Xiangyu Xu
Xiangyu Xu Zixia Shi1
Zixia Shi1 Depei Huang
Depei Huang Zheng Ma
Zheng Ma