- 1Department of Medical and Surgical Sciences, University of Catanzaro “Magna Graecia”, Catanzaro, Italy
- 2Physical and Rehabilitative Medicine, Department of Health Sciences, University of Eastern Piedmont “A. Avogadro”, Novara, Italy
- 3Division of Pathology, IEO, European Institute of Oncology IRCCS, Milan, Italy
- 4Department of Oncology and Hemato-Oncology, University of Milan, Milan, Italy
- 5Division of Early Drug Development, IEO, European Institute of Oncology IRCCS, Milan, Italy
- 6Physical Medicine and Rehabilitation Unit, Department of Neurosciences, ASST Carlo Poma, Mantova, Italy
- 7Translational Medicine, Dipartimento Attività Integrate Ricerca e Innovazione (DAIRI), Azienda Ospedaliera SS. Antonio e Biagio e Cesare Arrigo, Alessandria, Italy
Background: Cancer treatment-induced bone loss (CTIBL) is a frequent complication of breast cancer therapies affecting both disability and health-related quality of life (HRQoL). To date, there is still a lack of consensus about the most effective approach that would improve bone health and HRQoL. Therefore, the aim of this systematic review of randomized controlled trials (RCTs) was to summarize the evidence on the effects of antiresorptive drugs on CTIBL in patients with early breast cancer.
Methods: PubMed, Scopus, and Web of Science databases were systematically searched up to April 30, 2021 to identify RCTs satisfying the following PICO model: P) Participants: postmenopausal women with early breast cancer receiving adjuvant aromatase inhibitors (AI), age >18 years; I) Intervention: antiresorptive drugs (i.e. bisphosphonates and/or denosumab); C) Comparator: any comparator; O) Outcome: bone mineral density (BMD) modifications. Moreover, a quality assessment was performed according to the Jadad scale.
Results: Out of the initial 2415 records, 21 papers (15 studies) were included in the data synthesis. According to the Jadad scale, 6 studies obtained a score of 5, 1 study obtained a score of 4, 13 studies obtained a score of 3, and 1 study with score 1. Although both bisphosphonates and denosumab showed to increase BMD, only denosumab showed significant advantages on fractures.
Conclusions: Bone health management in patients with early breast cancer receiving adjuvant AIs remains challenging, and the optimal therapeutic approach is not standardized. Further studies are needed to investigate CTIBL, focusing on both the need for antiresorptive drugs and their duration based on individual patients’ characteristics.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero, identifier CRD42021267107.
Introduction
Breast cancer (BC) is the most prevalent malignancy in women worldwide, with incidence increasing in last decades (1). Oppositely, mortality from BC decreased in last years, due to the significant advancements in screening programs and therapeutical interventions (2). In response to the progressive increase of women living after a diagnosis of BC, survivorship issues related to cancer treatment and its impact on bone health and health-related quality of life (HRQoL) have progressively emerged (3–9).
Cancer treatment-induced bone loss (CTIBL) is a frequent side effect of the pharmacotherapy used for treating BC. While chemotherapy might lead to an unspecific increase in bone resorption, hormone therapies (HT) reduce residual serum endogenous estrogen levels, with a consequent decrease in bone mineral density (BMD) and an increase in fragility fracture risk (10–17). To date, aromatase inhibitors (AI) are considered the gold standard adjuvant therapy for postmenopausal women with hormone receptor (HR)-positive early BC (EBC) (18, 19). In such patients, a significant decrease in bone density has been observed (20, 21). To counter bone loss induced by AIs in BC patients, several anti-resorptive molecules have been investigated (22, 23). The ZO-FAST study supported the efficacy of zoledronic acid in increasing BMD in postmenopausal women receiving adjuvant AIs (24). In addition, the ABCSG-12 trial showed that zoledronic acid along with endocrine therapy could also increase disease-free survival (DFS) in premenopausal women with EBC (25). In 2015, the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) published a meta-analysis of individual patient data investigating bisphosphonates (BPs) in the adjuvant setting of EBC, including data from 18,766 women in 26 trials. All tumor subtypes and adjuvant treatments were considered. Use of BPs reduced both bone recurrence (rate ratio [RR] 0.83; p=0.004) and bone fractures (RR: 0.85; p=0.02), with a significant impact also on distant recurrence (RR 0.92; p=0.03) and BC mortality (RR 0.91; p=0.04). Notably, the subgroup analysis showed how the added value of bisphosphonate is limited in premenopausal patients, while postmenopausal patients derived a greater benefit in all outcomes.
Denosumab, a fully human IgG2 monoclonal antibody, has been proposed to treat CTIBL in BC patients undergoing HT not only by improving BMD but also by reducing the rate of clinical fragility fractures (both hip and vertebrae) (12, 26, 27).
Although the long-term management of bone health in BC patients through the combination of different pharmacological therapies is gaining interest, most studies conducted to date have only assessed the effects of a single drug in terms of BMD improvement or fracture risk reduction (28–30). Thus, the gap of knowledge about tailored and effective bone health interventions is far from being understood.
Therefore, this systematic review aims to summarize the current evidence on the efficacy of anti-resorptive agents and their impact on bone health and HRQoL in post-menopausal patients with EBC receiving adjuvant AIs.
Materials and Methods
Study Registration
This systematic review of randomized controlled trials (RCTs) has been performed ethically in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement (31). The PRISMA Checklist is provided as Supplementary Material. A protocol was developed before study initiation and submitted to PROSPERO (https://www.crd.york.ac.uk/prospero; registration number CRD42021267107).
Search Strategy
We systematically searched PubMed/Medline, Scopus, and Web of Science for RCTs published up to April 30, 2021. Two investigators independently searched the databases. The search strategy is reported in Table 1.
Selection Criteria
In accordance with the PICO model (32), we considered eligible RCTs satisfying the following criteria:
1. P) Participants: postmenopausal women with early BC receiving adjuvant AI, age >18 years;
2. I) Intervention: antiresorptive drugs (i.e. BPs and/or denosumab);
3. C) Comparator: any comparator;
4. O) Outcome: BMD modifications.
Only RCTs published in International journals in English language were included. The exclusion criteria were: i) studies involving animals; ii) language other than English; iii) participants with pregnancy; iv) cancer different of BC; v) studies involving patients with metastatic BC; vi) conference abstracts.
After duplication removal, two investigators independently reviewed the title and abstracts of retrieved articles to choose relevant articles. A third reviewer was asked in case of disagreement.
Data Extraction and Synthesis
Data were assessed and extracted from full-text documents by two independent reviewers (AdS and LL). Any disagreement was solved by discussion or consulting a third reviewer (MI).
The following data were extracted: 1) title and trial name; 2) authors; 3) publication year; 4) number of patients included; 5) intervention characteristics; 6) comparator arm(s); 7) bone-health related outcomes; 8) follow-up.
A descriptive approach was used to synthesize both study characteristics and data extracted. Subgroup analysis has been performed based on the specific drug assessed in the studies included.
Study Quality and Risk of Bias
Study quality was assessed according to the Jadad scale by two reviewers independently (33). In case of disagreement, a third reviewer was involved in the decisional process to achieve consensus. The clinical trials with a Jadad score between 3 and 5 points were considered as high-quality studies.
Results
Main Characteristics of the Included Studies
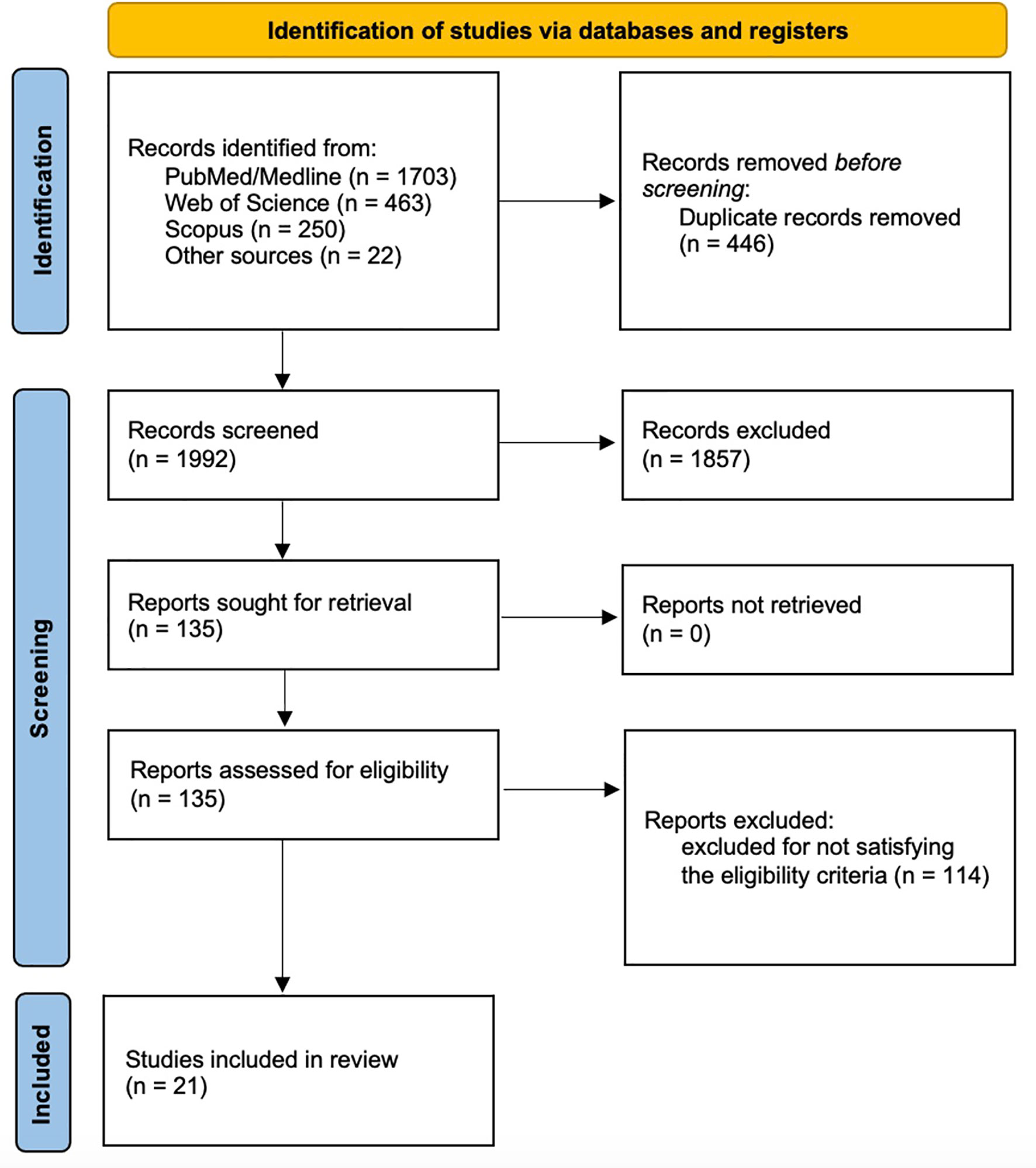
A total of 2416 records were identified from the search process (PubMed/Medline: 1703 records; Web of Science: 463 records; Scopus: 250 records) and 22 records were identified by reference lists of primary studies. After duplication removal, 1992 records were screened for title and abstract. Therefore, 1857 records were excluded, and 135 full-text studies were screened. One hundred and seventeen records were excluded for not satisfying the eligibility criteria. Finally, the following 21 papers (15 RCTs) were included in the present systematic review: Livi (2019) (29), Gnant (2015) (34), Gnant (2019) (35), Hines (2009) (36), Wagner-Johnston (2015) (37), Greenspan (2015) (38), Coleman (2013) (39), Rhee (2013) (40), Lester (2008) (41), Lester (2012) (42), Takahashi (2012) (43), Llombart (2012) (44), Van Poznak (2010) (45), Markopoulos (2010) (46), Eidtmann (2010) (47), Brufsky (2009) (48), Ellis (2008) (49), Bundred (2008) (24), Brufsky (2008) (50), Brufsky (2012) (51), Safra (2011) (52). Further details on the identification and inclusion/exclusion of the screened studies are reported in Figure 1.
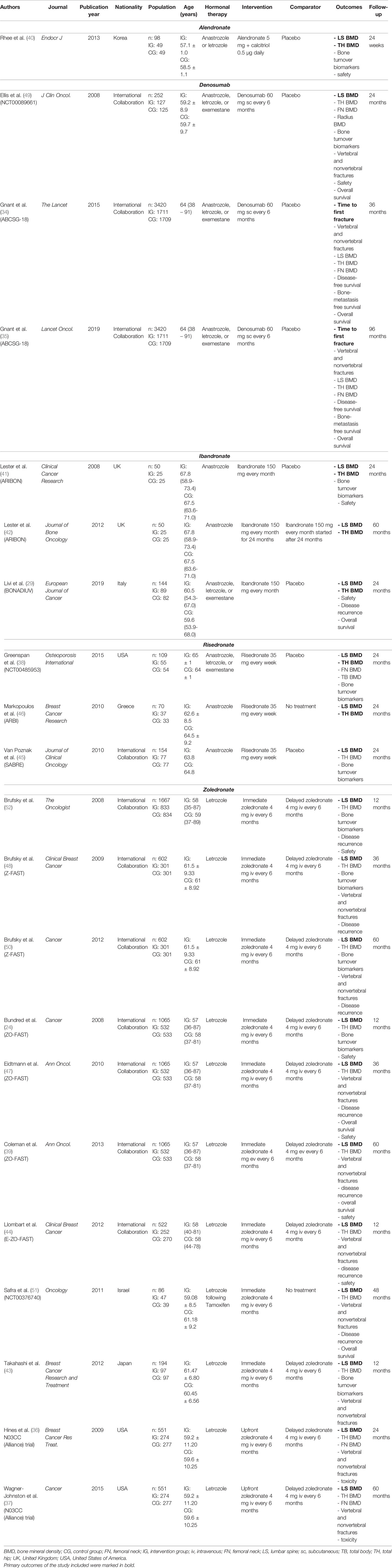
Main characteristics of the 15 clinical trials (21 papers) included (24, 29, 34, 35, 39–44, 46–52) are summarized in Table 2. These RCTs were published between 2008 (24, 41, 49, 50) and 2019 (29, 35). Most of them (7; 46.7%) were International collaborations (24, 34, 35, 39, 44, 45, 47–51), whereas 3 studies were carried out in Europe (1 in the United Kingdom (41, 42), 1 in Italy (35), 1 in Greece (46)), 3 in Asia (1 in Japan (43), 1 in Korea (40), 1 in Israel (52)) and 2 in the USA (36–38).
Number of patients included ranged from 50 (41) to 3420 (34, 35) subjects. Seven RCTs (24, 36, 37, 39, 43, 44, 47, 48, 50–52) assessed participants who were treated with letrozole, 3 RCTs (41, 42, 45, 46) enrolled patients receiving anastrozole, one RCT (40) included patients treated with anastrozole or letrozole, and in 4 RCTs (29, 34, 35, 38, 49) patients were treated with anastrozole, letrozole, or exemestane.
BC patients received denosumab in 2 studies (26, 34, 35), zoledronic acid in 7 studies (24, 36, 37, 39, 43, 44, 47, 48, 50–52), risedronate in 3 studies (38, 45, 46), ibandronate in 2 studies (29, 41, 42), and alendronate in only one study (40). The comparator arm consisted in no treatment in two studies (46, 52), delayed treatment in 6 studies (24, 36, 37, 39–44, 47, 48, 50–52), and placebo in 7 studies (29, 34, 35, 38, 45, 49).
Alendronate
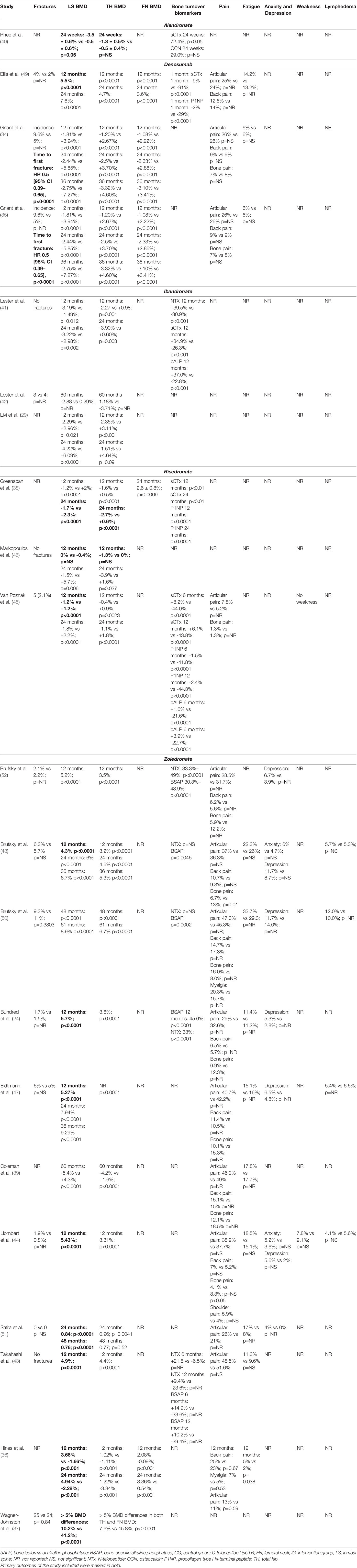
From the studies included in this systematic review, only one assessed oral alendronate 5 mg in addition to calcitriol 0.5 µg daily in patients with EBC receiving adjuvant anastrozole or letrozole (40). The study showed significant differences between alendronate and placebo groups in terms of lumbar BMD (-0.5 ± 0.6% vs -3.5 ± 0.6%; p=0.05) at 24 weeks, whereas non-significant improvements were observed in hip BMD (-0.5 ± 0.4% vs -1.3 ± 0.5%; p>0.05). Diverse expression levels were only found in sCTx (72.4%; p<0.05), whereas osteocalcin (OCN) did not show significant differences between groups (29.0%; p>0.05) (as shown by Table 3).
Denosumab
Three papers (2 studies) compared six-monthly denosumab 60 mg with placebo, reporting benefits in terms of fracture risk reduction or BMD improvement (34, 35, 49).
Gnant et al., in a collaborative study including 3420 patients, observed consistent differences in fracture incidence between patients treated with denosumab (5%) vs. untreated (9.6%) (34). Moreover, a significant difference in terms of time-to-first clinical fracture, the study primary endpoint, was observed between the two groups (HR 0.5, 95% CI 0.39–0.65, p<0.0001). Oppositely, the study by Ellis and colleagues (49) did not find major differences for fracture outcomes: no vertebral fractures were observed in both groups, the incidence of nonvertebral fractures was 6% in both arms, major nonvertebral fractures were observed in 3 women receiving denosumab (2%) and 5 women receiving placebo (4%).
Intriguingly, the two studies revealed significant differences between groups in terms of BMD. More in detail, Ellis et al. (49) reported significant differences between groups after 2 years of treatment (12 months: 5.5%; p<0.0001; 24 months: 7.6%; p<0.0001). On the other hand, hip BMD increased accordingly in both TH site (12 months: p<0.0001; 24 months: 4.7%; p<0.0001) and FN site (12 months: p<0.0001; 24 month: 3.6%; p<0.0001). Similarly, Gnant et al. (34, 35) underlined a significant difference between groups at 36 months (12 months: -1.81% vs +3.94%; p<0.0001; 24 months: -2.44% vs +5.85%; p<0.0001; 36 months: -2.75% vs +7.27%; p<0.0001). Hip BMD results were in line with the previous results with a significant increase in the denosumab group (12 months: -1.20% vs +2.67%; p<0.0001; 24 months: -2.5% vs +3.70%; p<0.0001; 36 months: -3.32% vs +4.60%; p<0.0001). Modifications in bone turnover were suggested by Ellis et al. (49), reporting significant differences between groups in C-telopeptide I (sCTx) and procollagen type I N-terminal peptide (P1NP), two markers of bone remodeling (1 month: CTX: -9% vs -91%; p<0.0001; P1NP: -2% vs -29%; p<0.0001). On the contrary, joint pain, back pain, bone pain and fatigue showed no differences when the two groups were compared. Outcomes are reported in detail in Table 3.
Ibandronate
The effect of another anti-resorptive drug (i.e., ibandronate 150 mg every month) was assessed in BC survivors receiving anastrozole (41, 42) and anastrozole, letrozole, and exemestane (29). The study of Lester et al. in 2008 assessed the effects of Ibandronate (150 mg every month) for 24 months compared to placebo in osteopenic patients (41). On the other hand, patients with normal BMD did not receive any therapy while patients with osteoporosis received Ibandronate 150 mg every month. Interestingly, no fractures were recorded during the first 2 years (41). After 2 years, 3/20 patients continued to receive BPs over the next 3 years, while 8 patients received delayed ibandronate treatment. At 60 months, BMD changes were reported without reporting significant differences between groups (LS BMD: -2.88 vs 0.29%; p=NR; TH BMD: 1.18% vs -3.71%; p=NR). On the other hand, the study conducted by Lester et al. in 2012 recorded 4 fractures in the group that received ibandronate for 2 years, while the group treated with ibandronate after 2 years showed 3 fractures (42). In total, 10 fragility fractures were recorded: 4 fractures in the group treated with ibandronate for 2 years, 3 fractures in the placebo group treated with ibandronate after 2 years, and further 3 fractures in the osteoporotic group treated with ibandronate for 5 years.
Variations in lumbar and hip BMD were chosen as primary outcomes in both the ARIBON (41, 42) and BONADIUV trials (29). In both of them significant differences were found between ibandronate and placebo treated patients at both lumbar BMD and hip BMD after 12 and 24 months (29, 41). In particular, Lester et al. (41, 42) reported significant differences between groups in LS BMD (12 months: -3.19% vs +1.49%; p=0.012; 24 months: -3.22% vs +2.98%; p=0.002) and in TH BMD (12 months: -2.27 vs +0.98; p=0.001; 24 months: -3.90% vs +0.60%; p=0.003). Accordingly, Livi et al. (29) reported significant differences between groups (LS BMD 12 months: -2.29% vs +2.96%; p=0.021; 24 months: -4.22% vs +6.09%; p<0.0001; TH BMD: 12 months: -2.35% vs +3.11%; p<0.001; 24 months: -1.51% vs +4.64%; p=0.09).
Bone turnover biomarkers (sCTx, NTX, and bALP) were assessed instead only in the ARIBON study, with significant differences (NTX 12 months: +39.5% vs -30.9%; p<0.001; sCTx 12 months: +34.9% vs -26.3%; p<0.001; bALP 12 months: +37.0% vs -22.8%; p<0.001) (41). Table 3 reported further details.
Risedronate
The effects of risedronate 35 mg weekly in BC patients treated with anastrozole or letrozole, or exemestane were assessed in three studies (38, 45, 46). No fragility fractures were reported by Markoupolos et al. (46). In the study by Von Poznak et al., four patients in the control arm had fractures versus none in the risedronate arm (45). Lumbar BMD, a primary outcome in all these studies, was significantly increased in all trials after 24 months of treatment with risedronate (38, 45, 46). Similarly, significant differences were reported in hip BMD (38, 45, 46).
When bone turnover biomarkers were evaluated, significant differences between the risedronate and placebo groups were seen in the expression of isoforms of alkaline phosphatase (bALP), sCTx, N-telopeptide (NTX), and P1NP (38, 45). Joint pain was reported only by Van Poznak et al. only (45), without significant differences between groups (see Table 3 for further details).
Zoledronate
Seven studies (24, 36, 37, 39, 43, 44, 47, 48, 50–52) assessed the effects of endovenous administration of zoledronate 4 mg every 6 months in BC women treated with adjuvant letrozole. Of note, the study of Wagner-Johnston et al. evaluated EBC patients starting letrozole after completing tamoxifen treatment (37). Six studies (24, 36, 37, 39, 43, 44, 47, 48, 50, 51) compared the bone protection effect of immediate-start to delayed-start of zoledronic acid administration. On the other hand, Safra et al. (52) compared zoledronic acid administration with a control group not receiving any treatment.
In the delayed arm, zoledronic acid was initiated when BMD decreased to less than -2.0 or when a fragility fracture occurred. Although no differences were detected between the randomized groups regarding fracture incidence, significant effects in terms of both lumbar, the primary endpoint, and hip BMD increase were reported in the early administration group after 12, 24, 36, and 60 months (24, 36, 37, 39, 43, 44, 47, 48, 50, 51) (see Table 3 for further details).
Bone turnover biomarkers were assessed in three studies, showing positive modifications in the early zoledronate group (24, 43, 48, 50, 51). Only one study did not record significant differences in sCTx concentrations after 36 months (48). Differences in terms of musculoskeletal pain, fatigue, anxiety, depression, weakness, and lymphedema were non-significant or not reported. Table 3 summarizes the main results of these studies.
Study Quality
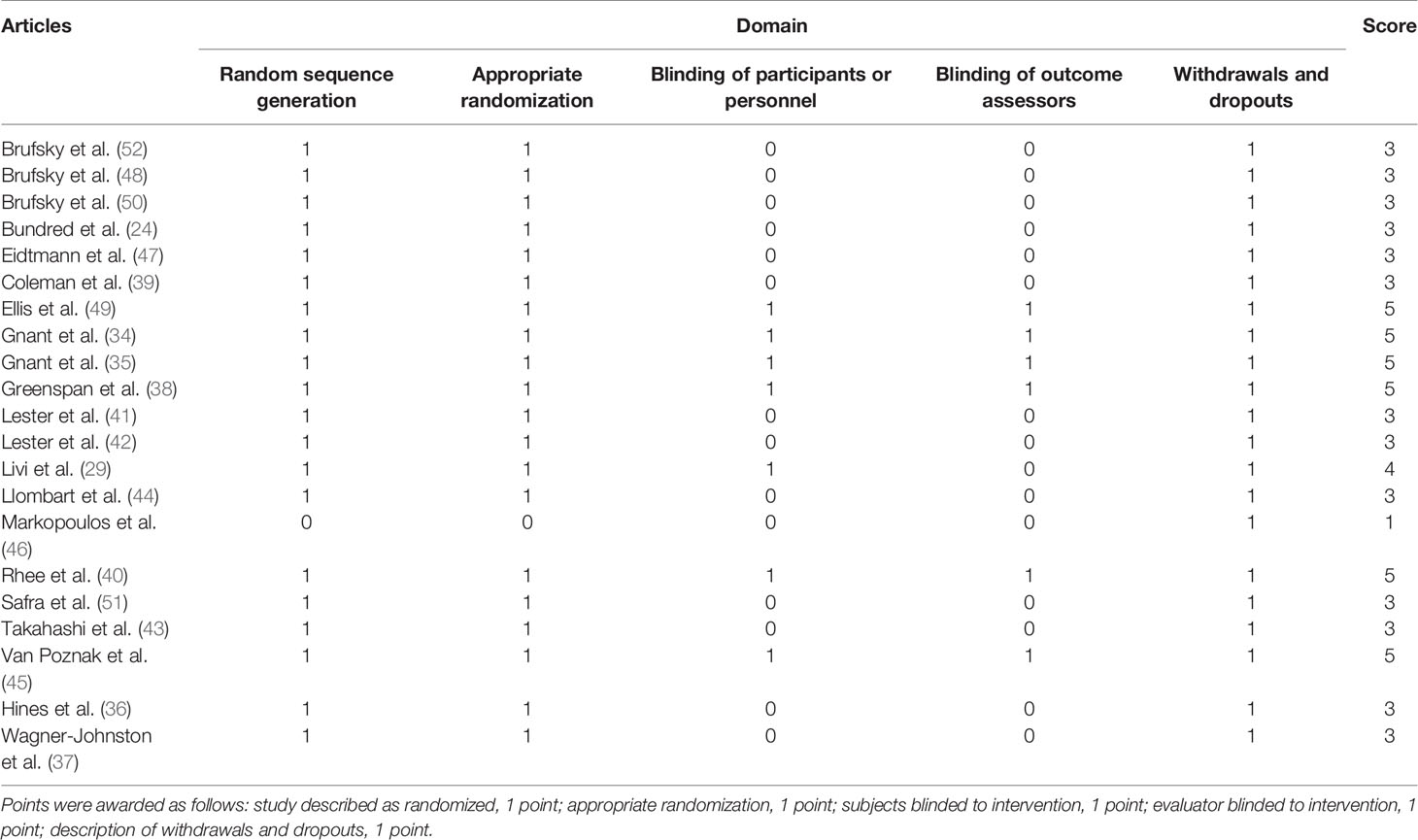
Out of 21 studies included in this analysis, 20 of them (24, 29, 34–44, 47–52) were classified as high quality according to the Jadad scale (53). In particular, 6 papers (28.6%) (34, 35, 38, 40, 45, 49) obtained a score of 5, 1 paper (4.8%) (29) obtained score 4, 13 papers (61.9%) (24, 36, 37, 39, 41–44, 47–52) obtained a score of 3 and 1 paper (4.8%) (46) obtained a score of 1 (further details are depicted in Table 4).
Discussion
AIs are considered the standard adjuvant therapy in postmenopausal women with early HR-positive BC (18, 19). However, the detrimental effect of AIs on bone health might significantly increase the risk of fractures, with negative consequences in terms of HRQoL and disability (54–56). Therefore, the implementation of tailored and effective interventions to reduce bone-related adverse events and preserve bone health is a crucial challenge in the complex management of patients with EBC receiving AIs. Thus, the present systematic review was aimed at summarizing the state of the art about bone-modifying agents to counteract Ais-induced bone loss, to provide data to guide the future research and clinical management of BC survivors.
Our findings pointed out the consistent improvement in BMD after 3 years of denosumab administration (34). Thus, denosumab could be considered among the most effective therapy to improve BMD and reduce fracture risk in EBC patients receiving AIs. Similarly, three RCTs provided long-term evidence (i.e., 5 years) about treatment with zoledronic acid, showing significant results in terms of lumbar and hip BMD improvement (37, 39, 51). Oral BPs also proved to be effective in enhancing BMD, even if the evidence supporting these drugs is weaker, given the smaller cohorts of patients, shorter treatment periods and less consistent results compared to those testing denosumab or zoledronic acid (29, 38, 40–42, 45, 46). Only the recent study from Livi et al. revealed a higher percentage of lumbar BMD improvement in BC survivors that were concomitantly treated with AIs and oral ibandronate compared with placebo (29). Yet, consistent data on the effectiveness of oral BPs on bone health in this setting are still lacking.
Interventions with anti-resorptive agents have also been found to have a positive impact on DFS. In particular, conflicting results were reported in the current literature with the ABCSG-18 trial (35) that underlined promising benefits of denosumab in DFS of post-menopausal early BC women receiving adjuvant aromatase inhibitor therapy. On the other hand, the D-CARE trial, which assessed the effects of denosumab in high-stage BC patients, did not report improvements in bone metastasis-free survival (57).
Similarly, controversial results were reported for BFs. In particular, the GAIN study showed no DFS benefits for both pre-menopausal and peri-menopausal BC patients who received oral ibandronate in the adjuvant treatment (58).
In accordance, large prospective studies assessing BPs failed to underline consistent effects on DFS endpoint in BC survivors (39, 51, 59) while positive data were provided by the EBCTCG meta-analysis reporting positive effects (RR for recurrence 0.86, 95% CI 0.78–0.94, p=0.002 in zoledronic acid arm) but restricted to postmenopausal women only (60). Therefore, to date, there is no consensus in terms of BPs prescription with the aim to improve DFS considering the large heterogeneous and discordant data.
On the other hand, a joint position statement of interdisciplinary cancer and bone societies suggested that adjuvant BPs should be considered in all postmenopausal women at risk for BC recurrence (61). Similarly, the Cancer Care Ontario and the American Society of Clinical Oncology (ASCO) guidelines recommended to consider BP prescription for all patients who are deemed at high enough risk of relapse (62). However, the authors underline that the lack of evidence did not allow a precise subgroups stratification for patients that might have major benefits from BP prescription (62).
Besides the role of BPs in overall and disease-free survival is still controversial, the cost-effectiveness of their routine use in clinical practice is far from being understood (63).
Taken together, these results suggest that the mechanisms underpinning the adjuvant effects of anti-resorptive drugs in patients with BC need to be further investigated.
Moreover, long-term effects of antiresorptive drugs also deserve to be considered. Although comprehensive management of AIs bone loss has been proposed to optimize bone health, to date, few evidence about the long-term effects of anti-osteoporotic treatments is available. International guidelines recommend the administration of anti-resorptive drugs for the whole duration of AIs therapy, but the optimal duration of these therapies is questionable (14, 64, 65). Moreover, it should be noted that AIs might be administered from 5 to more than 10 years (66), while studies assessing the long-term effects of denosumab or BPs in BC patients lasted 5-8 years (35, 39). Therefore, data supporting the long-term effects of anti-resorptive drugs on bone health in EBC patients receiving AIs are warranted.
This paper has some limitations which need to be taken into consideration. Firstly, only RCTs were included, thus excluding evidence provided by observational studies. Furthermore, because of statistical and methodologic heterogeneity among studies included, we did not carry out a pairwise or network meta-analysis.
In conclusion, bone health management is a cornerstone in the comprehensive management of patients with EBC receiving adjuvant AIs. Despite the remarkable advancements in understanding the mechanisms underpinning AI-induced bone loss, the optimal therapeutic framework for these patients remains a challenge for physicians.
This systematic review showed that denosumab and zoledronic acid might be considered the most effective anti-resorptive treatment options to improve BMD in patients with EBC on adjuvant AIs. However, robust data concerning the long-term effects of these drugs and their impact on the HRQoL are lacking. Thus, further studies addressing the long-term impact of these drugs are warranted. This could provide insightful evidence to guide clinicians in using tailored and effective treatments for BC survivors, to finally reduce their fracture risk and improve both HRQoL and long-term outcomes.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
Study design and conceptualization: AS and MI. Databases searching: AS. Data screening: AS, LL, and MI. Data extraction: AS, LL, and MI. Data synthesis and interpretation: AS, LL, and MI. Manuscript drafting: AS and LL. Critical revision: KV, SM, NF, and MI. Visualization: ES, CCu, AA, and CCr. Study supervision: AS and MI. Study submission: AS. All authors read and approved the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank Alberto Patella, MD for his support in this work.
Supplementary Material
The Supplementary Material for this article can be found onlineat: https://www.frontiersin.org/articles/10.3389/fonc.2021.829875/full#supplementary-material
References
1. Iacoviello L, Bonaccio M, de Gaetano G, Donati MB. Epidemiology of Breast Cancer, a Paradigm of the "Common Soil" Hypothesis. Semin Cancer Biol (2021) 72:4–10. doi: 10.1016/j.semcancer.2020.02.010
2. Lima SM, Kehm RD, Terry MB. Global Breast Cancer Incidence and Mortality Trends by Region, Age-Groups, and Fertility Patterns. E Clin Med (2021) 38:100985. doi: 10.1016/j.eclinm.2021.100985
3. de Sire A, Fusco N, Sajjadi E, Lippi L, Cisari C, Invernizzi M. Lymphedema Rehabilitation Using Self-Adaptive Inelastic Compression in Breast Cancer: A Proof-Of-Principle Study. Appl Sci (2021) 11(4):1901. doi: 10.3390/app11041901
4. de Sire A, Losco L, Cigna E, Lippi L, Gimigliano F, Gennari A, et al. Three-Dimensional Laser Scanning as a Reliable and Reproducible Diagnostic Tool in Breast Cancer Related Lymphedema Rehabilitation: A Proof-of-Principle Study. Eur Rev Med Pharmacol Sci (2020) 24(8):4476–85. doi: 10.26355/eurrev_202004_21030
5. Invernizzi M, de Sire A, Lippi L, Venetis K, Sajjadi E, Gimigliano F, et al. Impact of Rehabilitation on Breast Cancer Related Fatigue: A Pilot Study. Front Oncol (2020) 10:556718. doi: 10.3389/fonc.2020.556718
6. de Sire A, Losco L, Cisari C, Gennari A, Boldorini R, Fusco N, et al. Axillary Web Syndrome in Women After Breast Cancer Surgery Referred to an Oncological Rehabilitation Unit: Which Are the Main Risk Factors? A Retrospective Case-Control Study. Eur Rev Med Pharmacol Sci (2020) 24(15):8028–35. doi: 10.26355/eurrev_202008_22486
7. Invernizzi M, Lopez G, Michelotti A, Venetis K, Sajjadi E, De Mattos-Arruda L, et al. Integrating Biological Advances Into the Clinical Management of Breast Cancer Related Lymphedema. Front Oncol (2020) 10:422. doi: 10.3389/fonc.2020.00422
8. Invernizzi M, Kim J, Fusco N. Editorial: Quality of Life in Breast Cancer Patients and Survivors. Front Oncol (2020) 10:620574. doi: 10.3389/fonc.2020.620574
9. de Sire A, Ferrillo M, Gennari A, Cisari C, Pasqua S, Foglio Bonda PL, et al. Bone Health, Vitamin D Status and Oral Hygiene Screening in Breast Cancer Women Before Starting Osteoporosis Treatment: A Cross-Sectional Study. J Biol Regul Homeost Agents (2021) 35(1):397–402. doi: 10.23812/20-686-L
10. Burstein HJ, Temin S, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, et al. Adjuvant Endocrine Therapy for Women With Hormone Receptor-Positive Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline Focused Update. J Clin Oncol (2014) 32(21):2255–69. doi: 10.1200/JCO.2013.54.2258
11. Diana A, Carlino F, Giunta EF, Franzese E, Guerrera LP, Di Lauro V, et al. Cancer Treatment-Induced Bone Loss (CTIBL): State of the Art and Proper Management in Breast Cancer Patients on Endocrine Therapy. Curr Treat Options Oncol (2021) 22(5):45. doi: 10.1007/s11864-021-00835-2
12. Scaturro D, de Sire A, Terrana P, Curci C, Vitagliani F, Falco V, et al. Early Denosumab for the Prevention of Osteoporotic Fractures in Breast Cancer Women Undergoing Aromatase Inhibitors: A Case-Control Retrospective Study. J Back Musculoskelet Rehabil (2021) 1–6. doi: 10.3233/BMR-210012
13. Migliaccio S, de Sire A, Marocci C, Fornari R, Paoletta M, Greco EA, et al. Approach in Aromatase Inhibitors - Induced Osteoporosis: Results From an Italian Multicenter Observational Study. Clin cases Miner Bone Metab (2018) 15(3):334–9.
14. Hadji P, Coleman RE, Wilson C, Powles TJ, Clézardin P, Aapro M, et al. Adjuvant Bisphosphonates in Early Breast Cancer: Consensus Guidance for Clinical Practice From a European Panel. Ann Oncol (2016) 27(3):379–90. doi: 10.1093/annonc/mdv617
15. Kroep JR, Charehbili A, Coleman RE, Aft RL, Hasegawa Y, Winter MC, et al. Effects of Neoadjuvant Chemotherapy With or Without Zoledronic Acid on Pathological Response: A Meta-Analysis of Randomised Trials. Eur J Cancer (2016) 54:57–63. doi: 10.1016/j.ejca.2015.10.011
16. Zhao YY, Xue C, Hou X, Liao H, Li S, Zhao HY, et al. Changes of Bone Resorption Marker (NTX) in Chemotherapy Plus Zoledronic Acid Versus Chemotherapy Alone for Nasopharyngeal Cancer Patients With Bone Metastases. Eur J Cancer (2011) 47(6):848–53. doi: 10.1016/j.ejca.2010.12.004
17. Hadji P, Ziller M, Maskow C, Albert U, Kalder M. The Influence of Chemotherapy on Bone Mineral Density, Quantitative Ultrasonometry and Bone Turnover in Pre-Menopausal Women With Breast Cancer. Eur J Cancer (2009) 45(18):3205–12. doi: 10.1016/j.ejca.2009.09.026
18. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Aromatase Inhibitors Versus Tamoxifen in Early Breast Cancer: Patient-Level Meta-Analysis of the Randomised Trials. Lancet (2015) 386(10001):1341–52. doi: 10.1016/S0140-6736(15)61074-1
19. Senkus E, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rutgers E, et al. Primary Breast Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann Oncol (2015) 26 Suppl 5:v8–30. doi: 10.1093/annonc/mdv298
20. Hadji P. Cancer Treatment-Induced Bone Loss in Women With Breast Cancer. Bonekey Rep (2015) 4:692. doi: 10.1038/bonekey.2015.60
21. Rizzoli R, Body JJ, DeCensi A, Reginster JY, Piscitelli P, Brandi ML, et al. Guidance for the Prevention of Bone Loss and Fractures in Postmenopausal Women Treated With Aromatase Inhibitors for Breast Cancer: An ESCEO Position Paper. Osteoporos Int (2012) 23(11):2567–76. doi: 10.1007/s00198-011-1870-0
22. Cheung AM, Heisey R, Srighanthan J. Breast Cancer and Osteoporosis. Curr Opin Endocrinol Diabetes Obes (2013) 20(6):532–8. doi: 10.1097/01.med.0000436195.10599.dd
23. Paschou SA, Augoulea A, Lambrinoudaki I. Bone Health Care in Women With Breast Cancer. Hormones (Athens) (2020) 19(2):171–8. doi: 10.1007/s42000-019-00164-y
24. Bundred NJ, Campbell ID, Davidson N, DeBoer RH, Eidtmann H, Monnier A, et al. Effective Inhibition of Aromatase Inhibitor-Associated Bone Loss by Zoledronic Acid in Postmenopausal Women With Early Breast Cancer Receiving Adjuvant Letrozole: ZO-FAST Study Results. Cancer (2008) 112(5):1001–10. doi: 10.1002/cncr.23259
25. Gnant M, Mlineritsch B, Schippinger W, Luschin-Ebengreuth G, Pöstlberger S, Menzel C, et al. Endocrine Therapy Plus Zoledronic Acid in Premenopausal Breast Cancer. N Engl J Med (2009) 12;360(7):679–91. doi: 10.1056/NEJMoa0806285
26. Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, et al. Denosumab for Prevention of Fractures in Postmenopausal Women With Osteoporosis. N Engl J Med (2009) 361(8):756–65. doi: 10.1056/NEJMoa0809493
27. Lipton A, Smith MR, Ellis GK, Goessl C. Treatment-Induced Bone Loss and Fractures in Cancer Patients Undergoing Hormone Ablation Therapy: Efficacy and Safety of Denosumab. Clin Med Insights Oncol (2012) 6:287–303. doi: 10.4137/CMO.S8511
28. Lipton A, Fizazi K, Stopeck AT, Henry DH, Brown JE, Yardley DA, et al. Superiority of Denosumab to Zoledronic Acid for Prevention of Skeletal-Related Events: A Combined Analysis of 3 Pivotal, Randomised, Phase 3 Trials. Eur J Cancer (2012) 48(16):3082–92. doi: 10.1016/j.ejca.2012.08.002
29. Livi L, Scotti V, Desideri I, Saieva C, Cecchini S, Francolini G, et al. Phase 2 Placebo-Controlled, Single-Blind Trial to Evaluate the Impact of Oral Ibandronate on Bone Mineral Density in Osteopenic Breast Cancer Patients Receiving Adjuvant Aromatase Inhibitors: 5-Year Results of the Single-Centre BONADIUV Trial. Eur J Cancer (2019) 108:100–10. doi: 10.1016/j.ejca.2018.12.005
30. Yan T, Yin W, Zhou Q, Zhou L, Jiang Y, Du Y, et al. The Efficacy of Zoledronic Acid in Breast Cancer Adjuvant Therapy: A Meta-Analysis of Randomised Controlled Trials. Eur J Cancer (2012) 48(2):187–95. doi: 10.1016/j.ejca.2011.10.021
31. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ (2021) 372:n71. doi: 10.1136/bmj.n71
32. Huang X, Lin J, Demner-Fushman D. Evaluation of PICO as a Knowledge Representation for Clinical Questions. AMIA Annu Symp Proc (2006) 2006:359–63.
33. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the Quality of Reports of Randomized Clinical Trials: Is Blinding Necessary? Control Clin Trials (1996) 17(1):1–12. doi: 10.1016/0197-2456(95)00134-4
34. Gnant M, Pfeiler G, Dubsky PC, Hubalek M, Greil R, Jakesz R, et al. Adjuvant Denosumab in Breast Cancer (ABCSG-18): A Multicentre, Randomised, Double-Blind, Placebo-Controlled Trial. Lancet (2015) 386(9992):433–43. doi: 10.1016/S0140-6736(15)60995-3
35. Gnant M, Pfeiler G, Steger GG, Egle D, Greil R, Fitzal F, et al. Adjuvant Denosumab in Postmenopausal Patients With Hormone Receptor-Positive Breast Cancer (ABCSG-18): Disease-Free Survival Results From a Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Oncol (2019) 20(3):339–51. doi: 10.1016/S1470-2045(18)30862-3
36. Hines SL, Mincey B, Dentchev T, Sloan JA, Perez EA, Johnson DB, et al. Immediate Versus Delayed Zoledronic Acid for Prevention of Bone Loss in Postmenopausal Women With Breast Cancer Starting Letrozole After Tamoxifen-N03CC. Breast Cancer Res Treat (2009) 117(3):603–9. doi: 10.1007/s10549-009-0332-2
37. Wagner-Johnston ND, Sloan JA, Liu H, Kearns AE, Hines SL, Puttabasavaiah S, et al. 5-Year Follow-Up of a Randomized Controlled Trial of Immediate Versus Delayed Zoledronic Acid for the Prevention of Bone Loss in Postmenopausal Women With Breast Cancer Starting Letrozole After Tamoxifen: N03CC (Alliance) Trial. Cancer (2015) 121(15):2537–43. doi: 10.1002/cncr.29327
38. Greenspan SL, Vujevich KT, Brufsky A, Lembersky BC, van Londen GJ, Jankowitz RC, et al. Prevention of Bone Loss With Risedronate in Breast Cancer Survivors: A Randomized, Controlled Clinical Trial. Osteoporos Int (2015) 26(6):1857–64. doi: 10.1007/s00198-015-3100-7
39. Coleman R, de Boer R, Eidtmann H, Llombart A, Davidson N, Neven P, et al. Zoledronic Acid (Zoledronate) for Postmenopausal Women With Early Breast Cancer Receiving Adjuvant Letrozole (ZO-FAST Study): Final 60-Month Results. Ann Oncol (2013) 24(2):398–405. doi: 10.1093/annonc/mds277
40. Rhee Y, Song K, Park S, Park HS, Lim SK, Park BW. Efficacy of a Combined Alendronate and Calcitriol Agent (Maxmarvil®) in Korean Postmenopausal Women With Early Breast Cancer Receiving Aromatase Inhibitor: A Double-Blind, Randomized, Placebo-Controlled Study. Endocr J (2013) 60(2):167–72. doi: 10.1507/endocrj.ej12-0283
41. Lester JE, Dodwell D, Purohit OP, Gutcher SA, Ellis SP, Thorpe R, et al. Prevention of Anastrozole-Induced Bone Loss With Monthly Oral Ibandronate During Adjuvant Aromatase Inhibitor Therapy for Breast Cancer. Clin Cancer Res (2008) 14(19):6336–42. doi: 10.1158/1078-0432.CCR-07-5101
42. Lester JE, Dodwell D, Brown JE, Purohit OP, Gutcher SA, Ellis SP, et al. Prevention of Anastrozole Induced Bone Loss With Monthly Oral Ibandronate: Final 5 Year Results From the ARIBON Trial. J Bone Oncol (2012) 1(2):57–62. doi: 10.1016/j.jbo.2012.06.002
43. Takahashi S, Iwase T, Kohno N, Ishikawa T, Taguchi T, Takahashi M, et al. Efficacy of Zoledronic Acid in Postmenopausal Japanese Women With Early Breast Cancer Receiving Adjuvant Letrozole: 12-Month Results. Breast Cancer Res Treat (2012) 133(2):685–93. doi: 10.1007/s10549-012-1973-0
44. Llombart A, Frassoldati A, Paija O, Sleeboom HP, Jerusalem G, Mebis J, et al. Immediate Administration of Zoledronic Acid Reduces Aromatase Inhibitor-Associated Bone Loss in Postmenopausal Women With Early Breast Cancer: 12-Month Analysis of the E-ZO-FAST Trial. Clin Breast Cancer (2012) 12(1):40–8. doi: 10.1016/j.clbc.2011.08.002
45. Van Poznak C, Hannon RA, Mackey JR, Campone M, Apffelstaedt JP, Clack G, et al. Prevention of Aromatase Inhibitor-Induced Bone Loss Using Risedronate: The SABRE Trial. J Clin Oncol (2010) 28(6):967–75. doi: 10.1200/JCO.2009.24.5902
46. Markopoulos C, Tzoracoleftherakis E, Polychronis A, Venizelos B, Dafni U, Xepapadakis G, et al. Management of Anastrozole-Induced Bone Loss in Breast Cancer Patients With Oral Risedronate: Results From the ARBI Prospective Clinical Trial. Breast Cancer Res (2010) 12(2):R24. doi: 10.1186/bcr2565
47. Eidtmann H, de Boer R, Bundred N, Llombart-Cussac A, Davidson N, Neven P, et al. Efficacy of Zoledronic Acid in Postmenopausal Women With Early Breast Cancer Receiving Adjuvant Letrozole: 36-Month Results of the ZO-FAST Study. Ann Oncol (2010) 21(11):2188–94. doi: 10.1093/annonc/mdq217
48. Brufsky AM, Bosserman LD, Caradonna RR, Haley BB, Jones CM, Moore HC, et al. Zoledronic Acid Effectively Prevents Aromatase Inhibitor-Associated Bone Loss in Postmenopausal Women With Early Breast Cancer Receiving Adjuvant Letrozole: Z-FAST Study 36-Month Follow-Up Results. Clin Breast Cancer (2009) 9(2):77–85. doi: 10.3816/CBC.2009.n.015
49. Ellis GK, Bone HG, Chlebowski R, Paul D, Spadafora S, Smith J, et al. Randomized Trial of Denosumab in Patients Receiving Adjuvant Aromatase Inhibitors for Nonmetastatic Breast Cancer. J Clin Oncol (2008) 26(30):4875–82. doi: 10.1200/JCO.2008.16.3832
50. Brufsky AM, Harker WG, Beck JT, Bosserman L, Vogel C, Seidler C, et al. Final 5-Year Results of Z-FAST Trial: Adjuvant Zoledronic Acid Maintains Bone Mass in Postmenopausal Breast Cancer Patients Receiving Letrozole. Cancer (2012) 118(5):1192–201. doi: 10.1002/cncr.26313
51. Safra T, Bernstein-Molho R, Greenberg J, Pelles-Avraham S, Stephansky I, Sarid D, et al. The Protective Effect of Zoledronic Acid on Bone Loss in Postmenopausal Women With Early Breast Cancer Treated With Sequential Tamoxifen and Letrozole: A Prospective, Randomized, Phase II Trial. Oncology (2011) 81(5-6):298–305. doi: 10.1159/000334456
52. Brufsky A, Bundred N, Coleman R, Lambert-Falls R, Mena R, Hadji P, et al. Z-FAST and ZO-FAST Study Groups. Integrated Analysis of Zoledronic Acid for Prevention of Aromatase Inhibitor-Associated Bone Loss in Postmenopausal Women With Early Breast Cancer Receiving Adjuvant Letrozole. Oncologist (2008) 13(5):503–14. doi: 10.1634/theoncologist.2007-0206
53. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the Quality of Reports of Randomized Clinical Trials: Is Blinding Necessary? Control Clin Trials (1996) 17(1):1–12. doi: 10.1016/0197-2456(95)00134-4
54. Kelly RR, McDonald LT, Jensen NR, Sidles SJ, LaRue AC. Impacts of Psychological Stress on Osteoporosis: Clinical Implications and Treatment Interactions. Front Psychiatry (2019) 10:200. doi: 10.3389/fpsyt.2019.00200
55. Moretti A, de Sire A, Curci C, Toro G, Gimigliano F, Iolascon G. Effectiveness of Denosumab on Back Pain-Related Disability and Quality-of-Life in Patients With Vertebral Fragility Fractures. Curr Med Res Opin (2019) 35(1):151–5. doi: 10.1080/03007995.2018.1545636
56. Invernizzi M, de Sire A, Venetis K, Cigna E, Carda S, Borg M, et al. Quality of Life Interventions in Breast Cancer Survivors: State of the Art in Targeted Rehabilitation Strategies. Anticancer Agents Med Chem (2021). doi: 10.2174/1871520621666210609095602
57. Coleman R, Finkelstein DM, Barrios C, Martin M, Iwata H, Hegg R, et al. Adjuvant Denosumab in Early Breast Cancer (D-CARE): An International, Multicentre, Randomised, Controlled, Phase 3 Trial. Lancet Oncol (2020) 21(1):60–72. doi: 10.1016/S1470-2045(19)30687-4
58. von Minckwitz G, Möbus V, Schneeweiss A, Huober J, Thomssen C, Untch M, et al. German Adjuvant Intergroup Node-Positive Study: A Phase III Trial to Compare Oral Ibandronate Versus Observation in Patients With High-Risk Early Breast Cancer. J Clin Oncol (2013) 31(28):3531–9. doi: 10.1200/JCO.2012.47.2167
59. Coleman RE, Collinson M, Gregory W, Marshall H, Bell R, Dodwell D, et al. Benefits and Risks of Adjuvant Treatment With Zoledronic Acid in Stage II/III Breast Cancer. 10 Years Follow-Up of the AZURE Randomized Clinical Trial (BIG 01/04). J Bone Oncol (2018) 13:123–35. doi: 10.1016/j.jbo.2018.09.008
60. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Adjuvant Bisphosphonate Treatment in Early Breast Cancer: Meta-Analyses of Individual Patient Data From Randomised Trials. Lancet (2015) 386(10001):1353–61. doi: 10.1016/S0140-6736(15)60908-4
61. Hadji P, Aapro MS, Body JJ, Gnant M, Brandi ML, Reginster JY, et al. Management of Aromatase Inhibitor-Associated Bone Loss (AIBL) in Postmenopausal Women With Hormone Sensitive Breast Cancer: Joint Position Statement of the IOF, CABS, ECTS, IEG, ESCEO IMS, and SIOG. J Bone Oncol (2017) 7:1–12. doi: 10.1016/j.jbo.2017.03.001
62. Dhesy-Thind S, Fletcher GG, Blanchette PS, Clemons MJ, Dillmon MS, Frank ES, et al. Use of Adjuvant Bisphosphonates and Other Bone-Modifying Agents in Breast Cancer: A Cancer Care Ontario and American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol (2017) 20;35(18):2062–81. doi: 10.1200/JCO.2016.70.7257
63. National Guideline Alliance (UK). Early and Locally Advanced Breast Cancer: Diagnosis and Management. London: National Institute for Health and Care Excellence (UK (2018).
64. Coleman R, Hadji P, Body JJ, Santini D, Chow E, Terpos E, et al. Electronic Address:Q2xpbmljYWxndWlkZWxpbmVzQEVzbW8uT3Jn. Bone Health in Cancer: ESMO Clinical Practice Guidelines. Ann Oncol (2020) 31(12):1650–63. doi: 10.1016/j.annonc.2020.07.019
65. Dhesy-Thind S, Fletcher GG, Blanchette PS, Clemons MJ, Dillmon MS, Frank ES, et al. Use of Adjuvant Bisphosphonates and Other Bone-Modifying Agents in Breast Cancer: A Cancer Care Ontario and American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol (2017) 35(18):2062–81. doi: 10.1200/JCO.2016.70.7257
Keywords: breast cancer, early breast cancer, bone health, quality of life, osteoporosis, rehabilitation
Citation: de Sire A, Lippi L, Venetis K, Morganti S, Sajjadi E, Curci C, Ammendolia A, Criscitiello C, Fusco N and Invernizzi M (2022) Efficacy of Antiresorptive Drugs on Bone Mineral Density in Post-Menopausal Women With Early Breast Cancer Receiving Adjuvant Aromatase Inhibitors: A Systematic Review of Randomized Controlled Trials. Front. Oncol. 11:829875. doi: 10.3389/fonc.2021.829875
Received: 06 December 2021; Accepted: 30 December 2021;
Published: 21 January 2022.
Edited by:
Julio de la Torre, Comillas Pontifical University, SpainReviewed by:
Silvia Migliaccio, Foro Italico University of Rome, ItalyRichard De Boer, Peter MacCallum Cancer Centre, Australia
Copyright © 2022 de Sire, Lippi, Venetis, Morganti, Sajjadi, Curci, Ammendolia, Criscitiello, Fusco and Invernizzi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alessandro de Sire, YWxlc3NhbmRyby5kZXNpcmVAdW5pY3ouaXQ=
 Alessandro de Sire
Alessandro de Sire Lorenzo Lippi
Lorenzo Lippi Konstantinos Venetis
Konstantinos Venetis Stefania Morganti
Stefania Morganti Elham Sajjadi
Elham Sajjadi Claudio Curci
Claudio Curci Antonio Ammendolia
Antonio Ammendolia Carmen Criscitiello4,5
Carmen Criscitiello4,5 Nicola Fusco
Nicola Fusco Marco Invernizzi
Marco Invernizzi