
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Oncol. , 19 January 2022
Sec. Pharmacology of Anti-Cancer Drugs
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.801199
This article is part of the Research Topic Therapeutic Drug Monitoring and Clinical Toxicology of Anti-Cancer Drugs View all 25 articles
Introduction: Enfortumab vedotin (EV) has been demonstrated to have a significant response rate in early phase trials and is known for its tolerable side-effect profile. Emerging case reports have raised awareness of cutaneous toxicities, which may be a potentially fatal complication.
Objective: To assess the potential relevance between EV and cutaneous toxicities reports through data mining of the U.S. Food and Drug Administration (FDA) adverse event reporting system (FAERS).
Methods: Data from January 1, 2019, to November 4, 2021, in the FAERS database were retrieved. Information component (IC) and reporting odds ratio (ROR) were used to evaluate the association between EV and cutaneous toxicities events.
Results: EV was significantly associated with cutaneous toxicities in the database compared with both all other drugs (ROR 12.90 [10.62–15.66], IC 2.76 [2.52–3.01], middle signal) and platinum-based therapy (ROR 15.11 [12.43–18.37], IC 2.91 [2.66–3.15], middle signal) in the FAERS database. A significant association was detected between EV and all the cutaneous adverse effects (AEs) except erythema, palmar–plantar erythrodysesthesia syndrome, and dermatitis allergic. Both Stevens–Johnson syndrome and toxic epidermal necrolysis occurred 15 times as frequently for EV compared with all other drugs (ROR = 15.20; ROR = 15.52), while Stevens–Johnson syndrome occurred 18 times and toxic epidermal necrolysis occurred 7 times as frequently for EV compared with platinum-based therapy in the database (ROR = 18.74; ROR = 7.80). All groups that limited the gender and age showed a significant association between EV and cutaneous toxicities.
Conclusions: A significant signal was detected between EV use and cutaneous toxicities. It is worth noting that Stevens–Johnson syndrome and toxic epidermal necrolysis were significantly associated with EV use.
Urothelial cancer (UC) is the ninth most common cancer worldwide (1). At presentation, about 70% of patients have non-muscle-invasive disease and 25% muscle-invasive disease, and 5% will be metastatic (2). Early stages of disease (non-muscle-invasive UC and muscle-invasive disease UC) are often treated with cisplatin-based chemotherapy with objective response rates of approximately 50% (3). And the immune checkpoint inhibitor (ICI) is considered the standard of care in patients who are either cisplatin-unfit or platinum-refractory (4). However, patients with metastatic UC (mUC) with disease progression on both platinum-based chemotherapy and an ICI had few treatment options available and often have a dismal prognosis (5).
Enfortumab vedotin (EV) is an antimitotic antibody–drug conjugate (ADC) that inhibits microtubule assembly, which received Food and Drug Administration (FDA)-accelerated approval for the treatment of adult patients with locally advanced or mUC who had failed in the previous treatment of ICIs and platinum-based chemotherapeutic agents in 2019 (6). The drug has been demonstrated to have a significant response rate in early phase trials and is known for its tolerable side-effect profile (7–11). Common toxicities that have been attributed to EV were fatigue, peripheral neuropathy, skin rashes, gastrointestinal issues, and hematological suppression (12). The first case of cutaneous toxicities induced by EV was found in 2019 (13). Recently, emerging case reports have raised awareness of cutaneous toxicities, which may be a potentially fatal complication (14–17). But the precise descriptions of cutaneous toxicities were limited. Perhaps because of inadequate understanding as a form of EV-related cutaneous toxicities, data are derived primarily from case reports and clinical trials that may not correctly represent the real world. Moreover, the characteristics, outcomes, and types of EV-related cutaneous toxicities are still unknown.
Considering the wide clinical use of EV and the potentially fatal consequences of EV-associated cutaneous toxicities, it is important to identify its clinical manifestations. Therefore, we aim to assess the potential relevance between EV and cutaneous toxicities through data mining of the U.S. FDA adverse event (AE) reporting system (FAERS).
The data were obtained from the FAERS database, which is publicly available and contains spontaneous AE reports submitted to the U.S. FDA by healthcare professionals, consumers, drug manufacturers, and others. The FAERS database Quarterly Data Files (January 1, 2019, to November 4, 2021) were used. OpenVigil FDA, a validated pharmacovigilance tool, was adapted to access the FDA drug-event database with the additional openFDA drug mapping and duplicate detection functionality (18–20).
The reports in the FAERS database were coded using preferred terms (PTs) from the Medical Dictionary for Regulatory Activities. After literature review and summary of previous studies, we considered the following PTs as related to cutaneous toxicities: rash [10037844], rash pruritus [10037884], pruritus [10037087], rash erythematous [10037855], Stevens–Johnson syndrome [10042033], dry skin [10013786], toxic epidermal necrolysis [10044223], skin exfoliation [10040844], dermatitis bullous [10012441], rash maculopapular [10025423], skin discoloration [10040829], erythema [10015150], rash papular [10037876], skin reaction [10040914], skin toxicity [10059516], symmetrical drug-related intertriginous and flexural exanthema [10078325], dermatitis allergic [10012434], exfoliative rash [10064579], palmar–plantar erythrodysesthesia syndrome [10033553], and rash macular [10037867]. The clinical characteristics (gender, age, reporting time, etc.) of patients were collected.
Standard descriptive statistics were used to summarize the study population characteristics. We conducted a disproportionality analysis using the Bayesian confidence propagation neural network of information component (IC) and reporting odds ratio (ROR) to calculate disproportionality (21). ROR and IC are recognized disproportionality methods to identify whether a given AE (in this case, cutaneous toxicities) is reported more frequently than expected with a given drug (in this case, EV), which allows testing the possible disproportionate association between a drug and an AE (18). For IC, a significant signal was defined as the lower bound of the 95% CI (IC025) exceeded 0. If 0 < IC025 ≤ 1.5, then it is considered as weak signal; if 1.5 < IC025 ≤ 3.0, then it is considered as middle signal; if IC025 > 3.0, then it is considered as strong signal (22). Since IC-based signals were included in ROR-based ones (23), ROR was also calculated, and the significant signal was defined as the lower bound of the 95% CI (ROR025) exceeded 1, with at least 3 cases (24–26). All the analyses were performed using R version 3.2.5. The IC and ROR with 95% CI can be calculated by the following:
a = number of target AE of EV alone
b = number of other AEs of EV alone
c = number of target AE of other drugs except for EV
d = number of other AEs of other drugs except for EV
Overall, 409 AE reports related to EV and 212 AE reports related to cutaneous toxicities were submitted to the FAERS between January 1, 2004, and November 4, 2021. We screened all reported EV-related cutaneous toxicities, and the clinical characteristics are summarized in Table 1. Rash was the most common cutaneous toxicities related to EV. All the cases were reported between 2020 and 2021. Most cases were male (76.42%). The median age of cases was 74.5 (6–92) years. Most cases were EV monotherapy (83.49%), while only a few patients accepted combination therapy (Table 1).
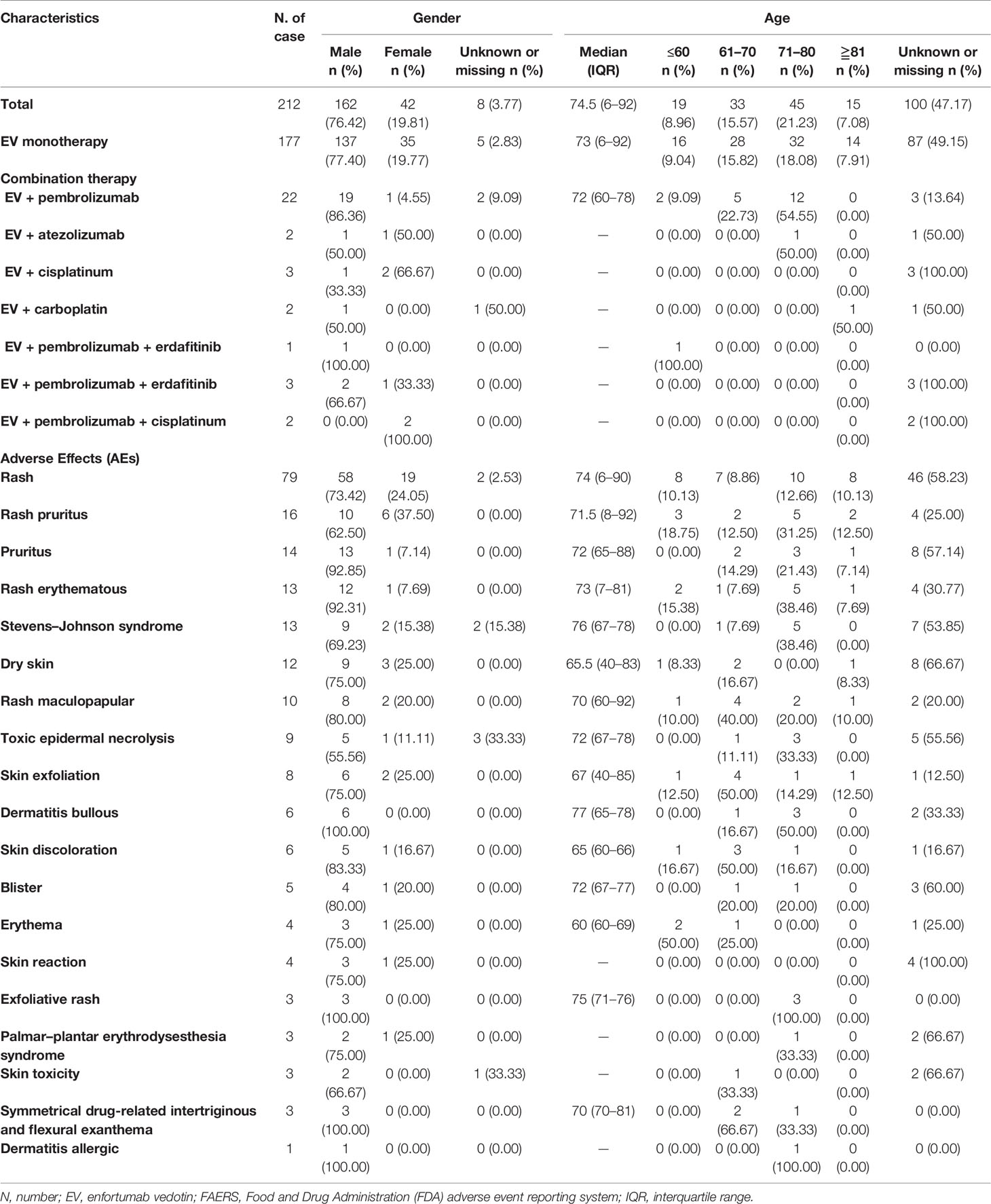
Table 1 Characteristics of patients with enfortumab vedotin associated cutaneous toxicities sourced from the FAERS database.
EV was significantly associated with cutaneous toxicities compared with both all other drugs (ROR 12.90 [10.62–15.66], IC 2.76 [2.52–3.01], middle signal, Table 2) and platinum-based therapy (ROR 15.11 [12.43–18.37], IC 2.91 [2.66–3.15], middle signal, Table 2). And significant association was detected between EV and all the cutaneous AEs except erythema, palmar–plantar erythrodysesthesia syndrome, and dermatitis allergic (Table 2). Nine AEs were detected as middle signal including rash (IC025 = 2.85), rash erythematous (IC025 = 2.49), Stevens–Johnson syndrome (IC025 = 2.96), dry skin (IC025 = 2.15), rash maculopapular (IC025 = 1.51), toxic epidermal necrolysis (IC025 = 2.02), skin exfoliation (IC025 = 1.57), dermatitis bullous (IC025 = 1.91), and blister (IC025 = 1.97) compared with platinum-based therapy in the database, while rash pruritus was detected as strong signal (IC025 = 3.32).
Stevens–Johnson syndrome and toxic epidermal necrolysis were the life-threatening AEs induced by EV. Those two AEs were all detected as middle signal and significantly associated with EV use. Both Stevens–Johnson syndrome and toxic epidermal necrolysis occurred 15 times as frequently for EV compared with all other drugs in the database (ROR = 15.20 and ROR = 15.52), while Stevens–Johnson syndrome occurred 18 times and toxic epidermal necrolysis occurred 7 times as frequently for EV compared with platinum-based therapy in the database (ROR = 18.74; ROR = 7.80).
Thirty-five death cases from all causes related to EV were submitted to the FAERS, and three cases were reported to be related to cutaneous toxicities (8.57%). It is worth noting that three cases were all related to Stevens–Johnson syndrome. The mortality rate of Stevens–Johnson syndrome related to EV was 13.64% in the FAERS.
We analyzed the association between EV and cutaneous toxicities in different groups that limited the gender and age. All groups showed significant association. Significant middle signals of cutaneous toxicities were shown in all groups (Table 3).
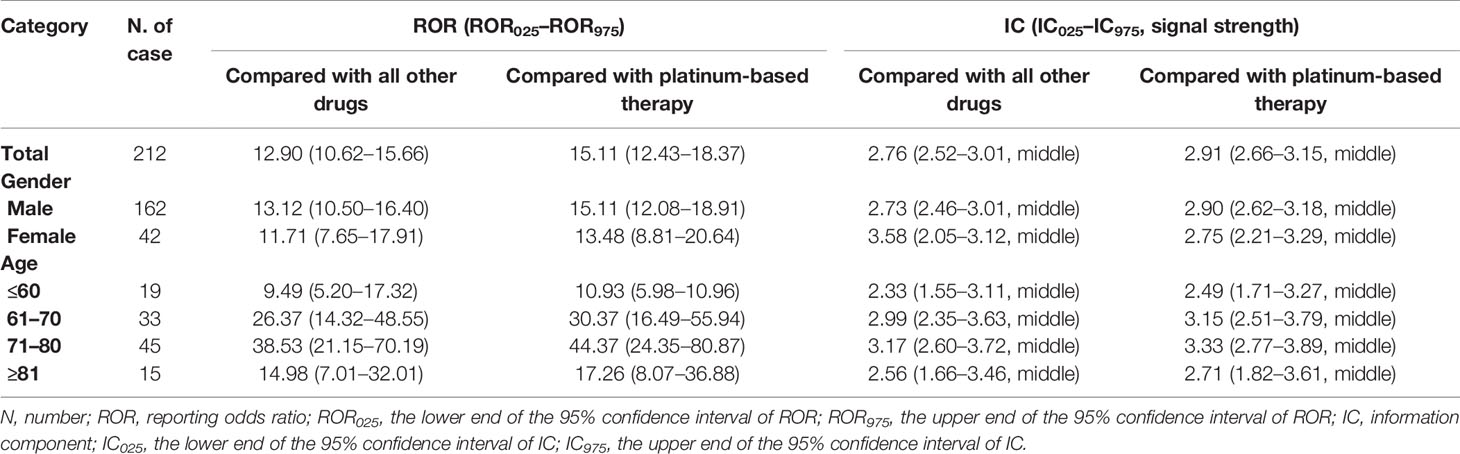
Table 3 Disproportionality analysis of enfortumab vedotin and cutaneous toxicities in different groups of cases.
To our knowledge, this is the first comprehensive pharmacovigilance study on cutaneous toxicities associated with EV based on the FAERS database. Our study included the largest such collection of cases to date, and 212 AE reports related to cutaneous toxicities were analyzed.
Our study detected a significant signal between EV use and cutaneous toxicities. The most well-recognized AE of EV is rash. The rate of rash was noted in 48% of patients in the previous clinical trial (8). The median time to onset of skin reactions has been estimated to be 1 month. Of patients who experienced rash, nearly two-thirds experienced complete resolution, and approximately one-fifth experienced partial improvement (27). Besides rash, our study detected other cutaneous AEs induced by EV including pruritus and Stevens–Johnson syndrome. The mechanism for the AEs is unclear now. EV is an ADC with a monomethyl auristatin E (MMAE) payload targeting Nectin-4, a protein widely expressed on UC cells (28). Nectin-4 is important in the skin, which has a role in cell–cell adhesion, and a functional disturbance could lead to impaired cell–cell attachment (29, 30). Besides that, cutaneous toxicities also appeared to be a common AE in studies involving other ADC that incorporate MMAE (31–33). Therefore, dermatologic sequelae observed could be attributed solely to the MMAE payload. Alternatively, the proposed mechanism is targeting Nectin-4 by EV with the delivery of the MMAE payload to the skin resulting in the observed keratinocyte apoptosis (16).
Stevens–Johnson syndrome and toxic epidermal necrolysis were the life-threatening AEs. Those two AEs have always been not a recognized side effect of EV. The first case report of a 71-year-old male who suffered from EV-induced toxic epidermal necrolysis was published in 2020 (15). And Viscuse et al. highlighted a case of Stevens–Johnson syndrome/toxic epidermal necrolysis following enfortumab infusions in 2021 (16). Unfortunately, both of the patients in these cases were dead after treatment. Those cases aroused our attention on EV-induced life-threatening cutaneous toxicity. Our study found that those two AEs were significantly associated with EV use. This reminded doctors that patients must be monitored for cutaneous toxicities with early involvement of dermatology.
Our study found a significant signal of cutaneous toxicities in all groups that limited the gender and age. All the groups were detected as middle signal. Young people (≤60 years old) had slightly lower reporting frequencies for cutaneous toxicities compared with old people.
Our study has limitations. First, the FAERS database was a spontaneous reporting system. Underreporting, selective reporting, and many missing data could bring reporting bias. Second, the limited data might not contribute to a better comprehensive evaluation of EV-induced cutaneous toxicities. Third, disproportionality analysis is a suitable tool to quantitate signals for the AE. But the causal relationship between drugs (EV) and the AE (cutaneous toxicities) cannot be verified without a clinically performed causality assessment, while confounders such as comorbidity and concomitant drugs cannot also be assessed properly.
Our study detected a significant signal between EV use and cutaneous toxicities. It is worth noting that Stevens–Johnson syndrome and toxic epidermal necrolysis were significantly associated with EV use. Patients must be monitored for cutaneous toxicities with early involvement of dermatology. Further study is required with better data sources and research design to draw conclusions on the strength of the relationships.
The datasets presented in this study can be found in FAERs database. Further inquiries can be directed to the corresponding authors.
HY was responsible for the study conception and design, data acquisition, data analysis and interpretation, manuscript preparation, and manuscript editing. XY was responsible for the data acquisition. ZA was responsible for the data analysis and interpretation. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.801199/full#supplementary-material
UC, urothelial cancer; ICI, immune checkpoint inhibitor; mUC, metastatic UC; EV, enfortumab vedotin; ADC, antibody–drug conjugate; FDA, Food and Drug Administration; FAERS, FDA adverse event reporting system; AEs, adverse events; PTs, preferred terms; IC, information component; ROR, reporting odds ratio; MMAE, monomethyl auristatin E.
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2018) 68:394–424. doi: 10.3322/caac.21492
2. Alt M, Stecca C, Tobin S, Jiang DM, Sridhar SS. Enfortumab Vedotin in Urothelial Cancer. Ther Adv Urol (2020) 12:1756287220980192. doi: 10.1177/1756287220980192
3. von der Maase H, Hansen SW, Roberts JT, Dogliotti L, Oliver T, Moore MJ, et al. Gemcitabine and Cisplatin Versus Methotrexate, Vinblastine, Doxorubicin, and Cisplatin in Advanced or Metastatic Bladder Cancer: Results of a Large, Randomized, Multinational, Multicenter, Phase III Study. J Clin Oncol (2000) 18:3068–77. doi: 10.1200/JCO.2000.18.17.3068
4. Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med (2017) 376:1015–26. doi: 10.1056/NEJMoa1613683
5. Sidaway P. Sacituzumab Govitecan is Safe and Effective. Nat Rev Clin Oncol (2021) 18(7):400. doi: 10.1038/s41571-021-00523-y
6. Hanna KS. Enfortumab Vedotin to Treat Urothelial Carcinoma. Drugs Today (Barc) (2020) 56(5):329–35. doi: 10.1358/dot.2020.56.5.3127027
7. Rosenberg J, Sridhar SS, Zhang J, Smith D, Ruether D, Flaig TW, et al. EV-101: A Phase I Study of Single-Agent Enfortumab Vedotin in Patients With Nectin-4–Positive Solid Tumors, Including Metastatic Urothelial Carcinoma. J Clin Oncol (2020) 38(10):1041–9. doi: 10.1200/JCO.19.02044
8. Rosenberg JE, O’Donnell PH, Balar AV, McGregor BA, Heath EI, Yu EY, et al. Pivotal Trial of Enfortumab Vedotin in Urothelial Carcinoma After Platinum and Anti-Programmed Death/Programmed Death Ligand 1 Therapy. J Clin Oncol (2019) 37(29):2592–600. doi: 10.1200/JCO.19.01140
9. Maas M, Stuhler V, Walz S, Stenzl A, Bedke J. Enfortumab Vedotin -Next Game- Changer in Urothelial Cancer. Expert Opin Biol Ther (2021) 21(7):801–9. doi: 10.1080/14712598.2021.1865910
10. Hoimes CJ, Rosenberg JE, Srinivas S, Petrylak DP, Flaig T. EV-103: Initial Results of Enfortumab Vedotin Plus Pembrolizumab for Locally Advanced or Metastatic Urothelial Carcinoma. Ann Oncol (2019) 30(suppl_5):v356–402. doi: 10.1093/annonc/mdz249
11. van der Heijden MS, Gupta S, Galsky MD, Derleth C, Steinberg J, Kataria R, et al. 798tip Study EV-302: A 3-Arm, Open-Label, Randomized Phase III Study of Enfortumab Vedotin Plus Pembrolizumab and/or Chemotherapy, Versus Chemotherapy Alone, in Untreated Locally Advanced or Metastatic Urothelial Cancer. Ann Oncol (2020) 31(suppl_4):S550–0. doi: 10.1016/j.annonc.2020.08.2069
12. Keerty D, Graham L, Haynes E, Hembree TN. Flexural Exanthema From Enfortumab Vedotin. Cureus (2020) 12(5):e8102. doi: 10.7759/cureus.8102
13. Wu S, Adamson AS. Cutaneous Toxicity Associated With Enfortumab Vedotin Treatment of Metastatic Urothelial Carcinoma. Dermatol Online J (2019) 25(2):13030/qt4j44w7w6. doi: 10.5070/D3252042890
14. Sasaki R, Fujimura T, Lyu C, Aiba S. Severe Eczematoid and Lichenoid Eruption With Full-Thickness Epidermal Necrosis Developing From Metastatic Urothelial Cancer Treated With Enfortumab Vedotin. J Dermatol (2020) 47(12):1436–8. doi: 10.1111/1346-8138.15577
15. Francis A, Jimenez A, Sundaresan S, Kelly B. A Rare Presentation of Enfortumab Vedotin-Induced Toxic Epidermal Necrolysis. JAAD Case Rep (2020) 7:57–9. doi: 10.1016/j.jdcr.2020.10.020
16. Viscuse PV, Marques-Piubelli ML, Heberton MM, Parra ER, Shah AY, Siefker-Radtke A, et al. Case Report: Enfortumab Vedotin for Metastatic Urothelial Carcinoma: A Case Series on the Clinical and Histopathologic Spectrum of Adverse Cutaneous Reactions From Fatal Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis to Dermal Hypersensitivity Reaction. Front Oncol (2021) 11:621591. doi: 10.3389/fonc.2021.621591
17. Dobry AS, Virgen CA, Hosking AM, Mar N, Doan L, Lee B, et al. Cutaneous Reactions With Enfortumab Vedotin: A Case Series and Review of the Literature. JAAD Case Rep (2021) 14:7–9. doi: 10.1016/j.jdcr.2021.05.020
18. Böhm R, von Hehn L, Herdegen T, Klein HJ, Bruhn O, Petri H, et al. OpenVigil FDA - Inspection of U.S. American Adverse Drug Events Pharmacovigilance Data and Novel Clinical Applications. PLoS One (2016) 11(6):e0157753. doi: 10.1371/journal.pone.0157753
19. Meng L, Yang B, Qiu F, Jia Y, Sun S, Yang J, et al. Lung Cancer Adverse Events Reports for Angiotensin-Converting Enzyme Inhibitors: Data Mining of the FDA Adverse Event Reporting System Database. Front Med (Lausanne) (2021) 8:594043. doi: 10.3389/fmed.2021.594043
20. Papazisis G, Spachos D, Siafis S, Pandria N, Deligianni E, Tsakiridis I, et al. Assessment of the Safety Signal for the Abuse Potential of Pregabalin and Gabapentin Using the FAERS Database and Big Data Search Analytics. Front Psychiatry (2021) 12:640264. doi: 10.3389/fpsyt.2021.640264
21. Andrews EB, Moore N. Mann\"s Pharmacovigilance || History of Pharmacovigilance. UK: John Wiley & Sons, Ltd (2014) p. 331–54.
22. Eudravigilance Expert Working Group (EV-EWG). European Medicine Agency Guidelines. London: Eudravigilance Expert Working Group (EV-EWG) (2006) p. 1–22.
23. Sakaeda T, Kadoyama K, Minami K, Okuno Y. Commonality of Drug-Associated Adverse Events Detected by 4 Commonly Used Data Mining Algorithms. Int J Med Sci (2014) 11(5):461–5. doi: 10.7150/ijms.7967
24. van Puijenbroek EP, Bate A, Leufkens HG, Lindquist M, Orre R, Egberts AC. A Comparison of Measures of Disproportionality for Signal Detection in Spontaneous Reporting Systems for Adverse Drug Reactions. Pharmacoepidemiol Drug Saf (2002) 11(1):3–10. doi: 10.1002/pds.668
25. Bate A, Evans SJ. Quantitative Signal Detection Using Spontaneous ADR Reporting. Pharmacoepidemiol Drug Saf (2009) 18(6):427–36. doi: 10.1002/pds.1742
26. Bate A. The Use of Bayesian Confidence Propagation Neural Network in Pharmacovigilance [Internet] [PhD Dissertation] (2003). Available at: http://urn.kb.se/resolve?urn=urn:nbn:se:umu:diva-83.
27. Yu EY, Petrylak DP, O'Donnell PH, Lee JL, van der Heijden MS, Loriot Y, et al. Enfortumab Vedotin After PD-1 or PD-L1 Inhibitors in Cisplatin-Ineligible Patients With Advanced Urothelial Carcinoma (EV−201): A Multicentre, Single-Arm, Phase 2 Trial. Lancet Oncol (2021) 22(6):872–82. doi: 10.1016/S1470-2045(21)00094-2
28. Hoffman-Censits J, Lombardo K, McConkey D, Hahn NM, Bashir B, Kelly WK, et al. New and Topics: Enfortumab Vedotin Mechanisms of Response and Resistance in Urothelial Cancer - What do We Understand So Far? Urol Oncol (2021) 39(10):619–22. doi: 10.1016/j.urolonc.2021.05.013
29. Fortugno P, Josselin E, Tsiakas K, Agolini E, Cestra G, Teson M, et al. Nectin-4 Mutations Causing Ectodermal Dysplasia With Syndactyly Perturb the Rac1 Pathway and the Kinetics of Adherens Junction Formation. J Invest Dermatol (2014) 134(8):2146–53. doi: 10.1038/jid.2014.119
30. Rikitake Y, Mandai K, Takai Y. The Role of Nectins in Different Types of Cellecell Adhesion. J Cell Sci (2012) 125(16):3713–22. doi: 10.1242/jcs.099572
31. de Claro RA, McGinn K, Kwitkowski V, Bullock J, Khandelwal A, Habtemariam B, et al. U.S. Food and Drug Administration Approval Summary: Brentuximab Vedotin for the Treatment of Relapsed Hodgkin Lymphoma or Relapsed Systemic Anaplastic Large-Cell Lymphoma. Clin Cancer Res (2012) 18(21):5845–9. doi: 10.1158/1078-0432.CCR-12-1803
32. Yardley DA, Weaver R, Melisko ME, Saleh MN, Arena FP, Forero A, et al. EMERGE: A Randomized Phase II Study of the Antibody-Drug Conjugate Glembatumumab Vedotin in Advanced Glycoprotein NMB-Expressing Breast Cancer. J Clin Oncol (2015) 33(14):1609–19. doi: 10.1200/JCO.2014.56.2959
33. Palanca-Wessels MC, Czuczman M, Salles G, Assouline S, Sehn LH, Flinn I, et al. Safety and Activity of the Anti-CD79B Antibody–Drug Conjugate Polatuzumab Vedotin in Relapsed or Refractory B-Cell non-Hodgkin Lymphoma and Chronic Lymphocytic Leukaemia: A Phase 1 Study. Lancet Oncol (2015) 16(6):704–15. doi: 10.1016/S1470-2045(15)70128-2
Keywords: cutaneous toxicity, EV, Food and Drug Administration Adverse Event Reporting System, disproportionality analysis, real-word study
Citation: Yang H, Yu X and An Z (2022) Cutaneous Toxicity Associated With Enfortumab Vedotin: A Real-Word Study Leveraging U.S. Food and Drug Administration Adverse Event Reporting System. Front. Oncol. 11:801199. doi: 10.3389/fonc.2021.801199
Received: 25 October 2021; Accepted: 22 December 2021;
Published: 19 January 2022.
Edited by:
Jennifer Martin, The University of Newcastle, AustraliaReviewed by:
Tapas Ranjan Behera, Cleveland Clinic, United StatesCopyright © 2022 Yang, Yu and An. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhuoling An, YW56aHVvbGluZ0AxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.