
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 19 October 2022
Sec. Thoracic Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1008326
Background: The purpose of this study was to analyze the clinical characteristics and prognosis of EPEC and to construct a prediction model based on the SEER database.
Methods: All EPECs from the SEER database were retrospectively analyzed. A comprehensive and practical nomogram that predicts the overall survival (OS) of EPEC was constructed. Univariate and multivariate Cox regression analysis was performed to explore the clinical factors influencing the prognosis of EPEC, and finally, the 1 -, 3 - and 5-year OS were predicted by establishing the nomogram. The discriminant and predictive ability of the nomogram was evaluated by consistency index (C-index), calibration plot, area under the curve (AUC), and receiver operating characteristic (ROC) curve. Decision curve analysis (DCA) was used to evaluate the clinical value of the nomogram.
Results: A total of 3478 patients diagnosed with EPEC were extracted from the SEER database, and the data were randomly divided into the training group (n=2436) and the validation group (n=1402). T stage, N stage, M stage, surgery, chemotherapy, radiotherapy, age, grade, and tumor size were independent risk factors for 1 -, 3 - and 5-year OS of EPEC (P< 0.05), and these factors were used to construct the nomogram prediction mode. The C-index of the validation and training cohorts was 0.718 and 0.739, respectively, which were higher than those of the TNM stage system. The AUC values of the nomogram used to predict 1-, 2-, and 3-year OS were 0.751, 0.744, and 0.786 in the validation cohorts (0.761, 0.777, 0.787 in the training cohorts), respectively. The calibration curve of 1-, 2-, and 3-year OS showed that the prediction of the nomogram was in good agreement with the actual observation. The nomogram exhibited higher clinical utility after evaluation with the 1-, 2-, and 3-year DCA compared with the AJCC stage system.
Conclusions: This study shows that the nomogram prediction model for EPEC based on the SEER database has high accuracy and its prediction performance is significantly better than the TNM staging system, which can accurately and individually predict the OS of patients and help clinicians to formulate more accurate and personalized treatment plans.
Esophageal cancer has become one of the most common malignant tumors in the world. According to global cancer statistics in 2020, the number of new cases of esophageal cancer reached 604,000 and the number of deaths reached 544,000 (1). The incidence of esophageal cancer in the aged gradually increases with the aging of the population (2). Most elderly patients often difficult to accept surgical treatment due to a lot of past medical history, organ function decline, poor physical condition, and other reasons, and even give up chemotherapy and choose radiotherapy as its radical treatment (3). Diabetes and hypertension are common medical diseases in the elderly, and their incidence continues to increase. There are few clinical studies on whether these basic diseases have an impact on the toxic side effects and efficacy of radiotherapy (4). Symptoms appear at an advanced stage due to a general lack of responsiveness in the elderly.
The prognostic factors of EPEC are still controversial. Currently, the TNM (Tumor-Node-Metastasis) staging system is considered the most widely used prognostic assessment system and clinical treatment of cancer patients, but it only includes the depth of local tumor invasion, the range of regional lymph node metastasis, and the state of distant metastasis (5). However, many important clinical features may potentially affect the prognosis of esophageal cancer. Therefore, the main aim of this study is to develop richer and more accurate prognostic models to guide survival.
The alignment diagram, also known as the nomogram diagram, is based on multi-factor regression analysis, integrating multiple prediction factors and drawing them in a certain proportion on the same plane with graduated line segments, so as to express the relationship between variables in the prediction model. In this study, based on the data of the SEER (Surveillance, Epidemiology, and End Results) database, the clinicopathological features affecting the prognosis of EPEC were discussed for the first time and the prognostic variables were further studied. Finally, we further construct a nomogram model to predict the prognosis of EPEC.
The study was based on clinical data from 18 (SEER) cancer registries. In this study, SEER*Stat software (version 8.4) was used to search the SEER database for patients older than 65 years of age with primary esophageal cancer from 2010 to 2015. Inclusion criteria of this study: (I) Primary esophageal cancer; (II) The years of diagnosis were from 2010 to 2015; (III) Single primary tumor; (IV) Pathological diagnosis is clear; (V). Older than 65. Exclusion criteria: (I) No follow-up time; (II) Incomplete data; (III) Younger than 65 years old. All data in this study were extracted from the SEER database free of charge.
Statistical analysis was performed using SPSS 25.0 software and R language 3.6.1. Patients were randomly divided into training set and validation set by 7∶3 to construct this nomogram. The cut-off values of continuous variables were determined by X-tile software and converted into classified variables. We performed a descriptive analysis of the clinical baseline data of the enrolled patients and used the Chi-square test to compare the characteristics of patients in the training and validation groups. COX hazard ratio model was used to analyze the factors influencing the survival and prognosis of patients in the training set. Factors of P<0.05 were included in the multifactor analysis to determine the final independent prognostic factors, and the nomogram containing these independent prognostic factors was constructed using R language. Internal and external validation was carried out in the training set and validation set, respectively.
The prediction effect of this model is evaluated by the area under (AUC) the receiver operating characteristic curve (ROC). The discriminative power of the model was evaluated by the concordance index (C-index). The clinical utility was analyzed using a decision curve analysis (DCA). DCA represents the net benefit of clinical decision-making. The Y-axis represents the net benefit and the X-axis represents the risk threshold. P < 0.05 was considered statistically significant.
Sex, age, race, T stage, N stage, M stage, pathological type, radiotherapy, chemotherapy, surgery, tumor location, pathological grade, and tumor size were included in the analysis. According to the inclusion and exclusion criteria, a total of 3478 eligible patients were screened from the SEER database between 2010 and 2015. A complete flow chart describing the selection process is shown on Figure 1. One-third of the patients were randomly assigned to the validation group and the rest were used to construct the nomogram prediction model. The detailed clinicopathological features of all cases were shown in Table 1.
The cut-off values of continuous variables were determined by X-tile software and converted into classified variables. Univariate Cox regression analysis showed that the factors influencing the prognosis of old esophageal cancer patients were race, tumor site, T stage, N stage, M stage, surgery, chemotherapy, radiotherapy, age (65-71 years, 72-83 years, and >83 years), histology, grade, and tumor size (<39mm, 39-62mm, and >62mm). The above 12 factors were again included in the multivariate Cox regression analysis, and the results showed that T stage, N stage, M stage, surgery, chemotherapy, radiotherapy, age, grade, and tumor size were independent factors influencing the prognosis of old esophageal cancer (Table 2).
Nine statistically significant independent prognostic factors were included in the above multivariate COX proportional regression model to construct a nomogram to predict 3-year and 5-year overall survival (Figure 2). Individual scores can be read for each clinicopathological indicator in each patient, and the scores are added together to obtain an overall score.
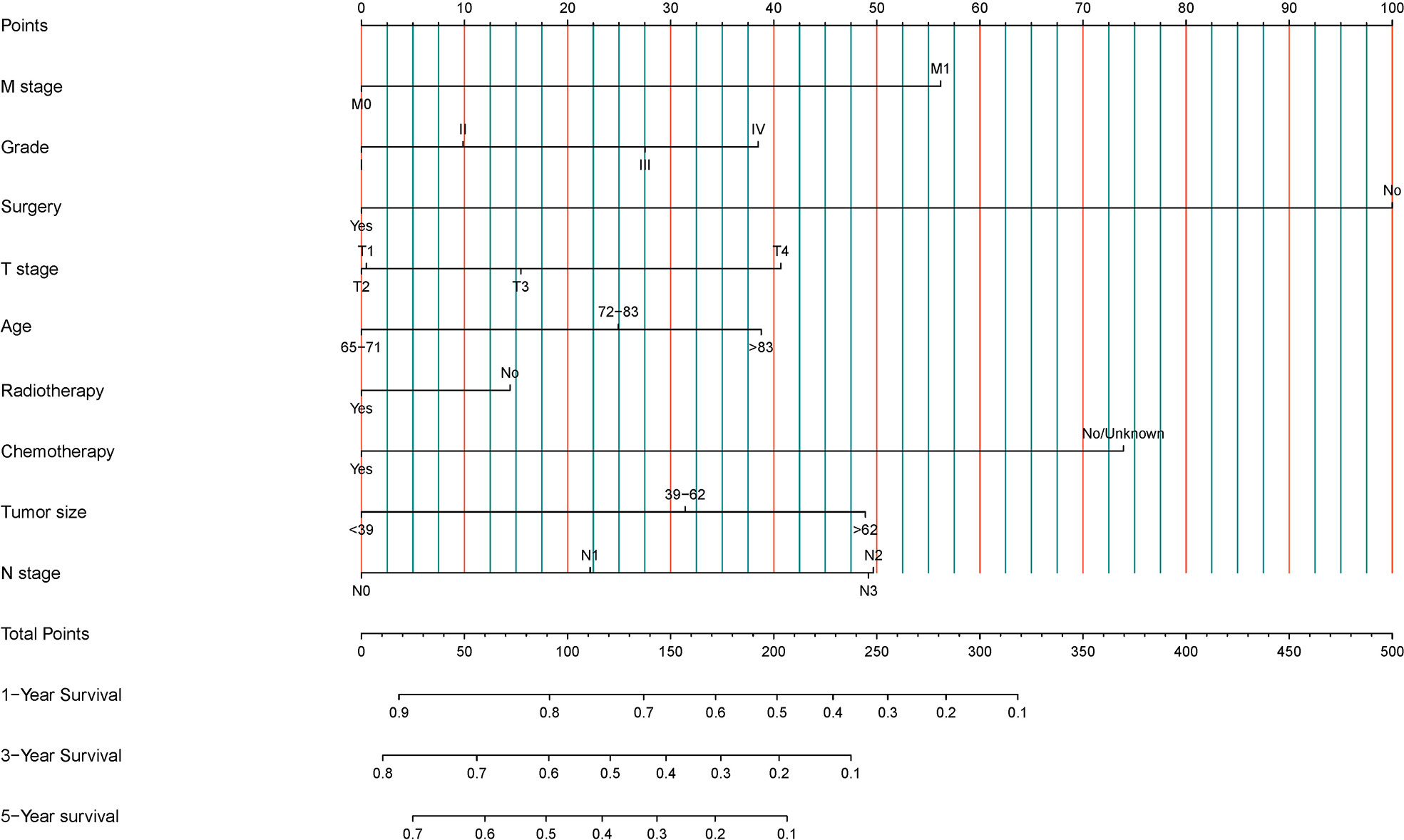
Figure 2 Development of a prognostic stratification nomogram and validation of the proposed nomogram.
Compared with the AJCC staging system, the C-index of the training cohorts and validation cohorts were 0.739 (95%CI: 0.727~0.750) and 0.718 (95%CI: 0.700~0.736), respectively, indicating that the nomogram had the good predictive ability, while C-indices of the AJCC stage system were 0.642 (95% CI: 0.627–0.656) and 0.630 (95% CI: 0.608–0.653) in the training cohorts and the validation cohorts, respectively.
For OS, this study draws the area under the ROC curve (AUC) of the nomogram prediction model and TNM staging system (as shown in Figure 3), which intuitively shows the performance of the nomogram prediction model is better than that of the TNM staging system. In the training cohorts, the 1-, 3-, and 5-year OS of AUC of this nomogram was superior to that of the AJCC stage system (1-year OS AUC: 0.8 vs. 0.689, 3-year OS AUC: 0.822 vs. 0.734, 5-year OS AUC: 0.824 vs. 0.752, respectively, Figures 3A–C), whereas the AUC of this nomogram and the AJCC stage system is shown in Figures 3D–F for the validation cohorts (1-year OS AUC: 0.772 vs. 0.752, 2-year OS AUC: 0.788 vs. 0.752, 3-year OS AUC: 0.784 vs. 0.752).
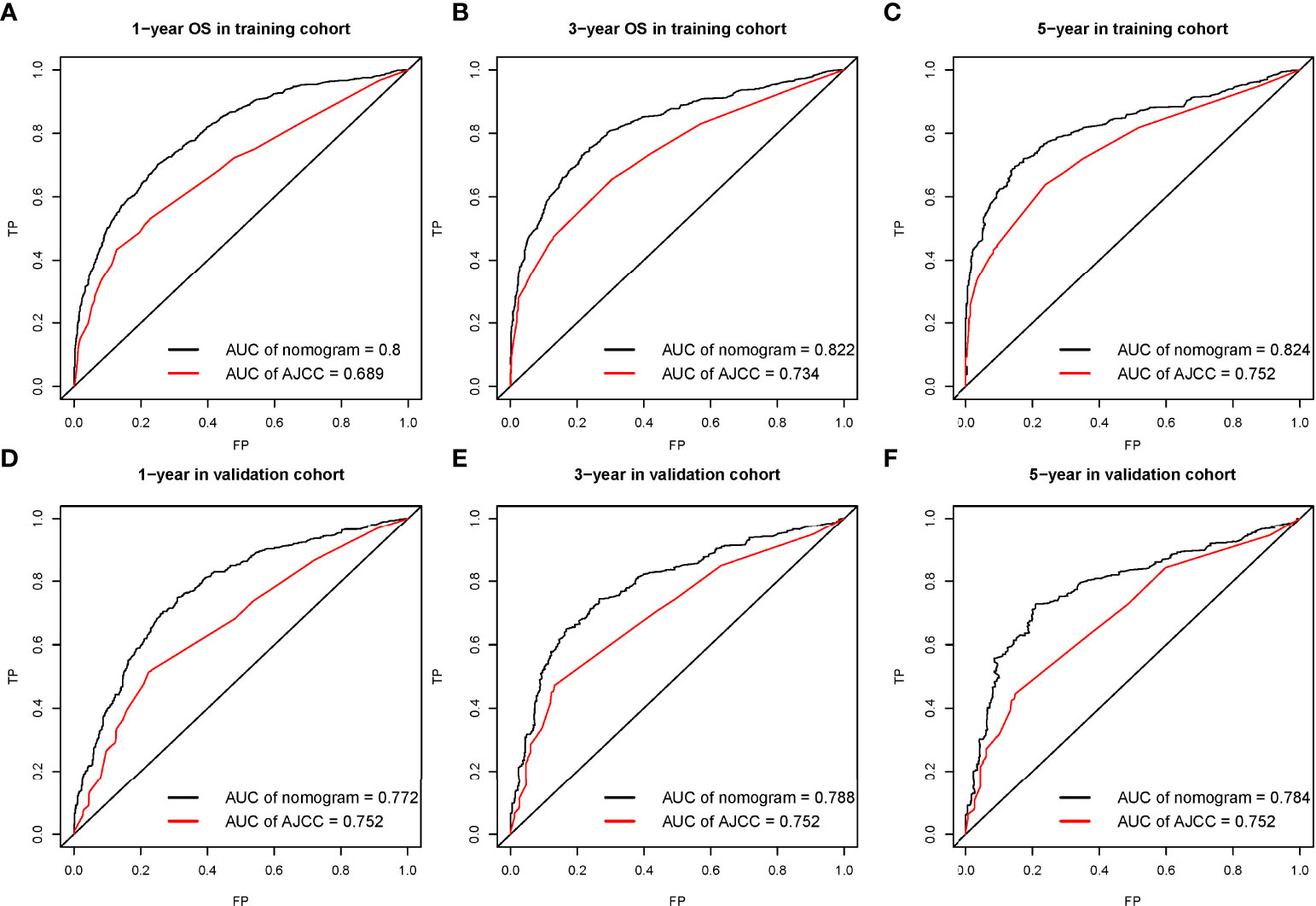
Figure 3 The receiver operating characteristic (ROC) curve for nomogram in the training cohort (A–C) and validation cohort (D–F) at 1-year, 3-year, and 5-year, respectively.
The calibration curve shows that there was a high degree of agreement between the nomogram prediction and the actual 1-, 3-, and 5-year OS in the training cohorts (Figures 3A–C) and the validation cohorts (Figures 4D–F).
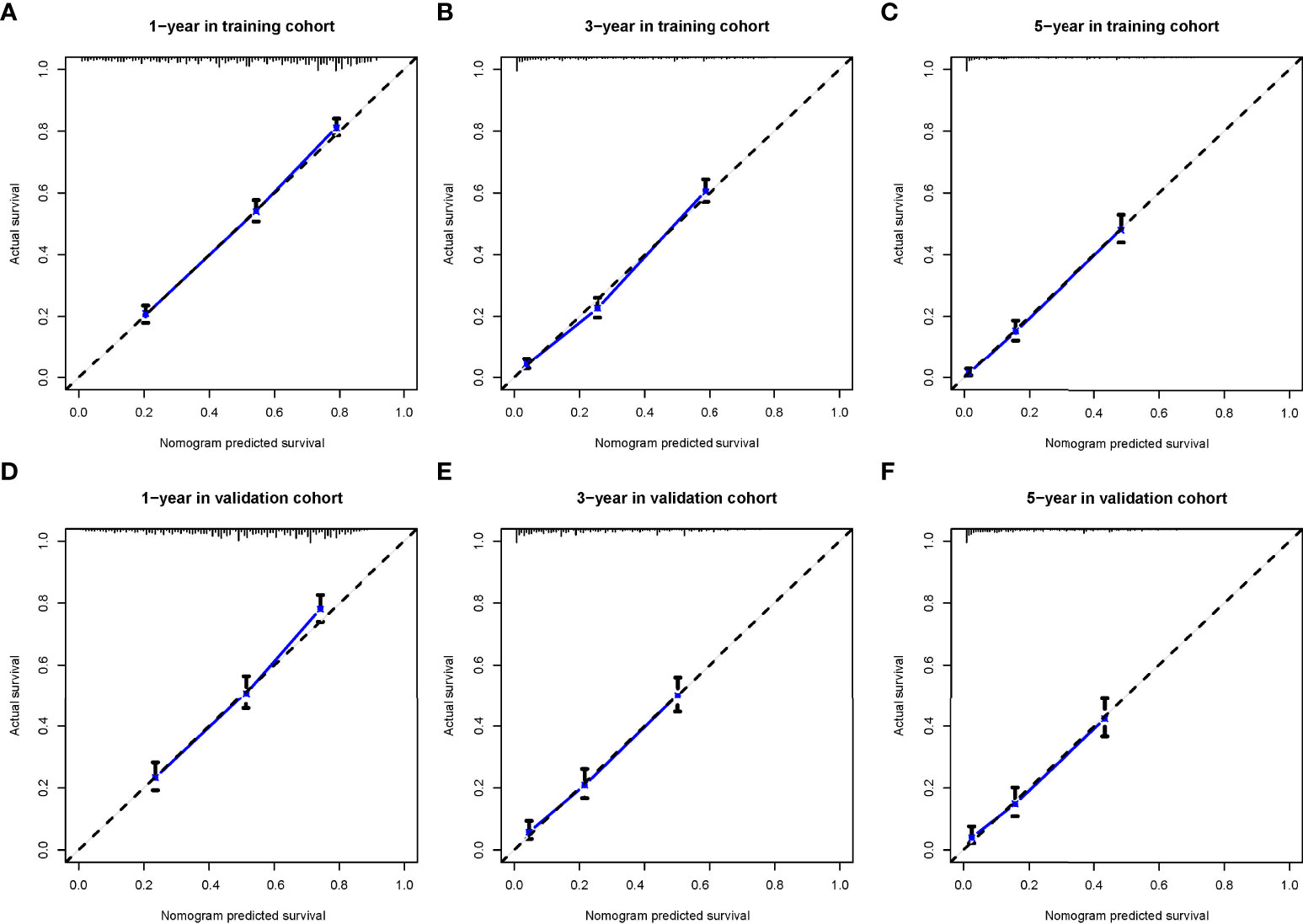
Figure 4 The calibration curves for predicting patients’ overall survival in the training cohort (A–C) and validation cohort (D–F) at 1-year, 3-year, and 5-year, respectively.
By drawing a decision Curve analysis (DCA) diagram (as shown in Figure 4) to further compare the clinical application value of the Nomogram prediction model with the TNM staging system, it is found that in almost all threshold probabilities at different points, The net return of Nomogram prediction model is better than TNM staging system, showing better clinical efficacy of the new model (Figure 5).
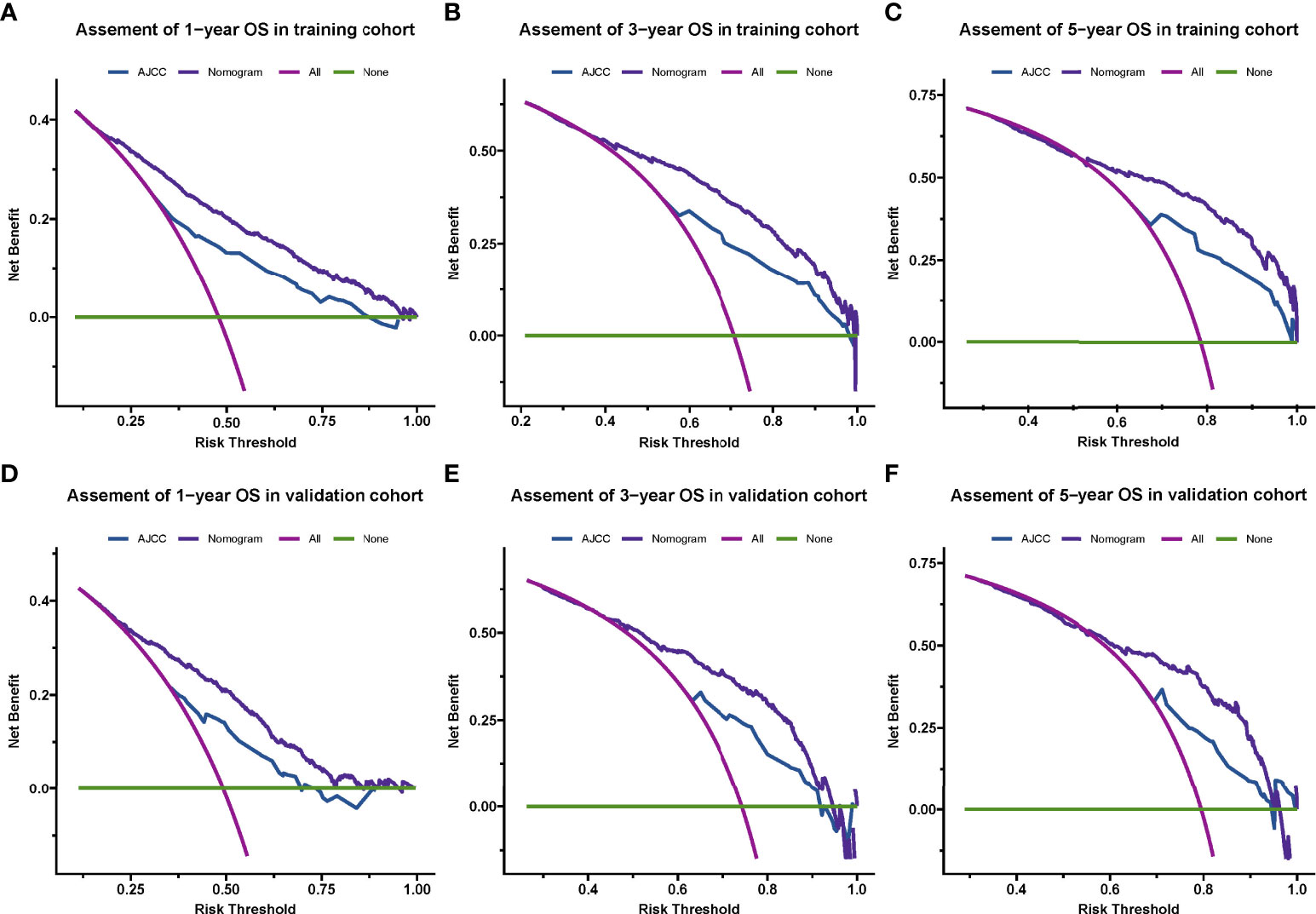
Figure 5 Decision curve analysis for the nomogram and AJCC stage in the training cohort (A–C) and validation cohort (D–F) at 1-year, 3-year, and 5-year, respectively.
For each variable in this nomogram, a total score is calculated for each patient and divided into 3 levels: low-risk (scores 0-185), intermediate-risk (scores 186-292), and high-risk (scores 293-437) group. Kaplan-Meier curves (Figure 6) show that this nomogram prediction ability is excellent and risk stratification is accurate.
The incidence of esophageal cancer began to rise rapidly after the age of 45, and with the increase of age, the incidence of esophageal cancer increased and reached a peak between 80 and 84 years old (6). Multiple retrospective analyses found that postoperative complications in elderly patients with esophageal cancer increased significantly, tolerance decreased, and perioperative mortality increased (7). For operable esophageal cancer, patients over 70 years old should be comprehensively evaluated before surgery (8). Patients with high surgical risk, complications, and poor cardiopulmonary function can be treated with radical radiotherapy. Radical radiotherapy is the main treatment for inoperable senile esophageal carcinoma (9). There are few reports on the results of high-grade randomized studies on EPEC only. Randomized clinical trials typically exclude patients over 70 years of age from esophageal cancer (10). Therefore, the present about the elderly esophagus. Most of the data on radiotherapy and chemotherapy for cancer come from retrospective studies, the number of cases is generally small, and the treatment standard has not been unified.
The TNM staging system is the most commonly used tumor staging system in the world, which helps doctors understand the progress of cancer, and can help doctors make treatment plans and judge the prognosis (11). Oncologists and patients alike want reliable prognostic information for each patient. The nomogram is more advantageous than the traditional TNM staging system, so it has been proposed as an alternative or even a new standard (12). The personalized predictive power of the nomogram allows it to be used to identify and stratify patients participating in clinical trials. The combination of friendly interfaces and extensive web availability makes them popular among oncologists and patients (13).
In this retrospective study, independent prognostic factors affecting survival in EPEC were obtained through univariate and multivariate analyses of SEER database data. Compared with the AJCC staging system, we constructed a new visual nomogram using these independent prognostic factors to predict the 1-, 3-, and 5-year overall survival with higher accuracy. The results of this study showed that T stage, N stage, M stage, tumor grade, tumor size, patient age, surgical status, radiotherapy status, and chemotherapy status were independent prognostic factors affecting EPEC.
With the improvement of esophageal surgery theory and technology, anesthesia technology, perioperative management, and the development and improvement of related disciplines and equipment, the surgical treatment effect of esophageal cancer has made great progress, and the safety factor of surgery has been greatly improved (14). Therefore, most scholars believe that surgery can completely remove the tumor, and as long as the patient can tolerate it, surgical treatment should be the first choice, and age should not be a limit for surgical treatment of esophageal cancer (15–17). Because elderly patients are often complicated with cardiovascular, cerebrovascular, and respiratory diseases, it is often believed that there are more postoperative complications, including surgery-related and non-surgery-related complications, which increase the perioperative mortality. The results of Tanja M (18) showed that there was no significant difference between the elderly patients (≥70 years old) and the young patients (< 70 years old) with surgery-related complications, which were 20% and 17%, respectively. The results of this study show that surgery can significantly prolong the overall survival of EPEC, which is consistent with the published literature.
To date, there are no guidelines for the treatment of EPEC. RTOG8501 compared the efficacy of 50Gy combined with cisplatin and fluorouracil combined with concurrent chemoradiotherapy plus chemotherapy and 64Gv alone in patients with esophageal cancer (23% of patients aged ≥70 years), and the results showed that the efficacy of concurrent chemoradiotherapy was significantly better than that of radiotherapy alone (5-year overall survival: 26%: 0), but concurrent chemoradiotherapy also resulted in severe acute side effects (grade 3-4 hematological side effects, 48% vs. 3%; grade 3-4 upper gastrointestinal reaction 33%: 18%); Among the patients who were subsequently enrolled in the concurrent chemoradiotherapy group, the completion rate of concurrent chemotherapy was only 68%. Therefore, the effect of concurrent chemoradiotherapy is better than radiotherapy alone.
With the progress and development of radiotherapy technology, the delineation of esophageal cancer radiotherapy target should be based on simulated positioning CT and enhanced contrast agent, so as to better confirm the target location. Intensity Modulated Radiation Therapy (IMRT), which is considered to be better than three-dimensional conformal radiotherapy (19, 20), is currently widely recommended. IMRT technology has better target conformal and can reduce the dose of important organs such as the heart, lung, and other tissues. In the treatment of esophageal cancer, the long-term damage of normal tissues is an important factor affecting the survival time and quality of life of patients in the later stage. Therefore, the application of IMRT technology provides a powerful technical condition to more strictly limit the dose of lung, heart and other important organs. Throughout the studies on esophageal cancer in recent years, a number of retrospective studies suggest that IMRT technology can improve the local control rate and survival of patients compared with 3DCT technology. With the help of IMRT, the 5-year overall survival for locally advanced esophageal cancer increased from 15% to 44% (21).
In the validation group, the calibration curve shows a high degree of agreement between nomogram predicted survival and actual survival. In addition, we find that the nomogram prediction model is superior to TNM staging system in terms of consistency index (C-index), area under ROC curve (AUC), and decision curve (DCA). Furthermore, in this study we attempt by nomogram prediction model to predict the total score, according to the scores of the risk is divided into three groups, low, medium and high risk through analysis showed the accuracy of the prediction model of risk stratification, such a high layer can effectively identify, between the survival outcomes for patients with low risk, which provide decision basis for the treatment of patients with different solutions.
Nonetheless, the study has several limitations. First, there may be selection bias because we excluded patients with incomplete information about variables. Secondly, the SEER database lacks some important parameters and specific information related to prognosis, such as the family history of esophageal cancer, radiotherapy, and chemotherapy. However, the nomogram of this study has been verified internally and has excellent clinical practicability. In conclusion, the nomogram prediction model constructed in this study can accurately predict the prognosis of EPEC and is superior to the TNM clinical staging system. It is expected that this model can be helpful to pathologists and oncologists in designing clinical strategies.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Study concept and design, SQ and HS. Analysis and interpretation of data, FW, FL, and JG. Drafting of the manuscript, SQ and XL. Critical revision of the manuscript for important intellectual content, YL and XS. All authors contributed to manuscript revision, read, and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Haefner MF, Lang K, Verma V, Koerber SA, Uhlmann L, Debus J, et al. Intensity-modulated versus 3-dimensional conformal radiotherapy in the definitive treatment of esophageal cancer: comparison of outcomes and acute toxicity. Radiat Oncol (2017) 12(1):131. doi: 10.1186/s13014-017-0863-3
3. Lester SC, Lin SH, Chuong M, Bhooshan N, Liao Z, Arnett AL, et al. A multi-institutional analysis of trimodality therapy for esophageal cancer in elderly patients. Int J Radiat Oncol Biol Phys (2017) 98(4):820–8. doi: 10.1016/j.ijrobp.2017.02.021
4. Lin SH, Zhang N, Godby J, Wang J, Marsh GD, Liao Z, et al. Radiation modality use and cardiopulmonary mortality risk in elderly patients with esophageal cancer. Cancer (2016) 122(6):917–28. doi: 10.1002/cncr.29857
5. Shimada H, Fukagawa T, Haga Y, Okazumi SI, Oba K. Clinical TNM staging for esophageal, gastric, and colorectal cancers in the era of neoadjuvant therapy: A systematic review of the literature. Ann Gastroenterol Surg (2021) 5(4):404–18. doi: 10.1002/ags3.12444
6. Markar SR, Karthikesalingam A, Thrumurthy S, Ho A, Muallem G, Low DE. Systematic review and pooled analysis assessing the association between elderly age and outcome following surgical resection of esophageal malignancy. Dis Esophagus. (2013) 26(3):250–62. doi: 10.1111/j.1442-2050.2012.01353.x
7. Tapias LF, Muniappan A, Wright CD, Gaissert HA, Wain JC, Morse CR, et al. Short and long-term outcomes after esophagectomy for cancer in elderly patients. Ann Thorac Surg (2013) 95(5):1741–8. doi: 10.1016/j.athoracsur.2013.01.084
8. Kumabe A, Zenda S, Motegi A, Onozawa M, Nakamura N, Kojima T, et al. Long-term clinical results of concurrent chemoradiotherapy for patients with cervical esophageal squamous cell carcinoma. Anticancer Res (2017) 37(9):5039–44. doi: 10.21873/anticanres.11919
9. Wang H, Li G, Chen L, Duan Y, Zou C, Hu C. Definitive concurrent chemoradiotherapy with s-1 and cisplatin in elderly esophageal squamous cell carcinoma patients. J Thorac Dis (2017) 9(3):646–54. doi: 10.21037/jtd.2017.03.105
10. Li B, Zhang Y, Miao L, Ma L, Luo X, Zhang Y, et al. Esophagectomy with three-field versus two-field lymphadenectomy for middle and lower thoracic esophageal cancer: Long-term outcomes of a randomized clinical trial. J Thorac Oncol (2021) 16(2):310–7. doi: 10.1016/j.jtho.2020.10.157
11. Zhang G, Zhang C, Wang L, Xue L, Jia J, Zuo Z, et al. The prognostic value of tumor deposits and the impact on the TNM classification system in esophageal cancer patients. J Surg Oncol (2021) 123(4):891–903. doi: 10.1002/jso.26376
12. Yu C, Zhang Y. Establishment of prognostic nomogram for elderly colorectal cancer patients: a SEER database analysis. BMC Gastroenterol (2020) 20(1):347. doi: 10.1186/s12876-020-01464-z
13. Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol (2015) 16(4):e173–80. doi: 10.1016/s1470-2045(14)71116-7
14. Borggreve AS, Kingma BF, Domrachev SA, Koshkin MA, Ruurda JP, van Hillegersberg R, et al. Surgical treatment of esophageal cancer in the era of multimodality management. Ann N Y Acad Sci (2018) 1434(1):192–209. doi: 10.1111/nyas.13677
15. Ruol A, Portale G, Zaninotto G, Cagol M, Cavallin F, Castoro C, et al. Results of esophagectomy for esophageal cancer in elderly patients: age has little influence on outcome and survival. J Thorac Cardiovasc Surg (2007) 133(5):1186–92. doi: 10.1016/j.jtcvs.2006.12.040
16. Kinugasa S, Tachibana M, Yoshimura H, Ueda S, Fujii T, Dhar DK, et al. Postoperative pulmonary complications are associated with worse short- and long-term outcomes after extended esophagectomy. J Surg Oncol (2004) 88(2):71–7. doi: 10.1002/jso.20137
17. Monson K, Litvak DA, Bold RJ. Surgery in the aged population: surgical oncology. Arch Surg (2003) 138(10):1061–7. doi: 10.1001/archsurg.138.10.1061
18. Cijs TM, Verhoef C, Steyerberg EW, et al. Outcome of esophagectomy for cancer in elderly patients. Ann Thorac Surg (2010) 90(3):900–7. doi: 10.1016/j.athoracsur.2010.05.039
19. Conroy T, Galais MP, Raoul JL, Bouché O, Gourgou-Bourgade S, Douillard JY, et al. Definitive chemoradiotherapy with FOLFOX versus fluorouracil and cisplatin in patients with oesophageal cancer (PRODIGE5/ACCORD17): final results of a randomised, phase 2/3 trial. Lancet Oncol (2014) 15(3):305–14. doi: 10.1016/S1470-2045(14)70028-2
20. van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med (2012) 366(22):2074–84. doi: 10.1056/NEJMoa1112088
21. Li CC, Chen CY, Chien CR. Comparison of intensity-modulated radiotherapy vs 3-dimensional conformal radiotherapy for patients with non-metastatic esophageal squamous cell carcinoma receiving definitive concurrent chemoradiotherapy: A population-based propensity-Score-Matched analysis. Med (Baltimore) (2018) 97(22):e10928. doi: 10.1097/MD.0000000000010928
Keywords: esophageal cancer, SEER, nomogram, survival analysis, elderly
Citation: Qie S, Shi H, Wang F, Liu F, Gu J, Liu X, Li Y and Sun X (2022) Construction of survival prediction model for elderly esophageal cancer. Front. Oncol. 12:1008326. doi: 10.3389/fonc.2022.1008326
Received: 31 July 2022; Accepted: 30 September 2022;
Published: 19 October 2022.
Edited by:
Stefan Rieken, University Medical Center Göttingen, GermanyReviewed by:
Martin Leu, University Medical Center Göttingen, GermanyCopyright © 2022 Qie, Shi, Wang, Liu, Gu, Liu, Li and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongyun Shi, Mjg1MDE4NDUyQHFxLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.