
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 29 November 2021
Sec. Skin Cancer
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.798385
This article is part of the Research Topic Environment and Skin Cancer View all 12 articles
Plasminogen activating inhibitor-1 (PAI-1) plays crucial roles in the development of various cancers, including melanomas. Indeed, various pro-tumorigenic functions of PAI-1 in cancer progression and metastasis have been widely reported. Among them, PAI-1 is also reported as a key regulator of PD-L1 expression on melanoma cells through endocytosis, leading to abrogating the efficacy of anti-PD1 antibodies (Abs). These findings suggested that PAI-1 expression might predict the efficacy of anti-PD1 Abs. In this report, the expression and production of PAI-1 in melanoma patients were evaluated, and the immunomodulatory effects of PAI-1 on tumor-associated macrophages were investigated in vitro. Immunohistochemical staining of PAI-1 showed that PAI-1 expression on melanoma cells was significantly decreased in responders compared to non-responders. Moreover, baseline serum levels of PAI-1 were significantly decreased in responders compared to non-responders. Notably, PAI-1 decreased the production of various chemokines from monocyte-derived M2 macrophages in vitro, suggesting that PAI-1 might decrease tumor-infiltrating lymphocytes to hamper the anti-tumor effects of anti-PD1 Abs. These results suggest that baseline serum levels of PAI-1 may be useful as a biomarker for identifying patients with advanced cutaneous melanoma most likely to benefit from anti-melanoma immunotherapy.
Plasminogen activating inhibitor-1 (PAI-1) is a serine protease that plays crucial roles in the development of various cancers, including melanomas (1–3). PAI-1 inhibits urokinase-type plasminogen activator and tissue type plasminogen activator, leading to attenuation of plasminogen activation and the development of thrombosis formation at tumor sites. PAI-1 increases the expression of focal adhesion kinase on tumor-associated macrophages (TAMs) to facilitate the migration of macrophages into tumor sites in a melanoma model (1). More recently, PAI-1 was found to facilitate PD-L1 endocytosis of melanoma cells to abrogate the efficacy of anti-PD-L1 antibodies (Abs) in mouse melanoma models (2). In addition, PAI-1 was found to induce resistance to chemotherapy in mouse B16F10 melanoma (3). These reports suggested that PAI-1 could play a significant role in maintaining the immunosuppressive microenvironment in melanoma through TAMs. In this study, the expression and production of PAI-1 were evaluated in melanoma patients, and the immunomodulatory effects of PAI-1 on TAMs were investigated in vitro.
The protocol for this human study was approved by the ethics committee of Tohoku University Graduate School of Medicine, Sendai, Japan (permit no. 2020-1-759). All methods were performed in accordance with the relevant guidelines and regulations. All patients provided written, informed consent prior to enrolment in the study.
Patients were eligible if they had unresectable stage III melanoma or if they had stage IV melanoma with accessible cutaneous, subcutaneous, and/or nodal lesions (staging was performed according to the AJCC Staging Manual, 8th edition, 2018). All patients received 2 mg/kg of nivolumab every three weeks, 3 mg/kg of nivolumab every two weeks, 240 mg of nivolumab every two weeks, 480 mg of nivolumab every four weeks, or 200 mg of pembrolizumab every three weeks. Serum was obtained from patients before the administration of anti-PD1 Abs. The response to anti-PD1 Abs was assessed according to the Response Evaluation Criteria In Solid Tumors.
Polyclonal rabbit Abs for human PAI-1 (Abcam, Tokyo, Japan) were used for immunohistochemical (IHC) staining. Archival formalin-fixed paraffin-embedded skin specimens were collected at the initial visit from 30 advanced melanoma patients who were treated with anti-PD1 Abs in the Department of Dermatology at Tohoku University Graduate School of Medicine. The patients’ characteristics are summarized in Table 1.
For semiquantitative analysis of IHC staining, PAI-1 expression on tumor cells was examined in more than 3 random, representative fields from each section. The expression levels were determined independently by two dermatologists. The expression level of PAI-1 was determined as follows: - (negative: Figure 1A), 1+ (weakly positive: Figure 1B), and 2+ (intensely positive): Figure 1C.

Figure 1 PAI-1-expressing cells in melanoma patients. Representative paraffin-embedded tissue samples from lesional skin of patients with melanoma. Sections were deparaffinized and stained with anti-PAI-1, then developed with liquid permanent red. PAI-1 negative (-) (A), weakly positive (+) (B), and intensely positive (++) (C). Scale bar, 100 µm. Pearson’s correlation coefficient was used to investigate the correlation between therapeutic effect and PAI-1 expression level (D). Sections of melanoma from an anti-PD1 Abs in a high PAI-1 expression patient (E) and a low PAI-1 expression patient (F) were deparaffinized and stained using anti-PD-L1 antibodies. Sections were developed with liquid permanent red. The percentages of PD-L1+ cells per all melanoma cells were automatically counted by BZ-X800 (G). Scale bar, 100 µm.
To quantify the IHC staining of each sample, the positive cells were counted using a BZ-X800 (KEYENCE, Tokyo, Japan). The percentage of IHC-positive cells per all tumor-infiltrating cells or melanoma cells was automatically counted.
The baseline serum PAI levels were evaluated in 49 patients with advanced melanoma. The serum PAI-1 levels were determined by an enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s protocol (R&D Systems, Minneapolis, MN). Data from each donor were obtained as the mean of duplicate assays. The protocol for this human study was approved by the ethics committee of Tohoku University Graduate School of Medicine, Sendai, Japan (approval no. 2020-1-759).
CD14+ monocytes were isolated from the peripheral blood mononuclear cells of healthy donors using MACS beads (CD14 microbeads, Miltenyi Biotec Inc., Sunnyvale, CA) according to the manufacturer’s protocol. The CD14+ monocytes (2 × 105 cells/ml) were cultured in complete medium containing 100 ng/ml of recombinant human macrophage colony-stimulating factor for 5 days, as previously reported (4). On the fifth day, monocyte-derived macrophages were treated with or without PAI-1 (0.1 µg/ml) (5) for 48 hours, and the culture supernatant was harvested. Each chemokine level was determined by an ELISA. This study was approved by the ethics committee of Tohoku University Graduate School of Medicine, Sendai, Japan (2019–1–925).
Pearson’s correlation coefficient was used to investigate the correlation between the therapeutic effect and PAI-1 expression levels. Receiver operating characteristic (ROC) curves were used to calculate cut-off values for serum levels of PAI-1 and areas under the curve (AUCs). Cut-offs were determined using Youden’s index (sensitivity + specificity -1) to determine the maximum index value. ROC curves were established to evaluate serum levels of PAI-1 in patients administered nivolumab. For a single comparison between two groups, the Mann-Whitney U-test was used. All statistical analyses were performed using JMP version 16.1 software (SAS Institute, Tokyo, Japan). The level of significance was set at p<0.05.
Patients’ demographic data of cohort 1 (IHC staining) and cohort 2 (serum PAI-1) are shown in Tables 1, 2, respectively.
Since PAI-1 is associated with a poor prognosis in various cancers, and since PAI-1 possesses immunomodulatory effects to polarize TAMs toward proinflammatory and immunosuppressive M2 phenotypes (6, 7), we hypothesized that PAI-1 expression correlates with the efficacies of anti-PD1 Abs for advanced melanoma patients. To test this hypothesis, IHC staining for PAI-1 and semiquantitative analysis of IHC staining of PAI-1 were used. The expression levels of PAI-1 and the best response to anti-PD1 Abs in each patient in cohort 1 are presented in Table 1. Pearson’s correlation coefficient showed a significant correlation between the PAI-1 expression level on tumor cells and the best response to anti-PD1 Abs (p=0.0029) (Figure 1).
As previous report suggested, PAI-1 facilitates PD-L1 endocytosis of melanoma cells to abrogate the efficacy of anti-PD-L1Abs (2), next we evaluated the PD-L1 expression on melanoma cells in each patient in cohort 1. IHC staining for PD-L1 was performed, and the percentage of PD-L1+ cells per all melanoma cells was automatically counted and quantitatively analyzed by BZ-X800. Percentage of PD-L1 expression on melanoma cells was significantly higher in the low PAI-1 expression group (Figure 1E) compared to that of the high PAI-1 expression group (Figure 1F) (p=0.0168) (Figure 1).
Next, to determine whether baseline serum levels of PAI-1 might be associated with early response in melanoma patients treated with anti-PD1 Abs, PAI-1 levels were evaluated in 49 patients (cohort 2) with advanced melanoma treated with anti-PD1 Abs. The baseline serum levels of PAI-1 and the best response to anti-PD1 Abs in each patient in cohort 2 are presented in Table 2. The threshold value of PAI-1 at baseline to distinguish responders from non-responders was 2.860 ng/ml. The sensitivity and specificity of the baseline serum PAI-1 in advanced melanoma were 71.4% and 76.5%, respectively (p=0.0016; Figure 2). High baseline serum levels of PAI-1 were significantly correlated with resistance to nivolumab in patients with advanced melanoma (p=0.0020) (Figure 2).
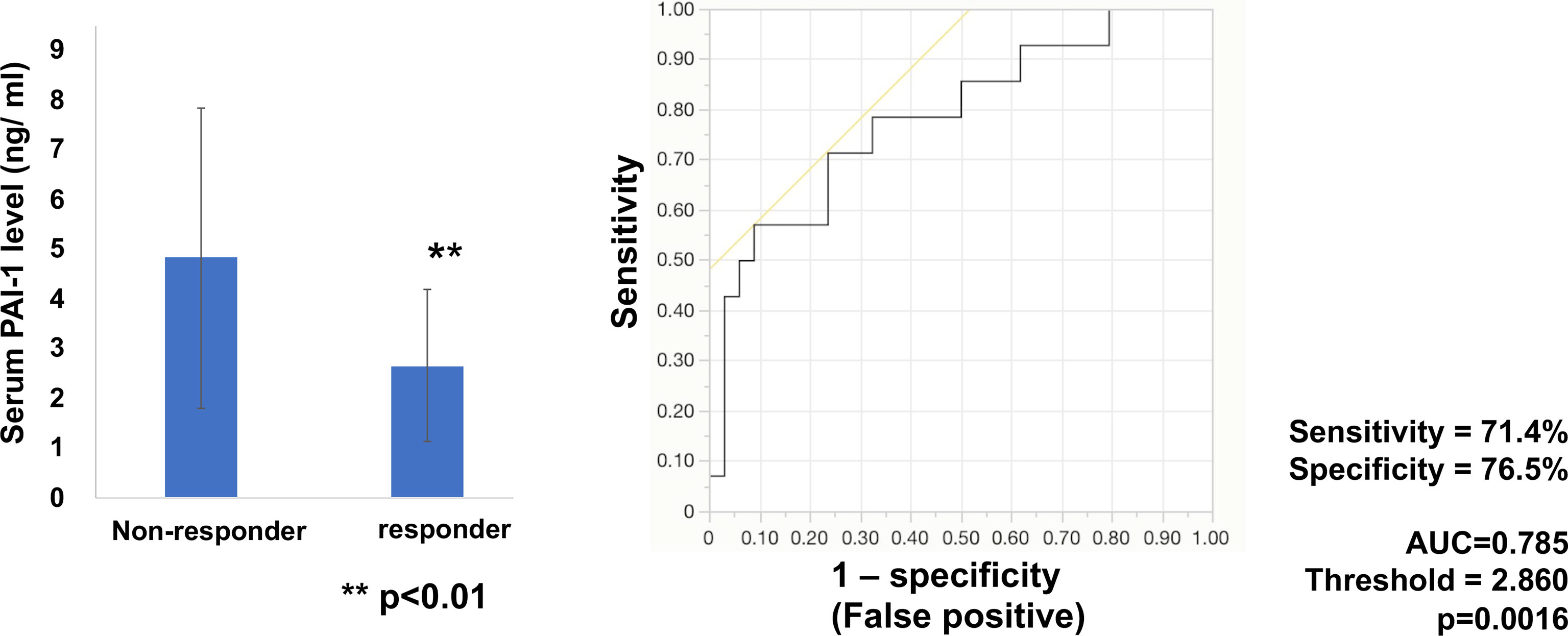
Figure 2 Serum levels of PAI-1 and the ROC curve in melanoma. Mean serum levels of PAI-1 in non-responders (n=34) and responders (n=14) at day 0. The ROC curve was used to calculate cut-offs for PAI-1 serum levels and the AUC. Cut-offs were determined to distinguish responders from non-responders using Youden’s index.
Since PAI-1 polarized TAMs toward proinflammatory and M2 phenotypes (6), the correlation between the ratio of TAMs among tumor-infiltrating lymphocytes (TILs) and the efficacies of anti-PD1 Abs were evaluated in melanoma patients. IHC staining for CD163, which is a commonly used marker for M2 macrophages (8), was performed, and the percentage of CD163+ TAMs in melanoma was quantitatively analyzed (Supplementary Figure 1). There was no significant correlation between the ratio of CD163+ macrophages among tumour-infiltrating cells and the efficacy of anti-PD1 Abs for melanoma (p=0.4288).
Since the ratio of CD163+ TAMs was not different between the responder group and the non-responder group, to further examine the immunomodulatory roles of PAI-1 on TAMs in the tumor microenvironment, CD163+ M2 macrophages were generated from CD14+ monocytes and stimulated by recombinant PAI-1 in vitro (4). PAI-1 decreased the production of CXCL10 and CCL22 (Figure 3), suggesting that PAI-1 might decrease the TILs in melanoma. In contrast, PAI-1 increased the production of CXCL5, suggesting that PAI-1 might increase the tumor infiltrating CXCR2+ myeloid-derived suppressor cells (MDSCs) and tumor-associated neutrophils (TANs) (Figure 3), both of which are known to be immunosuppressive cells 12. There was no significant difference of the production of CCL20, suggesting that PAI-1 might not effect on the production of CCL20 from TAMs (Figure 3).
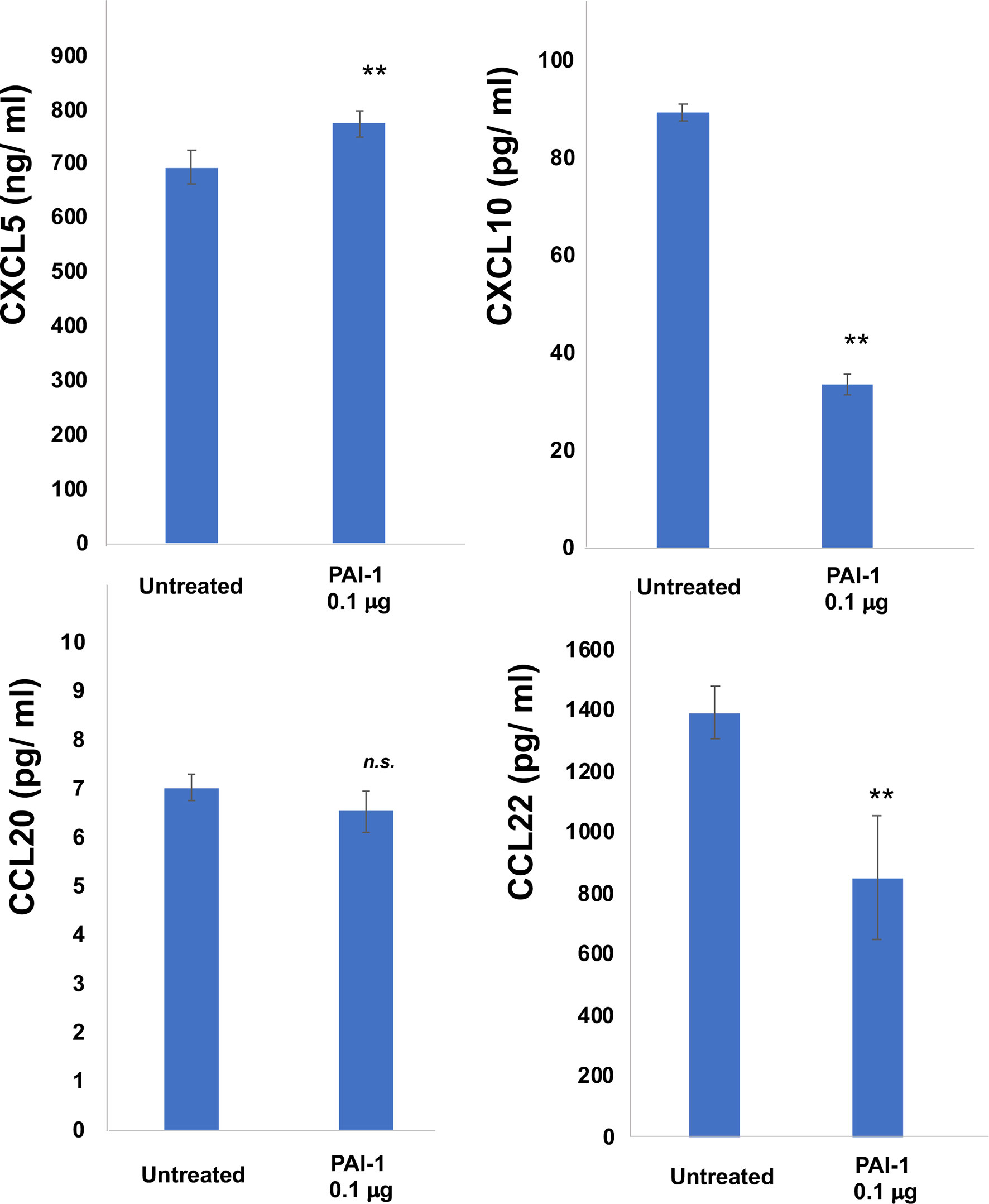
Figure 3 Production of CXCL5, CXCL10, CCL20, and CCL22 by M2 macrophages treated with PAI-1. Culture supernatant from M2 macrophages was harvested as described in Materials and Methods and measured by ELISA (n=3). Data from each donor were obtained from triplicate assays, and mean ± SD values were calculated. Representative data from at least three independent experiments are shown. **p < 0.01, Mann-Whitney U-test; n.s., not significant.
To further confirm immunomodulatory effects of PAI-1 on TAMs in melanoma patients, we employed IHC staining for CD8 in each patient in cohort 1. The percentage of CD8+ cells per all tumor-infiltrating leukocytes was automatically counted and quantitatively analyzed by BZ-X800. The absolute number of CD8+ cells per mm2 was automatically counted by BZ-X800. The absolute number of CD8+ cells per mm2 was significantly increased in a low PAI-1 expression group than that of a high PAI-1 expression group (Figure 4).
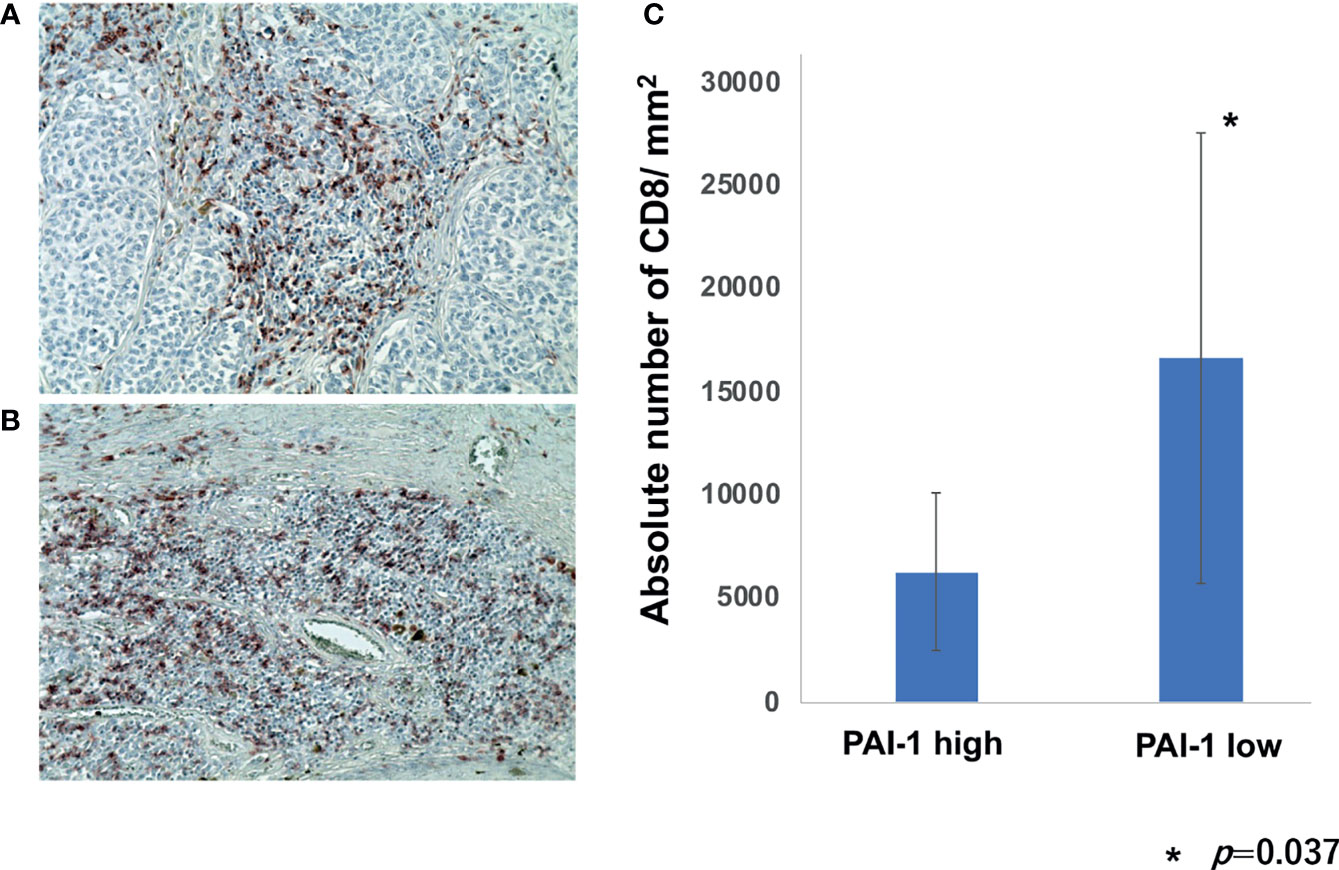
Figure 4 Percentage of CD8+ cells per TILs in melanoma patients. Sections of melanoma from an anti-PD1 Abs in a high PAI-1 expression patient (A) and a low PAI-1 expression patient (B) were deparaffinized and stained using anti-CD8 antibodies. Sections were developed with liquid permanent red. The percentages of CD8+ cells per TILs were automatically counted by BZ-X800 (C).
Plasminogen activator inhibitor-1 (PAI-1) is highly expressed in various types of tumors including melanoma (9), and various pro-tumorigenic functions of PAI-1 in cancer progression and metastasis have been widely reported (9). Among them, tumor-associated inflammation is one of the key pro-tumorigenic functions of PAI-1 (10). Indeed, PAI-1 stimulates the recruitment of fibrosis-inducing cells and macrophages. For example, tumor-derived PAI-1 promotes the migration of monocytes and polarizes tumor-associated macrophages (TAMs) towards proinflammatory phenotypes (7), leading to the production of proinflammatory and angiogenesis-promoting factors such as IL-1, IL-8, CCL2, CCL3, CCL5, and VEGF (11). Via an autocrine loop, IL-6 activates signal transduction and activator of transcription (STAT) 3 in monocytes, leading to an increase in the expression of arginase, IL-10, and CD163. Moreover, the expression of these M2-associated markers increases in parallel with immune checkpoints such as B7-homolog superfamily, including B7-H1 (PD-L1) (8). These reports suggested that PAI-1 promotes pro-tumorigenic, immunosuppressive functions through TAMs.
TAMs play various immunosuppressive roles in melanoma (8). For example, TAMs express immune checkpoint modulators that directly suppress activated T cells (12). In addition, TAMs produce various chemokines that attract other immunosuppressive cells such as Tregs and precursors of TAMs to maintain the immunosuppressive tumor microenvironment (8, 13, 14). Notably, TAM-related chemokines, such as CXCL5, CXCL10, and CCL22, as well as a TAM-activation marker, soluble (s)CD163, could be predictive markers for efficacy and immune-related adverse events (irAEs) in anti-PD1 Abs-treated advanced melanoma patients (15–17). In aggregate, TAMs and TAM-related factors could be important biomarkers to predict the efficacies of anti-PD1 Abs in advanced melanoma patients.
From the above findings, we hypothesized that PAI-1 expression on melanoma cells is correlated with the efficacy of anti-PD1 Abs for unresectable melanoma patients. Indeed, the present data suggested that both the expression levels of PAI-1 on melanoma cells and serum PAI-1 levels were significantly correlated with the efficacy of anti-PD1 Abs in advanced melanoma patients. Since the efficacy of anti-PD1 Abs correlates with the number of TILs at the tumor site in various cancers (5), the decreased chemokine production from TAMs might abrogate the anti-tumor immune response against melanoma. In addition, PAI-1 also increased CXCL5 production, suggesting that PAI-1 might increase the immunosuppressive CXCR2+ MDSCs and TANs. Moreover, as a previous report suggested, PAI-1 decreases PD-L1 expression on melanoma cells to abrogate the efficacy of anti-PD-L1 Abs in mouse melanoma models, suggesting another mechanism of inducing tolerance to anti-PD1 Abs (2). Indeed, The PD-L1 expression in a low PAI-1 expression group is significantly higher than that in a high PAI-1 expression group. Furthermore, the number of CD8+ cells in a low PAI-1 expression group was significantly increased than that in a high PAI-1 expression group. In aggregate, PAI-1 induces resistance to anti-PD1 Abs in melanoma, and co-administration of PAI-1 inhibitors might improve the anti-melanoma effects of anti-PD1 Abs. To prove this hypothesis, from September 2021, we have started a phase II study investigating the safety and efficacy of TM5614, a novel PAI-1 inhibitor with better oral bioavailability that selectively inhibits PAI-1 activity (18), in combination with nivolumab in the treatment of unresectable malignant melanoma (jRCT2021210029).
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The protocol was approved by the institutional review board of Tohoku University Hospital. This study was approved by the ethics committee of Tohoku University Graduate School of Medicine, Sendai, Japan (2020-1-759). The patients/participants provided their written informed consent to participate in this study.
TH and TF designed the research study. KO and TF collected and analyzed the data. KO, YK, and TF treated the patients and collected the clinical data and samples. TF wrote the manuscript. TF supervised the study. All authors contributed to the article and approved the submitted version.
This study was supported in part by the Japan Agency for Medical Research and Development (21ym0126041h0001).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.798385/full#supplementary-material
Supplementary Figure 1 | Sections of melanoma from an anti-PD1 Abs non-responding patient (A) and a responding patient (B) were deparaffinized and stained using anti-PAI-1 antibodies. Sections were developed with liquid permanent red. The percentages of IHC-positive cells per all tumor-infiltrating cells were automatically counted by BZ-X800 (C). Scale bar, 100 µm.
1. Thapa B, Koo BH, Kim YH, Kwon HJ, Kim DS. Plasminogen Activator Inhibitor-1 Regulates Infiltration of Macrophages Into Melanoma via Phosphorylation of FAK-Tyr925. Biochem Biophys Res Commun (2014) 450(4):1696–701. doi: 10.1016/j.bbrc.2014.07.070
2. Tseng YJ, Lee CH, Chen WY, Yang JL, Tzeng HT. Inhibition of PAI-1 Blocks PD-L1 Endocytosis and Improves the Response of Melanoma Cells to Immune Checkpoint Blockade. J Invest Dermatol (2021) 141(11):2690–98. doi: 10.1016/j.jid.2021.03.030
3. Tzeng HT, Yang JL, Tseng YJ, Lee CH, Chen WJ, Chyuan IT. Plasminogen Activator Inhibitor-1 Secretion by Autophagy Contributes to Melanoma Resistance to Chemotherapy Through Tumor Microenvironment Modulation. Cancers (Basel) (2021) 13(6):1253. doi: 10.3390/cancers13061253
4. Fujimura T, Kambayashi Y, Furudate S, Asano M, Kakizaki A, Aiba S. Receptor Activator of Nuclear Factor Kappa-B Ligand (RANKL) Promotes the Production of CCL17 From RANK+ M2 Macrophages. J Invest Dermatol (2015) 135(11):2884–7. doi: 10.1038/jid.2015.209
5. Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, et al. Association of PD-1, PD-1 Ligands, and Other Features of the Tumor Immune Microenvironment With Response to Anti-PD-1 Therapy. Clin Cancer Res (2014) 20(19):5064–74. doi: 10.1158/1078-0432
6. Chen S, Morine Y, Tokuda K, Yamada S, Saito Y, Nishi M, et al. Cancer-Associated Fibroblast-Induced M2-Polarized Macrophages Promote Hepatocellular Carcinoma Progression via the Plasminogen Activator Inhibitor-1 Pathway. Int J Oncol (2021) 59(2):59. doi: 10.3892/ijo.2021.5239
7. Kubala MH, Punj V, Placencio-Hickok VR, Fang H, Fernandez GE, Sposto R, et al. Plasminogen Activator Inhibitor-1 Promotes the Recruitment and Polarization of Macrophages in Cancer. Cell Rep (2018) 25(8):2177–2191.e7. doi: 10.1007/s10555-019-09806-4
8. Fujimura T, Kambayashi Y, Fujisawa Y, Hidaka T, Aiba S. Tumor-Associated Macrophages: Therapeutic Targets for Skin Cancer. Front Oncol (2018) 8:3. doi: 10.3389/fonc.2018.00003
9. Li S, Wei X, He J, Tian X, Yuan S, Sun L. Plasminogen Activator Inhibitor-1 in Cancer Research. BioMed Pharmacother (2018) 105:83–94. doi: 10.1016/j.biopha.2018.05.119
10. Kubala MH, DeClerck YA. The Plasminogen Activator Inhibitor-1 Paradox in Cancer: A Mechanistic Understanding. Cancer Metastasis Rev (2019) 38(3):483–92. doi: 10.1007/s10555-019-09806-4
11. Johnson DE, O’Keefe RA, Grandis JR. Targeting the IL-6/JAK/STAT3 Signalling Axis in Cancer. Nat Rev Clin Oncol (2018) 15(4):234–48. doi: 10.1038/nrclinonc.2018.8
12. Fujimura T, Ring S, Umansky V, Mahnke K, Enk AH. Regulatory T Cells Stimulate B7-H1 Expression in Myeloid-Derived Suppressor Cells in Ret Melanomas. J Invest Dermatol (2012) 132(4):1239–46. doi: 10.1038/jid.2011.416
13. Noy R, Pollard JW. Tumor-Associated Macrophages: From Mechanisms to Therapy. Immunity (2014) 41(1):49–61. doi: 10.1016/j.immuni.2014.06.010
14. Fujimura T, Aiba S. Significance of Immunosuppressive Cells as a Target for Immunotherapies in Melanoma and Non-Melanoma Skin Cancers. Biomolecules (2020) 10:E1087. doi: 10.3390/biom10081087
15. Fujimura T, Sato Y, Tanita K, Lyu C, Kambayash Y, Amagai R, et al. Association of Baseline Serum Levels of CXCL5 With the Efficacy of Nivolumab in Advanced Melanoma. Front Med (Lausanne) (2019) 6:86. doi: 10.3389/fmed.2019.00086
16. Fujimura T, Sato Y, Tanita K, Kambayashi Y, Otsuka A, Fujisawa Y, et al. Serum Soluble CD163 and CXCL5 Could be Predictive Markers for Immune Related Adverse Event in Patients With Advanced Melanoma Treated With Nivolumab. Oncotarget (2018) 9(21):15542–51. doi: 10.18632/oncotarget.24509
17. Fujimura T, Sato Y, Tanita K, Kambayash Y, Otsuka A, Fujisawa Y, et al. Serum Level of Soluble CD163 may be a Predictive Marker of the Effectiveness of Nivolumab in Patients With Advanced Cutaneous Melanoma. Front Oncol (2018) 8:530. doi: 10.3389/fonc.2018.00530
Keywords: melanoma, PAI-1, TAMs, Anti-PD1 Abs, efficacy
Citation: Ohuchi K, Kambayashi Y, Hidaka T and Fujimura T (2021) Plasminogen Activating Inhibitor-1 Might Predict the Efficacy of Anti-PD1 Antibody in Advanced Melanoma Patients. Front. Oncol. 11:798385. doi: 10.3389/fonc.2021.798385
Received: 20 October 2021; Accepted: 05 November 2021;
Published: 29 November 2021.
Edited by:
Motoki Nakamura, Nagoya City University, JapanReviewed by:
Tetsuya Magara, Nagoya City University, JapanCopyright © 2021 Ohuchi, Kambayashi, Hidaka and Fujimura. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Taku Fujimura, dGZ1amltdXJhMUBtYWMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.