
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 15 November 2022
Sec. Neuro-Oncology and Neurosurgical Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1001118
This article is part of the Research Topic Molecular Characteristics and Personalized Treatment for Pediatric Brain Tumors View all 6 articles
Ependymoma is the third most common pediatric primary brain tumor, with its most aggressive subtype being posterior fossa group A (PFA). Extraneural metastasis of pediatric PFA ependymoma is rare. Herein, we present a case of a 9-year-old girl with PFA ependymoma characterized by a lack of trimethylation of histone H3 at lysine 27 and elevated chromosome X open reading frame 67 expression. Despite multiple surgeries and radiotherapies, the patient had a rapid recurrence and developed osseous and pulmonary metastases, which may be attributed to the homozygous deletion of cyclin-dependent kinase (CDK) inhibitor 2A/B and CDK12 mutation. Importantly, the CDK12 mutation observed in the patient may be indicative of the need for further work-up to consider chemotherapy rather than administering poly (adenosine diphosphate-ribose) polymerase inhibitors. Taken together, this is the first report of pediatric PFA ependymoma with extraneural metastases, wherein we clarified the diagnostic procedures of this newly identified PFA ependymoma and provided new cues to study the invasiveness of this disease and treatment selection for such patients.
Ependymoma is the third most common pediatric primary brain tumor, and 90% of childhood ependymoma occurs intracranially, with one-third occurring in the supratentorial area and two-thirds occurring in the posterior fossa (PF) (1). The 2021 edition of the World Health Organization (WHO) classification of central nervous system (CNS) tumors refined approaches to classifying ependymoma based on gene expression profiles and deoxyribonucleic acid methylation status due to an increasing understanding of the genomic landscape of tumors of the CNS (2). PF ependymoma is classified into three subtypes, namely PF group A (PFA), PF group B, and PF subependymoma, each having different demographics, epigenetics, transcriptomes, and outcomes. Among these, PFA ependymoma, characterized by lack of trimethylation of histone H3 at lysine 27 (H3 K27-me3) and elevated chromosome X open reading frame 67 (CXorf67) expression, is the commonest and aggressive form (2, 3). Previous studies revealed that PFA ependymoma comprises ependymal cells with poor differentiation and active mitosis; therefore, the tumors have frequent local relapses and might metastasize to distant brain and spinal canal areas through the cerebrospinal fluid (1). However, extraneural metastasis of pediatric ependymoma is rare (4–6).
Herein, we report the case of a 9-year-old girl with PFA ependymoma characterized by lack of H3 K27-me3 and elevated CXorf67 expression. Despite undergoing multiple tumor resections and radiotherapies, the patient had a rapid recurrence and developed osseous and pulmonary metastases. Besides resecting the metastasis lesion in the sphenoid sinus by transnasal endoscopic surgery, we gathered tumor specimens to perform genomic analysis. Genomic analysis revealed that the patient harbored cyclin-dependent kinase (CDK) 12 mutation and homozygous deletion of CDK inhibitor 2A/B (CDKN2A/B). We also performed a systematic review of the literature to identify potential factors leading to extraneural metastasis of PFA ependymoma.
A 9-year-old girl visited our department in October 2021 owing to bilateral abducent paralysis. The timeline of the disease diagnosis and treatment are presented in Figure 1.
The patient first visited a local hospital on May 2019 owing to headache, nausea, and vomiting, and magnetic resonance imaging (MRI) revealed a tumor in the PF (Figures S1A-C). Ommaya reservoir implantation and subtotal resection of the tumor was performed at the local hospital (Figures S1D-F), and an initial pathological diagnosis of anaplastic ependymoma (WHO grade III) was established. The patient subsequently received adjuvant radiotherapy (50.4 Gy at 1.8 Gy per fraction over 4 weeks) in Japan. Unfortunately, an MRI performed in February 2021 revealed scalp and sacrococcygeal metastases (Figures S2A-D). Later in March 2021, the patient underwent metastases (scalp and sacrococcygeal) resection at the local hospital, followed by whole-spine radiotherapy (Figures S2E-H). When the patient had bilateral abducent paralysis in October 2021, she underwent whole-body positron emission tomography/computed tomography and brain MRI at our hospital. The results revealed sphenoid sinus, osseous, and pulmonary metastases (Figures S3A-D). The osseous metastasis was then confirmed by right femur biopsy, followed by histopathologic and immunohistochemical analysis (Figure S4). Furthermore, transnasal endoscopic surgery was performed to resect the sphenoid sinus metastasis (Figures S3E-G), and the specimen was sent for pathological examination and genomic analysis.
Histological examination revealed a tumor measuring 4×3×1.5 cm, with sheets of monotonous cells presenting as uniform, bland nuclei with evenly distributed chromatin on hematoxylin and eosin staining (Figure 2A). Perivascular pseudorosettes and necrotic zones were observed. The tumor cells showed dot-like epithelial membrane antigen immunoexpression (Figure 2B) and diffuse cytoplasmic glial fibrillary acidic protein immunoexpression (Figure 2C) suggestive of ependymal differentiation. Ki-67 showed a high proliferation fraction of the tumor cells (Figure 2D). Immunohistochemical staining was negative for H3 K27-me3 staining (Figure 2E) and positive for CXorf67 staining (Figure 2F). A final diagnosis of PFA ependymoma (WHO grade III) was established based on the histopathologic and immunohistochemical features, particularly the characteristic lack of H3 K27-me3 and elevated CXorf67 expression.
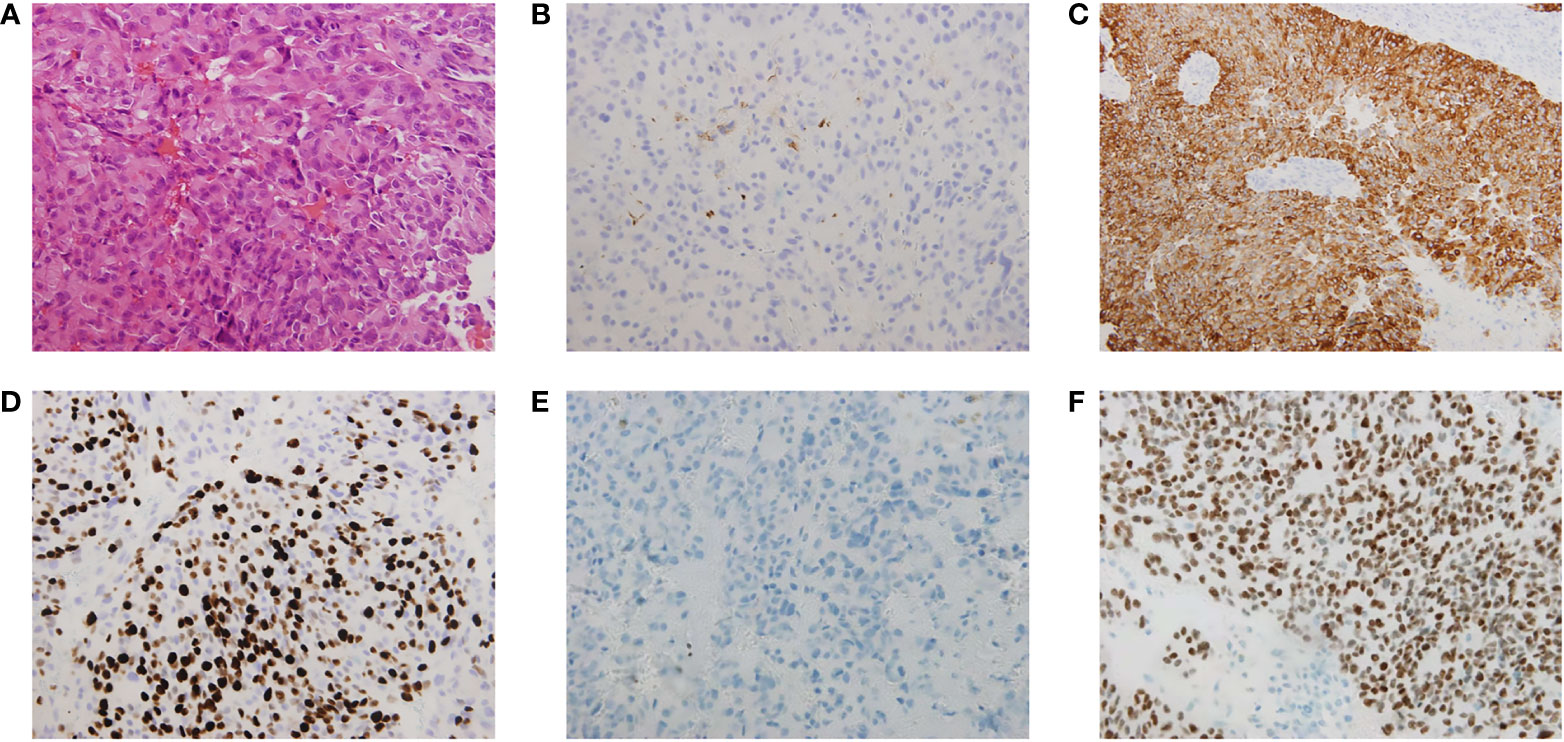
Figure 2 Histological and immunohistochemical analysis of the sphenoid sinus lesions. (A) Sheets of monotonous cells presenting as uniform, bland nuclei with evenly distributed chromatin, perivascular pseudorosettes and necrotic zones were observed on hematoxylin and eosin staining analysis. The tumor cells showed (B) dot-like epithelial membrane antigen (EMA) immunoexpression and (C) diffuse cytoplasmic glial fibrillary acidic protein (GFAP) immunoexpression suggestive of ependymal differentiation. (D) Ki-67 labelling index in the sphenoid sinus tumor was 60%, which showed a high proliferation fraction of the tumor cells. The tumor cells showed (E) immunonegative H3 K27-me3 staining and (F) immunopositive CXorf67 staining. Vascular endothelial cells in the tumor section were used as the positive control for H3 K27-me3 staining.
The tumor specimen removed from metastatic lesions of the sphenoid sinus was analyzed using next-generation sequencing to identify potential therapeutic targets. DNA extracted from plasma was used as methodological reference for eliminating the interference from germline mutation. The 833 genes based Onco PanScan™ panel identified CDK12: p.Gly1271AspfsTer (allelic frequency = 0.012) as a loss of function mutation. A homozygous deletion spanning the CDKN2A/B locus at 9p21 was also identified in the tumor sample using multicolor interphase fluorescence in situ hybridization analysis (Figure S5). The patient was then referred to a local oncology center, where she received conventional chemotherapy (etoposide + ifosfamide + cisplatin for five cycles and etoposide + ifosfamide + nedaplatin for seven cycles). After 12 cycles of chemotherapy, the patient had stable disease (SD).
Ependymoma, the third most common pediatric primary brain tumor, has a poor prognosis. Up to 50% of patients with ependymoma experience a relapse at the primary site, occasionally accompanied by metastasis within the CNS. However, based on our systematic literature review, pediatric ependymoma rarely metastasizes extraneurally, and to date, only 13 cases have been reported (including the present case) (5, 7–17) (Table S1). Of these, our report is the first and only case report of a PFA ependymoma with extraneural metastases. A lack of this finding might result in unnecessary diagnostic considerations, resulting in patients missing treatment opportunities. Herein, we reported a case that relapsed rapidly, with widespread osseous and pulmonary metastases from a PFA ependymoma with homozygous deletion of CDKN2A/B and CDK12 mutation.
Although several theories exist on the mechanism for extraneural spread, the exact mechanism by which ependymoma metastasizes extraneurally remains unknown. By performing a systematic review of the literature, we summarized the potential mechanisms for extraneural metastasis of ependymoma (Figure 3). Except for the molecular characteristics of tumor cells, including chromosomal abnormalities and genetic variations, four valuable potential extracellular mechanisms exist, which are as follows: (1) dissemination caused by tumor cells penetrating the scalp from the surgical site into the vascular or lymphatic system; (2) dissemination of tumor cells caused by the destruction of the blood-brain barrier following craniotomy and shunt surgery; (3) dissemination of tumor cells via the spread from the arachnoid villi of the CNS to the superior sagittal sinus and systemic venous circulation; (4) dissemination of tumor cells caused by the extension of the tumor to the skull structure and seeding into the lymphatic system (5, 16). Nevertheless, no study has identified the exact mechanism by which PFA ependymoma metastasizes. A recent study of a 7-year-old boy diagnosed with PF ependymoma reported lung metastases after surgical resection and ventriculoperitoneal shunt insertion and proposed that the lung metastasis might have been caused by tumor invasion into the dural venous sinus or diffusion after atrioventricular shunt implantation (5). In addition, recent studies reported that high-risk PFA ependymoma with poor outcomes, such as multiple recurrences, tended to have some genetic characteristics, including the gain of chromosome 1q, loss of chromosome 6q, and CDKN2A/B deletion (18–22). Among them, CDKN2A/B is the second most frequently inactivated tumor suppressor gene in human cancers, the inactivation of which reportedly affects tumor growth and metastasis.
In the current case, we speculated that osseous and pulmonary metastases might be attributed to the dissemination caused by tumor cells penetrating the scalp from the surgical site into the vascular or lymphatic system, considering that the patient had scalp occupation less than 2 years after the surgery. This is because the retrosigmoid approach for PF tumor resection was adopted during the first surgery at the local hospital. Furthermore, the high metastatic capacity of the tumor cells in the patient might theoretically be attributed to the homozygous deletion of CDKN2A/B and CDK12 mutation; however, no characteristic chromosomal abnormalities were observed. CDK12, as a member of the CDK family of serine/threonine protein kinases, regulates transcription and post-transcriptional processes, thereby regulating various cellular functions. Genomic alterations in CDK12 have been detected in breast, stomach, esophageal, and other cancers, with the characteristics of oncogenesis (23, 24). In addition, recent studies have demonstrated the aggressive behavior of CDK12 mutation. Melissa et al. reported that prostate cancer patients with CDK12 mutation undergo metastasis in a shorter time (25). Zhang et al. reported that CDK12 mutation is associated with osseous metastasis of non-small cell lung cancer (26). Therefore, we speculated that in addition to CDKN2A/B deletion, CDK12 mutation was associated with metastasis in pediatric PFA ependymoma.
Molecular profiling is gaining popularity in pediatric CNS tumors for supporting diagnosis, prognosis, and therapeutic decisions. Given that PFA ependymoma is known as epigenetically deregulated tumors, several studies proposed that epigenetic-related drugs, such as poly (adenosine diphosphate-ribose) polymerase (PARP) inhibitors, might serve as promising therapeutic drugs (3, 27). Our patient also met the inclusion criteria of a clinical trial on a PARP inhibitor (Nilaparil, ChiCTR2100051395) in China. However, due to the CDK12 mutation in our patient, the efficacy of PARP inhibitors is unknown. Although a preclinical study demonstrated that CDK12 deficiency might increase tumor cell sensitivity to PARP inhibitors in breast cancer gene (BRCA) 1- or BRCA2-mutant tumors via genetic synthetic lethality (28), recent studies revealed CDK12 mutation might correlate with inferior response to PARP inhibitors and increased sensitivity to platinum-based chemotherapy (29, 30). In addition, our patient and her family refused targeted therapy and received conventional chemotherapy owing to their reluctance to clinical trials. After 12 cycles of chemotherapy, the patient had SD.
To the best of our knowledge, this is the first study to report the case of a pediatric PFA ependymoma with extraneural metastases (osseous and pulmonary metastases). We further clarified the diagnostic procedures of this newly identified PFA ependymoma and provided new cues for the study of the disease invasiveness and treatment selection for such patients.
The raw data supporting the conclusions of this article will be made available by the authors.
The human tissue study protocol was approved by the Ethics Committee of Xuanwu Hospital, Capital Medical University. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
MZ: drafting/revision of the manuscript for content including medical writing for content. LW: interpretation of the pathological finding. PS: major role in the acquisition of data and performed the endoscopic surgery. YL: major role in the acquisition of data. GC: performed the endoscopic surgery and major role in the acquisition of data. GZ: study concept or design and Analysis or interpretation of data. All authors contributed to the article and approved the submitted version.
We are grateful to the patient and the families of the patient who have made this research possible. We thank Bullet Edits Limited for language editing of the manuscript. We also thank Genetron Health Inc., that supported genomic studies.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1001118/full#supplementary-material
Supplementary Table 1 | Summary of pediatric ependymoma cases with extraneural metastasis.
2. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro-oncology (2021) 23:1231–51. doi: 10.1093/neuonc/noab106
3. Han J, Yu M, Bai Y, Yu J, Jin F, Li C, et al. Elevated CXorf67 expression in PFA ependymomas suppresses DNA repair and sensitizes to PARP inhibitors. Cancer Cell (2020) 38:844–856.e847. doi: 10.1016/j.ccell.2020.10.009
4. Siada RG, Lu VM, Schwartz J, Daniels DJ. Recurring pediatric anaplastic ependymoma with rare peritoneal carcinomatosis: a case report and hypothesis of mechanism. Child's Nervous System ChNS Off J Int Soc Pediatr Neurosurg (2021) 37:1021–4. doi: 10.1007/s00381-020-04814-0
5. Alzahrani A, Alassiri A, Kashgari A, Alrehaili J, Alshaalan H, Zakzouk R. Extraneural metastasis of an ependymoma: a rare occurrence. Neuroradiol J (2014) 27:175–8. doi: 10.15274/NRJ-2014-10017
6. Yang X, Ren Y, Wu W, Wang X, Liu X, Zhang Y. Intracranial extra-axial ependymoma involving the petroclival region: a rare case report. Int J Clin Exp Pathol (2014) 7:9067–71.
7. Breslich PJ. Ependymoma of the right occipital lobe with death from extracranial metastases. Journal Lancet (1957) 77:99–103.
8. Newton HB, Henson J, Walker RW. Extraneural metastases in ependymoma. J Neuro Oncol (1992) 14:135–42. doi: 10.1007/BF00177617
9. Kinoshita M, Izumoto S, Kagawa N, Hashimoto N, Maruno M, Yoshimine T. Long-term control of recurrent anaplastic ependymoma with extracranial metastasis: Importance of multiple surgery and stereotactic radiosurgery procedures —Case report&mdash. Neurol Medico Chirurgica (2004) 44:669–73. doi: 10.2176/nmc.44.669
10. Varan A, Sari N, Akalan N, Söylemezoğlu F, Akyüz C, Kutluk T, et al. Extraneural metastasis in intracranial tumors in children: the experience of a single center. J Neuro Oncol (2006) 79:187–90. doi: 10.1007/s11060-006-9123-3
11. Kumar P, Rastogi N, Jain M, Chhabra P. Extraneural metastases in anaplastic ependymoma. J Cancer Res Ther (2007) 3:102–4. doi: 10.4103/0973-1482.34689
12. Hussain M, Mallucci C, Abernethy L, Godhamgaonkar V, Thorp N, Pizer B. Anaplastic ependymoma with sclerotic bone metastases. Pediatr Blood Cancer (2010) 55:1204–6. doi: 10.1002/pbc.22604
13. Chao MM, Packer RJ, Myseros JS, Rood BR. Isolated extracranial recurrence of anaplastic ependymoma. Pediatr Blood Cancer (2011) 56:317–8. doi: 10.1002/pbc.22764
14. Fischer C, Haque SS, Huse JT, Blochin E, Souweidane MM, Lis E, et al. Extraneural ependymoma: distant bone, lung, liver, and lymph node metastases following bevacizumab. Pediatr Blood Cancer (2013) 60:143–5. doi: 10.1002/pbc.24268
15. Kim SI, Lee Y, Kim SK, Kang HJ, Park SH. Aggressive supratentorial ependymoma, RELA fusion-positive with extracranial metastasis: A case report. J Pathol Trans Med (2017) 51:588–93. doi: 10.4132/jptm.2017.08.10
16. Umbach G, El Ahmadieh TY, Plitt AR, Aoun SG, Neeley OJ, Lyon KA, et al. Extraneural metastatic anaplastic ependymoma: a systematic review and a report of metastases to bilateral parotid glands. Neuro Oncol Pract (2020) 7:218–27. doi: 10.1093/nop/npz041
17. Joris V, Weil AG, Gennari A, Yuh SJ. Complete resection of dual ependymoma spinal metastasis using a fixed tubular retractor-a pediatric case report. Child's Nervous System ChNS Off J Int Soc Pediatr Neurosurg (2022) 38:1599–603. doi: 10.1007/s00381-022-05443-5
18. Baroni LV, Sundaresan L, Heled A, Coltin H, Pajtler KW, Lin T, et al. Ultra high-risk PFA ependymoma is characterized by loss of chromosome 6q. Neuro-oncology (2021) 23:1360–70. doi: 10.1093/neuonc/noab034
19. Pierce AM, Witt DA, Donson AM, Gilani A, Sanford B, Sill M, et al. Establishment of patient-derived orthotopic xenograft model of 1q+ posterior fossa group a ependymoma. Neuro-oncology (2019) 21:1540–51. doi: 10.1093/neuonc/noz116
20. Massimino M, Barretta F, Modena P, Witt H, Minasi S, Pfister SM, et al. Second series by the Italian association of pediatric hematology and oncology of children and adolescents with intracranial ependymoma: an integrated molecular and clinical characterization with a long-term follow-up. Neuro-oncology (2021) 23:848–57. doi: 10.1093/neuonc/noaa257
21. Korshunov A, Witt H, Hielscher T, Benner A, Remke M, Ryzhova M, et al. Molecular staging of intracranial ependymoma in children and adults. J Clin Oncol Off J Am Soc Clin Oncol (2010) 28:3182–90. doi: 10.1200/JCO.2009.27.3359
22. Lim KY, Lee K, Shim Y, Park JW, Kim H, Kang J, et al. Molecular subtyping of ependymoma and prognostic impact of ki-67. Brain Tumor Pathol (2022) 39:1–13. doi: 10.1007/s10014-021-00417-y
23. Lui GYL, Grandori C, Kemp CJ. CDK12: an emerging therapeutic target for cancer. J Clin Pathol (2018) 71:957–62. doi: 10.1136/jclinpath-2018-205356
24. Paculová H, Kohoutek J. The emerging roles of CDK12 in tumorigenesis. Cell Division (2017) 12:7. doi: 10.1186/s13008-017-0033-x
25. Reimers MA, Yip SM, Zhang L, Cieslik M, Dhawan M, Montgomery B, et al. Clinical outcomes in cyclin-dependent kinase 12 mutant advanced prostate cancer. Eur Urol (2020) 77:333–41. doi: 10.1016/j.eururo.2019.09.036
26. Zhang K, Zhang M, Zhu J, Hong W. Screening of gene mutations associated with bone metastasis in nonsmall cell lung cancer. J Cancer Res Ther (2016) 12:C186–c190. doi: 10.4103/0973-1482.200597
27. Panwalkar P, Tamrazi B, Dang D, Chung C, Sweha S, Natarajan SK, et al. Targeting integrated epigenetic and metabolic pathways in lethal childhood PFA ependymomas. Sci Trans Med (2021) 13:eabc0497. doi: 10.1126/scitranslmed.abc0497
28. Bajrami I, Frankum JR, Konde A, Miller RE, Rehman FL, Brough R, et al. Genome-wide profiling of genetic synthetic lethality identifies CDK12 as a novel determinant of PARP1/2 inhibitor sensitivity. Cancer Res (2014) 74:287–97. doi: 10.1158/0008-5472.CAN-13-2541
29. Antonarakis ES, Isaacsson Velho P, Fu W, Wang H, Agarwal N, Sacristan Santos V, et al. CDK12-altered prostate cancer: Clinical features and therapeutic outcomes to standard systemic therapies, poly (ADP-ribose) polymerase inhibitors, and PD-1 inhibitors. JCO Precis Oncol (2020) 4:370–81. doi: 10.1200/PO.19.00399
Keywords: PFA, pediatric ependymoma, extraneural metastases, CDK12, chemotherapy
Citation: Zhou M, Wang L, Sun P, Liu Y, Chen G and Zeng G (2022) Delineation of molecular characteristics in pediatric PFA ependymoma involving rare osseous and pulmonary metastases: A case report and literature review. Front. Oncol. 12:1001118. doi: 10.3389/fonc.2022.1001118
Received: 22 July 2022; Accepted: 27 October 2022;
Published: 15 November 2022.
Edited by:
Feng Wan, Huazhong University of Science and Technology, ChinaReviewed by:
Normand Garcia Hernandez, Pediatric Hospital “Dr. Silvestre Frenk Freud", MexicoCopyright © 2022 Zhou, Wang, Sun, Liu, Chen and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gao Zeng, emVuZ3JvZ29zc0AxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.