- 1Department of Surgical Oncology, Kanazawa Medical University, 1-1 Daigaku, Uchinada-machi, Kahoku-gun, Japan
- 2Department of General and Gastroenterologic Surgery, Kanazawa Medical University Himi Municipal Hospital, Himi City, Japan
The stomach exhibits abundant lymphatic flow, and metastasis to lymph nodes is common. In the case of gastric cancer, there is a regularity to the spread of lymph node metastasis, and it does not easily metastasize outside the regional nodes. Furthermore, when its extent is limited, nodal metastasis of gastric cancer can be cured by appropriate lymph node dissection. Therefore, identifying and determining the extent of lymph node metastasis is important for ensuring accurate diagnosis and appropriate surgical treatment in patients with gastric cancer. However, precise detection of lymph node metastasis remains difficult. Most nodal metastases in gastric cancer are microscopic metastases, which often occur in small-sized lymph nodes, and are thus difficult to diagnose both preoperatively and intraoperatively. Preoperative nodal diagnoses are mainly made using computed tomography, although the specificity of this method is low because it is mainly based on the size of the lymph node. Furthermore, peripheral nodal metastases cannot be palpated intraoperatively, nodal harvesting of resected specimens remains difficult, and the number of lymph nodes detected vary greatly depending on the skill of the technician. Based on these findings, gastrectomy with prophylactic lymph node dissection is considered the standard surgical procedure for gastric cancer. In contrast, several groups have examined the value of sentinel node biopsy for accurately evaluating nodal metastasis in patients with early gastric cancer, reporting high sensitivity and accuracy. Sentinel node biopsy is also important for individualizing and optimizing the extent of uniform prophylactic lymph node dissection and determining whether patients are indicated for function-preserving curative gastrectomy, which is superior in preventing post-gastrectomy symptoms and maintaining dietary habits. Notably, advancements in surgical treatment for early gastric cancer are expected to result in individualized surgical strategies with sentinel node biopsy. Chemotherapy for advanced gastric cancer has also progressed, and conversion gastrectomy can now be performed after downstaging, even in cases previously regarded as inoperable. In this review, we discuss the importance of determining lymph node metastasis in the treatment of gastric cancer, the associated difficulties, and the need to investigate strategies that can improve the diagnosis of lymph node metastasis.
Introduction
Lymph node metastasis is an important determinant of disease progression in patients with gastric cancer. The stage of gastric cancer without distant metastasis is determined based on the depth of invasion and the degree of lymph node metastasis (1–3). In recent years, determining the degree of lymph node metastasis after gastric cancer surgery has become essential for selecting appropriate adjuvant therapy and improving prognosis, as the effect of adjuvant chemotherapy in suppressing recurrence has become clear (4–8). In addition, given that it can be cured to some extent by prophylactic lymph node dissection, preoperative diagnosis of lymph node metastasis is an important factor when planning surgical treatment for patients with gastric cancer (9–11). For these reasons, numerous studies have investigated the diagnosis, pathophysiology, and treatment of lymph node metastasis in the context of gastric cancer (12–14). However, diagnosis of nodal metastasis remains difficult in these patients, and several problems with detection remain unresolved. In this review, we discuss the importance of determining lymph node metastasis in the treatment of gastric cancer and the associated difficulties.
Characteristics of Lymph Node Metastasis in Patients With Gastric Cancer
Lymph node metastasis is a common form of metastasis in patients with gastric cancer (9). Cancer that invades within the submucosa is defined as early gastric cancer, regardless of the presence or absence of metastasis (15). The rate of hematogenous metastasis in patients with early gastric cancer is approximately 0.2% (16), and peritoneal metastasis is unlikely to occur (17), whereas the incidence of lymph node metastasis is approximately 10% (18). The relatively high rate of lymph node metastasis in patients with gastric cancer can be attributed to the abundant lymphatic flow in the stomach (19, 20). Indeed, there is a rich lymphatic network in the submucosa, and immunohistological staining using D2-40 have revealed that there are abundant lymphatic vessels near the muscularis mucosae (21). This physiological environment explains the frequency of lymph node metastasis even in cases of early gastric cancer (19, 21).
The incidence of lymph node metastasis in gastric cancer is closely related to the depth of invasion (17). According to the database of the Cancer Institute Hospital of the Japan Foundation for Cancer Research, which is considered the most reliable large-scale database, the nodal metastasis rates according to pathological depth of invasion are 2.3% for mucosal, 21.9% for submucosal, 64.2% for proper muscle-subserosal, and 86.6% for serosal exposure cancers (17).
Previously, pioneers in gastric cancer surgery in Japan classified regional lymph nodes along the arteries, examined lymphatic flow using tracers, and tabulated the sites of lymph node metastasis (2, 3), revealing that there is a regularity in the spread of lymph node metastases in patients with gastric cancer. Table 1 shows that the rate of lymph node metastasis of early gastric cancer varies considerably according to station number (17, 22), based on data from representative articles. It can be seen that the lymph node metastasis rate varies considerably with the station number. Lymph nodes and lymphatic system of gastric cancer exhibit a stratified structure, consisting of three layers: the perigastric nodes and nodes along the left gastric artery; the nodes around the celiac artery, along the proper hepatic artery, and the suprapancreatic nodes; and the deeper para-aortic lymph nodes (Figure 1). The station numbers and definitions of the lymph nodes, which are important for gastric cancer staging and surgical treatment are precisely stated in Table 2. These nodes approximately correspond to group 1, 2, and 3 lymph nodes in the old Japanese Classification of Gastric Carcinoma, respectively (23). Most nodal metastases in early gastric cancer are confined to group 1 nodes, the rate of metastasis to group 2 nodes is low, and metastasis to group 3 nodes is rare. Lymph node metastases are thought to spread along with the lymphatic flow from group 1 to group 2 and then to group 3. In other words, lymph node metastasis of gastric cancer spreads from the perigastric nodes, via the suprapancreatic nodes and nodes around the celiac artery, to the para-aortic nodes, following which it flows out to the systemic circulation (Figure 2). Currently, the regional lymph nodes for gastric cancer are defined as No. 1 to No. 12 and No. 14v, and other nodal metastases are considered distant metastases (2, 3).
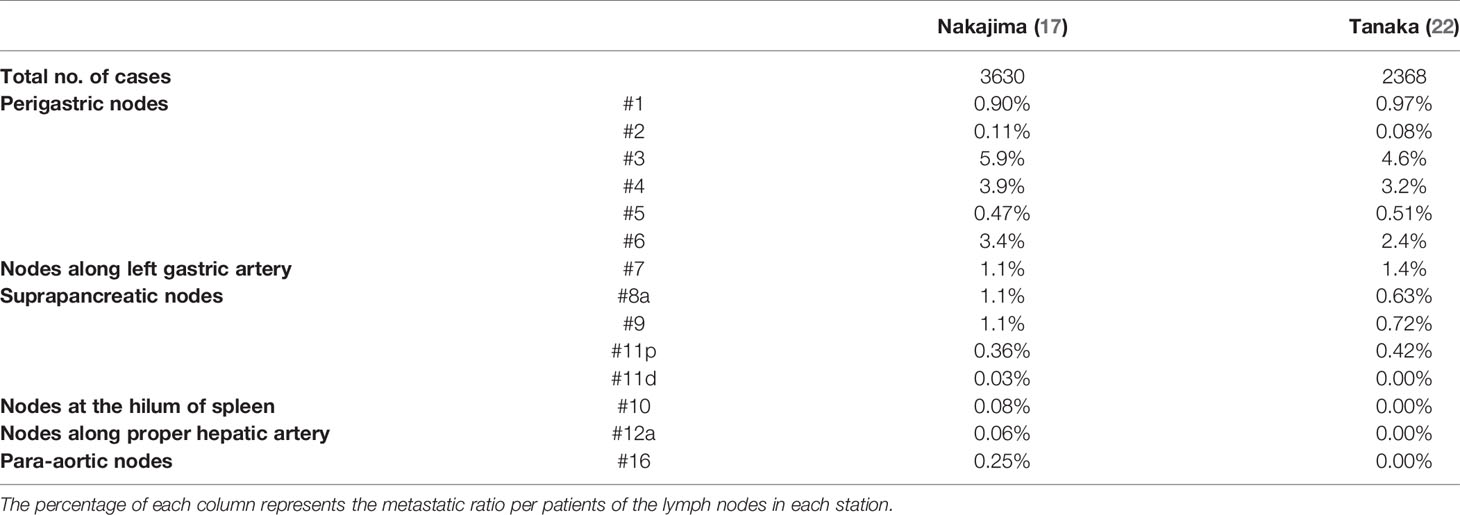
Table 1 The precise incidence of nodal metastasis of early gastric cancer in previous studies with large number of cases.
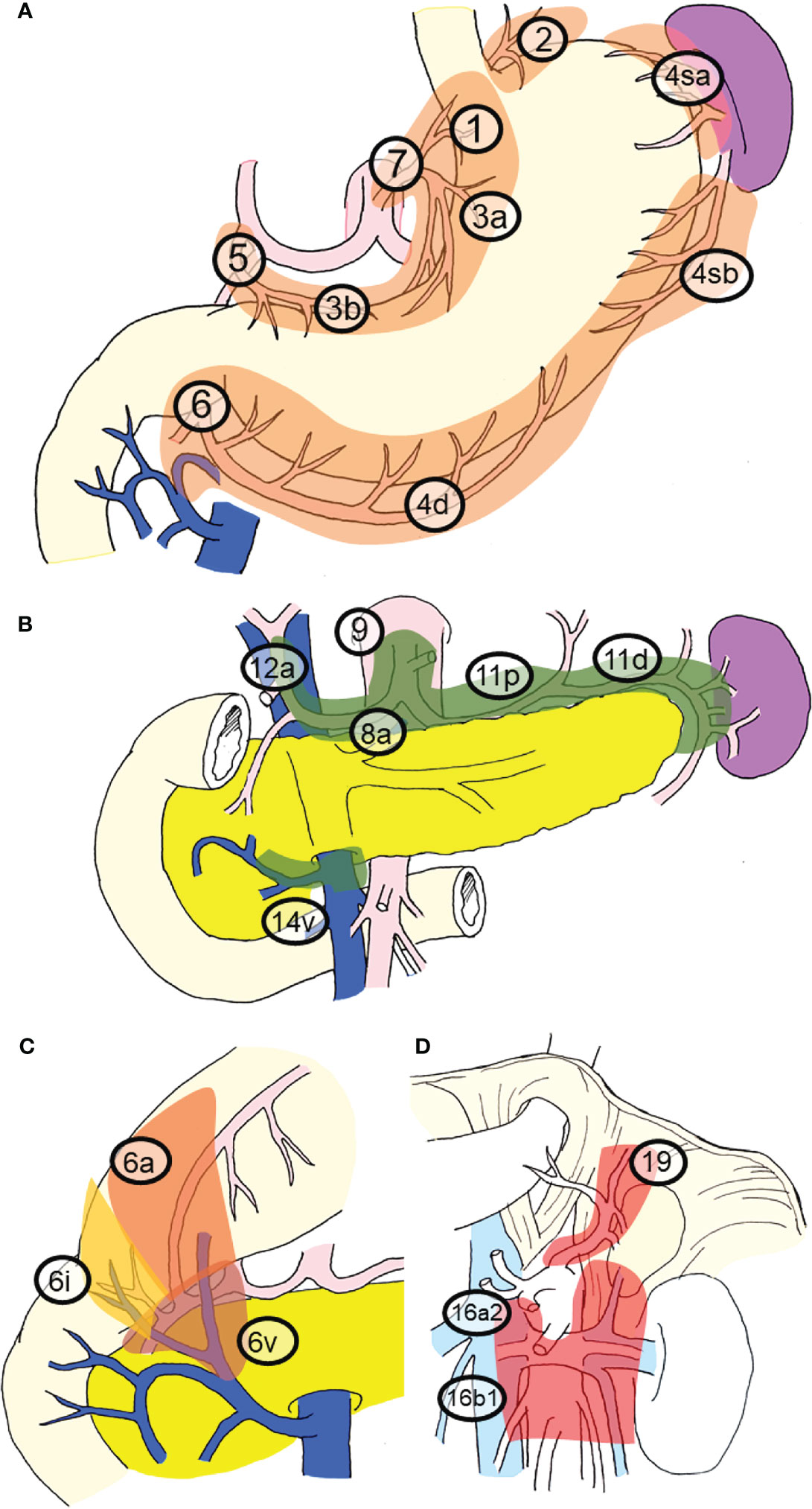
Figure 1 Regional lymph nodes and station numbers in gastric cancer. This classification and the station numbers are based on the Japanese Classification of Gastric Carcinoma. The details of the nodal station numbers have been described in Table 2. (A) Perigastric nodes and nodes along the left gastric artery. These nodes nearly correspond to group 1 nodes. (B) Nodes around the celiac artery, along the proper hepatic artery and suprapancreatic nodes. These nodes nearly correspond to the group 2 nodes. (C) The subcategory of No. 6 nodes. (D) Nodes in deeper layers. Para-aortic lymph nodes and No. 19 nodes. These nodes nearly correspond to group 3 nodes.

Table 2 The station numbers and the definitions of the lymph nodes which are important for gastric cancer staging and surgical treatment.
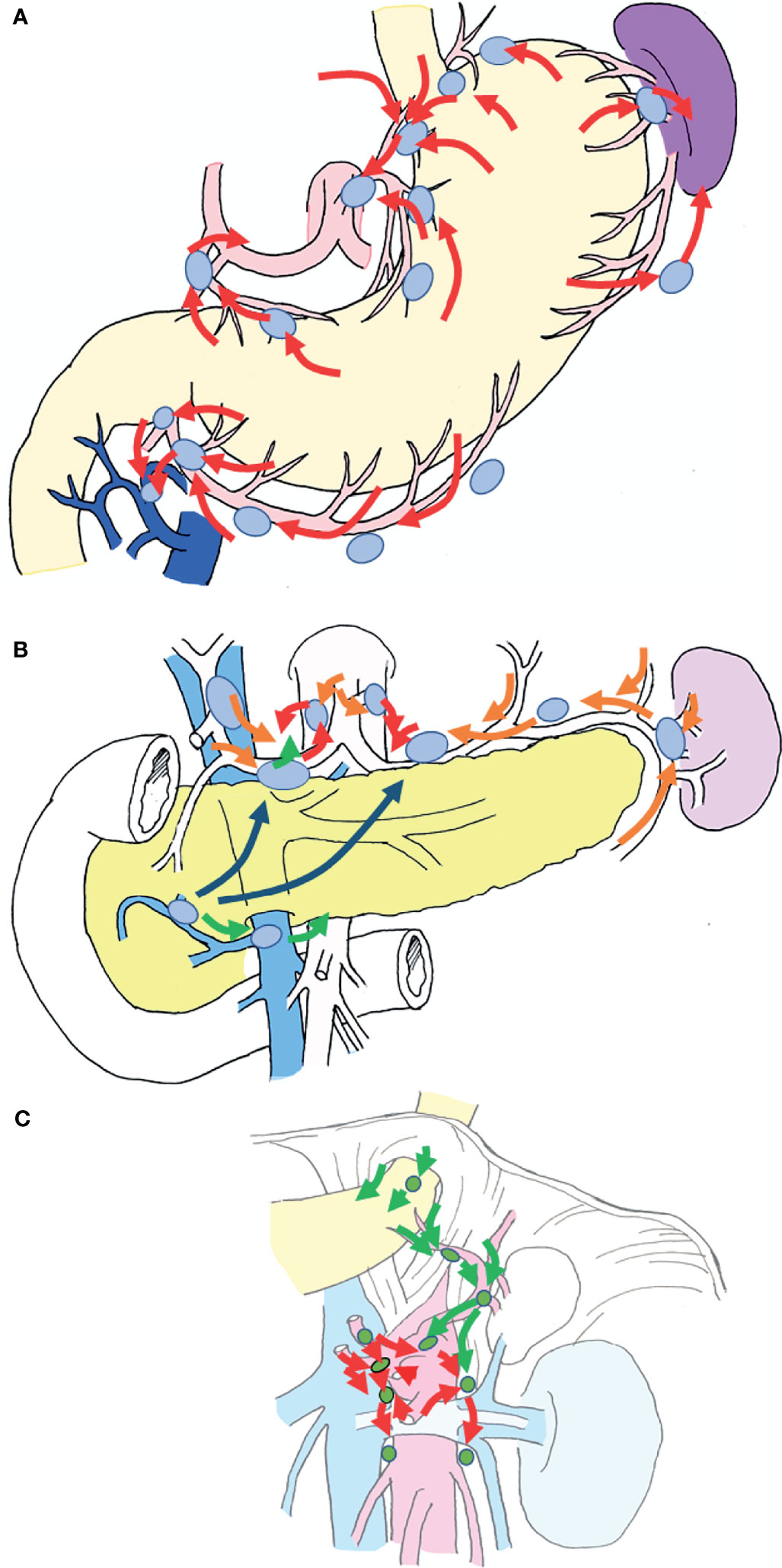
Figure 2 Schematic diagram of the lymphatic system of the stomach. The lymphatic flow of the stomach spreads from the perigastric nodes, via the suprapancreatic nodes and nodes around the celiac artery, to the para-aortic nodes, following which it enters the systemic circulation. (A) Lymphatic flow from the gastric wall is directed to the root of each artery via nearby perigastric nodes (red arrows). (B) The lymphatic flow into the root of each artery flows via suprapancreatic nodes (orange arrows) and out to the paraaortic nodes from the left and right of the celiac artery (red arrows). There are also routes from #8a to #8p (the posterior side of the common hepatic artery), and routes from #6 to the root of the superior mesenteric artery via #14v (green arrows). Routes from #6 to the suprapancreatic nodes via the lymphatics under the pancreatic capsule are also available (indigo arrows). (C) Lymphatic flow around the celiac artery and the superior mesenteric artery lead to the paraaortic nodes, which are the terminal lymph nodes of gastric cancer (red arrows). A route from the left dorsal side of the cardia to #16a2 lateral nodes via #19, along the left subphrenic artery also exists (green arrows).
In many cancers, lymph node metastasis is an important surrogate marker of survival prognosis and an important factor when considering postoperative adjuvant therapy. The prognosis of gastric cancer also worsens as the number of lymph node metastases increases (24–27). A previous study reported that survival curves clearly deviate according to the number of lymph node metastases, based on analysis of data in the registry of the Japanese Society of Gastric Cancer, a high-quality dataset that includes information for half of all patients undergoing gastric cancer surgery in Japan (9).
The Z0011 study demonstrated that additional axillary nodal dissection after sentinel node biopsy does not improve prognosis in patients with breast cancer (28–30). Similarly, in many carcinomas, lymph node metastasis is thought to be important for accurate staging, but prophylactic nodal dissection does not improve prognosis (31–35). In contrast, the therapeutic effect of prophylactic lymph node dissection in patients with gastric cancer has been verified. The rate of nodal metastasis for early gastric cancer is approximately 10% (18), but the 5-year survival rate after gastrectomy with prophylactic nodal dissection is as high as 98% (9–11). In cases of advanced gastric cancer, D2 (nodal dissection up to group 2 nodes) is associated with better prognosis than D1 (dissection of perigastric nodes only) (36, 37). This phenomenon seems to be a unique feature of gastric cancer. It is presumed that the major difference between gastric cancer and breast cancer is related to the anatomical location of the lymph nodes. Breast cancer that has metastasized to the axillary lymph nodes can easily develop systemic metastases from these nodes. In contrast, the lymphatic system of gastric cancer exhibits a stratified structure (Figures 1, 2), and there exists a state in which metastatic foci are localized around the stomach and not spread throughout the body, which is presumed to account for the prophylactic effect of lymph node dissection.
Previous studies have elucidated the molecular mechanisms involved in lymph node metastasis (38–41). Among them, the initial and most important process is lymphangiogenesis (42–44), which is regulated by members of the vascular endothelial growth factor (VEGF) family and their receptors (43–46). Cell migration is another important process in nodal metastasis, and Wnt-5a is thought to be among the cell migration-associated molecules involved in gastric cancer (47, 48). In addition, it is well accepted that cancer stem cells play a significant role in nodal metastasis of gastric cancer (48–50) (Figure 3).
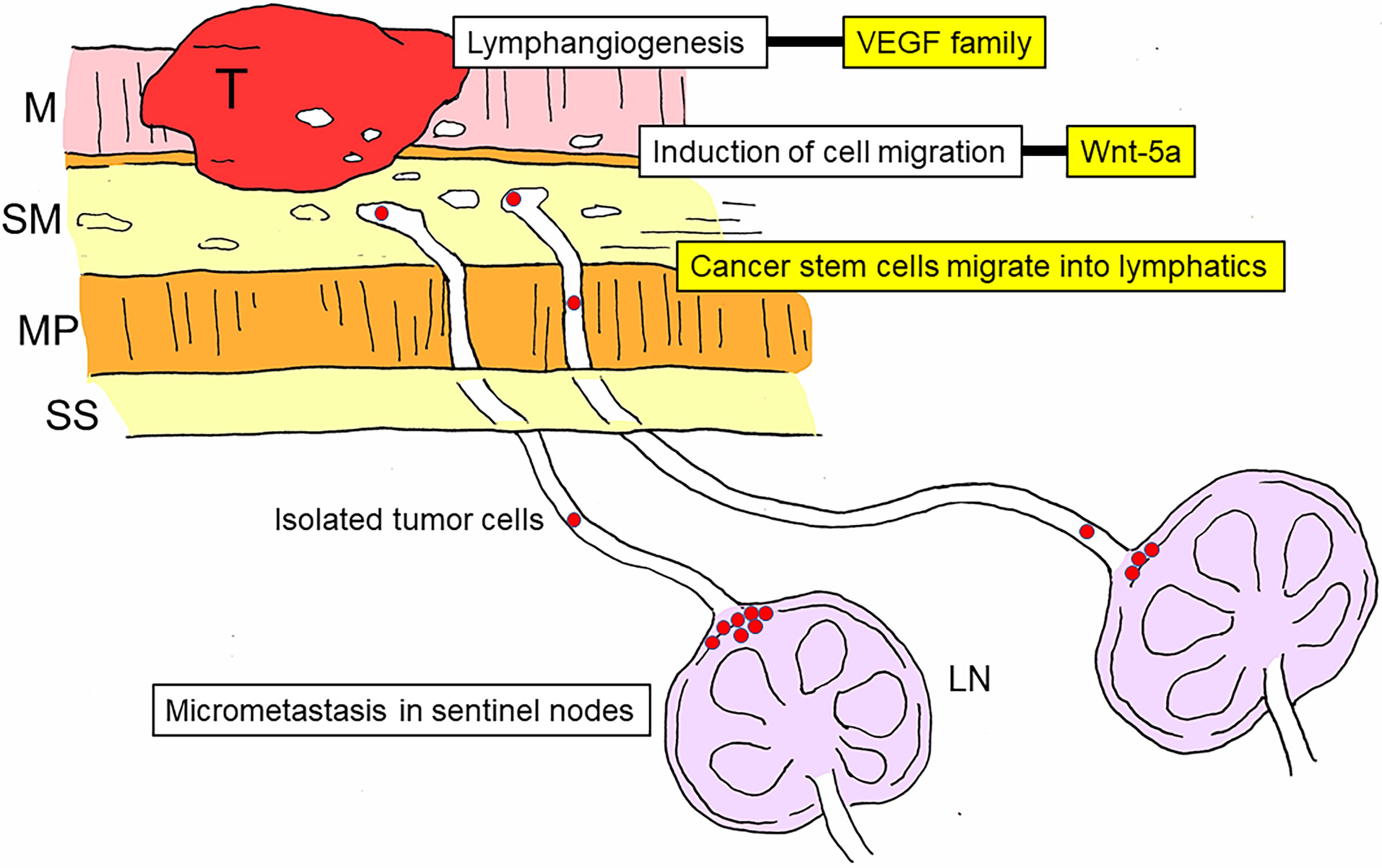
Figure 3 Schematic diagram of the development and molecular mechanisms of nodal metastasis in gastric cancer. Lymph node metastasis can be divided into multiple stages: lymphangiogenesis, induction of cell migration, invasion of cancer stem cells into the lymphatic system, arrival of cancer stem cells in sentinel lymph nodes, and establishment of micrometastasis in the marginal sinus. The vascular endothelial growth factor (VEGF) family is involved in lymphangiogenesis, and Wnt-5a is involved in the induction of cell migration. T, tumor; M, mucosal layer; SM, submucosal layer; MP, proper muscle layer; SS, subserosal layer; LN, lymph node; VEGF, vascular endothelial growth factor.
Standard Lymph Node Dissection Strategy for Gastric Cancer
Lymph node dissection for gastric cancer was well studied in Japan during the 20th century. Following the studies related to the frequency of lymph node metastasis and the prognosis of nodal dissection, the results were consolidated by the Japanese Research Society for Gastric Cancer, which revised the classification of station numbers and proposed extent of nodal dissection. In the past, the extent of nodal dissection was determined based on the location of gastric cancer (23), and strategies were based on the results of retrospective studies. The importance of prospective studies is now recognized in the field of surgical treatment. Since lymph node dissection allows for an accurate pathological diagnosis of nodal metastasis, leading to so-called stage migration (51–53), retrospective studies may overestimate the effect of lymph node dissection. Thus, the actual outcome of nodal dissection can only be evaluated in prospective studies.
Two prospective clinical trials were conducted to determine the influence of the extent of nodal dissection in gastric cancer surgery. One is JCOG9501 trial (53–56). At that time, approximately 10% of patients with para-aortic nodal metastasis survived for 5 years if these nodes were dissected (51). To verify the efficacy of para-aortic nodal dissection, a prospective randomized clinical trial was conducted, in which patients with advanced gastric cancer were randomly assigned to two treatment groups: the study group with lymph node dissection up to D2 plus para-aortic lymph node (PAN) dissection and the control group with nodal dissection up to D2 only. The results indicated that PAN dissection did not significantly influence outcomes, as there was little difference in the incidence of complications (55) or survival prognosis (54) between the two groups. In this study, metastases were histologically detected in 8.5% of patients who underwent D2 plus PAN dissection, and the 5-year overall survival rate was 18.2%. Given that nature of prospective randomized trials, these findings support the notion that some patients with latent pathological PAN metastases can survive for 5 years without dissection. In other words, although extended lymph node dissection may be effective for accurate staging, it does not improve survival.
Another important prospective trial was the aforementioned Dutch trial (36, 57), which compared the therapeutic effects of D1 and D2. This trial is significant in that a famous Japanese gastric surgeon provided guidance on the surgical techniques in the Netherlands. Initially, there was no difference in the 5-year survival rate between the D1 and D2 groups (57); however, after 15 years of follow-up, survival outcomes were better among patients who had undergone D2 than among those who had undergone D1 (36). The initial lack of difference in survival was attributed to the relatively greater invasiveness and apparently higher surgery-related mortality of D2 than D1. This study exposed the difficulties of prospective trials of surgical treatment in terms of quality control and evaluation methods. Nevertheless, it was important for demonstrating the superiority of D2 over D1 in patients with advanced gastric cancer. When the results of the JCOG9501 and Dutch trials are integrated, they demonstrate that D2 can improve the prognosis for advanced gastric cancer but that D2 plus PAN dissection has no effect. Furthermore, the integrated results indicate that Asian patients can tolerate the invasiveness of lymph node dissection while Western patients not.
After the clear survival advantages of adjuvant chemotherapy and adjuvant chemoradiotherapy for gastric cancer, prospective studies on lymph node dissection have become less common. Therefore, even though there is a consensus that appropriate prophylactic lymph node dissection improves prognosis, there is not enough evidence to determine the appropriate extent of dissection. The appropriate dissection range for gastric cancer in Western patients and patients with early gastric cancer, obesity, or comorbidities remains to be determined, and the superiority of D2 over D1+ remains unresolved. Currently, the range of D2 is specified for each gastrectomy method in the Japanese guidelines (58, 59), but the validity of each is unknown because no prospective studies have been conducted.
Challenges in the Preoperative Diagnosis of Lymph Node Metastasis in Gastric Cancer
Preoperative diagnosis of lymph node metastasis is essential for planning proper surgical treatment. However, it is difficult (60), and two factors make the preoperative diagnosis of nodal metastasis difficult: (a) The regional lymph nodes are in the abdominal cavity, and (b) approximately half of lymph node metastases are microscopic (61, 62). According to a recent study on diagnostic imaging (61), which classified metastatic lymph nodes based on microscopic metastatic morphology, 42.5% of metastatic lymph nodes in gastric cancer were of the small nodular type and peripheral type. Lesion volumes for these types are smaller than those for the large nodular and diffuse types.
Researchers have also examined the significance of ultrasonography for diagnosing lymph node metastasis; however, several studies have demonstrated that gastric cancer ultrasonography does not play a major role in diagnosing lymph node metastasis (60, 63, 64). The most important regional lymph nodes in breast cancer are the axillary lymph nodes, which are located under the skin, making ultrasonographic diagnosis easy and ultrasound-guided needle biopsy possible (65–67). On the other hand, since the regional lymph nodes of gastric cancer are in the abdominal cavity, it is difficult to make an ultrasonographic diagnosis from the body surface unless the patient has advanced metastasis, and needle biopsy is not possible. Endoscopic ultrasonography allows for observation within the lumen of the stomach and is more useful than ultrasonography on the body surface (68–70). In esophageal cancer, many of the regional lymph nodes are within the mediastinum around the esophagus; therefore, endoscopic ultrasonography, endoscopic ultrasound-guided elastography, and ultrasound-guided needle biopsy are extremely useful for preoperative diagnosis of lymph node metastasis (70–73). On the other hand, in gastric cancer, although most of the regional lymph nodes are located near the stomach, they lie within the perigastric mesentery (e.g., omentum), and the arteriovenous system runs in the immediate vicinity. Thus, even with endoscopic ultrasonography, it is difficult to visualize regional lymph nodes using ultrasound-guided needle biopsy (60, 74).
Computed tomography (CT) is the most important tool for preoperative nodal diagnosis of gastric cancer (60), and its diagnostic accuracy has increased with technological improvements in equipment (75). Currently, multi-detector spiral CT (MDCT) is widely used (75–82). The advantages of MDCT include objective anatomical imaging and superior spatial resolution. At present, with three-dimensional imaging and multi-planar reconstruction technology, it is possible to determine the precise position and shape of lymph nodes in all directions using different sections. However, even with MDCT, the preoperative nodal diagnosis of gastric cancer is not always satisfactory (81, 82). In a multicenter study concerning preoperative diagnosis of stage III gastric cancer, the sensitivity and specificity of the diagnostic method were 62.5% and 65.7%, respectively (81). A standard nodal diagnosis by MDCT is made based on the assumption that the size of the metastatic lymph node is large (60, 61, 75–82). However, metastatic nodes are not necessarily large (61, 62). In addition, the increased resolution does not mean that all regional lymph nodes can be visualized (61), and accurate calculation of diagnostic ability is difficult given the difficulty in achieving one-to-one correspondence between imaging and pathological diagnosis. In a recent article, the threshold from the receiver operating characteristic (ROC) curve was 7.6 mm in the long axis, and the sensitivity and specificity of the diagnostic method were 86.8% and 80.1%, respectively. However, after one-to-one correspondence based on accurate mapping and measurement of nodal size on resected specimens, the sensitivity and specificity were 91.4% and 47.3%, respectively, even when using the same threshold (61). The reason for such high sensitivity is that large nodes are often metastatic, while the low specificity can be explained by the fact that many metastatic nodes in gastric cancer are small. In this study, 56.3% of the metastatic nodes were below the threshold (61). Thus, patients diagnosed as positive for metastasis using MDCT are extremely likely to be positive for metastasis, but it is difficult to make a definitive diagnosis in node-negative patients. In other words, although MDCT diagnosis is beneficial for advanced gastric cancer, it is not suitable for confirming node-negative early gastric cancer (61, 82). One meta-analysis has indicated that the diagnostic ability of MDCT for lymph node metastasis of gastric cancer was greater for cases with exposed serosa than for cases without serosa exposure (75).
Positron emission tomography-CT (PET-CT) and magnetic resonance imaging (MRI) have also been used for preoperative imaging of lymph node metastasis (60, 80, 83–88), although neither has surpassed the diagnostic ability of MDCT (60). It is difficult to visualize microscopic metastases, even with PET-CT (83, 84). Although attempts have been made to diagnose nodal metastasis with MR lymphography using an ultrasmall superparamagnetic iron oxide (USPIO) contrast medium (88), no sufficient diagnostic results have been reported.
Some articles have reported attempts to improve the diagnostic imaging accuracy for nodal metastasis in gastric cancer. Since diagnostic imaging can determine not only the presence and size of lymph nodes but also the morphology, attempts have been made to improve its accuracy in diagnosing metastasis by taking these factors into consideration. EUS not only identifies perigastric lymph nodes, it also visualizes some internal structure of the lymph nodes, which can sometimes lead to the detection of the intranodal metastatic lesions (89–91). In MDCT, attempts are being made to recognize metastasis from the aspect ratio of lymph nodes and contrast pattern (60, 61, 76). Since the sizes of the regional lymph nodes in gastric cancer vary depending on the lymph node stations, attempt have been reported to set different threshold values for each station, without making it uniform (79).
An Attempt to Predict Lymph Node Metastasis in Early Gastric Cancer by Using Nomogram and Molecular Makers
There is a limit to diagnosing the presence of lymph node metastasis on diagnostic imaging for gastric cancer. However, if the presence of lymph node metastasis can be inferred from the state of the primary lesion, it may be possible to compensate for the uncertainty in diagnostic imaging. The rate of lymph node metastasis in advanced gastric cancer is high, and the degree of lymph node metastasis is of more importance than the presence or absence of lymph node metastasis. Therefore, it is more important to predict the presence or absence of lymph node metastasis in early gastric cancer than in advanced gastric cancer.
Attempts to calculate regression equations or nomograms for diagnosing the possibility of lymph node metastasis from the clinicopathological factors of early gastric cancer have been reported (92–95). However, unlike extracting the conditions for node-negative patients, attempts to diagnose node-positive patients are not always successful. This type of study generally analyzes patients (who have undergone surgical resection) based on tumor size, site of occupation, histology, depth of invasion, and lymphovascular invasion in resected specimens, but since many of these factors are known after resection, it is difficult to pin-point patients with metastases using only the factors available before surgery.
In order to solve this problem, an attempt to predict the presence of lymph node metastasis by adding molecular markers to clinicopathological factors has been reported. Microarray analysis has led to the observation of gene expressions involved in invasion and metastasis. In gastric cancer as well, upregulation and downregulation of many genes have been observed in relation to lymph node metastasis in basic studies (96–98). However, few molecular markers have proven useful in diagnosing lymph node metastasis in actual clinical specimens. These include VEGF-C (43, 44), EGFR (99), E-cadherin (100, 101), CD44v6 (102), and p53 (103, 104). Unfortunately, these are still in the research stage and have not yet been used in clinical practice. A reason for this is that the usefulness of these assays varies depending on the researcher (102); the heterogeneity of the expression site of the molecular marker may be another reason. In addition, a weak reason is that this is an indirect diagnosis, which does not directly diagnose lymph node metastasis.
Intraoperative Diagnosis of Lymph Node Metastasis in Gastric Cancer
Researchers have investigated the accuracy of evaluating lymph node metastasis in early gastric cancer via sentinel lymph node biopsy (105–108). A sentinel node is defined as a node that directly receives lymphatic drainage from a primary tumor (109). The results of a multicenter prospective study indicated that the sentinel node concept is also valid for early gastric cancer (106). The subject of sentinel node biopsy is a clinical node-negative patient, and although the spread of metastasis is unknown, sentinel node biopsy exhibits excellent performance, making it complementary to MDCT diagnosis (18, 110).
Unfortunately, sentinel node biopsy cannot overcome all the disadvantages of MDCT. First, sentinel node biopsy is beneficial only for patients within the indication, as it is feasible only for cT1N0 gastric cancer of less than 5 cm in size. There are also problems specific to gastric cancer sentinel node biopsy, including the use of lymphatic basin dissection as the standard method, which results in the need for dissection of some regional nodes. Therefore, unlike in cases of breast cancer, sentinel node biopsy is difficult to perform prior to gastrectomy (110). Another disadvantage is that it is technically difficult and requires extensive medical resources (18).
As with other carcinomas, sentinel node biopsy for gastric cancer is advantageous in its capacity for ultra-staging and omitting unnecessary nodal dissection in node-negative patients. As with axillary dissection in patients with breast cancer, researchers have investigated the value of sentinel node biopsy as an indicator for the application of function-preserving curative gastrectomy, which omits nodal dissection and reduces the extent of gastrectomy in patients with gastric cancer. A prospective clinical trial is currently ongoing (108, 111).
Challenges in the Pathological Investigation of Lymph Node Metastasis in Gastric Cancer
Although it is not often mentioned, there are some pitfalls in the pathological determination of lymph node metastases in gastric cancer, including the accuracy of the number of lymph nodes to be examined and the physical limitations in determining pathological metastasis.
In the old Japanese Classification of Gastric Carcinoma (112), the N stage was determined based on the location of lymph node metastasis. At this time, staging was possible when the presence or absence of metastasis of the most distal lymph node was known and harvesting all dissected lymph nodes was not always necessary. However, with this method, an accurate N stage cannot be determined until a certain extent of lymph node dissection is performed. Currently, the N stage is determined based on the number of metastatic lymph nodes (2, 3). This method is useful for generalization because it does not require complicated grouping or extended nodal dissection. However, all dissected lymph nodes must now be sent to pathology for staging, making it necessary to harvest all lymph nodes removed.
Gastric cancer has many regional lymph nodes, many of which are small, and metastases can occur even in these small lymph nodes (61, 62), which makes harvesting the nodes difficult. However, until now, the accuracy of node harvesting has been neglected (113, 114). Worldwide, harvested nodes are likely to be handled by pathologists. The “palpitation method” for discriminating lymph nodes is probably the most practiced method worldwide and can be performed by pathologists (113), but the number of lymph nodes is larger when examined by surgeons or at a specialized facility (115–117). In clinical practice, more than 15 lymph nodes are often targeted for harvesting (118). However, the actual number of affected lymph nodes is higher, and previous studies have reported that the number of lymph nodes harvested after D2 distal gastrectomy can exceed 40 (113, 119, 120). Many reports have suggested that a greater number of harvested lymph nodes is associated with better prognosis (121–123). In other words, although lymph node harvesting is an important prognostic factor, quality control remains inadequate. The packet submission method has been proposed as a strategy for improving accuracy (115, 124, 125). Although the detection accuracy of the fat-cleaning method has also been reported (126), the time and effort required for fixation, dyeing, and harvesting are extensive.
There are also physical limitations to pathological determination of nodal metastasis. In general, lymph node metastasis is determined via microscopic examination of the largest section containing the hilus after hematoxylin and eosin (H&E) staining (2, 3). However, this method can only detect metastatic lesions in the section and may not detect metastasis in the initial image. Lymph node metastasis is thought to progress from cancer cells that flow from the primary lesion into the lymphatic system and reach the marginal sinuses of nodes (110), and the initial images reflect isolated tumor cells (ITCs) and micrometastases. The IJCC staging manual defines an ITC as a metastatic lesion of 0.2 mm or less and a micrometastasis as a metastatic lesion of 2 mm or less (1). Although ITCs do not affect the prognosis of gastric cancer (127), micrometastasis is often reported to worsen the prognosis (128, 129). In other words, when metastasis is determined via microscopic examination of the H&E-stained section as usual, a certain degree of error must be considered when determining the number of metastases. For accurate detection of all micrometastases, all retrieved lymph nodes should be subjected to multiple sectioning at 2-mm intervals, which is not practical. An alternative to multiple sectioning is molecular diagnosis of homogenized whole lymph nodes (129). However, there are also challenges to overcome in molecular diagnosis, such as the selection of the gene amplification method, primer selection, contamination, pseudogenes, and cost, making it impractical for use in actual clinical practice. Furthermore, how the results of molecular diagnosis should be used to determine prognosis remains unknown.
Current State and Future Developments in the Evaluation of Lymph Node Metastasis in Gastric Cancer
To a certain extent, nodal metastasis of gastric cancer can be cured via lymph node dissection when limited to the perigastric nodes. Therefore, preoperative nodal diagnosis is important for planning surgical treatment in patients with gastric cancer. In the case of advanced gastric cancer, MDCT can be used for preoperative nodal diagnosis to some extent, although it is difficult to distinguish node-negative patients with early gastric cancer using this method. Sentinel node biopsy can overcome some of the disadvantages of MDCT.
Postoperative nodal metastatic status is also important when determining the strategy for adjuvant chemotherapy after gastrectomy. However, since the degree of nodal metastasis is determined based on the number of metastatic nodes, it should be noted that there are potential problems with the accuracy of harvesting and a possibility of underestimating micrometastasis. A practical solution to this problem would be to add a safety margin when performing lymph node dissection in patients undergoing gastrectomy.
In the remainder future developments surrounding lymph node metastasis in gastric cancer will be discussed.
Advancements in CT equipment and diagnosis are expected to continue. Improvements in artificial intelligence (AI) supported diagnosis will likely increase the accuracy of nodal diagnosis, rather than finer resolution and more detailed 3D construction (130–132). Although lymph node morphology and contrast patterns have been useful for nodal diagnosis (133), AI diagnosis is likely to surpass this. Advances in intraoperative nodal diagnosis are also expected. Indeed, recent studies have attempted to detect tumor antigens, enzymes produced by tumors, or stromal reactions surrounding metastases using fluorescence observation for rapid intraoperative diagnosis of metastasis (134–140).
Controlling the accuracy of harvesting remains critical, therefore, thorough analysis of the associated difficulties are required to develop simple standardized methods with better accuracy and objectivity than the palpitation method. The most promising method is the fat-dissociation method (113), which has been reported to be useful for shortening the time and improving the accuracy of node harvesting in patients with gastric cancer. Other methods such as indocyanine green fluorescence and methylene blue staining methods have also been proposed (141, 142).
In addition, therapeutic strategies targeting lymph node metastases, especially sentinel lymph node metastases in the case of molecular targeting therapy, have been considered (143). If such techniques prove useful, the significance of lymph node metastasis will extend beyond a mere basis for staging, and it will become an essential factor when planning more effective adjuvant therapy and treatment strategies for recurrence.
Chemotherapy for advanced gastric cancer has also progressed, and conversion gastrectomy can be performed after downstaging with chemotherapy, even in patients who are not eligible for radical resection (144, 145). It is important to diagnose nodal metastasis and determine its influence on therapeutic efficacy in such patients. To further enhance the effect of conversion gastrectomy following chemotherapy, prompt judgments of diagnosis and the chemotherapeutic effect are essential. Currently, PET-CT is useful; however, there are expectations for improved CT diagnosis using AI.
Author Contributions
SK was responsible for the scientific conception of the study and writing of the manuscript. All authors (SK, HS, and HT) contributed to the literature review, data analysis, drafting, editing, critical revision of the manuscript, and approval of the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Brierley J, Gospodarowicz M, Wittekind C. UICC TNM Classification of Malignant Tumours. 8th. Chichester: Wiley (2017).
2. Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma. 15th. Tokyo: Kanehara Shuppan (2017).
3. Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma: 3rd English Edition. Gastric Cancer (2011) 14(2):101–12. doi: 10.1007/s10120-011-0041-5
4. Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJHV, Nicolson M, et al. Perioperative Chemotherapy Versus Surgery Alone for Resectable Gastroesophageal Cancer. N Engl J Med (2006) 355(1):11–20. doi: 10.1056/NEJMoa055531
5. Ronellenfitsch U, Schwarzbach M, Hofheinz R, Kienle P, Kieser M, Slanger TE, et al. Perioperative Chemo(Radio)Therapy Versus Primary Surgery for Resectable Adenocarcinoma of the Stomach, Gastroesophageal Junction, and Lower Esophagus. Cochrane Database Syst Rev (2013) 31(5):CD008107. doi: 10.1002/14651858.CD008107.pub2
6. Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, et al. Five-Year Outcomes of a Randomized Phase III Trial Comparing Adjuvant Chemotherapy With S-1 Versus Surgery Alone in Stage II or III Gastric Cancer. J Clin Oncol (2011) 29(33):4387–93. doi: 10.1200/JCO.2011.36.5908
7. Noh SH, Park SR, Yang HK, Chung HC, Chung IJ, Kim SW, et al. Adjuvant Capecitabine Plus Oxaliplatin for Gastric Cancer After D2 Gastrectomy (Classic): 5-Year Follow-Up of an Open-Label, Randomised Phase 3 Trial. Lancet Oncol (2014) 15(12):1389–96. doi: 10.1016/S1470-2045(14)70473-5
8. Yoshida K, Kodera Y, Kochi M, Ichikawa W, Kakeji Y, Sano T, et al. Addition of Docetaxel to Oral Fluoropyrimidine Improves Efficacy in Patients With Stage III Gastric Cancer: Interim Analysis of JACCRO GC-07, a Randomized Controlled Trial. J Clin Oncol (2019) 37(15):1296–304. doi: 10.1200/JCO.18.01138
9. Katai H, Ishikawa T, Akazawa K, Isobe Y, Miyashiro I, Oda I, et al. Five-Year Survival Analysis of Surgically Resected Gastric Cancer Cases in Japan: A Retrospective Analysis of More Than 100,000 Patients From the Nationwide Registry of the Japanese Gastric Cancer Association(2001–2007). Gastric Cancer (2018) 21(1):144–54. doi: 10.1007/s10120-017-0716-7
10. Kikuchi S, Kuroda S, Nishizaki M, Kagawa T, Kanzaki H, Kawahara Y, et al. Management of Early Gastric Cancer That Meet the Indication for Radical Lymph Node Dissection Following Endoscopic Resection: A Retrospective Cohort Analysis. BMC Surg (2017) 17(1):72. doi: 10.1186/s12893-017-0268-0
11. Lee JH, Yom CK, Han HS. Comparison of Long-Term Outcomes of Laparoscopy-Assisted and Open Distal Gastrectomy for Early Gastric Cancer. Surg Endosc (2009) 23(8):1759–63. doi: 10.1007/s00464-008-0198-0
12. Deng JY, Liang H. Clinical Significance of Lymph Node Metastasis in Gastric Cancer. World J Gastroenterol (2014) 20(14):3967–75. doi: 10.3748/wjg.v20.i14.3967
13. Coburn N, Cosby R, Klein L, Knight G, Malthaner R, Mamazza J, et al. Staging and Surgical Approaches in Gastric Cancer: A Systematic Review. Cancer Treat Rev (2018) 63:104–15. doi: 10.1016/j.ctrv.2017.12.006
14. Kumagai K, Sano T, Natsugoe S eds. Part III Lymph Node Metastasis: Clinical Significance Gastric Cancer. In: Lymph Node Metastasis in Gastrointestinal Cancer. Singapore: Springer Nature Singapore Pte Ltd. p. 267–82. doi: 10.1007/978-981-10-4699-5_14
15. Tasaka S. National Survey of Early Gastric Cancer (Japanese). Gastroenterol Endosc (1962) 4:4–14.
16. Koufuji K, Takeda J, Hashimoto K, Tanaka T, Kakegawa T. Early Gastric Cancer Associated With Synchronous Liver Metastasis. Kurume Med J (1991) 38(4):271–4. doi: 10.2739/kurumemedj.38.271
17. Nakajima S, Yamaguchi T. Gastric Cancer Database of the Cancer Institute Hospital 1946–2004 (Japanese). Tokyo: Kanehara Shuppan (2006).
18. Kinami S, Nakamura N, Tomita Y, Miyata T, Fujita H, Ueda N, et al. Precision Surgical Approach With Lymph-Node Dissection in Early Gastric Cancer. World J Gastroenterol (2019) 25(14):1640–52. doi: 10.3748/wjg.v25.i14.1640
19. Lehnert T, Erlandson RA, Decosse JJ. Lymph and Blood Capillaries of the Human Gastric Mucosa. A Morphologic Basis for Metastasis in Early Gastric Carcinoma. Gastroenterology (1985) 89(5):939–50. doi: 10.1016/0016-5085(85)90192-1
20. Alexander JS, Ganta VC, Jordan PA, Witte MH. Gastrointestinal Lymphatics in Health and Disease. Pathophysiology (2010) 17(4):315–35. doi: 10.1016/j.pathophys.2009.09.003
21. Yonemura Y, Endou Y, Tabachi K, Kawamura T, Yun HY, Kameya T, et al. Evaluation of Lymphatic Invasion in Primary Gastric Cancer by a New Monoclonal Antibody, D2-40. Hum Pathol (2006) 37(9):1193–9. doi: 10.1016/j.humpath.2006.04.014
22. Tanaka N, Katai H, Taniguchi H, Saka M, Morita S, Fukagawa T, et al. Surgical Treatment for Early Gastric Cancer (in Japanese With English Abstract). Stomach Intest (2009) 44:700–6. doi: 10.11477/mf.1403101642
23. Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma. 13th. Tokyo: Kanehara Shuppan (1999).
24. Makino M, Moriwaki S, Yonekawa M, Oota M, Kimura O, Kaibara N. Prognostic Significance of the Number of Metastatic Lymph Nodes in Patients With Gastric Cancer. J Surg Oncol (1991) 47(1):12–6. doi: 10.1002/jso.2930470104
25. Ichikura T, Tomimatsu S, Okusa Y, Uefuji K, Tamakuma S. Comparison of the Prognostic Significance Between the Number of Metastatic Lymph Nodes and Nodal Stage Based on Their Location in Patients With Gastric Cancer. J Clin Oncol (1993) 11(10):1894–900. doi: 10.1200/JCO.1993.11.10.1894
26. Wu CW, Hsieh MC, Lo SS, Tsay SH, Lui WY, P’eng FK. Relation of Number of Positive Lymph Nodes to the Prognosis of Patients With Primary Gastric Adenocarcinoma. Gut (1996) 38(4):525–7. doi: 10.1136/gut.38.4.525
27. Karpeh MS, Leon L, Klimstra D, Brennan MF. Lymph Node Staging in Gastric Cancer: Is Location More Important Than Number? An Analysis of 1,038 Patients. Ann Surg (2000) 232(3):362–71. doi: 10.1097/00000658-200009000-00008
28. Giuliano AE, Hunt KK, Ballman KV, Beitsch PD, Whitworth PW, Blumencranz PW, et al. Axillary Dissection vs No Axillary Dissection in Women With Invasive Breast Cancer and Sentinel Node Metastasis: A Randomized Clinical Trial. JAMA (2011) 305(6):569–75. doi: 10.1001/jama.2011.90
29. Giuliano AE, Ballman KV, McCall L, Beitsch PD, Brennan MB, Kelemen PR, et al. Effect of Axillary Dissection vs No Axillary Dissection on 10-Year Overall Survival Among Women With Invasive Breast Cancer and Sentinel Node Metastasis: The ACOSOG Z0011 (Alliance) Randomized Clinical Trial. JAMA (2017) 318(10):918–26. doi: 10.1001/jama.2017.11470
30. Jatoi I, Benson JR, Toi M. De-Escalation of Axillary Surgery in Early Breast Cancer. Lancet Oncol (2016) 17(10):e430–41. doi: 10.1016/S1470-2045(16)30311-4
31. Anania G, Davies RJ, Arezzo A, Bagolini F, D’Andrea V, Graziosi L, et al. Rise and Fall of Total Mesorectal Excision With Lateral Pelvic Lymphadenectomy for Rectal Cancer: An Updated Systematic Review and Meta-Analysis of 11,366 Patients. Int J Colorectal Dis (2021) 36(11):2321–33. doi: 10.1007/s00384-021-03946-2
32. de Lima Vazquez V, Sachetto T, Perpetuo NM, Carvalho AL. Prognostic Factors for Lymph Node Metastasis From Advanced Squamous Cell Carcinoma of the Skin of the Trunk and Extremities. World J Surg Oncol (2008) 6(6: 73):73. doi: 10.1186/1477-7819-6-73
33. Panici PB, Maggioni A, Hacker N, Landoni F, Ackermann S, Campagnutta E, et al. Systematic Aortic and Pelvic Lymphadenectomy Versus Resection of Bulky Nodes Only in Optimally Debulked Advanced Ovarian Cancer: A Randomized Clinical Trial. J Natl Cancer Inst (2005) 97(8):560–6. doi: 10.1093/jnci/dji102
34. Nimura Y, Nagino M, Takao S, Takada T, Miyazaki K, Kawarada Y, et al. Standard Versus Extended Lymphadenectomy in Radical Pancreatoduodenectomy for Ductal Adenocarcinoma of the Head of the Pancreas: Long-Term Results of a Japanese Multicenter Randomized Controlled Trial. J Hepatobiliary Pancreat Sci (2012) 19(3):230–41. doi: 10.1007/s00534-011-0466-6
35. Hu H, Xu G, Du S, Luo Z, Zhao H, Cai J. The Role of Lymph Node Dissection in Intrahepatic Cholangiocarcinoma: A Multicenter Retrospective Study. BMC Surg (2021) 21(1):359. doi: 10.1186/s12893-021-01363-4
36. Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical Treatment of Gastric Cancer: 15-Year Follow-Up Results of the Randomised Nationwide Dutch D1D2 Trial. Lancet Oncol (2010) 11(5):439–49. doi: 10.1016/S1470-2045(10)70070-X
37. Wu CW, Hsiung CA, Lo SS, Hsieh MC, Chen JH, Li AF, et al. Nodal Dissection for Patients With Gastric Cancer: A Randomised Controlled Trial. Lancet Oncol (2006) 7(4):309–15. doi: 10.1016/S1470-2045(06)70623-4
38. Zhu T, Hu X, Wei P, Shan G. Molecular Background of the Regional Lymph Node Metastasis of Gastric Cancer. Oncol Lett (2018) 15(3):3409–14. doi: 10.3892/ol.2018.7813
39. Eccles SA, Welch DR. Metastasis: Recent Discoveries and Novel Treatment Strategies. Lancet (2007) 369(9574):1742–57. doi: 10.1016/S0140-6736(07)60781-8
40. Langheinrich MC, Schellerer V, Perrakis A, Lohmüller C, Schildberg C, Naschberger E, et al. Molecular Mechanisms of Lymphatic Metastasis in Solid Tumors of the Gastrointestinal Tract. Int J Clin Exp Pathol (2012) 5(7):614–23.
41. Achen MG, Stacker SA. Molecular Control of Lymphatic Metastasis. Ann NY Acad Sci (2008) 1131:225–34. doi: 10.1196/annals.1413.020
42. Sundar SS, Ganesan TS. Role of Lymphangiogenesis in Cancer. J Clin Oncol (2007) 25(27):4298–307. doi: 10.1200/JCO.2006.07.1092
43. Yonemura Y, Endo Y, Tabata K, Kawamura T, Yun HY, Bandou E, et al. Role of VEGF-C and VEGF-D in Lymphangiogenesis in Gastric Cancer. Int J Clin Oncol (2005) 10(5):318–27. doi: 10.1007/s10147-005-0508-7
44. Amioka T, Kitadai Y, Tanaka S, Haruma K, Yoshihara M, Yasui W, et al. Vascular Endothelial Growth Factor-C Expression Predicts Lymph Node Metastasis of Human Gastric Carcinomas Invading the Submucosa. Eur J Cancer (2002) 38(10):1413–9. doi: 10.1016/s0959-8049(02)00106-5
45. Kitadai Y. Angiogenesis and Lymphangiogenesis of Gastric Cancer. J Oncol (2010) 2010:468725. doi: 10.1155/2010/468725
46. Stacker SA, Achen MG, Jussila L, Baldwin ME, Alitalo K. Lymphangiogenesis and Cancer Metastasis. Nat Rev Cancer (2002) 2(8):573–83. doi: 10.1038/nrc863
47. Kurayoshi M, Oue N, Yamamoto H, Kishida M, Inoue A, Asahara T, et al. Expression of Wnt-5a Is Correlated With Aggressiveness of Gastric Cancer by Stimulating Cell Migration and Invasion. Cancer Res (2006) 66(21):10439–48. doi: 10.1158/0008-5472.CAN-06-2359
48. Yamamoto H, Kitadai Y, Yamamoto H, Oue N, Ohdan H, Yasui W, et al. Laminin Gamma2 Mediates Wnt5a-Induced Invasion of Gastric Cancer Cells. Gastroenterology (2009) 137(1):242–52, 252.e1, 252.e1–252.e6. doi: 10.1053/j.gastro.2009.02.003
49. Takaishi S, Okumura T, Tu S, Wang SS, Shibata W, Vigneshwaran R, et al. Identification of Gastric Cancer Stem Cells Using the Cell Surface Marker CD44. Stem Cells (2009) 27(5):1006–20. doi: 10.1002/stem.30
50. Wakamatsu Y, Sakamoto N, Oo HZ, Naito Y, Uraoka N, Anami K, et al. Expression of Cancer Stem Cell Markers ALDH1, CD44 and CD133 in Primary Tumor and Lymph Node Metastasis of Gastric Cancer. Pathol Int (2012) 62(2):112–9. doi: 10.1111/j.1440-1827.2011.02760.x
51. Takashima S, Kosaka T. Results and Controversial Issues Regarding a Para-Aortic Lymph Node Dissection for Advanced Gastric Cancer. Surg Today (2005) 35(6):425–31. doi: 10.1007/s00595-004-2976-1
52. Sano T, Katai H, Sasako M, Maruyama K. Problems of International Standardization of Gastric Cancer Surgery (Japanese). Nihon Geka Gakkai Zasshi (2001) 102(10):758–63.
53. Yoshikawa T, Sasako M, Sano T, Nashimoto A, Kurita A, Tsujinaka T, et al. Stage Migration Caused by D2 Dissection With Para-Aortic Lymphadenectomy for Gastric Cancer From the Results of a Prospective Randomized Controlled Trial. Br J Surg (2006) 93(12):1526–9. doi: 10.1002/bjs.5487
54. Sasako M, Sano T, Yamamoto S, Kurokawa Y, Nashimoto A, Kurita A, et al. D2 Lymphadenectomy Alone or With Para-Aortic Nodal Dissection for Gastric Cancer. N Engl J Med (2008) 359(5):453–62. doi: 10.1056/NEJMoa0707035
55. Sano T, Sasako M, Yamamoto S, Nashimoto A, Kurita A, Hiratsuka M, et al. Gastric Cancer Surgery: Morbidity and Mortality Results From a Prospective Randomized Controlled Trial Comparing D2 and Extended Para-Aortic Lymphadenectomy - Japan Clinical Oncology Group Study 9501. J Clin Oncol (2004) 22(14):2767–73. doi: 10.1200/JCO.2004.10.184
56. Tsujinaka T, Sasako M, Yamamoto S, Sano T, Kurokawa Y, Nashimoto A, et al. Influence of Overweight on Surgical Complications for Gastric Cancer: Results From a Randomized Control Trial Comparing D2 and Extended Para-Aortic D3 Lymphadenectomy (JCOG9501). Ann Surg Oncol (2007) 14(2):355–61. doi: 10.1245/s10434-006-9209-3
57. Bonenkamp JJ, Hermans J, Sasako M, van de Velde CJ, Welvaart K, Songun I, et al. Extended Lymph-Node Dissection for Gastric Cancer. N Engl J Med (1999) 340(12):908–14. doi: 10.1056/NEJM199903253401202
58. Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2021. 6th. Tokyo: Kanehara Shuppan (2021).
59. Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2018 5th Ed. Gastric Cancer (2021) 24:1–21. doi: 10.1007/s10120-020-01042-y
60. Kwee RM, Kwee TC. Imaging in Assessing Lymph Node Status in Gastric Cancer. Gastric Cancer (2009) 12(1):6–22. doi: 10.1007/s10120-008-0492-5
61. Jiang ZY, Kinami S, Nakamura N, Miyata T, Fujita H, Takamura H, et al. Diagnostic Ability of Multi-Detector Spiral Computed Tomography for Pathological Lymph Node Metastasis of Advanced Gastric Cancer. World J Gastrointest Oncol (2020) 12(4):435–46. doi: 10.4251/wjgo.v12.i4.435
62. Noda N, Sasako M, Yamaguchi N, Nakanishi Y. Ignoring Small Lymph Nodes can be a Major Cause of Staging Error in Gastric Cancer. Br J Surg (1998) 85(6):831–4. doi: 10.1046/j.1365-2168.1998.00691.x
63. Smeets AJ, Zonderland HM, van der Voorde F, Laméris JS. Evaluation of Abdominal Lymph Nodes by Ultrasound. J Ultrasound Med (1990) 9(6):325–31. doi: 10.7863/jum.1990.9.6.325
64. Liao SR, Dai Y, Huo L, Yan K, Zhang L, Zhang H, et al. Transabdominal Ultrasonography in Preoperative Staging of Gastric Cancer. World J Gastroenterol (2004) 10(23):3399–404. doi: 10.3748/wjg.v10.i23.3399
65. Alvarez S, Añorbe E, Alcorta P, López F, Alonso I, Cortés J. Role of Sonography in the Diagnosis of Axillary Lymph Node Metastases in Breast Cancer: A Systematic Review. AJR (2006) 186(5):1342–8. doi: 10.2214/AJR.05.0936
66. Swinson C, Ravichandran D, Nayagam M, Allen S. Ultrasound and Fine Needle Aspiration Cytology of the Axilla in the Pre-Operative Identification of Axillary Nodal Involvement in Breast Cancer. Eur J Surg Oncol (2009) 35(11):1152–7. doi: 10.1016/j.ejso.2009.03.008
67. Gipponi M, Fregatti P, Garlaschi A, Murelli F, Margarino C, Depaoli F, et al. Axillary Ultrasound and Fine-Needle Aspiration Cytology in the Preoperative Staging of Axillary Node Metastasis in Breast Cancer Patients. Breast (2016) 30:146–50. doi: 10.1016/j.breast.2016.09.009
68. Mocellin S, Marchet A, Nitti D. EUS for the Staging of Gastric Cancer: A Meta-Analysis. Gastrointest Endosc (2011) 73(6):1122–34. doi: 10.1016/j.gie.2011.01.030
69. Mocellin S, Pasquali S. Diagnostic Accuracy of Endoscopic Ultrasonography (EUS) for the Preoperative Locoregional Staging of Primary Gastric Cancer. Cochrane Database Syst Rev (2015) 6(2):CD009944. doi: 10.1002/14651858.CD009944.pub2
70. Tamanini G, Cominardi A, Brighi N, Fusaroli P, Lisotti A. Endoscopic Ultrasound Assessment and Tissue Acquisition of Mediastinal and Abdominal Lymph Nodes. World J Gastrointest Oncol (2021) 13(10):1475–91. doi: 10.4251/wjgo.v13.i10.1475
71. Paterson S, Duthie F, Stanley AJ. Endoscopic Ultrasound-Guided Elastography in the Nodal Staging of Oesophageal Cancer. World J Gastroenterol (2012) 18(9):889–95. doi: 10.3748/wjg.v18.i9.889
72. Harrington C, Smith L, Bisland J, López González EL, Jamieson N, Paterson S, et al. Mediastinal Node Staging by Positron Emission Tomography-Computed Tomography and Selective Endoscopic Ultrasound With Fine Needle Aspiration for Patients With Upper Gastrointestinal Cancer: Results From a Regional Centre. World J Gastrointest Endosc (2018) 10(1):37–44. doi: 10.4253/wjge.v10.i1.37
73. Sazuka T, Akai T, Uesato M, Horibe D, Kuboshima M, Kitabayashi H, et al. Assessment for Diagnosis of Lymph Node Metastasis in Esophageal Cancer Using Endoscopic Ultrasound Elastography. Esophagus (2016) 13:254–63. doi: 10.1007/s10388-016-0521-0
74. Bentrem D, Gerdes H, Tang L, Brennan M, Coit D. Clinical Correlation of Endoscopic Ultrasonography With Pathologic Stage and Outcome in Patients Undergoing Curative Resection for Gastric Cancer. Ann Surg Oncol (2007) 14(6):1853–9. doi: 10.1245/s10434-006-9037-5
75. Luo M, Lv Y, Guo X, Song H, Su G, Chen B. Value and Impact Factors of Multidetector Computed Tomography in Diagnosis of Preoperative Lymph Node Metastasis in Gastric Cancer: A PRISMA-Compliant Systematic Review and Meta-Analysis. Medicine (2017) 96(33):e7769. doi: 10.1097/MD.0000000000007769
76. Wang J, Zhong L, Zhou X, Chen D, Li R. Value of Multiphase Contrast-Enhanced CT With Three-Dimensional Reconstruction in Detecting Depth of Infiltration, Lymph Node Metastasis, and Extramural Vascular Invasion of Gastric Cancer. J Gastrointest Oncol (2021) 12(4):1351–62. doi: 10.21037/jgo-21-276
77. Jiang M, Wang X, Shan X, Pan D, Jia Y, Ni E, et al. Value of Multi-Slice Spiral Computed Tomography in the Diagnosis of Metastatic Lymph Nodes and N-Stage of Gastric Cancer. J Int Med Res (2019) 47(1):281–92. doi: 10.1177/0300060518800611
78. Kubota K, Suzuki A, Shiozaki H, Wada T, Kyosaka T, Kishida A. Accuracy of Multidetector-Row Computed Tomography in the Preoperative Diagnosis of Lymph Node Metastasis in Patients With Gastric Cancer. Gastrointest Tumors (2017) 3(3–4):163–70. doi: 10.1159/000454923
79. Saito T, Kurokawa Y, Takiguchi S, Miyazaki Y, Takahashi T, Yamasaki M, et al. Accuracy of Multidetector-Row CT in Diagnosing Lymph Node Metastasis in Patients With Gastric Cancer. Eur Radiol (2015) 25(2):368–74. doi: 10.1007/s00330-014-3373-9
80. Choi JI, Joo I, Lee JM. State-Of-the-Art Preoperative Staging of Gastric Cancer by MDCT and Magnetic Resonance Imaging. World J Gastroenterol (2014) 20(16):4546–57. doi: 10.3748/wjg.v20.i16.4546
81. Fukagawa T, Katai H, Mizusawa J, Nakamura K, Sano T, Terashima M, et al. A Prospective Multi-Institutional Validity Study to Evaluate the Accuracy of Clinical Diagnosis of Pathological Stage III Gastric Cancer (JCOG1302A). Gastric Cancer (2018) 21(1):68–73. doi: 10.1007/s10120-017-0701-1
82. Ohashi M, Morita S, Fukagawa T, Wada T, Kushima R, Onaya H, et al. Evaluation of 64-Channel Contrast-Enhanced Multi-Detector Row Computed Tomography for Preoperative N Staging in Ct2-4 Gastric Carcinoma. World J Surg (2016) 40(1):165–71. doi: 10.1007/s00268-015-3318-8
83. Yang QM, Kawamura T, Itoh H, Bando E, Nemoto M, Akamoto S, et al. Is PET-CT Suitable for Predicting Lymph Node Status for Gastric Cancer? Hepatogastroenterology (2008) 55(82–83):782–5.
84. Park K, Jang G, Baek S, Song H. Usefulness of Combined PET/CT to Assess Regional Lymph Node Involvement in Gastric Cancer. Tumori (2014) 100(2):201–6. doi: 10.1700/1491.16415
85. Kawanaka Y, Kitajima K, Fukushima K, Mouri M, Doi H, Oshima T, et al. Added Value of Pretreatment (18)F-FDG PET/CT for Staging of Advanced Gastric Cancer: Comparison With Contrast-Enhanced MDCT. Eur J Radiol (2016) 85(5):989–95. doi: 10.1016/j.ejrad.2016.03.003
86. Choi JY, Shim KN, Kim SE, Jung HK, Jung SA, Yoo K. The Clinical Value of 18F-Fluorodeoxyglucose Uptake on Positron Emission Tomography/Computed Tomography for Predicting Regional Lymph Node Metastasis and Non-Curative Surgery in Primary Gastric Carcinoma. Korean J Gastroenterol (2014) 64(6):340–7. doi: 10.4166/kjg.2014.64.6.340
87. Borggreve AS, Goense L, Brenkman HJF, Mook S, Meijer GJ, Wessels FJ, et al. Imaging Strategies in the Management of Gastric Cancer: Current Role and Future Potential of MRI. Br J Radiol (2019) 92(1097):20181044. doi: 10.1259/bjr.20181044
88. Tokuhara T, Tanigawa N, Matsuki M, Nomura E, Mabuchi H, Lee SW, et al. Evaluation of Lymph Node Metastases in Gastric Cancer Using Magnetic Resonance Imaging With Ultrasmall Superparamagnetic Iron Oxide (USPIO): Diagnostic Performance in Post-Contrast Images Using New Diagnostic Criteria. Gastric Cancer (2008) 11(4):194–200. doi: 10.1007/s10120-008-0480-9
89. Catalano MF, Sivak MV, Rice T, Gragg LA, Van Dam JV. Endosonographic Features Predictive of Lymph Node Metastasis. Gastrointest Endosc (1994) 40(4):442–6. doi: 10.1016/s0016-5107(94)70206-3
90. Faige DO. EUS in Patients With Benign and Malignant Lymphadenopathy. Gastrointest Endosc (2001) 53(6):593–8. doi: 10.1067/mge.2001.114060
91. Shimoyama S, Yasuda H, Hashimoto M, Tatsutomi Y, Aoki F, Mafune K, et al. Accuracy of Linear-Array EUS for Preoperative Staging of Gastric Cardia Cancer. Gastrointest Endosc (2004) 60(1):50–5. doi: 10.1016/s0016-5107(04)01312-4
92. Shida A, Fujioka S, Kawamura M, Takahashi N, Ishibashi Y, Nakada K, et al. Prediction of Lymph Node Metastasis in Patients With Submucosa-Invading Early Gastric Cancer. Anticancer Res (2014) 34(8):4471–4.
93. Fujii M, Egashira Y, Akutagawa H, Nishida T, Nitta T, Edagawa G, et al. Pathological Factors Related to Lymph Node Metastasis of Submucosally Invasive Gastric Cancer: Criteria for Additional Gastrectomy After Endoscopic Resection. Gastric Cancer (2013) 16(4):521–30. doi: 10.1007/s10120-012-0215-9
94. Zheng Z, Zhang Y, Zhang L, Li Z, Wu X, Liu Y, et al. A Nomogram for Predicting the Likelihood of Lymph Node Metastasis in Early Gastric Patients. BMC Cancer (2016) 16:92. doi: 10.1186/s12885-016-2132-5
95. Zhao LY, Yin Y, Li X, Zhu CJ, Wang YG, Chen XL, et al. A Nomogram Composed of Clinicopathologic Features and Preoperative Serum Tumor Markers to Predict Lymph Node Metastasis in Early Gastric Cancer Patients. Oncotarget (2016) 7(37):59630–9. doi: 10.18632/oncotarget.10732
96. Hippo Y, Taniguchi H, Tsutsumi S, Machida N, Chong JM, Fukayama M, et al. Global Gene Expression Analysis of Gastric Cancer by Oligonucleotide Microarrays. Cancer Res (2002) 62(1):233–40.
97. Inoue H, Matsuyama A, Mimori K, Ueo H, Mori M. Prognostic Score of Gastric Cancer Determined by cDNA Microarray. Clin Cancer Res (2002) 8(11):3475–9.
98. Oue N, Hamai Y, Mitani Y, Matsumura S, Oshimo Y, Aung PP, et al. Gene Expression Profile of Gastric Carcinoma: Identification of Genes and Tags Potentially Involved in Invasion, Metastasis, and Carcinogenesis by Serial Analysis of Gene Expression. Cancer Res (2004) 64(7):2397–405. doi: 10.1158/0008-5472.can-03-3514
99. Jin EH, Lee DH, Jung SA, Shim KN, Seo JY, Kim N, et al. Clinicopathologic Factors and Molecular Markers Related to Lymph Node Metastasis in Early Gastric Cancer. World J Gastroenterol (2015) 21(2):571–7. doi: 10.3748/wjg.v21.i2.571
100. Yoshii T, Miyagi Y, Nakamura Y, Kobayashi O, Kameda Y, Ohkawa S. Pilot Research for the Correlation Between the Expression Pattern of E-Cadherin-β-Catenin Complex and Lymph Node Metastasis in Early Gastric Cancer. Tumori (2013) 99(2):234–8. doi: 10.1700/1283.14198
101. Piccolo G, Zanghı` A, Di Vita M, Bisagni P, Lecchi F, Cavallaro A, et al. The Role of E-Cadherin Expression in the Treatment of Western Undifferentiated Early Gastric Cancer: Can a Biological Factor Predict Lymph Node Metastasis? PloS One (2020) 15(4):e0232429. doi: 10.1371/journal.pone.0232429
102. Eom BW, Joo J, Park B, Jo MJ, Choi SH, Cho SJ, et al. Nomogram Incorporating CD44v6 and Clinicopathological Factors to Predict Lymph Node Metastasis for Early Gastric Cancer. PloS One (2016) 11(8):e0159424. doi: 10.1371/journal.pone.0159424
103. Wang YW, Zhu ML, Wang RF, Xue WJ, Zhu XR, Wang LF, et al. Predictable Factors for Lymph Node Metastasis in Early Gastric Cancer Analysis of Clinicopathologic Factors and Biological Markers. Tumour Biol (2016) 37(7):8567–78. doi: 10.1007/s13277-015-4721-3
104. Yin XY, Pang T, Liu Y, Cui HT, Luo TH, Lu ZM, et al. Development and Validation of a Nomogram for Preoperative Prediction of Lymph Node Metastasis in Early Gastric Cancer. World J Surg Oncol (2020) 18(1):2. doi: 10.1186/s12957-019-1778-2
105. Miwa K, Kinami S, Taniguchi K, Fushida S, Fujimura T, Nonomura A. Mapping Sentinel Nodes in Patients With Early-Stage Gastric Carcinoma. Br J Surg (2003) 90(2):178–82. doi: 10.1002/bjs.4031
106. Kitagawa Y, Takeuchi H, Takagi Y, Natsugoe S, Terashima M, Murakami N, et al. Sentinel Node Mapping for Gastric Cancer: A Prospective Multicenter Trial in Japan. J Clin Oncol (2013) 31(29):3704–10. doi: 10.1200/JCO.2013.50.3789
107. Takeuchi H, Goto O, Yahagi N, Kitagawa Y. Function-Preserving Gastrectomy Based on the Sentinel Node Concept in Early Gastric Cancer. Gastric Cancer (2017) 20(Suppl 1):53–9. doi: 10.1007/s10120-016-0649-6
108. Park JY, Kim YW, Ryu KW, Nam BH, Lee YJ, Jeong SH, et al. Assessment of Laparoscopic Stomach Preserving Surgery With Sentinel Basin Dissection Versus Standard Gastrectomy With Lymphadenectomy in Early Gastric Cancer-A Multicenter Randomized Phase III Clinical Trial (SENORITA Trial) Protocol. BMC Cancer (2016) 16(16):340. doi: 10.1186/s12885-016-2336-8
109. Morton DL, Wen DR, Wong JH, Economou JS, Cagle LA, Storm FK, et al. Technical Details of Intraoperative Lymphatic Mapping for Early Stage Melanoma. Arch Surg (1992) 127(4):392–9. doi: 10.1001/archsurg.1992.01420040034005
110. Kinami S, Kosaka T, Natsugoe S eds. Part IV Sentinel Node Navigation Surgery: Methodology: Dye and Isotope Method. In: Lymph Node Metastasis in Gastrointestinal Cancer. Singapore: Springer Nature Singapore Pte Ltd. p. 305–21. doi: 10.1007/978-981-10-4699-5_16
111. Kamiya S, Takeuchi H, Fukuda K, Kawakubo H, Takahashi N, Mitsumori N, et al. A Multicenter Non-Randomized Phase III Study of Sentinel Node Navigation Surgery for Early Gastric Cancer. Jpn J Clin Oncol (2021) 51(2):305–9. doi: 10.1093/jjco/hyaa179
112. Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition. Gastric Cancer (1998) 1(1):10–24. doi: 10.1007/s101209800016
113. Kinami S, Ohnishi T, Nakamura N, Jiang ZY, Miyata T, Fujita H, et al. Efficacy of the Fat-Dissociation Method for Nodal Harvesting in Gastric Cancer. World J Gastrointest Surg (2020) 12(6):277–86. doi: 10.4240/wjgs.v12.i6.277
114. Ambrosio MRA, Perotti B, Cavazzana A, Arganini M. How Surgeon and Pathologist Cooperation may Drive Toward a More Efficient Nodes Harvesting in Gastric Cancer Surgery. Updates Surg (2021) 73(5):2025–8. doi: 10.1007/s13304-021-01030-6
115. Ikoma N, Estrella JS, Hofstetter WL, Ajani JA, Fournier KF, Mansfield PF, et al. Surgeon Assessment of Gastric Cancer Lymph Node Specimens With a Video of Technique. J Gastrointest Surg (2018) 22(11):2013–9. doi: 10.1007/s11605-018-3880-0
116. Morgan JW, Ji L, Friedman G, Senthil M, Dyke C, Lum SS. The Role of the Cancer Center When Using Lymph Node Count as a Quality Measure for Gastric Cancer Surgery. JAMA Surg (2015) 150(1):37–43. doi: 10.1001/jamasurg.2014.678
117. Bunt AM, Hermans J, van de Velde CJ, Sasako M, Hoefsloot FA, Fleuren G, et al. Lymph Node Retrieval in a Randomized Trial on Western-Type Versus Japanese-Type Surgery in Gastric Cancer. J Clin Oncol (1996) 14(8):2289–94. doi: 10.1200/JCO.1996.14.8.2289
118. Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, et al. AJCC Cancer Staging Manual. 8th. New York: Springer (2017).
119. Inaki N, Etoh T, Ohyama T, Uchiyama K, Katada N, Koeda K, et al. A Multi-Institutional, Prospective, Phase II Feasibility Study of Laparoscopy-Assisted Distal Gastrectomy With D2 Lymph Node Dissection for Locally Advanced Gastric Cancer (JLSSG0901). World J Surg (2015) 39(11):2734–41. doi: 10.1007/s00268-015-3160-z
120. Hu Y, Huang C, Sun Y, Su X, Cao H, Hu J, et al. Morbidity and Mortality of Laparoscopic Versus Open D2 Distal Gastrectomy for Advanced Gastric Cancer: A Randomized Controlled Trial. J Clin Oncol (2016) 34(12):1350–7. doi: 10.1200/JCO.2015.63.7215
121. Smith DD, Schwarz RR, Schwarz RE. Impact of Total Lymph Node Count on Staging and Survival After Gastrectomy for Gastric Cancer: Data From a Large US-Population Database. J Clin Oncol (2005) 23(28):7114–24. doi: 10.1200/JCO.2005.14.621
122. Macalindong SS, Kim KH, Nam BH, Ryu KW, Kubo N, Kim JY, et al. Effect of Total Number of Harvested Lymph Nodes on Survival Outcomes After Curative Resection for Gastric Adenocarcinoma: Findings From an Eastern High-Volume Gastric Cancer Center. BMC Cancer (2018) 18(1):73. doi: 10.1186/s12885-017-3872-6
123. Lu J, Wang W, Zheng CH, Fang C, Li P, Xie JW, et al. Influence of Total Lymph Node Count on Staging and Survival After Gastrectomy for Gastric Cancer: An Analysis From a Two-Institution Database in China. Ann Surg Oncol (2017) 24(2):486–93. doi: 10.1245/s10434-016-5494-7
124. Cao Y, Xiong L, Deng S, Shen L, Li J, Wu K, et al. The Effect of Perigastric Lipolymphatic Tissue Grouping by Surgeon on the Number of Pathologic Sampled Lymph Nodes After Radical Gastrectomy. Med (Baltimore) (2018) 97(27):e11411. doi: 10.1097/MD.0000000000011411
125. Wang P, Zhang K, Xi H, Liang W, Xie T, Gao Y, et al. Lymph Node Yield Following Packet Submission After Isolation by Surgeon During Gastrectomy. Cancer Manag Res (2019) 11:9871–81. doi: 10.2147/CMAR.S211218
126. Candela FC, Urmacher C, Brennan MF. Comparison of the Conventional Method of Lymph Node Staging With a Comprehensive Fat-Clearing Method for Gastric Adenocarcinoma. Cancer (1990) 66(8):1828–32. doi: 10.1002/1097-0142(19901015)66:8<1828::aid-cncr2820660830>3.0.co;2-z
127. Fukagawa T, Sasako M, Ito S, Nakanishi H, Iinuma H, Natsugoe S, et al. The Prognostic Significance of Isolated Tumor Cells in the Lymph Nodes of Gastric Cancer Patients. Gastric Cancer (2010) 13(3):191–6. doi: 10.1007/s10120-010-0556-1
128. Huang JY, Xu YY, Li M, Sun Z, Zhu Z, Song YX, et al. The Prognostic Impact of Occult Lymph Node Metastasis in Node-Negative Gastric Cancer: A Systematic Review and Meta-Analysis. Ann Surg Oncol (2013) 20(12):3927–34. doi: 10.1245/s10434-013-3021-7
129. Arigami T, Uenosono Y, Yanagita S, Ishigami S, Natsugoe S. Gastric Cancer in Lymph Node Metastasis in Gastrointestinal Cancer. In: Natsugoe S, editor. Part II Lymph Node Micrometastasis: Clinical Aspect. Springer: Springer Nature Singapore Pte Ltd (2019). p. 209–33. doi: 10.1007/978-981-10-4699-5_10
130. Gao Y, Zhang ZD, Li S, Guo YT, Wu QY, Liu SH, et al. Deep Neural Network-Assisted Computed Tomography Diagnosis of Metastatic Lymph Nodes From Gastric Cancer. Chin Med J (Engl) (2019) 132(23):2804–11. doi: 10.1097/CM9.0000000000000532
131. Li C, Zhang S, Zhang H, Pang L, Lam K, Hui C, et al. Using the K-Nearest Neighbor Algorithm for the Classification of Lymph Node Metastasis in Gastric Cancer. Comput Math Methods Med (2012) 2012:876545. doi: 10.1155/2012/876545
132. Niu PH, Zhao LL, Wu HL, Zhao DB, Chen YT. Artificial Intelligence in Gastric Cancer: Application and Future Perspectives. World J Gastroenterol (2020) 26(36):5408–19. doi: 10.3748/wjg.v26.i36.5408
133. Liu S, Shi H, Ji C, Zheng H, Pan X, Guan W, et al. Preoperative CT Texture Analysis of Gastric Cancer: Correlations With Postoperative TNM Staging. Clin Radiol (2018) 73(8):756.e1–9. doi: 10.1016/j.crad.2018.03.005
134. Matsumoto T, Murayama Y, Matsuo H, Okochi K, Koshiishi N, Harada Y, et al. 5-ALA-Assistant Automated Detection of Lymph Node Metastasis in Gastric Cancer Patients. Gastric Cancer (2020) 23(4):725–33. doi: 10.1007/s10120-020-01044-w
135. Tsujimoto H, Morimoto Y, Takahata R, Nomura S, Yoshida K, Hiraki S, et al. Theranostic Photosensitive Nanoparticles for Lymph Node Metastasis of Gastric Cancer. Ann Surg Oncol (2015) 22(Suppl 3):S923–8. doi: 10.1245/s10434-015-4594-0
136. Eisenberg DP, Adusumilli PS, Hendershott KJ, Chung S, Yu Z, Chan MK, et al. Real-Time Intraoperative Detection of Breast Cancer Axillary Lymph Node Metastases Using a Green Fluorescent Protein-Expressing Herpes Virus. Ann Surg (2006) 243(6):824–830; discussion 830. doi: 10.1097/01.sla.0000219738.56896.c0
137. Tummers WS, Miller SE, Teraphongphom NT, van den Berg NS, Hasan A, Longacre TA, et al. Detection of Visually Occult Metastatic Lymph Nodes Using Molecularly Targeted Fluorescent Imaging During Surgical Resection of Pancreatic Cancer. HPB (Oxf) (2019) 21(7):883–90. doi: 10.1016/j.hpb.2018.11.008
138. Shinden Y, Ueo H, Tobo T, Gamachi A, Utou M, Komatsu H, et al. Rapid Diagnosis of Lymph Node Metastasis in Breast Cancer Using a New Fluorescent Method With γ-Glutamyl Hydroxymethyl Rhodamine Green. Sci Rep (2016) 9:6, 27525. doi: 10.1038/srep27525
139. Cho HJ, Lee S, Park SJ, Lee YD, Jeong K, Park JH, et al. Tumor Microenvironment-Responsive Fluorogenic Nanoprobe for Ratiometric Dual-Channel Imaging of Lymph Node Metastasis. Colloids Surf B Biointerf (2019) 179:9–16. doi: 10.1016/j.colsurfb.2019.03.047
140. Mieog JSD, Achterberg FB, Zlitni A, Hutteman M, Burggraaf J, Swijnenburg RJ, et al. Fundamentals and Developments in Fluorescence-Guided Cancer Surgery. Nat Rev Clin Oncol (2022) 19:9–22. doi: 10.1038/s41571-021-00548-3
141. Kusano M, Ono H, Danjo Y, Kawamata F, Tajima Y, Ohtsubo S, et al. Chapter14: Fluorescent Navigation Surgery for Gastrointestinal Tract Cancers: Detection of Sentinel Nodes, Tumor Tattooing, and Harvesting of Lymph Nodes. In: Kusano M, Kokudo N, Toi M, Kaibori M, editors. ICG Fluorescence Imaging and Navigation Surgery. Tokyo: Springer Nature Japan (2016). p. 165–74. doi: 10.1007/978-4-431-55528-5_14
142. Aoyama T, Fujikawa H, Cho H, Ogata T, Shirai J, Hayashi T, et al. A Methylene Blue–Assisted Technique for Harvesting Lymph Nodes After Radical Surgery for Gastric Cancer: A Prospective, Randomized, Controlled Study. Am J Surg Pathol (2015) 39(2):266–73. doi: 10.1097/PAS.0000000000000336
143. Mansouri N, Movafagh A, Sayad A, Heidary Pour A, Taheri M, Soleimani S, et al. Targeting of BUB1b Gene Expression in Sentinel Lymph Node Biopsies of Invasive Breast Cancer in Iranian Female Patients. Asian Pac J Cancer Prev (2016) 17(S3):317–21. doi: 10.7314/apjcp.2016.17.s3.317
144. Yoshida K, Yamaguchi K, Okumura N, Tanahashi T, Kodera Y. Is Conversion Therapy Possible in Stage IV Gastric Cancer: The Proposal of New Biological Categories of Classification. Gastric Cancer (2016) 19(2):329–38. doi: 10.1007/s10120-015-0575-z
Keywords: gastric cancer, lymph node metastasis, MDCT, sentinel node, staging
Citation: Kinami S, Saito H and Takamura H (2022) Significance of Lymph Node Metastasis in the Treatment of Gastric Cancer and Current Challenges in Determining the Extent of Metastasis. Front. Oncol. 11:806162. doi: 10.3389/fonc.2021.806162
Received: 31 October 2021; Accepted: 13 December 2021;
Published: 07 January 2022.
Edited by:
Sanjit Mukherjee, National Institutes of Health (NIH), United StatesCopyright © 2022 Kinami, Saito and Takamura. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shinichi Kinami, a2luYW1pQGthbmF6YXdhLW1lZC5hYy5qcA==
 Shinichi Kinami
Shinichi Kinami Hitoshi Saito2
Hitoshi Saito2