- 1Department of Thoracic Oncology, West China Hospital, Sichuan University, Chengdu, China
- 2Radiation Physics Center, West China Hospital, Sichuan University, Chengdu, China
- 3Reproductive Medical Center, Department of Obstetrics and Gynecology, West China 2nd University Hospital, Sichuan University, Chengdu, China
- 4Key laboratory of Birth Defects and Related Diseases of Women and Children (Sichuan University), Ministry of Education, Chengdu, China
Objective: The purpose of this study was to initially investigate the effect of previous antiangiogenic therapy (bevacizumab and endostatin) on the efficacy of anlotinib in patients with advanced or metastatic lung cancer (LC).
Methods: We retrospectively collected the clinical data of patients with LC treated with anlotinib and divided them into group A (treated with anlotinib after the failure of previous antiangiogenic drugs and group B (no prior use of antiangiogenic drugs). We used propensity score matching (PSM) for confounding factors between the groups. Progression-free survival (PFS) and overall survival (OS) were also recorded.
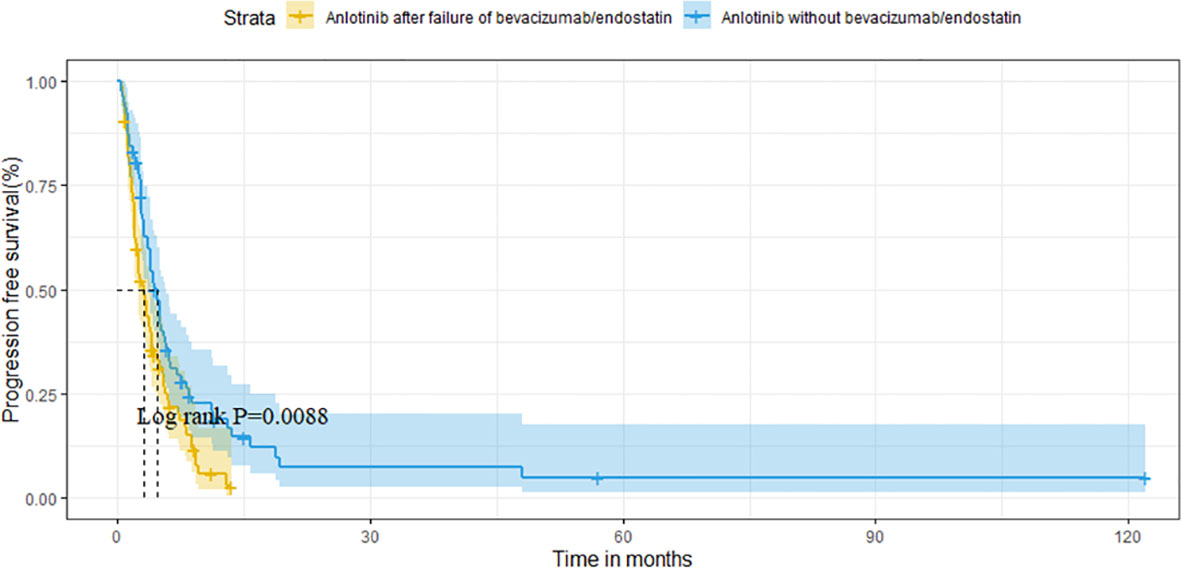
Results: A total of 160 patients were included in the analysis. The median OS in groups A and group B was 11.8 months and 16.1 months (P=0.120), whereas the median PFS was 3.1 months and 4.7 months (P=0.009), respectively. Moreover, the objective response rate (ORR) of the two groups was 9.6% and 10.4% (P=0.874), and the disease control rate (DCR) was 71.1% and 80.5% (P=0.165).
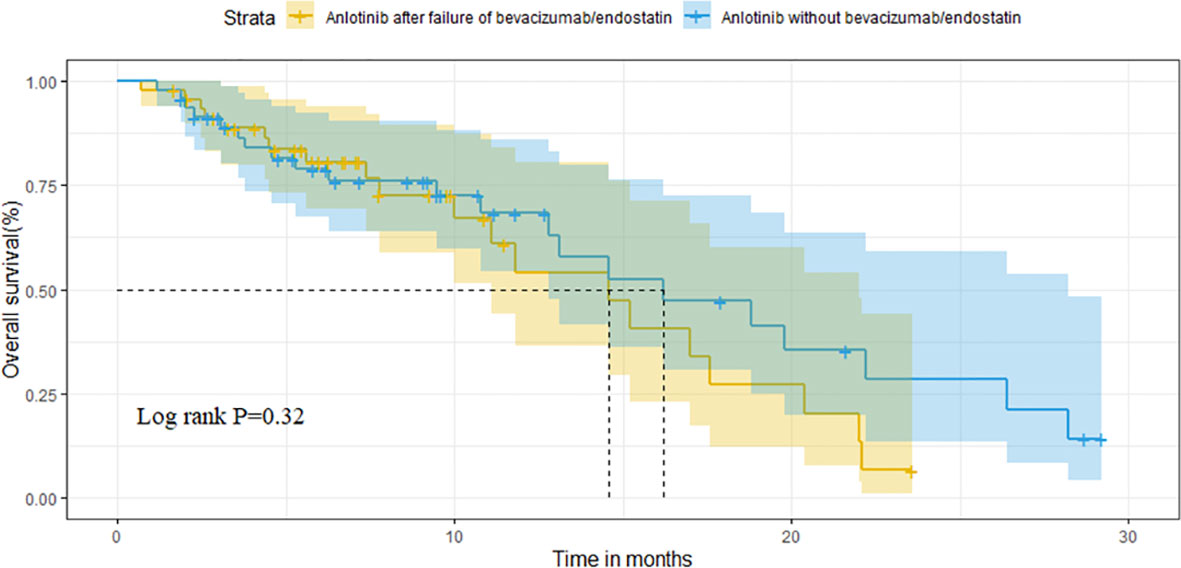
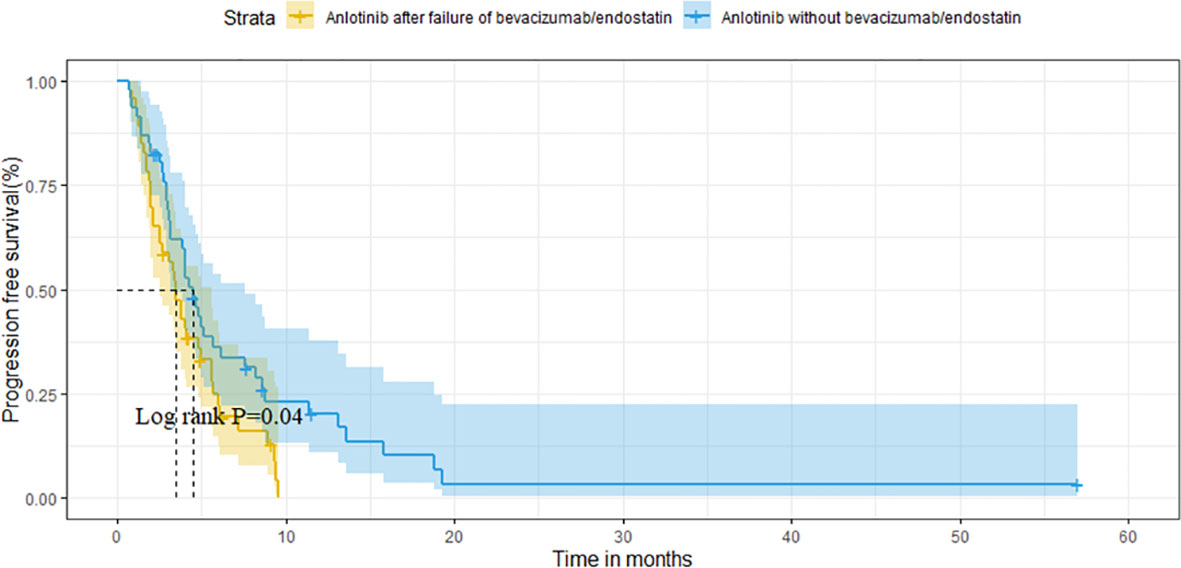
After PSM (n=46), baseline characteristics were comparable between groups A and B. Furthermore, the median OS of the two groups was 14.6 months and 16.2 months (P=0.320), whereas the median PFS was 3.5 months and 4.5 months (P=0.040), respectively. Moreover, the ORR of the two groups were 13.0% and 10.9% (P=0.748), and the DCR were 78.3% and 82.6% (P=0.599), respectively.
Conclusions: Previous antiangiogenic treatments may affect the PFS of patients who receive anlotinib later, but it might not affect the patient’s ORR and OS.
Introduction
In recent years, malignant tumors have become a major global public health problem, among which lung cancer (LC) is one of the most common malignant tumors (1). In 2020, the newly diagnosed patients with LC accounted for 11.4% of all malignant tumors, and 1.8 million people died of LC, which posed a great burden to individuals, families, and society (2).
The NCCN guidelines recommend first- and second-line treatment for patients with LC; however, there are fewer options for third- and further- line treatments (3). More patients with LC remain in good physical condition after receiving the recommended standard treatment, and they need safe and more effective third- and further-line treatments. Among these few treatment options is anlotinib (4–7).
Currently, a significant number of patients were treated with bevacizumab or endostatin antiangiogenic therapy prior to anlotinib (8, 9). A retrospective study of 118 patients with advanced LC demonstrated that there is no benefit from continued second-line bevacizumab treatment after progression of first-line treatment with bevacizumab or endostatin (10). After the failure of single-target bevacizumab therapy, the effectiveness of switching to another small-molecule multi-target antiangiogenic agent deserves further investigation. Retrospective studies have shown that previous antiangiogenic therapies (bevacizumab or endostatin) may not affect the efficacy of retro-line anlotinib therapy, suggesting that there may be no cross-resistance between anlotinib and other antiangiogenic agents (11, 12).The possible mechanism is that anlotinib not only inhibits the VEGF pathway, but also inhibited other angiogenic bypasses. In LC, due to the small sample size of existing studies, it is unclear whether antiangiogenic drugs can be cross-line used to induce sustained vascular inhibition. Therefore, the present study retrospectively analyzed the efficacy of anlotinib in patients with advanced LC, and preliminarily observed whether previous antiangiogenic drugs affected anlotinib efficacy.
Materials and Methods
Patients
We scanned the medical data of patients with LC treated with anlotinib who were admitted at the West China Hospital from June 2018 to January 2021. Patients treated with anlotinib after the failure of previous antiangiogenic therapy (bevacizumab or endostatin), were assigned to group A (83 patients). By contrast, patients treated with anlotinib without any previous antiangiogenic therapy, were assigned to group B (77 patients).
Therapeutic Regimen
The initial dose of anlotinib was 12mg or 10mg orally once daily on days 1-14, every 3 weeks. The dose for some patients was reduced to 10mg/day or 8mg/day when they became intolerant to adverse reactions. Anlotinib was continued until disease progression or when patients remained intolerant to adverse reactions. The anlotinib regimens include:(1)anlotinib monotherapy,(2)anlotinib combined with chemotherapy (no restriction on chemotherapy regimen), (3) anlotinib combined with immune checkpoint inhibitors (anti-PD-1/L1 antibodies), (4) anlotinib combined with targeted agents (including epidermal growth factor receptor TKI and ALK/ROS inhibitors), and (5) anlotinib combined with local radiotherapy (no limitation on the radiotherapy site and dose).
Efficacy Evaluation
Radiographic examinations were performed to evaluate the efficacy two cycles after initiation, and then every two cycles or periodically according to clinical conditions. Efficacy evaluation was directly performed by the researchers using the Picture Archiving and Communication Systems in the hospital according to the Response Evaluation Criteria In Solid Tumor 1.1, with reference to imaging reports and clinical practice. The best response evaluation was the best response record from the beginning to the end of anlotinib treatment. Progression-free survival (PFS) was defined as the time from anlotinib initiation to the presence of objective evidence of disease progression (or death for any reason). Overall survival (OS) was defined as the time from anlotinib initiation until death, or loss to follow-up or reaching the study observation deadline. Adverse events were documented according to the electronic medical records of our hospital, and the incidence of adverse events was lower than the actual incidence.
Statistical Analysis
Statistical analyses were performed using R version 4.0.5. Categorical variables were compared using Pearson’s chi-square test or Fisher’s exact test. Survival curves were created using the Kaplan–Meier method. The log-rank test was used for univariate analysis of PFS and OS. Propensity score matching (PSM) was performed using 1:1 nearest neighbor matching (caliper 0.2). The matching factors were sex, age (< 60 years old, ≥ 60 years old), histological subtype (adenocarcinoma, squamous cell carcinoma, others), clinical-stage (stage IV), and the number of previous treatment lines (<3 lines, ≥3 lines). Statistical significance was set at P<0.05 (both sides).
Results
Baseline Patient Characteristics
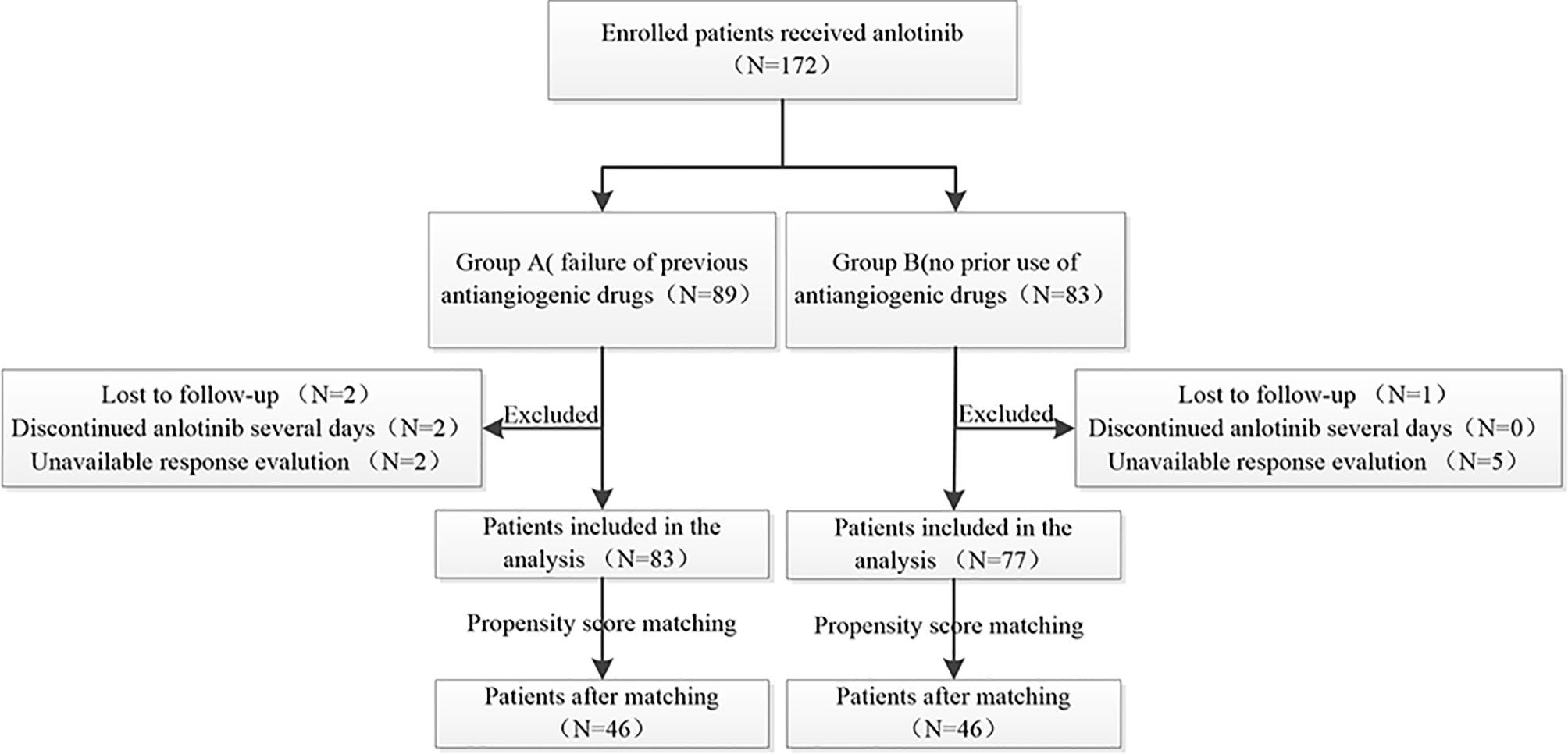
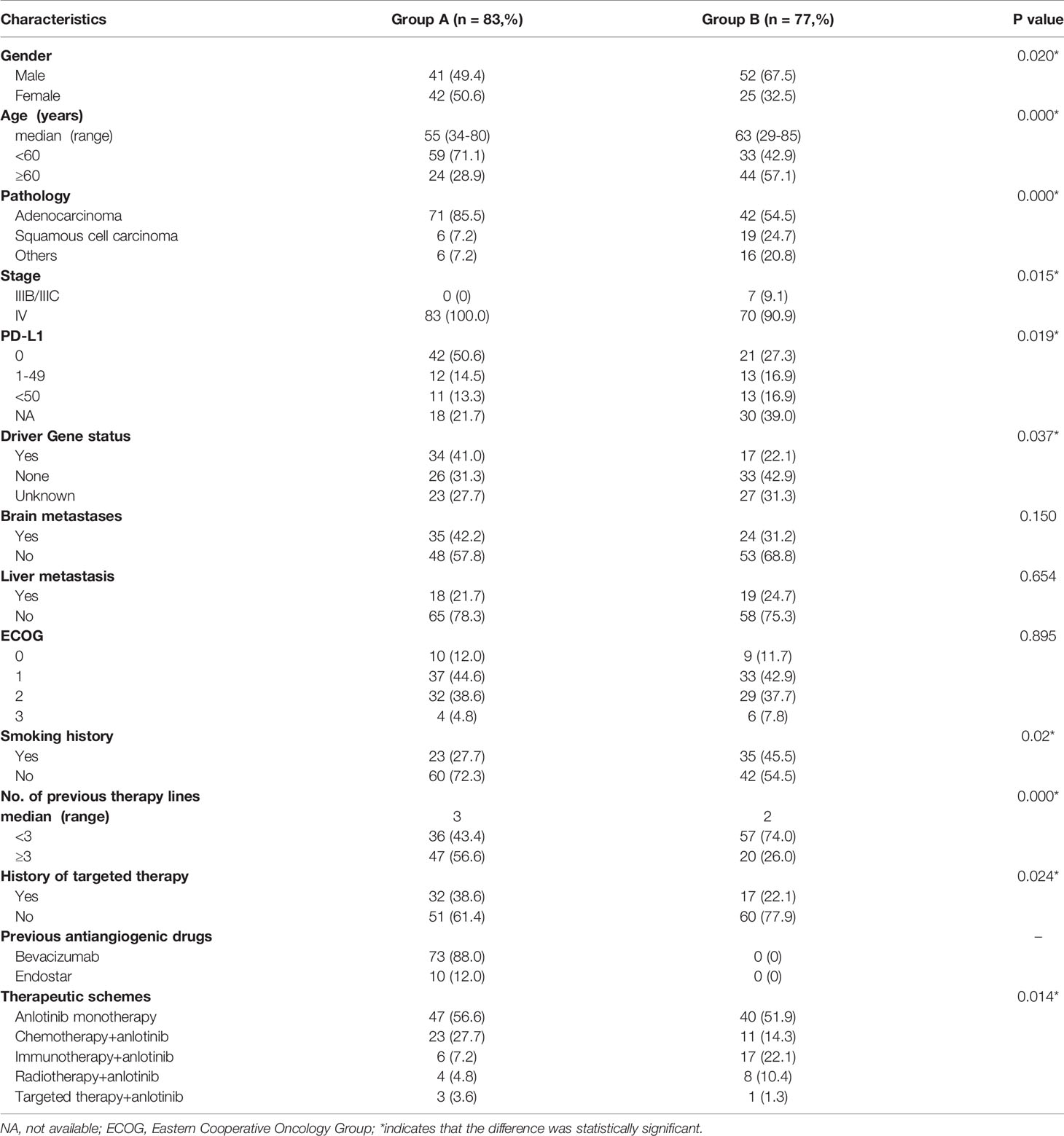
A total of 172 patients were treated with anlotinib. Three patients were lost to follow-up, two cases were discontinued anlotinib after several days, one of which was due to dizziness, and the other was due to fatigue, hypertension, and proteinuria. Efficacy evaluation could not be performed in seven patients due to imaging inaccessibility, therefore they were excluded. A total of 160 patients were included in the analysis, 83 of whom had received prior antiangiogenic therapy (bevacizumab or endostatin) (group A) and 77 of whom did not receive antiangiogenic therapy (group B).The research flowchart is shown in Figure 1, and the baseline patient characteristics are shown in Table 1. As seen in the table, the two patients groups were not balanced in terms of sex (P=0.020), age (P=0.000), pathological type (P=0.000), clinical-stage (P=0.015), PD-L1 expression level (P=0.019), smoking history (P=0.02), number of previous treatment lines (P=0.000), history of targeted therapy (P=0.024), and therapeutic schemes (P=0.014) (Table 1). PSM was used to control the influence of confounding factors between the two groups. After matching, the baseline characteristics of the two groups became comparable (Table 2).
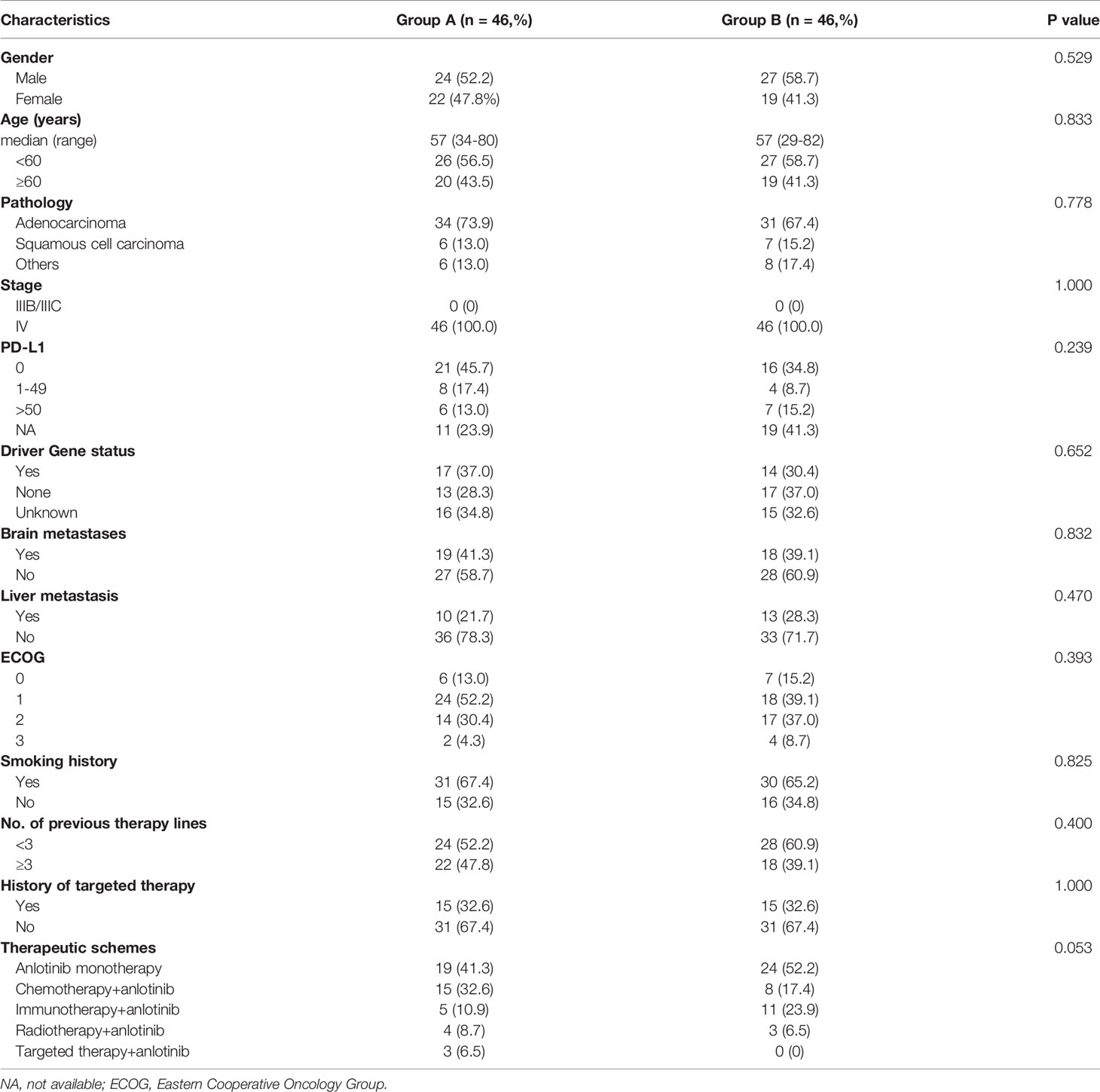
Table 2 Comparison of baseline characteristics of patients after 1:1 matching of propensity score matching.
Comparison of Efficacy Between the Two Groups
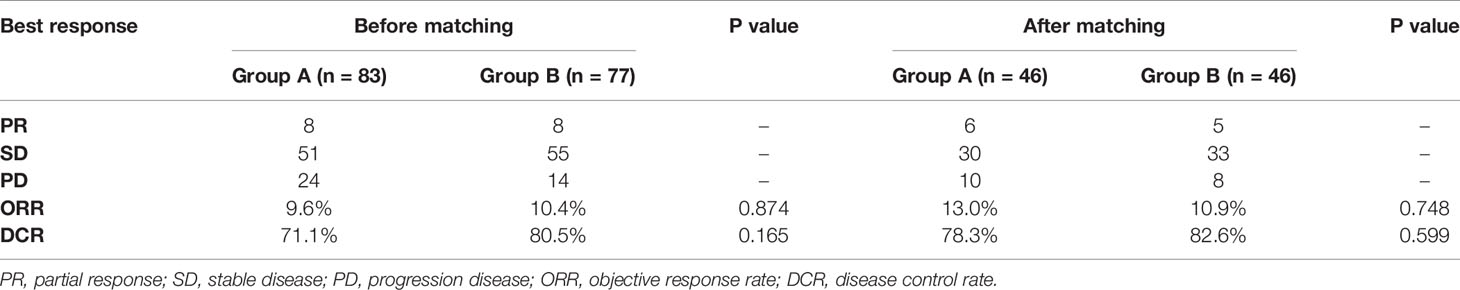
The best response in group A was partial response (PR) (n =8), stable disease (SD) (n = 51) and progressive disease (PD) (n =24). In group B, the best response PR (n =8), SD (n = 55) and PD (n =14). The objective response rate (ORR) and disease control rate (DCR) of the two groups were 9.6% in group A vs. 10.4% in group B (P=0.874), and 71.1% in group A vs. 80.5% in group B (P=0.165), respectively (Table 3).
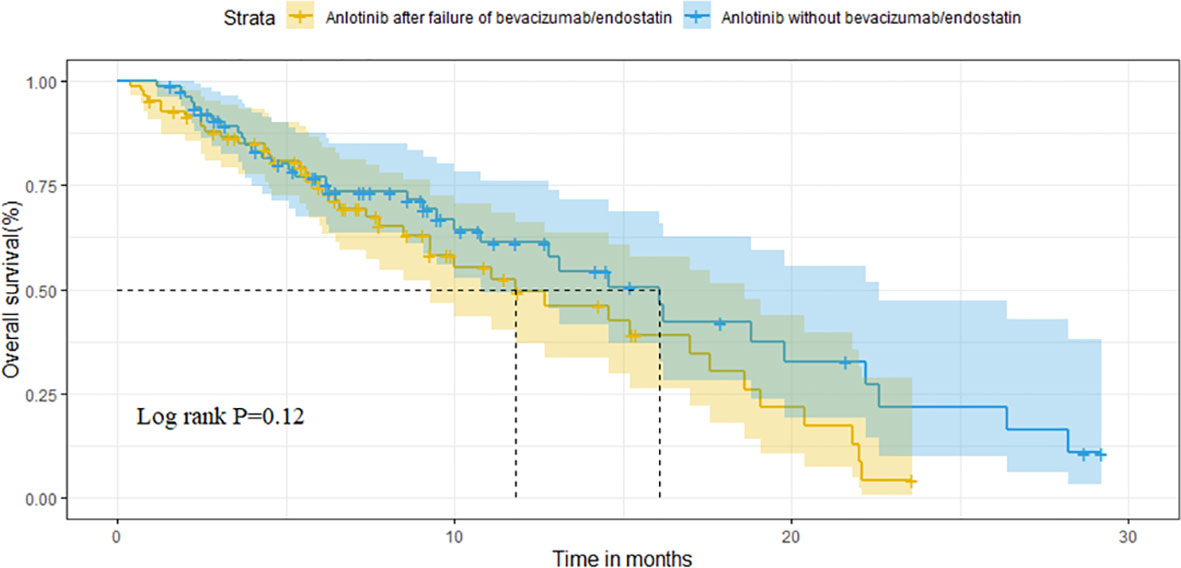
For the overall population, the median OS and PFS were was 14.6 months (95%CI 11.1-18.1) and 3.8 months (95%CI 3.1-4.5), respectively. The median OS was 11.8 months (95%CI 6.7-16.9) in group A vs. 16.1 months (95%CI 12.6-19.6) in group B (P=0.121) (Figure 2). Median PFS was 3.1 months vs. 4.7 months (95%CI 3.9-5.5),(P=0.009; Figure 3).
After matching, the ORR of the two groups was 13.0% vs. 10.9% (P=0.748). The DCR of two groups was 78.3% vs. 82.6% (P=0.599), respectively (Table 3). The median OS of the two groups was 14.6 months (95%CI 10.1-19.1) in group A vs. 16.2 months (95%CI 9.2-23.2) in group B (P=0.320; Figure 4). Median PFS was 3.5 months (95%CI 2.7-4.3) and 4.5 months (95%CI 3.7-5.3) (P=0.040; Figure 5).
Analysis of the Subsequent Treatment After Anlotinib Progression
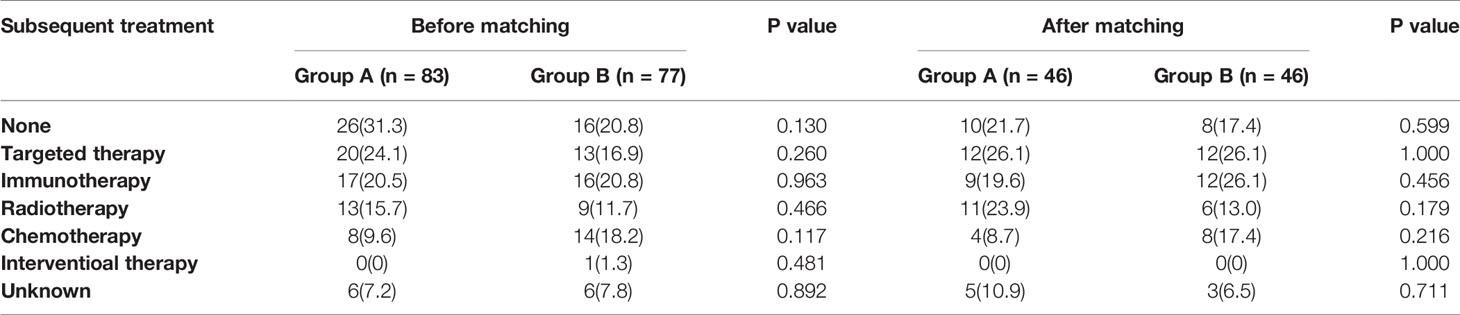
After anlotinib progression, 57 patients (68.7%) in group A and 61 patients (79.2%) in group B received subsequent therapy. However, there was no statistically significant difference in subsequent line therapy between the two groups before and after matching. The most common posterior line treatments in both groups were targeted therapy, immunotherapy, and radiotherapy, whereas the other relatively uncommon treatments included chemotherapy and liver interventional therapy. However, data regarding the posterior line treatment of a few patients were unavailable (Table 4).
Effect of Previous Antiangiogenic Therapy on Anlotinib
The effect of previous antiangiogenic treatment on anlotinib in group A was further analyzed, the Kaplan-Meier analysis showed that the PFS for anlotinib in the bevacizumab and endostatin groups were 3.2 and 2.1 months, respectively (P =0.973). The PFS for anlotinib in the PR, SD, and PD groups that received prior antiangiogenic therapy were 2.7 months, 3.1 months and 3.8 months (P =0.918), respectively. Similarly, after grouping according to the PFS achieved from previous antiangiogenic treatment, the PFS for anlotinib in the shorter (≤6 months) and longer (>6 months) groups were 2.9 and 3.3 months (P=0.592), respectively. The OS data for the anlotinib group after the progression of previous antiangiogenic therapy are detailed in Supplementary Table 1.
Univariate and Multivariate Analyses of Anlotinib Clinical Factors
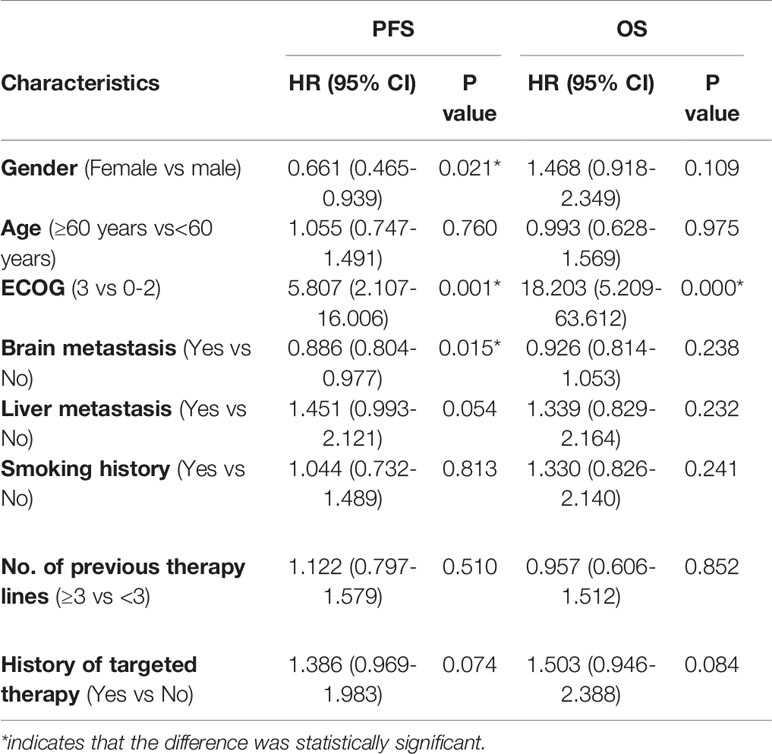
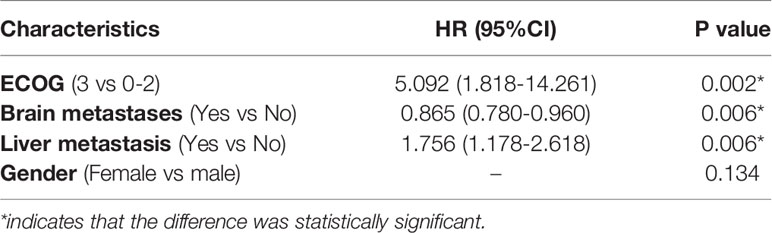
The univariate analysis showed that female patients (P=0.021), patients with ECOG scores of 0-2 (P=0.001), and patients with brain metastases (P=0.015) had longer PFS (Table 5). Factors with P<0.05 in the univariate analysis and those clinically associated with prognostic factors (liver metastasis) were included in the multivariate analysis. ECOG score (P=0.002), brain metastasis (P=0.006), and liver metastasis (P=0.006) were factors found to independently influence PFS (Table 6). In terms of OS, the univariate analysis showed that patients with an ECOG score of 0-2 had significantly longer OS than those with an ECOG score of 3, whereas other factors did not affect the OS of the patients (Table 5).
Safety Analysis
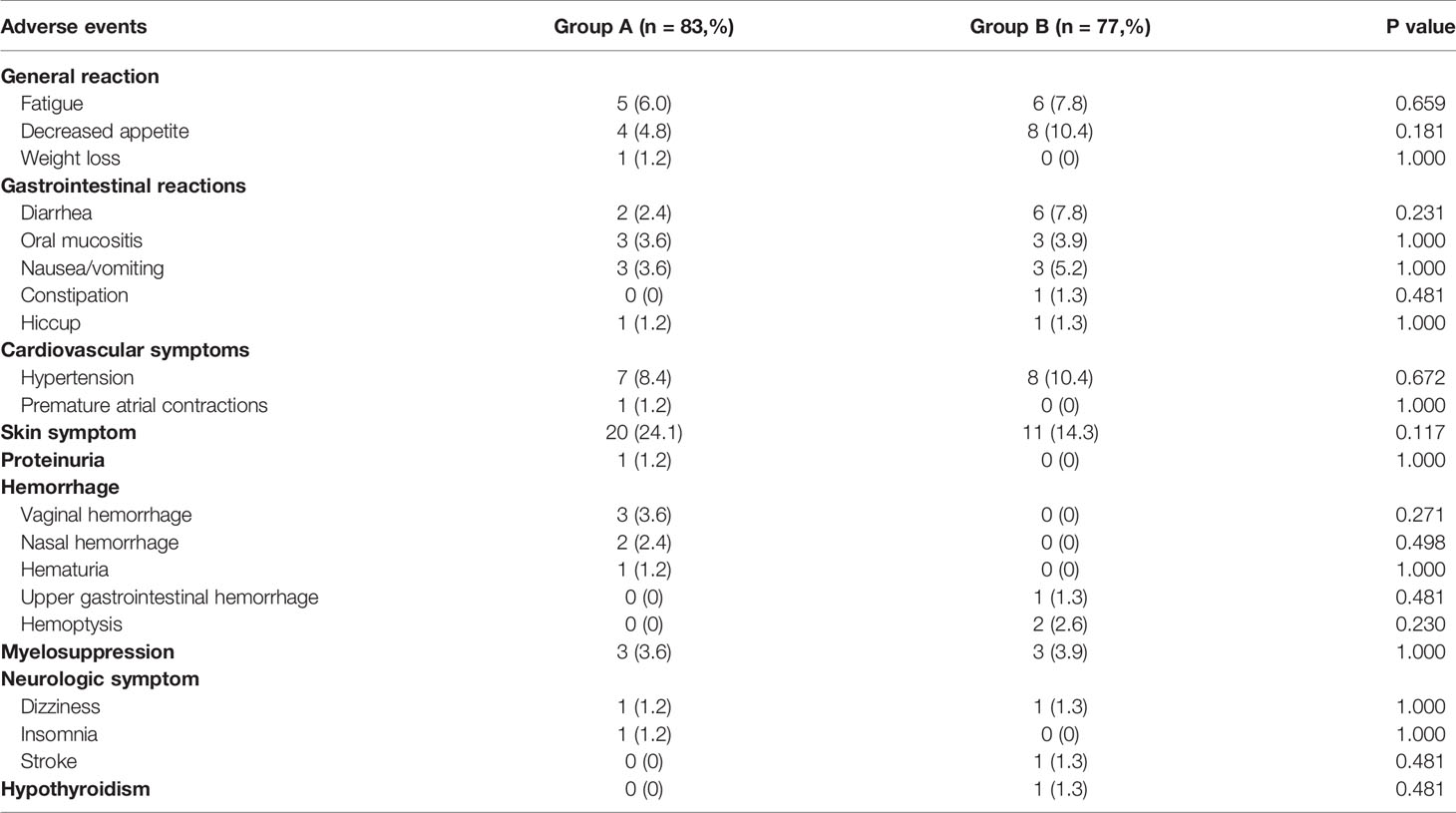
No life-threatening adverse events associated with anlotinib were documented in this study. However, six patients were spontaneously discontinued in group A due to being intolerant to adverse reactions, including one patient who experienced vaginal bleeding and fatigue, one who experienced hematuria, three who experienced skin symptoms, and one who experienced nausea and vomiting. In group B, four patients were discontinued due to adverse reaction intolerance, including one who experienced nausea and vomiting, one who had hand and foot syndrome, one who had an acute stroke, and one who had upper gastrointestinal bleeding after an esophageal cancer diagnosis.
There was no statistically significant difference in the incidence of adverse events between the two groups. The most common adverse events in group A were skin symptoms (24.1%), including rash, pruritus, hand and foot syndrome, followed by hypertension (8.4%) and fatigue (6.0%). The other relatively rare adverse events of concern included vaginal bleeding (3.6%), nosebleeds (2.4%), and hematuria (1.2%). In group B, the most common adverse events were skin symptoms (14.3%), hypertension (10.4%), and anorexia (10.4%), followed by diarrhea (7.8%) and fatigue (7.8%). The other relatively uncommon adverse events of concern included hemoptysis (2.6%), upper gastrointestinal bleeding (1.3%), and stroke (1.3%) (Table 7).
Discussion
Angiogenesis plays an important role in tumor growth, proliferation, and metastasis of tumors (13). Excluding nintedanib (14) and anlotinib (5, 7), most small-molecule TKIs, such as sorafenib, sunitinib and apatinib, can significantly prolong the PFS of patients, but do not significantly increase the OS (13, 15–19). By contrast, anlotinib has become one of the few multi-target angiogenesis inhibitors with survival benefits (5). In the ALTER0303 trial, the vast majority of patients had not previously received antiangiogenic drugs; therefore, it is worth studying whether similar survival benefits can be achieved with anlotinib in these patients after progression of antiangiogenic therapy. The present study therefore aimed to answer the question of whether prior use of antiangiogenic therapy influences anlotinib efficacy and whether the efficacy of previous antiangiogenic therapy affects the efficacy of anlotinib antiangiogenic therapy.
In this study, the median PFS of the entire cohort was 3.8 months, whereas the median OS was 14.6 months, which also confirmed that in the real world, anlotinib could bring PFS and OS benefits among patients with advanced LC; furthermore, and the benefit seemed not inferior to that of previously reported second-line docetaxel and immune checkpoint inhibitors (20, 21). In addition, the PFS in our study was similar to that reported in the ALTER0303 trial, with a slightly longer OS than that in ALTER0303, which might be due to the higher subsequent-line treatment rate in our study.
We also found that patients who had previously received bevacizumab and endostatin had a shorter PFS (3.1 months vs. 4.7 months); however, the ORR, DCR, and OS were similar to those who had received the first antiangiogenic therapy (anlotinib). Considering that the baseline characteristics and subsequent treatment imbalance between our two patient groups may lead to results bias, we used PSM to reduce intergroup confounders. After PSM, we also found that previous antiangiogenic therapy only affected PFS in patients treated with anlotinib, but could not affect OS. Failure of previous antiangiogenic therapy may be accompanied by resistance to VEGF pathways and the emergence of new resistance mechanisms, which may be related to the shorter PFS of some patients receiving anlotinib therapy; moreover, this survival benefit might be due to the multi-target inhibition of anlotinib (22).
Furthermore, it is of interest whether the efficacy of previous antiangiogenic therapy predicts anlotinib efficacy. We found that previous drug therapy (bevacizumab or endostatin), the best response (PR, SD or PD) of the prior antiangiogenic therapy, and PFS (≤6 months or > 6 months) related to prior antiangiogenic therapy all have no significant effect on the efficacy (PFS and OS) among patients receiving anlotinib (P>0.05). These results suggest that anlotinib efficacy may be independent of the previous antiangiogenic therapy and the sensitivity of previous antiangiogenic therapy. Notably, our multivariate analysis found that patients with brain metastasis had longer PFS than those with other metastatic sites (without brain metastasis), hence we hypothesized that a small -molecule agent (anlotinib) could enter the blood-brain barrier to function.
The incidence of adverse events recorded in both of our patient groups was low and similar to that reported in the literature (23–26). In this study, the proportion of patients that required rechecking of ECG, thyroid function and urine routine was small. Therefore, the incidence of abnormal ECG, increased thyroid-stimulating hormone (TSH) and proteinuria was lower than the actual situation and the incidence reported in previous studies. Therefore, it is necessary to further strengthen the monitoring of adverse reactions during treatment.
In this study, the most common subsequent therapy after anlotinib failure was targeted therapy and immunotherapy. With the popularity of more targeted agents and immunotherapy drugs, as well as their lower toxicity compared to chemoradiotherapy, targeted therapy and immunotherapy have become the alternatives for patients with poor ECOG scores after multiline therapy. This may be one reason why the median OS in our study was slightly longer than that in the ALTER0303 trail.
Limitations
Our study has several limitations. The results of this study were based on the experience of a single-center and a small sample size. The baseline characteristics of the patients in the two groups were influenced by confounding factors before matching. After PSM, the number of patients available for analysis was halved, which reduced the representativeness of our cohort, and could also have introduced certain limitations in the results. The variety of drugs used in combination with anlotinib might have also affected the outcomes.
Conclusions
Based on the limited data available in this study, anlotinib may bring PFS and OS benefits to patients with advanced LC. Previous antiangiogenic treatments may affect the PFS of patients who receive anlotinib later, but this might not affect the patient’s ORR and OS. Therefore, the use of anlotinib in patients with advanced LC after the progression of antiangiogenic therapy is worthy of subsequent prospective, large-sample clinical studies.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Biomedical Research Ethics Committee, West China Hospital, Sichuan University(approval number: 2020162). The ethics committee waived the requirement of written informed consent for participation.
Author Contributions
All authors contributed to the study conception and design. JS, YS, and YF: Data collection, analysis and drafting. WX and XZ: Data analysis and essay structure and revision of manuscripts. YW: Determination of topics, essay structure and revision of manuscripts. JZ: In charge of the whole study, perform the final revision of the manuscript. All authors commented on previous versions of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.788837/full#supplementary-material
References
1. International Agency for Research on Cancer (IARC) [EB/OL]. Available at: https://www.iarc.fr/faq/latest-global-cancer-data-2020-qa/.
2. Bade BC, Dela Cruz CS. Lung Cancer 2020: Epidemiology, Etiology, and Prevention. Clin Chest Med (2020) 41(1):1–24. doi: 10.1016/j.ccm.2019.10.001
3. NCCN Clinical Practice Guidelines in Oncology. Non Small Cell Lung Cancer(2021 Version I)[Db/Ol]. Available at: http://www.nccn.org.
4. Si X, Zhang L, Wang H, Zhang X, Wang M, Han B, et al. Quality of Life Results From a Randomized, Double-Blinded, Placebo-Controlled, Multi-Center Phase III Trial of Anlotinib in Patients With Advanced non-Small Cell Lung Cancer. Lung Cancer (2018) 122:32–7. doi: 10.1016/j.lungcan.2018.05.013
5. Han B, Li K, Wang Q, Zhang L, Shi J, Wang Z, et al. Effect of Anlotinib as a Third-Line or Further Treatment on Overall Survival of Patients With Advanced non-Small Cell Lung Cancer: The ALTER 0303 Phase 3 Randomized Clinical Trial. JAMA Oncol (2018) 4(11):1569–75. doi: 10.1001/jamaoncol.2018.3039
6. Han B, Li K, Zhao Y, Li B, Cheng Y, Zhou J, et al. Anlotinib as a Third-Line Therapy in Patients With Refractory Advanced non-Small-Cell Lung Cancer: A Multicentre, Randomised Phase II Trial (ALTER0302). Br J Cancer (2018) 118(5):654–61. doi: 10.1038/bjc.2017.478
7. Cheng Y, Wang Q, Li K, Shi J, Liu Y, Wu L, et al. Overall Survival (OS) Update in ALTER 1202: Anlotinib as Third-Line or Further-Line Treatment in Relapsed Small-Cell Lung Cancer (SCLC). Ann Oncol (2019) 30. doi: 10.1093/annonc/mdz264.002
8. Garcia J, Hurwitz H, Sandler A, Miles D, Coleman R, Deurloo R, et al. Bevacizumab (Avastin®) in Cancer Treatment: A Review of 15 Years of Clinical Experience and Future Outlook. Cancer Treat Rev (2020) 86:102017. doi: 10.1016/j.ctrv.2020.102017
9. Wang J, Sun Y, Liu Y, Yu Q, Zhang Y, Li K, et al. [Results of Randomized, Multicenter, Double-Blind Phase III Trial of Rh-Endostatin (YH-16) in Treatment of Advanced non-Small Cell Lung Cancer Patients]. Zhongguo Fei Ai Za Zhi (2005) 8(4):283–90. doi: 10.3779/j.issn.1009-3419.2005.04.07
10. Xing P, Mu Y, Wang Y, Hao X, Zhu Y, Hu X, et al. Real World Study of the Continuation of Bevacizumab Beyond Disease Progression After First-Line Treatment Containing Bevacizumab in Chinese Patients With Advanced non-Small Cell Lung Cancer. Thorac Cancer (2018) 9(12):1716–24. doi: 10.1111/1759-7714.12886
11. Wang L, He Z, Yang S, Tang H, Wu Y, Li S, et al. The Impact of Previous Therapy Strategy on the Efficiency of Anlotinib Hydrochloride as a Third-Line Treatment on Patients With Advanced non-Small Cell Lung Cancer (NSCLC): A Subgroup Analysis of ALTER0303 Trial. Transl Lung Cancer Res (2019) 8(5):575–83. doi: 10.21037/tlcr.2019.09.21
12. Wu D, Nie J, Dai L, Hu W, Zhang J, Chen X, et al. Salvage Treatment With Anlotinib for Advanced non-Small Cell Lung Cancer. Thorac Cancer (2019) 10(7):1590–6. doi: 10.1111/1759-7714.13120
13. Qiang H, Chang Q, Xu J, Qian J, Zhang Y, Lei Y, et al. New Advances in Antiangiogenic Combination Therapeutic Strategies for Advanced non-Small Cell Lung Cancer. J Cancer Res Clin Oncol (2020) 146(3):631–45. doi: 10.1007/s00432-020-03129-6
14. Reck M, Kaiser R, Mellemgaard A, Douillard J-Y, Orlov S, Krzakowski M, et al. Docetaxel Plus Nintedanib Versus Docetaxel Plus Placebo in Patients With Previously Treated non-Small-Cell Lung Cancer (LUME-Lung 1): A Phase 3, Double-Blind, Randomised Controlled Trial. Lancet Oncol (2014) 15(2):143–55. doi: 10.1016/s1470-2045(13)70586-2
15. Paz-Ares L, Hirsh V, Zhang L, de Marinis F, Yang JC, Wakelee HA, et al. Monotherapy Administration of Sorafenib in Patients With non-Small Cell Lung Cancer (MISSION) Trial: A Phase III, Multicenter, Placebo-Controlled Trial of Sorafenib in Patients With Relapsed or Refractory Predominantly Nonsquamous non-Small-Cell Lung Cancer After 2 or 3 Previous Treatment Regimens. J Thorac Oncol: Off Publ Int Assoc Study Lung Cancer (2015) 10(12):1745–53. doi: 10.1097/jto.0000000000000693
16. Baggstrom MQ, Socinski MA, Wang XF, Gu L, Stinchcombe TE, Edelman MJ, et al. Maintenance Sunitinib Following Initial Platinum-Based Combination Chemotherapy in Advanced-Stage IIIB/IV non-Small Cell Lung Cancer: A Randomized, Double-Blind, Placebo-Controlled Phase III Study-CALGB 30607 (Alliance). J Thorac Oncol: Off Publ Int Assoc Study Lung Cancer (2017) 12(5):843–9. doi: 10.1016/j.jtho.2017.01.022
17. Villaruz LC, Socinski MA. The Role of Anti-Angiogenesis in non-Small-Cell Lung Cancer: An Update. Curr Oncol Rep (2015) 17(6):26. doi: 10.1007/s11912-015-0448-y
18. Hall RD, Le TM, Haggstrom DE, Gentzler RD. Angiogenesis Inhibition as a Therapeutic Strategy in non-Small Cell Lung Cancer (NSCLC). Transl Lung Cancer Res (2015) 4(5):515–23. doi: 10.3978/j.issn.2218-6751.2015.06.09
19. Zhang N, Guo N, Tian L, Miao Z. Systematic Review and Meta-Analysis of Third-Line Salvage Therapy for the Treatment of Advanced non-Small-Cell Lung Cancer: A Meta-Analysis of Randomized Controlled Trials. Oncotarget (2018) 9(83):35439–47. doi: 10.18632/oncotarget.24967
20. Gubens MA, Wakelee HA. Docetaxel in the Treatment of non-Small Cell Lung Carcinoma: An Update and Analysis. Lung Cancer (Auckland NZ) (2010) 1:63–76. doi: 10.2147/lctt.s6499
21. Lee C, Man J, Lord S, Cooper W, Links M, Gebski V, et al. Clinical and Molecular Characteristics Associated With Survival Among Patients Treated With Checkpoint Inhibitors for Advanced non-Small Cell Lung Carcinoma: A Systematic Review and Meta-Analysis. JAMA Oncol (2018) 4(2):210–6. doi: 10.1001/jamaoncol.2017.4427
22. Lin B, Song X, Yang D, Bai D, Yao Y, Lu N. Anlotinib Inhibits Angiogenesis via Suppressing the Activation of VEGFR2, Pdgfrbeta and FGFR1. Gene (2018) 654:77–86. doi: 10.1016/j.gene.2018.02.026
23. Zhang K, Ma X, Gao H, Wang H, Qin H, Yang S, et al. Efficacy and Safety of Anlotinib in Advanced non-Small Cell Lung Cancer: A Real-World Study. Cancer Manage Res (2020) 12:3409–17. doi: 10.2147/CMAR.S246000
24. Cheng JD, Chai LX, Zhao ZP, Hao YY, Li S. Efficacy and Safety of Anlotinib for Patients With Advanced NSCLC Who Progressed After Standard Regimens and the Preliminary Analysis of an Efficacy Predictor. Cancer Manage Res (2020) 12:5641–50. doi: 10.2147/CMAR.S253366
25. Shao L, Wang W, Song Z, Zhang Y. The Efficacy and Safety of Anlotinib Treatment for Advanced Lung Cancer. OncoTargets Ther (2019) 12:6549–54. doi: 10.2147/OTT.S205674
Keywords: lung cancer, antiangiogenic, efficacy, survival, anlotinib
Citation: Suo J, Sun Y, Fu Y, Xiu W, Zhang X, Wang Y and Zhu J (2021) A Retrospective Analysis of the Effect of Anlotinib in Patients With Lung Cancer With or Without Previous Antiangiogenic Therapy. Front. Oncol. 11:788837. doi: 10.3389/fonc.2021.788837
Received: 03 October 2021; Accepted: 24 November 2021;
Published: 23 December 2021.
Edited by:
Christian Rolfo, University of Maryland Medical System, United StatesReviewed by:
Francesco Cortiula, Maastricht University Medical Centre, NetherlandsAlberto Pavan, Azienda ULSS 3 Serenissima, Italy
Copyright © 2021 Suo, Sun, Fu, Xiu, Zhang, Wang and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Wang, d2FuZ3l5MTIxMEAxNjMuY29t; Jiang Zhu, emh1amlhbmdAd2Noc2N1LmNu
†These authors have contributed equally to this work and share first authorship
 Jiaojiao Suo
Jiaojiao Suo Yu Sun2†
Yu Sun2† Weigang Xiu
Weigang Xiu