
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 07 November 2022
Sec. Gynecological Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1003465
Background: Ovarian cancer is the leading cause of cancer-related death among gynecologic malignancies. With much evidence suggesting that 18F-FDG PET/CT may be an excellent imaging test for the diagnosis of epithelial ovarian cancer recurrence, we conducted a systematic review and meta-analysis to summarize relevant studies and evaluate the accuracy and application value of 18F-FDG PET/CT in the diagnosis of recurrence of epithelial ovarian cancer.
Materials and methods: Clinical trials of 18F-FDG PET/CT for the diagnosis of recurrence of epithelial ovarian cancer were systematically searched in PubMed, Embase, Cochrane Library, Web of Science and OVID database. The relevant literature was searched until May 22, 2022. Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) was used to evaluate the quality of the included original studies, and the meta-analysis was performed using a bivariate mixed-effects model and completed in Stata 15.0.
Results: A total of 17 studies on 18F-FDG PET/CT for the diagnosis of epithelial ovarian cancer recurrence were included in this systematic review, involving 639 patients with epithelial ovarian cancer. Meta-analysis showed that the sensitivity, specificity and area under the curve of 18F-FDG PET/CT for the diagnosis of epithelial ovarian cancer recurrence were 0.88 (95% CI: 0.79 - 0.93), 0.89 (95% CI: 0.72 - 0.96) and 0.94 (95% CI: 0.91- 0.96), respectively. Subgroup analysis showed higher diagnostic efficacy in prospective studies than in retrospective studies, and no significant publication bias was observed in Deeks’ funnel plot, with sensitivity analysis revealing the stability of results. Meta regression shows that the heterogeneity of this study comes from study type.
Conclusion: 18F-FDG PET/CT has good diagnostic value in the recurrence of epithelial ovarian cancer.
Ovarian cancer ranks as the eighth leading gynecologic malignancy in terms of both incidence and cause of death in women (1), with more than 220,000 cases of ovarian cancer and approximately 160,000 cancer-related deaths worldwide in 2010 (2, 3). Epithelial ovarian cancer is the most common pathological type of ovarian cancer with the highest mortality, accounting for about 85-90% of ovarian malignancies (4, 5), and 70% of patients with epithelial ovarian cancer have a 5-year survival rate of less than 30% and an extremely poor prognosis due to the diagnosis at an advanced stage (6). Although surgery and first-line chemotherapy improve the prognosis of epithelial ovarian cancer to some extent, 70-80% of patients still experience recurrence. The recurrence rate is 23% within 6 months after the end of the initial chemotherapy and up to 60% after 6 months (7). Disease recurrence is an important factor affecting clinical decision-making and survival prognosis of patients with epithelial ovarian cancer. Therefore, the search for imaging methods to accurately diagnose the recurrence of epithelial ovarian cancer has become an urgent clinical problem at present. Fluorine-18 fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (18F-FDG PET/CT) is an imaging method with increasing relevance in this clinical context.
A previous meta-analysis revealed that FDG PET showed good sensitivity and moderate specificity, which may be a potentially effective method for identifying recurrent ovarian cancer (8). In order to update this meta-analysis and perform additional calculations, such as the area under the curve, a new meta-analysis on this topic was carried out. An international guideline on FDG PET/CT in ovarian cancer was published in 2021 by the European Association of Nuclear Medicine (EANM) and other international associations. It concluded that 18F-FDG PET/CT is effective in initial ovarian cancer detection, disease staging, and outcome determination, especially with class I evidence for recurrence detection (9).
In recent years, 18F-FDG PET/CT has gradually attracted attention for diagnosis and recurrence assessment in gynecologic tumors (10, 11). It also has significant advantages in the diagnosis of epithelial ovarian cancer recurrence due to its function of simultaneously providing information on glucose metabolism of tumor cells and anatomical structure of tumor lesions, as well as detection of systemic metastases in a single imaging session (12, 13).
Currently, there have been several international studies on the diagnosis of epithelial ovarian cancer recurrence by 18F-FDG PET/CT, whereas the conclusions are not consistent. Hence, we conducted this systematic review and meta-analysis to assess the overall diagnostic value of 18F-FDG PET/CT for the recurrence of epithelial ovarian cancer and to provide a reference for the clinical application of 18F-FDG PET/CT.
We conducted this meta-analysis according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (14).
Studies on the diagnostic value of 18F-FDG PET/CT for epithelial ovarian cancer were searched in PubMed, Embase, Web of Science, OVID and Cochrane Library. Subject terms combined with free words were used as search criteria (MeSH in PubMed and Emtree in Embase), with the search period up to May 22, 2022.
We also reviewed references of relevant reviews and meta-analyses to identify eligible studies. The specific search strategy is described in Appendix 1. In addition, we supplemented the manual search to find relevant literature.
Inclusion criteria: (a) the study included patients have diagnosed or suspected epithelial ovarian cancer. Additionally, they are suspected to suffer from recurrence and metastasis more than six months after the preoperative imaging examination, or six months after the first cytoreductive surgery and standard chemotherapy for ovarian cancer achieved clinical response; (b) the study used 18F-FDG PET/CT in the diagnosis of epithelial ovarian cancer recurrence; (c) the article was published in English.
Exclusion criteria: (a) letters, case reports, reviews, systematic review, meta-analysis and conference abstracts; (b) data that could not be extracted for true positives (TP), false positives (FP), false negatives (FN) and true negatives (TN); (c) duplicate studies.
We performed an initial search to remove duplicate records, filter titles and abstracts for relevance, and identify records for inclusion, exclusion, or uncertainty. For uncertain studies, the full text was obtained to determine whether the requirements were met. Data were extracted as follows: (1) first author, year of publication, country, number of recurrent patients, the total number of patients, age, type of study design, the dose of imaging agent used, and threshold; (2) outcome indicators of TP, FP, FN, and TN. Two investigators (WXY and YLF) performed data extraction independently, and the third investigator (WY) was consulted to resolve discrepancies.
As in a previous meta-analysis by Delgado-Bolton et al. (15), on the basis of their design, 2 types of studies were differentiated; in some, 18F-FDG PET CT was performed when epithelial ovarian cancer was diagnosed recurrence(type I); whereas, in others (type II), 18F-FDG PET CT was compared with CT or MRI or tumor markers or ultrasound in related study that included epithelial ovarian cancer patients who had presented results for recurrence detection in all of the following diagnostic procedures (if performed in each particular patient): (a) careful clinical history and complete physical examination; (b) laboratory analysis(tumor markers); (c) radiologic or isotopic procedures except 18F-FDG PET CT, CT, MRI, and ultrasound; or even (d) surgical exploration, biopsy, or fine-needle aspiration cytology of suspicious lesions.
The methodological quality of the selected eligible articles was assessed using the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) scoring system. Two authors (WXY and YLF) independently assessed the methodological quality of the included studies and, in case of disagreement, the third investigator (WY) was consulted to resolve the dispute.
Risk of bias assessment plots for the included studies were completed using RevMan 5.4 software. Stata 15 software (Stata Corp LP, College Station, TX, USA) was adopted to perform additional statistical analyses. Diagnostic threshold effects were evaluated using the typical “shoulder-arm shape” of the summary receiver operating characteristic (SROC) curve, and a bivariate mixed-effects model was used to analyze the data. Sensitivity (Sen), specificity (Spe), positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic odds ratio (DOR) were combined, and the corresponding forest plots were drawn to calculate the area under the curve (AUC) values. Moreover, the inconsistency index (I2) and P-value were used to evaluate the heterogeneity among studies. Heterogeneity was considered significant when I2 > 50% or P > 0.05.
The relationship between the prior probability, likelihood ratio and posterior probability was evaluated using Fagan’s nomogram (16). Meta-regression and subgroup analysis were employed to analyze the sources of heterogeneity of the included studies, which were performed according to the design type (prospective or retrospective study), area (Asia or Europe or North America), study type (type I or type II). Sensitivity analysis was adopted to verify the robustness of the study results, and Deeks’ funnel plot was used to assess publication bias.
The flow chart of the selected studies is shown in Figure 1. A total of 667 articles were retrieved from the initial literature search, and 218 duplicate articles were removed. Of the remaining 449 articles, 388 records were excluded by title and abstract screening, and then the full text of 61 articles was screened, 44 of which were excluded. A total of 17 articles were eventually included in the meta-analysis (17–33).
A total of 920 cases were included in the present study, and recurrence was confirmed in 639 patients by pathology and clinical follow-up. The basic characteristics and more details of the included literature are shown in Table 1. The literature was published from 2002 to 2021; four were prospective studies (17, 18, 22, 32) and thirteen were retrospective studies (19–21, 23–31, 33); eleven of the sample sources were in Asian countries (17, 20–22, 24–26, 28, 29, 32, 33), four in Europe (18, 19, 23, 27), and two in North America (30, 31); nine articles mentioned image use doses (17, 19–24, 27, 29–33) and only three addressed diagnostic thresholds (19, 28, 32). With regard to the types of studies described in the methods, 7 studies were type I (18, 22–24, 26, 30, 33), 10 studies were type II (17, 19–21, 25, 27–29, 31, 32).
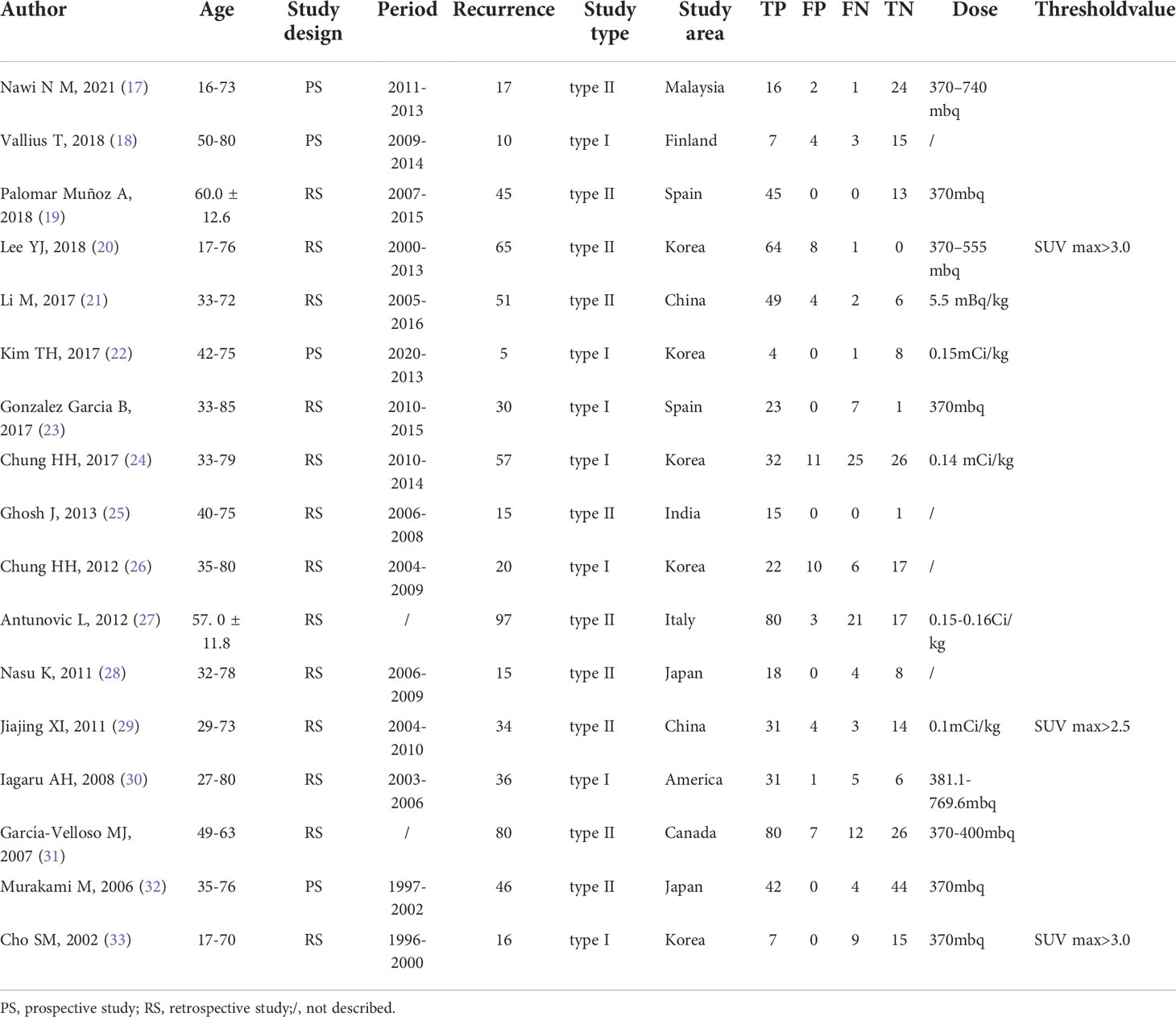
Table 1 Basic information of the literature on 18F-FDG PET/CT for the diagnosis of epithelial ovarian cancer recurrence.
QUADAS-2 was used to assess the quality of the included literature (Figures 2A, B). As shown in Figure 2B, the quality of reporting on the selection of included cases, the gold standard, and the case flow and progression of cases is good, while the quality of reporting on the implementation of diagnostic tests is poor and uneven. This is mainly related to inappropriate inclusion of study subjects (18, 20, 21, 23, 24, 26, 27, 29–33), unclear implementation of literature blinding (17, 18, 20–33) and reporting of thresholds (17–19, 21–28, 30–32), and unclear risk of bias or high risk of bias in the time interval between the trial to be evaluated and the gold standard (18–33).
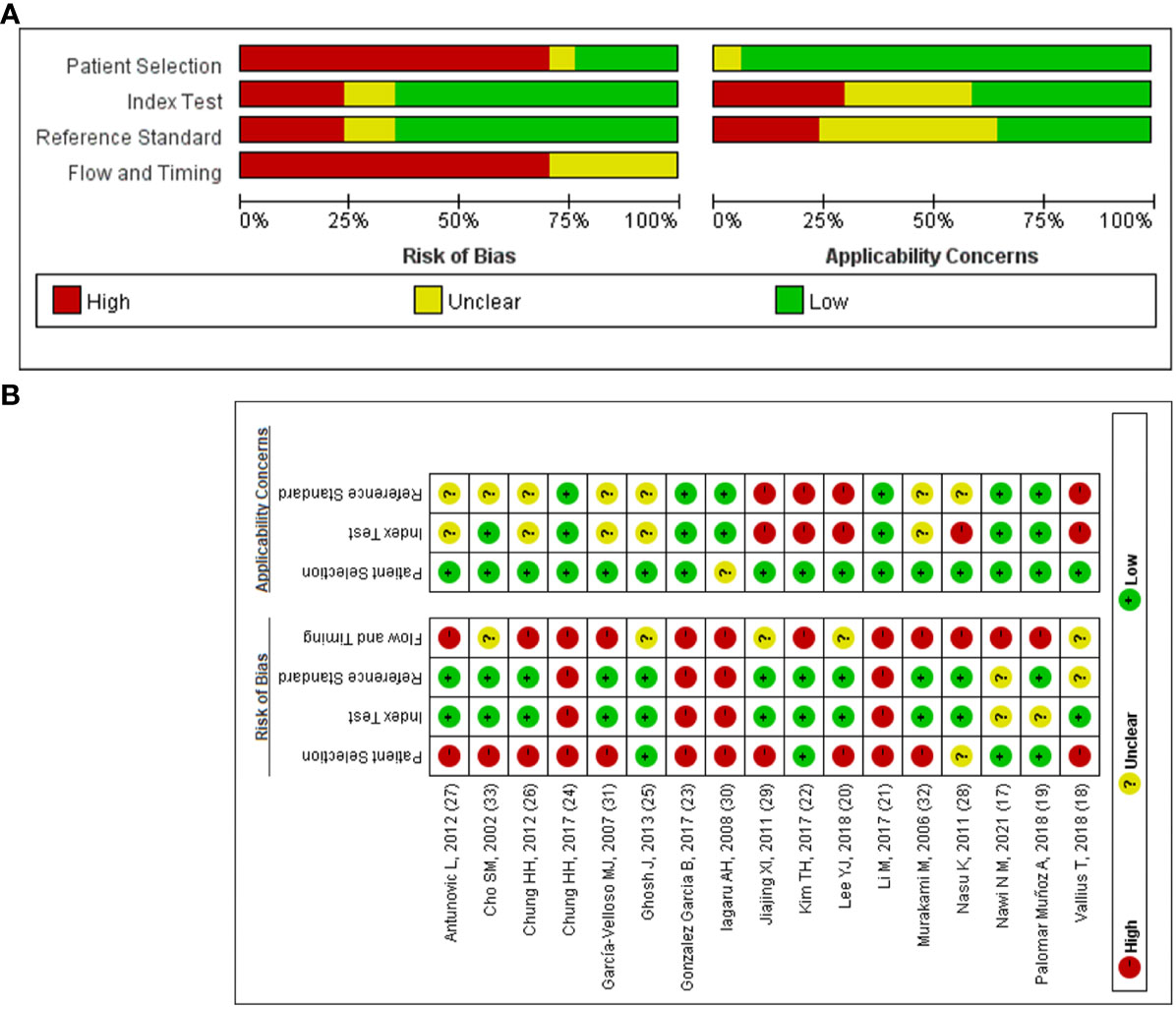
Figure 2 The quality of included articles according to the QUADAS-2 guidelines. (A) Risk of bias summary; (B) Risk of bias graph.
Figures 3 A–D shows the forest plots and SROC curve of the diagnostic accuracy of 18F-FDG PET/CT. The SROC curve (Figure 3D) does not show the typical “shoulder-arm shape”, indicating that there is no threshold effect in the diagnostic test. The results of the bivariate mixed-effects model showed that the combined sensitivity (Figure 3A), specificity (Figure 3A), positive likelihood ratio (Figure 3B), negative likelihood ratio (Figure 3B), and diagnostic odds ratio (Figure 3C) were 0.88 (95% CI: 0.79-0.93), 0.89 (95% CI: 0.72-0.96), 7.73 (95% CI: 2.86-20.89), 0.14 (95% CI: 0.08-0.24), and 4.02 (95% CI: 2.82-5.22), respectively. In addition, the AUC value was 0.94 (95% CI: 0.91-0.96). The predictive probability plot of Fagan’s nomogram shows that if the pre-test probability ratio is 20%, the post-test probability is 66% for PLR (Figure 4)and 3% for NLR (Figure 4), suggesting the good value of 18F-FDG PET/CT for the diagnosis of epithelial ovarian cancer.
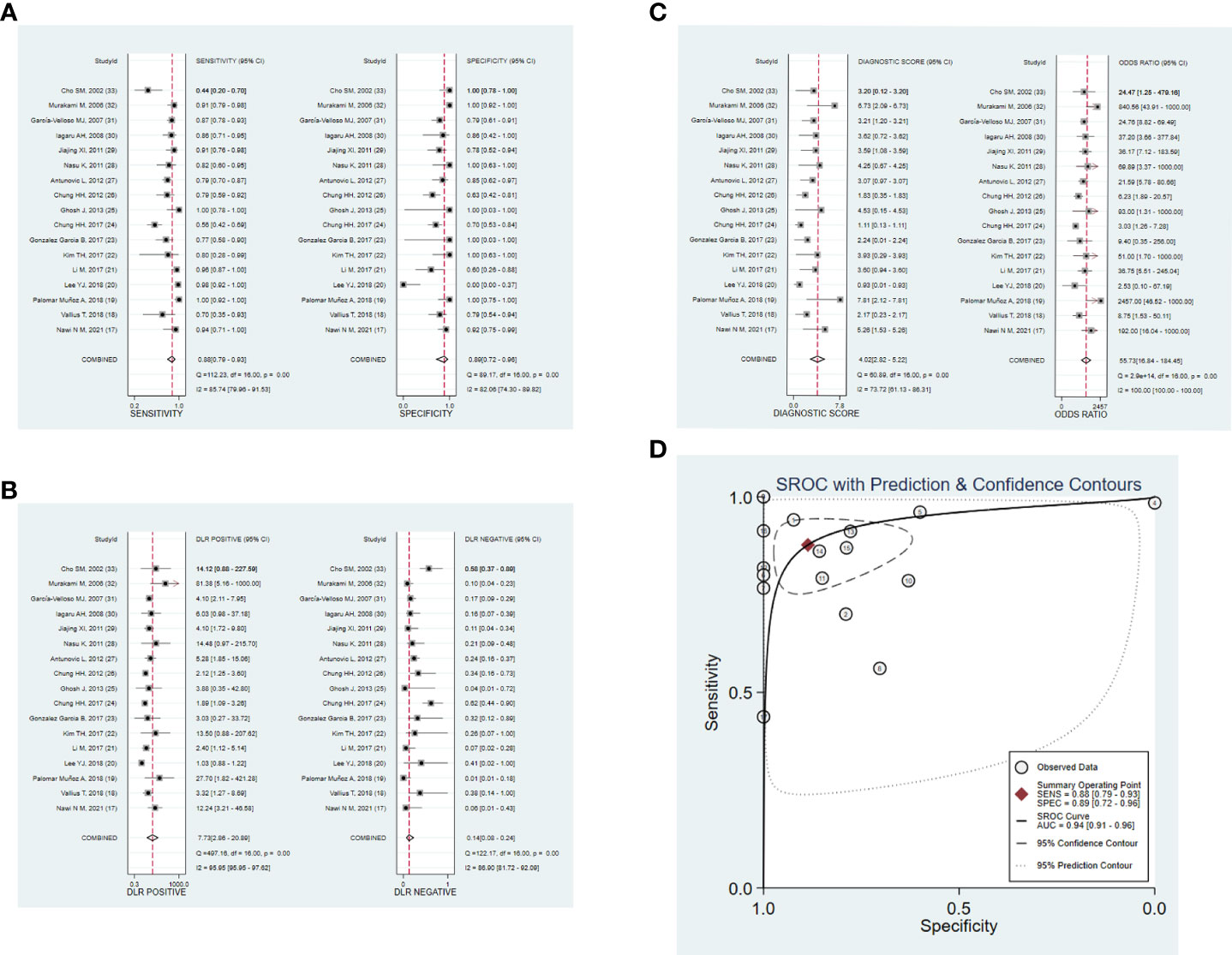
Figure 3 Forest plots of 18F-FDG PET/CT for the diagnostic value of epithelial ovarian cancer. (A) sensitivity and specificity. (B) PLR and NLR. (C) DOR. (D) SROC. DOR, diagnostic odds ratio; PLR, positive likelihood ratio; NLR, negative likelihood ratio; SROC, summary receiver operating characteristic.
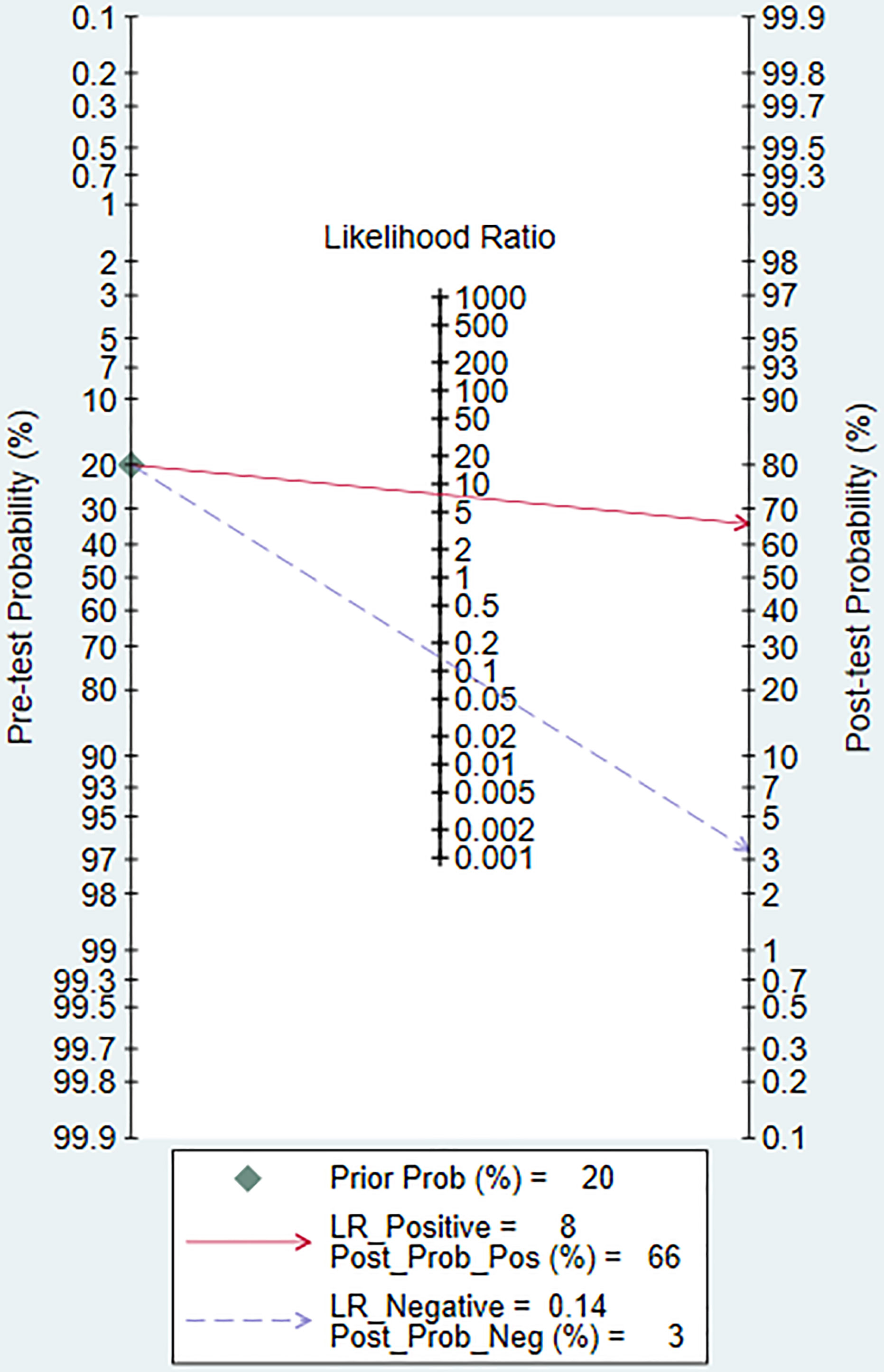
Figure 4 Fagan’s plot of PLR and NLR to evaluate the clinical utility of 18F-FDG PET/CT in the diagnosis of epithelial ovarian cancer. PLR, positive likelihood ratio; NLR, negative likelihood ratio.
Design type, study area and study type were included in the regression model. There was no significant difference in the combined sensitivity and specificity of different design types and areas (P > 0.05). In the study type, the combined sensitivity of type I was statistically significant (P < 0.05). It is speculated that the heterogeneity of this study is from study type. Details of the meta-regression are shown in Table 2 and Figure 5, and details of the subgroup analysis are shown in Table 3.
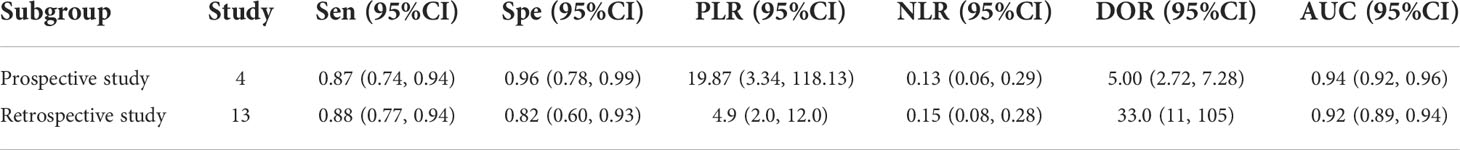
Table 2 Summary estimated of diagnostic performance of 18F-FDG PET/CT for epithelial ovarian cancer based on design type.
Sensitivity analysis showed a good fit for the goodness of fit and binary normality (Figure 6A, B). There were four articles weighted (Figure 6C) (34), which may be a source of heterogeneity shown by outlier detection (Figure 6D). After the exclusion of abnormal studies, sensitivity decreased slightly from 0.88 to 0.85, specificity decreased from 0.89 to 0.80, AUC value decreased from 0.94 to 0.87, and DOR decreased from 4.02 to 3.12.
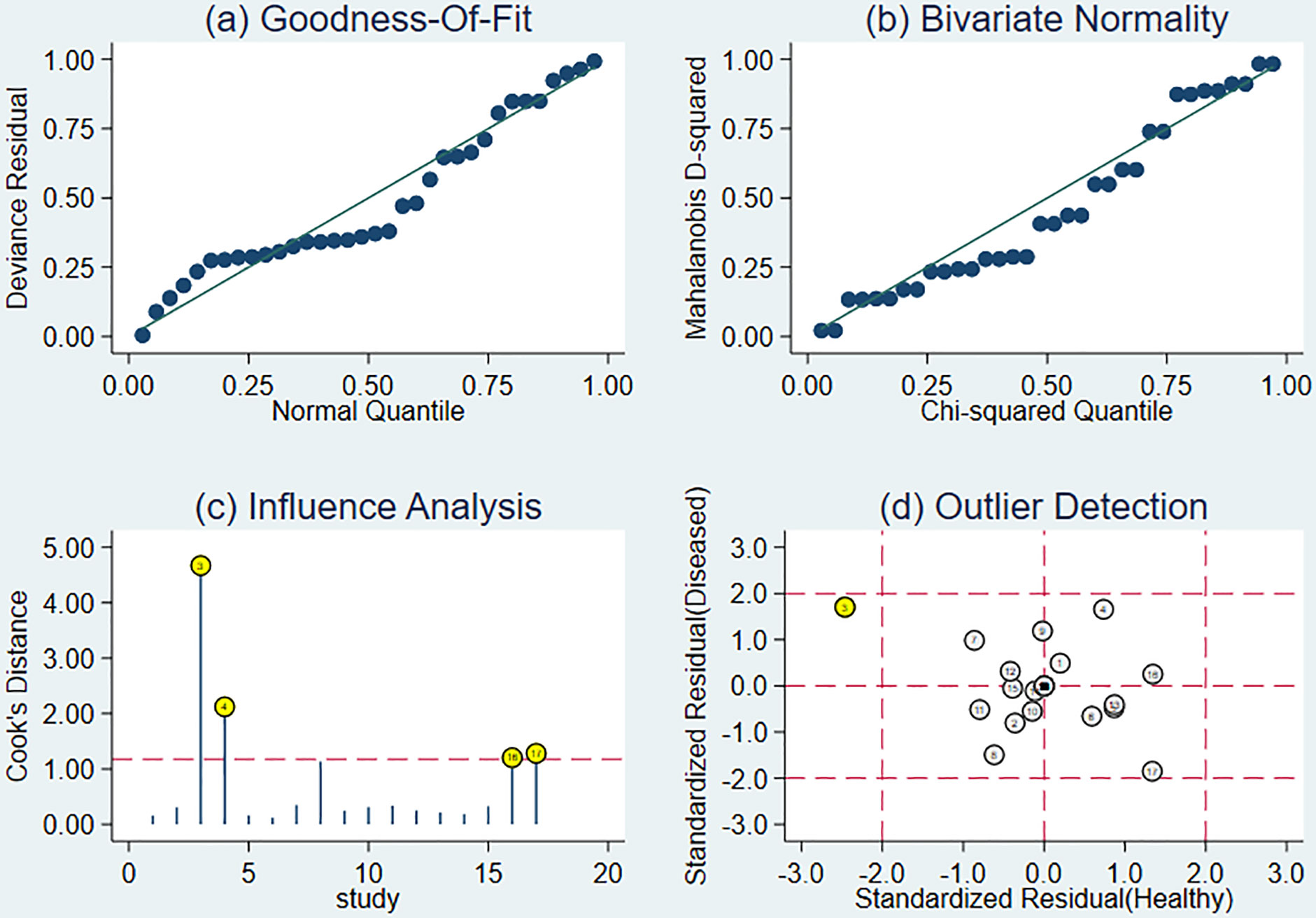
Figure 6 The results of sensitivity analysis. (A) Goodness-of-ft. (B) Bivariate normality. (C) Influence analysis. (D) Outlier detection.
The Deeks’ funnel plot showed a P-value of 0.93, indicating that there was no publication bias in this meta-analysis (Figure 7).
With a total of 17 articles included in this study, the results showed that the sensitivity and specificity of 18F-FDG PET/CT in diagnosing recurrence of epithelial ovarian cancer were 88% and 89%, and the AUC value of 18F-FDG PET/CT in assessing recurrence of epithelial ovarian cancer was 0.94, suggesting its high diagnostic efficacy. Through reviewing previous meta-analyses, the AUC values for single ctDNA and microRNA to detect recurrence of epithelial ovarian cancer were 0.884 and 0.894 (35), and the AUC values for ROMA values and HE 4 to detect recurrence of epithelial ovarian cancer were 0.93 and 0.91, respectively (36, 37). In the combined serum tumor marker test, the diagnostic AUC value of the combined serum CA125, CA19.9 and CEA test was 0.92 (38), all of which were lower than the diagnostic ability of 18F-FDG PET/CT.
However, the false-negative rate (1-Sen) and misdiagnosis rate (1-Spe) of 18F-FDG PET/CT were 12% and 11%, respectively, suggesting that there were certain missed diagnoses and misdiagnoses in 18F-FDG PET/CT diagnosis. According to relevant literature and clinical experience, infection or inflammation is one of the common causes of misdiagnosis (17, 20). Strong uptake of the tracer 18F-FDG by anti-inflammatory cells such as macrophages or granulation tissue activated in areas of inflammation leads to active inflammatory lesions or abscesses that may be misinterpreted as malignant tumors. Other causes include ureteral stasis, atherosclerotic plaque, dislocations due to peristalsis, bladder filling or diverticulitis. Moreover, some benign gynecologic diseases and peritoneal disease may also lead to false-positive results of 18F-FDG PET/CT (39), including endometritis, follicular cyst, functional corpus luteum cyst, tubal oophoritis, fibroids, cystadenofibroma, teratoma, endometriosis, oviduct ovarian abscess, benign meningioma, schwannoma, and pelvic tuberculosis. Meanwhile, given the usually low FDG uptake of malignant peritoneal disease, it is recommended to study in detail both the CT component of the FDG PET/CT and previous contrast enhanced CTs to improve the accuracy (9, 40). Common reasons for missed findings are first, the location of the lesion is too close to the bladder, which contains high concentrations of 18F-FDG and may be confused with urinary secretions (41); second, the resolution of the lesion is low when it is in a hypometabolic state (42), and third, the size of the lesion can also affect the accuracy of diagnosis of ovarian cancer. Pannu et al. (43) reported a sensitivity of 50-90% for 18F-FDG PET/CT for the diagnosis of recurrent ovarian cancer lesions >1 cm (44) and 13% for lesions <1 cm (45). Therefore, in clinical practice, in addition to the causes of missed diagnosis and misdiagnosis, the possibility of reducing the rate of missed diagnosis and misdiagnosis by combining serum tumor marker detection should also be considered. The use of serum tumor marker testing alone is prone to false positives, while in the diagnosis of recurrence of ovarian cancer, the sensitivity of CA 125 and HE 4 combined with 18F-FDG PET/CT was increased to 98% - 100% (46), and the specificity of CA 125 combined with 18F-FDG PET/CT was increased to 91.8% (21). We recommend the use of 18F-FDG PET/C in combination with serum tumor markers for monitoring the recurrence of epithelial ovarian cancer.
Early diagnosis and treatment can improve the survival rate and prolong the survival time of ovarian cancer patients. At present, clinical diagnosis of epithelial ovarian cancer recurrence mainly relies on imaging examination and histopathological examination. Histopathological examination is the gold standard for the diagnosis of malignant tumors, but it has not been widely used in cancer diagnosis due to its invasiveness and high cost (47). The commonly used imaging examination methods are ultrasound, CT and MRI. Ultrasonography is simple and safe, but it is easily affected by the patient’s body shape, abdominal fluid, and abdominal wall thickness, leading to missed diagnoses. CT and MRI examinations provide an effective reference for accurate localization, measurement and efficacy evaluation of lesions, but the degree of detection of lesions by CT and MRI is proportional to the size of lesions. The imaging visibility is better when the lesions are more than 5-10mm, while the false negative rate increases when the diameter of the recurrence lesion in the pelvic cavity is less than 1 cm. Due to the susceptibility to physiological imaging of the intestinal and urinary systems, CT and MRI may also have some difficulties in differentiating abdominal and pelvic lesions from tumor recurrence (48, 49), and thus ordinary imaging examination should not be used as the primary examination to determine the recurrence of epithelial ovarian cancer.
PET is a clinical molecular imaging test that can detect positron-emitting radionuclides. It uses positron nuclides or positron nuclide-labeled compounds (such as ligands, antibodies, and enzymes) as tracers to detect the equal and opposite γ-ray produced by the combination and annihilation of positron and negative electrons in the living body (50). PET-CT is a PET-based integrated device that utilizes the X-ray CT in the same device to perform attenuation correction on the γ-ray detected by PET. In this way, it can provide high-resolution anatomical structure information of molecular-level tissue cells obtained by PET. Meanwhile, it can not only fuse PET and CT images but also offer independent CT diagnosis function (51). As PET-CT equipment keeps updating and improving, now it can detect specific physiological components inside the human body. Therefore, PET-CT has been widely recognized as one of the most advanced clinical molecular imaging equipment.
Ovarian tumor imaging usually uses 18 FDG as a tracer, whose molecular structure is similar to glucose. After entering the body, it is taken up by cells through the glucose transport mechanism and retained in cells but will not be further metabolized, and the glucose metabolism rate of ovarian malignant tumors is significantly higher than that of normal tissues. Compared with other imaging examinations, 18F-FDG PET/CT can more accurately display the concentration of imaging agent uptake and the metabolism and recurrence of the imaging agent in the lesion. Meanwhile, 18F-FDG PET/CT imaging is superior to other imaging examinations in diagnosing ovarian cancer metastases because of the lower location of the ovary in the pelvis, less fusion error between PET and CT images, and less interference by respiratory motion during imaging (52–54). Relevant studies have also demonstrated (27, 55) that the diagnostic value of 18F-FDG PET/CT for suspected epithelial ovarian cancer recurrence is higher than that of conventional imaging examination.
Furthermore, the results of subgroup analysis and meta-regression analysis showed that the diagnostic efficacy of 18F-FDG PET/CT for the assessment of epithelial ovarian cancer recurrence was less influenced by study design, study area and study type. Meta regression shows that the heterogeneity of this study comes from study type, which is caused by differences between studies. Subgroup analysis of our results based on different study designs found that 18F-FDG PET/CT was more effective in the prospective study than the retrospective study in the diagnosis of epithelial ovarian cancer recurrence, with an AUC value of 0.94 (95%CI: 0.92-0.96), the sensitivity of 0.87 and specificity of 0.96, indicating that the diagnostic ability of this interval was higher than that of other intervals.
This study also has the following limitations which need to be improved: first, most of the studies included in this study were retrospective, thus preventing an accurate evaluation of the blinding method; second, the 17 included studies had different follow-up times and doses of imaging agents used, and only three articles addressed the threshold for 18F-FDG PET/CT use, leading to possible heterogeneity.
In conclusion, our analysis suggests that the overall diagnostic value and accuracy of 18F-FDG PET/CT for recurrence in patients with epithelial ovarian cancer may be quite high. It can be used as an effective imaging method to diagnose the recurrence of epithelial ovarian cancer. We look forward to further studies to confirm our analysis.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
XW and LY conceived and designed the study. XW and LY selected the studies and collected the data. XW analyzed data. All authors interpreted the results. YW drafted the paper. All authors revised the draft paper. All authors contributed to the article and approved the submitted version.
This study was supported by the Fifth Ningxia Youth Science and Technology Talents Project Acknowledgments.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1003465/full#supplementary-material
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet (2014) 384(9951):1376–88. doi: 10.1016/S0140-6736(13)62146-7
3. Perrone AM, Girolimetti G, Procaccini M, Marchio L, Livi A, Borghese G, et al. Potential for mitochondrial DNA sequencing in the differential diagnosis of gynaecological malignancies. Int J Mol Sci (2018) 19(7):2048. doi: 10.3390/ijms19072048
4. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin (2015) 65(2):87–108. doi: 10.3322/caac.21262
5. Colombo N, Lorusso D, Scollo P. Impact of recurrence of ovarian cancer on quality of life and outlook for the future. Int J Gynecol Cancer. (2017) 27(6):1134–40. doi: 10.1097/IGC.0000000000001023
6. du Bois A, Reuss A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the arbeitsgemeinschaft gynaekologische onkologie studiengruppe ovarialkarzinom (AGO-OVAR) and the groupe d'Investigateurs nationaux pour les etudes des cancers de l'Ovaire (GINECO). Cancer (2009) 115(6):1234–44. doi: 10.1002/cncr.24149
7. Vahidfar N, Farzanefar S, Ahmadzadehfar H, Molloy EN, Eppard E. A review of nuclear medicine approaches in the diagnosis and the treatment of gynecological malignancies. Cancers (Basel). (2022) 14(7):1779. doi: 10.3390/cancers14071779
8. Ruiz-Hernández G, Delgado-Bolton RC, Fernández-Pérez C, Lapeña-Gutiérrez L, Carreras-Delgado JL. Meta-análisis de la eficacia diagnóstica de la PET-FDG en pacientes con sospecha de recurrencia por cáncer de ovario [Meta-analysis of the diagnostic efficacy of FDG-PET in patients with suspected ovarian cancer recurrence]. Rev Esp Med Nucl (2005) 24(3):161–73. doi: 10.1157/13073787
9. Delgado Bolton RC, Aide N, Colletti PM, Ferrero A, Paez D, Skanjeti A, et al. EANM guideline on the role of 2-[18F]FDG PET/CT in diagnosis, staging, prognostic value, therapy assessment and restaging of ovarian cancer, endorsed by the American college of nuclear medicine (ACNM), the society of nuclear medicine and molecular imaging (SNMMI) and the international atomic energy agency (IAEA). Eur J Nucl Med Mol Imaging. (2021) 48(10):3286–302. doi: 10.1007/s00259-021-05450-9
10. Slart RHJA, Writing group, Reviewer group, Members of EANM Cardiovascular; Members of EANM Infection & Inflammation, Members of Committees, SNMMI Cardiovascular, Members of Council, PET Interest Group, et al. FDG-PET/CT (A) imaging in large vessel vasculitis and polymyalgia rheumatica: joint procedural recommendation of the EANM, SNMMI, and the PET interest group (PIG), and endorsed by the ASNC. Eur J Nucl Med Mol Imaging. (2018) 45(7):1250–69. doi: 10.1007/s00259-018-3973-8
11. Adam JA, Loft A, Chargari C, Delgado Bolton RC, Kidd E, Schöder H, et al. EANM/SNMMI practice guideline for [18F]FDG PET/CT external beam radiotherapy treatment planning in uterine cervical cancer v1.0. Eur J Nucl Med Mol Imaging. (2021) 48(4):1188–99. doi: 10.1007/s00259-020-05112-2
12. Liu S, Feng Z, Wen H, Jiang Z, Pan H, Deng Y, et al. 18F-FDG PET/CT can predict chemosensitivity and proliferation of epithelial ovarian cancer via SUVmax value. Jpn J Radiol (2018) 36(9):544–50. doi: 10.1007/s11604-018-0755-y
13. Nakajima R, Matsuo Y, Kondo T, Abe K, Sakai S. Prognostic value of metabolic tumor volume and total lesion glycolysis on preoperative 18F-FDG PET/CT in patients with renal cell carcinoma. Clin Nucl Med (2017) 42(4):e177–82. doi: 10.1097/RLU.0000000000001552
14. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (2009) 339:b2700. doi: 10.1136/bmj.b2700
15. Delgado-Bolton RC, Fernández-Pérez C, González-Maté A, Carreras JL. Meta-analysis of the performance of 18F-FDG PET in primary tumor detection in unknown primary tumors. J Nucl Med (2003) 44(8):1301–14. Available at: https://jnm.snmjournals.org/content/44/8/1301.long.
16. Fagan TJ. Letter: Nomogram for bayes theorem. N Engl J Med (1975) 293(5):257. doi: 10.1056/NEJM197507312930513
17. Nawi NM, Mohamad I, Khader M, Shuaib IL. Diagnostic accuracy of 18F-FDG PET/CT in the evaluation of recurrent ovarian cancer. Bangladesh J Med Sci (2021) 20(2):302–12. doi: 10.3329/bjms.v20i2.51539
18. Vallius T, Hynninen J, Kemppainen J, Alves V, Auranen K, Matomäki J, et al. 18F-FDG-PET/CT based total metabolic tumor volume change during neoadjuvant chemotherapy predicts outcome in advanced epithelial ovarian cancer. Eur J Nucl Med Mol Imaging. (2018) 45(7):1224–32. doi: 10.1007/s00259-018-3961-z
19. Palomar Muñoz A, Cordero García JM, Talavera Rubio MDP, García Vicente AM, Pena Pardo FJ, Jiménez Londoño GA, et al. Value of [18F]FDG-PET/CT and CA125, serum levels and kinetic parameters, in early detection of ovarian cancer recurrence: Influence of histological subtypes and tumor stages. Med (Baltimore) (2018) 97(17):e0098. doi: 10.1097/MD.0000000000010098
20. Lee YJ, Kim YM, Jung PS, Lee JJ, Kim JK, Kim YT, et al. Diagnostic value of integrated 18F-fluoro-2-deoxyglucose positron emission tomography/computed tomography in recurrent epithelial ovarian cancer: accuracy of patient selection for secondary cytoreduction in 134 patients. J Gynecol Oncol (2018) 29(3):e36. doi: 10.3802/jgo.2018.29.e36
21. Li M, Li X, Yan S, Zhong F, Wu D. Value of ~(18)F-fluorodeoxyglucose PET/CT combined with serum carbohydrate antigen 125 in detecting the recurrence and metastasis of postoperative ovarian cancer. Jounral Chongqing Med Univ (2017) 42(12):1635–8. doi: 10.13406/j.cnki.cyxb.001417
22. Kim TH, Kim J, Kang YK, Lee M, Kim HS, Cheon GJ, et al. Identification of metabolic biomarkers using serial 18F-FDG PET/CT for prediction of recurrence in advanced epithelial ovarian cancer. Transl Oncol (2017) 10(3):297–303. doi: 10.1016/j.tranon.2017.02.001
23. González García B, García Vicente AM, Jiménez Londoño GA, Pena Pardo FJ, Bellón Guardia ME, Talavera Rubio MP, et al. 18F-FDG PET/CT as predictor of tumour biology and prognosis in epithelial ovarian carcinoma. Rev Esp Med Nucl Imagen Mol (2017) 36(4):233–40. doi: 10.1016/j.remn.2017.01.004
24. Chung HH, Lee M, Kim HS, Kim JW, Park NH, Song YS, et al. Prognostic implication of the metastatic lesion-to-ovarian cancer standardised uptake value ratio in advanced serous epithelial ovarian cancer. Eur Radiol (2017) 27(11):4510–5. doi: 10.1007/s00330-017-4883-z
25. Ghosh J, Thulkar S, Kumar R, Malhotra A, Kumar A, Kumar L. Role of FDG PET-CT in asymptomatic epithelial ovarian cancer with rising serum CA-125: a pilot study. Natl Med J India. (2013) 26(6):327–31. Available at: https://pubmed.ncbi.nlm.nih.gov/25073988/.
26. Chung HH, Kwon HW, Kang KW, Kim JW, Park NH, Song YS, et al. Preoperative [F]FDG PET/CT predicts recurrence in patients with epithelial ovarian cancer. J Gynecol Oncol (2012) 23(1):28–34. doi: 10.3802/jgo.2012.23.1.28
27. Antunovic L, Cimitan M, Borsatti E, Baresic T, Sorio R, Giorda G, et al. Revisiting the clinical value of 18F-FDG PET/CT in detection of recurrent epithelial ovarian carcinomas: correlation with histology, serum CA-125 assay, and conventional radiological modalities. Clin Nucl Med (2012) 37(8):e184–8. doi: 10.1097/RLU.0b013e31825b2583
28. Nasu K, Abe W, Takai N, Tomonari K, Narahara H. Impact of positron emission tomography/computed tomography in the management of patients with epithelial ovarian carcinoma after treatment. Arch Gynecol Obstet. (2011) 283(5):1121–6. doi: 10.1007/s00404-010-1568-0
29. Jiajing XI, Xiaoli LAN, Guoxiang CAO, Yongxue Z, Shanshan L. Value of 18f-FDG PET/CT in the follow-up and clinical-decision of postoperation patients with epithelial ovarian cancer. Chin J Med Imaging Technol (2011) 27(7):1451–4. doi: 10.1631/jzus.B1000197
30. Iagaru AH, Mittra ES, McDougall IR, Quon A, Gambhir SS. 18F-FDG PET/CT evaluation of patients with ovarian carcinoma. Nucl Med Commun (2008) 29(12):1046–51. doi: 10.1097/MNM.0b013e32831089cb
31. García-Velloso MJ, Jurado M, Ceamanos C, Aramendía JM, Garrastachu MP, López-García G, et al. Diagnostic accuracy of FDG PET in the follow-up of platinum-sensitive epithelial ovarian carcinoma. Eur J Nucl Med Mol Imaging. (2007) 34(9):1396–405. doi: 10.1007/s00259-007-0366-9
32. Murakami M, Miyamoto T, Iida T, Tsukada H, Watanabe M, Shida M, et al. Whole-body positron emission tomography and tumor marker CA125 for detection of recurrence in epithelial ovarian cancer. Int J Gynecol Cancer. (2006) 16 Suppl 1:99–107. doi: 10.1111/j.1525-1438
33. Cho SM, Ha HK, Byun JY, Lee JM, Kim CJ, Nam-Koong SE, et al. Usefulness of FDG PET for assessment of early recurrent epithelial ovarian cancer. AJR Am J Roentgenol (2002) 179(2):391–5. doi: 10.2214/ajr.179.2.1790391
34. Mahmoud EH, Fawzy A A, Elshimy RA. Serum microRNA-21 negatively relates to expression of programmed cell death-4 in patients with epithelial ovarian cancer. Asian Pac J Cancer Prev (2018) 19(1):33–8. doi: 10.22034/APJCP.2018.19.1.33
35. Fu X, Liu Y. A systematic review and meta-analysis of indirect comparison between miRNA and ctDNA in diagnosis of epithelial ovarian cancer. Transl Cancer Res (2021) 10(12):5372–82. doi: 10.21037/tcr-21-2609
36. Li F, Tie R, Chang K, Wang F, Deng S, Lu W, et al. Does risk for ovarian malignancy algorithm excel human epididymis protein 4 and CA125 in predicting epithelial ovarian cancer: a meta-analysis. BMC Cancer. (2012) 12:258. doi: 10.1186/1471-2407-12-258
37. Suri A, Perumal V, Ammalli P, Suryan V, Bansal SK. Diagnostic measures comparison for ovarian malignancy risk in epithelial ovarian cancer patients: a meta-analysis. Sci Rep (2021) 11(1):17308. doi: 10.1038/s41598-021-96552-9
38. Guo J, Yu J, Song X, Mi H. Serum CA125, CA199 and CEA combined detection for epithelial ovarian cancer diagnosis: a meta-analysis. Open Med (Wars). (2017) 12:131–7. doi: 10.1515/med-2017-0020
39. Lerman H, Metser U, Grisaru D, Fishman A, Lievshitz G, Even-Sapir E. Normal and abnormal 18F-FDG endometrial and ovarian uptake in pre- and postmenopausal patients: assessment by PET/CT. J Nucl Med (2004) 45(2):266–71. Available at: https://jnm.snmjournals.org/content/45/2/266.long.
40. Delgado Bolton RC, Calapaquí Terán AK, Pellet O, Ferrero A, Giammarile F. The search for new 2-18F-FDG PET/CT imaging biomarkers in advanced ovarian cancer patients: focus on peritoneal staging for guiding precision medicine and management decisions. Clin Nucl Med (2021) 46(11):906–7. doi: 10.1097/RLU.0000000000003784
41. Anjos DA, Etchebehere EC, Ramos CD, Santos AO, Albertotti C, Camargo EE. 18F-FDG PET/CT delayed images after diuretic for restaging invasive bladder cancer. J Nucl Med (2007) 48(5):764–70. doi: 10.2967/jnumed.106.036350
42. Nakamoto Y, Saga T, Fujii S. Positron emission tomography application for gynecologic tumors. Int J Gynecol Cancer. (2005) 15(5):701–9. doi: 10.1111/j.1525-1438.2005.00245.x
43. Pannu HK, Cohade C, Bristow RE, Fishman EK, Wahl RL. PET-CT detection of abdominal recurrence of ovarian cancer: radiologic-surgical correlation. Abdom Imaging. (2004) 29(3):398–403. doi: 10.1007/s00261-003-0118-7
44. Sironi S, Messa C, Mangili G, Zangheri B, Aletti G, Garavaglia E, et al. Integrated FDG PET/CT in patients with persistent ovarian cancer: correlation with histologic findings. Radiology (2004) 233(2):433–40. doi: 10.1148/radiol.2332031800
45. Kim CK, Park BK, Choi JY, Kim BG, Han H. Detection of recurrent ovarian cancer at MRI: comparison with integrated PET/CT. J Comput Assist Tomogr. (2007) 31(6):868–75. doi: 10.1097/rct.0b013e31803e8c45
46. Sun J, Cui XW, Li YS, Wang SY, Yin Q, Wang XN, et al. The value of 18F-FDG PET/CT imaging combined with detection of CA125 and HE4 in the diagnosis of recurrence and metastasis of ovarian cancer. Eur Rev Med Pharmacol Sci (2020) 24(13):7276–83. doi: 10.26355/eurrev_202007_21882
47. Wittmann J, Jäck HM. Serum microRNAs as powerful cancer biomarkers. Biochim Biophys Acta (2010) 1806(2):200–7. doi: 10.1016/j.bbcan.2010.07.002
48. Castellucci P, AM P, Picchio M, Ghi T, Farsad M, Nanni C, et al. Diagnostic accuracy of 18F-FDG PET/CT in characterizing ovarian lesions and staging ovarian cancer: correlation with transvaginal ultrasonography, computed tomography, and histology. Nucl Med Commun (2007) 28(8):589–95. doi: 10.1097/MNM.0b013e3281afa256
49. Yuan Y, Gu ZX, Tao XF, Liu SY. Computer tomography, magnetic resonance imaging, and positron emission tomography or positron emission tomography/computer tomography for detection of metastatic lymph nodes in patients with ovarian cancer: a meta-analysis. Eur J Radiol (2012) 81(5):1002–6. doi: 10.1016/j.ejrad.2011.01.112
50. Fonti R, Conson M, Del Vecchio S. PET/CT in radiation oncology. Semin Oncol (2019) 46(3):202–9. doi: 10.1053/j.seminoncol.2019.07.001
51. Dongyan WANG, Chenghai SU. Progression of comparison study between PET or PET-CT and magnetic resonance diffusion-weighted imaging in the investigation of tumor. Int J Radiat Med Nucl Med (2011) 06):339–46. doi: 10.3760/cma.j.issn.1673-4114.2011.06.005
52. Vargas HA, IA B, DA G, Miccò M, RE S, Weber W, et al. Volume-based quantitative FDG PET/CT metrics and their association with optimal debulking and progression-free survival in patients with recurrent ovarian cancer undergoing secondary cytoreductive surgery. Eur Radiol (2015) 25(11):3348–53. doi: 10.1007/s00330-015-3729-9
53. Yamamoto M, Tsujikawa T, Fujita Y, Chino Y, Kurokawa T, Kiyono Y, et al. Metabolic tumor burden predicts prognosis of ovarian cancer patients who receive platinum-based adjuvant chemotherapy. Cancer Sci (2016) 107(4):478–85. doi: 10.1111/cas.12890
54. Bono Y, Mizumoto Y, Nakamura M, Iwadare J, Obata T, Fujiwara H. FDG-PET-positive ovarian thecoma with GLUT5 expression: five cases. J Obstet Gynaecol Res (2017) 43(3):599–603. doi: 10.1111/jog.13243
Keywords: Epithelial ovarian cancer, recurrence, 18F-FDG PET/CT, diagnosis, meta-analysis
Citation: Wang X, Yang L and Wang Y (2022) Meta-analysis of the diagnostic value of 18F-FDG PET/CT in the recurrence of epithelial ovarian cancer. Front. Oncol. 12:1003465. doi: 10.3389/fonc.2022.1003465
Received: 26 July 2022; Accepted: 30 September 2022;
Published: 07 November 2022.
Edited by:
Anna Myriam Perrone, Sant’Orsola-Malpighi Polyclinic, ItalyReviewed by:
Roberto C. Delgado Bolton, Hospital San Pedro, SpainCopyright © 2022 Wang, Yang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Wang, WWFuY2V5X3dnQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.