
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 18 November 2022
Sec. Surgical Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1004108
This article is part of the Research Topic Case Reports in Surgical Oncology: 2022 View all 56 articles
Hepatic undifferentiated embryonal sarcoma of the liver (UESL) is a rare hepatic malignancy found more commonly in pediatric patients. It has been associated with poor outcomes in adults and the role and timing of systemic therapy is unclear. There have been very few case reports detailing combination neoadjuvant and adjuvant chemotherapy use for hepatic undifferentiated embryonal sarcoma in adults. In this report, a 22-year-old male admitted with right upper quadrant pain was diagnosed with a 20 x 10 x 10 cm well-circumscribed, highly vascularized hepatic mass in the entirety of the left lobe. Biopsy confirmed the diagnosis of UESL. PET/CT showed no evidence of metastatic disease, and he received four cycles of Doxorubicin and Ifosfamide with demonstrated reduction in size and decrease in PET avidity. He underwent left hepatectomy with periportal lymphadenectomy, cholecystectomy, and partial gastrectomy with negative margins and received adjuvant Doxorubicin, Ifosfamide and Mesna. At 48 months, the patient was alive without evidence of disease. We hereby emphasize the potential advantages of combination chemotherapy and surgical resection in the management of UESL in adults.
First described in 1978, undifferentiated embryonal sarcoma (UESL) of the liver is a rare mesenchymal tumor, almost exclusively found in pediatric age patients (1–3). The average age of presentation is approximately 6 to 10 years of age with over 88% of cases occurring in patients 15 years of age or younger (1, 4). Diagnosis can be difficult, as there are shared clinical and radiological findings with other hepatic tumors. The pathogenesis is not well-described but is possibly associated with mesenchymal hamartoma as both tumors have been associated with similar cytogenetic abnormalities (3).
Accounting for less than 1% of all primary liver neoplasms in adults, an optimal treatment regimen for UESL is still being determined (5). Surgical resection of the tumor was previously the mainstay of treatment for UESL (3). Recently, several studies have indicated multimodal therapy with surgery, radiation, and chemotherapy can significantly improve patient prognosis (3). Despite aggressive treatment, a diagnosis of UESL carries an overall 5-year survival rate estimated between 65% to 72% (6, 7). Adults have been reported to have a worse prognosis than in pediatric patients with a reported survival of 48.2% (7). Given the high rates of regional and local recurrence, a strategy employing early systemic therapy may have the potential to improve outcomes. There is limited data on the efficacy and feasibility of multimodal therapy in this rare tumor type. This report outlines the successful treatment of a male adult patient with UESL treated with neoadjuvant and adjuvant chemotherapy in combination with surgical resection.
A 22-year-old male presented with one month of decreased appetite and sharp, intermittent epigastric pain radiating to the right upper quadrant. The patient did have a history of anemia but was otherwise healthy with no significant family history. Physical exam revealed abdominal distension, with visible protrusion in the LUQ, and no tenderness. Laboratory tests revealed anemia and neutropenia with elevated alkaline phosphatase, lipase, lactate dehydrogenase and bilirubin (WBC 2.1, normal 4 -11 g/L; Hemoglobin 8.0, normal 13.5 - 17.5 g/dL; Alkaline phosphatase 179, normal 39 - 117; lipase 1140, normal 16 - 63 U/L; LDH 569, normal 94 - 250 U/L, Bilirubin 1.0, normal < 0.3 mg/dL). Alpha Fetoprotein, AST and ALT levels were normal. Hepatitis serologies were negative.
Abdominal computed tomography (CT) revealed a solitary 20 x 10 x 10 cm well-circumscribed, highly vascularized hepatic mass in the entirety of the left lobe with relative sparing of the right lobe (Figure 1). Positron emission tomography (PET) Scan redemonstrated this hepatic mass demonstrating intense FDG uptake of SUV 11.5 and areas of necrosis (Figure 1). There was no evidence of metastatic disease and no evidence of invasion into surrounding structures. Ultrasound guided core needle biopsy identified a malignant spindle cell neoplasm consistent with diffuse embryonal sarcoma (Figure 2). Biopsy results combined with imaging were diagnostic of UESL.
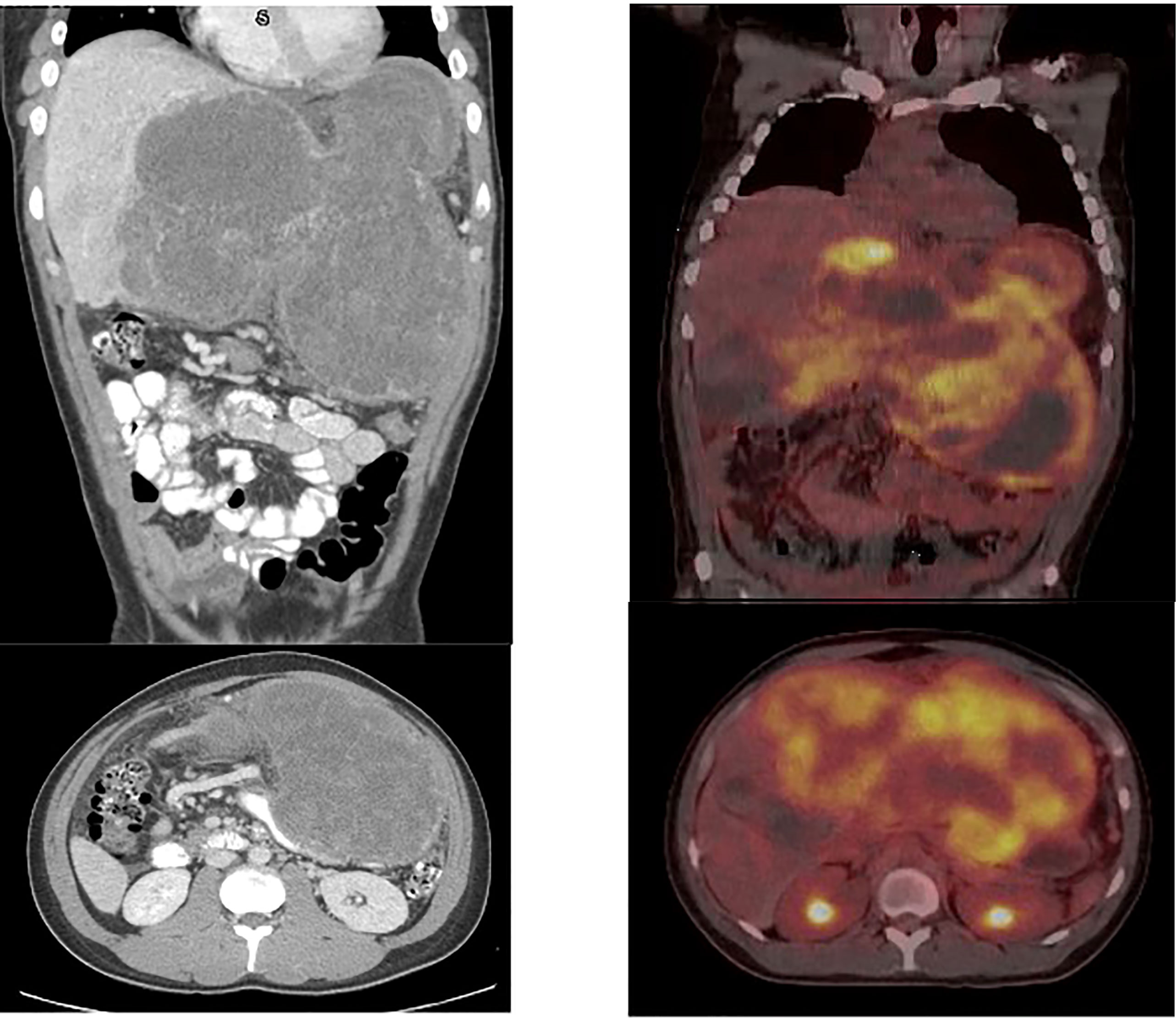
Figure 1 Initial CT scan demonstrating UESL. 20 x 10 x 10 cm well-circumscribed, solid mass of the entirety of the left lobe of the liver with relative sparing of the right lobe. Initial PET/CT demonstrating large hepatic mass measuring 25 x 13 cm with maximal SUV 11.5 as well as areas of necrosis, demonstrated by lack of FDG avidity.
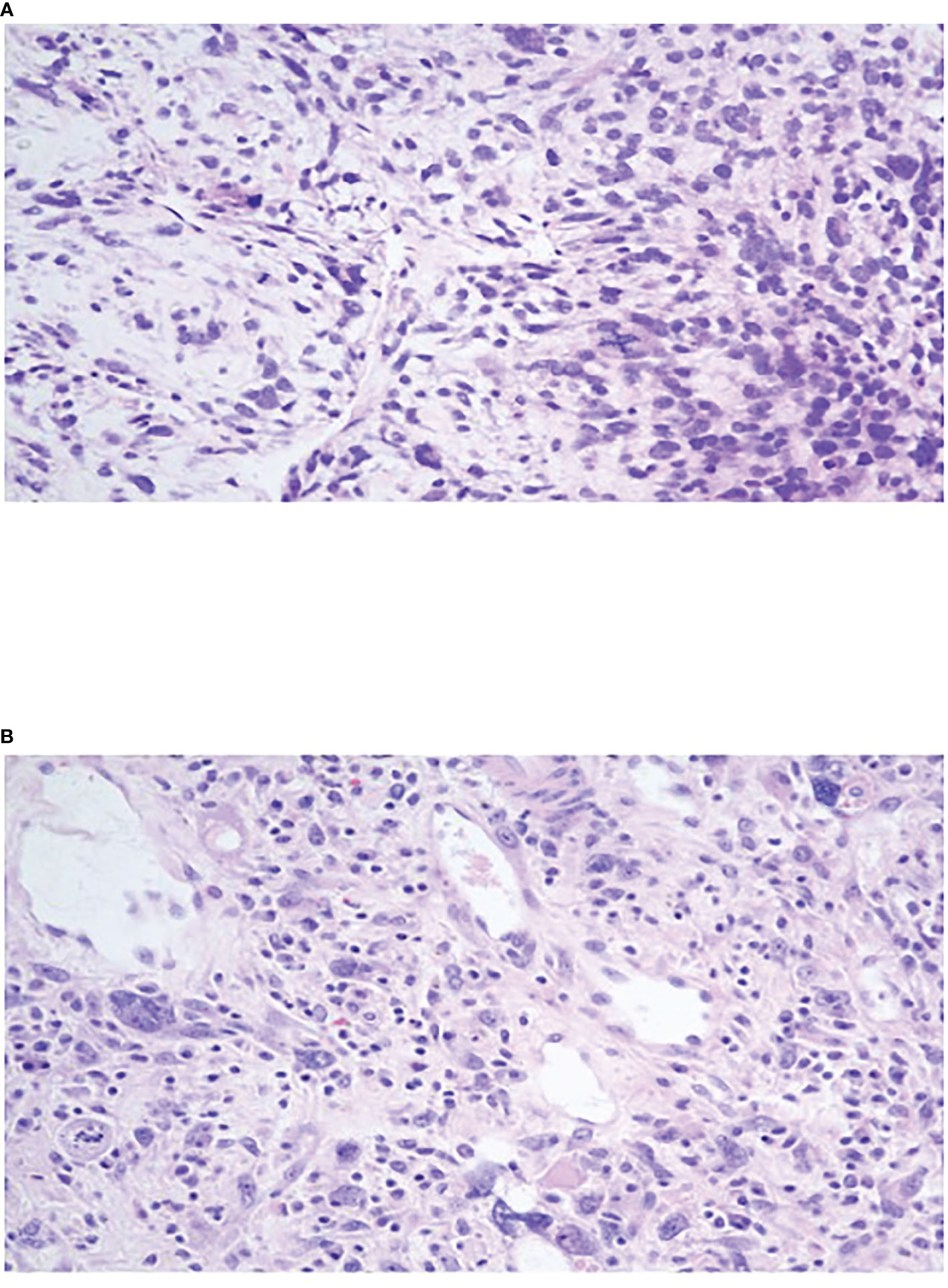
Figure 2 (A) Oval to stellate tumor cells with ill-defined cell borders loosely distributed in myxoid stroma with marked nuclear pleomorphism, hyperchromasia and brisk mitotic activity. (B) Tumor cells within a fibroinflammatory background showing marked nuclear pleomorphism, hyperchromasia, and frequent multinucleated and bizarre giant cells. Cytoplasmic and extracellular eosinophilic globules noted.
Patient was treated with neoadjuvant chemotherapy modeled after protocol ARST 1321 Regimen B by the Children’s Oncology Group. Preoperative chemotherapy consisted of Ifosfamide (2.5g/m^2) per dose intravenously on days 1 to 3) with MESNA and doxorubicin (37.5 mg/m^2 per dose intravenously on days 1 to 2) at 3-week intervals for 4 cycles. Beyond generalized malaise, the patient did not experience any adverse side effects to chemotherapy. Repeat CT scan indicated a decrease in tumor size to 15.2 x 7.7 x 8.1 cm. Repeat PET scan corroborated the decrease in tumor size and indicated a significant decrease in FDG uptake, with a maximum SUV of 5.4 (Figure 3).
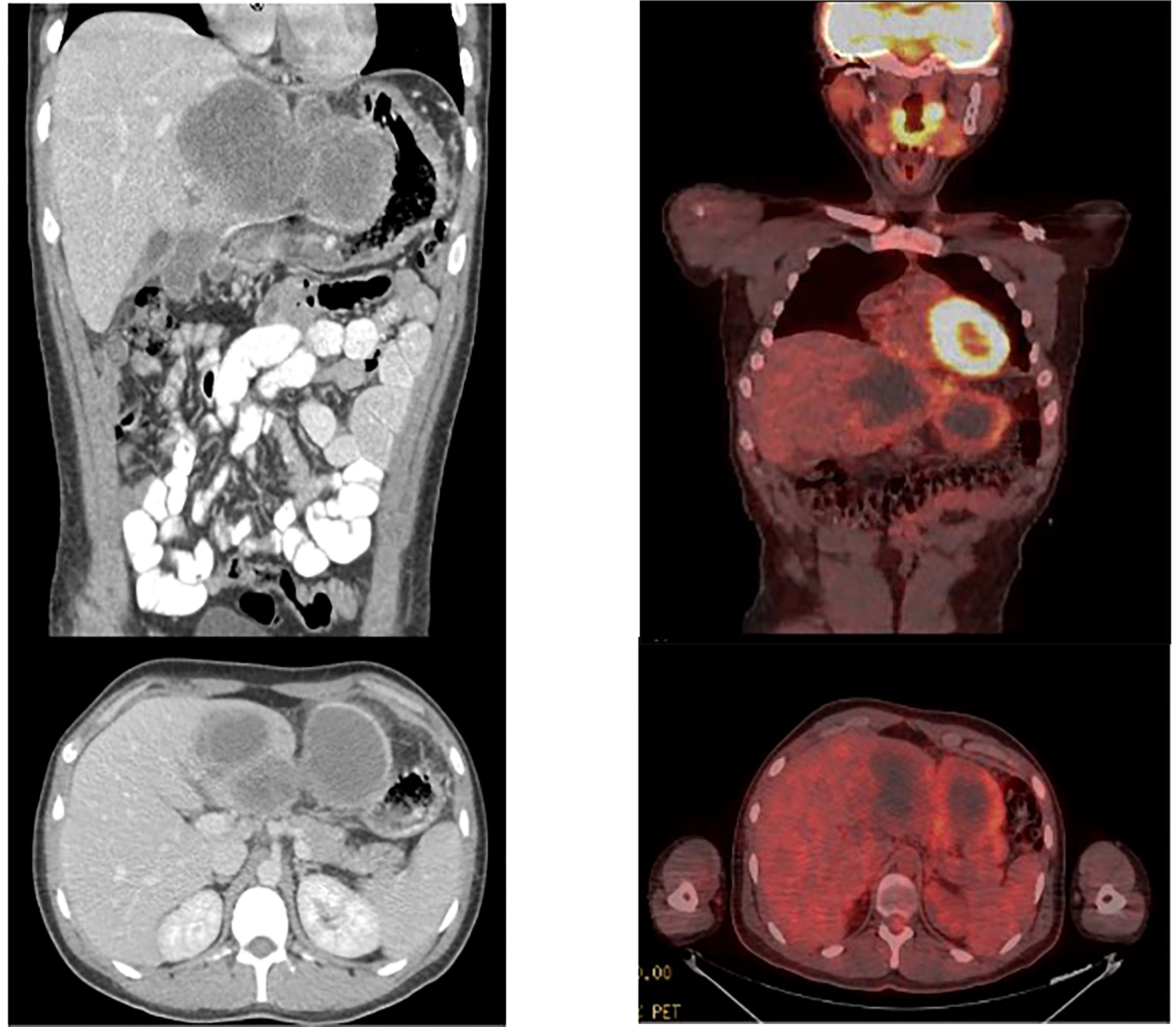
Figure 3 CT scan after completion of neodjuvant chemotherapy. Significant improvement was visualized with a decrease in lesion size to 15 x 7.7 x 8.1 cm. PET scan after completion of neodjuvant chemotherapy demonstrating reduction in size of mass to 14.5 x 6.8 x 8.9 cm as well as decreased FDG avidty of SUV 5.4.
Intraoperatively, the hepatic mass of the left lobe was identified with significant omental and gastric adhesions. The tumor was also invading the medial gallbladder wall. Intraoperative ultrasound was used to delineate intraparenchymal tumor borders and ultimately the patient underwent left hepatectomy with periportal lymphadenectomy, cholecystectomy, and partial gastrectomy. Surgical margin was negative defined as greater than 1mm. The patient recovered with no surgical complications. Postoperatively, the patient underwent an additional two cycles of adjuvant Doxorubicin, Ifosfamide and Mesna. 3 weeks after surgery the patient received 2 cycles of Doxorubicin/Ifosfamide and one of Doxorubicin only at 3 week intervals, with or without Pazopanib, completing all therapy at week 25 (cumulative doses: Ifosfamide 45 g/m^2, Doxorubicin 375 mg/m^2).
For surveillance the patient underwent CT or MRI of the abdomen and pelvis plus a chest X-ray or CT. Surveillance protocol included CT scan every three months for the first year, every four months the second year, every six months through the third year and then once yearly beyond three years. The patient has since been noted to be in good health with no evidence of local or distant recurrence at present, 4 years post-resection.
At 2 to 15% of cases, UESL is the third most common hepatic malignancy in pediatric populations after hepatoblastoma and hepatocellular carcinoma (1, 4, 8). It is estimated that only 47 cases of UESL in patients older than 15 years old have been reported in the literature from 1978 to 2007 (9). A more recent review from 1973-2019 reported less than 90 adult UESL cases and a recent NCDB study only identified 41 adult patients (6, 7). Patients with UESL can present with nonspecific symptoms including abdominal pain, fever, weight loss diarrhea or vomiting (1, 4, 9–11). Laboratory evaluation may reveal leukocytosis and anemia; however no specific serum markers identify UESL (1, 3). The differential diagnosis of UESL includes abscess, mesenchymal hamartoma, hydatid cyst, hepatocellular carcinoma, hepatoblastoma, biliary tract rhabdomyosarcoma, cystic metastasis, angiolipoma, leiomyosarcoma, liposarcoma, epithelioid hemangioendothelioma, and malignant melanoma (1, 3, 8). UESL does share chromosomal abnormalities seen with mesenchymal hamartoma, with some suggesting malignant transformation of mesenchymal hamartoma to develop UESL; abnormalities associated with 19q13.4 including balanced translocations t(11;19)(q13;q13.4) and t(15;19)(q15;q13.4) (1, 3, 12).
UESL can have a differential appearance on imaging. Ultrasound demonstrates a solid hepatic mass, generally isoechoic to normal liver, with anechoic areas corresponding to areas of tumor necrosis or degeneration (1, 13). CT demonstrates a well-defined hypodense mass with internal septations, almost cystic in appearance (1, 13). With contrast administration the tumor does progressively enhance (1, 13). MRI demonstrates low signal intensity on T1 weighted images and high signal intensity on T2 weighted images, again with enhancement on contrast administration (1, 13). Due to its differential appearance, image-guided biopsy is the mainstay of diagnosis, however cases have been reported of tumor rupture after biopsy (14). Rupture prior to complete surgical resection can be a consideration for treatment of residual disease and higher risk of recurrence (2, 4). The prognosis of UESL with rupture into the peritoneal cavity is less favorable in comparison to cancerous counterparts including hepatocellular carcinoma secondary to factors including increased risk of systemic metastases (15).
Histopathology demonstrates that UESL is composed of undifferentiated spindle cells with mitotic figures and myxoid stroma (9, 16). The high water content of the myxoid stroma is thought to be the reason for the differential appearance of UESL on imaging (1, 13). UESL tumors also show characteristic intracellular hyaline globules and anaplasia on a mesenchymal background (8, 17). These tumors tend to have a pseudocapsule made of compressed hepatic parenchyma, leading to a well circumscribed hepatic mass (9).
Prognosis for UESL has historically been considered dismal with reported 5-year overall survival rate of approximately 65% in all patients with worse outcomes reported in adults (6, 18). Factors that have been associated with improved survival are margin negative resection, receipt of chemotherapy and childhood. Treatment options for UESL include surgery, chemotherapy, and liver transplantation (1, 19–21). One retrospective study of UESL patients from 1975 to 2015 using the SEER database showed while 84% of patients underwent surgery, only 65% underwent chemotherapy and 9% underwent radiation therapy (18). While surgical resection of the primary tumor was previously the mainstay of treatment, recent studies have shown success with use of adjuvant chemotherapy and neoadjuvant chemotherapy, particularly in initially unresectable cases (3). Tumors are generally considered unresectable due to size, amount of liver involvement, and invasion into surrounding structures (14). Given the poorer outcome in adults and the propensity for early local and distant metastases a strategy employing combined neoadjuvant and adjuvant chemotherapy may result in improved outcomes. A recent review of NCDB patients with UESL from 2004 – 2015 showed that 92.7% of pediatric patients received chemotherapy as opposed to 65.9% of adults (7). Only 1 out of 41 adult patients receiving both surgery and chemotherapy also received neoadjuvant therapy as opposed to adjuvant therapy (7).
As shown in one study, 12 of 17 children with UESL treated with a multimodal approach between 1979 and 1995 were alive at 2.4 to 20 years follow-up (4). A separate study of five pediatric patients treated with resection followed by adjuvant chemotherapy demonstrated no disease recurrence at a median of 53 months post-treatment (22). A third study of five pediatric patients ages 10 to 19 were also treated with multimodal therapy, including orthotopic liver transplantation in unresectable cases, demonstrating 100% survival at 21 to 68 months of follow-up with instances of recurrence (23). Combinations of Vincristine, Cyclophosphamide, Dactinomycin, Doxorubicin, Etoposide and Ifosfamide are associated with longer survival and disease-free prognosis (4, 8, 10, 24). Chemotherapy for UESL is modeled after established regimens for pediatric rhabdomyosarcoma or Ewing sarcoma without cisplatin (8, 17, 25). UESL metastases have been reported in up to 15% of pediatric patients and are mainly seen during primary diagnosis in which sites most commonly include lungs, adrenal glands, peritoneum and extension into the heart (23, 26, 27). Available data regarding metastatic disease in UESL is limited but is a critical consideration for future optimization of multimodal treatment options.
A review of multimodal treatment regimens for UESL are outlined in Table 1. Also included are some cases of patients undergoing surgery alone with no chemotherapy, highlighting the improved success with neoadjuvant and adjuvant treatment strategies. Given the reported improvements associated with chemotherapy in pediatric patients, we elected to treat our patient with multimodal chemotherapy and surgical resection. The intended goal of this approach in our patient was decrease tumor size to enable resection, start early systemic therapy for a disease with a propensity for early metastases, and to assess tumor response in vivo. These advantages have been reported for other cancers such as gastric, gastroesophageal and pancreatic cancers (34, 35). Our patient thus far appears to have achieved some of the intended benefits of combination chemotherapy as well as disease free survival of 48 months. In conclusion, we believe this approach is well suited for this cancer given its underlying biology i.e. a propensity for early local and distant recurrence, and therefore warrants further study. This case study adds to the growing body of literature favoring combination of combined neoadjuvant and adjuvant chemotherapy with hepatic resection in treatment of UESL.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
This case report is exempt from IRB approval. Patient did provide consent to publication of this manuscript.
RV and SK completed review of the literature, acquisition of data, drafting and completion of the manuscript. SK and GM participated in the critical review of the paper. All authors contributed to the article and approved the submitted version.
Funding for publication was obtained from the Western Michigan University Homer Stryker MD School of Medicine Department of Surgery research fund.
We would like to thank Bronson Methodist Hospital, West Michigan Cancer Center, and Western Michigan University for allowing us to conduct this project.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; CT, Computed tomography; FDG, F-18-Deoxyglucose or Fluorodeoxyglucose; NCDB, National Cancer Database; PET, Positron emission tomography; SEER, Surveillance, Epidemiology and End Results database; SUV, Standard uptake value; UESL, Undifferentiated embryonic sarcoma.
1. Gomes F, Melo D, Esteves C, Lima B, Carneiro F, Oliveira P. Undifferentiated embryonal sarcoma of the liver: A rare hepatic tumor and its related characteristic radiological features. Radiol Case Rep (2021) 16(3):646–50. doi: 10.1016/j.radcr.2020.12.017
2. Stocker JT. Hepatic tumors in children. Clin Liver Dis (2001) 5(1):259–ix. doi: 10.1016/s1089-3261(05)70163-x
3. Putra J, Ornvold K. Undifferentiated embryonal sarcoma of the liver: A concise review. Arch Pathol Lab Med (2015) 139(2):269–73. doi: 10.5858/arpa.2013-0463-RS
4. Bisogno G, Pilz T, Perilongo G, Ferrari A, Harms D, Ninfo V, et al. Undifferentiated sarcoma of the liver in childhood: A curable disease. Cancer. (2002) 94(1):252–7. doi: 10.1002/cncr.10191
5. Noguchi K, Yokoo H, Nakanishi K, Kakisaka T, Tsuruga Y, Kamachi H, et al. A long-term survival case of adult undifferentiated embryonal sarcoma of liver. World J Surg Oncol (2012) 10:65. doi: 10.1186/1477-7819-10-65
6. Wu Z, Wei Y, Cai Z, Zhou Y. Long-term survival outcomes of undifferentiated embryonal sarcoma of the liver: A pooled analysis of 308 patients. ANZ J Surg (2020) 90(9):1615–20. doi: 10.1111/ans.15684
7. Ziogas IA, Zamora IJ, Lovvorn Iii HN, Bailey CE, Alexopoulos SP. Undifferentiated embryonal sarcoma of the liver in children versus adults: A national cancer database analysis. Cancers (Basel) (2021) 13(12):2918. doi: 10.3390/cancers13122918
8. Childhood liver cancer treatment (PDQ)–health professional version Targeted Therapy to Treat Cancer (2022). (Bethesda, Maryland: National Cancer Institute). Available at: https://www.cancer.gov/types/liver/hp/child-liver-treatment-pdq.
9. Pachera S, Nishio H, Takahashi Y, Yokoyama Y, Oda K, Ebata T, et al. Undifferentiated embryonal sarcoma of the liver: Case report and literature survey. J Hepatobiliary Pancreat Surg (2008) 15(5):536–44. doi: 10.1007/s00534-007-1265-y
10. Walther A, Geller J, Coots A, Towbin A, Nathan J, Alonso M, et al. Multimodal therapy including liver transplantation for hepatic undifferentiated embryonal sarcoma. Liver Transpl (2014) 20(2):191–9. doi: 10.1002/lt.23773
11. Yu Y, Zhong Y, Wang J, Wu D. Sarcomatoid hepatocellular carcinoma (SHC): a case report. World J Surg Oncol (2017) 15(1):219. doi: 10.1186/s12957-017-1286-1
12. Stringer MD, Alizai NK. Mesenchymal hamartoma of the liver: a systematic review. J Pediatr Surg (2005) 40(11):1681–90. doi: 10.1016/j.jpedsurg.2005.07.052
13. Techavichit P, Masand PM, Himes RW, Abbas R, Goss JA, Vasudevan SA, et al. Undifferentiated embryonal sarcoma of the liver (UESL): A single-center experience and review of the literature. J Pediatr Hematol/Oncol (2016) 38(4):261–8. doi: 10.1097/MPH.0000000000000529
14. Babu BI, Bigam DL, Gilmour SM, Dajani KZ, Shapiro AMJ, Kneteman NM. Liver transplantation in locally unresectable, undifferentiated embryonal cell sarcoma. Transplant Direct (2021) 7(2):e654. doi: 10.1097/TXD.0000000000001106
15. Pandit N, Jaiswal LS, Shrestha V, Awale L, Adhikary S. Undifferentiated embryonal sarcoma of liver in an adult with spontaneous rupture and tumour thrombus in the right atrium. ANZ J Surg (2019) 89(9):E396–7. doi: 10.1111/ans.14670
16. Almogy G, Pappo O, Gips M, Lieberman S, Edden Y, Eid A. Improved survival with surgery and systemic chemotherapy for undifferentiated embryonal sarcoma of the liver. Isr Med Assoc J (2005) 7(10):672–3.
17. Stocker JT, Ishak KG. Undifferentiated (embryonal) sarcoma of the liver: report of 31 cases. Cancer. (1978) 42(1):336–48. doi: 10.1002/1097-0142(197807)42:1<336::aid-cncr2820420151>3.0.co;2-v
18. Pan L, Yin L, Liu XC, Ying RC, Kong WC. Adult versus paediatric undifferentiated embryonal sarcoma of the liver: a SEER database analysis. ANZ J Surg (2021) 91(12):2690–4. doi: 10.1111/ans.17290
19. Yu XH, Huang J, Ge NJ, Yang YF, Zhao JY. Recurrent undifferentiated embryonal sarcoma of the liver in adult patient treated by pembrolizumab: A case report. World J Clin Cases (2021) 9(10):2281–8. doi: 10.12998/wjcc.v9.i10.2281
20. Pandit N, Deo KB, Jaiswal LS, Pradhan A, Adhikary S. Hanging undifferentiated embryonal sarcoma of the liver in adult: an unusual presentation of an aggressive tumor. J Gastrointest Canc (2019) 50(3):689–92. doi: 10.1007/s12029-018-0119-y
21. Esteban SG, Emilio CU, Emmanuel AF, Oscar SJ, Paulina CE, Angel MM. Undifferentiated embryonal sarcoma of the liver in adult patient: A report of two cases. Ann Hepatobiliary Pancreat Surg (2018) 22(3):269–73. doi: 10.14701/ahbps.2018.22.3.269
22. May LT, Wang M, Albano E, Garrington T, Dishop M, Macy ME. Undifferentiated sarcoma of the liver: A single institution experience using a uniform treatment approach. J Pediatr Hematol Oncol (2012) 34(3):e114–6. doi: 10.1097/MPH.0b013e3182331fbe
23. Plant AS, Busuttil RW, Rana A, Nelson SD, Auerbach M, Federman NC. A single-institution retrospective cases series of childhood undifferentiated embryonal liver sarcoma (UELS): Success of combined therapy and the use of orthotopic liver transplant. J Pediatr Hematol Oncol (2013) 35(6):451–5. doi: 10.1097/MPH.0b013e318271c948
24. Mathias MD, Ambati SR, Chou AJ, Slotkin EK, Wexler LH, Meyers PA, et al. A single-center experience with undifferentiated embryonal sarcoma of the liver. Pediatr Blood Canc (2016) 63(12):2246–8. doi: 10.1002/pbc.26154
25. Ismail H, Dembowska-Bagińska B, Broniszczak D, Kaliciński P, Maruszewski P, Kluge P, et al. Treatment of undifferentiated embryonal sarcoma of the liver in children–single center experience. J Pediatr Surg (2013) 48(11):2202–6. doi: 10.1016/j.jpedsurg.2013.05.020
26. Shi M, Xu H, Sangster GP, Gu X. Pulmonary metastases from an undifferentiated embryonal sarcoma of the liver: A case report and review. Case reports in oncological medicine. 2018 (2018) 7840865:1–6. doi: 10.1155/2018/7840865
27. Shi Y, Rojas Y, Zhang W, Beierle EA, Doski JJ, Goldfarb M, et al. Characteristics and outcomes in children with undifferentiated embryonal sarcoma of the liver: A report from the national cancer database. Pediatr Blood Cancer (2017) 64(4):e26272. doi: 10.1002/pbc.26272
28. Pinamonti M, Vittone F, Ghiglione F, Borasi A, Silvestri S, Coverlizza S. Unexpected liver embryonal sarcoma in the adult: Diagnosis and treatment. Case Rep Surg (2018) 2018:8362012. doi: 10.1155/2018/8362012
29. Khan ZH, Ilyas K, Khan HH, Ghazanfar H, Hussain Q, Inayat F, et al. Unresectable undifferentiated embryonal sarcoma of the liver in an adult Male treated with chemotherapy and orthotopic liver transplantation. Cureus. (2017) 9(10):e1759. doi: 10.7759/cureus.1759
30. Giakoustidis DE, Gargavanis AA, Katsiki ED, Salveridis NT, Antoniadis NA, Papanikolaou V. Undifferentiated embryonal sarcoma of the liver in a young female: treatment with portal vein embolization and liver trisectonectomy. Korean J Hepatobiliary Pancreat Surg (2016) 20(3):144–7. doi: 10.14701/kjhbps.2016.20.3.144
31. Zanwar S, Goel M, Patkar S, Ramaswamy A, Shetty N, Ramadwar M, et al. A case of ruptured adult embryonal sarcoma of the liver with excellent outcome after neoadjuvant chemotherapy. J Gastrointest Canc (2017) 48(1):100–2. doi: 10.1007/s12029-016-9877-6
32. Kim HH, Kim JC, Park EK, Hur YH, Koh YS, Cho CK, et al. Undifferentiated embryonal sarcoma of the liver presenting as a hemorrhagic cystic tumor in an adult. Hepatobiliary Pancreat Dis Int (2011) 10(6):657–60. doi: 10.1016/S1499-3872(11)60112-4
33. Ma L, Liu YP, Geng CZ, Tian ZH, Wu GX, Wang XL. Undifferentiated embryonal sarcoma of liver in an old female: case report and review of the literature. World J Gastroenterol (2008) 14(47):7267–70. doi: 10.3748/wjg.14.7267
34. Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, et al. Perisoperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. (2019) 393(10184):1948–57. doi: 10.1016/S0140-6736(18)32557-1
Keywords: embryonal sarcoma of adult liver, hepatectomy, neoadjuvant chemotherapy, undifferentiated embryonal hepatic sarcoma, UESL
Citation: Vergara R, Khalil S and Munene G (2022) Case report: Multimodal neoadjuvant and adjuvant chemotherapy for hepatic undifferentiated embryonal sarcoma in a young adult. Front. Oncol. 12:1004108. doi: 10.3389/fonc.2022.1004108
Received: 26 July 2022; Accepted: 13 October 2022;
Published: 18 November 2022.
Edited by:
Akinfemi Akingboye, Dudley Group NHS Foundation Trust, United KingdomReviewed by:
Gunes Guner, Hacettepe University, TurkeyCopyright © 2022 Vergara, Khalil and Munene. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sarah Khalil, c2FyYWgua2hhbGlsQG1lZC53bWljaC5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.