
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 24 January 2023
Sec. Gastrointestinal Cancers: Hepato Pancreatic Biliary Cancers
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1007374
This article is part of the Research Topic Clinical Trials, Practice and Design in Gastrointestinal Cancers View all 23 articles
 Zhi-Hua Xie1†
Zhi-Hua Xie1† Xuebing Shi1†
Xuebing Shi1† Ming-Qi Liu1†
Ming-Qi Liu1† Jinghan Wang2
Jinghan Wang2 Yong Yu1
Yong Yu1 Ji-Xiang Zhang1
Ji-Xiang Zhang1 Kai-Jian Chu1
Kai-Jian Chu1 Wei Li1
Wei Li1 Rui-Liang Ge1
Rui-Liang Ge1 Qing-Bao Cheng1*
Qing-Bao Cheng1* Xiao-Qing Jiang1*
Xiao-Qing Jiang1*Objective: The aim of this study was to develop and validate a nomogram to predict the overall survival of incidental gallbladder cancer.
Methods: A total of 383 eligible patients with incidental gallbladder cancer diagnosed in Shanghai Eastern Hepatobiliary Surgery Hospital from 2011 to 2021 were retrospectively included. They were randomly divided into a training cohort (70%) and a validation cohort (30%). Univariate and multivariate analyses and the Akaike information criterion were used to identify variables independently associated with overall survival. A Cox proportional hazards model was used to construct the nomogram. The C-index, area under time-dependent receiver operating characteristic curves and calibration curves were used to evaluate the discrimination and calibration of the nomogram.
Results: T stage, N metastasis, peritoneal metastasis, reresection and histology were independent prognostic factors for overall survival. Based on these predictors, a nomogram was successfully established. The C-index of the nomogram in the training cohort and validation cohort was 0.76 and 0.814, respectively. The AUCs of the nomogram in the training cohort were 0.8, 0.819 and 0.815 for predicting OS at 1, 3 and 5 years, respectively, while the AUCs of the nomogram in the validation cohort were 0.846, 0.845 and 0.902 for predicting OS at 1, 3 and 5 years, respectively. Compared with the 8th AJCC staging system, the AUCs of the nomogram in the present study showed a better discriminative ability. Calibration curves for the training and validation cohorts showed excellent agreement between the predicted and observed outcomes at 1, 3 and 5 years.
Conclusions: The nomogram in this study showed excellent discrimination and calibration in predicting overall survival in patients with incidental gallbladder cancer. It is useful for physicians to obtain accurate long-term survival information and to help them make optimal treatment and follow-up decisions.
Gallbladder cancer (GBC) is a rare malignancy with a documented incidence of 1.13 per 100,000 (1). Most patients are diagnosed with advanced incurable disease with a poor prognosis. The 5-year overall survival (OS) for stage III was 22.1% to 25.7% and 6.7% to 15.7% for stage IV patients (2). Radical resection is the only potential cure for GBC patients, especially those in early stages, who are most frequently diagnosed incidentally. In particular, with the widespread adoption of laparoscopic cholecystectomy, the number of incidentally gallbladder cancers (IGBCs) discovered after cholecystectomy for presumed benign disease has increased dramatically, accounting for 1.6% of all cholecystectomies (3). Due to the predisposition of port-site metastasis, peritoneal metastasis and the possibility of tumor residual in the liver bed and/or regional lymph nodes after initial cholecystectomy, the optimal management of IGBCs after the index cholecystectomy is a challenge which has attracted physicians’ attention.
Although the extent and timing of reresection for IGBC remain controversial, reoperation has been recommended because of improved survival in retrospective studies. The rationale behind reresection is not only to remove any residual disease but also to restage the disease accurately, which may be instrumental in achieving tumor-free margins, guiding adjuvant therapy and predicting prognosis (4–6). However, the existing survival prediction models of GBC do not take its specific characteristics (such as reresection and time to reoperation) into account due to its low incidence (7–13), and may not be able to provide accurate survival predictions for patients with IGBC and reduce the prognostic value of the 8th edition of the American Joint Committee on Cancer (AJCC) staging system model. Therefore, in this special group of patients, it is necessary to identify independent prognostic factors associated with IGBC survival and develop an appropriate model to accurately predict the survival rate of IGBC.
Recently, user-friendly and intuitive nomograms that can accurately predict overall survival have been widely used to evaluate the prognosis of various cancers. In this study, univariate and multivariate analyses were used to explore the independent prognostic factors of IGBC based on the clinicopathological data collected from our center in the past decade. Next, a nomogram was established to predict OS, and the accuracy and precision of the nomogram in the training and validation sets were evaluated by receiver operating characteristic (ROC) curves and calibration curves, respectively.
This study was approved by the institutional Review Board of our Ethics Committee, and informed patient consent was obtained (No. EHBHKY2022-K-025). The patients who underwent index cholecystectomy and were diagnosed with IGBC in Eastern Hepatobiliary Surgery Hospital from 2011 to 2021 and those who were first diagnosed with IGBC in other hospitals and underwent reresection for curable purposes in our hospital during the period were retrospectively analyzed. All enrolled cases were randomly divided into two datasets: 70% of eligible cases were allocated to the training cohort (n=269), and 30% were allocated to the validation cohort (n=114). The inclusion criteria for both cohorts were all patients diagnosed with incidental gallbladder cancer, defined as patients with no preoperative suspicion of GBC but pathologically confirmed gallbladder malignant tumor after cholecystectomy. Patients who were under the age of 18 years at diagnosis or lacked follow-up information were excluded. Clinical information such as sex, age at diagnosis, histology type, T stage, N metastasis, peritoneal metastasis, etc., were reviewed from medical records. The cutoff value of the time to reoperation was defined as the median time (19 days). The histological classification was adenocarcinoma or nonadenocarcinoma (adenosquamous or squamous). N metastasis was described as either negative or positive lymph node status. M metastasis was described as either negative or positive distant metastasis. Resection margin R was described as either negative (R0) or positive (R1/R2). Overall survival was chosen as the endpoint of interest, with dates calculated from the time of first surgery to death from any cause or the last follow-up on January 1, 2022.
Descriptive statistics were used to summarize all clinical features. χ2 or Fisher’s exact tests were performed to assess the distribution of basic categorical variables of patients in the training and validation cohorts, as appropriate. Potential prognostic variables with p values <0.1 identified in univariable Cox analyses were further selected and included in multivariable Cox regression analyses. Stepwise backward model selection was performed based on Akaike information criterion (AIC) values. Variables with two-sided p values <0.05 were considered as statistically significant and were identified as independent prognostic factors to construct a nomogram of the prediction model. In the training and validation cohorts, the nomogram was validated both internally and externally with 500-bootstrap resampling.
Discrimination and calibration were used to evaluate the predicted OS performance of the nomogram. Harrell’s concordance index (C-index) was calculated to measure the difference between the observed outcomes and the nomogram predictions on a scale of 0.5 to 1.0, where 0.5 indicated no discrimination at all and 1.0 indicated a perfect fit. Calibration curves were visualized to compare the predicted and observed probabilities of OS at 1, 3 and 5 years. Furthermore, time-dependent ROC curves were generated to compare the power of the nomogram model with the 8th edition of the AJCC TNM staging system model. Missing data were completed with multiple imputation using the ‘mice’ package with default values. Statistical analyses were performed using version R 4.1.1 (R Foundation for Statistical Computing, Vienna, Austria). The related R packages ‘rms’, ‘foreign’, ‘VIM’, ‘epiDisplay’, ‘dplyr’, ‘mice’, ‘survival’, ‘survivalROC’, ‘forestplot’, and ‘caret’ were applied to create and evaluate the nomogram. This study was designed according to the Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD) guidelines.
A total of 383 patients with incidental gallbladder cancer who met the inclusion criteria were identified from 2011 to 2021. They were randomly divided into the training cohort (n=269, 70%) and validation cohort (n=114, 30%). All patients underwent surgical resection, of whom approximately 84.1% underwent reresection. The median follow-up was 31.3 months, with a range of 1.3 to 136 months. Detailed baseline characteristics of patients in each cohort are shown in Table 1. Approximately 64% of the cases were female, and 31.6% were male. There were slightly more patients under the age of 60 than those over the age of 60 (51.4% vs. 48.6%). Most of the patients had T2-T3 stages (315 cases, 82.2%) and adenocarcinomas (340 cases, 88.8%). More importantly, in these 383 patients, upon reresection, 9.7% developed distant metastases, 19.3% developed lymph node metastases, and 6.3% developed peritoneal metastases. Among the 383 cases, 141 (36.8%) had chronic disease. In addition, the median time to reoperation was 19 [InterQuartile Range (IQR), 12-26.5] days, and a total of 182 (47.5%) patients underwent reoperation within 19 days from their initial cholecystectomy.
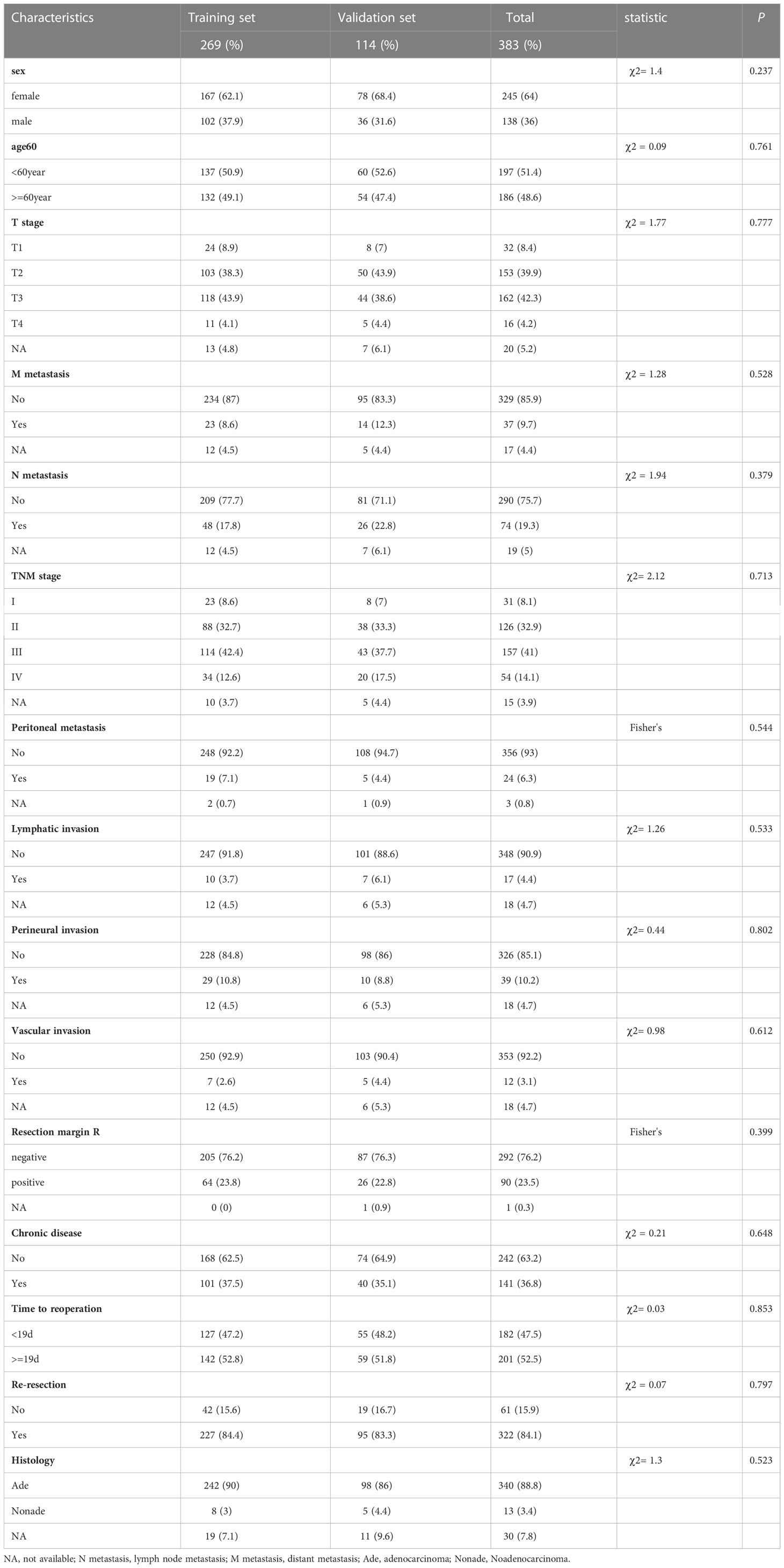
Table 1 Characteristics of incidental gallbladder cancer patients in the Training and Validation set.
To identify prognostic factors associated with OS before constructing a nomogram model, we employed univariate and multivariate Cox regression analyses. Table 2 and Figure 1 show the detailed results of the univariate and multivariate analyses in the training cohort. Univariate analysis found that histology, M metastasis, N metastasis, perineural invasion, peritoneal metastasis, resection margin R, reresection, T stage, TNM stage and vascular invasion were associated with OS. Variables with P values <0.1 were considered as statistically significant. Subsequently, these ten meaningful variables were put into a multivariate Cox regression model using a backward stepwise method. Based on multivariate analysis, five variables (histology, N metastasis, peritoneal metastasis, reresection and T stage) were finally considered as independent prognostic factors with a P value <0.05 and a minimum AIC value of 1001.06.
Next, we successfully developed a nomogram model to predict OS at 1, 3, and 5 years based on the above five identified independent variables, as shown in Figure 2. According to the total subscale at the bottom, the probabilities of 1-, 3-, and 5-year OS were simply calculated from the sum of the scores for each individual variable. Harrell’s C-index, time-dependent ROC curves (Figure 3) and calibration curves (Figure 4) were used to evaluate the established nomogram model. The C-index value of the nomogram was 0.76 [95% confidence interval (CI), 0.72-0.80] in the training cohort and 0.814 (95% CI, 0.76-0.87) in the validation cohort. Time-dependent ROC curves were used to compare the sensitivity and specificity between the predictive model and the TNM staging model. The areas under the curve (AUCs) of the nomogram for predicting OS at 1, 3, and 5 years were 0.8, 0.819 and 0.815 in the training cohort and 0.846, 0.845 and 0.902 in the validation cohort, respectively. Meanwhile, the 1-, 3- and 5-year AUC values of the TNM staging model were 0.722, 0.781 and 0.785 in the training cohort and 0.777, 0.822 and 0.874 in the validation cohort, respectively. As shown in Figure 3, in the training and validation cohorts of 1-, 3- and 5-year OS, the nomogram model showed better discriminative power and larger AUCs than the TNM staging model, illustrating that the nomogram model exhibited a more powerful discrimination. Meanwhile, calibration curves illustrating the relationship between predicted and actual OS probabilities were tested with 500 bootstrap resamples in both the training and validation cohorts. Calibration plots showed that OS prediction at 1, 3, and 5 years for both cohorts was in excellent agreement with actual observations. Taken together, the nomogram model demonstrated good discriminative and calibration power for predicting 1-, 3-, and 5-year OS in IGBC.
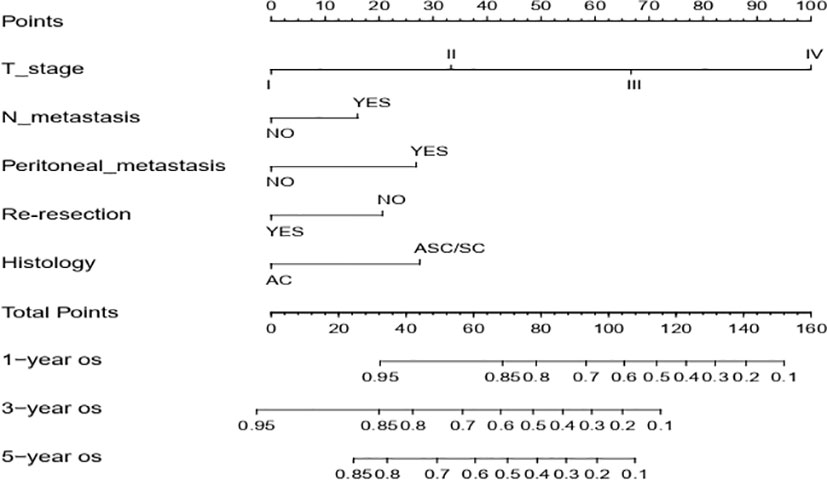
Figure 2 Nomogram for estimating the 1-, 3-, 5-year OS of IGBC patients. N metastasis, lymph node metastasis; AC, adenocarcinoma; ASC/SC, adenosquamous or squamous; OS, overall survival.
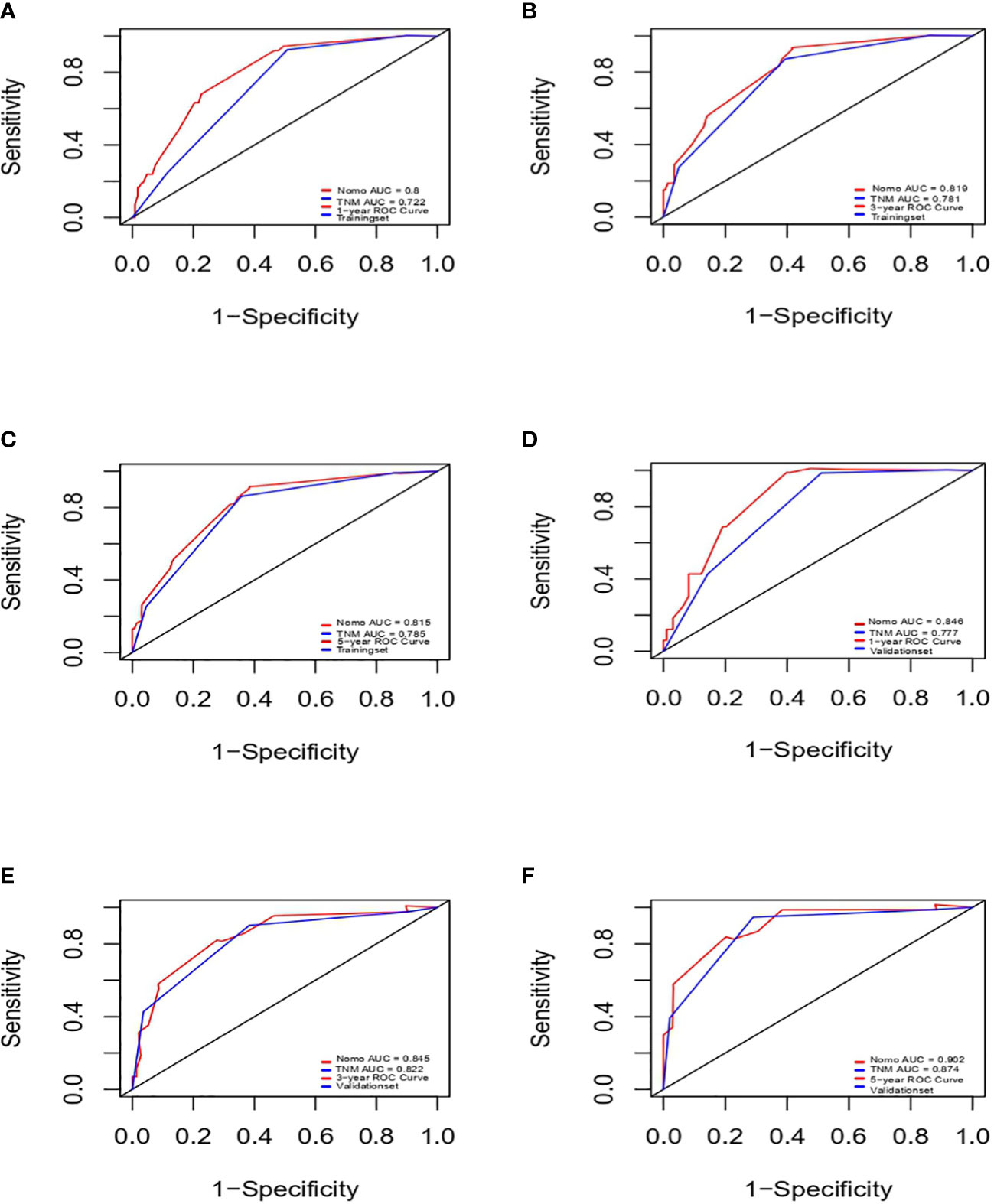
Figure 3 ROCs of IGBC for predicting OS at 1-, 3-, 5-year in the training (A–C) and validation set (D–F), respectively. ROC, receiver operating characteristic; AUC, area under the curve; IGBC, incidentally gallbladder cancer; OS, overall survival.
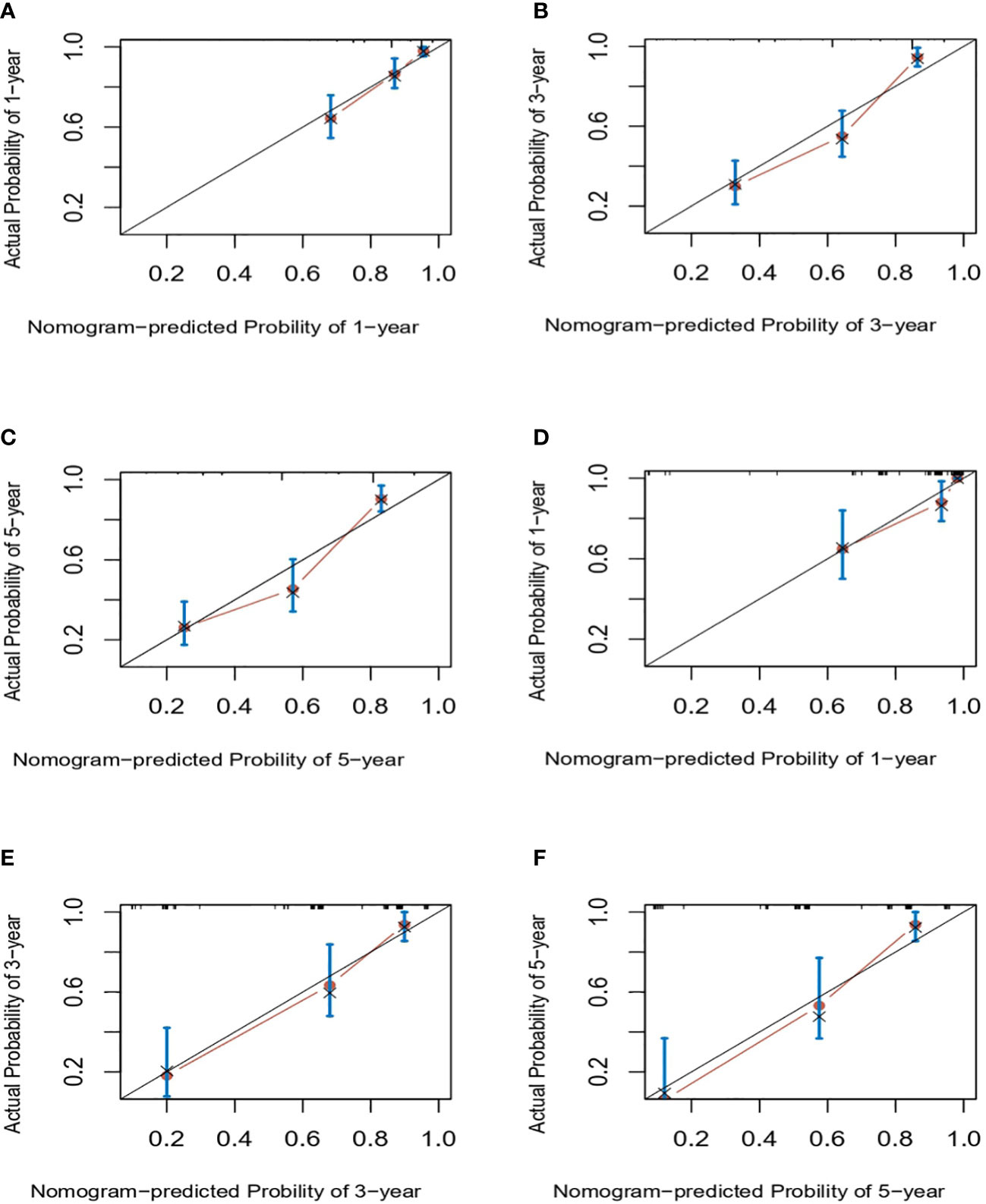
Figure 4 Calibration curves for predicting IGBC OS at 1-, 3-, 5-year in the training (A–C) and validation set (C–E), respectively. IGBC, incidentally gallbladder cancer; OS, overall survival.
Gallbladder cancer is an aggressive disease with a dismal prognosis. It is usually occult onset with an asymptomatic course that is not easily discovered in the early stages before operation. Most incidental gallbladder cancers were occasionally diagnosed after cholecystectomy, and a few were discovered during surgery. In recent decades, with the rapid increase in the number of patients undergoing laparoscopic cholecystectomy, the gradual increase in the incidence of IGBC is of concern. However, existing models and the latest AJCC TNM staging system for predicting survival in GBC that do not specifically consider IGBC may not be applicable to IGBC (7–14). Due to its user-friendly graphical interface and the integration of multiple easily accessible variables, the nomogram has been increasingly popular and widely used for personalized cancer prediction of various cancers. In this study, we first developed a nomogram model to predict survival for IGBC. Based on univariate and multivariate analyses, we identified five factors (T stage, N metastasis, peritoneal metastasis, reresection, histology) that were independently associated with overall survival.
T stage in our study was one of the top five independent prognostic factors that has also been identified in previous studies of IGBC (6, 15–17). Residual disease was considered as one of the most important characteristics of IGBC; in statistics, approximately 35% ~ 50.8% of patients had residual disease (RD) (4, 18), and T stage was closely associated with residual disease and proved to be an excellent predictor of residual disease in IGBC18. It was reported that approximately 20% of T1b, 23.8% of T2, and 71.7% of T3 of IGBC patients had accompanying RD (19). Evidently, R0 resection represents the strongest long-term prognostic factor and chance for cure. To remove microscopic or macroscopic RD, reoperation is recommended for T1b or higher IGBC by international guidelines (14). Consistent with previous studies, reresection is beneficial and associated with improved survival for patients with IGBC (4–6). Interestingly, resection margin status in our cohort did not show significant differences in prognosis for patients with IGBC after reoperation, which was also observed in the study of Vega and colleagues (20). However, the opposite conclusion can also be drawn from the work of de Savornin Lohman (4). The paradoxical results aroused our attention. Despite the improved survival observed in the reresection group, patients with RD have been shown to have shorter survival times than those without RD (4, 19). It is now evident that patients without residual disease or with disseminated disease cannot benefit from reoperation. Ramos’ group (18) showed that only the patients with local RD that isolated nondiscontinuous involvement of the vesicular bed or the cystic stump were found to have acquired more benefit from reoperation compared with regional or distant RD. Similarly, the conclusion that reresection may be beneficial solely for patients with microscopic RD undetected by the pathologist was made by the de Savornin Lohman group (4). They perceived that the tumor may have already progressed beyond potential curation when macroscopic RD was found. In this regard, we presume that the difference in predictive prognosis efficacy of resection margin status may be due to the varying proportion of patients who can potentially benefit from reresection. Consequently, the survival benefit of reoperation for T1b IGBC remains controversial (21, 22). The survival benefit of reoperation for T2/T3 IGBC patients has reached an expert consensus (6, 22).
Additionally, peritoneal metastasis occurred frequently in IGBC, mainly due to bile spillage of the gallbladder during initial cholecystectomy, particularly in minimally invasive approaches on various conditions. It was an important factor for IGBC patients in losing the chance of radical reoperation. Statistically, approximately 7-7.6% of patients with peritoneal metastasis were found to have reoperation (23, 24), which was similar to our results (6.3% of patients with peritoneal metastases during reoperation). Evidently, the poor prognosis association with peritoneal metastasis has also been demonstrated in multiple abdominal cancers, such as colorectal, gastric and liver cancers (25–27).
In addition, adenosquamous or squamous cell carcinoma represents a minority (2%) histological type of gallbladder cancer. Studies have shown that it is commonly larger and more aggressive than adenocarcinoma, with a significantly shorter median overall survival than adenocarcinoma, and is an independent prognostic factor for GBC (28, 29), which was similar to and supported our results.
In the present study, the proposed nomogram, which incorporated 5 comprehensive variables (including T stage, N metastasis, peritoneal metastasis, reresection and histology), performed well, as supported by the C index values of 0.76 and 0.814 in the training and validation cohorts, respectively, and the calibration curves showed excellent agreement between predicted and observed outcomes in the 1-, 3-, and 5-year OS. Remarkably, IGBC has unique characteristics, such as a few patients with distant metastasis and iatrogenic peritoneal metastasis often derived from bile spillage that occurred at initial surgery. Therefore, M status was excluded from the nomogram, while peritoneal metastasis and the other 4 variables were included in the nomogram, and the nomogram was more accurate than the AJCC TNM staging system for predicting the prognosis of patients with IGBC.
However, some limitations need to be considered in this study. First, it was a retrospective single-center study without external data validation, which may result in some bias and low accuracy, and further large-scale multicenter cohort studies are needed to validate our results. Second, the lack of relevant information on postoperative adjuvant chemotherapy and serum tumor markers may reduce the accuracy of our predictions, and future studies need to consider these variables. Despite these limitations, the nomogram model constructed in this study has excellent AUC values and calibration curves, making it an excellent model to provide physicians with accurate survival prediction.
In conclusion, based on the variables identified in this study, we successfully established a nomogram of IGBC for the first time. Well-calibrated nomogram survival curves can help physicians to make appropriate clinical decisions for individual IGBC patients.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
This study was approved by the institutional Review Board of Shanghai Eastern Hepatobiliary Surgery Hospital Ethics Committee (No. EHBHKY2022-K-025) and complied with its ethical standards. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Acquisition of data, analysis and interpretation of data: Z-HX, XS, M-QL, JW, J-XZ, K-JC, WL, R-LG. Writing-original draft: Z-HX, XS, M-QL. Writing-review and editing: allauthors. Supervision: X-QJ, Q-BC. All authors contributed to thearticle and approved the submitted version.
This study was supported by a grant from the National Natural Science Foundation of China (No. 81972256).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Henley SJ, Weir HK, Jim MA, Watson M, Richardson LC. Gallbladder cancer incidence and mortality, united states 1999-2011. Cancer Epidemiol Biomarkers Prev (2015) 24:1319–26. doi: 10.1158/1055-9965.EPI-15-0199
2. Lee AJ, Chiang Y-J, Lee JE, Conrad C, Chun Y-S, Aloia TA, et al. Validation of American joint committee on cancer eighth staging system for gallbladder cancer and its lymphadenectomy guidelines. J Surg Res (2018) 230:148–54. doi: 10.1016/j.jss.2018.04.067
3. Ahn Y, Park C-S, Hwang H, Jang H-J, Choi K-M, Lee S-G. Incidental gallbladder cancer after routine cholecystectomy: when should we suspect it preoperatively and what are predictors of patient survival? Ann Surg Treat Res (2016) 90:131–8. doi: 10.4174/astr.2016.90.3.131
4. de Savornin Lohman EAJ, van der Geest LG, de Bitter TJJ, Nagtegaal ID, van Laarhoven CJHM, van den Boezem P, et al. Re-resection in incidental gallbladder cancer: Survival and the incidence of residual disease. Ann Surg Oncol (2020) 27:1132–42. doi: 10.1245/s10434-019-08074-4
5. Søreide K, Guest RV, Harrison EM, Kendall TJ, Garden OJ, Wigmore SJ. Systematic review of management of incidental gallbladder cancer after cholecystectomy. Br J Surg (2019) 106:32–45. doi: 10.1002/bjs.11035
6. Lundgren L, Muszynska C, Ros A, Persson G, Gimm O, Andersson B, et al. Management of incidental gallbladder cancer in a national cohort. Br J Surg (2019) 106:1216–27. doi: 10.1002/bjs.11205
7. de Savornin Lohman EAJ, de Bitter TJJ, Hannink G, Wietsma MFT, Vink-Börger E, Nagtegaal ID, et al. Development and external validation of a model to predict overall survival in patients with resected gallbladder cancer. Ann Surg (2021). doi: 10.1097/SLA.0000000000005154
8. Wu Y, Li Q, Cai Z, Zhang Y, Qiu Y, Yang N, et al. Survival prediction for gallbladder carcinoma after curative resection: Comparison of nomogram and Bayesian network models. Eur J Surg Oncol (2020) 46:2106–13. doi: 10.1016/j.ejso.2020.07.009
9. He C, Cai Z, Zhang Y, Lin X. Prognostic model to predict cancer-specific survival for patients with gallbladder carcinoma after surgery: A population-based analysis. Front Oncol (2019) 9:1329. doi: 10.3389/fonc.2019.01329
10. Chen M, Cao J, Zhang B, Pan L, Cai X. A nomogram for prediction of overall survival in patients with node-negative gallbladder cancer. J Cancer (2019) 10:3246–52. doi: 10.7150/jca.30046
11. Xiao Z, Shi Z, Hu L, Gao Y, Zhao J, Liu Y, et al. A new nomogram from the SEER database for predicting the prognosis of gallbladder cancer patients after surgery. Ann Transl Med (2019) 7:738. doi: 10.21037/atm.2019.11.112
12. Bai Y, Liu Z-S, Xiong J-P, Xu W-Y, Lin J-Z, Long J-Y, et al. Nomogram to predict overall survival after gallbladder cancer resection in China. World J Gastroenterol (2018) 24:5167–78. doi: 10.3748/wjg.v24.i45.5167
13. Zhang W, Hong HJ, Chen Y-L. Establishment of a gallbladder cancer-specific survival model to predict prognosis in non-metastatic gallbladder cancer patients after surgical resection. Dig Dis Sci (2018) 63:2251–8. doi: 10.1007/s10620-018-5103-7
14. Benson AB, D’Angelica MI, Abbott DE, Anaya DA, Anders R, Are C, et al. Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw (2021) 19:541–65. doi: 10.6004/jnccn.2021.0022
15. Addeo P, Centonze L, Locicero A, Faitot F, Jedidi H, Felli E, et al. Incidental gallbladder carcinoma discovered after laparoscopic cholecystectomy: Identifying patients who will benefit from reoperation. J Gastrointest Surg (2018) 22:606–14. doi: 10.1007/s11605-017-3655-z
16. Ethun CG, Postlewait LM, Le N, Pawlik TM, Buettner S, Poultsides G, et al. Association of optimal time interval to re-resection for incidental gallbladder cancer with overall survival: A multi-institution analysis from the US extrahepatic biliary malignancy consortium. JAMA Surg (2017) 152:143–9. doi: 10.1001/jamasurg.2016.3642
17. Ethun CG, Postlewait LM, Le N, Pawlik TM, Poultsides G, Tran T, et al. Routine port-site excision in incidentally discovered gallbladder cancer is not associated with improved survival: A multi-institution analysis from the US extrahepatic biliary malignancy consortium. J Surg Oncol (2017) 115:805–11. doi: 10.1002/jso.24591
18. Ramos E, Lluis N, Llado L, Torras J, Busquets J, Rafecas A, et al. Prognostic value and risk stratification of residual disease in patients with incidental gallbladder cancer. World J Surg Oncol (2020) 18:18. doi: 10.1186/s12957-020-1794-2
19. Gil L, de Aretxabala X, Lendoire J, Duek F, Hepp J, Imventarza O. Incidental gallbladder cancer: How residual disease affects outcome in two referral HPB centers from south America. World J Surg (2019) 43:214–20. doi: 10.1007/s00268-018-4762-z
20. Vega EA, De Aretxabala X, Qiao W, Newhook TE, Okuno M, Castillo F, et al. Comparison of oncological outcomes after open and laparoscopic re-resection of incidental gallbladder cancer. Br J Surg (2020) 107:289–300. doi: 10.1002/bjs.11379
21. Kim B-H, Kim S-H, Song I-S, Chun G-S. The appropriate surgical strategy for T1b gallbladder cancer incidentally diagnosed after a simple cholecystectomy. Ann Hepatobiliary Pancreat Surg (2019) 23:327–33. doi: 10.14701/ahbps.2019.23.4.327
22. Matsuyama R, Yabusita Y, Homma Y, Kumamoto T, Endo I. Essential updates 2019/2020: Surgical treatment of gallbladder cancer. Ann Gastroenterol Surg (2021) 5:152–61. doi: 10.1002/ags3.12434
23. Kumar S, Bhoriwal S, Muduly D, Kar M, Sharma A, Pathy S, et al. Multimodality management of incidentally detected gall bladder cancer: long term results from a tertiary care cancer centre. J Gastrointest Oncol (2019) 10:128–33. doi: 10.21037/jgo.2018.09.10
24. Ethun CG, Postlewait LM, Le N, Pawlik TM, Buettner S, Poultsides G, et al. A novel pathology-based preoperative risk score to predict locoregional residual and distant disease and survival for incidental gallbladder cancer: A 10-institution study from the U.S. extrahepatic biliary malignancy consortium. Ann Surg Oncol (2017) 24:1343–50. doi: 10.1245/s10434-016-5637-x
25. Bae SJ, Shin US, Ki Y-J, Cho SS, Moon SM, Park SH. Role of peritoneal lavage cytology and prediction of prognosis and peritoneal recurrence after curative surgery for colorectal cancer. Ann Coloproctol (2014) 30:266–73. doi: 10.3393/ac.2014.30.6.266
26. Arigami T, Matsushita D, Okubo K, Kawasaki Y, Iino S, Sasaki K, et al. Indication and prognostic significance of conversion surgery in patients with liver metastasis from gastric cancer. Oncology (2020) 98:273–9. doi: 10.1159/000505555
27. Iida H, Tani M, Aihara T, Hasegawa K, Eguchi H, Tanabe M, et al. New metastasectomy criteria for peritoneal metastasis of hepatocellular carcinoma: A study of the Japanese society of hepato-Biliary-Pancreatic surgery. J Hepatobiliary Pancreat Sci (2020) 27:673–81. doi: 10.1002/jhbp.796
28. Murimwa G, Hester C, Mansour JC, Polanco PM, Porembka MR, Wang SC, et al. Comparative outcomes of adenosquamous carcinoma of the gallbladder: an analysis of the national cancer database. J Gastrointest Surg (2021) 25:1815–27. doi: 10.1007/s11605-020-04729-w
Keywords: gallbladder cancer, re-resection, nomogram, overall survival, incidental gallbladder cancer
Citation: Xie Z-H, Shi X, Liu M-Q, Wang J, Yu Y, Zhang J-X, Chu K-J, Li W, Ge R-L, Cheng Q-B and Jiang X-Q (2023) Development and validation of a nomogram to predict overall survival in patients with incidental gallbladder cancer: A retrospective cohort study. Front. Oncol. 12:1007374. doi: 10.3389/fonc.2022.1007374
Received: 30 July 2022; Accepted: 28 December 2022;
Published: 24 January 2023.
Edited by:
Alberto Puccini, Ospedale Policlinico San Martino IRCCS, ItalyCopyright © 2023 Xie, Shi, Liu, Wang, Yu, Zhang, Chu, Li, Ge, Cheng and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondenc: Xiao-Qing Jiang, anhxMTIyNUBzaW5hLmNvbQ==; Qing-Bao Cheng, MTM5MTYzMDk1MDBAMTI2LmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.