- 1Department of Hepatobiliary Surgery, State Key Laboratory of Molecular Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 2Department of New Drug Registration, Hebei Immune Cell Application Engineering Research Center/Baoding Newish Technology Co., LTD/Newish Technology (Beijing) Co., LTD, Beijing, China
- 3School of Pharmaceutical Sciences, Tsinghua University, Beijing, China
- 4State Key Laboratory of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, China
Background: Postoperative adjuvant transcatheter arterial chemoembolization (TACE) following curative hepatectomy has been reported to improve the clinical outcomes of hepatocellular carcinoma (HCC) patients with microvascular invasion (MVI), but more endeavors are required to achieve greater clinical benefit. Central memory T-cell (Tcm) self-transfusion has shown superior antitumor activity in several preclinical studies; however, clinical studies are rare. The aim of this study was to evaluate the clinical benefit and safety of combination treatment with Tcm self-transfusion and TACE as adjuvant treatment in HCC patients with MVI after curative hepatectomy.
Methods: From October 2016 to September 2018, primary HCC patients with histologically confirmed MVI who underwent curative hepatectomy at the Cancer Hospital of the Chinese Academy of Medical Sciences were recruited for this study. The patients were divided into a Tcm group (combined Tcm self-transfusion with TACE treatment) or a control group (TACE treatment alone) according to their willingness. The recurrence-free survival (RFS), quality-of-life (QOL) score, and adverse events of each patient were recorded within 2 years.
Results: A total of 52 patients were enrolled, and 48 were eligible for the final data analysis. The median follow-up time was 20.5 months (95% CI: 17.05–22.55 months). The median RFS time was 9.5 months in the control group; the cutoff date was not reached in the Tcm group (when the follow-up duration was 12 months, p = 0.049, HR = 0.40; 95% CI: 0.16–0.99). Compared with the control group, 1- and 2-year RFS rates were higher in the Tcm group (72.0% vs. 46.4% and 58.18% vs. 39.14%, respectively). Multivariate analysis did not indicate that Tcm treatment was an independent prognostic factor associated with HCC recurrence (p = 0.107, HR = 2.312; 95% CI: 0.835–6.400), which might be due to the small sample size of this study. Nevertheless, Tcm treatment effectively improved a reduced QOL due to HCC and liver function injury. Finally, the safety profile of Tcm treatment in this study was good, without any serious adverse events.
Conclusions: This pilot study showed that Tcm self-transfusion combined with TACE treatment might be a beneficial adjuvant therapy with good safety for primary HCC patients with MVI after curative hepatectomy.
Trial registration number: NCT03575806
Introduction
Hepatocellular carcinoma (HCC) accounts for approximately 90% of liver cancer cases and is the sixth most common cancer and fourth leading cause of cancer-related death worldwide, with an estimated 841,080 new cases and 781,631 deaths in 2018 (1, 2). The highest incidence and mortality rates of HCC are reported in Eastern Asia and sub-Saharan Africa, where the main risk factor is cirrhosis caused by hepatitis B virus (HBV) or hepatitis C virus (HCV) infection (2). Currently, hepatectomy is one of the most reliable therapies for HCC (3, 4), though, high postoperative recurrence remains a serious problem (5). In China, the 5-year survival rate for liver cancer is only 12.1%. The rate of tumor recurrence and metastasis 5 years after hepatocellular carcinoma resection is as high as 40%–70%; approximately 50% of HCC patients experience recurrence within 2 years (6–8). Yet, there is no recognized drug or treatment to prevent recurrence of liver cancer in the world at present.
Several major risk factors have been identified as being closely linked to the postoperative recurrence of HCC, including blood vessel invasion, the number of nodules, tumor size, preoperative glutamic oxaloacetic aminotransferase (AST) elevation, resection margin, and liver capsule invasion; blood vessel invasion includes macrovascular invasion/portal vein tumor thrombosis (PVTT) and microvascular invasion (MVI) (3, 7, 9–11). MVI is defined as invasion of the intrahepatic portal vein or hepatic vein branches by tumor cells; the incidence rate of MVI is 15%~57.1% in HCC patients, and MVI indicates aggressive tumor behavior, with a greater tumor burden (12–14). MVI is generally considered an independent risk factor for intrahepatic metastasis and early tumor recurrence (15, 16). It has been demonstrated that the degree of MVI described by invaded vessels, invading carcinoma cells, and the distance of invasion from the tumor edge is valuable for predicting prognosis after curative hepatectomy (17, 18). Statistically, the time of recurrence-free survival (RFS) in HCC patients with MVI is shorter than that in HCC patients without blood vessel invasion (19). As there is no established standard adjuvant treatment for HCC patients with MVI following hepatectomy, developing effective modalities to prevent the postoperative recurrence of HCC in patients with MVI is of great significance (20).
Current treatments to prevent postoperative recurrence in patients with HCC with MVI include postoperative adjuvant transarterial chemoembolization (pa-TACE), postoperative radiotherapy, radiofrequency ablation (RFA), and sorafenib, and pa-TACE is the most common adjuvant therapy after curative resection (21). Through arterial injection of chemotherapeutic drugs and embolizing agents, TACE decreases blood flow to the tumor and induces tumor ischemic necrosis (22). Many studies have reported that pa-TACE improves the overall survival (OS) and RFS of HCC patients with blood vessel invasion after curative hepatectomy (20, 23–26). However, due to controversial reports on the clinical benefits of pa-TACE (27, 28), the optimal postoperative adjuvant treatment for preventing HCC recurrence in patients with MVI reaches no consensus and requires further investigation. Thus, it remains an area where clinical needs are not being met. It is urgent to conduct clinical trials to evaluate the efficacy of adjuvant therapy for patients with high risk of recurrence after radical HCC surgery, which has become the primary task in the postoperative treatment of HCC.
Recently, immunotherapy for cancer treatment has gained growing attention, and adoptive cell transfer of immune effector cells has been shown to have clinical benefit in postoperative HCC patients (29, 30). For instance, a meta-analysis including 11 clinical studies reported that dendritic cell and cytokine-induced killer cell (DC-CIK cell) transfusion combined with TACE/TACE+RFA markedly improved RFS and OS compared with simple TACE/TACE+RFA (31). Moreover, a phase 3 randomized controlled trial in Korea demonstrated that postoperative adjuvant transfusion of active CIK cells notably increased the RFS and OS of HCC patients (32, 33), with efficacy lasting for more than 5 years (34). Despite these positive results, the clinical outcomes of CIK cell-based immunotherapy remain controversial, and its effect in postoperative HCC patients with MVI has not been exclusively studied (35, 36). Other immune cells with high cytotoxicity, such as tumor-infiltrating lymphocytes and peripheral blood T cells (37, 38), might be ideal sources for adoptive cell therapy to prevent postoperative recurrence in HCC patients with MVI.
In this study, we focused on central memory T cells (Tcms), which can quickly differentiate and proliferate into effector T cells, extensively secrete effector cytokines, and strongly activate the memory immune response when again encountering the same antigen (39, 40). Different from effector T cells, memory T cells express CD45RO and can be divided into two populations according to the expression of the lymphoid-homing molecule CD62L, with Tcms being CD62L positive and effector memory T cells (Tems) CD62L negative (41). For this reason, Tcms home to and reside in lymph nodes, while Tems reside in blood, spleen, and peripheral tissues. Tcms have a long life and can rapidly proliferate and differentiate upon secondary response, thus possess superior immune activity than Tems and effector T cells in immune therapy. Using Tem transfusion as a control, Tcm transfusion together with tumor vaccination effectively inhibits tumor growth in a mouse xenograft melanoma model (42), and mesothelin-specific Tcm infusion significantly extends the survival of mesothelioma-bearing NSG mice (43). Furthermore, Busch et al. found that successive transfer of a single Tcm was able to rebuild the lymphoid population and phenotypical diversity of T cells after homing to lymphoid or nonlymphoid organs; moreover, Tcm descendants ultimately reconstituted immunocompetence against lethal infection with bacterial pathogens (44). Overall, the advantages of memorization, homing, and the immune reconstitution capacity indicate that Tcms might be more persistent and effective than CIK cells in HCC patients. Therefore, our study aimed to explore the efficacy and safety of Tcm transfusion combined with TACE in postoperative HCC patients with MVI, which has not been reported in previous studies.
Materials and Methods
Additional information is provided in Supplementary File S1 (Trial registration number NCT03575806).
Patient Selection
A total of 52 HCC patients who underwent curative hepatectomy at the Department of Hepatobiliary Surgery, Cancer Hospital of Chinese Academy of Medical Sciences between October 2016 and September 2018 were enrolled in this study. All subjects met the following criteria: (1) pathological diagnosis of primary HCC with microvascular invasion; (2) R0 tumor resection; (3) Child-Pugh grade A; (4) Eastern Cooperative Oncology Group (ECOG) performance status score of 0; (5) adequate liver, kidney, and marrow functions based on routine blood tests; (6) qualified radiography data within 4 weeks (28 ± 7 days) after enrollment; and (7) no evidence of recurrent HCC or PVTT. The present study was carried out in accordance with the Declaration of Helsinki revised in 1983. The prospective study was approved by the Committee on Medical Ethics of the Cancer Hospital of Chinese Academy of Medical Sciences.
This study did not employ a randomized design, and patients were recruited according to their willingness to receive Tcm transfusion as adjuvant therapy after being screened and meeting the eligibility criteria. Patients who consented to participate in the trial and underwent Tcm infusion combined with TACE were assigned to the Tcm group. Patients who refused Tcm infusion were assigned to the control group and received only TACE. The patients in both groups underwent anatomical hepatectomy or nonanatomical hepatectomy, as decided by the principal investigator. The tumor size and resection margin were measured before specimen fixation.
Both MVI and histological differentiation were examined by microscopy. We evaluated the degree of MVI according to the following three risk factors based on all sections in each case: the number of invaded vessels (≤5 and >5); the number of invading carcinoma cells (≤50 and >50); and the distance of invasion from the tumor edge (≤1 and >1 cm). Cases with no risk factors were classified as M0; those with one risk factor were classified as M1; and those with two or three risk factors as M2 (45).
Therapeutic Regimens
TACE
At 1 month after curative hepatectomy, after examination by radiography (enhanced computed tomography (CT)/magnetic resonance imaging (MRI)) and determination of liver function recovery, a hepatic arterial catheter was placed into the proper hepatic artery through the femoral artery using the Seldinger technique. A mixed emulsion of fluorouracil (1.0 g), adriamycin (40 mg), cisplatin (50 mg), and lipiodol (10–20 ml) was infused into the remnant liver via the catheter.
Tcm Therapy
Two or 3 days before TACE, 80–100 ml of the patient’s venous blood was collected for monocyte extraction by Ficoll-Paque™ PLUS (GE Healthcare, Chicago, IL, USA) according to manufacturer’s protocol. The collected cells were seeded at an initial density of 1–1.5 × 106 cells/ml into a new 75-cm2 culture flask, which had been pretreated with anti-CD3 (3 μg/ml, Acro Biosystems, Newark, DE, USA) and anti-CD28 (1 μg/ml, Acro Biosystems) with GT-T551 H3 serum-free medium (TAKARA, Kusatsu, Japan) containing IL-2 (200 IU/ml, Jiangsu Kingsley Pharm, Wuxi, China), IL-7 (5 ng/ml, Novus, St. Louis, MO, USA) and IL-15 (2.5 ng/ml, Novus) at 37℃, 5% CO2 in the GMP laboratory. On day 4, the cells were transferred from cell culture flask to cell culture bag and fresh medium was added to the culture on day 4/7/10. Tcm cells were harvested on day 14 and confirmed negative for bacteria, fungi, and mycoplasma; the viability, quantity, and purity of Tcms (CD45RO+CD62L+CD3+/CD45+) were measured by qualified methods. In general, 2–5 × 109 cells could be harvested after culture for 14 ± 1 days, and the proportion of Tcms were more than 80% for most patients. The characteristics of Tcms were analyzed in a preclinical study (Figure 1), as described in the Supplementary Methods; the surface marker expression profiles of cultured Tcms from two selected patients are shown (Supplementary Figure S1). For intravenous administration, Tcms harvested on day 14 were resuspended in 100 ml of cool saline with 1% human serum albumin. Blood collection and Tcm transfusion were performed again in the next month, and the procedures were the same as those for the first transfusion. Acute adverse events, such as fever and rash that occurred during cell transfusion were recorded.
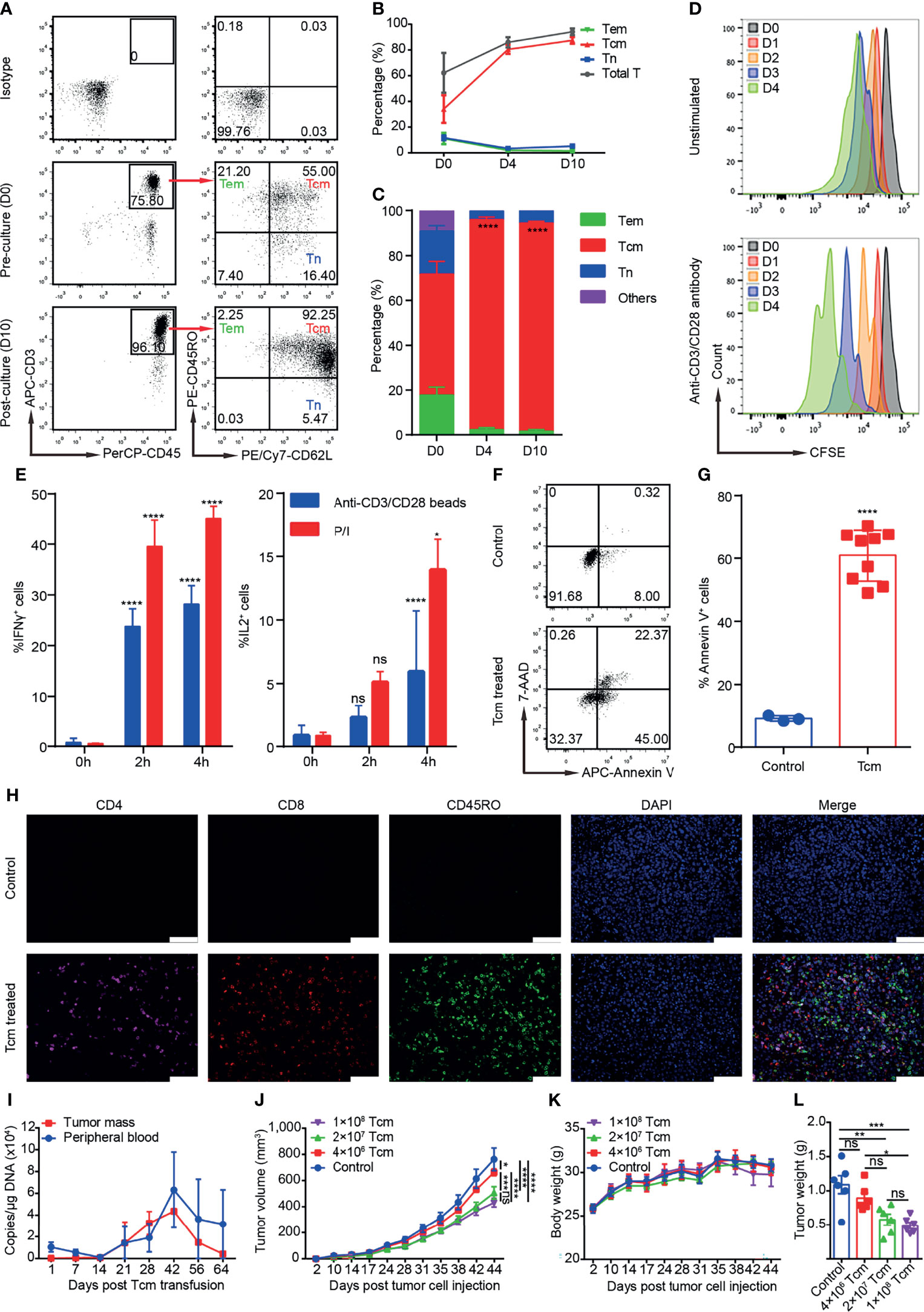
Figure 1 Phenotypes, cytotoxicity, and safety profiles of Tcms in the preclinical study, which was performed as described in the Supplementary Methods. (A) Representative flow plots of Tcm (central memory T cell), Tem (effector memory T cell), and Tn (naïve T cell) surface marker expression in cultured monocytes on D0 and D10. (B) Percentage of the indicated T-cell subsets among monocytes cultured from D0 to D10. Data are shown as the mean ± SD (n = 3). (C) Percentage of Tcms, Tems, and Tns in the CD3+ T-cell population among monocytes cultured from D0 to D10. Data are shown as the mean ± SD (n = 3). (D) Proliferation rate of cultured Tcms on D14 stimulated with anti-CD3/CD28 antibody, shown as carboxyfluorescein succinimidyl ester (CFSE) staining and cultured for over 4 consecutive days; unstimulated Tcms are shown as a control. (E) Cytokine secretion of cultured Tcms on D14 shown as the percentage of IFNγ+ and IL2+ cells measured at 0, 2, and 4 h after stimulation with phorbol myristate acetate/ionomycin (P/I) or anti-CD3/CD28 beads. Data are shown as the mean ± SD (n = 3). (F, G) Cytotoxicity (in vitro killing) of cultured Tcms on D14 against human hepatocellular carcinoma cell line QGY-7703, as measured by targeted cell stained with 7-AAD and Annexin V, detected by flow cytometry. Tcms and QGY-7703 cells were coincubated for 6 h at an effector:target ratio of 1:5. Representative flow plots and the percentage of Annexin V+ cells in target cells are shown in (F, G), respectively. (H) Representative immunohistochemistry-paraffin (IHC-P) images of tumor tissue slices immunostained with antibodies against CD4, CD8, and CD45RO and DAPI. Mice were inoculated subcutaneously with QGY-7703 tumor cells on D0 and then treated with Tcms or PBS (control) by tail vein injection, and on D42, the subcutaneous tumor was stripped for IHC-P. (I) Tcm persistence (shown as copies/μg DNA) in the tumor mass and peripheral blood of NPG mouse recipients measured by qPCR at the indicated time after Tcm transfusion by tail vein injection. Data are shown as the mean ± SD (n = 6 mice). (J–L) Different doses of Tcms were transfused into QGY-7703 tumor-bearing mice, and the (J) tumor volume and (K) body weight were measured on the indicated days. (L) The tumor weight of each mouse was measured on D42 posttumor cell injection. Animals in the control group received only PBS. Data are shown as the mean ± SEM (n = 6 mice). ns, not significant; *p < 0.5; **p < 0.01; ***p < 0.001; ****p < 0.0001.
Follow-Up Evaluation and Therapeutic Efficacy Analysis
All enrolled patients were evaluated for recurrence in the first 1 month after hepatectomy by enhanced CT or MRI. The patients were then reevaluated every 3 months by enhanced CT or MRI and determination of the serum alpha-fetoprotein (AFP) level until death or withdrawal from the follow-up program. The recurrence rates reported are based on radiography results.
RFS was defined as the interval (in months) between hepatectomy and the diagnosis of recurrence using either intrahepatic recurrence or extrahepatic metastasis as the primary end point. OS was defined as the interval (in months) from the date of hepatectomy to the date of death. This study was terminated on October 1, 2019, and the last follow-up was considered the end of the study.
Analysis of Adverse Events and Quality of Life
During Tcm treatment, the patients were followed up every 4 weeks with routine blood tests and liver and kidney function tests for safety monitoring until 4 weeks after the completion of both Tcm transfusion regimens. Thereafter, the patients underwent further routine blood tests and liver and kidney function tests every 12 weeks for 48 weeks or until the last follow-up before October 1, 2019. At every visit, the patients were also requested to complete the Functional Assessment of Cancer Therapy-Hepatobiliary (FACT-Hep) table to evaluate quality-of-life (QOL) (46).
Adverse events resulting from the administration of more than one Tcm transfusion were evaluated using Common Terminology Criteria Adverse Events, version 4.0 (CTCAE v4.0).
Statistical Analysis
The SPSS 22.0 statistical software package (IBM, Armonk, NY, USA) was used for data analyses. Data are expressed as the mean and range. Quantitative data and percent data were compared using t-tests and Chi-square tests, respectively. Discrete variables were compared with Chi-square tests. RFS time is presented by Kaplan-Meier survival curves, and survival analysis was performed using the Gehan-Breslow-Wilcoxon test. Univariate and multivariate Cox proportional hazard models were applied for prognostic risk factor analysis. Seventeen factors associated with RFS after hepatectomy were identified by univariate analysis, and significant factors (p < 0.15) were evaluated by multivariate analysis to identify valuable independent factors for predicting RFS. For all tests, p < 0.05 was considered statistically significant.
Results
Patient Characteristics
From October 2016 to September 2019, we recruited 52 HCC patients with MVI who underwent curative hepatectomy for this study. Patients were excluded from the final analysis if they had metastatic HCC (one patient in the Tcm group), voluntarily withdrew (two patients in the Tcm group), or were ineligible for curative hepatectomy with HCC recurrence within 1 month after hepatectomy (one patient in the Tcm group). Ultimately, 48 patients were enrolled, including 23 in the Tcm group and 25 in the control group, according to their willingness.
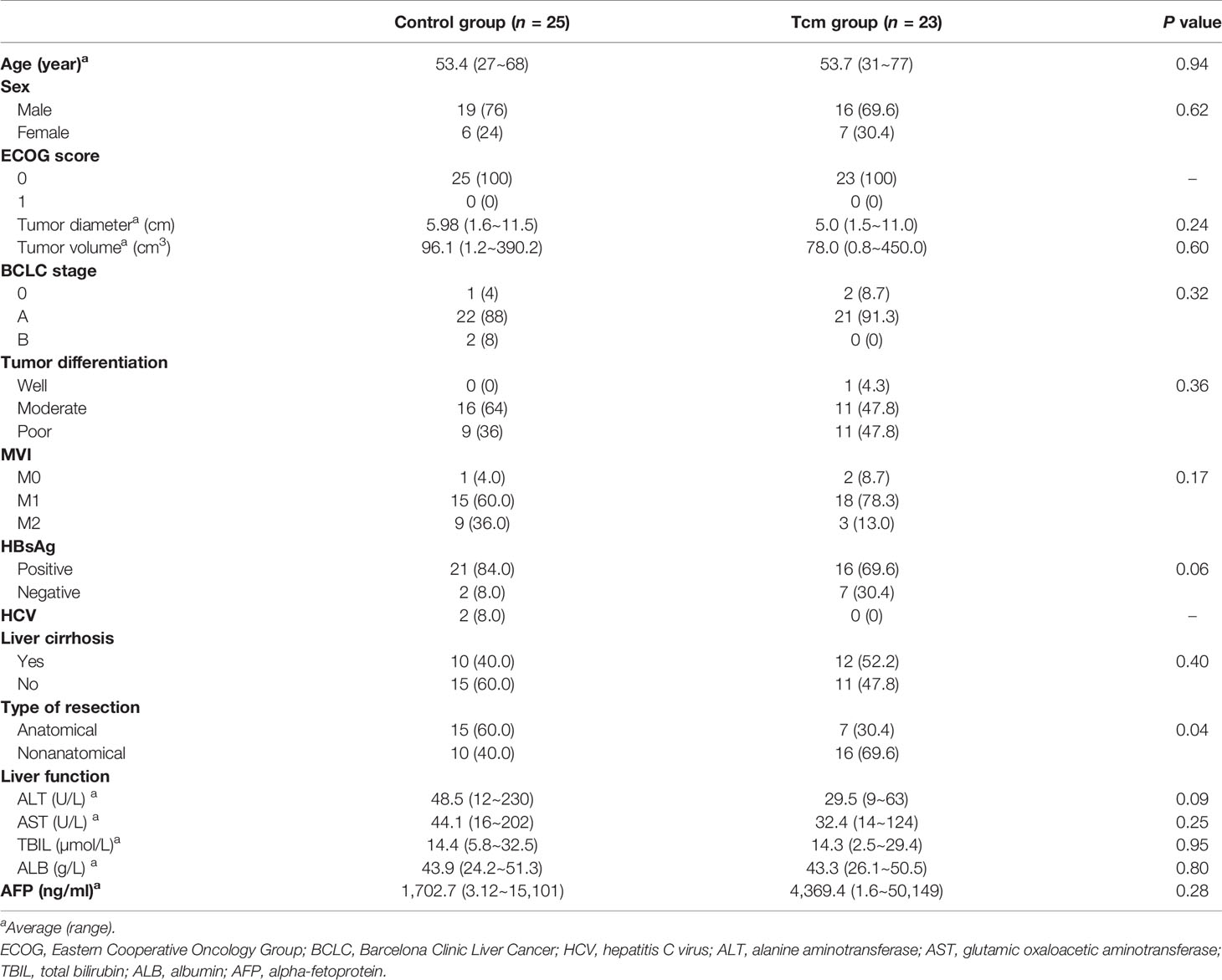
The baseline characteristics of all HCC patients with MVI are presented in Table 1. Overall, the median age was 53.5 years (range: 27–77 years). Of the 48 HCC patients, 35 (72.9%) were male and 13 (27.1%) female. All patients had an ECOG score of 0 points before hepatectomy. In addition, 37 (68.6%) and 22 (25.0%) patients were positive for hepatitis B surface antigen (HbsAg) and had liver cirrhosis. A total of 22 patients (45.8%) underwent anatomical liver resection, and the average tumor size was 5.51 cm (range: 1.5–11.5 cm). Twenty (41.7%) and 27 (56.3%) patients were diagnosed with poorly and moderately differentiated HCC, respectively, and 33 (65.1%) and 12 (27.9%) were diagnosed with M1 and M2, respectively, according to MVI classification criteria.
Comparison of RFS According to Tcm Treatment
The median follow-up time was 20.5 months (95% CI: 17.05–22.55 months) in all patients, 21.7 months (95% CI: 16.17–24.16 months) in the Tcm group and 18.43 months (95% CI: 15.40–23.54 months) in the control group. No in-hospital death occurred.
Kaplan-Meier curves and the Gehan-Breslow-Wilcoxon test were used to analyze RFS in the two groups, which was higher in the Tcm group than in the control group (72.0% vs. 46.4% for 1 year; 58.18% vs. 39.14% for 2 years) (Figure 2). In addition, the median RFS time was 9.5 months in the control group, whereas the cutoff date was not reached in the Tcm group because both 12- and 24-month RFS rates were still greater than 50% (Figure 2). The Gehan-Breslow-Wilcoxon test revealed a significantly better RFS in the Tcm group when the follow-up duration was 12 months (p = 0.049; HR = 0.40; 95% CI: 0.16–0.99), but the difference was not statistically significant at 24 months (p = 0.06; HR = 0.49; 95% CI: 0.21–1.12). Overall, Tcm combined with TACE obviously extended the RFS time in the early period after hepatectomy, with protective efficacy possibly lasting for 12 months. However, because the AFP level gradually decreased after hepatectomy (returning to normal in almost all patients within 2 months after hepatectomy) and the AFP level increased upon relapse, the change in the latter was not significant between the twbo groups (Supplementary Figure S2).
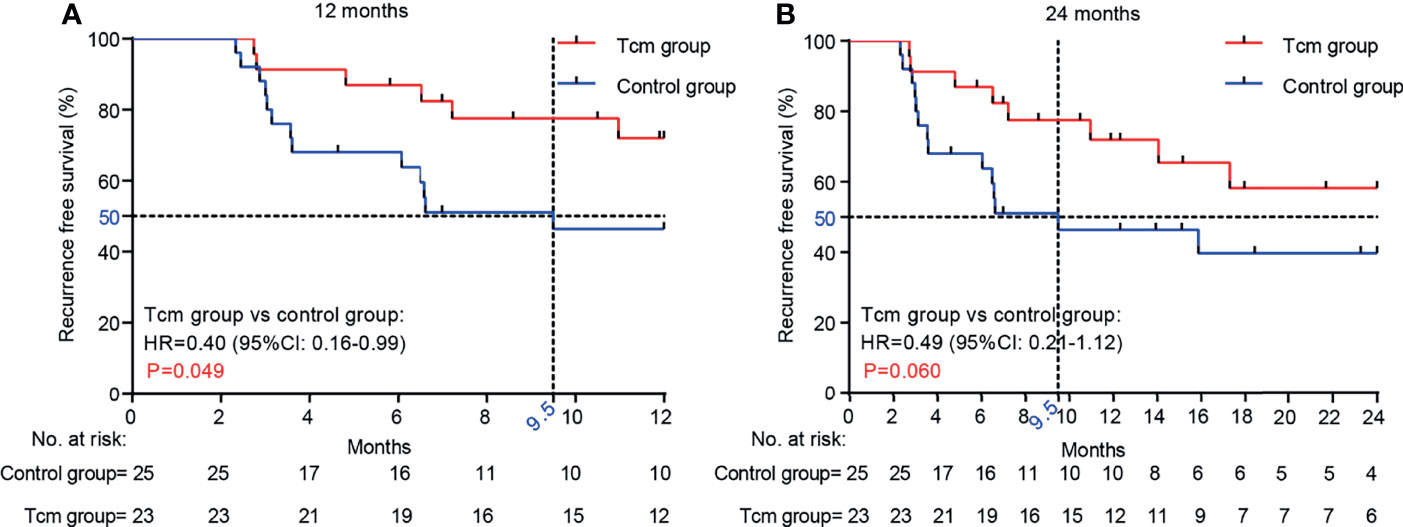
Figure 2 Comparison of RFS between the Tcm group and the control group at (A) 12 months (p = 0.049) and (B) 24 months (p = 0.060). p-values were calculated with the Gehan-Breslow-Wilcoxon test.
Impact of Tcm Therapy on the RFS of Postoperative HCC Patients
In the entire cohort, tumor recurrence developed in 22 (45.8%) patients, including eight (34.8%) in the Tcm group and 14 (56.0%) in the control group. The significant predictors (p < 0.15) obtained by univariate analysis, such as Tcm therapy, tumor diameter, and tumor volume, were entered into the multivariate logistic regression model to identify valuable independent predictors for RFS. Nonetheless, no significant independent predictors (p < 0.05) for RFS in postoperative HCC patients were found; the p-value of Tcm therapy was 0.107 (HR = 2.312, 95% CI: 0.835–6.400). A reasonable explanation is the small sample size and the small difference in tumor diameter and tumor volume at baseline between the two groups. The results of univariate and multivariate analyses for RFS are shown in Table 2.
Patient-Reported Outcome (FACT-Hep) Analyses
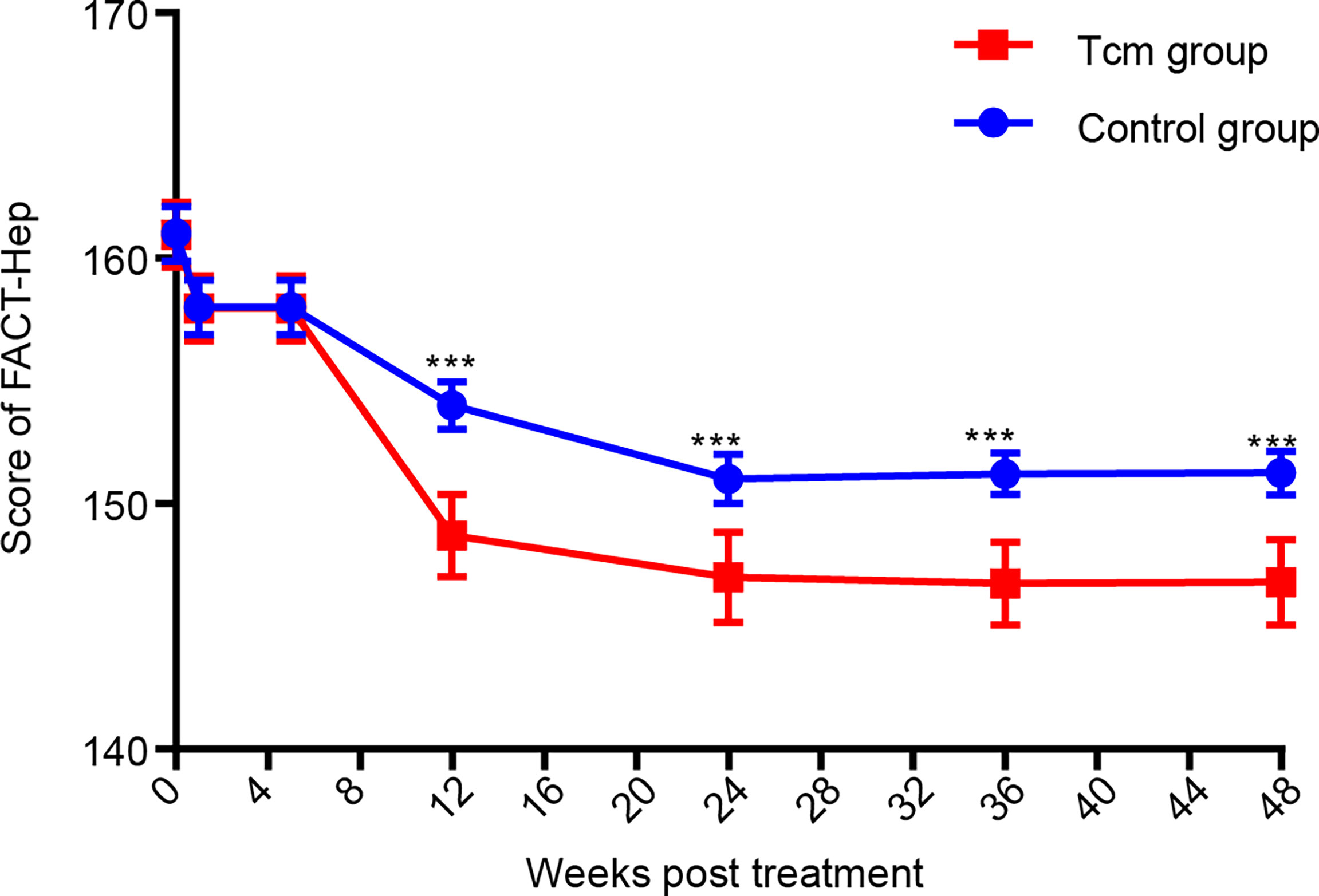
Functional Assessment of Cancer Therapy-General (FACT-G) contains 27 items for assessing four main domains: physical wellbeing, social and family well-being (range = 0–28), emotional well-being (range = 0–24), and functional well-being (range = 0–28). The scores for the FACT-G and the hepatobiliary cancer subscale (HCS), including 18 items (range = 0–72) for assessing specific concerns and issues in patients with HCC, were summed to obtain the FACT-Hep total score, which ranged from 0 to 180. High scores for all FACT-Hep dimensions are interpreted as high QOL (47). After collecting all patient FACT-Hep scores during the 48-week follow-up program, the data showed that the QOL of patients in the Tcm group was obviously better than that of those in the control group (Figure 3). In detail, the subscores of the FACT-G and HCS at the time of the 2nd Tcm transfusion and at 4 weeks after completing the 2nd Tcm transfusion demonstrated that Tcm transfusion mainly reduced the median HCS score (61.0 vs. 65.0 and 54.1 vs. 63.1, see Supplementary Table S1). It was concluded that the antitumor efficacy of Tcm treatment can relieve symptoms due to HCC as well as liver function injury.
Analysis of Adverse Events After Tcm Therapy
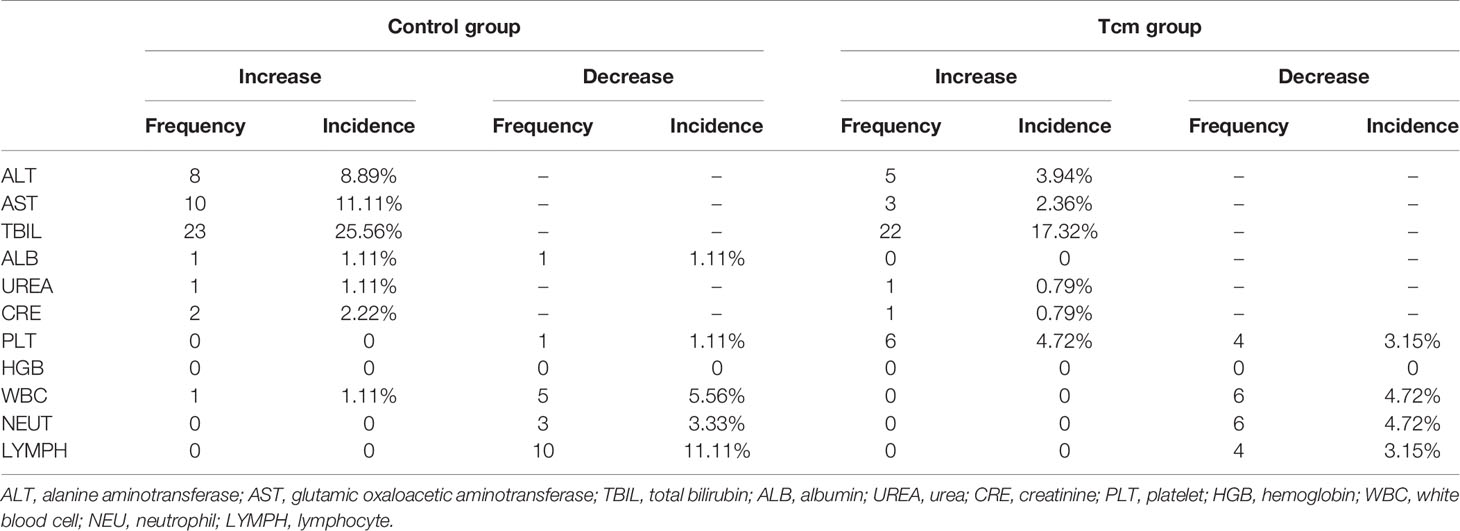
Twenty-six patients administered more than one Tcm transfusion and 25 patients in the control group were included in safety analysis, and the main clinical symptom after Tcm treatment was transient fever (incidence rate: 3.08%). The results of blood tests and liver and kidney function tests for all patients were collected every 4 weeks during Tcm treatment and every 12 weeks after Tcm treatment for 48 weeks or the last follow-up before October 1, 2019. The most common adverse reaction was an increase in bilirubin, occurring in 17.32% of patients in the Tcm group and in 25.56% of patients in the control group, indicating that bilirubin elevation might be caused by hepatectomy and not Tcm treatment. In addition, no grade 3 or 4 adverse events or deaths occurred in either group. After statistical calculation of other test indices, mean aminotransferase, albumin, urea, creatinine, platelet, hemoglobin, white blood cell lymphocyte, and neutrophil results were all within normal limits (Figure 4), and nearly no difference in the incidence of abnormal results was found between the groups (Table 3). Nonetheless, we did find that only 3.15% of patients in the Tcm group but that 11.11% of patients in the control group experienced a decrease in the number of lymphocytes, indicating that Tcm transfusion efficiently relieved lymphocyte deficiency in postoperative HCC patients.
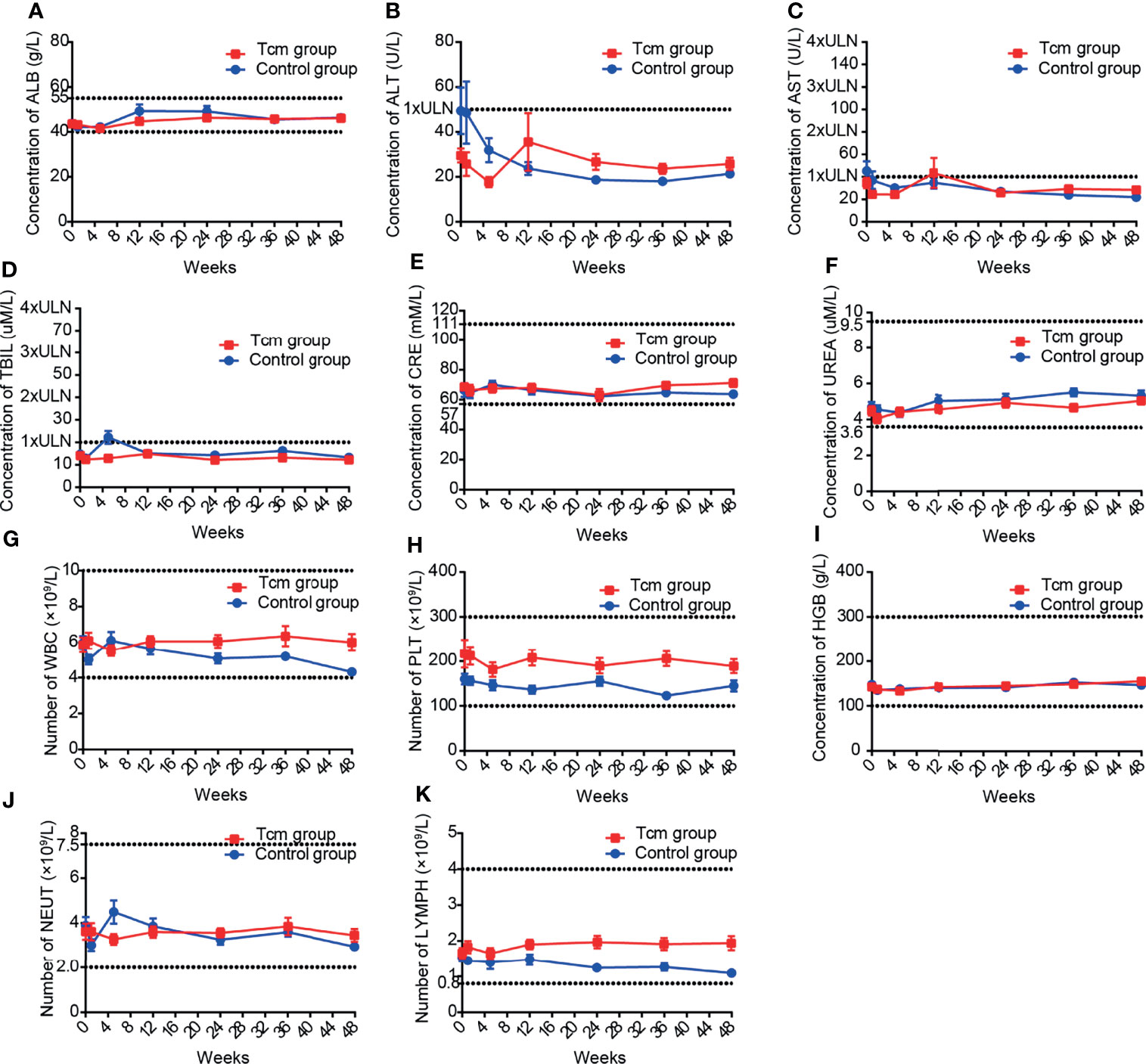
Figure 4 Mean laboratory results of (A–F) liver and kidney function tests and (G–K) blood tests in the Tcm group and control group during the follow-up period. The upper and lower dotted lines in each graph are the upper and lower limits of normal, respectively; for graph with only one dotted line, the lower limits of normal is 0. ( ALB, albumin; ALT, alanine aminotransferase; AST, glutamic oxaloacetic aminotransferase; TBIL, total bilirubin; CRE, creatinine; UREA, urea; WBC, white blood cell; PLT, platelet; HGB, hemoglobin; NEU, neutrophil; LYMPH, lymphocyte; ULN, upper limit of normal).
Discussion
MVI is an important independent predictor of recurrent HCC after hepatectomy (15, 16), and pa-TACE is recommended for adjuvant treatment to prevent recurrence in HCC patients with MVI after hepatectomy (23). Regardless, the limited and controversial clinical benefits of pa-TACE necessitate improvement of this therapy, and combined therapy is an approach worth considering.
The pathogenesis of MVI is closely related to the immunosuppressive tumor microenvironment, in which tumor-infiltrating lymphocytes display characteristics of exhaustion. Adoptive cell therapies, including autologous transfer of lymphokine-activated killer (LAK) cells (48), tumor-infiltrating lymphocytes (49) and CIK cells (32), have exhibited antitumor effects in preventing the postoperative recurrence of HCC. However, their clinical effects are limited due to short-term in vivo persistence, reliance on multiple cytokine boosts and a lack of focused analysis involving HCC patients with MVI. For example, at least four CIK transfusions are required to prevent short-term recurrence in postoperative HCC patients (32, 35). In contrast, the potent ability of Tcms for rapid activation, self-renewal, lymphatic homing and immunological reconstruction confers them with the capacity for long-term retention in vivo with strong antitumor activity (40). Indeed, our study was the first to find that Tcm treatment combined with TACE significantly prolonged the median RFS time compared with TACE treatment alone (>24 vs. 9.5 months) and largely improved patient-reported outcomes. In addition, Tcm treatment did not cause obvious damage in terms of liver or kidney function, blood indices, or systemic response markers, even in HCC patients with reduced liver function. Overall, the liver function of patients who incurred previous damage recovered almost to normal by 2–3 weeks after completing Tcm treatment, which indicates the safety of this treatment. The failure of this study to identify Tcm treatment as an independent prognostic factor associated with HCC recurrence might be due to the small sample size, nonrandomized design, short follow-up period, and/or single-centered nature, and further investigations are needed to demonstrate efficacy.
In conclusion, this pilot study for the first time expands the indications for Tcm treatment combined with TACE as an adjuvant therapy in postoperative HCC patients with MVI. The clinical outcome is encouraging but still speculative for limited number of patients and short follow-up period. Additional phase II studies should be performed to evaluate the efficacy of this treatment on more HCC patients. Longer follow-up period (4–5 years) may be warranted to explore whether this treatment could improve OS of postoperative HCC patients with MVI. Also, examination of cytokine profile (IL-6, IFN-γ, IL-10, TGF-β) and HCC-associated antigen-reactive lymphocyte populations could be performed to obtain more information to optimize the treatment formula. In all, further clinical trials are needed to test the efficacy of Tcm treatment and to identify the most suitable patient population.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Committee on Medical Ethics of the Cancer Hospital of Chinese Academy of Medical Sciences. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
HZ, JC, and JM contributed to the study design. HZ, JC, and JZ contributed to patient enrollment and study management. HZ, JM, DL and HX contributed to data analysis. JM, HX, DL and HZ wrote the manuscript. All authors contributed to data interpretation and planning and critical review of the manuscript content. All authors were responsible for the final decision to submit.
Funding
This study was sponsored by Newish Technology (Beijing) Co. Ltd, S&T program of Hebei (program No. 20372403D), National Science and Technology Major Projects for Major New Drugs Innovation and Develop (grant No. 2018ZX09711003-004-002), the National Natural Science Foundation of China (81972311), the Non-profit Central Research Institution Fund of Chinese Academy of Medical Sciences (2019PT310026), and Sanming Project of Medicine in Shenzhen (No. SZSM202011010). Tcm treatment was sponsored by Newish Technology (Beijing) Co. Ltd.
Conflict of Interest
DL and HQ are current employees of Newish Technology (Beijing) Co., LTD.
The authors declare that this study received funding from Newish Technology Co. LTD. The funder had the following involvement in the study: DL and HQ were involved in the preclinical study design, collection, analysis, interpretation of the preclinical data and the revision of the manuscript. Tcm cells were manufactured in the GMP laboratory of Newish Technology.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.781029/full#supplementary-material
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: Cancer J Clin (2018) 68:394–424. doi: 10.3322/caac.21492
2. Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular Carcinoma. Nat Rev Dis Primers (2021) 7:6. doi: 10.1038/s41572-020-00240-3
3. Lopez PM, Villanueva A, Llovet JM. Systematic Review: Evidence-Based Management of Hepatocellular Carcinoma–an Updated Analysis of Randomized Controlled Trials. Aliment Pharmacol Ther (2006) 23:1535–47. doi: 10.1111/j.1365-2036.2006.02932.x
4. Bruix J, Sherman M. Management of Hepatocellular Carcinoma: An Update. Hepatol (Baltimore Md) (2011) 53:1020–2. doi: 10.1002/hep.24199
5. Colecchia A, Schiumerini R, Cucchetti A, Cescon M, Taddia M, Marasco G, et al. Prognostic Factors for Hepatocellular Carcinoma Recurrence. World J Gastroenterol (2014) 20:5935–50. doi: 10.3748/wjg.v20.i20.5935
6. Chun JM, Kwon HJ, Sohn J, Kim SG, Park JY, Bae HI, et al. Prognostic Factors After Early Recurrence in Patients Who Underwent Curative Resection for Hepatocellular Carcinoma. J Surg Oncol (2011) 103:148–51. doi: 10.1002/jso.21786
7. Cucchetti A, Piscaglia F, Caturelli E, Benvegnu L, Vivarelli M, Ercolani G, et al. Comparison of Recurrence of Hepatocellular Carcinoma After Resection in Patients With Cirrhosis to its Occurrence in a Surveilled Cirrhotic Population. Ann Surg Oncol (2009) 16:413–22. doi: 10.1245/s10434-008-0232-4
8. Llovet JM, Schwartz M, Mazzaferro V. Resection and Liver Transplantation for Hepatocellular Carcinoma. Semin Liver Dis (2005) 25:181–200. doi: 10.1055/s-2005-871198
9. Lim KC, Chow PK, Allen JC, Chia GS, Lim M, Cheow PC, et al. Microvascular Invasion is a Better Predictor of Tumor Recurrence and Overall Survival Following Surgical Resection for Hepatocellular Carcinoma Compared to the Milan Criteria. Ann Surg (2011) 254:108–13. doi: 10.1097/SLA.0b013e31821ad884
10. Fan ST, Poon RT, Yeung C, Lam CM, Lo CM, Yuen WK, et al. Outcome After Partial Hepatectomy for Hepatocellular Cancer Within the Milan Criteria. Br J Surg (2011) 98:1292–300. doi: 10.1002/bjs.7583
11. Shindoh J, Hasegawa K, Inoue Y, Ishizawa T, Nagata R, Aoki T, et al. Risk Factors of Post-Operative Recurrence and Adequate Surgical Approach to Improve Long-Term Outcomes of Hepatocellular Carcinoma. HPB Off J Int Hepato Pancreato Biliary Assoc (2013) 15:31–9. doi: 10.1111/j.1477-2574.2012.00552.x
12. Toyosaka A, Okamoto E, Mitsunobu M, Oriyama T, Nakao N, Miura K. Pathologic and Radiographic Studies of Intrahepatic Metastasis in Hepatocellular Carcinoma; the Role of Efferent Vessels. HPB Surg (1996) 10:97–103. doi: 10.1155/1996/75210
13. Rodriguez-Peralvarez M, Luong TV, Andreana L, Meyer T, Dhillon AP, Burroughs AK. A Systematic Review of Microvascular Invasion in Hepatocellular Carcinoma: Diagnostic and Prognostic Variability. Ann Surg Oncol (2013) 20:325–39. doi: 10.1245/s10434-012-2513-1
14. Lee YH, Hsu CY, Huang YH, Hsia CY, Chiou YY, Su CW, et al. Vascular Invasion in Hepatocellular Carcinoma: Prevalence, Determinants and Prognostic Impact. J Clin Gastroenterol (2014) 48:734–41. doi: 10.1097/MCG.0b013e3182a8a254
15. Lauwers GY, Terris B, Balis UJ, Batts KP, Regimbeau JM, Chang Y, et al. Prognostic Histologic Indicators of Curatively Resected Hepatocellular Carcinomas: A Multi-Institutional Analysis of 425 Patients With Definition of a Histologic Prognostic Index. Am J Surg Pathol (2002) 26:25–34. doi: 10.1097/00000478-200201000-00003
16. Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, et al. Predicting Survival After Liver Transplantation in Patients With Hepatocellular Carcinoma Beyond the Milan Criteria: A Retrospective, Exploratory Analysis. Lancet Oncol (2009) 10:35–43. doi: 10.1016/s1470-2045(08)70284-5
17. Roayaie S, Blume IN, Thung SN, Guido M, Fiel MI, Hiotis S, et al. A System of Classifying Microvascular Invasion to Predict Outcome After Resection in Patients With Hepatocellular Carcinoma. Gastroenterology (2009) 137:850–5. doi: 10.1053/j.gastro.2009.06.003
18. Zhang X, Li J, Shen F, Lau WY. Significance of Presence of Microvascular Invasion in Specimens Obtained After Surgical Treatment of Hepatocellular Carcinoma. J Gastroenterol Hepatol (2018) 33:347–54. doi: 10.1111/jgh.13843
19. Zhao H. Prognostic Value and Preoperative Predictors of Microvascular Invasion in Solitary Hepatocellular Carcinoma </= 5 Cm Without Macrovascular Invasion. Oncotarget (2017) 8:61203–14. doi: 10.18632/oncotarget.18049
20. Andreana L, Burroughs AK. Treatment of Early Hepatocellular Carcinoma: How to Predict and Prevent Recurrence. Dig Liver Dis Off J Ital Soc Gastroenterol Ital Assoc Study Liver (2010) 42(Suppl 3):S249–57. doi: 10.1016/s1590-8658(10)60513-0
21. Yang J, Liang H, Hu K, Xiong Z, Cao M, Zhong Z, et al. The Effects of Several Postoperative Adjuvant Therapies for Hepatocellular Carcinoma Patients With Microvascular Invasion After Curative Resection: A Systematic Review and Meta-Analysis. Cancer Cell Int (2021) 21:92. doi: 10.1186/s12935-021-01790-6
22. Llovet JM, Bruix J. Systematic Review of Randomized Trials for Unresectable Hepatocellular Carcinoma: Chemoembolization Improves Survival. Hepatol (Baltimore Md) (2003) 37:429–42. doi: 10.1053/jhep.2003.50047
23. Peng BG, He Q, Li JP, Zhou F. Adjuvant Transcatheter Arterial Chemoembolization Improves Efficacy of Hepatectomy for Patients With Hepatocellular Carcinoma and Portal Vein Tumor Thrombus. Am J Surg (2009) 198:313–8. doi: 10.1016/j.amjsurg.2008.09.026
24. Sun JJ, Wang K, Zhang CZ, Guo WX, Shi J, Cong WM, et al. Postoperative Adjuvant Transcatheter Arterial Chemoembolization After R0 Hepatectomy Improves Outcomes of Patients Who Have Hepatocellular Carcinoma With Microvascular Invasion. Ann Surg Oncol (2016) 23:1344–51. doi: 10.1245/s10434-015-5008-z
25. Wang YY, Wang LJ, Xu D, Liu M, Wang HW, Wang K, et al. Postoperative Adjuvant Transcatheter Arterial Chemoembolization Should be Considered Selectively in Patients Who Have Hepatocellular Carcinoma With Microvascular Invasion. HPB Off J Int Hepato Pancreato Biliary Assoc (2019) 21:425–33. doi: 10.1016/j.hpb.2018.08.001
26. Liang L, Li C, Diao YK, Jia HD, Xing H, Pawlik TM, et al. Survival Benefits From Adjuvant Transcatheter Arterial Chemoembolization in Patients Undergoing Liver Resection for Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Ther Adv Gastroenterol (2020) 13:1756284820977693. doi: 10.1177/1756284820977693
27. Wang L, Chen B, Li Z, Yao X, Liu M, Rong W, et al. Optimal Postoperative Adjuvant Treatment Strategy for HBV-Related Hepatocellular Carcinoma With Microvascular Invasion: A Propensity Score Analysis. OncoTargets Ther (2019) 12:1237–47. doi: 10.2147/ott.s179247
28. Wang L, Wang W, Yao X, Rong W, Wu F, Chen B, et al. Postoperative Adjuvant Radiotherapy is Associated With Improved Survival in Hepatocellular Carcinoma With Microvascular Invasion. Oncotarget (2017) 8:79971–81. doi: 10.18632/oncotarget.20402
29. Thanendrarajan S, Nowak M, Abken H, Schmidt-Wolf IG. Combining Cytokine-Induced Killer Cells With Vaccination in Cancer Immunotherapy: More Than One Plus One? Leuk Res (2011) 35:1136–42. doi: 10.1016/j.leukres.2011.05.005
30. Sangro B, Sarobe P, Hervas-Stubbs S, Melero I. Advances in Immunotherapy for Hepatocellular Carcinoma. Nat Rev Gastroenterol Hepatol (2021) 18:525–43. doi: 10.1038/s41575-021-00438-0
31. Li X, Dai D, Song X, Liu J, Zhu L, Xu W. A Meta-Analysis of Cytokine-Induced Killer Cells Therapy in Combination With Minimally Invasive Treatment for Hepatocellular Carcinoma. Clinics Res Hepatol Gastroenterol (2014) 38:583–91. doi: 10.1016/j.clinre.2014.04.010
32. Lee JH, Lee JH, Lim YS, Yeon JE, Song TJ, Yu SJ, et al. Adjuvant Immunotherapy With Autologous Cytokine-Induced Killer Cells for Hepatocellular Carcinoma. Gastroenterology (2015) 148:1383–1391.e1386. doi: 10.1053/j.gastro.2015.02.055
33. Zhong JH, Deng L, Tan JT, Li LQ. Adjuvant Immunotherapy for Postoperative Hepatocellular Carcinoma. Gastroenterology (2015) 149:1639–40. doi: 10.1053/j.gastro.2015.06.056
34. Lee JH, Lee JH, Lim YS, Yeon JE, Song TJ, Yu SJ, et al. Sustained Efficacy of Adjuvant Immunotherapy With Cytokine-Induced Killer Cells for Hepatocellular Carcinoma: An Extended 5-Year Follow-Up. Cancer Immunol Immunother CII (2019) 68:23–32. doi: 10.1007/s00262-018-2247-4
35. Xu L, Wang J, Kim Y, Shuang ZY, Zhang YJ, Lao XM, et al. A Randomized Controlled Trial on Patients With or Without Adjuvant Autologous Cytokine-Induced Killer Cells After Curative Resection for Hepatocellular Carcinoma. Oncoimmunology (2016) 5:e1083671. doi: 10.1080/2162402x.2015.1083671
36. Hui D, Qiang L, Jian W, Ti Z, Da-Lu K. A Randomized, Controlled Trial of Postoperative Adjuvant Cytokine-Induced Killer Cells Immunotherapy After Radical Resection of Hepatocellular Carcinoma. Dig Liver Dis Off J Ital Soc Gastroenterol Ital Assoc Study Liver (2009) 41:36–41. doi: 10.1016/j.dld.2008.04.007
37. Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, et al. Intratumoral Balance of Regulatory and Cytotoxic T Cells is Associated With Prognosis of Hepatocellular Carcinoma After Resection. J Clin Oncol Off J Am Soc Clin Oncol (2007) 25:2586–93. doi: 10.1200/jco.2006.09.4565
38. Cariani E, Pilli M, Zerbini A, Rota C, Olivani A, Pelosi G, et al. Immunological and Molecular Correlates of Disease Recurrence After Liver Resection for Hepatocellular Carcinoma. PloS One (2012) 7:e32493. doi: 10.1371/journal.pone.0032493
39. Mueller SN, Gebhardt T, Carbone FR, Heath WR. Memory T Cell Subsets, Migration Patterns, and Tissue Residence. Annu Rev Immunol (2013) 31:137–61. doi: 10.1146/annurev-immunol-032712-095954
40. Liu Q, Sun Z, Chen L. Memory T Cells: Strategies for Optimizing Tumor Immunotherapy. Protein Cell (2020) 11:549–64. doi: 10.1007/s13238-020-00707-9
41. Busch DH, Fräßle SP, Sommermeyer D, Buchholz VR, Riddell SR. Role of Memory T Cell Subsets for Adoptive Immunotherapy. Semin Immunol (2016) 28:28–34. doi: 10.1016/j.smim.2016.02.001
42. Klebanoff CA, Gattinoni L, Torabi-Parizi P, Kerstann K, Cardones AR, Finkelstein SE, et al. Central Memory Self/Tumor-Reactive CD8+ T Cells Confer Superior Antitumor Immunity Compared With Effector Memory T Cells. Proc Natl Acad Sci USA (2005) 102:9571–6. doi: 10.1073/pnas.0503726102
43. Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, et al. A Human Memory T Cell Subset With Stem Cell-Like Properties. Nat Med (2011) 17:1290–7. doi: 10.1038/nm.2446
44. Graef P, Buchholz VR, Stemberger C, Flossdorf M, Henkel L, Schiemann M, et al. Serial Transfer of Single-Cell-Derived Immunocompetence Reveals Stemness of CD8(+) Central Memory T Cells. Immunity (2014) 41:116–26. doi: 10.1016/j.immuni.2014.05.018
45. Sumie S, Nakashima O, Okuda K, Kuromatsu R, Kawaguchi A, Nakano M, et al. The Significance of Classifying Microvascular Invasion in Patients With Hepatocellular Carcinoma. Ann Surg Oncol (2014) 21:1002–9. doi: 10.1245/s10434-013-3376-9
46. Heffernan N, Cella D, Webster K, Odom L, Martone M, Passik S, et al. Measuring Health-Related Quality of Life in Patients With Hepatobiliary Cancers: The Functional Assessment of Cancer Therapy-Hepatobiliary Questionnaire. J Clin Oncol Off J Am Soc Clin Oncol (2002) 20:2229–39. doi: 10.1200/jco.2002.07.093
47. Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, et al. The Functional Assessment of Cancer Therapy Scale: Development and Validation of the General Measure. J Clin Oncol Off J Am Soc Clin Oncol (1993) 11:570–9. doi: 10.1200/jco.1993.11.3.570
48. Rosenberg SA, Lotze MT, Muul LM, Leitman S, Chang AE, Ettinghausen SE, et al. Observations on the Systemic Administration of Autologous Lymphokine-Activated Killer Cells and Recombinant Interleukin-2 to Patients With Metastatic Cancer. N Engl J Med (1985) 313:1485–92. doi: 10.1056/nejm198512053132327
Keywords: hepatocellular carcinoma, microvascular invasion, adjuvant therapy, Tcm treatment, clinical study
Citation: Cai J, Zhao J, Liu D, Xie H, Qi H, Ma J, Sun Z and Zhao H (2021) Efficacy and Safety of Central Memory T Cells Combined With Adjuvant Therapy to Prevent Recurrence of Hepatocellular Carcinoma With Microvascular Invasion: A Pilot Study. Front. Oncol. 11:781029. doi: 10.3389/fonc.2021.781029
Received: 05 October 2021; Accepted: 08 November 2021;
Published: 03 December 2021.
Edited by:
Manel Juan, Hospital Clínic de Barcelona, SpainReviewed by:
Sanjay Rathod, University of Pittsburgh, United StatesMeiyu Peng, Weifang Medical University, China
Copyright © 2021 Cai, Zhao, Liu, Xie, Qi, Ma, Sun and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhongjie Sun, c3pqQG5ld2lzaGVzLmNvbQ==; Hong Zhao, cHVtY3poYW9ob25nQDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
 Jianqiang Cai1†
Jianqiang Cai1† Defang Liu
Defang Liu Junfan Ma
Junfan Ma Hong Zhao
Hong Zhao