- 1Department of Pancreatic Surgery, West China Hospital, Sichuan University, Chengdu, China
- 2Department of Pathology, West China Hospital, Sichuan University, Chengdu, China
- 3Department of Radiology, The First People’s Hospital of Chengdu, Chengdu, China
Vemurafenib and trametinib have a lot of successful experiences in the treatment of unresectable or metastatic melanoma with BRAF V600E mutation. However, they have not been reported in the treatment of advanced pancreatic ductal adenocarcinoma (PDAC). We report here a 66-year-old male who was diagnosed as PDAC with multiple metastases of the abdominal cavity and liver according to pathological examination. After three cycles of gemcitabine plus nab-paclitaxel (GA) regimen chemotherapy, the liver metastasis of the patient progressed, and the patient could not continue to receive chemotherapy because of poor physical condition. BRAF V600E mutation was found by genetic detection in this patient, so targeted therapy with vemurafenib combined with trametinib was performed and the follow-up period was up to 24 months. To the best of our knowledge, this is a rare report that patients with stage IV PDAC with BRAF V600E mutation can receive significantly survival benefits from targeted therapy with vemurafenib combined with trametinib. This report provides experience for the use of these two drugs in patients with advanced PDAC.
Introduction
The incidence of pancreatic ductal adenocarcinoma (PDAC) is increasing at a rate of 0.5%–1% per year. It is estimated that pancreatic cancer will become the second-leading cause of cancer death in the United States by 2030, and 50% of PDAC patients have already metastasized at diagnosis (1, 2). The current first-line chemotherapy treatment for metastatic patients is gemcitabine plus nab-paclitaxel (GA) or modified FOLFIRINOX regimen (1). GA regimen is a suitable choice for the elderly or poor physical condition patients (3), but its curative effect is not optimistic (4).
Patients with advanced PDAC are in urgent need of new treatment. Erlotinib, a tyrosine kinase inhibitor of epidermal growth factor receptor, is considered to be a promising drug for the treatment of advanced pancreatic cancer. Nevertheless, compared with gemcitabine alone, erlotinib combined with gemcitabine did not lead to a significant increase in progression-free survival (PFS) and overall survival (OS) (3, 5). Olaparib, a poly(adenosine diphosphate [ADB]-ribose) polymerase inhibitor, improved PFS but not OS in patients with BRCA1/2 mutation and metastatic PDAC after platinum-based chemotherapy (6). However, germline or somatic mutations of the BRCA1/2 exist only in 5% of patients with pancreatic cancer (7). Therefore, diverse treatments for advanced pancreatic cancer are needed.
Whole-exome sequencing showed that there were 92% PDAC patients who have KRAS mutation, and the mutation rate of BRAF V600E in KRAS wild-type patients was about 3%, which was mutually exclusive with KRAS mutations (8). This mutation greatly increased the activity of many carcinogenic-associated kinases and induced the proliferation of cancer cells (8). This mutation occurs in about half of melanomas and is prevalent in many other cancers (9). Vemurafenib affects the transduction of RAF signal pathway in BRAF mutant cells by affecting the dimerization of RAF, which plays an important role in the treatment of metastatic melanoma (10). However, since the drug abnormally activates the MAPK pathway in active cells driven by RAF (or other upstream signals), it is necessary to combine MEK inhibitors to overcome the drug resistance (11). Trametinib can non-competitively inhibit the activation and kinase activity of MEK1 and MEK2 (12). In combination with BRAF inhibitors, it delays drug resistance due to reactivation of the MAPK pathway during BRAF or MEK monotherapy and ultimately prolongs patient survival (11). To sum up, we report a case of vemurafenib combined with trametinib targeting therapy for advanced PDAC patients with BRAF V600E mutations after failed chemotherapy with the GA regimen. This patient benefited significantly from this combined targeting therapy.
Case Description
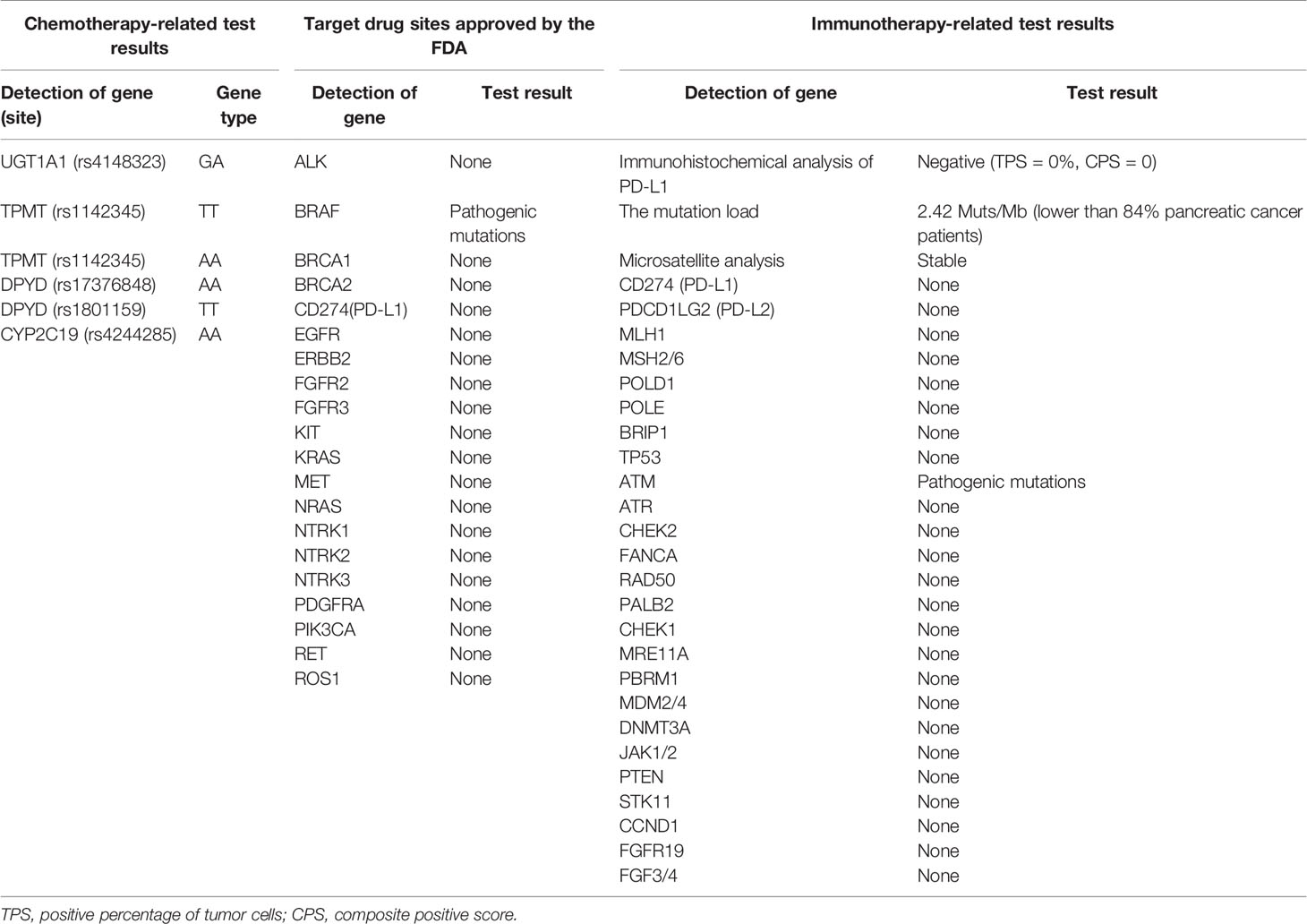
A 66-year-old male received epigastric enhanced CT due to complaint of intermittent mid-upper abdominal pain. The CT showed 2.8×2.6 cm isodensity nodules in the tail of the pancreas. Enhanced CT scan showed that the enhancement degree was lower than normal pancreatic parenchyma (Figure 1, left). At the same time, multiple nodular low-density masses (Figure 1C, left) were detected in the liver. The tumor markers showed CA19-9 11.31 U/ml and CA125 74.73 U/ml. This patient received exploratory laparotomy. We found multiple metastases in the liver, peritoneum, and omentum during the operation. Rapid pathology revealed that the metastatic nodule was adenocarcinoma, and further immunohistochemistry showed pancreatic ductal adenocarcinoma (Figure 2). This patient received his first chemotherapy (GA regimen: gemcitabine 1,700 mg, nab-paclitaxel 200 mg) 20 days after operation; however, myelosuppression appeared after chemotherapy. This patient received subsequent two courses of chemotherapies after the physical condition was improved. At this time, whole abdominal enhanced CT showed the progression of liver metastasis (70 days after the operation) (Figure 1B, middle). The captured libraries from the abdominal metastasis of the patient were loaded onto a NovaSeq 6000 platform (Illumina) for 100 bp paired-end sequencing. The results showed that the patient had ATM germline mutation (gene type: heterozygote) and BRAF V600E mutation (mutation rate: 19.38%). In addition to the above two genes, no other common mutant genes were detected in this patient (Table 1). We decided to use the combined targeted therapy of vemurafenib and trametinib at the initial dose of vemurafenib 960 mg twice a day and trametinib 2 mg once a day. Initially, the therapy was tolerated well by the patient, but he began to develop rash and pruritus, which aggravated gradually after 3 months, so we stopped the treatment temporarily. After a week, his symptoms were relieved. At this time, the patient was given combined targeted therapy again, but the dose was adjusted to vemurafenib 720 mg twice a day and trametinib 2 mg once a day. As before, the patient showed tolerance at the beginning, and then the patient gradually developed rash and pruritus again, accompanied by oral ulcer and constipation. When the symptoms of the patient gradually worsened to intolerance, we stopped the drug again. This treatment cycle lasted 3 months, but at this time, the recovery time of the patient was significantly longer than before. Fourteen days later, we adjusted the dose of vemurafenib 480 mg twice a day and trametinib 2 mg once a day. This course lasted for 3 months, and the response of the patient was similar to that before. After this treatment, the patient recovered for 3 weeks and received combined targeted therapy for 3 months again. The dose was vemurafenib 240 mg twice a day and trametinib 2 mg once a day. Although the dose was gradually decreasing, the degree of complications was becoming more and more serious and the time for recovery was getting longer and longer. The patient had repeated fever and incomplete bowel obstruction due to constipation. After this treatment, the patient rested for 1 month, and then received vemurafenib 240 mg twice a day and trametinib 2 mg once a day again. However, this course lasted only 2 months, because the patient could no longer stand it. There was a decrease in glomerular filtration rate (low to 60 ml/min) and a continuous increase in urinary protein (up to 5.1 g/24 h). Finally, the patient rested for 2 months and received vemurafenib 240 mg twice a day and trametinib 2 mg once every 2 days for 1 month. At this time, we evaluated the tumor and found that the primary tumor was enlarged (Figure 1, right) and the liver had a new metastatic nodule (Figure 1), so the therapy was stopped. The patient was still in follow-up, and the tumor marker test results are shown in Figure 3. This patient received a total of 13 months of combined targeted therapy, and the treatment course lasted for 17 months. Excitingly, he had been followed for 24 months.

Figure 1 Contrast-enhanced CT of the whole abdomen and pelvic cavity of the patient in different periods. Enhanced scanning was performed using Omnipaque (300 mg/ml) with an injection rate of 3 ml/s. The arterial phase was scanned 28–30 s after injection, the portal phase 48–50 s, and the delayed phase 60–70 s. (A) Primary tumor: from left to right are preoperative, after chemotherapy, and after targeted treatment (the following sequence is from left to right). (B) No metastases before surgery, new metastases after chemotherapy, and metastases disappeared after target therapy. (C) Preoperative metastases, metastases increased after chemotherapy and decreased after targeted therapy. (D) Preoperative metastases, new metastases after chemotherapy and disappeared after targeted therapy. (E) No metastasis here before surgery, no metastasis here after chemotherapy, and new recurrence after targeted therapy.
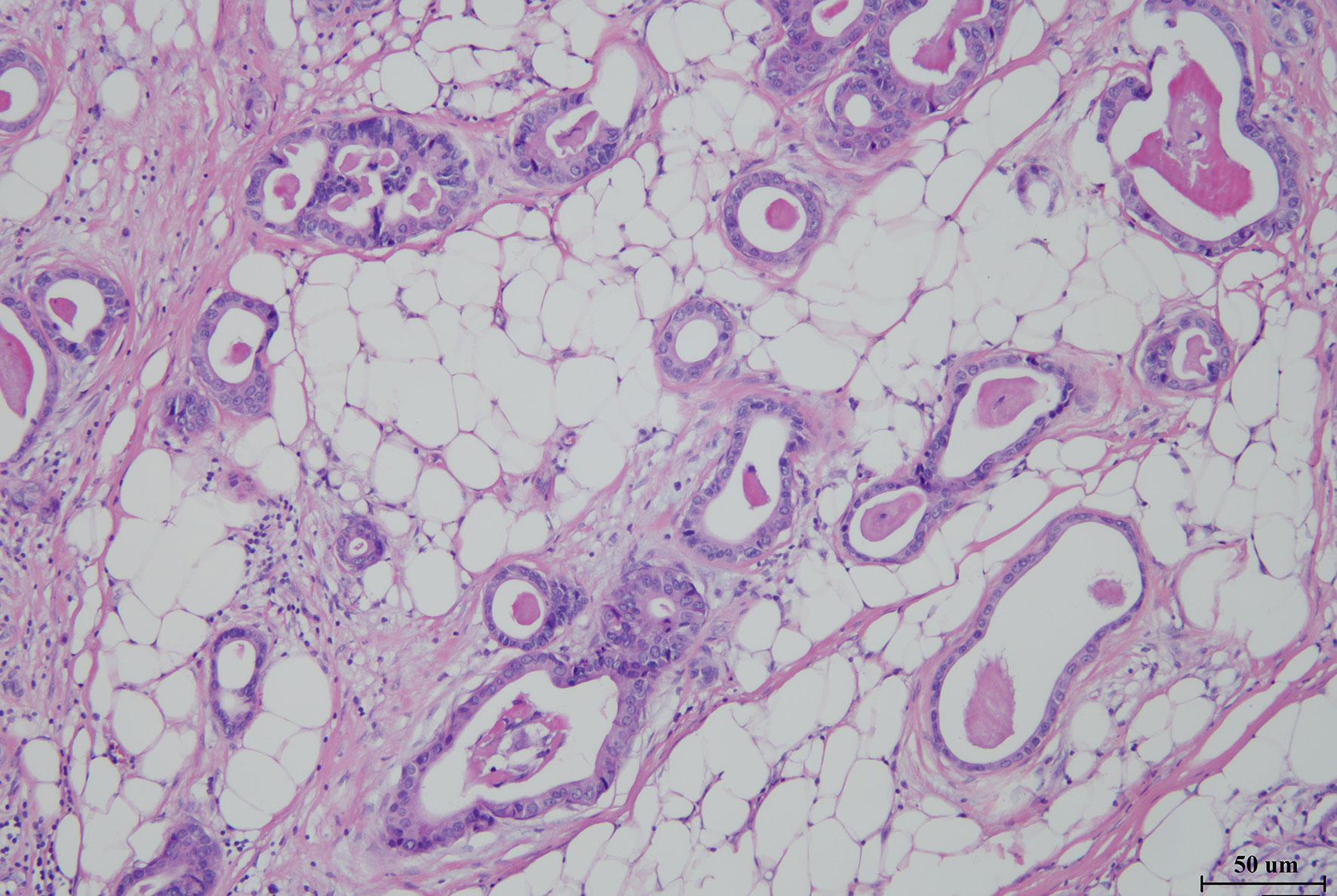
Figure 2 Hematoxylin–eosin staining image of metastases from the abdominal cavity (×100) of the patient, showing adenocarcinoma derived from pancreatic ducts.
Discussion
Over the past decade, the 5-year survival rate of PDAC has increased slightly from 5% to 8%, which almost results from the use of adjuvant and combination therapy rather than surgical techniques (13). However, due to the lack of effective targeted drugs, the precision medicine of PDAC obviously lags behind other malignant tumors (14). PDAC has four major gene mutations: KRAS, TP53, CDKN2A, and Smad4 (14). The carcinogenic mutation of KRAS was found in more than 90% of PDAC (7, 15). This high prevalence rate has led to a considerable interest among scientists. Unfortunately, the KRAS protein has a high affinity for GTP and/or GDP, so it is difficult to be a target (16). For TP53, CDKN2A, and Smad4, there is also a lack of effective targeted therapy (17). In view of the lack of precision treatment for common mutations in PDAC, we believe that partial patients who have specific genetic mutations are the key for the precision treatment of PDAC. Although some PDAC patients, especially those with germline BRCA mutations, have received unprecedented benefits from Olaparib (18), the studies on PARP inhibitor mentioned so far have only focused on patients with germline or somatic BRCA1/2 or PALB2 mutations. This subgroup includes only about 14% of PDAC patients (19). Therefore, it is necessary for us to learn from the breakthrough findings of other cancers to benefit partial PDAC patients.
In 2002, whole-genome sequencing identified the BRAF gene as an oncogene and a potential therapeutic target for the first time (20). BRAF mutations occur in about 8% of human cancers, including melanoma, colorectal cancer, glioma, thyroid cancer, non-small cell lung cancer, cholangiocarcinoma, and several hematological malignancies (20, 21). Most mutations are caused by a missense mutation V600E in the kinase domain (22). BRAF V600E mutations increase the kinase activity of RAS and activate the MEK-ERK signal pathway (23). The first successful treatment for BRAF mutation was vemurafenib in melanoma (24, 25). Although the early and effective response to vemurafenib is common, drug resistance generally appears and largely limits the treatment time to 12 months (25). The drug resistance of BRAF inhibitors is largely due to the reactivation of MAPK signaling pathway (26), so combined targeted therapy is a reasonable approach. The specific method is to block the downstream signal of the RAS pathway by suppressing MEK1/2. Trametinib is the only MEK inhibitor approved as a monotherapy for metastatic melanoma (27). The combination of BRAF and MEK inhibitors has become the standard treatment for metastatic melanoma.
BRAF V600E mutation was found in about 3% of PDAC patients (8). At present, only a few data have reported the clinical experience of targeted therapy of BRAF mutation in advanced PDAC patients. Seghers et al. reported that a patient with PDAC in uncinate process of the pancreas with diffuse liver metastasis was treated with vemurafenib separately after failure of the first-line chemotherapy, resulting in a PFS of 9 months. Because the patient cannot tolerate the side effects of the MEK inhibitor cobimetinib, the PFS of the patient was shorter than that of the patient in this report (28). One case reported that a patient with stage IV pancreatic cancer with BRAF V600E mutation received combined targeted therapy with dabrafenib and trametinib, but the patient died of acute abdomen (29) after only 19 days of treatment, while the therapeutic effect of vemurafenib on advanced pancreatic cancer was not observed in another case (30). In addition, a patient with acinar cell carcinoma in the tail of the pancreas and BRAF V600E mutation obtained 8 months of PFS by combined target therapy with dabrafenib and trametinib, but the patient received chemotherapy for more than 5 years before the target therapy. Besides, the BRAF V600E mutation rate was only 1.4% (31), which was significantly lower than the 19.38% we reported here. In view of the fact that the patient has received combined targeted therapy and other treatments, it is difficult to attribute the benefit of the patient entirely to targeted therapy. A recent case also showed a positive response to combined targeted therapy in patients with pancreatic acinar cell carcinoma with BRAF V600E mutations, who achieved almost complete remission for 12 months (32). Considering that acinar cell carcinoma of the pancreas is a rare pancreatic exocrine tumor with more favorable prognosis than PDAC (33), the significance of combined targeted therapy for this tumor is less exciting than PDAC.
In this case, it is reported for the first time that the combination of BRAF inhibitors and MEK inhibitors can significantly benefit the survival of patients with stage IV PDAC, which makes us see the light of precision medicine in the treatment of PDAC. This case suggests that we should change the one-size-fits-all treatment concept of PDAC, and it is necessary to learn from the breakthrough findings of other cancer types. Compared with a previous similar report (28), our patient received a combination therapy, which is an important reason for prolonging the survival time (11). Current studies have not shown that intermittent treatment course is more beneficial to patients than continuous dosing (20). So, we think continuous dosing may be one of the reasons for the survival benefits of the patients. Based on this case, we found that the adverse reactions in the course of combination therapy were an important factor affecting the compliance of the patient. Although there were fewer adverse skin events in the full dose combination of the two drugs compared with BRAF inhibitor monotherapy, the addition of trametinib increased pyrexia, interstitial lung disease, venous thromboembolism, gastrointestinal bleeding, and heart (cardiomyopathy, decreased left ventricular ejection fraction) or ocular (retinal vein occlusion, uveitis) toxicity (34, 35). In this case, the patient showed repeated fever (remission after drug withdrawal), and after excluding the factors of infection, we believed that it is caused by the combination of drugs. In addition, patients with advanced melanoma who received combined targeted therapy showed a better response to PD-1 therapy (36), so triple therapy to combining targeted and immunotherapy may be a positive attempt.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics Statement
Ethical review and approval were not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
NK and XL contributed to the study concept and design. ZW contributed to the investigation and writing of the original draft. DH contributed to the collection of pathology data and analysis. CC contributed to the collection of CT image data and analysis. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by Sichuan Province Science and Technology Planning Project (2020YFS0262), West China Hospital Clinical Research Incubation Project (21HXFH058), and the 1·3·5 Project for Disciplines of Excellence–Clinical Research Incubation Project (ZY2017302 and ZYJC21037), West China Hospital, Sichuan University.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to express our heartfelt gratitude to all the experts participating in the multidisciplinary consultation.
References
1. Park W, Chawla A, O'Reilly EM. Pancreatic Cancer: A Review. Jama (2021) 326(9):851–62. doi: 10.1001/jama.2021.13027
2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin (2021) 71:7–33. doi: 10.3322/Caac.21654
3. Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic Cancer. Lancet. (2020) 395:2008–20. doi: 10.1016/S0140-6736(20)30974-0
4. Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased Survival In Pancreatic Cancer With Nab-Paclitaxel Plus Gemcitabine. N Engl J Med (2013) 369:1691–703. doi: 10.1056/Nejmoa1304369
5. Benzel J, Fendrich V. Familial Pancreatic Cancer. Oncol Res Treat (2018) 41:611–8. doi: 10.1159/000493473
6. Shroff RT, Hendifar A, Mcwilliams RR, Geva R, Epelbaum R, Rolfe L, et al. Rucaparib Monotherapy In Patients With Pancreatic Cancer And A Known Deleterious Brca Mutation. Jco Precis Oncol (2018) 2018:PO.17.00316. doi: 10.1200/Po.17.00316
7. Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, et al. Genomic Analyses Identify Molecular Subtypes Of Pancreatic Cancer. Nature. (2016) 531:47–52. doi: 10.1038/Nature16965
8. Witkiewicz AK, Mcmillan EA, Balaji U, Baek G, Lin WC, Mansour J, et al. Whole-Exome Sequencing Of Pancreatic Cancer Defines Genetic Diversity And Therapeutic Targets. Nat Commun (2015) 6:6744. doi: 10.1038/Ncomms7744
9. Pollock PM, Meltzer PS. A Genome-Based Strategy Uncovers Frequent Braf Mutations In Melanoma. Cancer Cell (2002) 2:5–7. doi: 10.1016/S1535-6108(02)00089-2
10. Bollag G, Tsai J, Zhang J, Zhang C, Ibrahim P, Nolop K, et al. Vemurafenib: The First Drug Approved For Braf-Mutant Cancer. Nat Rev Drug Discov (2012) 11:873–86. doi: 10.1038/Nrd3847
11. Lugowska I, Koseła-Paterczyk H, Kozak K, Rutkowski P. Trametinib: A Mek Inhibitor For Management Of Metastatic Melanoma. Onco Targets Ther (2015) 8:2251–9. doi: 10.2147/Ott.S72951
12. Yamaguchi T, Kakefuda R, Tajima N, Sowa Y, Sakai T. Antitumor Activities Of Jtp-74057 (Gsk1120212), A Novel Mek1/2 Inhibitor, On Colorectal Cancer Cell Lines In Vitro And In Vivo. Int J Oncol (2011) 39:23–31. doi: 10.3892/Ijo.2011.1015
13. He J, Ahuja N, Makary MA, Cameron JL, Eckhauser FE, Choti MA, et al. 2564 Resected Periampullary Adenocarcinomas At A Single Institution: Trends Over Three Decades. Hpb (Oxford) (2014) 16:83–90. doi: 10.1111/Hpb.12078
14. Herbst B, Zheng L. Precision Medicine In Pancreatic Cancer: Treating Every Patient As An Exception. Lancet Gastroenterol Hepatol (2019) 4:805–10. doi: 10.1016/S2468-1253(19)30175-X
15. Nevala-Plagemann C, Hidalgo M, Garrido-Laguna I. From State-Of-The-Art Treatments To Novel Therapies For Advanced-Stage Pancreatic Cancer. Nat Rev Clin Oncol (2020) 17:108–23. doi: 10.1038/S41571-019-0281-6
16. Mccormick F, Kras AS. A Therapeutic Target. Clin Cancer Res (2015) 21:1797–801. doi: 10.1158/1078-0432.Ccr-14-2662
17. Pihlak R, Weaver J, Valle JW, Mcnamara MG. Advances In Molecular Profiling And Categorisation Of Pancreatic Adenocarcinoma And The Implications For Therapy. Cancers (Basel) (2018) 10(1):17. doi: 10.3390/Cancers10010017
18. Golan T, Hammel P, Reni M, Van Cutsem E, Macarulla T, Hall MJ, et al. Maintenance Olaparib For Germline Brca-Mutated Metastatic Pancreatic Cancer. N Engl J Med (2019) 381:317–27. doi: 10.1056/Nejmoa1903387
19. Dreyer SB, Chang DK, Bailey P, Biankin AV. Pancreatic Cancer Genomes: Implications For Clinical Management And Therapeutic Development. Clin Cancer Res (2017) 23:1638–46. doi: 10.1158/1078-0432.Ccr-16-2411
20. Halle BR, Johnson DB. Defining And Targeting Braf Mutations In Solid Tumors. Curr Treat Options Oncol (2021) 22:30. doi: 10.1007/S11864-021-00827-2
21. Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations Of The Braf Gene In Human Cancer. Nature. (2002) 417:949–54. doi: 10.1038/Nature00766
22. Gorden A, Osman I, Gai W, He D, Huang W, Davidson A, et al. Analysis Of Braf And N-Ras Mutations In Metastatic Melanoma Tissues. Cancer Res (2003) 63:3955–7.
23. Wellbrock C, Ogilvie L, Hedley D, Karasarides M, Martin J, Niculescu-Duvaz D, et al. V599eb-Raf Is An Oncogene In Melanocytes. Cancer Res (2004) 64:2338–42. doi: 10.1158/0008-5472.Can-03-3433
24. Haugh AM, Johnson DB. Management Of V600e And V600k Braf-Mutant Melanoma. Curr Treat Options Oncol (2019) 20:81. doi: 10.1007/S11864-019-0680-Z
25. Flaherty KT, Puzanov I, Kim KB, Ribas A, Mcarthur GA, Sosman JA, et al. Inhibition Of Mutated, Activated Braf In Metastatic Melanoma. N Engl J Med (2010) 363:809–19. doi: 10.1056/Nejmoa1002011
26. Johnson DB, Menzies AM, Zimmer L, Eroglu Z, Ye F, Zhao S, et al. Acquired Braf Inhibitor Resistance: A Multicenter Meta-Analysis Of The Spectrum And Frequencies, Clinical Behaviour, And Phenotypic Associations Of Resistance Mechanisms. Eur J Cancer (2015) 51:2792–9. doi: 10.1016/J.Ejca.2015.08.022
27. Robert C, Flaherty K, Nathan P, Hersey P, Garbe C, Milhem M, et al. Five-Year Outcomes From A Phase 3 Metric Study In Patients With Braf V600 E/K-Mutant Advanced Or Metastatic Melanoma. Eur J Cancer (2019) 109:61–9. doi: 10.1016/J.Ejca.2018.12.015
28. Seghers AK, Cuyle PJ, Van Cutsem E. Molecular Targeting Of A Braf Mutation In Pancreatic Ductal Adenocarcinoma: Case Report And Literature Review. Target Oncol (2020) 15:407–10. doi: 10.1007/S11523-020-00727-9
29. Grinshpun A, Zarbiv Y, Roszik J, Subbiah V, Hubert A. Beyond Kras: Practical Molecular Targets In Pancreatic Adenocarcinoma. Case Rep Oncol (2019) 12:7–13. doi: 10.1159/000496018
30. Hyman DM, Puzanov I, Subbiah V, Faris JE, Chau I, Blay JY, et al. Vemurafenib In Multiple Nonmelanoma Cancers With Braf V600 Mutations. N Engl J Med (2015) 373:726–36. doi: 10.1056/Nejmoa1502309
31. Sasankan S, Rebuck L, Darrah G, Harari Turquie M, Rabinowitz I. Metastatic Pancreatic Cancer With Braf And P53 Mutations: Case Report Of Therapeutic Response To Doublet Targeted Therapy. Case Rep Oncol (2020) 13:1239–43. doi: 10.1159/000510096
32. Busch E, Kreutzfeldt S, Agaimy A, Mechtersheimer G, Horak P, Brors B, et al. Successful Braf/Mek Inhibition In A Patient With Braf (V600e)-Mutated Extrapancreatic Acinar Cell Carcinoma. Cold Spring Harb Mol Case Stud (2020) 6(4):a005553. doi: 10.1101/Mcs.A005553
33. Al-Hader A, Al-Rohil RN, Han H, Von Hoff D. Pancreatic Acinar Cell Carcinoma: A Review On Molecular Profiling Of Patient Tumors. World J Gastroenterol (2017) 23:7945–51. doi: 10.3748/Wjg.V23.I45.7945
34. Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J, et al. Combined Braf And Mek Inhibition In Melanoma With Braf V600 Mutations. N Engl J Med (2012) 367:1694–703. doi: 10.1056/Nejmoa1210093
35. Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, et al. Improved Overall Survival In Melanoma With Combined Dabrafenib And Trametinib. N Engl J Med (2015) 372:30–9. doi: 10.1056/Nejmoa1412690
Keywords: vemurafenib, trametinib, BRAF V600E, PDAC, survival, benefit
Citation: Wang Z, He D, Chen C, Liu X and Ke N (2022) Vemurafenib Combined With Trametinib Significantly Benefits the Survival of a Patient With Stage IV Pancreatic Ductal Adenocarcinoma With BRAF V600E Mutation: A Case Report. Front. Oncol. 11:801320. doi: 10.3389/fonc.2021.801320
Received: 25 October 2021; Accepted: 27 December 2021;
Published: 25 January 2022.
Edited by:
Husain Yar Khan, Wayne State University, United StatesReviewed by:
Maria Diab, Emory University, United StatesFaisal M. Nimri, Henry Ford Hospital, United States
Vineet Kumar Gupta, University of Miami, United States
Copyright © 2022 Wang, He, Chen, Liu and Ke. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nengwen Ke, a2VuZW5nd2VuQHNjdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Ziyao Wang
Ziyao Wang Du He
Du He Chen Chen
Chen Chen Xubao Liu
Xubao Liu Nengwen Ke
Nengwen Ke