- 1Center for Proton Therapy, National Cancer Center, Goyang, South Korea
- 2Center for Breast Cancer, National Cancer Center, Goyang, South Korea
Background: Few studies of proton beam therapy (PBT) for patients with liver metastasis from breast cancer (LMBC) are available to date. The aim of the present study was to evaluate the clinical effectiveness of PBT for patients with LMBC.
Material and Methods: Seventeen patients with LMBC treated with PBT were included in this study. The median prescribed dose of PBT was 66 GyE (range, 60–80) in 10 fractions, 5 times a week. In patients with LMBC receiving PBT, freedom from local progression (FFLP), progression-free survival (PFS), and overall survival (OS) rates were assessed.
Results: The median follow-up time was 34.2 months (range, 11.5–56.1). The median FFLP time was not yet reached, and the 3-year FFLP rates were 94.1% (95% confidence interval [CI], 82.9–105.3). The median times of PFS and OS were 7.9 months (95% CI, 5.3–10.5) and 39.3 months (95% CI, 33.2–51.9), respectively, and the 3-year PFS and OS rates were 19.6% (95% CI, -1.8–41.0) and 71.7% (95% CI, 46.8–96.6), respectively. Grade 3 or higher adverse events were not observed.
Conclusion: PBT for patients with LMBC showed promising FFLP and OS with safe toxicity profiles. These findings suggest that PBT can be considered a local treatment option in patients with LMBC.
Introduction
Patients with liver metastases from breast cancer (LMBC), considered a manifestation of incurable systemic disease, have a poor prognosis of 4–8 months’ survival, if untreated, and 18-24 months, even with systemic chemotherapy and/or hormonal treatments (1). The role of local treatments, including hepatic resection, is controversial in patients with LMBC, but hepatic resection is still a potentially curative treatment and could increase survival in selected patients who have metastatic disease confined within the liver or stable extrahepatic disease after systemic treatments (2, 3). However, most patients with LMBC remain ineligible for hepatic resection, which has raised the need for other local treatments, such as radiofrequency ablation (RFA), cryoablation, transarterial chemoembolization (TACE), transarterial radioembolization (TARE), stereotactic body radiotherapy (SBRT), and proton beam therapy (PBT) (4–27). Local treatments have the potential to improve survival by reducing the tumor burden and allowing subsequent systemic treatments to be more effective, but these treatments have a low level of evidence and could delay or interrupt systemic treatments. Thus, the ideal local treatment for patients with LMBC would be minimally invasive with a low morbidity and mortality rate and could minimize the delay or interruption of systemic treatments.
With technological advances in radiotherapy, SBRT has a growing role as non-invasive treatment option in the treatment for patients with metastatic liver tumors (26, 28, 29). In particular, the recent introduction of magnetic resonance imaging (MRI) guided radiotherapy has made it possible to precisely identify and verify the target before and/or during treatment (28). Although MRI guidance technology has not been applied to PBT yet, PBT has the potential to allow safe dose escalation in the target volumes while sparing uninvolved liver tissue due to the unique property of proton beams (called ‘Bragg peak’) when compared to radiotherapy with X-ray; moreover, PBT has been proven to be safe and effective as a local treatment for primary liver cancer (30–34). In addition, compared with conventional fractionated radiotherapy, hypofractionated radiotherapy has potential theoretical and practical advantages in terms of improvement of the therapeutic ratio by reducing cancer cell proliferation in involved tissues within the tolerance of surrounding uninvolved normal tissues and minimizing the delay or interruption of systemic treatments by shortening the overall duration of radiotherapy. Based on this background, hypofractionated PBT with various sequences and/or regimens of systemic treatments depending on the response to systemic treatments to LMBC and/or extrahepatic disease has been applied for patients with LMBC in our institution. The purpose of this study was to evaluate the clinical effectiveness of hypofractionated PBT for patients with LMBC.
Material And Methods
Patients
Patients with LMBC treated with proton beam therapy (PBT) between February 2013 and August 2019 were registered, and their database was reviewed. Treatment strategy was decided through discussion in a multidisciplinary team considering the patient’s performance status, location and size of tumor, and status of extrahepatic disease. Medical records (including admission information and summaries, discharge summaries, surgical notes, physician and nursing notes, laboratory reports, radiologic imaging and reports, and pathologic reports) of each patient were evaluated, and clinicopathologic data of each patient (such as age, histology, grade, clinical and pathological stage, pre-treatments prior to PBT, post-treatments after PBT, sites and times of disease progression, and follow-up data) were obtained. The collected data were managed by assigning case numbers anonymizing them, and then, data analyses were conducted according to the relevant guidelines and regulations. This study complied with the Declaration of Helsinki and Good Clinical Practice guidelines and was approved by the institutional review board of the National Cancer Center in Korea (20210266). Written informed consent was not required because the design of this study was retrospective.
Treatment
The PBT procedures for liver tumors have been previously reported (30–34). In brief, a contrast-enhanced four-dimensional CT scan was obtained for each patient while monitoring the respiratory signals by a real-time position management system (Varian Medical Systems, Palo Alto, CA, USA). The gross tumor volume (GTV) was delineated in the average intensity projection CT images during exhalation (gated) phases (30% of the total respiratory cycle) fused with magnetic resonance imaging (MRI) and/or positron emission tomography (PET), and no margin was added for the clinical target volume (26, 27, 30). The internal target volume (ITV) and contours of organs at risk (OARs) were defined as the sum of the GTVs and each OAR in each CT image during gated (exhalation) phases, respectively, and the planning target volume (PTV) was defined as the ITV plus 5–7 mm margins in all directions. The PBT plan was designed with the intention of delivering 100% of the prescribed doses to at least 90% of the PTV using 2–3 (median, 3) non- or coplanar beams of 230 MeV passively double-scattered proton beams (Proteus 235; Ion Beam Applications, S.A., Louvain-la-Neuve, Belgium). Gray equivalent (GyE = proton physical dose [Gray] × relative biologic effectiveness [1.1]) was used to describe the radiation doses of PBT, and the median prescribed dose to the PTV was 66 GyE (range, 60–80) in 10 fractions, 5 times a week (Figure 1). Previously published dose-fractionations of PBT and dose-volume constraints for the OARs in liver and abdominal tumors were used (31, 32, 34–36). Briefly, dose-fractionations of PBT were decided by the tumor location as follow: i) 60GyE in 10 fractions was administered for tumor which was located within 2cm from gastrointestinal organs to avoid toxicity of gastrointestinal organs; ii) 66GyE in 10 fractions was administered for tumor which was located within 2cm from the hepatic hilum and more than 2cm from gastrointestinal organs; and iii) 70-80 GyE in 10 fractions was administered for tumor which was located more than 2cm from gastrointestinal organs and hepatic hilum. In dose-volume constraints for the OARs, the maximum dose for the spinal cord was <30 GyE; the radiation doses in 2 cm3 (D2cc) for the stomach, duodenum, and bowel were ≤39 GyE, ≤ 37 GyE, and ≤ 37 GyE, respectively; the relative liver volume receiving ≥27 GyE was limited to <60%; and the kidney volume receiving ≥18 GyE was <35%. Fasting for at least 4 hours prior to PBT was required for all patients at each treatment, and PBT radiation was delivered during gated phases after verifying each patient’s position and isocenter considering the liver, diaphragm, bones, etc., under the image guidance (AdapPT Insight; Ion Beam Applications, S.A., Louvain-la-Neuve, Belgium) using X-ray and/or cone beam CT images.
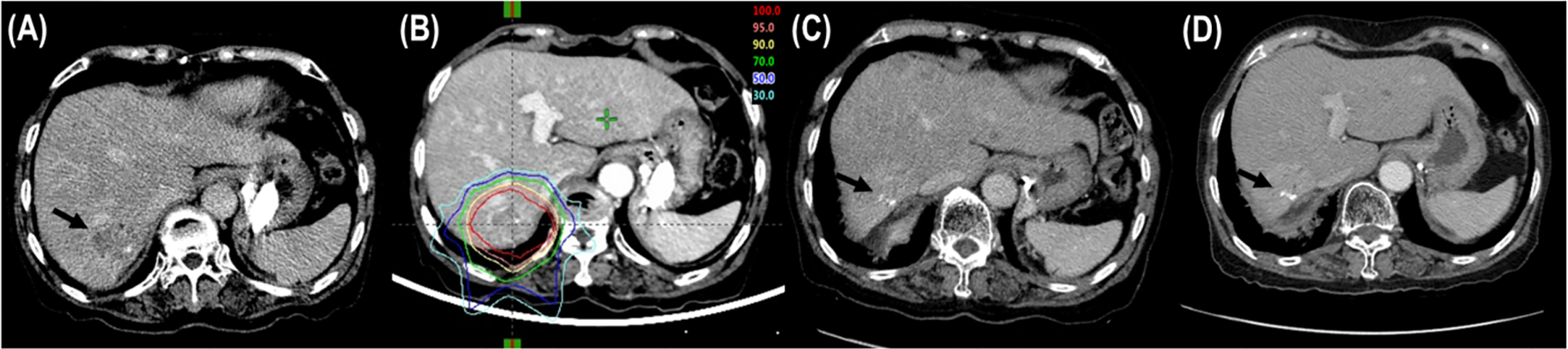
Figure 1 Tumor response after proton beam therapy (PBT). (A) CT scans prior to PBT showing the tumor (arrow). (B) The patient was treated with PBT. (C) CT scans at 6 months after PBT showing shrinkage of the tumor (arrow). (D) CT scans at 12 months after PBT showing complete response of the tumor (arrow).
Assessments and Statistical Analysis
Follow-up examinations with routine laboratory tests, tumor markers, and contrast-enhanced CT and/or MRI were performed at 1 month after PBT and every 3-4 months thereafter. Disease progression was determined by pathologic and/or radiological findings showing an increase in size over time. The appearance of regrowth or a new tumor within the PTV to target lesion(s) was defined as local progression, while the appearance of regrowth of previously untreated nontarget lesion(s) or a new tumor within the liver outside of the PTV and at extrahepatic sites was defined as intrahepatic and extrahepatic progression, respectively. The tumor responses of LMBC(s) treated with PBT and the adverse events (AEs) related to PBT were assessed according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 (37) and the Common Terminology Criteria for Adverse Events version 4.03 (https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_4.03.xlsx), respectively. The freedom from local progression (FFLP), progression-free survival (PFS) and overall survival (OS) were calculated from the commencement date of PBT until the date of local progression, the date of any disease progression or death, and the date of death from any cause or the last follow-up (i.e., censoring), respectively. The probability of FFLP, PFS, and OS was calculated using the Kaplan–Meier method. The log-rank test was used to compare survival differences, and a p value <0.05 was considered statistically significant. All statistical analyses were conducted using STATA software (version 14.0; StataCorp, College Station, TX).
Results
A total of 17 LMBC patients receiving PBT between February 2013 and August 2019 were registered. Patient characteristics at the time of PBT are summarized in Table 1. The median size of LMBCs treated with PBT was 2.4 cm (range, 1.0–4.0), and the number of LMBCs treated with PBT was one and two in 16 patients and 1 patient, respectively. Three patients (17.6%) had de novo LMBC(s), and 14 patients (82.4%) had LMBC(s) at disease progression (Table 1). The median interval between the occurrence of LMBC(s) and initial diagnosis was 33.1 months (range, 0–184.8). All patients received systemic treatments and/or radiofrequency ablation for a median of 16.2 months (range, 2.2–84.2) prior to PBT to LMBC(s) (Table 1). At the time of PBT to LMBC(s), four patients (23.5%) had only LMBC(s) treated with PBT, but 13 patients (76.5%) had more than one intrahepatic and/or extrahepatic metastatic disease(s) other than LMBC(s) treated with PBT (Table 1). After PBT to LMBC(s), subsequent maintenance treatments were continued in 13 patients (76.5%) until disease progression (Table 2).
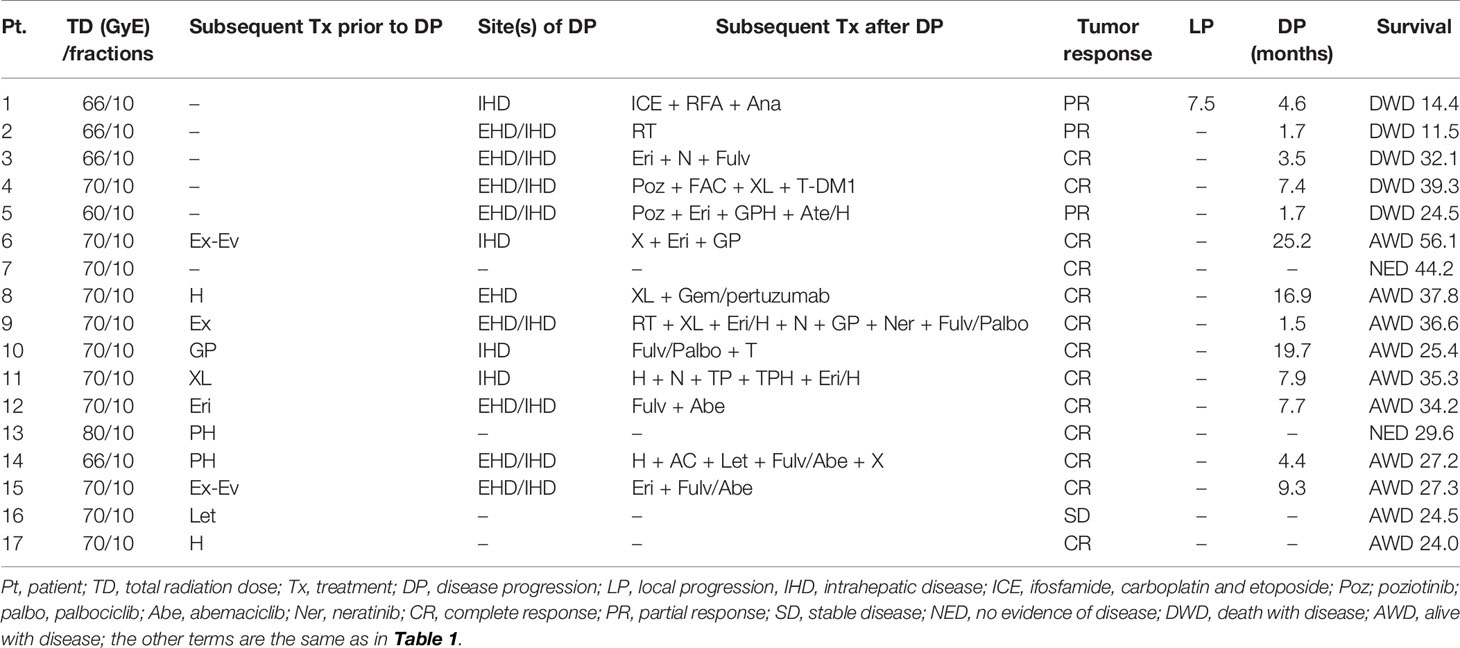
Table 2 Treatment details and outcomes of patients with liver metastasis from breast cancer receiving proton beam therapy.
The median follow-up time was 34.2 months (range, 11.5–56.1). During follow-up, the best tumor responses of LMBC(s) after PBT were complete response (CR) in 13 patients (76.5%), partial response (PR) in 3 patients (17.6%), stable disease (SD) in 1 patient (5.9%), and progressive disease (PD) in no patient (Figure 1). Of 17 patients, local progression of LMBC(s) treated with PBT was observed in 1 patient (5.9%) at 7.5 months after PBT, while local progression of LMBC(s) was not observed in the remaining 16 patients (94.1%) for a median follow-up time of 30.9 months (range, 11.5–56.1). The first sites of disease progressions were local in 0 patients (0%), intrahepatic in 8 patients (47.1%), and extrahepatic in 7 patients (41.2%), and the cumulative sites of disease progressions were local in 1 patient (5.9%), intrahepatic in 10 patients (58.8%), and extrahepatic in 11 patients (64.7%) (Figure 2). After the development of disease progression, subsequent salvage treatments were performed (Table 2). At the time of analysis, 12 patients were alive, and 5 patients died from disease progression. The median time of FFLP was not yet reached, and the 1-, 2-, 3-, and 4-year FFLP rates were 94.1% (95% confidence interval [CI], 82.9–105.3), 94.1% (95% CI, 82.9–105.3), 94.1% (95% CI, 82.9–105.3), and 94.1% (95% CI, 82.9–105.3), respectively (Figure 3A). The median time of PFS was 7.9 months (95% CI, 5.3–10.5), and the 1-, 2-, and 3-year PFS rates were 41.2% (95% CI, 17.8–64.5), 29.4% (95% CI, 7.6–51.1), and 19.6% (95% CI, -1.8–41.0), respectively (Figure 3B). The median OS time was 39.3 months (95% CI, 33.2–51.9), and the 1-, 2-, 3-, and 4-year OS rates were 94.1% (95% CI, 82.9–105.3), 88.2% (95% CI, 72.9–103.5), 71.7% (95% CI, 46.8–96.6), and 47.8% (95% CI, 6.1–89.5), respectively (Figure 3C). The median time of OS from the date of diagnosis of LMBC was 117.5 months, and the 1-, 2-, 3-, 4- and 5-year OS rates were 100%, 94.1% (95% CI, 82.9–105.3), 94.1% (95% CI, 82.9–105.3), 79% (95% CI, 57.6–100.4), and 79% (95% CI, 57.6–100.4), respectively. Patients who had only LMBC(s) treated with PBT (n=4) had significantly longer PFS than patients who had metastatic disease other than LMBC(s) treated with PBT (n=13) (2-year: 75.0% vs. 15.4%, p=0.034), while patients who had only LMBC(s) had a trend of higher OS than patients who had metastatic disease other than LMBC(s), but these findings were without statistical significance (2-year, 100% vs. 84.6%, p=0.074) due to the small size of the study population (n=17).
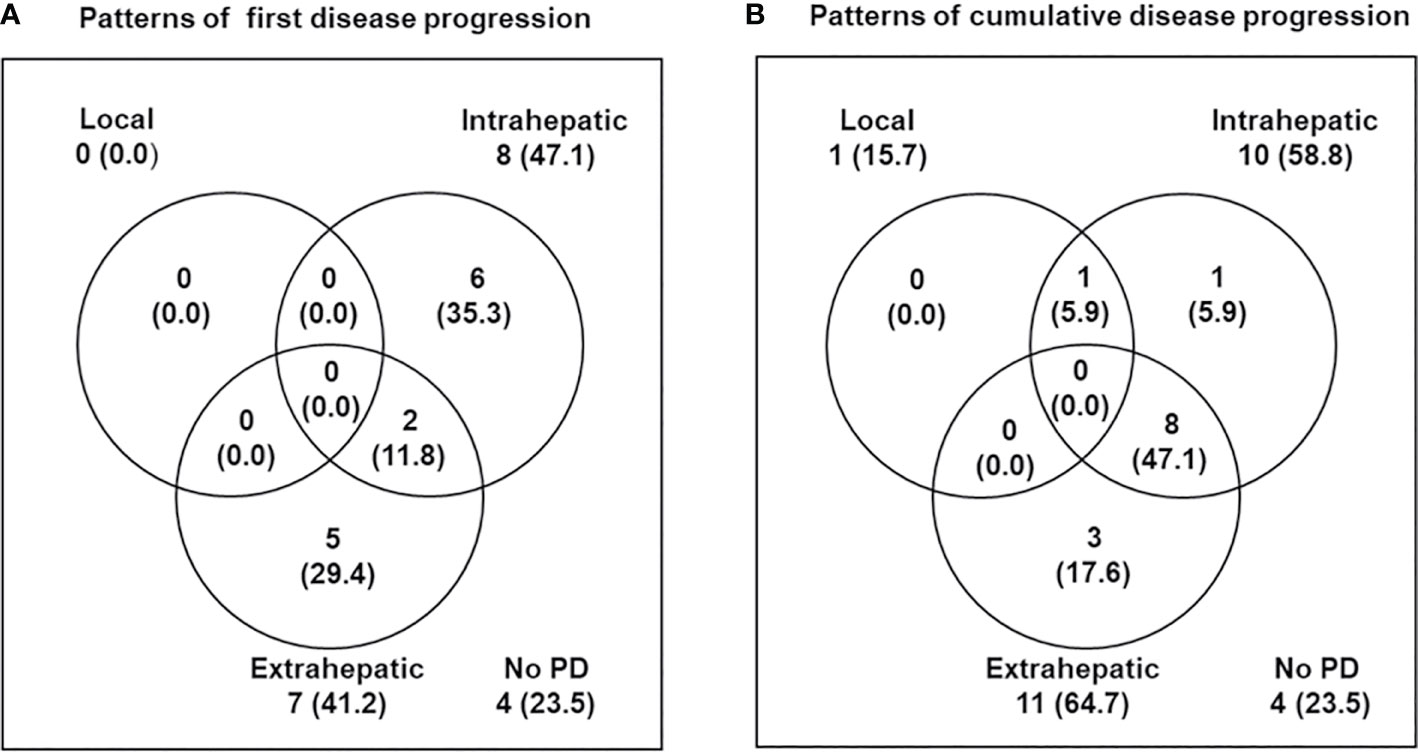
Figure 2 Patterns of disease progression. (A) First and (B) cumulative disease progression at the time of analysis.
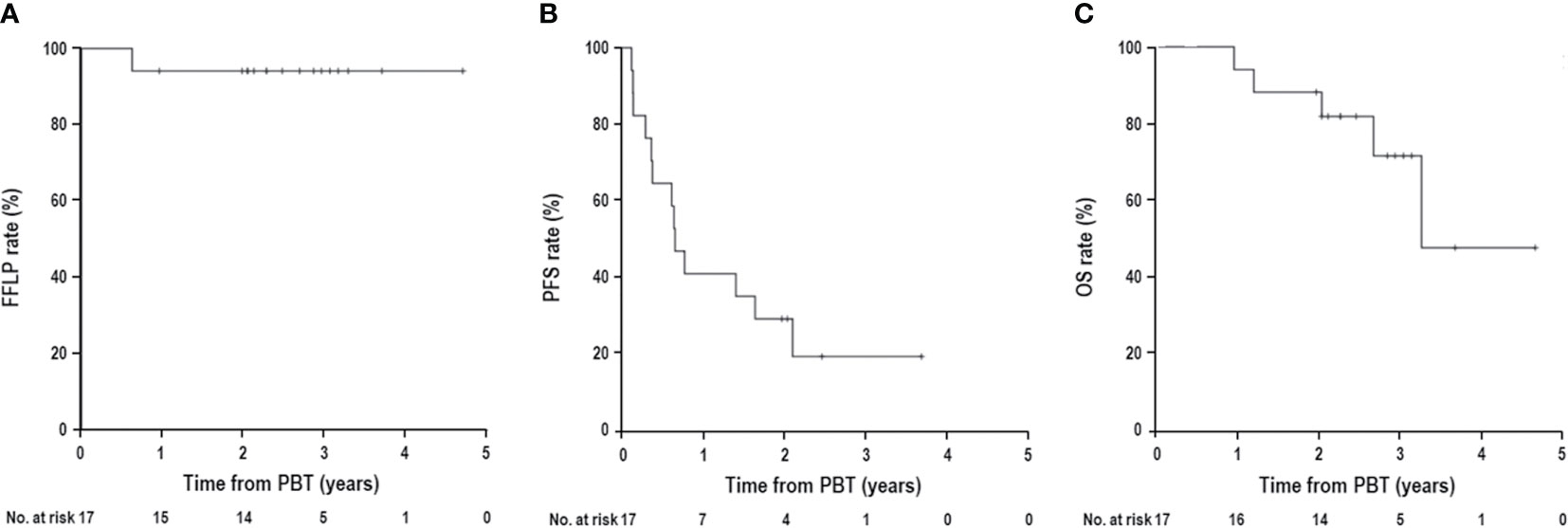
Figure 3 (A) Freedom from local progression (FFLP), (B) progression-free survival (PFS), and (C) overall survival (OS) curves in patients with liver metastasis from breast cancer receiving proton beam therapy (PBT).
Within 3 months after PBT, 4 patients (23.5%) experienced dermatitis (grade 1, 3 [17.6%], and grade 2, 1 [5.9%]); two patients (11.8%) experienced grade 1 elevated alanine aminotransferase levels without evidence of disease progression; 3 patients (17.6%) experienced leukopenia (grade 1, 2 [11.8%], and grade 2, 1 [5.9%]); and one patient (5.9%) experienced grade 1 thrombocytopenia. Three months after PBT, 7 patients (41.2%) experienced grade 1 radiation pneumonitis, and one patient (5.9%) experienced grade 2 gastric ulcers managed by medication without invasive intervention. Treatment-related death and hepatic failure without evidence of disease progression were not observed in the subsequent follow-up period.
Discussion
In breast cancer, tumor cells spread to the liver via the systemic circulation from the primary breast site; thus, isolated hepatic metastasis without multiorgan spread is rare, in contrast with colorectal cancer, in which tumor cells spread via the portal vein so that the liver is the first and principal metastatic site. Thus, systemic chemotherapy and/or hormonal treatments are currently the standard treatment for patients with LMBC, and even with recent advances in systemic treatments, the median OS time is 18-24 months. In addition, long-term survival of >5 years after systemic treatments alone has rarely been achieved (1). To date, the role of hepatic resection in patients with LMBC remains unclear, but this approach has been tried because hepatic resection is the only potentially curative treatment for primary or metastatic liver tumors. In a recent systemic review including 33 studies on hepatic resection of LMBC (n=965) (2), the median OS time was 35.1 months (range, 22.5–74), with a median 1-, 2-, 3-, and 5-year OS of 84.6% (range, 60–100), 71.4% (range, 40–90), 52.9% (range, 31-80), and 33% (range, 11.1-80), respectively. Although surgical series included a small, highly selected, and heterogeneous population, these results have suggested that despite LMBC being a systemic disease, local treatments have the potential to improve survival in selected patients who have LMBC confined to the liver or stable extrahepatic disease (3). However, hepatic resection is a potentially morbid procedure with a mortality rate of 0–5.9% and a median morbidity rate of 15% (2); thus, an effective local treatment option for LMBC that is less invasive and less influential on systemic treatments is needed.
Various nonsurgical local treatments, such as RFA, cryoablation, TACE, TARE, SBRT, and PBT, have been attempted (Table 3) (4–27). Local transarterial treatments, such as TACE (14–18) and TARE (14, 19–25), have been applied in LMBC patients with no or limited systemic treatment options or during holidays/breaks from systemic treatments. TACE has shown an objective response rate of 7-35.7% and a median OS time of 4.6-28.0 months (14–18); similarly, TARE has also shown an objective response rate of 28.9-56% and a median OS time of 4.0-13.6 months (14, 19–25) (Table 3). Although direct comparison among these studies is difficult due to different tumor burdens of intrahepatic and extrahepatic disease, patient characteristics, and selection criteria, these data suggest that transarterial local treatments, such as TACE or TARE, might be considered palliative treatment options rather than equivalent alternatives to hepatic resection for LMBC patients. Ablative treatments, such as RFA and cryoablation, are well-known minimally invasive and potentially curative local treatments for small sized (i.e., less than 2-3 cm) primary liver tumors, and they have also been applied for patients with LMBC (Table 3) (4–13). Ablative treatments of patients with LMBC have shown promising outcomes, with median OS times of 10.9–58.6 months and 1-, 3-, and 5-year OS rates of 68-90%, 25.3–49.3%, and 11–29%, respectively (Table 3). These results of ablative treatments suggest that ablative treatments are probably less effective at local tumor control than surgical resection, but they might be considered reasonable alternatives to surgical resection in selected patients who have LMBC confined to the liver or stable extrahepatic disease. However, the local progression rate after ablative treatment is relatively high, i.e., 7.3-53.8% (Table 3) (4–13). Even with complex planning, ultrasound and CT guidance and the use of multiple electrodes, the application and effectiveness of ablative procedures have been limited due to the size (i.e., >3 cm) of LMBC, proximity to major vessels and bile ducts, deep or subcapsular location, and visibility of the tumor with imaging guidance. Thus, another local treatment option for patients with LMBC is needed to overcome the technical limitations of ablative treatments and to achieve local tumor control comparable with that by hepatic resection.
With technical advances in radiotherapy, a number of studies have shown that SBRT and PBT are effective and safe local treatment options for liver tumors (30–34, 38), but few studies have focused on SBRT and PBT for LMBC alone (26, 27). Onal et al. (26) analyzed 22 LMBC patients treated with both SBRT to LMBC and systemic treatment and reported promising outcomes in terms of 1- and 2-year FFLP rates of 100% and 88%, respectively, and 1- and 2-year OS rates of 87% and 57%, respectively. Fukumitsu et al. (27) analyzed 8 patients with LMBC without extrahepatic disease treated with PBT and reported 1-, 3- and 5-year FFLP rates of 86%, 86%, and 86%, respectively, and 1-, 3-, and 5-year OS rates of 88%, 73%, and 58%, respectively. In the present study, which analyzed 17 patients treated with PBT, the local progression rate was 5.9% with 1-, 3-, and 4-year FFLP rates of 94.1%, 94.1%, and 94.1%, respectively, and the median OS time was 39.3 months with 1-, 3-, and 4-year OS rates of 94.1%, 79.8%, and 47.8%, respectively. To date, there are no data from randomized study comparing PBT with SBRT, so it remains unanswered whether PBT is truly equivalent or superior to SBRT in these patients. In SBRT, the image guidance techniques using fiducials, surface guidance, stereotactic X-ray, CT, and MRI can allow a precise identification and verification of target before and/or during treatment and also reduce the target volume by minimizing the PTV margin (26, 28, 29, 38). MRI guidance technology is more helpful for tumors in the liver, frequently poorly visualized on the CT images, than those in other anatomical sites (28). To date, MRI guidance technology has not been applied to PBT in clinical practice, but several dosimetric studies comparing PBT with radiotherapy with X-rays in primary liver tumors showed that PBT can reduce the irradiated volume of remaining liver and allow dose escalation for tumors (39–41). Meta-analysis for primary liver tumors (42) showed a similar FFLP and OS and lower rate of toxicity in PBT compared to SBRT. In addition, although direct comparison of PBT with other local treatments was difficult due to heterogeneity of the study population among the studies, PBT yielded comparable or superior FFLP and OS to those of ablative treatments in previous studies (Table 3) (4–13) and comparable OS to that of hepatic resection (2).
The present study has several inherent limitations due to its relatively small (n=17) and retrospective nature. First, this study included a heterogeneous population with respect to factors such as tumor biology (i.e., status of hormonal receptors and human epidermal growth factor receptor), tumor burdens in intrahepatic and extrahepatic disease, responses to systemic treatments, and various histories of pre- and post-treatments; thus, potential selection bias and confounding factors related to prognosis were not thoroughly evaluated. In the present study, the patients who had only LMBC(s) treated with PBT had significantly longer PFS (2-year: 75.0% vs. 15.4%, p=0.034) and showed a trend toward longer OS (2-year, 100% vs. 84.6%, p=0.074) than patients who had other metastatic diseases. This finding implied that patients with LMBC confined to the liver or stable extrahepatic disease after systemic treatments may be subgroups that could benefit from local treatments, including PBT. Second, PBT for LMBC showed a safety profile, without ≥grade 3 AEs in the present study and other studies (27), but the interpretation of data should be carefully performed due to the probability of underestimation of AEs in retrospective studies due to the incompleteness of medical records, recall bias, etc. Third, the effect of the PBT dose on local tumor control was not evaluated due to the small size of the study population (n=17), but in the present study, PBT showed promising outcomes in terms of a local progression rate of 5.9% and 3-year FFLP rates of 94.1%. In addition, to the best of our knowledge, few reports of PBT for LMBC with a smaller study population (n=8) than that of the present study are available to date (27). However, further, large-scale studies are needed to confirm the effectiveness of PBT for LMBC and to select patients who will benefit from PBT.
In conclusion, the present study showed that PBT can yield promising FFLP and OS rates similar to those resulting from hepatic resection and ablative treatments in patients with LMBC, with a safe profile of toxicity. These findings suggest that PBT may be considered a local treatment option for patients with LMBC confined to the liver or stable extrahepatic disease after systemic treatments.
Data Availability Statement
All data used in this study are available upon request to the corresponding author.
Ethics Statement
This study was approved by the Ethical Committee of the National Cancer Center (20210266), and written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
TK, KL, and SS: conceptualization. TK, KL, and SS: data collection. TK: formal analysis. TK, KL SS, Y-JK, DK, HC, E-GL, JH, SJ, SL, HK and ESL: investigation. TK: writing the original draft. TK, KL, and SS: supervision. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by National Cancer Center Grants (2110351 and 1910300). The funders had no role in the design of this study; curation, analysis, and interpretation of data; writing of the manuscript; or decision to publish this study.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
All authors thank Eun-Young Park, Ph.D., Biostatistics Collaboration Team, National Cancer Center, Goyang, Korea, for assistance during the statistical analyses of this study.
References
1. Eng LG, Dawood S, Sopik V, Haaland B, Tan PS, Bhoo-Pathy N, et al. Ten-Year Survival in Women With Primary Stage IV Breast Cancer. Breast Cancer Res Treat (2016) 160(1):145–52. doi: 10.1007/s10549-016-3974-x
2. Fairhurst K, Leopardi L, Satyadas T, Maddern G. The Safety and Effectiveness of Liver Resection for Breast Cancer Liver Metastases: A Systematic Review. Breast (2016) 30:175–84. doi: 10.1016/j.breast.2016.09.011
3. Cardoso F, Paluch-Shimon S, Senkus E, Curigliano G, Aapro MS, Andre F, et al. 5th ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 5). Ann Oncol (2020) 31(12):1623–49. doi: 10.1016/j.annonc.2020.09.010
4. Bai XM, Yang W, Zhang ZY, Jiang AN, Wu W, Lee JC, et al. Long-Term Outcomes and Prognostic Analysis of Percutaneous Radiofrequency Ablation in Liver Metastasis From Breast Cancer. Int J Hyperthermia (2019) 35(1):183–93. doi: 10.1080/02656736.2018.1488279
5. Carrafiello G, Fontana F, Cotta E, Petulla M, Brunese L, Mangini M, et al. Ultrasound-Guided Thermal Radiofrequency Ablation (RFA) as an Adjunct to Systemic Chemotherapy for Breast Cancer Liver Metastases. Radiol Med (2011) 116(7):1059–66. doi: 10.1007/s11547-011-0697-2
6. Jakobs TF, Hoffmann RT, Schrader A, Stemmler HJ, Trumm C, Lubienski A, et al. CT-Guided Radiofrequency Ablation in Patients With Hepatic Metastases From Breast Cancer. Cardiovasc Intervent Radiol (2009) 32(1):38–46. doi: 10.1007/s00270-008-9384-7
7. Kumler I, Parner VK, Tuxen MK, Skjoldbye B, Bergenfeldt M, Nelausen KM, et al. Clinical Outcome of Percutaneous RF-Ablation of non-Operable Patients With Liver Metastasis From Breast Cancer. Radiol Med (2015) 120(6):536–41. doi: 10.1007/s11547-014-0489-6
8. Lawes D, Chopada A, Gillams A, Lees W, Taylor I. Radiofrequency Ablation (RFA) as a Cytoreductive Strategy for Hepatic Metastasis From Breast Cancer. Ann R Coll Surg Engl (2006) 88(7):639–42. doi: 10.1308/003588406X149129
9. Meloni MF, Andreano A, Laeseke PF, Livraghi T, Sironi S, Lee FT Jr. Breast Cancer Liver Metastases: US-Guided Percutaneous Radiofrequency Ablation–Intermediate and Long-Term Survival Rates. Radiology (2009) 253(3):861–9. doi: 10.1148/radiol.2533081968
10. Schullian P, Johnston E, Laimer G, Putzer D, Eberle G, Scharll Y, et al. Stereotactic Radiofrequency Ablation of Breast Cancer Liver Metastases: Short- and Long-Term Results With Predicting Factors for Survival. Cardiovasc Intervent Radiol (2021) 44(8):1184–93. doi: 10.1007/s00270-021-02820-6
11. Tasci Y, Aksoy E, Taskin HE, Aliyev S, Moore H, Agcaoglu O, et al. A Comparison of Laparoscopic Radiofrequency Ablation Versus Systemic Therapy Alone in the Treatment of Breast Cancer Metastasis to the Liver. HPB (Oxford) (2013) 15(10):789–93. doi: 10.1111/hpb.12133
12. Veltri A, Gazzera C, Barrera M, Busso M, Solitro F, Filippini C, et al. Radiofrequency Thermal Ablation (RFA) of Hepatic Metastases (METS) From Breast Cancer (BC): An Adjunctive Tool in the Multimodal Treatment of Advanced Disease. Radiol Med (2014) 119(5):327–33. doi: 10.1007/s11547-013-0354-z
13. Zhang W, Yu H, Guo Z, Li B, Si T, Yang X, et al. Percutaneous Cryoablation of Liver Metastases From Breast Cancer: Initial Experience in 17 Patients. Clin Radiol (2014) 69(3):231–8. doi: 10.1016/j.crad.2013.09.014
14. Chang J, Charalel R, Noda C, Ramaswamy R, Kim SK, Darcy M, et al. Liver-Dominant Breast Cancer Metastasis: A Comparative Outcomes Study of Chemoembolization Versus Radioembolization. Anticancer Res (2018) 38(5):3063–8. doi: 10.21873/anticanres.12563
15. Eichler K, Jakobi S, Gruber-Rouh T, Hammerstingl R, Vogl TJ, Zangos S. Transarterial Chemoembolisation (TACE) With Gemcitabine: Phase II Study in Patients With Liver Metastases of Breast Cancer. Eur J Radiol (2013) 82(12):e816–22. doi: 10.1016/j.ejrad.2013.08.046
16. Li XP, Meng ZQ, Guo WJ, Li J. Treatment for Liver Metastases From Breast Cancer: Results and Prognostic Factors. World J Gastroenterol (2005) 11(24):3782–7. doi: 10.3748/wjg.v11.i24.3782
17. Lin YT, Medioni J, Amouyal G, Dean C, Sapoval M, Pellerin O. Doxorubicin-Loaded 70-150 Mum Microspheres for Liver-Dominant Metastatic Breast Cancer: Results and Outcomes of a Pilot Study. Cardiovasc Intervent Radiol (2017) 40(1):81–9. doi: 10.1007/s00270-016-1465-4
18. Vogl TJ, Naguib NN, Nour-Eldin NE, Eichler K, Zangos S, Gruber-Rouh T. Transarterial Chemoembolization (TACE) With Mitomycin C and Gemcitabine for Liver Metastases in Breast Cancer. Eur Radiol (2010) 20(1):173–80. doi: 10.1007/s00330-009-1525-0
19. Cianni R, Pelle G, Notarianni E, Saltarelli A, Rabuffi P, Bagni O, et al. Radioembolisation With (90)Y-Labelled Resin Microspheres in the Treatment of Liver Metastasis From Breast Cancer. Eur Radiol (2013) 23(1):182–9. doi: 10.1007/s00330-012-2556-5
20. Fendler WP, Lechner H, Todica A, Paprottka KJ, Paprottka PM, Jakobs TF, et al. Safety, Efficacy, and Prognostic Factors After Radioembolization of Hepatic Metastases From Breast Cancer: A Large Single-Center Experience in 81 Patients. J Nucl Med (2016) 57(4):517–23. doi: 10.2967/jnumed.115.165050
21. Gordon AC, Gradishar WJ, Kaklamani VG, Thuluvath AJ, Ryu RK, Sato KT, et al. Yttrium-90 Radioembolization Stops Progression of Targeted Breast Cancer Liver Metastases After Failed Chemotherapy. J Vasc Interv Radiol (2014) 25(10):1523–32, 1532 e1-2. doi: 10.1016/j.jvir.2014.07.007
22. Haug AR, Tiega Donfack BP, Trumm C, Zech CJ, Michl M, Laubender RP, et al. 18f-FDG PET/CT Predicts Survival After Radioembolization of Hepatic Metastases From Breast Cancer. J Nucl Med (2012) 53(3):371–7. doi: 10.2967/jnumed.111.096230
23. Jakobs TF, Hoffmann RT, Fischer T, Stemmler HJ, Tatsch K, La Fougere C, et al. Radioembolization in Patients With Hepatic Metastases From Breast Cancer. J Vasc Interv Radiol (2008) 19(5):683–90. doi: 10.1016/j.jvir.2008.01.009
24. Pieper CC, Meyer C, Wilhelm KE, Block W, Nadal J, Ahmadzadehfar H, et al. Yttrium-90 Radioembolization of Advanced, Unresectable Breast Cancer Liver Metastases-A Single-Center Experience. J Vasc Interv Radiol (2016) 27(9):1305–15. doi: 10.1016/j.jvir.2016.05.028
25. Saxena A, Kapoor J, Meteling B, Morris DL, Bester L. Yttrium-90 Radioembolization for Unresectable, Chemoresistant Breast Cancer Liver Metastases: A Large Single-Center Experience of 40 Patients. Ann Surg Oncol (2014) 21(4):1296–303. doi: 10.1245/s10434-013-3436-1
26. Onal C, Guler OC, Yildirim BA. Treatment Outcomes of Breast Cancer Liver Metastasis Treated With Stereotactic Body Radiotherapy. Breast (2018) 42:150–6. doi: 10.1016/j.breast.2018.09.006
27. Fukumitsu N, Okumura T, Numajiri H, Takizawa D, Ohnishi K, Mizumoto M, et al. Follow-Up Study of Liver Metastasis From Breast Cancer Treated by Proton Beam Therapy. Mol Clin Oncol (2017) 7(1):56–60. doi: 10.3892/mco.2017.1283
28. Boldrini L, Corradini S, Gani C, Henke L, Hosni A, Romano A, et al. MR-Guided Radiotherapy for Liver Malignancies. Front Oncol (2021) 11:616027. doi: 10.3389/fonc.2021.616027
29. Mahadevan A, Blanck O, Lanciano R, Peddada A, Sundararaman S, D'Ambrosio D, et al. Stereotactic Body Radiotherapy (SBRT) for Liver Metastasis - Clinical Outcomes From the International Multi-Institutional RSSearch(R) Patient Registry. Radiat Oncol (2018) 13(1):26. doi: 10.1186/s13014-018-0969-2
30. Kim TH, Koh YH, Kim BH, Kim MJ, Lee JH, Park B, et al. Proton Beam Radiotherapy vs. Radiofrequency Ablation for Recurrent Hepatocellular Carcinoma: A Randomized Phase III Trial. J Hepatol (2021) 74(3):603–12. doi: 10.1016/j.jhep.2020.09.026
31. Kim TH, Park JW, Kim BH, Kim H, Moon SH, Kim SS, et al. Does Risk-Adapted Proton Beam Therapy Have a Role as a Complementary or Alternative Therapeutic Option for Hepatocellular Carcinoma? Cancers (Basel) (2019) 11(2):230. doi: 10.3390/cancers11020230
32. Kim TH, Park JW, Kim BH, Oh ES, Youn SH, Moon SH, et al. Phase II Study of Hypofractionated Proton Beam Therapy for Hepatocellular Carcinoma. Front Oncol (2020) 10:542. doi: 10.3389/fonc.2020.00542
33. Kim TH, Park JW, Kim YJ, Kim BH, Woo SM, Moon SH, et al. Phase I Dose-Escalation Study of Proton Beam Therapy for Inoperable Hepatocellular Carcinoma. Cancer Res Treat (2015) 47(1):34–45. doi: 10.4143/crt.2013.218
34. Kim DY, Park JW, Kim TH, Kim BH, Moon SH, Kim SS, et al. Risk-Adapted Simultaneous Integrated Boost-Proton Beam Therapy (SIB-PBT) for Advanced Hepatocellular Carcinoma With Tumour Vascular Thrombosis. Radiother Oncol (2017) 122(1):122–9. doi: 10.1016/j.radonc.2016.12.014
35. Kim TH, Lee WJ, Woo SM, Oh ES, Youn SH, Jang HY, et al. Efficacy and Feasibility of Proton Beam Radiotherapy Using the Simultaneous Integrated Boost Technique for Locally Advanced Pancreatic Cancer. Sci Rep (2020) 10(1):21712. doi: 10.1038/s41598-020-78875-1
36. Kim TH, Lee WJ, Woo SM, Kim H, Oh ES, Lee JH, et al. Effectiveness and Safety of Simultaneous Integrated Boost-Proton Beam Therapy for Localized Pancreatic Cancer. Technol Cancer Res Treat (2018) 17:1533033818783879. doi: 10.1177/1533033818783879
37. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur J Cancer (2009) 45(2):228–47. doi: 10.1016/j.ejca.2008.10.026
38. Wahl DR, Stenmark MH, Tao Y, Pollom EL, Caoili EM, Lawrence TS, et al. Outcomes After Stereotactic Body Radiotherapy or Radiofrequency Ablation for Hepatocellular Carcinoma. J Clin Oncol (2016) 34(5):452–9. doi: 10.1200/JCO.2015.61.4925
39. Gandhi SJ, Liang X, Ding X, Zhu TC, Ben-Josef E, Plastaras JP, et al. Clinical Decision Tool for Optimal Delivery of Liver Stereotactic Body Radiation Therapy: Photons Versus Protons. Pract Radiat Oncol (2015) 5(4):209–18. doi: 10.1016/j.prro.2015.01.004
40. Kim JY, Lim YK, Kim TH, Cho KH, Choi SH, Jeong H, et al. Normal Liver Sparing by Proton Beam Therapy for Hepatocellular Carcinoma: Comparison With Helical Intensity Modulated Radiotherapy and Volumetric Modulated Arc Therapy. Acta Oncol (2015) 54(10):1827–32. doi: 10.3109/0284186X.2015.1009637
41. Wang X, Krishnan S, Zhang X, Dong L, Briere T, Crane CH, et al. Proton Radiotherapy for Liver Tumors: Dosimetric Advantages Over Photon Plans. Med Dosim (2008) 33(4):259–67. doi: 10.1016/j.meddos.2007.04.008
Keywords: liver metastasis, breast cancer, freedom from local progression rate, overall survival, proton beam therapy, radiotherapy
Citation: Kim TH, Lee KS, Sim SH, Kim YJ, Kim DY, Chae H, Lee EG, Han JH, Jung SY, Lee S, Kang HS and Lee ES (2021) Clinical Effectiveness of Hypofractionated Proton Beam Therapy for Liver Metastasis From Breast Cancer. Front. Oncol. 11:783327. doi: 10.3389/fonc.2021.783327
Received: 26 September 2021; Accepted: 20 October 2021;
Published: 03 November 2021.
Edited by:
David Krug, University Medical Center Schleswig-Holstein, GermanyReviewed by:
Marta Scorsetti, Humanitas Research Hospital, ItalyFabian Weykamp, Heidelberg University Hospital, Germany
Copyright © 2021 Kim, Lee, Sim, Kim, Kim, Chae, Lee, Han, Jung, Lee, Kang and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tae Hyun Kim, azJvbmNvQG5jYy5yZS5rcg==
†These authors have contributed equally to this work
 Tae Hyun Kim
Tae Hyun Kim Keun Seok Lee2†
Keun Seok Lee2† Sung Hoon Sim
Sung Hoon Sim Eun-Gyeong Lee
Eun-Gyeong Lee Jai Hong Han
Jai Hong Han So Youn Jung
So Youn Jung Eun Sook Lee
Eun Sook Lee