
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 05 January 2022
Sec. Cancer Genetics
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.810301
Background: Lung cancer is the leading cause of cancer-related mortality worldwide. Although cigarette smoking is an established risk factor for lung cancer, few reliable smoking-related biomarkers for non-small-cell lung cancer (NSCLC) are available. An improved understanding of these biomarkers would further the development of new biomarker-targeted therapies and lead to improvements in overall patient survival.
Methods: We performed bioinformatic analysis to screened potential target genes, then quantitative PCR, western, siRNA, CCK-8, flow cytometry, tumorigenicity assays in nude mice were performed to validated the function.
Results: In this study, we identified 83 smoking-related genes (SRGs) based on an integration analysis of two Gene Expression Omnibus (GEO) datasets, and 27 hub SRGs with potential carcinogenic effects by analyzing a dataset of smokers with NSCLC in The Cancer Genome Atlas (TCGA) database. A survival analysis revealed three genes with potential prognostic value, namely SRXN1, KRT6A and JAKMIP3. A univariate Cox analysis revealed significant associations of elevated SRXN1 and KRT6A expression with prognosis. A receiver operating characteristic (ROC) curve analysis indicated the high diagnostic value of SRXN1 and KRT6A for smoking and cancer. Quantitative PCR and western blotting validated the increased expression of SRXN1 and KRT6A mRNA and protein, respectively, in lung cancer cell lines and NSCLC tissues. In patients with NSCLC, SRXN1 and KRT6A expression was associated with the tumor–node–metastasis (TNM) stage, presence of metastasis, history of smoking and daily smoking consumption. Furthermore, inhibition of SRXN1 or KRT6A suppressed viability and enhanced apoptosis in the A549 human lung carcinoma cell line. Tumorigenicity assays in nude mice confirmed that the siRNA-mediated downregulation of SRXN1 and KRT6A expression inhibited tumor growth in vivo.
Conclusions: In summary, SRXN1 and KRT6A act as oncogenes in NSCLC and might be potential biomarkers of smoking exposure and the early diagnosis and prognosis of NSCLC in smokers, which is vital for lung cancer therapy.
Lung cancer is currently a leading cause of death in both men and women worldwide, and the combined lung cancer-related death rate exceeds that of the three most common incident cancers (colon, breast and pancreatic) combined. NSCLC accounts for approximately 84% of lung cancer diagnoses; at diagnosis, approximately 57% of these tumors have metastasized, 22% have spread to regional lymph nodes and 16% remain localized (1). Clinically, only a small proportion of NSCLC patients are diagnosed at an early stage (stage І or П), when the tumor can be treated by surgical resection (2).
The status of lung cancer as one of the most common causes of cancer death persists, despite an understanding of the major etiology. Epidemiological studies have identified cigarette smoking as the most important risk factor for lung cancer, and 80% and 50% of lung cancers in male and female patients, respectively, are associated with cigarette smoking. Particularly, smoking is associated with a 5- to 10-fold increase in the risk of lung cancer, and this relationship exhibits a clear dose–response pattern (3). In other words, an increase in the number of smoking years or the number of packs smoked per day increases the degree of lung cancer risk (4). Additionally, exposure to environmental tobacco smoke increases the lung cancer risk by approximately 20% among non-smokers (3). In summary, the vast majority of lung cancers occur in people aged >50 years with a history of cigarette smoking (5), whereas only 10–15% of cases involve non-smokers (6). An understanding of the epidemiology and causal factors of lung cancer can provide an additional foundation for disease prevention.
Although most countries have long made efforts to reduce tobacco consumption and exposure, the 2015 global report on trends in the prevalence of tobacco smoking by the World Health Organization revealed that there are 1.1 billion adult smokers and at least 367 million smokeless tobacco users worldwide, and that more than 6 million people die from tobacco-related causes every year (7). Today, passive exposure to tobacco smoke (i.e., passive smoking) is recognized as an important environmental risk factor for the development of lung cancer, asthma and fetal growth (8–10). For example, one meta-analysis and comprehensive review determined that the relative risk of developing lung cancer from passive smoking ranged from 1.14 to 5.20 among people who had never smoked but lived with a smoker (11). According to the U.S. Surgeon General, living with a smoker can increase a non-smoker’s risk of developing lung cancer by approximately 20–30% (12). In China, approximately 16% of lung cancer cases among never-smokers are potentially attributable to passive smoking (13).
Globally, smoking is a major public health problem, and exposure to environmental tobacco smoke (ETS) affects both smokers and passive smokers. ETS is known as an important source of multiple pollutants, and 73 of the more than 5,000 compounds identified in cigarette smoke are considered carcinogenic to either laboratory animals or humans by the International Agency for Research on Cancer (14, 15). Although many components of tobacco smoke contribute to lung cancer, nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) is a key ingredient with a major role in carcinogenesis (16). Studies on tobacco smoke have confirmed NNK as the most potent lung carcinogen and, to date, it is the only tobacco carcinogen that systemically induces lung tumors in rats, mice and hamsters (i.e., three of the most common rodent models) (17).
The relevance of NNK in triggering lung tumorigenesis has been described consistently and confirmed in numerous studies. It not only mutates or activates oncogenes and tumor suppressor genes such as Adrb2, Kras, Tp53 and Txa2 (18–21), but also induces hypermethylation of the promoters of multiple tumor suppressor genes. Specifically, DNMT1 is overexpressed in the lung, and the resulting hypermethylation reduces the expression of tumor suppressor genes such as IGFBP-3, CDKN2A, death-associated protein kinase 1 (Dapk1), retinoic acid receptor β (Rar-β) and runt-related transcription factor 3 (Runx) (22–25). Another study suggested that NNK stimulated the Erk signaling pathway and induced cell transformation and proliferation via the epidermal growth factor receptor signaling pathway (26). Furthermore, NNK can significantly stimulate thromboxane synthase activity and enhance LUSC generation, as indicated by the upregulated expression of CD133 and ALDH1A1 and increases in the tumor sphere number and size (27). NNK also promotes the expression of LCSC-related molecules, including β-catenin and Nanog (27). Therefore, NNK, as the dominant carcinogen in tobacco smoke, is the key factor in smoking-related lung cancer, and it is necessary to disrupt the key mechanisms linking its effects with lung cancer development.
This study aimed to identify novel biomarkers for the early diagnosis of smoking-related NSCLC. To address this issue, we extracted data from GEO and TCGA databases and compared the differentially expressed genes (DEGs) between lung cancer and normal tissues from smoking and non-smoking subjects. Notably, we identified SRXN1 and KRT6A as pivotal smoking-related genes (SRGs) with important roles in lung cancer carcinogenesis through subsequent experimental validation in vitro and in vivo.
The raw data and clinical information of healthy participants were downloaded from the GEO (https://www.ncbi.nlm.nih.gov/geo/). Gene expression profiling of the GSE18385 (28) and GSE76324 (29) datasets was conducted using the GPL570 platform (Affymetrix Human Genome U133 plus 2.0 Array). The GSE18385 series comprised samples from 72 never-smokers and 89 smokers, and the GSE76324 series comprised samples from 97 never-smokers and 120 smokers. Samples with >20% missing expression values were excluded. Finally, 60 and 84 samples from never-smokers and smokers, respectively, in the GSE18385 dataset and 83 and 76 samples from never-smokers and smokers, respectively, in the GSE76324 dataset were enrolled. The processing of mRNA-seq data was based on the normalized chip values from the pre-processed data that were subjected to log2 transformation and submitted for analysis.
The raw data and clinical information of currently smoking patients with NSCLC were downloaded from TCGA (https://cancergenome.nih.gov). Supplementary Table S1 summarizes the clinical characteristics of 256 current smoking patients with NSCLC up to September 2019. Of these patients, 226 patients whose clinical files unambiguously indicated the smoking status were selected based on the described neoplastic and histological information. Patients with a previous history of cancer and whose tissues were fixed with formalin and embedded in paraffin were excluded. The samples with >20% missing expression values were excluded. The processing of mRNA-seq data was based on the normalized count reads from pre-processed data subjected to log2 transformation after adding a 0.5 pseudocount.
After data pre-processing, the differential gene expression analysis was conducted by using the limma and DESeq2 packages in R (v. 3.6; R Project for Statistical Computing, Vienna, Austria) (Love et al., 2014). An absolute log2 fold change value >1 and cut-off value of 0.05 were applied for the both raw P-value and Benjamini–Hochberg (BH) adjusted P-value (Benjamini and Hochberg, 1995). Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses were conducted using the DAVID platform (https://david.ncifcrf.gov/). KEGG pathways with P-values <0.05 were selected for bar chart in R.
For gene expression and patient survival analyses, the lung cancer patients were classified into high and low gene expression groups according to the median expression value. We used the survival and survMine tools on the R platform to conduct the survival analysis and generate the Kaplan–Meier (KM) survival plots. We additionally performed a univariate Cox regression analysis and log-odd tests for genes in the context of significant difference in patient survival in the high and low expression group. Hazard ratios (HRs) >1 and <1 indicated low and high rates of survival, respectively, among patients with high expression of the target gene.
We conducted a logistic regression using the lm function in R to classify the samples from smokers and never-smokers based on the gene expression data. Similarly, we used the same way to classify the tumor and normal samples on the basis of their gene expression data. The R package ROCR was used to assess the logistic regression performance, plot receiver operating characteristic (ROC) curves and calculate the areas under the curves (AUC).
Seventy-five pairs of NSCLC and normal adjacent non-tumor tissue samples were obtained by surgical resection between August 2017 and June 2019 at the General Hospital of Southern Theater Command (Guangzhou, China). Patients who received preoperative treatments such as radiation or chemotherapy were excluded. The paired adjacent non-tumor tissues were sampled at a distance of 3 cm from the tumor. Both the cancer tissues and matched normal tissues were histologically confirmed. All tissues were snap-frozen in liquid nitrogen and stored at -80°C. The clinicopathological characteristics of the 75 NSCLC patients, including sex, age, smoking history, daily smoking consumption, histology, TNM stage, tumor size, lymph node stage and distant metastasis status, are summarized in Supplementary Table S2. All clinicopathological data were obtained from the patients’ clinical and pathologic records.
The study protocol was approved by the Research Ethics Committee of Guangzhou Medical University and the General Hospital of Southern Theater Command. Written informed consent was obtained from all patients.
The human bronchial epithelial cell line Beas-2B and the NSCLC cell lines A549 and 95D were obtained from the Chinese Academy of Sciences’ typical culture preservation committee cell bank (Shanghai, China). Beas-2B cells were induced to undergo malignant transformation by exposure to NNK in our laboratory as previous report (designated as BEAS-2B-NNK) (30). Beas-2B and BEAS-2B-NNK cells were cultured in bronchial epithelial basal medium (BEBM, Clonetics/Lonza, Basel, Switzerland) supplemented with SingleQuots (Clonetics). A549 and 95D cells were cultured respectively in F-12 Kaighn’s Modification (HyClone, Logan, UT, USA) and RPMI-1640 (HyClone) media supplemented with 10% fetal bovine serum (FBS; Gibco, Thermo Fisher Scientific, Inc., Chicago, IL, USA). All cells were cultured at 37°C in a humidified incubator in an atmosphere of 95% air and 5% CO2.
Total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. Next, cDNA was prepared from total RNA via reverse-transcription (RT), using the GoScript Reverse Transcription System (Promega, Madison, WI, USA) according to the manufacturer’s instructions. Quantitative real-time PCR (qPCR) was performed on an Applied Biosystems™ 7500 Fast Dx Real-Time PCR Instrument (Foster City, CA, USA) with GoTaq qPCR Master Mix (Promega, Madison, WI, USA) according to the following program: denaturation, 95°C for 10 min and 36 cycles of denaturation at 95°C for 25 s, annealing at 60°C for 1 min and extension at 72°C for 30 s. The expression data were calculated using the 2-ΔΔCt method. The results were normalized to the expression of the housekeeping gene, GAPDH. All primers were synthesized by Invitrogen (Shanghai, China). The primer sequences for RT-qPCR were shown in Table 1.
Cells were harvested, lysed with RIPA buffer (Beyotime, Jiangsu, China) on ice for 30 min and centrifuged at 14,000 rpm and 4°C for 20 min. The total protein concentrations in the lysates were quantified using a BCA protein assay kit (Beyotime). The proteins were then separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to PVDF membranes (Millipore, Burlington, MA, USA). The membranes were blocked in buffer containing 5% skim milk and 0.1% Tween-20 and then incubated overnight at 4°C with the primary antibodies specific for SRXN1 (Abcam, Cambridge, UK) or KRT6A (Cell Signaling Technology, Danvers, MA, USA). β-Actin (Abcam) was used as the protein loading control. The membranes were then washed with (Tris-buffered saline with 0.1% Tween-20) TBST for 10 min, and incubated for 1 h at room temperature with the appropriate secondary antibody: IRDye 800 CW-conjugated anti-rabbit IgG (Li-Cor, Lincoln, NE, USA). After washing with TBST, the labeled protein bands on the membranes were quantified using an Odyssey infrared Imaging System (Li-Cor).
Small interfering RNAs (siRNAs) were designed to knock down the expression of SRXN1 and KRT6A. In this experiment, the siRNA molecules were synthesized by Ribobio Co., Ltd. (Guangzhou, China) as double-stranded RNA oligonucleotides with proprietary chemical modifications. The target sequences of the six siRNAs used to target SRXN1 and KRT6A were as follows:
si-SRXN1-1: GGAGGTGACTACTTCTACT
si-SRXN1-2: CGATGTCCTCTGGATCAAA
si-SRXN1-3: CAGACCTAAGGGTGTACCT
si-KRT6A-1: GAGGAGATTGCTCAGAGAA
si-KRT6A-2: CCAGCAGGAAGAGCTATAA
si-KRT6A-3: TGCCAAGAACAAGCTGGAA
The sense strand of each siRNA is the RNA transcribed from target sequences, and the antisense strand is the complementary RNA of the target sequence. A dTdT sequence was added to the end of each strand to achieve siRNA stability.
Cells were cultured in 6-well plates to 50–60% confluency and then transfected with siRNAs using Lipofectamine® 2000 Reagent (Thermo Fisher, Inc., Chicago, IL, USA) according to the manufacturer’s instructions. Total RNA was isolated from the cells at 48 h post-transfection and subjected to qRT-PCR to measure the efficiency of siRNA-based interference. These cells were also harvested and subjected to western blotting, Cell Counting Kit-8 (CCK-8) assays, flow cytometric analyses and tumorigenicity assays in nude mice.
Cell proliferation was assessed using CCK-8 (Dojindo Laboratories, Tokyo, Japan) according to the manufacturer’s protocol. In brief, approximately 5.0 × 103 cells were plated into each well of a 96-well plate, followed by incubation for 0, 24, 48 or 72 h. At the indicated time point, 10 μL of CCK-8 solution was added to each well, followed by another 1 h incubation at 37°C. The cell proliferation curves were plotted by measuring the absorbance at 450 nm in each well at each indicated time point. Three wells were used per experimental condition, and all experiments were performed in triplicate.
Apoptosis was assessed using the Annexin V-PE/7AAD Apoptosis Detection Kit (KeyGen Biotech, Nanjing, China). Briefly, cells were harvested using EDTA-free trypsin, washed twice with ice-cold phosphate-buffered saline (PBS), collected, and counted. Next, 50 μL of binding buffer and 5 μL of 7-AAD were added to aliquots of 1 × 105 cells in flow cytometry tubes, which were then incubated for 10 min in the dark. Then, 450 μL of Binding Buffer and 1 μL of Annexin V-PE were added to each tube. After a 10 min incubation in the dark, the cells were analyzed on a CytoFLEX Cytometer (Beckman-Coulter, CA, USA).
BALB/c nude mice (age, 4–5 weeks; body weight, 20–25 g) were obtained from Guangdong Medical Laboratory Animal Center (Guangzhou, China). All experimental procedures involving animals were in accordance with the Guide for the Care and Use of Laboratory Animals and the institutional ethical guidelines for experiments involving animals. A549 cells in the logarithmic growth phase were collected for inoculation. Mice were randomly divided into 3 groups to test the roles of SRXN1 and KRT6A: control, negative control (NC) and siRNA (3 groups for each gene, giving 6 groups in total; n = 6 per group, including 3 male and 3 female). Briefly, 2.0 × 106 cells were suspended in 200 µL of a 1:1 mixture of Matrigel® Basement Membrane Matrix High Concentration (Corning, NY, USA) and complete medium, and then injected into the right lower groin of each mouse. The length (a) and width (b) of each xenograft tumor were measured every 4 days with a vernier caliper. Tumor volumes (mm3) were calculated using the following formula: volume = ab2/2. Mice were sacrificed 4 weeks after inoculation by cervical dislocation, and the tumors were excised, weighed and subjected to an immunohistochemical (IHC) analysis.
Histological sections of the tumor xenografts were excised, fixed in 4% paraformaldehyde for 24 h and embedded in paraffin. Subsequently, 5-µm tissue sections were used in a diagnostic examination of tumor pathology. First, the paraffinized sections were dewaxed by washing with xylene, absolute ethyl alcohol, 75% alcohol and distilled water in sequence. Next, hematoxylin–eosin (H&E) staining of some sections was performed to visualize morphological alterations. Other dewaxed sections were put in to microwave oven, BSA seal. After examination under a microscope, the sections were incubated with a primary antibody specific for Ki-67 (dilution, 1:100; Abcam) overnight at 4°C, and subsequently with a secondary antibody labeled with horseradish peroxidase (Beyotime) for 1 h at 37°C. The DAB Horseradish Peroxidase Color Development Kit (Beyotime) and TMB chromogenic substrate were used to enable the peroxidase-catalyzed final brown coloration of the labeled areas.
All statistical analyses were performed using the SPSS version 25 software package (SPSS, Inc., Chicago, IL, USA). Values are expressed as means ± standard deviations . Continuous variables are displayed as means and standard deviations, whereas categorical variables are presented as proportions. In univariate analyses, the qualitative variables were compared using a t-test (or non-parametric test when necessary). The mean values of quantitative variables were compared using Student’s t-test, and differences between the groups were analyzed using an ANOVA. The Shapiro–Wilk and Levene tests were used to test the normality of distribution of the residuals and the homogeneity of variances, respectively. The Wilcoxon–Mann–Whitney test was performed when the basic assumptions of Student’s t-test were not satisfied. A P-value <0.05 was considered to indicate a statistically significant difference.
As shown in the flowchart in Figure 1, two training cohorts of healthy human participants, GSE18385 and GSE76324, were downloaded from the GEO database for the differential gene expression analysis. Using the cut-off criteria of an adjusted P-value <0.05 and |log2FC| >1, 83 SRGs were detected by overlapping the differential expression profiles of the two cohorts (Figures 2A–C). As shown in Figures 2D, E, 62 and 21 of these SRGs were upregulated and downregulated, respectively.
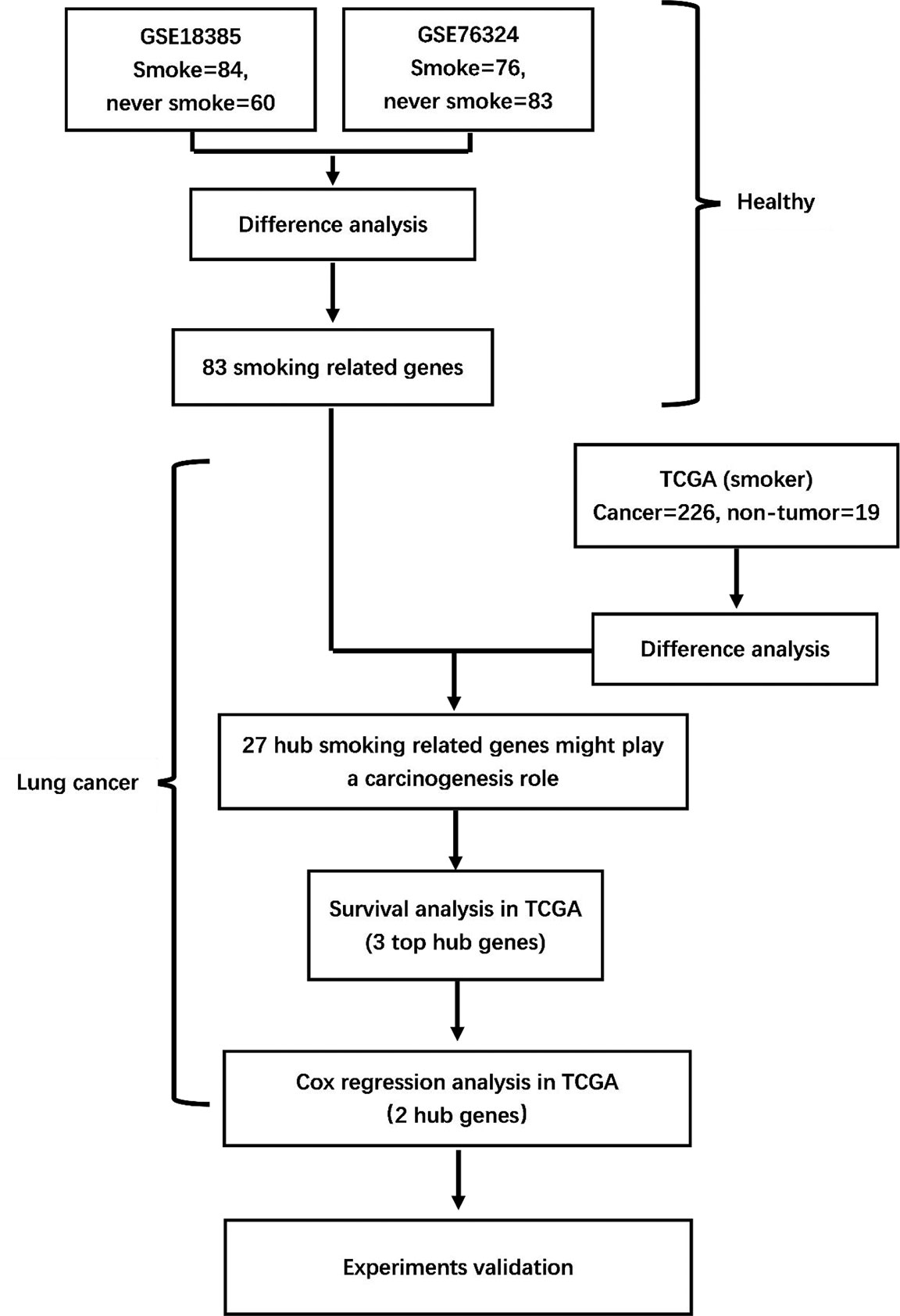
Figure 1 Flowchart of the study. GSE18385 and GSE76324, Gene Expression Omnibus cohorts; TCGA, The Cancer Genome Atlas.
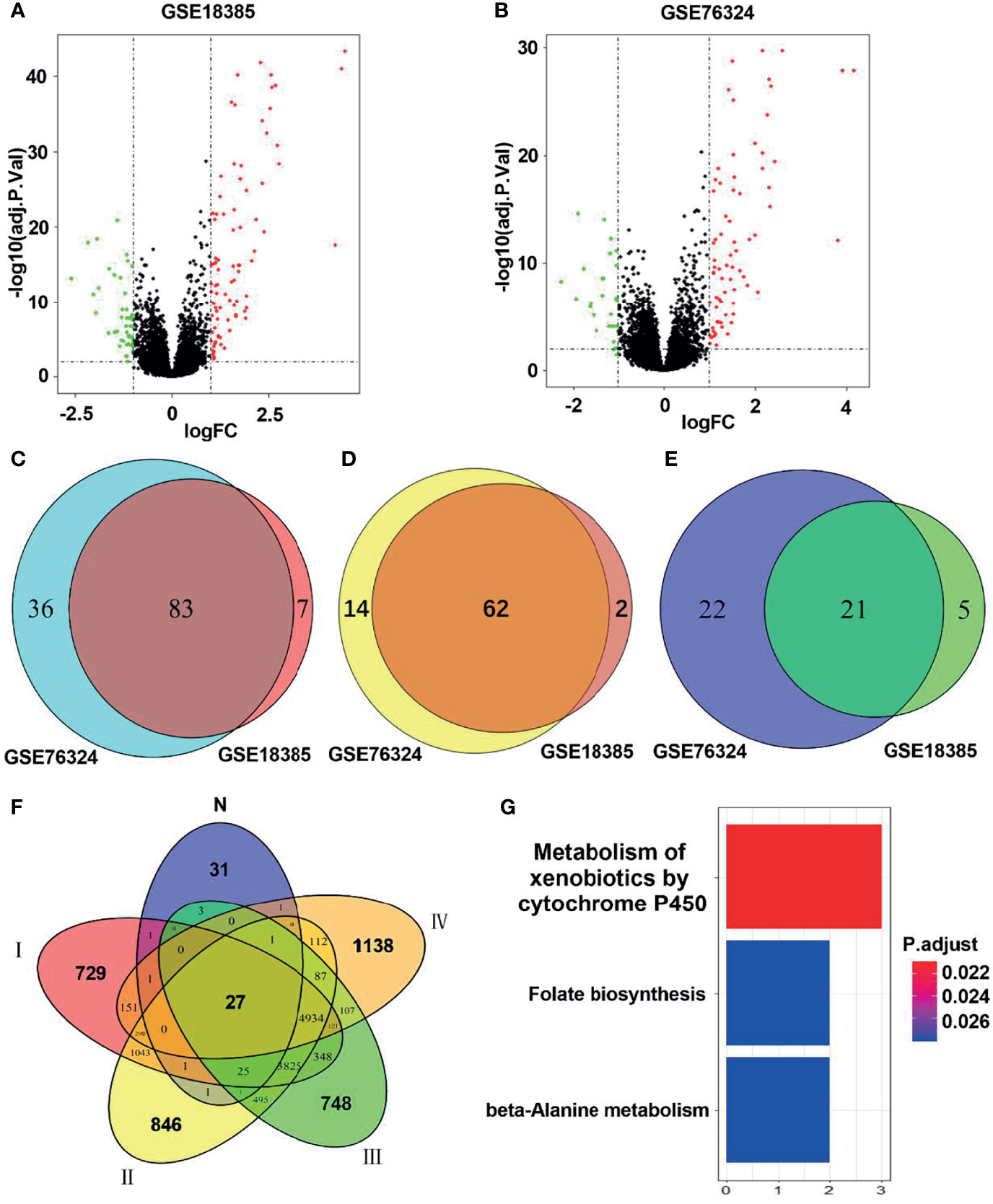
Figure 2 Identification of hub smoking-related genes (SRGs). (A, B) Volcano plots of differentially expressed genes in the GSE18385 and GSE76324 cohorts. Log2 (FC) vs. -log10 (adj.P.Val) for differentially expressed genes. Red and blue dots represent upregulated and downregulated genes, respectively (log2|FC| > 1, adj.P.Val < 0.05). (C) Venn diagram of SRGs in GSE18385 and GSE76324. (D) Venn diagram of upregulated differentially expressed genes in GSE18385 and GSE76324. (E) Venn diagram of downregulated differentially expressed genes in GSE18385 and GSE76324. (F) Venn diagrams plotted to showcase overlaps between the 83 SRGs and TCGA dataset. N represents 83 SRGs from healthy smokers, while I, II, III and IV represent clinical stages I, II, III and IV among smokers with lung cancer, respectively. (G) Significantly enriched Kyoto Encyclopedia of Genes and Genomes pathways corresponding to the 27 hub SRGs.
To identify hub SRGs that might contribute to lung cancer development, we evaluated the expression of these 83 SRGs in lung cancers, using data from TCGA. We identified potential oncogenes and tumor suppressor genes by calculating the differential gene expression between non-tumor samples and samples of each clinical stage of NSCLC. We identified 27 hub SRGs that were significantly correlated with lung cancer development through our integrated analysis of the 83 SRGs and the differentially expressed genes identified from TCGA (Figure 2F). As shown in Table 2, 19 of these 27 hub SRGs exhibited upregulated expression in smokers, with further increases observed in smokers with NSCLC, whereas 7 exhibited downregulated expression in smokers and were further reduced in smokers with NSCLC. Only KLHDC8A was downregulated by smoking but upregulated in smokers with NSCLC. A KEGG analysis indicated that the 27 hub SRGs were significantly enrichened in the pathways of “metabolism of xenobiotics by cytochrome P450” and “folate biosynthesis and beta-alanine metabolism” (Figure 2G). All of these results indicate that these 27 SRGs might be the key genes contributing to smoking-related lung cancer.
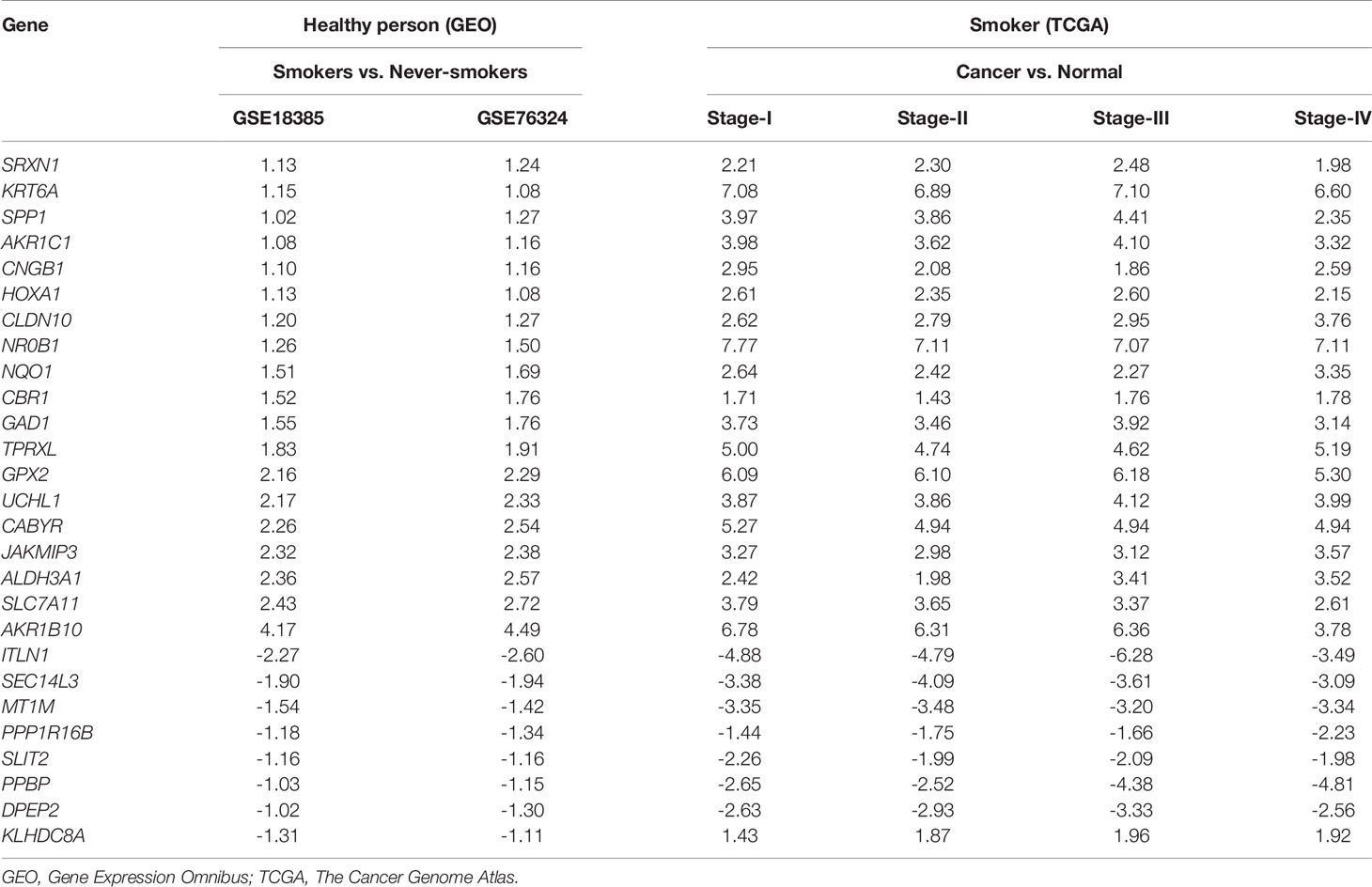
Table 2 Differential expression levels of 27 hub smoking-related genes between healthy smokers and those with lung cancer.
To identify the potential prognostic value of these 27 hub SRGs, we evaluated the relationship between the expression of each SRG and overall survival (OS) among smokers with NSCLC. These patients were stratified into high and low expression groups according to the median expression of each SRG. The results of a univariate survival analysis demonstrated that the expression of SRXN1, KRT6A and JAKMIP3 was negatively correlated with OS (both P < 0.05, Figure 3A). The univariate Cox regression analysis revealed that the expression of SRXN1 (HR = 1.70, P < 0.05) and KRT6A (HR = 1.71, P < 0.05) was significantly associated with the prognosis of patients with NSCLC; no significant association was observed with the expression of JAKMIP3 (P > 0.05, Table 4). Moreover, an ROC analysis revealed that SRXN1 and KRT6A were both highly sensitive and specific, suggesting that both had a high diagnostic value for distinguishing smokers from never-smokers (AUC = 92.3% in GSE18385 and 93.7% in GSE76324 for SRXN1 and 68.2% in GSE18385 and 67.0% in GSE76324 for KRT6A, Figures 3B, C). Another ROC analysis showed that SRXN1 and KRT6A could clearly distinguish patients with NSCLC from healthy individuals (AUC = 74.8% in TCGA for SRXN1 and 76.9% in TCGA for KRT6A, Figures 3D, E). In summary, these results suggest that SRXN1 and KRT6A might be potential biomarkers of smoking exposure and early lung cancer diagnosis and prognosis.
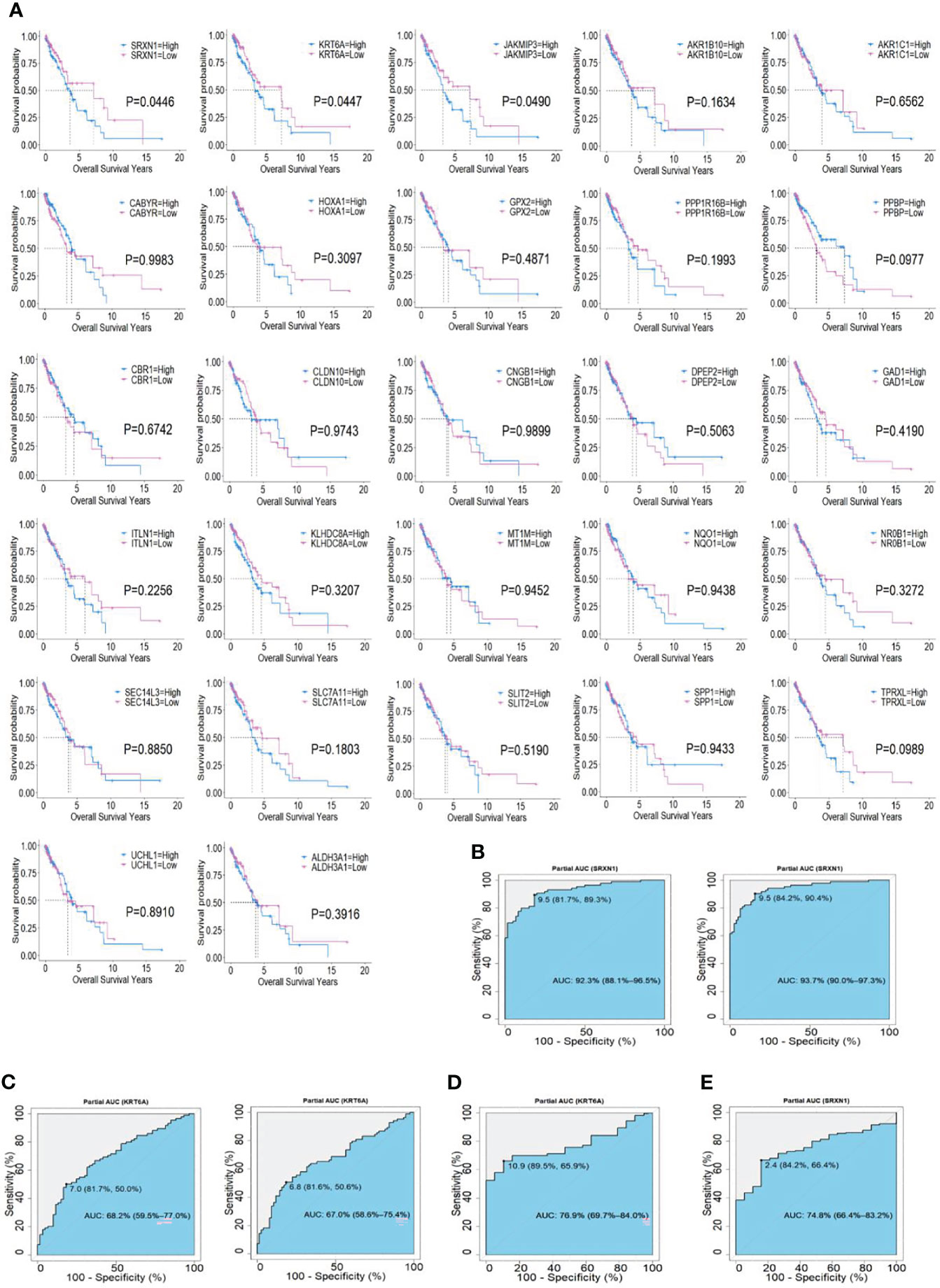
Figure 3 Associations of two top hub smoking-related genes (SRGs) with overall survival in lung cancer patients via Kaplan–Meier and Cox regression analyses. (A) Kaplan–Meier curves of 27 hub SRGs; (B) receiver operating characteristic (ROC) curves of SRXN1 in the GSE18385 and GSE76324 datasets. (C) ROC curves of KRT6A in the GSE18385 and GSE76324 datasets. (D) ROC curves of SRXN1 in The Cancer Genome Atlas (TCGA). (E) ROC curves of KRT6A in TCGA.
To verify the carcinogenic roles of SRXN1 and KRT6A in NSCLC, we measured the expression of these genes in 75 paired NSCLC and non-tumor tissues by RT-qPCR. Both SRXN1 and KRT6A were significantly upregulated in NSCLC tissues relative to non-tumor tissues (5.07 ± 3.65 fold and 6.80 ± 4.53 fold, respectively; both P < 0.001, Figures 4A, B). Correlations were observed between increasing expression levels of SRXN1 and KRT6A and an advanced TNM stage (P < 0.001 for both, Figures 4C, D), distant metastasis (P = 0.001 and P < 0.001, respectively, Figures 4E, F), lymph node metastasis (P < 0.001 and P = 0.003, respectively, Figures 4G, H) and smoking (P = 0.001 and P = 0.03, respectively, Figures 4I, J). Furthermore, higher SRXN1 expression levels were observed in squamous patients than in adenocarcinoma patients (P < 0.05, Figure 4K), suggesting that SRXN1 might be correlated with the lung cancer subtype. A Spearman rank correlation analysis revealed that the expression levels of SRXN1 and KRT6A were significantly correlated with daily smoking consumption (r = 0.453 and r = 0.445, respectively, both P < 0.05, Figures 4L, M). However, neither SRXN1 nor KRT6A expression was correlated significantly with age, sex or tumor size (P > 0.05, Table 3).
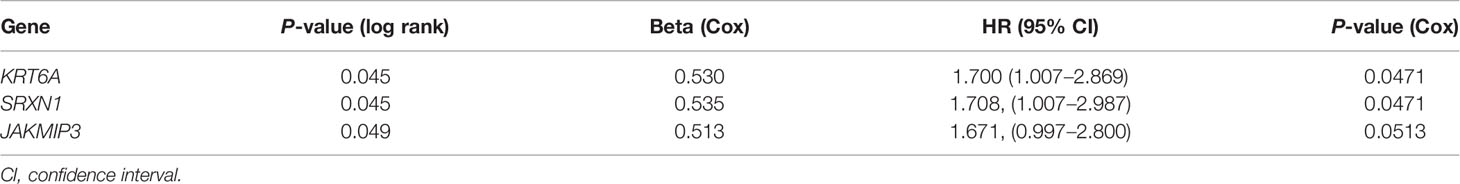
Table 3 Univariate Cox regression analysis of smoking-related genes (SRGs) in The Cancer Genome Atlas (TCGA).
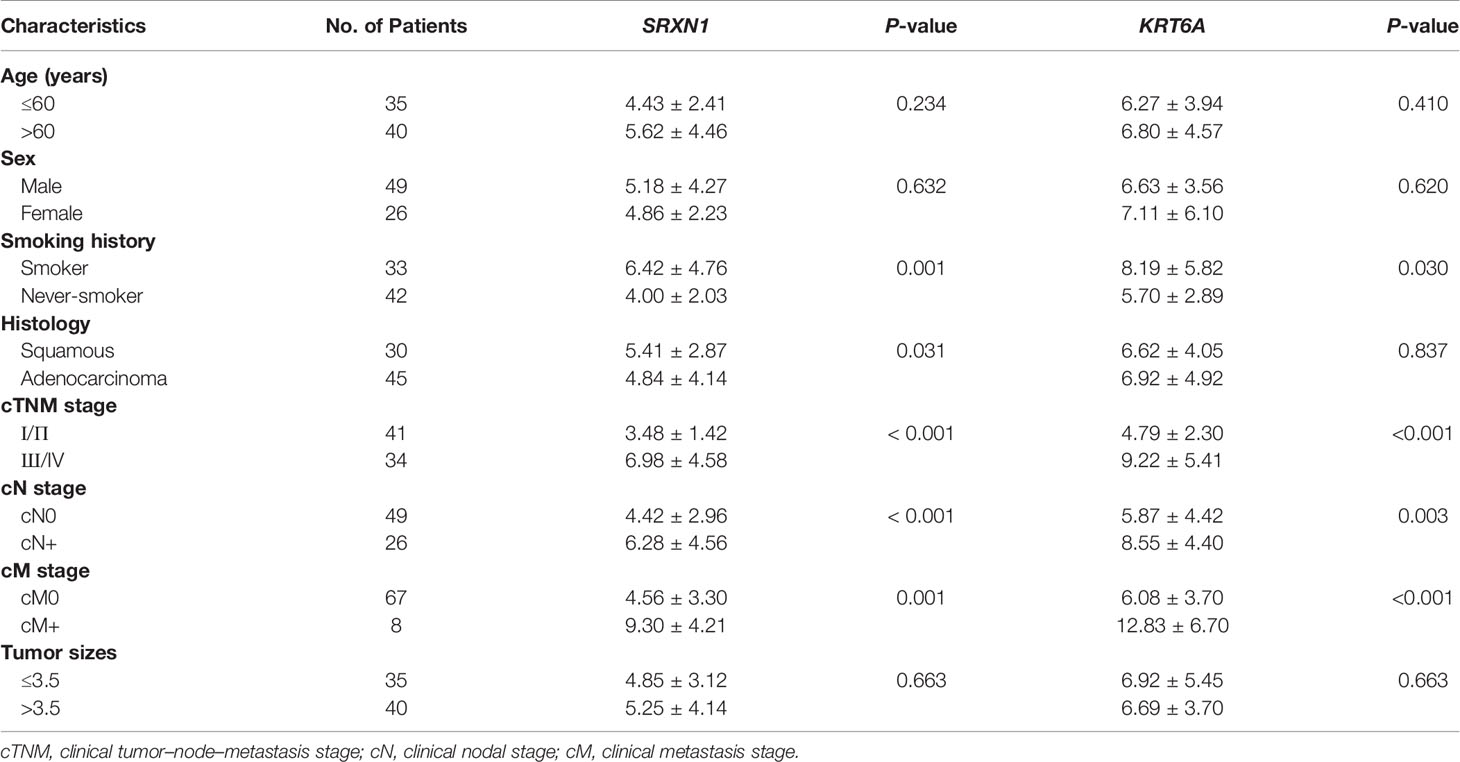
Table 4 Non-parametric assessment of the correlations between clinicopathological factors and SRXN1 and KRT6A expression levels in 75 patients with non-small-cell lung cancer.
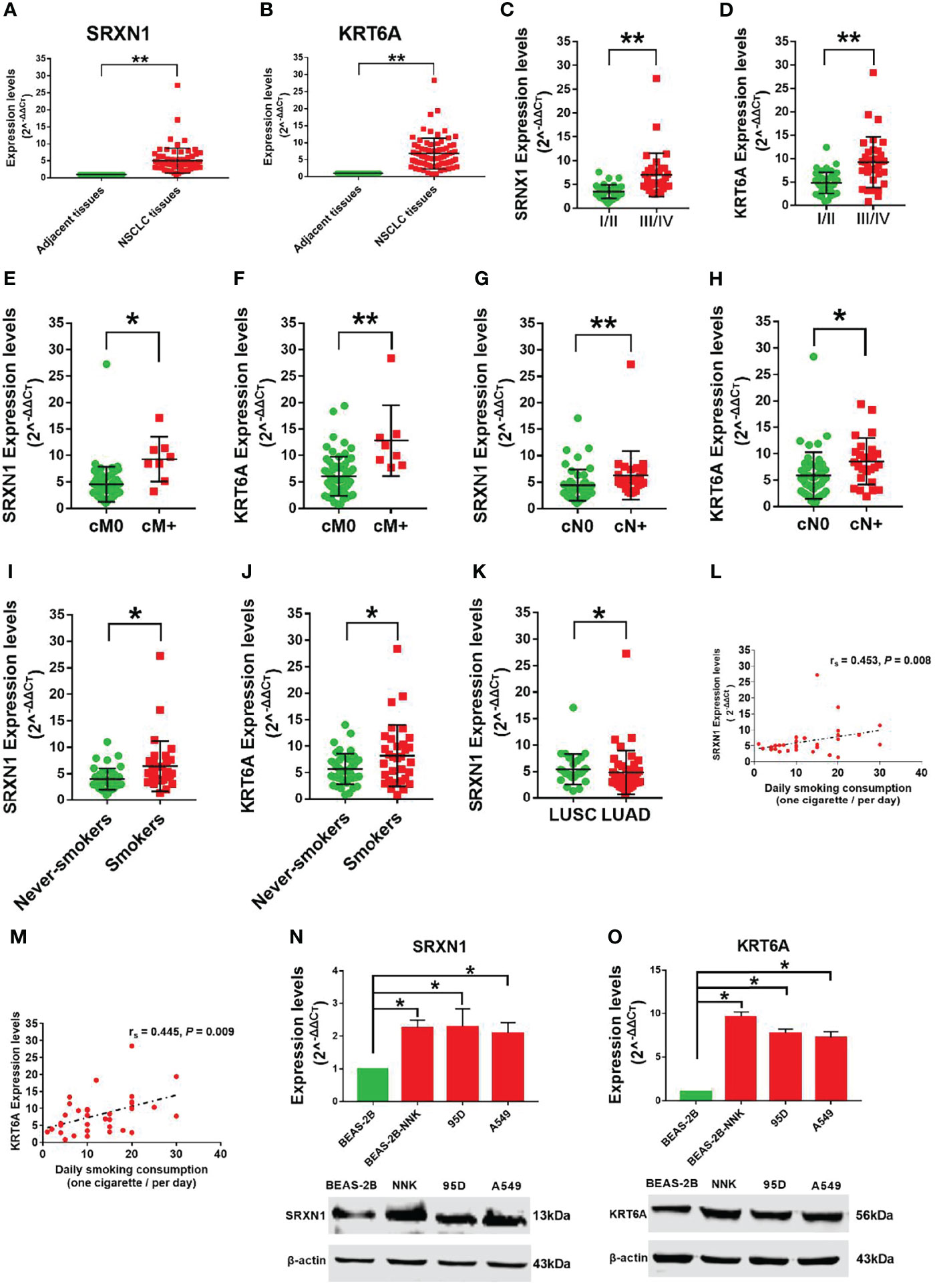
Figure 4 Strong expression of SRXN1 and KRT6A in non-small-cell lung cancer (NSCLC) tissues and lung cancer cell lines. (A) SRXN1 expression was detected in NSCLC and normal tissues by RT-qPCR. (B) KRT6A expression was detected in NSCLC and normal tissues by RT-qPCR. (C, D) Higher expression of SRXN1 and KRT6A in patients with clinical stage III/IV NSCLC vs. those with clinical stage I/II NSCLC. (E, F) Higher expression of SRXN1 and KRT6A in patients with cM+ NSCLC vs. patients with cM0 NSCLC. (G, H) Higher expression of SRXN1 and KRT6A in patients with cN+ NSCLC vs. patients with cN0 NSCLC. (I, J) Higher expression of SRXN1 and KRT6A in smokers with NSCLC vs. never-smokers with NSCLC. (K) Higher expression of SRXN1 in LUAD patients vs. LUSC patients. (L, M) Scatter plots of the correlations between SRXN1 and KRT6A expression and daily smoking consumption. (N, O) RT-qPCR and western blot analyses of the SRXN1 and KRT6A mRNA and protein levels in 95D, A549 and Beas-2B-NNK cells vs. with Beas-2B cells. *P < 0.05, **P < 0.01.
Furthermore, we observed increased expression of SRXN1 and KRT6A mRNA and protein in lung cancer cell lines (95D, A549) and BEAS-2B-NNK cells, compared with normal BEAS-2B cells (both P < 0.05, Figures 4N, O). These results imply that SRXN1 and KRT6A might play a role in the carcinogenesis of NSCLC and are closely related to smoking.
Because SRXN1 and KRT6A expression was upregulated in lung cancers, we designed three siRNAs to suppress the endogenous expression of SRXN1 and KRT6A transcripts. Both RT-qPCR and western blotting were used to examine the effectiveness of siRNA interference. The levels of SRXN1 or KRT6A mRNAs and proteins were obviously decreased in A549 cells after transfection with si-SRXN1-1 or si-KRT6A-1, respectively, in comparison with the NC and control groups. However, si-SRXN1-2, si-SRXN1-3, si-KRT6A-2 and si-KRT6A-3 had no significant effects on SRXN1 or KRT6A expression (Figures 5A, B). Therefore, si-SRXN1-1 and si-KRT6A-1 were selected for use in subsequent experiments.
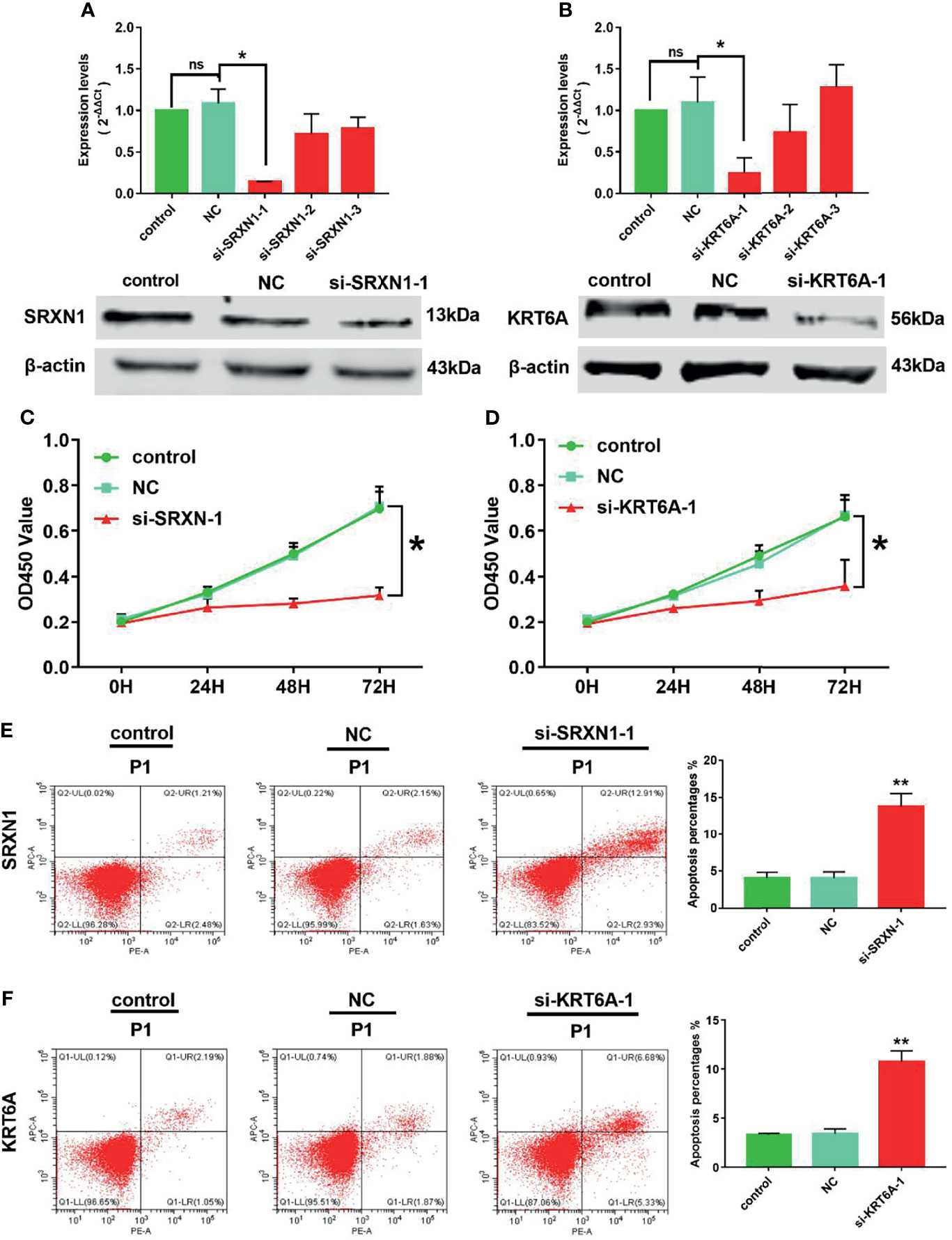
Figure 5 SRXN1 or KRT6A inhibition suppressed cell viability and promoted cell apoptosis. (A) RT-qPCR and western blot analyses were performed to test the effect of SRXN1 interference. (B) RT-qPCR and western blot analyses were performed to test the effect of KRT6A interference. (C) Cell viability in response to SRXN1 depletion was monitored using a CCK-8 assay. (D) Cell viability in response to KRT6A depletion was monitored using a CCK-8 assay. (E) Representative flow cytometry plots of cell apoptosis in response to SRXN1 depletion and a graph of apoptosis rates per group. (F) Representative flow cytometry plots of cell apoptosis in response to KRT6A depletion and a graph of apoptosis rates per group. *P < 0.05, **P < 0.01. NC, negative control; ns, no significance.
CCK-8 proliferation assays were performed to assess the viability of the cells subjected to knockdown. We observed a significant reduction in the viability of A549 cells subjected to SRXN1 or KRT6A silencing, compared with the NC and control groups, respectively. At 72 h after siRNA transfection, the cell viability in the si-SRXN1-1 and si-KRT6A-1 groups were reduced by 54.5% (P < 0.05, Figure 5C) and 46.1% (P < 0.05, Figure 5D), respectively. A flow cytometry analysis revealed that A549 cells exhibited enhanced apoptosis under SRXN1 or KRT6A suppression (both P < 0.05, Figures 5E, F), compared with the NC and control groups.
The significant effects of SRXN1 or KRT6A knockdown on cell growth in vitro warranted an exploration of the roles of SRXN1 and KRT6A in vivo. Nude mice xenograft models were generated by the subcutaneous injection of si-SRXN1- or si-KRT6A-transfected A549 cells, siRNA NC-transfected A549 cells or control A549 cells. The tumor growth curves indicated significant growth inhibition in the si-SRXN1-1 and si-KRT6A-1 groups (both P < 0.05, Figures 6A, B), compared with the NC and control groups, respectively. The tumor volumes and weights in the si-SRXN1-1 and si-KRT6A-1 groups were obviously reduced relative to those in the NC and control groups (both P < 0.05), but there was no difference between the NC and control groups (Figures 6C, D).
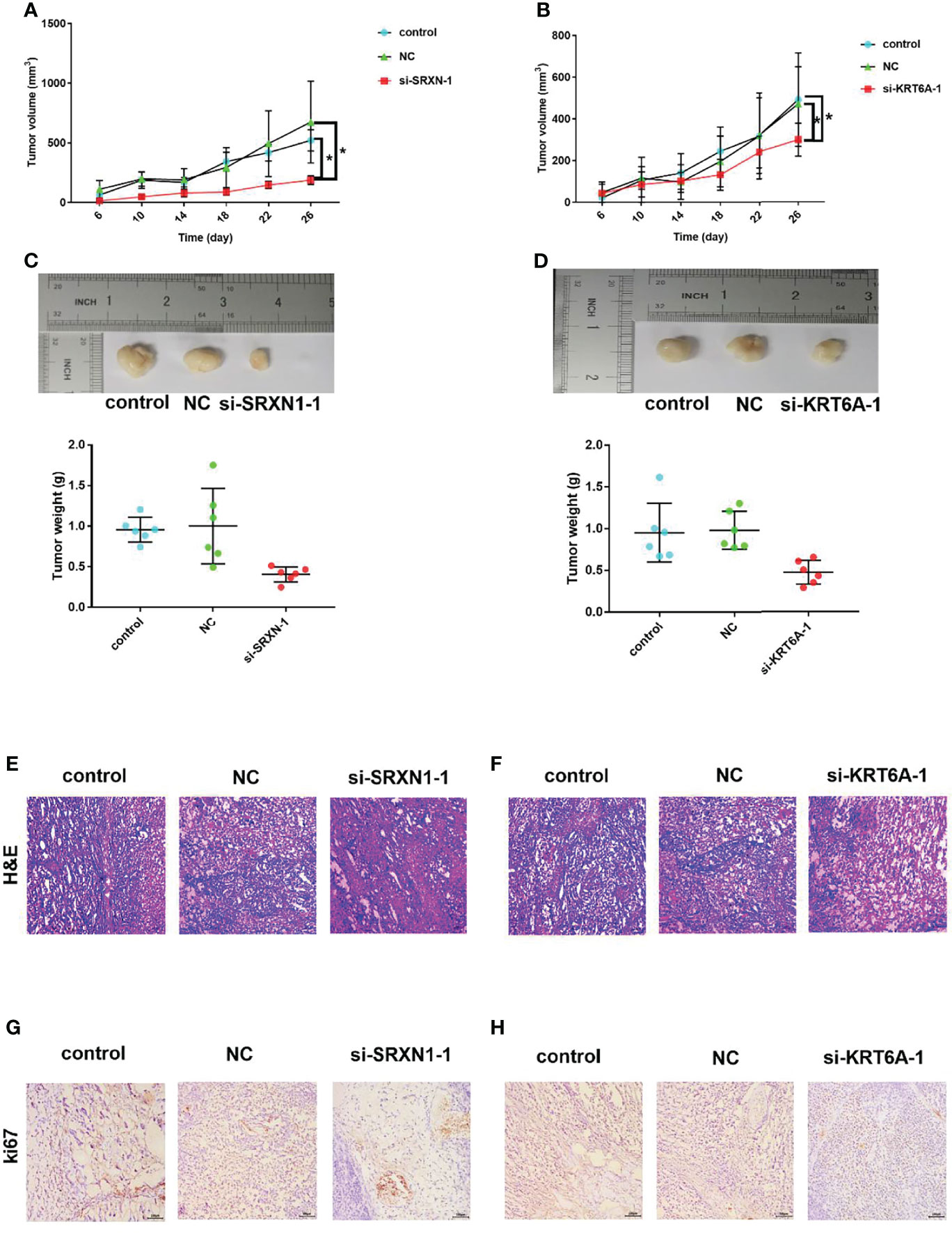
Figure 6 SRXN1 or KRT6A knockdown suppressed tumor growth in vivo. (A) Tumor growth curve in response to SRXN1 depletion. (B) Tumor growth curve in response to KRT6A depletion. (C) Representative images of xenograft tumors from nude mice and comparison of tumor weights in response to SRXN1 depletion. (D) Representative images of xenograft tumors from nude mice and comparison of tumor weights in response to KRT6A depletion. (E, F) Representative images of hematoxylin–eosin-stained tumor samples from SRXN1 and KRT6A knockdown samples, respectively, relative to controls and negative controls (NC). (G, H) Representative images of Ki67-immunostained tumor samples from SRXN1 and KRT6A knockdown samples, respectively, relative to controls and NCs. *P < 0.05.
To delineate the underlying mechanism, the harvested tumors were further subjected to H&E staining and IHC analysis. H&E staining of the xenograft tumors revealed spherical cells with large, deeply stained nuclei (Figures 6E, F). The average percentages of Ki67-positive cells in the si-SRXN1-1 and si-KRT6A-1 groups were significantly lower than those in the NC and control groups (Figures 6G, H). This suppressive effect of SRXN1 or KRT6A knockdown on tumor growth was consistent with the results in vitro.
Cigarette smoking is the most common etiology of NSCLC, accounting for the vast majority of cases worldwide (31), and remains one of the strongest risk factors for NSCLC occurrence and development. Additionally, a large majority of lung cancer deaths are attributable to cigarette smoking. Cigarettes contain a complex mixture of components and are associated with complex molecular mechanisms of carcinogenesis (32, 33). It is therefore imperative to curb the rates of cigarette smoking. Developments in gene sequencing technology have enabled the identification of some potential gene markers with predictive value in cigarette smokers and patients with NSCLC (34, 35). However, few reliable markers are available. Therefore, the identification of reliable biomarkers that more accurately predict the early diagnosis and prognosis of NSCLC is urgently needed.
Significant developments in high-throughput sequencing technology have led more researchers to devote close attention to bioinformatics as a means of identifying the key genes in cigarette-related carcinogenesis. For instance, Wang et al. (36) identified three smoking-related genes, namely GYPC (glycophorin C), NME1 (NME/NM23 nucleoside diphosphate kinase 1) and SLIT2 (slit guidance ligand 2), that were significantly associated with cigarette smoke-induced LUAD, based on an integration analysis of four GEO datasets and an mRNA sequencing analysis. Ren et al. (37) reported that the expression of nine key genes, namely UBE2T, EXO1, TOP2A, CDCA7, HMMR, ANLN, RAD54L, DEPDC1 and CDCA8, was correlated with an adverse prognosis in patients with smoking-related LUAD. Moreover, Huang et al. (38) identified several candidate microRNAs that might be useful for assessing the risk of smoking-related lung diseases based on an integrated analysis of four GEO datasets. Chawsheen et al. (39) reported that lung cancer patients with high sulfiredoxin level was associated with a significantly shorter survival duration in a bioinformatics analysis of lung cancer patients. Sarill et al. (40) reported that exposure to cigarette smoke extract induced a significant increase in the expression of SRXN1 mRNA. Xiao et al. (41) observed that high KRT6A expression was correlated with an unfavorable prognosis in a bioinformatics analysis of lung adenocarcinoma patients. These previous studies confirm the important roles of SRXN1 and KRT6A in lung cancer. Consistently, we identified both genes as the top two SRGs with potential carcinogenic effects and high predictive values with respect to smoking exposure and the early diagnosis and prognosis of NSCLC through our bioinformatics analyses.
SRXN1, an endogenous antioxidant, has been shown to protect against exogenous compound-induced oxidative stress in vitro and in vivo (42, 43). However, numerous studies reported that the anti-oxidative stress effect of SRXN1 could not protect against pulmonary disease (44). Jiang et al. (45) reported that SRXN1 promoted colorectal cancer cell invasion and metastasis by enhancing EGFR signaling. KRT6A, a member of the keratin protein family, plays an important role in squamous epithelial epidermalization. Besides, cancer-related studies identified associations between the expression of KRT6A and several diseases and cancers, such as pachyonychia congenita, oral squamous cell carcinoma, lung cancer, renal carcinoma and progressive breast cancer (46–48). In one study, KRT6A silencing suppressed nasopharyngeal carcinoma cell invasion and metastasis via the β−catenin cascade (49).
Cigarette smoking, which is preventable, remains the main risk factor for lung cancer worldwide (50). Changes in gene expression in response to environmental contact may signal exposure to toxins. To identify genes with altered expression levels in response to cigarette smoking, we compared the transcriptomes of lung tissues from smokers and never-smokers from two GEO datasets and a TCGA dataset using a bioinformatics analysis. We found that SRXN1 and KRT6A were differentially expressed between both groups in all three datasets, with concordant higher expression in ever-smokers. Meanwhile, we observed the smoking-induced upregulation of SRXN1 and KRT6A expression in 75 matched tumor–normal tissue pairs from patients with NSCLC who were enrolled in our study. More importantly, both SRXN1 and KRT6A expression were significantly correlated with smoking so that we proposed an assumption that SRXN1 and KRT6A might be the key for preventing smoke cause lung cancer. In summary, the findings suggested that SRXN1 and KRT6A might be potential biomarkers of the extent of exposure to environmental tobacco smoke. We believed that SRXN1 and KRT6A might be the early diagnostic biomarkers and therapy targets on clinical therapy of smoke cause lung cancer with further validated study in future.
In conclusion, SRXN1 and KRT6A expression might be potential biomarkers of smoking exposure and the early diagnosis and prognosis of NSCLC. Our findings shed light on the novel molecular mechanisms underlying the pathophysiology of smoking-related lung cancer, and reveal new pathways that might be therapeutically exploitable.
Publicly available datasets were analyzed in this study. This data can be found here: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi GEO database: GSE18385, GSE76324. The data of TCGA can be found in TCGA database by the following link: https://portal.gdc.cancer.gov/repository.
The studies involving human participants were reviewed and approved by the Ethics Committee of Guangzhou medical university. The patients/participants provided their written informed consent to participate in this study. The animal study was reviewed and approved by the Ethics Committee of Guangzhou medical university. Written informed consent was obtained from the owners for the participation of their animals in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
QY was responsible for the concept. QY was responsible for funding acquisition. QY designed the experiments. JZZ, GJ, and EX collected and analyzed data. JXZ and LL did data interpretation and discussions. JZZ and GJ initially drafted the paper. All co-authors reviewed and edited the manuscript and approved the submission.
This work was supported by the National Natural Science Foundation of China (Grant Number: 81773385 and 81472937 to QY), Natural Science Foundation of Guangdong Province (Grant Number: 2019A1515011298 to QY).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
This work benefited from the Gene Expression Omnibus (GEO) database and the Cancer Genome Atlas (TCGA) database. We were grateful to the access to the resources and the efforts of the staff to expand and improve the two databases.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.810301/full#supplementary-material
1. Siegel Y, Kuker R, Danton G, Gonzalez J. Occult Lung Cancer Occluding a Pulmonary Vein With Suspected Venous Infarction, Mimicking Pneumonia and a Pulmonary Embolus. J Emerg Med (2016) 51(2):e11–14. doi: 10.1016/j.jemermed.2015.12.019
2. Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol (2015) 10(9):1243–60. doi: 10.1097/JTO.0000000000000630
3. Schwartz AG, Cote ML. Epidemiology of Lung Cancer. Adv Exp Med Biol (2016) 893:21–41. doi: 10.1007/978-3-319-24223-1_2
4. Hecht SS. Tobacco Smoke Carcinogens and Lung Cancer. J Natl Cancer Inst (1999) 91(14):1194–210. doi: 10.1093/jnci/91.14.1194
5. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2016. CA Cancer J Clin (2016) 66(1):7–30. doi: 10.3322/caac.21332
6. Thun MJ, Hannan LM, Adams-Campbell LL, Boffetta P, Buring JE, Feskanich D, et al. Lung Cancer Occurrence in Never-Smokers: An Analysis of 13 Cohorts and 22 Cancer Registry Studies. PloS Med (2008) 5(9):e185. doi: 10.1371/journal.pmed.0050185
7. World Health Organization. WHO Global Report on Trends in Prevalence of Tobacco Smoking 2000–2025. 2nd ed. UC San Francisco: Center for Tobacco Control Research and Education (2018). Available at: https://escholarshiporg/uc/item/4dc4t5d9.
8. Chen MM, Chiu CH, Yuan CP, Liao YC, Guo SE. Influence of Environmental Tobacco Smoke and Air Pollution on Fetal Growth: A Prospective Study. Int J Environ Res Public Health (2020) 17(15):5139. doi: 10.3390/ijerph17155319
9. de Andrade M, Ebbert JO, Wampfler JA, Miller DL, Marks RS, Croghan GA, et al. Environmental Tobacco Smoke Exposure in Women With Lung Cancer. Lung Cancer (2004) 43(2):127–34. doi: 10.1016/j.lungcan.2003.08.025
10. Holst GJ, Pedersen CB, Thygesen M, Brandt J, Geels C, Bonlokke JH, et al. Air Pollution and Family Related Determinants of Asthma Onset and Persistent Wheezing in Children: Nationwide Case-Control Study. BMJ (2020) 370:m2791. doi: 10.1136/bmj.m2791
11. Whitrow MJ, Smith BJ, Pilotto LS, Pisaniello D, Nitschke M. Environmental Exposure to Carcinogens Causing Lung Cancer: Epidemiological Evidence From the Medical Literature. Respirology (2003) 8(4):513–21. doi: 10.1046/j.1440-1843.2003.00497.x
13. Du Y, Cui X, Sidorenkov G, Groen HJM, Vliegenthart R, Heuvelmans MA, et al. Lung Cancer Occurrence Attributable to Passive Smoking Among Never Smokers in China: A Systematic Review and Meta-Analysis. Transl Lung Cancer Res (2020) 9(2):204–17. doi: 10.21037/tlcr.2020.02.11
14. Hecht SS. Research Opportunities Related to Establishing Standards for Tobacco Products Under the Family Smoking Prevention and Tobacco Control Act. Nicotine Tob Res (2012) 14(1):18–28. doi: 10.1093/ntr/ntq216
15. Hoffmann D, Rathkamp G, Rubin J. Chemical Studies on Tobacco Smoke. II. Comparison of the Yields of Several Selected Components in the Smoke From Five Major Turkish Tobacco Varieties. Food Cosmet Toxicol (1967) 5(1):37–8. doi: 10.1016/S0015-6264(67)82884-0
16. Akopyan G, Bonavida B. Understanding Tobacco Smoke Carcinogen NNK and Lung Tumorigenesis. Int J Oncol (2006) 29(4):745–52. doi: 10.3892/ijo.29.4.745
17. Hecht SS. Carcinogen Derived Biomarkers: Applications in Studies of Human Exposure to Secondhand Tobacco Smoke. Tob Control (2004) 13 Suppl 1:i48–56. doi: 10.1136/tc.2002.002816
18. Huang RY, Chen GG. Cigarette Smoking, Cyclooxygenase-2 Pathway and Cancer. Biochim Biophys Acta (2011) 1815(2):158–69. doi: 10.1016/j.bbcan.2010.11.005
19. Zheng HC, Takano Y. NNK-Induced Lung Tumors: A Review of Animal Model. J Oncol (2011) 2011:635379. doi: 10.1155/2011/635379
20. Jin H, Li Q, Cao F, Wang SN, Wang RT, Wang Y, et al. miR-124 Inhibits Lung Tumorigenesis Induced by K-Ras Mutation and NNK. Mol Ther Nucleic Acids (2017) 9:145–54. doi: 10.1016/j.omtn.2017.09.005
21. Shao L, Zuo X, Yang Y, Zhang Y, Yang N, Shen B, et al. The Inherited Variations of a P53-Responsive Enhancer in 13q12.12 Confer Lung Cancer Risk by Attenuating TNFRSF19 Expression. Genome Biol (2019) 20(1):103. doi: 10.1186/s13059-019-1696-1
22. Harada A, Jogie-Brahim S, Oh Y. Tobacco Specific Carcinogen 4-(Methylnitrosamino)-1-(3-Pyridyl)-1-Butanone Suppresses a Newly Identified Anti-Tumor IGFBP-3/IGFBP-3R System in Lung Cancer Cells. Lung Cancer (2013) 80(3):270–7. doi: 10.1016/j.lungcan.2013.02.016
23. Pulling LC, Klinge DM, Belinsky SA. P16ink4a and Beta-Catenin Alterations in Rat Liver Tumors Induced by NNK. Carcinogenesis (2001) 22(3):461–6. doi: 10.1093/carcin/22.3.461
24. Vuillemenot BR, Hutt JA, Belinsky SA. Gene Promoter Hypermethylation in Mouse Lung Tumors. Mol Cancer Res (2006) 4(4):267–73. doi: 10.1158/1541-7786.MCR-05-0218
25. Hutt JA, Vuillemenot BR, Barr EB, Grimes MJ, Hahn FF, Hobbs CH, et al. Life-Span Inhalation Exposure to Mainstream Cigarette Smoke Induces Lung Cancer in B6C3F1 Mice Through Genetic and Epigenetic Pathways. Carcinogenesis (2005) 26(11):1999–2009. doi: 10.1093/carcin/bgi150
26. Laag E, Majidi M, Cekanova M, Masi T, Takahashi T, Schuller HM. NNK Activates ERK1/2 and CREB/ATF-1 via Beta-1-AR and EGFR Signaling in Human Lung Adenocarcinoma and Small Airway Epithelial Cells. Int J Cancer (2006) 119(7):1547–52. doi: 10.1002/ijc.21987
27. Liu Y, Yang S, Li MY, Huang R, Ng CS, Wan IY, et al. Tumorigenesis of Smoking Carcinogen 4-(Methylnitrosamino)-1-(3-Pyridyl)-1-Butanone is Related to its Ability to Stimulate Thromboxane Synthase and Enhance Stemness of non-Small Cell Lung Cancer Stem Cells. Cancer Lett (2016) 370(2):198–206. doi: 10.1016/j.canlet.2015.10.017
28. Wang R, Wang G, Ricard MJ, Ferris B, Strulovici-Barel Y, Salit J, et al. Smoking-Induced Upregulation of AKR1B10 Expression in the Airway Epithelium of Healthy Individuals. Chest (2010) 138(6):1402–10. doi: 10.1378/chest.09-2634
29. Wang G, Zhou H, Strulovici-Barel Y, Al-Hijji M, Ou X, Salit J, et al. Role of OSGIN1 in Mediating Smoking-Induced Autophagy in the Human Airway Epithelium. Autophagy (2017) 13(7):1205–20. doi: 10.1080/15548627.2017.1301327
30. Chen E, Zhou J, Xu E, Zhang C, Liu J, Zhou J, et al. A Genome-Wide Screen for Differentially Methylated Long Noncoding RNAs Identified That Lncac007255.8 is Regulated by Promoter DNA Methylation in Beas-2B Cells Malignantly Transformed by NNK. Toxicol Lett (2021) 346:34–46. doi: 10.1016/j.toxlet.2021.04.013
31. Hecht SS. Lung Carcinogenesis by Tobacco Smoke. Int J Cancer (2012) 131(12):2724–32. doi: 10.1002/ijc.27816
32. Milenkovic U, Albersen M. Chapter 6.1 - Smoking and Men’s Health. In: Yafi FA, Yafi NR, editors. Effects of Lifestyle on Men's Health. Leuven, Belgium: Academic Press (2019). p. 303–19.
33. Kapp R. Tobacco Smoke. In: Wexler P, editor. Encyclopedia of Toxicology, 2nd ed. New York: Elsevier (2005). p. 200–2.
34. Guo W, Hu M, Wu J, Zhou A, Liao Y, Song H, et al. Gprc5a Depletion Enhances the Risk of Smoking-Induced Lung Tumorigenesis and Mortality. BioMed Pharmacother (2019) 114:108791. doi: 10.1016/j.biopha.2019.108791
35. Zhang MY, Liu XX, Li H, Li R, Liu X, Qu YQ. Elevated mRNA Levels of AURKA, CDC20 and TPX2 are Associated With Poor Prognosis of Smoking Related Lung Adenocarcinoma Using Bioinformatics Analysis. Int J Med Sci (2018) 15(14):1676–85. doi: 10.7150/ijms.28728
36. Wang J, Chen T, Yu X, OU N, Tan L, Jia B, et al. Identification and Validation of Smoking-Related Genes in Lung Adenocarcinoma Using an In Vitro Carcinogenesis Model and Bioinformatics Analysis. J Transl Med (2020) 18(1):313. doi: 10.1186/s12967-020-02474-x
37. Ren C, Sun W, Lian X, Han C. Identification of Nine Key Genes by Bioinformatics Analysis for Predicting Poor Prognosis in Smoking-Induced Lung Adenocarcinoma. Lung Cancer Manag (2020) 9(2):LMT30. doi: 10.2217/lmt-2020-0009
38. Huang J, Jiang W, Tong X, Zhang L, Zhang Y, Fan H. Identification of Gene and microRNA Changes in Response to Smoking in Human Airway Epithelium by Bioinformatics Analyses. Med (Baltimore) (2019) 98(38):e17267. doi: 10.1097/MD.0000000000017267
39. Chawsheen HA, Jiang H, Ying Q, Ding N, Thapa P, Wei Q. The Redox Regulator Sulfiredoxin Forms a Complex With Thioredoxin Domain-Containing 5 Protein in Response to ER Stress in Lung Cancer Cells. J Biol Chem (2019) 294(22):8991–9006. doi: 10.1074/jbc.RA118.005804
40. Sarill M, Zago M, Sheridan JA, Nair P, Matthews J, Gomez A, et al. The Aryl Hydrocarbon Receptor Suppresses Cigarette-Smoke-Induced Oxidative Stress in Association With Dioxin Response Element (DRE)-Independent Regulation of Sulfiredoxin 1. Free Radic Biol Med (2015) 89:342–57. doi: 10.1016/j.freeradbiomed.2015.08.007
41. Xiao J, Lu X, Chen X, Zou Y, Liu A, Li W, et al. Eight Potential Biomarkers for Distinguishing Between Lung Adenocarcinoma and Squamous Cell Carcinoma. Oncotarget (2017) 8(42):71759–71. doi: 10.18632/oncotarget.17606
42. Li L, Lin G, Gu H, Yu L, Ni C. Effects of Srxn1 on Growth and Notch Signalling of Astrocyte Induced by Hydrogen Peroxide. Artif Cells Nanomed Biotechnol (2019) 47(1):1917–23. doi: 10.1080/21691401.2019.1614016
43. Lv X, Yu H, Zhang Q, Huang Q, Hong X, Yu T, et al. SRXN1 Stimulates Hepatocellular Carcinoma Tumorigenesis and Metastasis Through Modulating ROS/p65/BTG2 Signalling. J Cell Mol Med (2020) 24(18):10714–29. doi: 10.1111/jcmm.15693
44. Rogers S, de Souza AR, Zago M, Iu M, Guerrina N, Gomez A, et al. Aryl Hydrocarbon Receptor (AhR)-Dependent Regulation of Pulmonary miRNA by Chronic Cigarette Smoke Exposure. Sci Rep (2017) 7:40539. doi: 10.1038/srep40539
45. Jiang H, Wu L, Chen J, Mishra M, Chawsheen HA, Zhu H, et al. Sulfiredoxin Promotes Colorectal Cancer Cell Invasion and Metastasis Through a Novel Mechanism of Enhancing EGFR Signaling. Mol Cancer Res (2015) 13(12):1554–66. doi: 10.1158/1541-7786.MCR-15-0240
46. Bu W, Chen J, Morrison GD, Huang S, Creighton CJ, Huang J, et al. Keratin 6a Marks Mammary Bipotential Progenitor Cells That can Give Rise to a Unique Tumor Model Resembling Human Normal-Like Breast Cancer. Oncogene (2011) 30(43):4399–409. doi: 10.1038/onc.2011.147
47. da Rocha RG, Santos EMS, Santos EM, Gomes ESB, Ramos GV, Aguiar KM, et al. Leptin Impairs the Therapeutic Effect of Ionizing Radiation in Oral Squamous Cell Carcinoma Cells. J Oral Pathol Med (2019) 48(1):17–23. doi: 10.1111/jop.12786
48. Hu J, Zhang LC, Song X, Lu JR, Jin Z. KRT6 Interacting With Notch1 Contributes to Progression of Renal Cell Carcinoma, and Aliskiren Inhibits Renal Carcinoma Cell Lines Proliferation In Vitro. Int J Clin Exp Pathol (2015) 8(8):9182–8.
49. Chen C, Shan H. Keratin 6A Gene Silencing Suppresses Cell Invasion and Metastasis of Nasopharyngeal Carcinoma via the Betacatenin Cascade. Mol Med Rep (2019) 19(5):3477–84. doi: 10.3892/mmr.2019.10055
Keywords: lung cancer, non-small-cell lung cancer, smoking-related gene, KRT6A, SRXN1
Citation: Zhou J, Jiang G, Xu E, Zhou J, Liu L and Yang Q (2022) Identification of SRXN1 and KRT6A as Key Genes in Smoking-Related Non-Small-Cell Lung Cancer Through Bioinformatics and Functional Analyses. Front. Oncol. 11:810301. doi: 10.3389/fonc.2021.810301
Received: 06 November 2021; Accepted: 14 December 2021;
Published: 05 January 2022.
Edited by:
Elzbieta Pluciennik, Medical University of Lodz, PolandReviewed by:
Ishtiaque Ahammad, National Institute of Biotechnology, BangladeshCopyright © 2022 Zhou, Jiang, Xu, Zhou, Liu and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiaoyuan Yang, cWlhb3l1YW5feWFuZ0BnemhtdS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.