
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 26 October 2022
Sec. Surgical Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1007296
This article is part of the Research Topic Application of Molecular Genetics for the Diagnosis and Classification of Rare Cancers in Surgical Pathology View all 13 articles
ALK rearrangements have rarely been reported in S100- and CD34-co-expressing soft tissue neoplasms with lipofibromatosis-like neural tumor (LPFNT) pattern or stromal and perivascular hyalinization, mimicking NTRK-rearranged spindle cell tumors. Here, we reported ALK fusions involving related partner genes in two adult soft tissue tumors with S100 and CD34 co-expression, and conducted a literature review of mesenchymal tumors harboring ALK or other kinase fusions. Case 1 was a 25-year-old female who underwent excision of a soft tissue mass in the anterior thigh region. Morphologically, the tumor was composed of spindle cells adjacent to epithelioid cells embedded in myxedematous and hyalinized stroma, with infiltrative boundary. Spindle cells mixed with inflammatory infiltration resembling inflammatory myofibroblastic tumor (IMT) were seen sporadically. However, brisk mitosis and focal necrosis was also observed, indicating an intermediate-grade sarcoma. In case 2, the left side of the neck of a 34-year-old man was affected. The tumor was composed of monomorphic spindle cells arranged in fascicular growth or patternless pattern, with stromal and perivascular hyalinization. Sparse inflammatory cell infiltration was also observed. Both tumors showed CD34, S100, and ALK-D5F3 immunoreactivity. Next generation sequencing (NGS) test identified a PLEKHH2::ALK fusion in case 1, which was confirmed by RT-PCR and Sanger sequencing, whereas the RT-PCR (ARMS method) test detected an EML4::ALK fusion in case 2. In conclusion, this study expands the morphological and genetic landscape of tumors with S100 and CD34 co-expression harboring kinase fusions, and suggests that kinase fusion–positive mesenchymal neoplasms are becoming an enlarging entity with a variety of morphological patterns.
According to the 2020 WHO classification of soft tissue tumors (STTs), NTRK-rearranged spindle cell tumors are an emerging entity, which spans a wide spectrum of morphologies and histologic grades, with frequent immunohistochemical co-expression of S100 and CD34. Notably, the family of this entity is expanding, as tumors with similar clinicopathological features and morphology but alternative kinase genes fusions are constantly identified; among them, STTs with ALK gene rearrangement have emerged as a recent hot spot (1–20).
The ALK gene (2p23) encodes a cell membrane receptor tyrosine kinase (RTK), which plays an important role in brain development and specific neurons in the nervous system. Oncogenic activation of ALK kinase following ALK rearrangement has been reported in a variety of tumors, including non-small cell lung cancer (NSCLC), anaplastic large cell lymphoma (ALCL), IMT, epithelioid fibrous histiocytoma (EFH) (21), ALK-positive histiocytosis (22), renal cell carcinoma (23), thyroid cancer (24), secretory carcinomas (25), and gastrointestinal stromal tumor (GIST) (26). Recently, ALK rearrangements have been reported in S100- and CD34-co-expressing soft tissue tumors (5–14). A provisionally termed entity, superficial ALK-rearranged myxoid spindle cell neoplasm, has been coined to emphasize the characteristic swirling pattern of spindle cells arranged in myxoid or myohyaline stroma (6). Later, Kao YC et al. reported an additional case of superficial ALK-rearranged spindle cell neoplasm, which showed ovoid tumor cells predominantly arranged in reticular and cord-like patterns in a hyalinized stroma, with only focal presence of whorl-like pattern (7). However, the emerging tumor was also characterized by frequent S100 protein and CD34 co-expression, perivascular hyalinization, and collagenous stroma, and it partly showed LPFNT pattern, which could not sufficiently distinguish it from other ALK-rearranged tumors with S100 and CD34 co-expression. Furthermore, infantile fibrosarcoma (IFS)-like pattern, which is normally reported in the wide morphological spectrum of NTRK-rearranged STTs, including infantile fibrosarcoma and NTRK-rearranged spindle cell tumors, has also been documented with ALK rearrangements (8, 27).
Therefore, more cases are needed to recognize the innate character of such soft tissue tumors with ALK rearrangements and improve their classification and nomenclature. In this study, we identified two S100- and CD34-co-expressing STTs with ALK rearrangement and summarized the clinicopathological characteristics of the reported kinase fusion–positive mesenchymal neoplasms, hoping to enlighten new ideas.
Case 1 was a 25-year-old woman with an egg-sized, movable, painless mass in the left anterior thigh region for more than 1 year, with a gradual increase in size associated with pain for 2 months. Magnetic resonance imaging (MRI) suggested an intramuscular mass between the anterior rectus and vastus lateralis muscles in the left thigh (Supplementary Figure 1). The patient underwent resection of the mass. Macroscopically, the resected specimen comprised a soft solid tumor mass measuring 6.5 × 3.5 × 2.8 cm with a gray-white, fleshy, or myxoid cut surface. Microscopically, the tumor was composed of spindle cells juxtaposed with epithelioid cells embedded in myxedematous and hyalinized stroma (Figure 1A), partially infiltrating surrounding striated muscles and adipose tissue. The spindle cells were arranged in sheet-like, intersecting fascicles, or in patternless patterns, showing indistinct cytoplasmic borders and moderate nuclear pleomorphism (Figure 1B). The epithelioid cells were arranged in a nest- or cord-like pattern in a myohyaline background with ample eosinophilic cytoplasm and round to ovoid nuclei (Figure 1C). The mitotic figures (MFs) were plentiful, especially in the cellular area (about 8 MFs/10 high-power fields (HPFs)) (Figure 1D). Focal hemorrhage and necrosis were also observed in the spindle cell area (Figure 1E). Prominent branching of thin-walled blood vessels of different sizes was also found (Figure 1F). At the periphery, some spindle tumor cells admixed with infiltrating inflammatory cells, closely resembling IMT (Figure 1G). According to the French Federation of Cancer Centers Sarcoma Group (FNCLCC) grading, the morphology of the neoplasm was intermediate grade. The tumor cells were immunohistochemically positive for CD34 (Figure 1H), S100 (Figure 1I), ALK-D5F3 (Figure 1J), H3K27me3, vimentin, and CD99 (paranuclear dot-like staining), and they were negative for STAT6, CK-pan, EMA, desmin, SMA, CD31, WT-1, and pan-TRK. The average Ki-67 index was 35%. The patient underwent postoperative radiotherapy(70Gy/35F), and there were no signs of recurrence or metastasis 48 months after surgery.
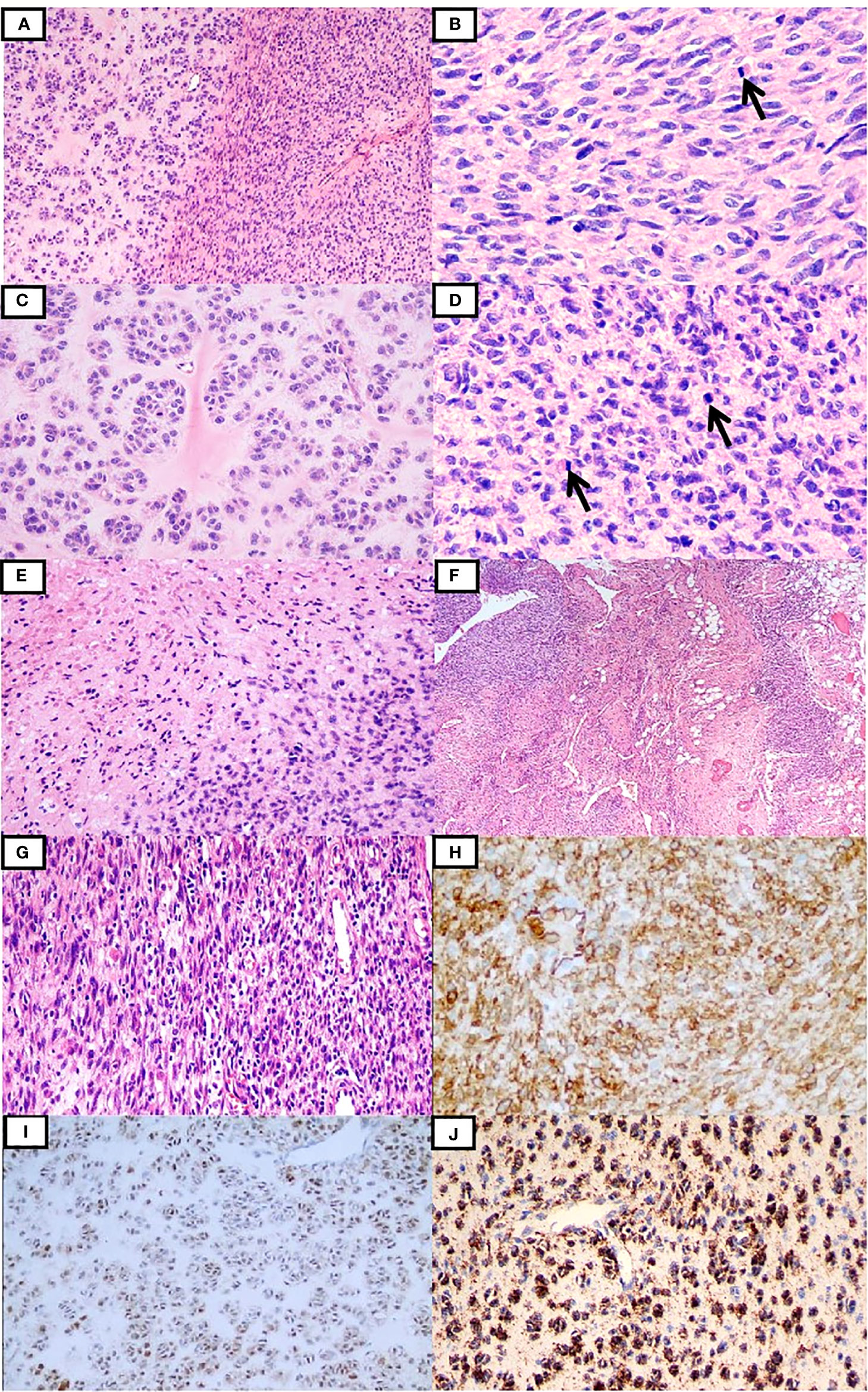
Figure 1 Clinicopathological findings of the tumor in case 1. The tumor was composed of the spindle and epithelioid cells embedded in myxedematous and hyalinized stroma (A). The spindle cells were focally arranged in the intersecting fascicles with frequently observed mitotic figures (B), while the epithelioid cells were arranged in the nest- or cord-like pattern in the myohyaline background (C). In some areas, the tumor cells were more cellular with relatively brisk mitoses (D). Focal necrosis was recognized (E). Tumor cells infiltrating the surrounding adipose tissues and thin-walled branching vessels were seen (F). Inflammatory cell infiltration was observed locally (G). Tumor cells were positive for CD34 (H), S100 (I), and ALK-D5F3 (J).
Case 2 was a 34-year-old man with a mass on the left side of his neck. The tumor was marginally removed without further treatment. Grossly, the mass was partially encapsulated measuring 8 × 5 × 4 cm in size. The texture was soft, and the cut surface was gray-white to gray-yellow. Microscopically, the lesion was composed of spindle-shaped mesenchymal cells infiltrating the fat tissue and striated muscle, with stromal and perivascular hyalinization (Figures 2A, B). A higher-power view showed bland spindle cells arranged in a patternless pattern with fusiform nuclei and fine chromatin. Focal clusters of cells showed clear cytoplasm (Figure 2C). Pleomorphic and multinucleate cells were occasionally seen. Sparse inflammatory cell infiltration was also observed (Figure 2D). The mitotic count was 1 MF/10 HPFs. Necrosis was not found. According to FNCLCC grading, the morphology of the neoplasm was low grade. The tumor cells were immunohistochemically positive for CD34 (Figure 2E), S100 (Figure 2F), and ALK-D5F3 (Figure 2G), and negative for AE1/3, SMA, desmin, STAT6, and SOX10. H3K27me3 staining was retained. The available clinical follow-up information of the patient revealed that the tumor recurred at the original site 27 months after surgery. Pathologically, the relapsed tumor showed similar morphology and immunophenotype to the original tumor, with more compact tumor cells (Figure 2H).
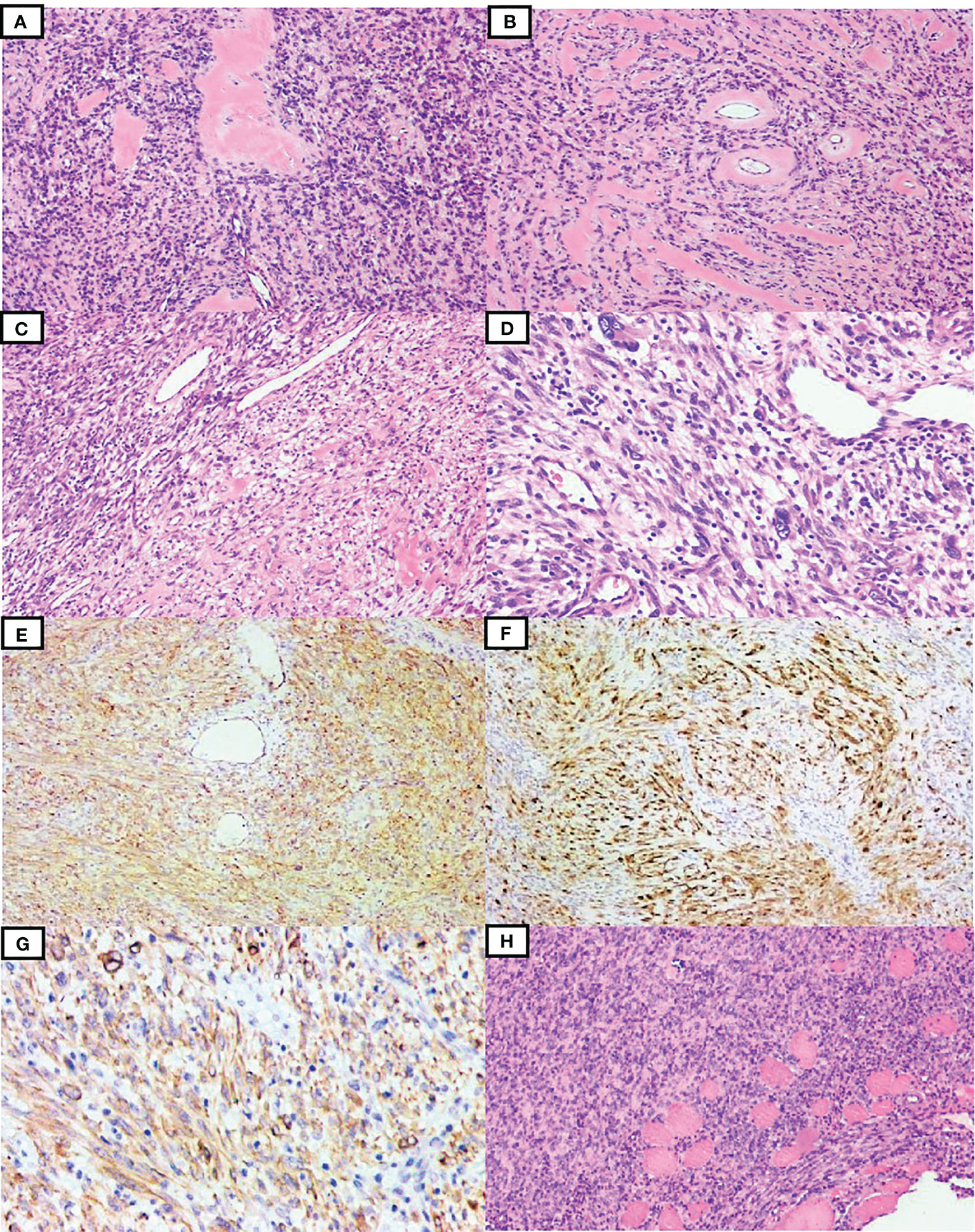
Figure 2 Clinicopathological findings of the tumor in case 2. The tumor consisted of spindle-shaped mesenchymal cells with stromal and perivascular hyalinization (A, B). Focal staghorn vessels and clusters of clear cytoplasmic cells were observed (C). Giant multinucleated tumor cells and inflammatory infiltration were also seen (D). The tumor cells were diffusely positive for CD34 (E), S100 (F), and ALK-D5F3 (G). The relapsed tumor showed diffuse proliferation of compact spindle cells, also infiltrating striated muscles (H).
Genomic DNA was extracted from formaldehyde-fixed paraffin-embedded (FFPE) tumor tissues using the QIAamp DNA mini kit (Qiagen, Hilden, Germany). Targeted deep sequencing of mutational hot spots was conducted using a capture-based targeted sequencing panel (Burning Rock Biotech, Guangzhou, China), including a panel of 520 genes to detect genomic alterations including single base substitution, short and long insertions/deletions, copy number variations, gene fusions, and rearrangement. NGS test identified a transcript comprising intron 6 of PLEKHH2 and intron 20 of ALK in case 1, which was validated by RT-PCR and Sanger sequencing (Figure 3A).
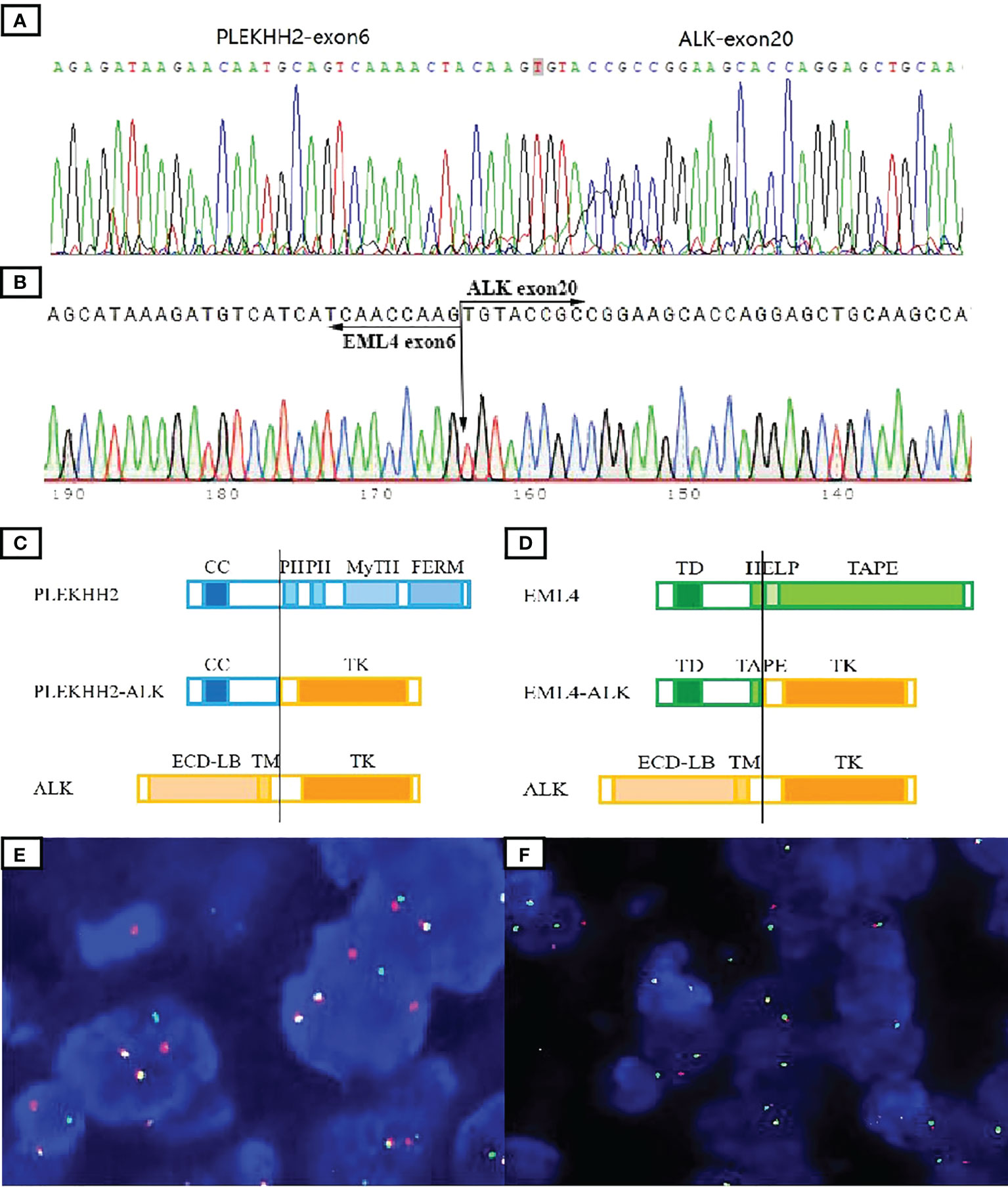
Figure 3 Molecular tests to validate ALK fusions. The presence of the PLEKHH2::ALK fusion transcript was validated by Sanger sequencing (A). Direct sequencing of the purified RT-PCR products revealed the chimeric transcripts between exon 6 of EML4 and exon 20 of ALK (B). Schematic representation of the predicted chimeric proteins (C, D). By ALK break-apart FISH test, most tumor cells in both cases demonstrated a signal pattern consisting of isolated 5′ (green) and isolated 3′ (orange), along with fused 3′/5′ signals (E, F). TD, trimerization domain; HELP, hydrophobic motif in EML proteins; TAPE, tandem atypical propeller domain; ECD-LB, extracellular ligand-binding domain; TM, transmembrane domain; TK, tyrosine kinase domain; CC, a-helical coiled-coil domain; PH, Pleckstrin homology domain; MyTH, MyTH4 domain; FERM, four-point-one ezrin radxin mesoin family.
Genomic RNA was extracted from tumor FFPE tissues using RNeasy FFPE (Qiagen, Hilden, Germany) and reverse transcribed using SuperScript IV First-Strand Synthesis System (Invitrogen, Carlsbad, CA, USA). The mutation of EML4::ALK was detected according to the ARMS methods using a human multigene mutation detection kit (PCR fluorescence probe method) (Amoy Diagnostics Co. Ltd., Xiamen, China). The PCR product was analyzed by Sanger sequencing using Big Dye Terminator Sequencing kit (Applied Biosystems, Foster City, CA, USA). ARMS test detected a transcript comprising exon 6 of EML4 and exon 20 of ALK in case 2 (Figure 3B).
The predicted chimeric proteins consisted of an N-terminal part with the coiled-coil domains of PLEKHH2 or EML4 and a C-terminal part with the complete kinase domain of ALK (Figures 3C, D).
Fluorescence in situ hybridization (FISH) analysis was performed on 3-µm-thick FFPE tumor sections using the dual-color break-apart probe of ALK (Abbot Molecular, Abbott Park, IL, USA). A hundred nonoverlapping cells were scored, and more than 20% of tumor cells with abnormal signals were considered positive for gene rearrangement. FISH results confirm ALK rearrangements in both cases (Figures 3E, F).
In this study, we reported two cases of STTs with S100 and CD34 co-expression harboring ALK gene rearrangements with some distinct features. Morphologically, case 1 was intermediate-grade sarcoma composed of uniform spindle cells and epithelioid cells arranged in myxedematous and hyalinized stroma, with brisk mitosis, focal necrosis, and inflammatory cell infiltration. Although most of the kinase fusion–positive STTs were defined as spindle cell tumors, epithelioid cells have been observed in some areas of S100- and CD34-co-expressing tumors harboring RAF1, BRAF, and ALK gene rearrangements (3, 9, 19). Myxedematous stroma has been found in some cases of ALK-rearranged STTs with S100 and CD34 co-expression (6–11). However, tumor cells with brisk mitosis and focal necrosis, which were the features of intermediate- to high-grade sarcoma, have rarely been reported in ALK-rearranged STTs. The tumor in case 2 showed moderate to high cellular proliferation and stromal and perivascular hyalinization, which are consistent with morphological features reported by Suurmeijer et al. (15). Similar to other reported S100- and CD34-co-expressing mesenchymal tumors harboring ALK rearrangement (6, 8, 9, 12, 13), inflammatory infiltration was found in both our cases. However, case 1 even showed IMT-like morphology, suggesting IMT in the differential diagnosis, but then we discarded the hypothesis due to the absence of myogenic expression and S100 and CD34 co-expression. To the best of our knowledge, IMT-like morphology has not been revealed in S100- and CD34-co-expressing mesenchymal tumors harboring ALK rearrangement. Nevertheless, it has been reported in NTRK-rearranged spindle cell tumors, presented primarily (28) or as a morphological transformation after chemotherapy (29). Based on the case reported here and the literature reviewed in the Introduction section, we speculate that similar to NTRK-rearranged spindle cell tumors, ALK-rearranged soft tissue tumors also span a wide spectrum of morphologies and histologic grades. Furthermore, the IMT-like pattern, analogous to the LPFNT pattern, might overlap with other patterns in the wide spectrum of kinase fusion–positive mesenchymal neoplasms.
Genetically, the tumor in case 1 was identified to harbor PLEKHH2::ALK fusion gene, whereas the tumor in case 2 showed EML4::ALK gene fusion. The PLEKHH2 gene (2p21) encodes an intracellular protein highly enriched in renal glomerular podocytes, which plays a structural and functional role in the podocyte foot processes. The presence of a putative a-helical coiled-coil domain was observed in the N-terminus of PLEKHH2 (30). The EML4 gene (2p21) encodes a microtubule-associated protein with a coiled-coil domain and may generate abnormal fusion with ALK, which has been identified in lung adenocarcinoma, breast cancer, colorectal cancer, IMT, and S100- and CD34-co-expressing neoplasms. Commonly, ALK fusions could activate the ALK kinase domain without a ligand through autophosphorylation due to dimerization. Both of the fusion genes in our study contained the entire intracellular kinase domain of ALK and the coiled-coil domain of the fusion partner genes, which mediated dimerization and activation of the ALK kinase domain. Therefore, the fusion proteins were presumed to have an oncogenic function.
Recently, an emerging class of spindle cell tumors characterized by frequent S100 protein and/or CD34 co-expression and recurrent tyrosine kinase fusions, including BRAF, RAF1, RET, MET, ROS1, and ALK, has been documented, although it is unclear whether these tumors should be classified into one category (31). We tried to summarize their features and find some commonalities listed hereafter. First, the related kinase fusion genes are predominantly tyrosine kinase genes, which regulate downstream signaling pathways, including the MAPK/ERK, PI3K/AKT, and JAK3-STAT3. BRAF and RAF1 even constitute the MAPK pathway components. Second, most of the oncogenic activation of kinase genes is through rearrangement. The kinase domain is reserved, and the partner gene is responsible for dimerization or other ways to mediate the activation of the kinase domain. Third, kinase fusion–positive neoplasms have been proven to be effective for targeted therapy (13, 27), not only in mesenchymal tumors but also in various epithelial neoplasms. Finally, these kinase fusion–positive mesenchymal neoplasms share similar clinicopathological features with NTRK-rearranged spindle cell tumors.
We have generalized the features of 47 mesenchymal neoplasms with oncogenic kinase alterations akin to NTRK-rearranged mesenchymal neoplasms searched in the available published literature, including 10 cases positive for RAF1, eight for RET, four for BRAF, 21 for ALK, one for MET, one for ROS1, and two for ABL1 gene rearrangements (1–20). Among them, 15 were found in children (<10 years), seven were found in adolescents (age range of 10–20 years), and 25 were found in adult patients (>20 years old). Both sexes were affected (27 females and 20 males). The tumors were most commonly located in soft tissues of the trunk and extremities, while a few occurred in the head and neck region, viscera, and even skeleton. Tumor size ranged from 0.5 cm to 14 cm in 29 tumors with available data. The 47 tumors spanned a wide spectrum of morphologies and histologic grades, showing monomorphic spindle cell proliferation in a haphazard arrangement with occasional components of epithelioid or pleomorphic cells. In addition to the unified features mentioned above, some cases seem to show overlapped characteristics. Myxoid stroma was observed in some cases (14/47) and seemed to be more frequently present in ALK-rearranged tumors (6–11, 16). Some ALK-, RAF1-, or RET-rearranged tumors were characterized by the presence of tumor cells arranged in concentric whorls, which was also observed in NTRK-rearranged tumors (6, 7, 17, 18, 32). Staghorn or hemangiopericytoma-like vessels were also observed in some ALK-, RET-, RAF1-, or BRAF-rearranged tumors (8/47), which has also been recognized as one of the characteristics of NTRK-rearranged STTs (6, 14, 16, 18, 19). Inflammatory infiltration was readily witnessed in nearly one-third of cases, closely correlating with LPFNT morphology (20). Tumors with low-grade morphological features were common (32/47, 68.1%), while intermediate- to advanced-grade tumors were relatively rare. Certainly, with the deepening understanding of these tumors, some less common features will be reported and summarized, even under the name of other provisionally termed entities. The prognosis of the tumors appears to be related to histologic grade. Low-grade tumors with positive margins showed a propensity for local recurrence, whereas high-grade tumors showed aggressive clinical behavior and metastasized to lungs or other organs. Thus, in view of increasing cases of kinase fusion–positive mesenchymal neoplasms, we believe that the emerging entity of mesenchymal neoplasms with oncogenic kinase alterations akin to NTRK-rearranged spindle cell tumors could develop into a constantly expanding family of kinase fusion–positive soft tissue tumors.
Herein, we reported two spindle and epithelioid cell neoplasms with S100 and CD34 co-expression showing recurrent ALK rearrangements. Our report adds to the morphological and genetic spectrum of the novel, recently described entity with S100 and CD34 co-expression harboring kinase fusions. We believe that more reported cases will unveil the panoramic view of the clinicopathological features of kinase fusion–positive STTs and improve patient treatment strategies and prognosis via targeted therapies.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University. The patients/participants provided their written informed consent to participate in this study.
S-JS, J-ML, Q-HF, MZ, and Q-XG designed the study. S-JS, J-ML and YL participated in patient treatment and analyzed clinical data. S-YC performed molecular testing and analyzed the data. S-JS and J-ML drafted the manuscript. Q-XG and MZ supervised the work and revised the manuscript. Q-HF helped revised the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by Technology Support Programs of Suqian city (S201519), Natural Science Foundation of Zhejiang Province (LY21H160052), and Zhejiang Provincial Medicine and Health Research Foundation (2023KY040). The funders did not have any role in the design and conduct of the study, the analysis and interpretation of the data, and preparation of the manuscript.
Author S-YC was employed by Guangzhou LBP Medicine Science & Technology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1007296/full#supplementary-material
Supplementary Figure 1 | Imaging studies of the left thigh in case 1. The lesion situated between the anterior rectus and vastus lateralis muscles (A) and was slightly hypointense in T1 weighted imaging (B) and significant hyperintense in T2 weighted imaging (C).
1. Subbiah V, Westin SN, Wang K, Araujo D, Wang WL, Miller VA, et al. Targeted therapy by combined inhibition of the RAF and mTOR kinases in malignant spindle cell neoplasm harboring the KIAA1549-BRAF fusion protein. J Hematol Oncol (2014) 7:8. doi: 10.1186/1756-8722-7-8
2. Flucke U, van Noesel MM, Wijnen M, Zhang L, Chen CL, Sung YS, et al. TFG-MET fusion in an infantile spindle cell sarcoma with neural features. Genes Chromosomes Cancer (2017) 56(9):663–7. doi: 10.1002/gcc.22470
3. Hicks JK, Henderson-Jackson E, Duggan J, Joyce DM, Brohl AS. Identification of a novel MTAP-RAF1 fusion in a soft tissue sarcoma. Diagn Pathol (2018) 13(1):77. doi: 10.1186/s13000-018-0759-z
4. Antonescu CR, Dickson BC, Swanson D, Zhang L, Sung YS, Kao YC, et al. Spindle cell tumors with RET gene fusions exhibit a morphologic spectrum akin to tumors with NTRK gene fusions. Am J Surg Pathol (2019) 43(10):1384–91. doi: 10.1097/PAS.0000000000001297
5. Abs D, Landman S, Osio A, Lepesant P, Schneider P, Obadia D, et al. Spindle cell tumor with CD34 and S100 co-expression and distinctive stromal and perivascular hyalinization showing EML4-ALK fusion. J Cutan Pathol (2021) 48(7):896–901. doi: 10.1111/cup.13926
6. Dermawan JK, Azzato EM, Goldblum JR, Rubin BP, Billings SD, Ko JS, et al. Superficial ALK-rearranged myxoid spindle cell neoplasm: a cutaneous soft tissue tumor with distinctive morphology and immunophenotypic profile. Mod Pathol (2021) 34(9):1710–8. doi: 10.1038/s41379-021-00830-w
7. Kao YC, Lee PH, Wu CL, Yu SC, Huang HY. Superficial ALK-rearranged myxoid spindle cell neoplasm with a novel FMR1-ALK fusion gene. Mod Pathol (2022) 35(3):438–41. doi: 10.1038/s41379-021-00952-1
8. Tan SY, Al-Ibraheemi A, Ahrens WA, Oesterheld JE, Fanburg-Smith JC, Liu YJ, et al. ALK rearrangements in infantile fibrosarcoma-like spindle cell tumours of soft tissue and kidney. Histopathology (2022) 80(4):698–707. doi: 10.1111/his.14603
9. Coppock JD, Schneider MA, Surrey LF, Karakousis GC, Maki RG, Cooper K, et al. S100 and CD34 expressing mesenchymal neoplasm with rare PLEKHH2::ALK fusion and response to ALK inhibition. Am J Surg Pathol (2022) 46(9):1309–13. doi: 10.1097/PAS.0000000000001887
10. Lopez-Nunez O, Surrey LF, Alaggio R, Fritchie KJ, John I. Novel PPP1CB-ALK fusion in spindle cell tumor defined by S100 and CD34 coexpression and distinctive stromal and perivascular hyalinization. Genes Chromosomes Cancer (2020) 59(8):495–9. doi: 10.1002/gcc.22844
11. Mantilla JG, Cheung H, Ha AS, Hoch BL, Liu YJ, Ricciotti RW, et al. Spindle cell neoplasm with EML4-ALK gene fusion presenting as an intraosseous vertebral mass. Genes Chromosomes Cancer (2021) 60(4):282–6. doi: 10.1002/gcc.22917
12. Kao YC, Suurmeijer AJH, Argani P, Ickson BC, Zhang L, Sung YS, et al. Soft tissue tumors characterized by a wide spectrum of kinase fusions share a lipofibromatosis-like neural tumor pattern. Genes Chromosomes Cancer (2020) 59(10):575–83. doi: 10.1002/gcc.22877
13. Rakheja D, Park JY, Fernandes NJ, Watt TC, Laetsch TW, Collins RRJ, et al. Pediatric non-myofibroblastic primitive spindle cell tumors with ALK gene rearrangements and response to crizotinib. Int J Surg Pathol (2022) 30(6):706–15. doi: 10.1177/10668969221080072
14. Agaram NP, Zhang L, Sung YS, Chen CL, Chung CT, Antonescu CRc, et al. Recurrent NTRK1 gene fusions define a novel subset of locally aggressive lipofibromatosis-like neural tumors. Am J Surg Pathol (2016) 40:1407–16. doi: 10.1097/PAS.0000000000000675
15. Suurmeijer AJH, Dickson BC, Swanson D, Zhang L, Sung YS, Cotzia P, et al. A novel group of spindle cell tumors defined by S100 and CD34 co-expression shows recurrent fusions involving RAF1, BRAF, and NTRK1/2 genes. Genes Chromosomes Cancer (2018) 57(12):611–21. doi: 10.1002/gcc.22671
16. Loong S, Lian DWQ, Kuick CH, Lim TH, Nah SA, Wong KPL, et al. Novel TFG-RET fusion in a spindle cell tumour with S100 and CD34 coexpresssion. Histopathology (2020) 76(2):333–6. doi: 10.1111/his.13971
17. Michal M, Ptáková N, Martínek P, Gatalica Z, Kazakov DV, Michalová K, et al. S100 and CD34 positive spindle cell tumor with prominent perivascular hyalinization and a novel NCOA4-RET fusion. Genes Chromosomes Cancer (2019) 58(9):680–5. doi: 10.1002/gcc.22758
18. Mok Y, Kimpo MS, Chen H, Kuick CH, Chang KT, Lee VKM, et al. Spindle cell tumour with S100 and CD34 co-expression showing PDZRN3-RAF1 rearrangement–a recently described entity. Histopathology (2019) 74(7):1109–11. doi: 10.1111/his.13841
19. Sheng SJ, Li JM, Zou YF, Peng XJ, Wang QY, Fang HS, et al. A low-grade malignant soft tissue tumor with S100 and CD34 co-expression showing novel CDC42SE2-BRAF fusion with distinct features. Genes Chromosomes Cancer (2020) 59(10):595–600. doi: 10.1002/gcc.22875
20. Choo F, Rakheja D, Davis LE, Davare M, Park JY, Timmons CF, et al. GAB1-ABL1 fusions in tumors that have histologic overlap with NTRK-rearranged spindle cell tumors. Genes Chromosomes Cancer (2021) 60(9):623–30. doi: 10.1002/gcc.22972
21. Dickson BC, Swanson D, Charames GS, Fletcher CD, Hornick JL. Epithelioid fibrous histiocytoma: molecular characterization of ALK fusion partners in 23 cases. Mod Pathol (2018) 31(5):753–62. doi: 10.1038/modpathol.2017.191
22. Kemps PG, Picarsic J, Durham BH, Hélias-Rodzewicz Z, Hiemcke- Jiwa L, van den Bos C, et al. ALK-positive histiocytosis: a new clinicopathologic spectrum highlighting neurologic involvement and responses to ALK inhibition. Blood (2022) 139(2):256–80. doi: 10.1182/blood.2021013338
23. Kuroda N, Trpkov K, Gao Y, Tretiakova M, Liu YJ, Ulamec M, et al. ALK rearranged renal cell carcinoma (ALK-RCC): a multi-institutional study of twelve cases with identification of novel partner genes CLIP1, KIF5B and KIAA1217. Mod Pathol (2020) 33(12):2564–79. doi: 10.1038/s41379-020-0578-0
24. Panebianco F, Nikitski AV, Nikiforova MN, Kaya C, Yip L, Condello V, et al. Characterization of thyroid cancer driven by known and novel ALK fusions. Endocr Relat Cancer (2019) 26(11):803–14. doi: 10.1530/ERC-19-0325
25. Sasaki E, Masago K, Fujita S, Suzuki H, Hanai N, Hosoda W. Salivary secretory carcinoma harboring a novel ALK fusion: Expanding the molecular characterization of carcinomas beyond the ETV6 gene. Am J Surg Pathol (2020) 44(7):962–9. doi: 10.1097/PAS.0000000000001471
26. Zhao L, Nathenson MJ, Nowak JA, Fairweather M, Hornick JL. ALK rearrangement in a gastrointestinal stromal tumour of the small bowel. Histopathology (2020) 77(3):513–5. doi: 10.1111/his.14133
27. Chen T, Wang Y, Goetz L, Corey Z, Dougher MC, Smith JD, et al. Novel fusion sarcomas including targetable NTRK and ALK. Ann Diagn Pathol (2021) 54:151800. doi: 10.1016/j.anndiagpath.2021.151800
28. Wong DD, Vargas AC, Bonar F, Maclean F, Kattampallil J, Stewart C, et al. NTRK-rearranged mesenchymal tumours: diagnostic challenges, morphological patterns and proposed testing algorithm. Pathology (2020) 52(4):401–9. doi: 10.1016/j.pathol.2020.02.004
29. Pavlick D, Schrock AB, Malicki D, Stephens PJ, Kuo DJ, Ahn H, et al. Identification of NTRK fusions in pediatric mesenchymal tumors. Pediatr Blood Cancer (2017) 64(8):e26433. doi: 10.1002/pbc.26433
30. Perisic L, Lal M, Hulkko J, Hultenby K, Önfelt B, Sun Y, et al. Plekhh2, a novel podocyte protein downregulated in human focal segmental glomerulosclerosis, is involved in matrix adhesion and actin dynamics. Kidney Int (2012) 82(10):1071–83. doi: 10.1038/ki.2012.252
31. Antonescu CR. Emerging soft tissue tumors with kinase fusions: An overview of the recent literature with an emphasis on diagnostic criteria. Genes Chromosomes Cancer (2020) 59(8):437–44. doi: 10.1002/gcc.22846
Keywords: ALK, PLEKHH2, EML4, S100 and CD34 co-expression, soft tissue tumor
Citation: Sheng S-J, Li J-M, Fan Q-H, Liu Y, Chen S-Y, Zhao M and Gong Q-X (2022) Case report: ALK-rearranged spindle and epithelioid cell neoplasms with S100 and CD34 co-expression: Additional evidence of kinase fusion–positive soft tissue tumors. Front. Oncol. 12:1007296. doi: 10.3389/fonc.2022.1007296
Received: 30 July 2022; Accepted: 10 October 2022;
Published: 26 October 2022.
Edited by:
Jen-Chieh Lee, National Taiwan University Hospital, TaiwanReviewed by:
Husan-Ying Huang, Kaohsiung Chang Gung Memorial Hospital, TaiwanCopyright © 2022 Sheng, Li, Fan, Liu, Chen, Zhao and Gong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qi-Xing Gong, Z29uZ3FpeGluZ0Bob3RtYWlsLmNvbQ==; Ming Zhao, emhhb21pbmdwYXRob2xAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.