
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 12 January 2022
Sec. Hematologic Malignancies
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.799721
This article is part of the Research Topic Advances in the Treatment of Primary Central Nervous System Lymphoma View all 6 articles
Objective: The reviewed literature supports a treatment regimen for primary central nervous system lymphoma (PCNSL) that includes induction chemotherapy, followed by one consolidation therapy. High-dose chemotherapy supported by autologous stem-cell transplantation (ASCT) is the most studied option, but its effects are controversial. The aim of this study was to evaluate the efficacy and safety of ASCT for newly diagnosed PCNSL by means of a meta-analysis.
Methods: The PubMed, Embase, and Cochrane Library databases were systematically searched for studies published until May 20, 2021. Included studies were prospective studies of patients with newly diagnosed PCNSL treated with ASCT. The pooled rates and 95% confidence intervals (CIs) were determined for all outcomes. Subgroup analysis was conducted to compare the relative risk (RR) with 95% CIs for the complete remission (CR) rate and the hazard ratios (HRs) with 95% CIs for progression-free survival (PFS) and overall survival (OS).
Results: Thirteen prospective studies including 348 patients were analyzed. The pooled CR rate, overall response rate, and relapse rate were 80% (95% CI, 71–88%, I2 = 67.06%, p = 0.00), 95% (95% CI, 87–100%, I2 = 73.65%, p= 0.00), and 19% (95% CI, 15–24%, I2 = 76.18%, p = 0.00), respectively. The pooled 2- and 5-year PFS and OS rates were 74% (95% CI, 68–80%, I2 = 3.90%), 65% (95% CI, 51–77%, I2 = 74.61%), 80% (95% CI, 72–88%, I2 = 57.54%), and 69% (95% CI, 53–83%, I2 = 83.89%), respectively. Hematological toxicity and infections were more common adverse events above grade 3. The pooled treatment-related mortality was 3% (95% CI, 1–6%, I2 = 28.18%, p = 0.16). In the group analysis of ASCT compared with whole-brain radiotherapy, there were no significant differences in the CR rate (RR, 1.00, 95% CI, 0.88–1.14, p = 0.971), relapse rate (RR, 0.44, 95% CI, 0.06–3.10, p = 0.408), PFS (HR, 1.28, 95% CI, 0.81–2.01, p = 0.29), or OS (HR, 1.62, 95% CI, 0.97–2.69, p = 0.06). Cognitive functions were preserved or improved after ASCT.
Conclusions: ASCT is a feasible approach for consolidation with good tolerability for newly diagnosed PCNSL patients. High-quality randomized controlled trials are still needed to confirm the effects of ASCT.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42021268422.
Primary central nervous system lymphoma (PCNSL) is a rare form of aggressive extranodal non-Hodgkin lymphoma located in the brain, leptomeninges, spinal cord, and intraocular structures (1, 2). The incidence of PCNSL has markedly increased in immunocompetent patients over the previous decades (3). Although high-dose methotrexate (HD-MTX)-based chemotherapy regimens significantly improved the prognosis of PCNSL, the duration of the remission period was short, and the disease easily progressed and recurred. The main therapeutic goals in the treatment of PCNSL are to further control the disease and prolong survival with consolidation therapy after induced remission.
Both whole-brain radiotherapy (WBRT) and high-dose chemotherapy (HDC) are important consolidation therapies in the management of PCNSL (4–9). Compared with WBRT alone, HD-MTX-based chemotherapy combined with WBRT extended the median progression-free survival (PFS) and overall survival (OS) by two to three times. However, WBRT can lead to irreversible neurotoxicity, such as brain dysfunction, progressive dementia, and urinary incontinence (10, 11). PCNSL mostly occurs in elderly individuals who have poor tolerance to high-dose combined chemotherapy (12, 13). Alternative strategies are being investigated to improve disease outcomes and mitigate neurocognitive side effects in patients. These include reduced-dose WBRT, nonmyeloablative high-dose chemotherapy, and HDC with autologous stem-cell transplantation (ASCT). There are no randomized studies that have compared all of these consolidation regimens head to head.
Different alternatives have been proposed, with ASCT being the most investigated strategy. A number of studies suggesting the high efficacy of HDC supported by ASCT, with acceptable tolerability, mostly using thiotepa-based conditioning regimens, have been reported (14, 15). However, the level of evidence in this field is still low, and the existing literature mostly comprises retrospective studies and small study samples. In the current study, we conducted a meta-analysis using the available data on the complete remission (CR) rate, overall response rate (ORR), relapse rate, grade 3–4 toxic effect rate, treatment-related mortality (TRM), PFS, and OS to assess the efficacy of ASCT as part of the first-line treatment for newly diagnosed PCNSL.
This systematic review and meta-analysis was registered with the International Prospective Register of Systematic Reviews (PROSPERO, number CRD42021268422), and the study was carried out in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement (16, 17).
We carefully searched academic databases (PubMed, EMBASE, and the Cochrane Library) to identify relevant studies from the date the database was established until May 20, 2021. The search typically included two key terms: “primary central nervous system lymphoma” and “autologous stem-cell transplantation.” The search strategy for each database is shown in Supplementary Table 1. The search was not restricted by region, race, age, or payment method. In addition, we searched the reference lists of the identified articles, original studies, and previous meta-analyses to identify other potential studies. The databases were searched, and the results were imported to EndNote software (X9 version).
Studies were included according to the following criteria: (1) the study type included randomized clinical trials (RCTs), prospective cohort studies, and single-arm studies; (2) the patients were newly diagnosed with PCNSL confirmed by histopathology; (3) studies included PCNSL patients treated with ASCT as part of first-line treatment; (4) all the included studies reported sufficient data on survival outcomes, such as CR, PFS, and OS; and (5) for duplicate data or data from sequential publications, only the largest or newest research study was included.
Studies were excluded based on the following criteria: (1) written in a language other than English; (2) incomplete data for the targeted outcomes; (3) reviews, letters, reports, conference abstracts or papers, mail articles, editorials, and cellular or animal studies; (4) sample cases from a database; and (5) not related to the topic.
Two investigators (JL and JG) independently extracted the data using a standardized form and subsequently validated the extracted data through discussion and consensus. For each study, the following data were collected: (1) last name of the first author, publication year, and study design; (2) study population location, sample size, median age, the sex of patients, induction chemotherapy, and median follow-up time; (3) toxicities associated with ASCT and TRM; and (4) survival outcomes, including CR, ORR (including the CR rate and partial remission rate), PFS, and OS. The data were directly collected from the article if the hazard ratios (HRs), 95% confidence intervals (CIs), and p-values were reported; otherwise, we extracted the data from Kaplan–Meier curves using Engauge Digitizer version 13.0 (18) or contacted the corresponding authors to obtain these data.
The quality of the included articles was assessed by two investigators (JL and JG) independently. We used the Cochrane Collaboration risk of bias tool to evaluate the included RCTs according to the Cochrane Handbook recommendations (19). The quality assessment of prospective cohort studies were evaluated by the modified Newcastle–Ottawa scale (20). Prospective nonrandomized studies were evaluated by the methodological index for nonrandomized studies (MINORS) (21).
Statistical analysis was performed with STATA SE 15.1 (StataCorp, College Station, TX, USA) and RevMan 5.3 by two investigators (JL and JG) independently. Disagreements were resolved by discussion with the third investigator (XS). For the pooled rates, a random-effect model or a fixed-effect model with double arcsine transformation was used. The effect size (ES) of all combined results is represented by the 95% CI (with upper and lower limits).
Heterogeneity was assessed by the chi-square test (Q-statistic) and I2 statistic. If p > 0.10 and/or I2 < 50%, the heterogeneity was deemed to be low, and a fixed-effect (Mantel–Haenszel method) model was used; otherwise, we selected a random-effect (Mantel–Haenszel method) model because of the presence of significant heterogeneity. The ES for each meta-analysis was calculated as follows: (1) For all included studies, the CR rate, ORR, relapse rate, grade 3–4 toxic effect rate, and TRM are represented as pooled rates with corresponding 95% CIs. (2) For RCTs, the CR rate is represented as the relative risk (RR) with 95% CIs and the PFS and OS rates are represented as HRs with 95% CIs; if RR > 1.0, HR < 1.0, and p < 0.05, the results favored ASCT therapy and were considered statistically significant. In addition, we assessed publication bias by funnel plots, Begg’s test and Egger’s test.
The flow chart of the screening process is shown in Figure 1. A total of 1,344 articles were screened by the search strategy, and 144 duplicate articles were excluded. Then, 1,200 articles underwent a title and abstract review, and 1,129 were excluded for the following reasons: obvious irrelevance (n = 1,001) and reviews, letters, conference abstracts or papers, mail articles, and editorials (n = 128). The remaining 71 articles were comprehensively reviewed, 55 of which were excluded for the following reasons: letter to the editor (n = 3), protocol (n = 8), retrospective study (n = 39), allogeneic HSCT for PCNSL (n = 3), and ASCT for relapse/refractory PCNSL (n = 2). The 16 remaining studies fulfilled the eligibility criteria for qualitative synthesis. After further evaluation, one study involved an error correction (22), and two studies updated the results of three studies (23, 24). Our meta-analysis ultimately included 13 articles, including 1 RCT (25), 1 randomized noncomparative phase II trial (26), 8 single-arm phase II trials (15, 27–33), 1 prospective cohort study (34), and 2 pilot trials (35, 36).
In the included studies, a total of 524 newly diagnosed PCNSL patients were included, 348 of whom received therapy with ASCT. The classification and features of the included studies, including the study design, publication year, recruitment period, country, sample size (total/number of patients who underwent ASCT), sex (male/female), median age, induction therapy, ASCT setting, and outcome indicators, are shown in Table 1. The 13 prospective studies were conducted in different countries: three were conducted in the United States, one was conducted in Europe, one was conducted in South Korea, two were conducted in France, five were conducted in Germany, and one was conducted in Canada. The MTX-based regimen was the most common induction therapy. The conditioning regimens included three studies with TBC (thiotepa, busulfan, cyclophosphamide), one study with rituximab-TBC, six studies with thiotepa combined with other drugs, one study with BUCYE (busulfan, cyclophosphamide, etoposide), and two studies with BEAM (carmustine, etoposide, cytarabine, melphalan) regimens, followed by ASCT.
The Cochrane Collaboration risk of bias tool was used to assess the quality of one included RCT (Supplementary Figure 1). The risk of bias graph showed that the included RCT was a high-quality study. Eight single-arm studies, one randomized noncomparative phase II trial, and two pilot trials were assessed using the MINORS index score, which ranges from 10 to 20 points and showed that these studies were acceptable for the present meta-analysis (Table 2A). In addition, we used the modified Newcastle–Ottawa scale for the quality assessment of one included prospective cohort study. This study was considered to be of high quality, with a rating of eight stars (Table 2B).
The 13 included studies reported the CR rate and relapse rate, and 12 studies reported the ORR.
The pooled CR rate, ORR, and relapse rate after treatment with ASCT were 80% (95% CI, 71–88%, I2 = 67.06%, p = 0.00), 95% (95% CI, 87–100%, I2 = 73.65%, p = 0.00), and 19% (95% CI, 15–24%, I2 = 76.18%, p = 0.00), respectively (Figure 2 and Figure 3A).
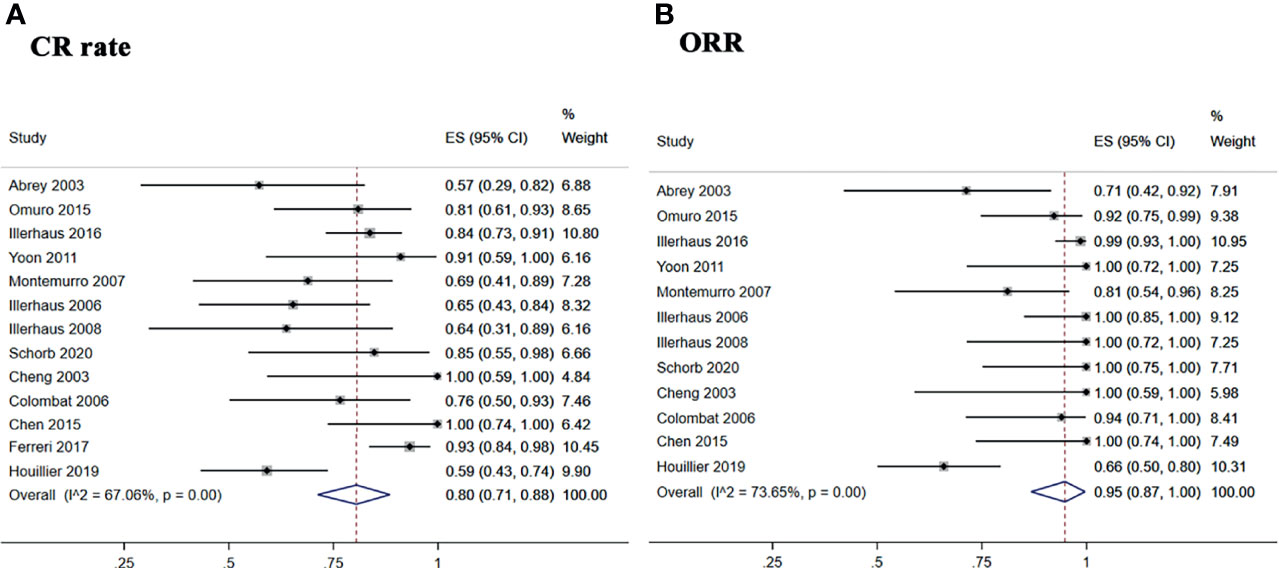
Figure 2 Forest plot of pooled response rate. (A) Complete remission rate. (B) overall response rate.
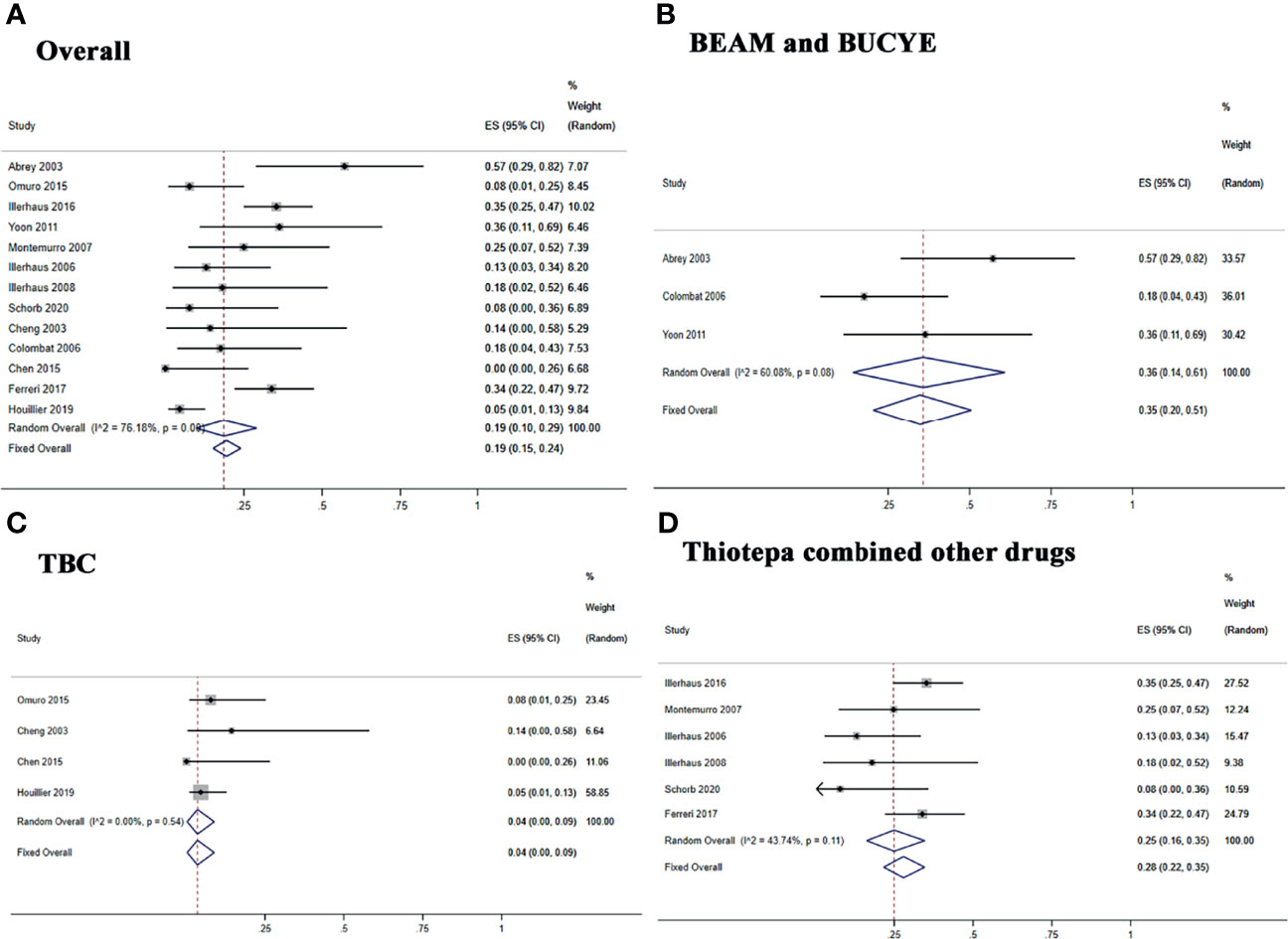
Figure 3 Forest plot of the relapse rate for treatment with the ASCT. (A) Overall relapse rete. (B) BEAM and BUCYE conditioning regimens. (C) TBC conditioning regimen. (D) Thiotepa combined other drugs.
The subgroup analysis was stratified according to the different conditioning regimens. Three studies (29, 32, 34) investigated 42 PCNSL patients treated with the BEAM or BUCYE conditioning regimen, and the pooled relapse rate was 35% (95% CI, 20–51%, I2 = 60.08%, p = 0.08). Four studies (26, 30, 31, 35) treated 111 patients with the TBC regimen, and the pooled relapse rate was 4% (95% CI, 0–9%, I2 = 0%, p = 0.54). Six studies (15, 25, 27, 28, 33, 36) showed 195 patients with thiotepa combined with other drugs; the pooled relapse rate was 28% (95% CI, 22–35%, I2 = 43.74%, p = 0.11) (Figures 3B–D).
Five studies reported 1-, 2-, and 3-year PFS, and four studies reported 5-year PFS Kaplan–Meier curves. Ten studies reported 1-, 2-, and 3-year OS, and six studies reported 5-year OS Kaplan–Meier curves.
The 1-, 2-, 3-, and 5-year pooled PFS rates for newly diagnosed PCNSL with ASCT were 79% (95% CI, 73–84%, I2 = 61.93%, p = 0.03), 74% (95% CI, 68–80%, I2 = 3.90%, p = 0.38), 71% (95% CI, 64–76%, I2 = 48.89%, p = 0.10), and 65% (95% CI, 51–77%, I2 = 74.61%, p = 0.01), respectively (Figure 4). The 1-, 2-, 3-, and 5-year pooled OS rates for newly diagnosed PCNSL with ASCT were 88% (95% CI, 78–95%, I2 = 73.86%, p = 0.00), 80% (95% CI, 72–88%, I2 = 57.54%, p = 0.01), 77% (95% CI, 69–85%, I2 = 50.37%, p = 0.03), and 69% (95% CI, 53–83%, I2 = 83.89%, p = 0.00), respectively (Figure 5).
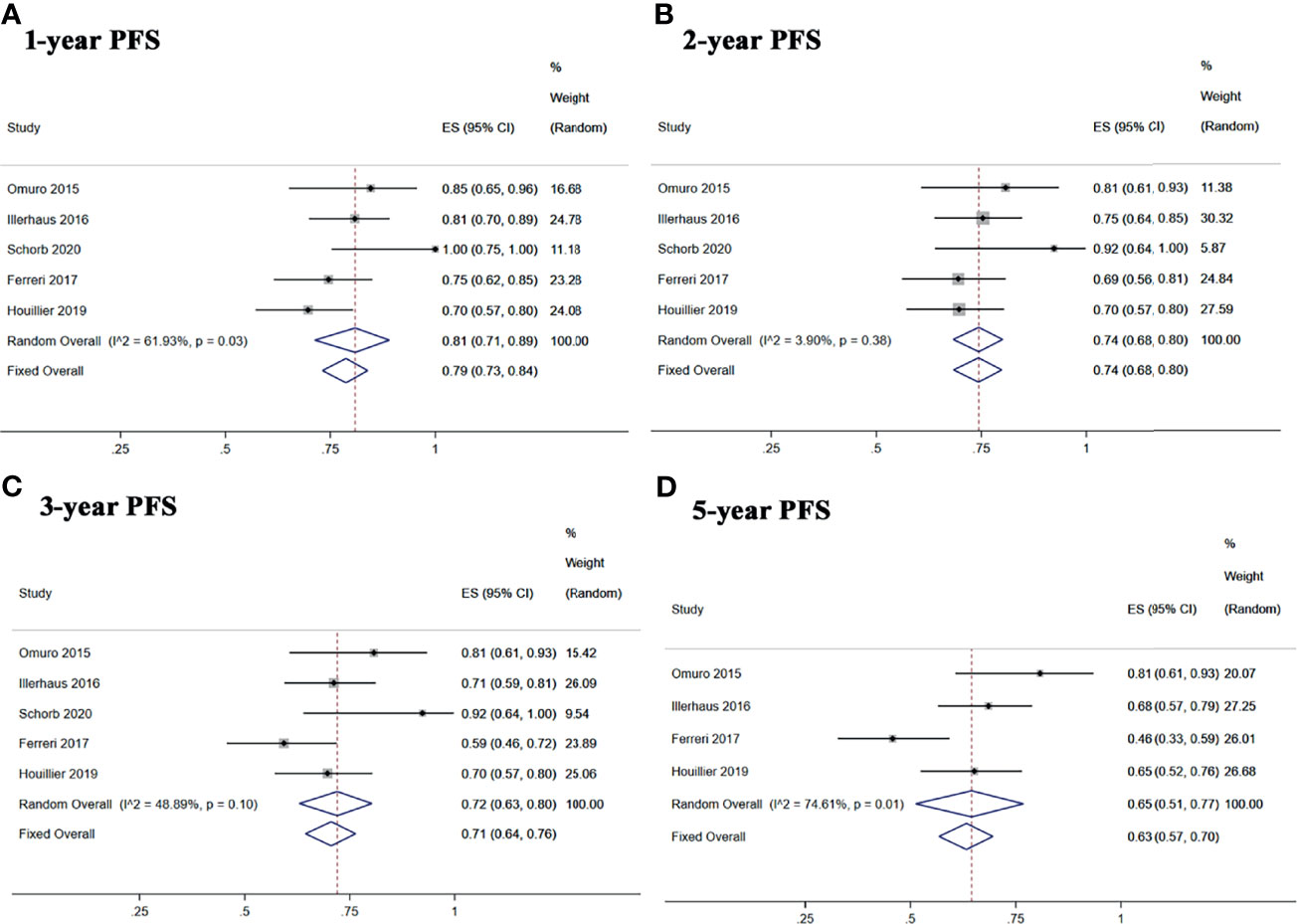
Figure 4 Forest plot of progression-free survival for treatment with ASCT. (A) 1-year progression-free survival. (B) 2-year progression-free survival. (C) 3-year progression-free survival. (D) 5-year progression-free survival.
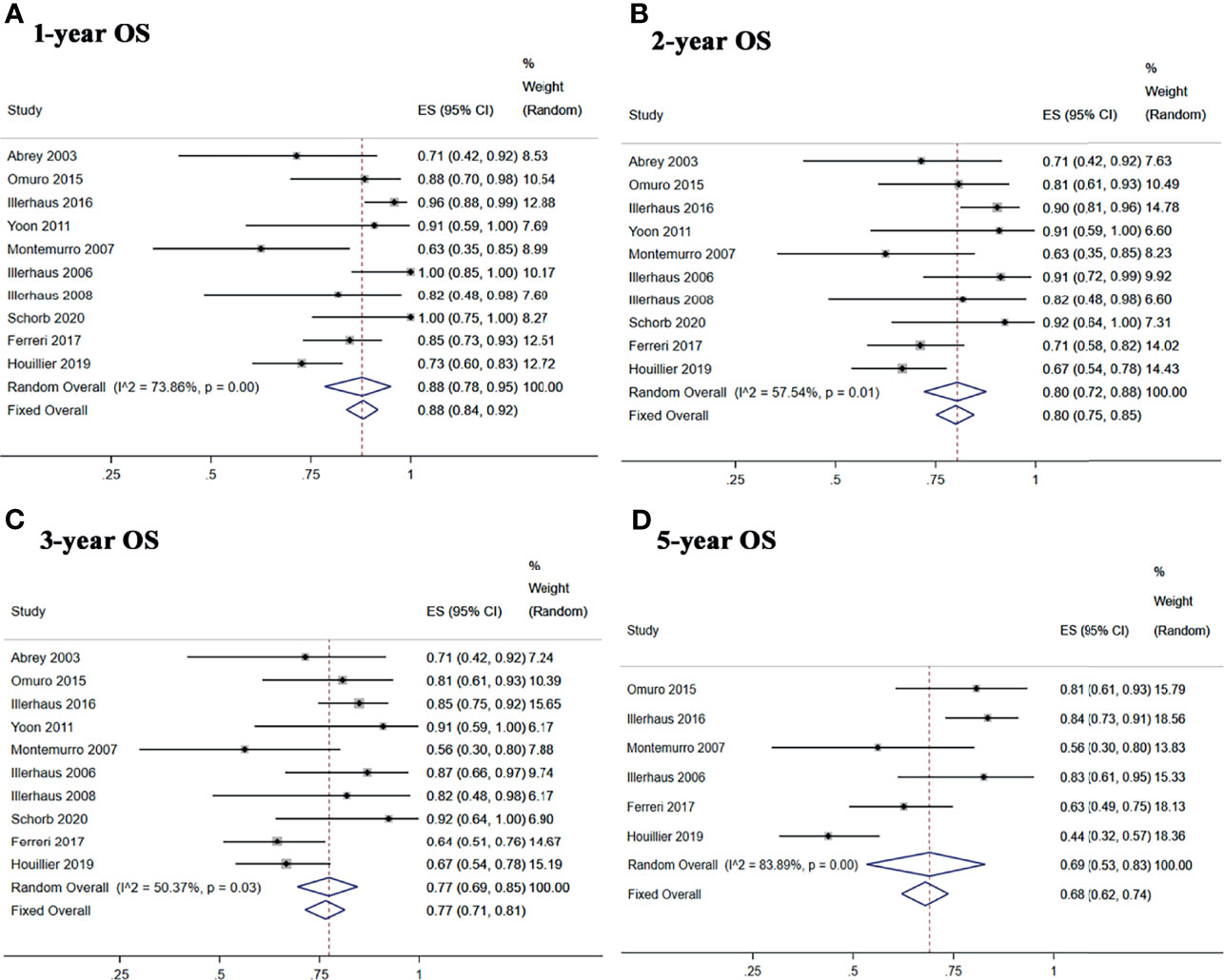
Figure 5 Forest plot of overall survival for treatment with ASCT. (A) 1-year overall survival. (B) 2-year overall survival. (C) 3-year overall survival. (D) 5-year overall survival.
We also performed a subgroup analysis of the two randomized phase II trials (25, 26) comparing the treatment with ASCT and WBRT for newly diagnosed PCNSL patients. We chose the Mantel–Haenszel fixed-effects model for the CR rate analysis because of the low heterogeneity among the included studies (I2 = 0.0%, p = 0.618) and chose the Mantel–Haenszel random-effects model for the relapse rate analysis because of the high heterogeneity (I2 = 89.4%, p = 0.002). The pooled data of the studies showed no significant difference in the CR rate (RR: 1.00, 95% CI, 0.88–1.14, p = 0.971) or the relapse rate (RR: 0.44, 95% CI, 0.06–3.10, p = 0.408) (Figure 6).
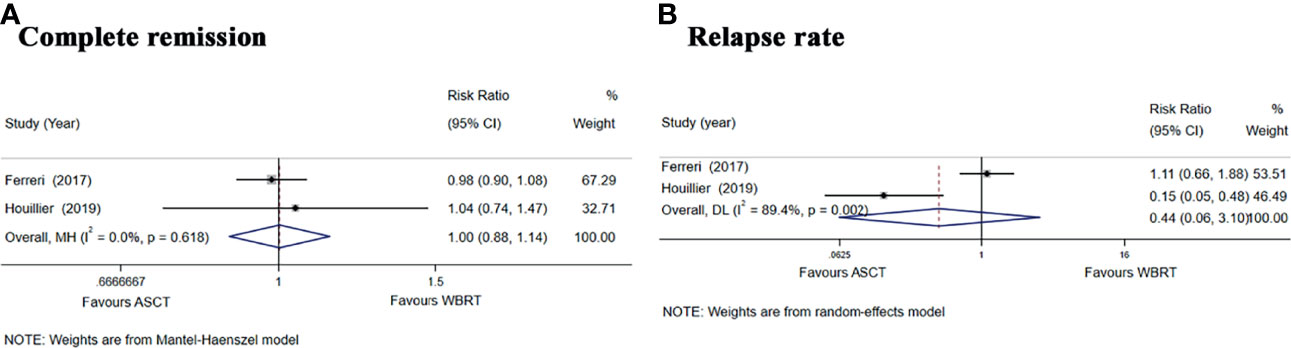
Figure 6 Forest plot of the CR and relapse rate for treatment with the ASCT group vs. WBRT group. (A) Complete remission rate. (B) Relapse rate.
In the intention-to-treat analyses, the pooled analysis showed no significant differences in the PFS (HR = 1.28, 95% CI, 0.81–2.01, p = 0.29), with low heterogeneity (I2 = 0%, p = 0.66) and OS (HR = 1.62, 95% CI, 0.97–2.69, p = 0.06), with low heterogeneity (I2 = 0%, p = 0.80) (Figures 7A, B). For the 1-, 2-, and 5-year PFS, the RR for the ASCT group vs. WBRT group were 0.93 (95% CI, 0.80–1.07, p = 0.307), with low heterogeneity (I2 = 0.0%, p = 0.462), 1.02 (95% CI, 0.74–1.40, p = 0.912), with high heterogeneity (I2 = 72.5%, p = 0.057), and 1.34 (95% CI, 0.54–5.35, p = 0.531), with high heterogeneity (I2 = 91.2%, p = 0.001) (Supplementary Figure 2). For 1- and 2-year OS, the pooled results favored the WBRT group (RR = 0.87, 95% CI, 0.78–0.97, p = 0.04), with low heterogeneity (I2 = 32.1%, p = 0.225), and (RR = 0.86, 95% CI, 0.74–0.99, p = 0.038), with low heterogeneity (I2 = 0.00%, p = 0.756). The pooled results showed no significant difference in the 5-year OS (RR = 0.86, 95% CI, 0.69–1.07, p = 0.156), with low heterogeneity (I2 = 0.0%, p = 0.602) (Supplementary Figure 3).
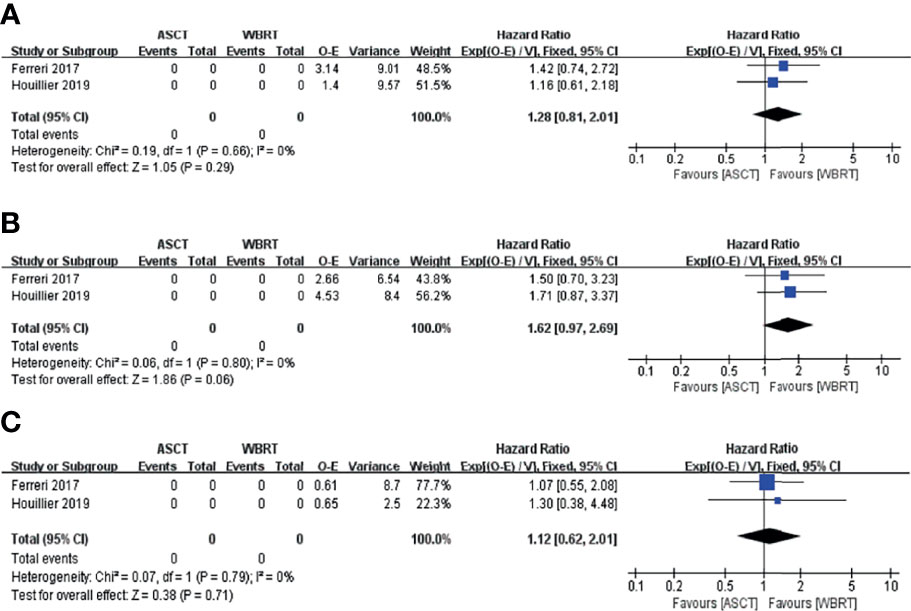
Figure 7 Forest plot of the survival for the treatment with the ASCT group vs. WBRT group. (A) Progression-free survival in the intention-to-treat analyses. (B) Overall survival in the intention-to-treat analyses. (C) Progression-free survival in the per-protocol analyses.
In the per-protocol analyses, the pooled analysis showed no significant differences in the PFS (HR = 1.12, 95% CI, 0.62–2.01, p = 0.71), with low heterogeneity (I2 = 0%, p = 0.79; Figure 7C). For the 1-, 2-, and 5-year PFS, the RR for the ASCT group vs. WBRT group were 0.99 (95% CI, 0.88–1.12, p = 0.878), with low heterogeneity (I2 = 0.0%, p = 0.455), 1.14 (95% CI, 0.87–1.49, p = 0.356), with high heterogeneity (I2 = 67.7%, p = 0.078), and 1.23 (95% CI, 0.65–2.34, p = 0.523), with high heterogeneity (I2 = 85.8%, p = 0.008), respectively (Supplementary Figure 4).
For the grade 3–4 toxic effect rate, we performed a subgroup analysis of the included studies based on the type of side effect. Hematologic toxicities mainly included neutropenia/leukopenia, thrombocytopenia, and anemia. The pooled rate of grade 3–4 hematologic toxicities was 99% (95% CI, 97–100%, I2 = 0.0%, p = 0.68). Severe nonhematologic toxicities mainly included infection, febrile neutropenia, hepatotoxicity, gastrointestinal toxicities, mucositis, and acute neurotoxicity/encephalopathy. The pooled rate of grade 3–4 febrile neutropenia or infection was 59% (95% CI, 53–65%, I2 = 94.15%, p = 0.00). The pooled rate of grade 3–4 hepatotoxicity was 4% (95% CI, 1–8%, I2 = 0.0%, p = 0.75). The pooled rate of grade 3–4 gastrointestinal toxicities was 16% (95% CI, 11–22%, I2 = 0.0%, p = 0.62). The pooled rates of grade 3–4 mucositis and acute neurotoxicity/encephalopathy were 28% (95% CI, 22–34%, I2 = 92.55%, p = 0.00) and 4% (95% CI, 1–8%, I2 = 67.09%, p = 0.00), respectively (Table 3). The pooled TRM was 3% (95% CI, 1–6%, I2 = 28.18%, p = 0.16) (Figure 8).
We conducted sensitivity analysis by removing individual studies one by one from the pooled results with high heterogeneity to check the influence of the removed data set on the overall ES. The results of the sensitivity analysis indicated that the pooled result was stable in terms of the CR rate, ORR, toxic effect, PFS, and OS analyses when studies were omitted, indicating that our combined results are reliable (Supplementary Figures 5–9).
We used the funnel plot and Egger’s and Begg’s tests to evaluate the publication bias in studies assessing the CR rate (p = 0.714 for Begg’s test and p = 0.776 for Egger’s test), recurrence rate (p = 1.000 for Begg’s test and p = 0.643 for Egger’s test), TRM (p = 0.714 for Begg’s test and p = 0.438 for Egger’s test) and 2-year OS rate (p = 0.788 for Begg’s test and p = 0.669 for Egger’s test), which did not demonstrate significant publication bias (Figure 9).
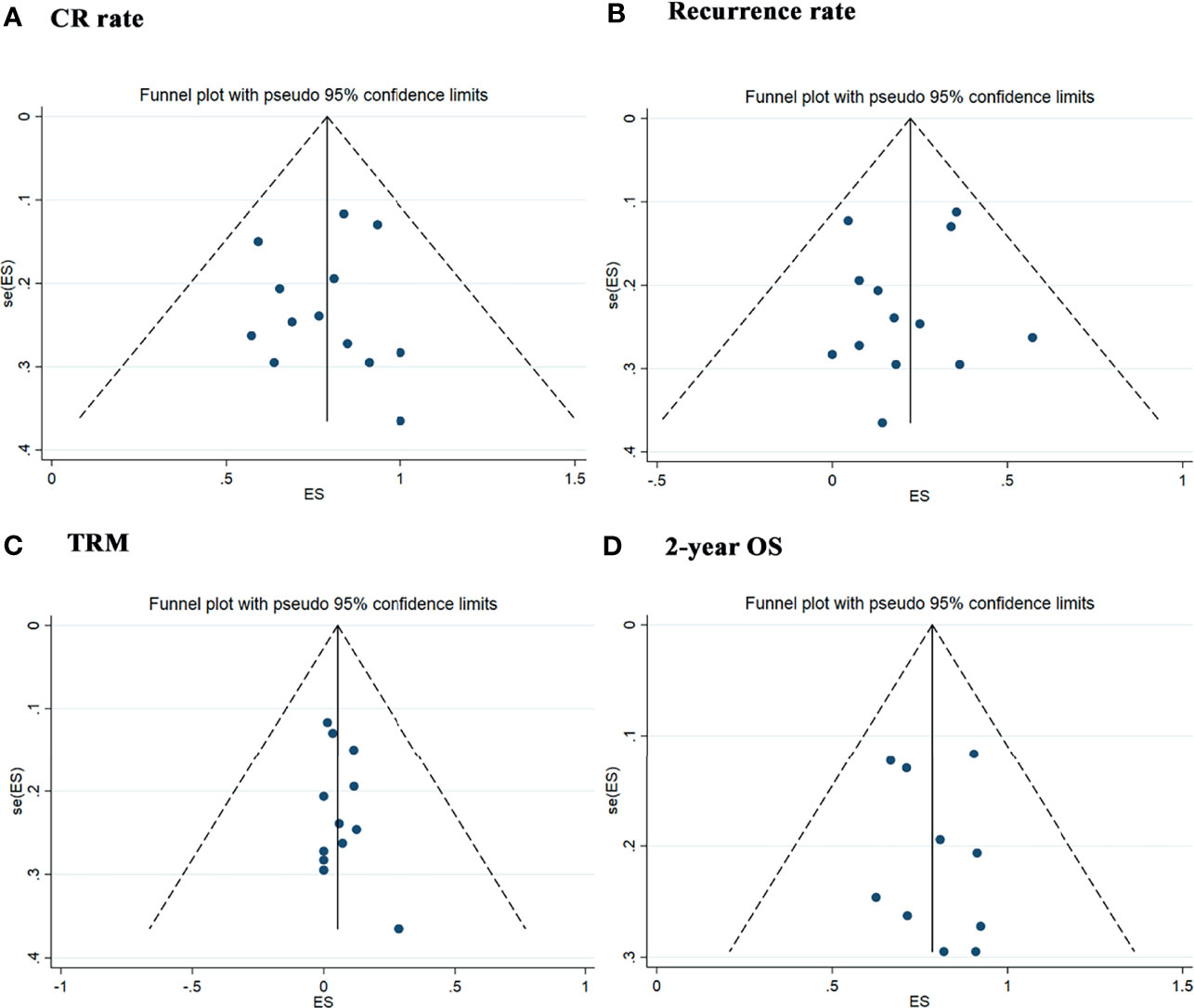
Figure 9 Publication Bias with the funnel plot. (A) Assessment result of the complete remission rate. (B) Assessment result of the recurrence rate. (C) Assessment result of the treatment-related mortolity. (D) Assessment result of the 2-year overall survival.
PCNSL has a dismal prognosis. Although there are therapeutic progress and improvement in survival figures (37), the outcomes of patients with PCNSL remain poor, with 20–30% survival at 5 years and 10–20% survival at 10 years (38–40). Induction treatment with HD-MTX-based chemotherapy is standard for PCNSL and can achieve a CR rate of more than 40% and an ORR of 60% or higher (12). Despite high response rates with initial HD-MTX-based treatment, approximately 50% responders are still at risk for progression or relapse. Moreover, approximately 25% patients fail to respond to the initial treatment (41). In a retrospective study, the median OS from the time of progression was 3.7 months for patients who relapsed within the first year of the initial therapy and 2 months for primary refractory patients (42).
Induction chemotherapy followed by consolidation therapy has been proposed. Consolidation treatment includes WBRT, HDC supported by ASCT, and nonmyeloablative chemotherapy. WBRT in the management of PCNSL has the risk of severe neurotoxicity observed in patients treated with chemoradiotherapy (43, 44). HDC supported by ASCT is the most commonly investigated alternative to WBRT in patients with PCNSL (45). Data are currently limited to retrospective studies and several single-arm phase II trials with few subjects enrolled, and the effects of ASCT on PCNSL are controversial.
In the current study, we conducted a meta-analysis to evaluate the efficacy and safety of HDC followed by ASCT as first-line treatment in newly diagnosed PCNSL patients. In all included studies, induction chemotherapy was based on HD-MTX either as monotherapy or combination, using drugs including cytarabine, carmustine, etoposide, procarbazine, rituximab, temozolomide, pemetrexed, thiotepa, and so on. Intensive chemotherapy mainly contained a thiotepa-based regimen. Our analysis of the efficacy of ASCT in the treatment of newly diagnosed PCNSL patients showed that the pooled CR rate and ORR could reach 80 and 95%, respectively. Only one included study (36) conducted the ASCT in elderly patients with a median age of 74 years (range, 69–79 years). This study showed an increase in CR rate from 28.6 to 84.6% pre- and post-ASCT. This finding shows that ASCT could achieve satisfactory results in the treatment of PCNSL. The relapse rate was 19% after ASCT treatment. HD-MTX-based chemotherapy followed by ASCT as consolidate treatment with higher response rate and lower relapse rate compared with induction chemotherapy alone.
However, the I2 values for the overall CR rate, ORR, and relapse rate were 67.06, 73.65, and 76.18%, which was quite heterogeneous. The heterogeneity of response rate may be related to the difference of including induction chemotherapy and/or conditioning regimens: MTX-based combination chemotherapy and high-dose MTX (≥3.5 g/m2) with higher response rate than MTX monotherapy and MTX dose below 3.5 g/m2. The subgroup analysis stratified according to the different conditioning regimens significantly reduced the heterogeneity of relapse rate, indicating that the heterogeneity was derived from the differences in the conditioning treatment plan. The subgroup analysis showed that the relapse rate from BEAM/BUCYE, TBC, and thiotepa combined with other drugs (busulfan/carmustine/cytarabine) were 35, 4, and 28% respectively. The BEAM regimen is a feasible approach with good tolerability, especially for elderly patients with PCNSL. However, these drugs have poor blood–brain barrier permeability with higher relapse rate. Thiotepa, busulfan, and cyclophosphamide can penetrate the blood–brain barrier (46–48). Compared to the BEAM regimen, theoretically, TBC drugs should produce better effects because of good blood–brain barrier penetration. On one study in 2003 by Abrey et al. (29), 14 patients received the BEAM regimen, followed by ASCT. Eight patients (57.1%) experienced relapse a median of 2.3 months after transplantation (range, 1.3–29.6 months), all but one patient within 7 months. In 2011, Yoon et al. (34) reported that 11 patients received BUCYE as a conditioning regimen; four (36.3%) patients experienced relapse within 1 year. Although effective for systemic lymphoma, these findings have questioned the efficacy of BEAM as consolidation for PCNSL. The relapse rate ranged from 0 to 14.3% with TBC regimen (26, 30, 31, 35), which is much lower than that of the thiotepa combined with busulfan/carmustine/cytarabine (7.7–35%) (15, 25, 27, 28, 33, 36) and BEAM/BUCYE regimens (17.6–57.1%) (29, 32, 34). The best results in the Chen et al. study (30) are the TBC combined with rituximab as a conditioning regimen for 12 newly diagnosed PCNSL patients, none of whom experienced relapse or death.
The subgroup analysis of the two included randomized phase II studies, the pooled analysis of CR rate and relapse rate in the ASCT group compared with the WBRT group, suggested that there were no significant differences. In the International Extranodal Lymphoma Study Group-32 (IELSG32) study (25), 20 patients relapsed after ASCT, while only 3 relapsed in the PRECIS trial (26). The chemotherapy regimen was more intensive in the PRECIS trial, which might explain the apparently lower number of relapses after ASCT in the PRECIS study. The number of relapsed patients in the WBRT group (36 vs. 40 Gy) in the two trials was similar: 18 and 20.
After HD-MTX-based induction therapy, only 20–30% of patients achieve durable long-term remission (49, 50). In our study, the pooled survival analysis of ASCT treatment showed promising results. The pooled 1-, 2-, 3-, and 5-year PFS rates were 84, 80, 77, and 69%. The pooled 1-, 2-, 3-, and 5-year OS rates were 90, 85, 83, and 78%. The heterogeneity of 1- and 5-year PFS and 1-, 2-, 3-, and 5-year OS was relatively significant, which may be due to the limited sample size, various induction or intensive chemotherapy regimens used, and the different characteristics of the patients [age, Eastern Cooperative Oncology Group (ECOG) status, lesion depth, and so on]. The subgroup analysis of ASCT group compared with the WBRT group, the intention-to-treat analyses of the pooled 1- and 2-year OS rates showed higher OS rates in the WBRT group than in the ASCT group. Early ASCT treatment-related mortality because of hematological toxicities might explain the shorter OS than the WBRT group in the first and second years. In the per-protocol analyses, the pooled results of 2- and 5-year PFS tended to favor the ASCT group but with no significant differences.
We also conducted a meta-analysis of grade 3–4 toxicities and found that the most common toxicities were hematological toxicities and febrile neutropenia or infections. Hematological toxicities mainly included neutropenia or leukopenia, thrombocytopenia, and anemia, with an incidence of 99%. The nonhematological toxicities with the highest incidences were infection or febrile neutropenia (59%) and mucositis (28%). Some studies reported that WBRT had a high risk of neurotoxicity (6, 51). In this study, acute neurotoxicity/encephalopathy after ASCT treatment occurred in only 4% of patients. The TRM of ASCT was 3%. Nevertheless, the results of our study still suggest that clinicians should pay attention to the prevention of toxicities such as bone marrow suppression, infection, mucositis, hepatotoxicity, and gastrointestinal tract dysfunction when treating patients with ASCT.
Regarding the cognitive function of PCNSL patients after treatment, the result was consistent in the included studies (25, 26, 28, 29, 31, 35), suggesting that the cognitive function improved over time post-transplantation. Cognitive and quality-of-life (QoL) measures were proposed to be used in prospective clinical trials. Neuropsychological tests usually covered attention, executive functions, memory, and psychomotor speed (10). The Phase II trial in 2015 by Omuro et al. (31) evaluated neurotoxicity by neuropsychological testing, mood/QoL scores, and white matter changes on MRI over time (at baseline, after induction chemotherapy, and before transplant, every 6 months after transplant). Neuropsychological testing included TMTA (Trail-Making Test Part A), TMTB (TMT Part B), BTA (Brief Test of Attention), COWA (Controlled Word Association Test), HVLT-RTL (Hopkins Verbal Learning Test–Revised–Total Learning), HVLT-R-DEL (HVLT–Revised–Delayed Recall), HVLT-R-DI (HVLT–Revised–Discrimination Index), GPT-D (Grooved Pegboard Test–Dominant Hand), and GPT-ND (GPT–Non-Dominant Hand). Results of the HVLT-R-DEL and HVLT-R-DI tests indicated continuous improvement in scores from baseline over time. All of the other tests suggested that the rate of cognitive improvement slowed by 12–18 months post-transplant. Mood/QoL scores used BDI (Beck Depression Inventory) and FACT-BR (Functional Assessment of Cancer Therapy–Brain Cancer). BDI scores significantly and linearly decreased over time. FACT-BR scores significantly improved from baseline, with slowed improvement by 12–18 months post-transplant. An analysis of white matter abnormalities (modified Fazekas scale) showed an improvement (62%) after induction chemotherapy compared with baseline. Following transplant, there was an increase (25%) in white matter abnormalities compared with induction chemotherapy, and then they remained stable over time. In 2016, Illerhaus et al. (28) reported that the mean MMSE (Mini–Mental State Examination) score and the summary measure of Global Health Status Score (European Organisation for Research and Treatment of Cancer QoL core questionnaire) increased over time. Abrey et al. (29) showed that prospective neuropsychologic evaluations for patients with continued follow-up after ASCT displayed no evidence of significant delayed treatment-related neurocognitive decline. Cheng et al. (35) showed that patients experienced an improvement in their neurological performance and QoL post-transplantation. The two included randomized clinical trials demonstrated that cognitive impairment was observed after WBRT, whereas cognitive functions were preserved or improved after ASCT (25, 26). In the PRECIS trial (26), patients were evaluated on global cognitive function [MMSE and Mattis Dementia Rating Scale (MDRS)], episodic verbal memory [free and cued selective reminding test (FCSRT)], attention and mental flexibility (executive function; TMTA and TMTB), and psychoaffective status (motivation; Marin’s apathy scale) at baseline, after induction, and every 6 months after consolidation treatment, respectively. Neuropsychological testing showed an improvement in cognitive function at the end of induction chemotherapy in both groups. However, over time, patients presented a poorer score after WBRT and an improved score after ASCT in executive functions (MDRS, FCSRT total free recall, and TMT), whereas no significant change in hippocampus functions (FCSRT total free and cued recall) was observed in either group. In the IELSG32 trial (25), patients receiving consolidation treatment were assessed on cognitive functions and QoL. An analysis of delta values between baseline and post-treatment scores presented a significant improvement in attention and executive functions (TMT, phonemic verbal fluency) and visuoconstructive abilities (Rey Complex Figure Copy Test) in ASCT treatment. Moreover, the differences in scores between post-treatment immediately and at 2 years of follow-up after treatment showed a significant impairment in some attention and executive functions [Wisconsin card sorting test (WCST)] in the WBRT group, a remarkable improvement in these attention and executive functions, memory (Rey auditory verbal learning test–delayed recall), and QoL figures in the ASCT group. However, one study showed that the use of reduced-dose WBRT (23.4 Gy) resulted in stable cognitive functions for up to 2 years (52). As consolidation therapy, radiation doses are different in different studies, from 23 to 45 Gy (25, 26, 43, 52, 53), suggesting that the radiation dose is proportionately associated with the risk of neurotoxicity, whereas the relation between radiation dose reduction and efficacy remains to be defined.
Morris et al. (52) treated 52 newly diagnosed PCNSL patients with rituximab, HD-MTX, procarbazine, and vincristine as induction chemotherapy who achieved a CR received reduced-dose WBRT (23.4 Gy) and Ara-C. This trial showed high response rates, long-term disease control, and minimal neurotoxicity. In another trial [Radiation Therapy Oncology Group (RTOG) 0227] conducted by Glass et al. (54), patients received HD-MTX, rituximab, and temozolomide, followed by reduced-dose WBRT (36 Gy) and temozolomide with a favorable 2-year OS and PFS as well as the low incidence of late neurotoxicity, cognitive decline, decreased QoL, and leukoencephalopathy compared with the RTOG-9310 trial (WBRT: 45 Gy) (55). These results suggested that reduced-dose WBRT is feasible and effective as consolidation therapy after HD-MTX-based chemotherapy. However, the optimal dose remains to be defined and studies identifying the patients at greatest risk for neurotoxicity are clearly needed.
This meta-analysis has several limitations (1): There were only two randomized clinical trials (one RCT and one randomized noncomparative phase II trial) included because of the rarity of PCNSL. Single-arm trials make it difficult to compare ASCT treatment with other consolidation treatments. (2) All prospective studies were phase II clinical trials. (3) Some data extracted from the Kaplan–Meier curve may be biased. (4) The induction therapy or intensive chemotherapy regimens used among different institutions were significantly different. (5) Due to language limitations, this study only included studies in English.
At present, it is supported that PCNSL treatment includes induction chemotherapy, followed by one consolidation therapy. The HD-MTX-based regimen is the standard induction chemotherapy; however, it is not clear which consolidation therapy should be selected and which is the optimal choice. ASCT as a consolidation strategy is the most investigated approach. Through a meta-analysis of existing prospective studies on ASCT in newly diagnosed PCNSL patients, we found that ASCT can be safe and effective. The results are based on up-to-date information and a high quality of evidence according to the objective of this analysis. Nevertheless, in terms of the limitations of our study, it is essential to update and confirm the analysis in the future when there are more well-controlled, large-sample randomized clinical trials. Further studies are needed to determine the optimal intensive chemotherapy regimen, followed by ASCT and the optimal strategy for relapse after ASCT in the treatment of PCNSL.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
YL and CG designed the study. JL, JG, and XS conducted literature search and data extraction and performed the statistical analysis. JL wrote the manuscript. CG assisted in writing the manuscript. YL and CG reviewed and edited the manuscript in detail. All authors contributed to the article and approved the submitted version.
This work was supported by Capital’s Funds for Health Improvement and Research, NO.2020-2-2049.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.799721/full#supplementary-material
1. Wang CC, Carnevale J, Rubenstein JL. Progress in Central Nervous System Lymphomas. Br J Haematol (2014) 166(3):311–25. doi: 10.1111/bjh.12938
2. Batchelor T, Loeffler JS. Primary CNS Lymphoma. J Clin Oncol (2006) 24(8):1281–8. doi: 10.1200/JCO.2005.04.8819
3. Mendez JS, Ostrom QT, Gittleman H, Kruchko C, DeAngelis LM, Barnholtz-Sloan JS, et al. The Elderly Left Behind-Changes in Survival Trends of Primary Central Nervous System Lymphoma Over the Past 4 Decades. Neuro Oncol (2018) 20(5):687–94. doi: 10.1093/neuonc/nox187
4. Abrey LE, Yahalom J, DeAngelis LM. Treatment for Primary CNS Lymphoma: The Next Step. J Clin Oncol (2000) 18(17):3144–50. doi: 10.1200/JCO.2000.18.17.3144
5. DeAngelis LM, Seiferheld W, Schold SC, Fisher B, Schultz CJ. Combination Chemotherapy and Radiotherapy for Primary Central Nervous System Lymphoma: Radiation Therapy Oncology Group Study 93-10. J Clin Oncol (2002) 20(24):4643–8. doi: 10.1200/JCO.2002.11.013
6. Thiel E, Korfel A, Martus P, Kanz L, Griesinger F, Rauch M, et al. High-Dose Methotrexate With or Without Whole Brain Radiotherapy for Primary CNS Lymphoma (G-PCNSL-SG-1): A Phase 3, Randomised, Non-Inferiority Trial. Lancet Oncol (2010) 11(11):1036–47. doi: 10.1016/S1470-2045(10)70229-1
7. Wieduwilt MJ, Valles F, Issa S, Behler CM, Hwang J, McDermott M, et al. Immunochemotherapy With Intensive Consolidation for Primary CNS Lymphoma: A Pilot Study and Prognostic Assessment by Diffusion-Weighted MRI. Clin Cancer Res (2012) 18(4):1146–55. doi: 10.1158/1078-0432.CCR-11-0625
8. Rubenstein JL, Hsi ED, Johnson JL, Jung SH, Nakashima MO, Grant B, et al. Intensive Chemotherapy and Immunotherapy in Patients With Newly Diagnosed Primary CNS Lymphoma: CALGB 50202 (Alliance 50202). J Clin Oncol (2013) 31(25):3061–8. doi: 10.1200/JCO.2012.46.9957
9. Birsen R, Willems L, Pallud J, Blanc E, Burroni B, Legoff M, et al. Efficacy and Safety of High-Dose Etoposide Cytarabine as Consolidation Following Rituximab Methotrexate Temozolomide Induction in Newly Diagnosed Primary Central Nervous System Lymphoma in Immunocompetent Patients. Haematologica (2018) 103(7):e296–9. doi: 10.3324/haematol.2017.185843
10. Correa DD, Maron L, Harder H, Klein M, Armstrong CL, Calabrese P, et al. Cognitive Functions in Primary Central Nervous System Lymphoma: Literature Review and Assessment Guidelines. Ann Oncol (2007) 18(7):1145–51. doi: 10.1093/annonc/mdl464
11. Hottinger AF, DeAngelis LM, Yahalom J, Abrey LE. Salvage Whole Brain Radiotherapy for Recurrent or Refractory Primary CNS Lymphoma. Neurology (2007) 69(11):1178–82. doi: 10.1212/01.wnl.0000276986.19602.c1
12. Ferreri AJ, Reni M, Foppoli M, Martelli M, Pangalis GA, Frezzato M, et al. High-Dose Cytarabine Plus High-Dose Methotrexate Versus High-Dose Methotrexate Alone in Patients With Primary CNS Lymphoma: A Randomised Phase 2 Trial. Lancet (2009) 374(9700):1512–20. doi: 10.1016/S0140-6736(09)61416-1
13. Ferreri AJ, Licata G, Foppoli M, Corazzelli G, Zucca E, Stelitano C, et al. Clinical Relevance of the Dose of Cytarabine in the Upfront Treatment of Primary CNS Lymphomas With Methotrexate-Cytarabine Combination. Oncologist (2011) 16(3):336–41. doi: 10.1634/theoncologist.2010-0361
14. Welch MR, Sauter CS, Matasar MJ, Faivre G, Weaver SA, Moskowitz CH, et al. Autologous Stem Cell Transplant in Recurrent or Refractory Primary or Secondary Central Nervous System Lymphoma Using Thiotepa, Busulfan and Cyclophosphamide. Leuk Lymphoma (2015) 56(2):361–7. doi: 10.3109/10428194.2014.916800
15. Illerhaus G, Müller F, Feuerhake F, Schäfer AO, Ostertag C, Finke J. High-Dose Chemotherapy and Autologous Stem-Cell Transplantation Without Consolidating Radiotherapy as First-Line Treatment for Primary Lymphoma of the Central Nervous System. Haematologica (2008) 93(1):147–8. doi: 10.3324/haematol.11771
16. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ (Clin Res ed) (2021) 372:n71. doi: 10.1136/bmj.n71
17. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ (Clin Res ed) (2021) 372:n160. doi: 10.1136/bmj.n160
18. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical Methods for Incorporating Summary Time-to-Event Data Into Meta-Analysis. Trials (2007) 8:16. doi: 10.1186/1745-6215-8-16
19. Cochrane. Cochrane Methods: Bias . Available at: http://methods.cochrane.org/bias/assessing-risk-bias-included-studies (Accessed 5 June 2020).
20. Wells GA, Shea BJ, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis. The Ottawa Hospital ohri.ca/programs/clinical_epidemiology/oxford/htm.
21. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological Index for Non-Randomized Studies (Minors): Development and Validation of a New Instrument. ANZ J Surg (2003) 73(9):712–6. doi: 10.1046/j.1445-2197.2003.02748.x
22. Kasenda B, Schorb E, Fritsch K, Finke J, Illerhaus G. Prognosis After High-Dose Chemotherapy Followed by Autologous Stem-Cell Transplantation As First-Line Treatment in Primary CNS Lymphoma–a Long-Term Follow-Up Study. Ann Oncol (2015) 26(3):608–11. doi: 10.1093/annonc/mdv002
23. Kiefer T, Hirt C, Späth C, Schüler F, Al-Ali HK, Wolf HH, et al. Long-Term Follow-Up of High-Dose Chemotherapy With Autologous Stem-Cell Transplantation and Response-Adapted Whole-Brain Radiotherapy for Newly Diagnosed Primary CNS Lymphoma: Results of the Multicenter Ostdeutsche Studiengruppe Hamatologie Und Onkologie OSHO-53 Phase II Study. Ann Oncol (2012) 23(7):1809–12. doi: 10.1093/annonc/mdr553
24. Kasenda B, Schorb E, Fritsch K, Finke J, Illerhaus G. Prognosis After High-Dose Chemotherapy Followed by Autologous Stem-Cell Transplantation as First-Line Treatment in Primary CNS Lymphoma–a Long-Term Follow-Up Study. Ann Oncol (2012) 23(10):2670–5. doi: 10.1093/annonc/mds059
25. Ferreri AJM, Cwynarski K, Pulczynski E, Fox CP, Schorb E, La Rosée P, et al. Whole-Brain Radiotherapy or Autologous Stem-Cell Transplantation as Consolidation Strategies After High-Dose Methotrexate-Based Chemoimmunotherapy in Patients With Primary CNS Lymphoma: Results of the Second Randomisation of the International Extranodal Lymphoma Study Group-32 Phase 2 Trial. Lancet Haematol (2017) 4(11):e510–23. doi: 10.1016/S2352-3026(17)30174-6
26. Houillier C, Taillandier L, Dureau S, Lamy T, Laadhari M, Chinot O, et al. Radiotherapy or Autologous Stem-Cell Transplantation for Primary CNS Lymphoma in Patients 60 Years of Age and Younger: Results of the Intergroup ANOCEF-GOELAMS Randomized Phase II PRECIS Study. J Clin Oncol (2019) 37(10):823–33. doi: 10.1200/JCO.18.00306
27. Illerhaus G, Marks R, Ihorst G, Guttenberger R, Ostertag C, Derigs G, et al. High-Dose Chemotherapy With Autologous Stem-Cell Transplantation and Hyperfractionated Radiotherapy as First-Line Treatment of Primary CNS Lymphoma. J Clin Oncol (2006) 24(24):3865–70. doi: 10.1200/JCO.2006.06.2117
28. Illerhaus G, Kasenda B, Ihorst G, Egerer G, Lamprecht M, Keller U, et al. High-Dose Chemotherapy With Autologous Haemopoietic Stem Cell Transplantation for Newly Diagnosed Primary CNS Lymphoma: A Prospective, Single-Arm, Phase 2 Trial. Lancet Haematol (2016) 3(8):e388–97. doi: 10.1016/S2352-3026(16)30050-3
29. Abrey LE, Moskowitz CH, Mason WP, Crump M, Stewart D, Forsyth P, et al. Intensive Methotrexate and Cytarabine Followed by High-Dose Chemotherapy With Autologous Stem-Cell Rescue in Patients With Newly Diagnosed Primary CNS Lymphoma: An Intent-to-Treat Analysis. J Clin Oncol (2003) 21(22):4151–6. doi: 10.1200/JCO.2003.05.024
30. Chen YB, Batchelor T, Li S, Hochberg E, Brezina M, Jones S, et al. Phase 2 Trial of High-Dose Rituximab With High-Dose Cytarabine Mobilization Therapy and High-Dose Thiotepa, Busulfan, and Cyclophosphamide Autologous Stem Cell Transplantation in Patients With Central Nervous System Involvement by Non-Hodgkin Lymphoma. Cancer (2015) 121(2):226–33. doi: 10.1002/cncr.29023
31. Omuro A, Correa DD, DeAngelis LM, Moskowitz CH, Matasar MJ, Kaley TJ, et al. R-MPV Followed by High-Dose Chemotherapy With TBC and Autologous Stem-Cell Transplant for Newly Diagnosed Primary CNS Lymphoma. Blood (2015) 125(9):1403–10. doi: 10.1182/blood-2014-10-604561
32. Colombat P, Lemevel A, Bertrand P, Delwail V, Rachieru P, Brion A, et al. High-Dose Chemotherapy With Autologous Stem Cell Transplantation as First-Line Therapy for Primary CNS Lymphoma in Patients Younger Than 60 Years: A Multicenter Phase II Study of the GOELAMS Group. Bone Marrow Transplant (2006) 38(6):417–20. doi: 10.1038/sj.bmt.1705452
33. Montemurro M, Kiefer T, Schüler F, Al-Ali HK, Wolf HH, Herbst R, et al. Primary Central Nervous System Lymphoma Treated With High-Dose Methotrexate, High-Dose Busulfan/Thiotepa, Autologous Stem-Cell Transplantation and Response-Adapted Whole-Brain Radiotherapy: Results of the Multicenter Ostdeutsche Studiengruppe Hamato-Onkologie OSHO-53 Phase II Study. Ann Oncol (2007) 18(4):665–71. doi: 10.1093/annonc/mdl458
34. Yoon DH, Lee DH, Choi DR, Sohn BS, Kim S, Kim SW, et al. Feasibility of BU, CY and Etoposide (BUCYE), and Auto-SCT in Patients With Newly Diagnosed Primary CNS Lymphoma: A Single-Center Experience. Bone Marrow Transplant (2011) 46(1):105–9. doi: 10.1038/bmt.2010.71
35. Cheng T, Forsyth P, Chaudhry A, Morris D, Glück S, Russell JA, et al. High-Dose Thiotepa, Busulfan, Cyclophosphamide and ASCT Without Whole-Brain Radiotherapy for Poor Prognosis Primary CNS Lymphoma. Bone Marrow Transplant (2003) 31(8):679–85. doi: 10.1038/sj.bmt.1703917
36. Schorb E, Kasenda B, Ihorst G, Scherer F, Wendler J, Isbell L, et al. High-Dose Chemotherapy and Autologous Stem Cell Transplant in Elderly Patients With Primary CNS Lymphoma: A Pilot Study. Blood Adv (2020) 4(14):3378–81. doi: 10.1182/bloodadvances.2020002064
37. Shiels MS, Pfeiffer RM, Besson C, Clarke CA, Morton LM, Nogueira L, et al. Trends in Primary Central Nervous System Lymphoma Incidence and Survival in the U.S. Br J Haematol (2016) 174(3):417–24. doi: 10.1111/bjh.14073
38. Batchelor TT. Primary Central Nervous System Lymphoma. Hematol Am Soc Hematol Educ Program (2016) 2016(1):379–85. doi: 10.1182/asheducation-2016.1.379
39. Ferreri AJ, Reni M, Pasini F, Calderoni A, Tirelli U, Pivnik A, et al. A Multicenter Study of Treatment of Primary CNS Lymphoma. Neurology (2002) 58(10):1513–20. doi: 10.1212/WNL.58.10.1513
40. Zeremski V, Koehler M, Fischer T, Schalk E. Characteristics and Outcome of Patients With Primary CNS Lymphoma in a "Real-Life" Setting Compared to a Clinical Trial. Ann Hematol (2016) 95(5):793–9. doi: 10.1007/s00277-016-2602-5
41. Han CH, Batchelor TT. Diagnosis and Management of Primary Central Nervous System Lymphoma. Cancer (2017) 123(22):4314–24. doi: 10.1002/cncr.30965
42. Langner-Lemercier S, Houillier C, Soussain C, Ghesquières H, Chinot O, Taillandier L, et al. Primary CNS Lymphoma at First Relapse/Progression: Characteristics, Management, and Outcome of 256 Patients From the French LOC Network. Neuro Oncol (2016) 18(9):1297–303. doi: 10.1093/neuonc/now033
43. Correa DD, Shi W, Abrey LE, Deangelis LM, Omuro AM, Deutsch MB, et al. Cognitive Functions in Primary CNS Lymphoma After Single or Combined Modality Regimens. Neuro Oncol (2012) 14(1):101–8. doi: 10.1093/neuonc/nor186
44. Gaut D, Schiller GJ. Hematopoietic Stem Cell Transplantation in Primary Central Nervous System Lymphoma: A Review of the Literature. Int J Hematol (2019) 109(3):260–77. doi: 10.1007/s12185-019-02594-1
45. Ferreri AJ, Illerhaus G. The Role of Autologous Stem Cell Transplantation in Primary Central Nervous System Lymphoma. Blood (2016) 127(13):1642–9. doi: 10.1182/blood-2015-10-636340
46. Wiebe VJ, Smith BR, DeGregorio MW, Rappeport JM. Pharmacology of Agents Used in Bone Marrow Transplant Conditioning Regimens. Crit Rev Oncol Hematol (1992) 13(3):241–70. doi: 10.1016/1040-8428(92)90092-5
47. Heideman RL, Cole DE, Balis F, Sato J, Reaman GH, Packer RJ, et al. Phase I and Pharmacokinetic Evaluation of Thiotepa in the Cerebrospinal Fluid and Plasma of Pediatric Patients: Evidence for Dose-Dependent Plasma Clearance of Thiotepa. Cancer Res (1989) 49:736–41.
48. Hassan M, Oberg G, Ericson K, Ehrsson H, Eriksson L, Ingvar M, et al. In Vivo Distribution of [11C]- Busulfan in Cynomolgus Monkey and in the Brain of a Human Patient. Cancer Chemother Pharmacol (1992) 30:81–5. doi: 10.1007/BF00686397
49. Jahnke K, Thiel E, Martus P, Herrlinger U, Weller M, Fischer L, et al. Relapse of Primary Central Nervous System Lymphoma: Clinical Features, Outcome and Prognostic Factors. J Neurooncol (2006) 80(2):159–65. doi: 10.1007/s11060-006-9165-6
50. Langner-Lemercier S, Houillier C, Soussain C, Ghesquières H, Chinot O, Taillandier L, et al. Primary CNS Lymphoma at First Relapse/Progression: Characteristics, Management, and Outcome of 256 Patients From the French LOC Network. Neuro Oncol (2016) 18(9):1297–303. doi: 10.1093/neuonc/now033
51. Korfel A, Thiel E, Martus P, Möhle R, Griesinger F, Rauch M, et al. Randomized Phase III Study of Whole-Brain Radiotherapy for Primary CNS Lymphoma. Neurology (2015) 84(12):1242–8. doi: 10.1212/WNL.0000000000001395
52. Morris PG, Correa DD, Yahalom J, Raizer JJ, Schiff D, Grant B, et al. Rituximab, Methotrexate, Procarbazine, and Vincristine Followed by Consolidation Reduced-Dose Whole-Brain Radiotherapy and Cytarabine in Newly Diagnosed Primary CNS Lymphoma: Final Results and Long-Term Outcome. J Clin Oncol (2013) 31(31):3971–9. doi: 10.1200/JCO.2013.50.4910
53. Doolittle ND, Korfel A, Lubow MA, Schorb E, Schlegel U, Rogowski S, et al. Long-Term Cognitive Function, Neuroimaging, and Quality of Life in Primary CNS Lymphoma. Neurology (2013) 81(1):84–92. doi: 10.1212/WNL.0b013e318297eeba
54. Glass J, Won M, Schultz CJ, Brat D, Bartlett NL, Suh JH, et al. Phase I and II Study of Induction Chemotherapy With Methotrexate, Rituximab, and Temozolomide, Followed By Whole-Brain Radiotherapy and Postirradiation Temozolomide for Primary CNS Lymphoma: NRG Oncology RTOG 0227. J Clin Oncol (2016) 34(14):1620–5. doi: 10.1200/JCO.2015.64.8634
55. DeAngelis LM, Seiferheld W, Schold SC, Fisher B, Schultz CJ. Radiation Therapy Oncology Group Study 93-10. Combination Chemotherapy and Radiotherapy for Primary Central Nervous System Lymphoma: Radiation Therapy Oncology Group Study 93-10. J Clin Oncol (2002) 20(24):4643–8. doi: 10.1200/JCO.2002.11.013
Keywords: primary central nervous system lymphoma, autologous stem-cell transplantation, meta-analysis, prospective studies, whole-brain radiotherapy, relapse
Citation: Liu J, Guo J, Sun X, Liu Y and Gao C (2022) Efficacy and Safety of Autologous Stem-Cell Transplantation as Part of First-Line Treatment for Newly Diagnosed Primary Central Nervous System Lymphoma: A Systematic Review and Meta-Analysis. Front. Oncol. 11:799721. doi: 10.3389/fonc.2021.799721
Received: 22 October 2021; Accepted: 14 December 2021;
Published: 12 January 2022.
Edited by:
Mario Tiribelli, University of Udine, ItalyReviewed by:
Lucien A. Noens, Ghent University Hospital, BelgiumCopyright © 2022 Liu, Guo, Sun, Liu and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuanbo Liu, eXVhbmJvbEBjY211LmVkdS5jbg==; Chunji Gao, Z2FvY2h1bmppMzAxQDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.