- 1Department of Internal Medicine, Albert Schweitzer Hospital, Dordrecht, Netherlands
- 2Laboratory Medical Immunology, Department of Immunology, Erasmus MC, Rotterdam, Netherlands
- 3Department of Research and Development, Netherlands Comprehensive Cancer Organisation (IKNL), Utrecht, Netherlands
- 4Department of Hematology, Amsterdam University Medical Center, University of Amsterdam, Cancer Center Amsterdam, Lymphoma and Myeloma Center Amsterdam, Amsterdam, Netherlands
The clinical course of chronic lymphocytic leukemia (CLL) is highly variable. Over the past decades, several cytogenetic, immunogenetic and molecular features have emerged that identify patients suffering from CLL with high-risk molecular features. These biomarkers can clearly aid prognostication, but may also be capable of predicting the efficacy of various treatment strategies in subgroups of patients. In this narrative review, we discuss treatment approaches to CLL with high-risk molecular features. Specifically, we review and provide a comprehensive overview of clinical trials evaluating the efficacy of chemotherapy, chemoimmunotherapy and novel agent-based treatments in CLL patients with TP53 aberrations, deletion of the long arm of chromosome 11, complex karyotype, unmutated IGHV, B cell receptor stereotypy, and mutations in NOTCH1 or BIRC3. Furthermore, we discuss future pharmaceutical and immunotherapeutic perspectives for CLL with high-risk molecular features, focusing on agents currently under investigation in clinical trials.
Introduction
Chronic lymphocytic leukemia (CLL) is the most prevalent form of leukemia in the Western world with an age-standardized incidence rate of 4-5 cases per 100,000 persons per year (1–5). The clinical course of CLL is characterized by marked heterogeneity, with some patients surviving for more than 10 years without treatment, whereas others suffer rapid disease progression and poor outcome, in spite of the availability of effective treatment regimens. Historically, prognostication in CLL has relied on clinical staging: the Rai and Binet staging systems, developed approximately 40 years ago, are still frequently used in clinical practice (6, 7). However, as advanced molecular techniques such as fluorescence in situ hybridization (FISH), next-generation sequencing (NGS) and microarray-based genomic profiling have provided greater insight into the biology of CLL cells, the prognostication paradigm has shifted towards a perspective that not only relies on clinical features, but also incorporates high-risk genetic and molecular biomarkers. Indeed, the CLL international prognostic index (CLL-IPI) incorporates cytogenetic, immunogenetic and molecular features to predict the survival of CLL patients (8). In addition to prognostication, the presence of certain cytogenetic, immunogenetic and molecular features can predict differential responses to treatment. Several of the more newly discovered biomarkers are not routinely measured in clinical research and patient care, despite evidence of their predictive impact. Conversely, some routinely measured biomarkers that were historically associated with poor prognosis in the chemo(immuno)therapy era may have lost their predictive value in the context of novel agent-based therapies. Presently, the international workshop on CLL (iwCLL) guidelines recommend assessment of immunoglobulin heavy chain gene (IGHV) mutational status and TP53 aberrations in every CLL patient before the initiation of treatment (9). TP53 aberrations should be assessed by both targeted FISH and either Sanger sequencing or NGS. Whereas the IGHV mutational status is stable over time, additional (cyto)genetic abnormalities with therapeutic implications may be acquired over the course of the disease, necessitating reassessment at every subsequent line of therapy (9).
This narrative review discusses such therapeutic implications of CLL with high-risk molecular features. Specifically, we review the predictive impact, if any, of TP53 aberrations, deletion of the long arm of chromosome 11, complex karyotype or genomic complexity, unmutated IGHV, B cell receptor stereotypy, mutated NOTCH1 and mutated BIRC3. Furthermore, we discuss future perspectives for CLL with high-risk molecular features, focusing on upcoming agents in the therapeutic armamentarium of CLL.
TP53 Aberrations
Among the numerous prognostic and predictive biomarkers that have been identified over the previous decades, TP53 aberrations indisputably remain the single most impactful genetic lesion in CLL. The TP53 locus encodes the tumor-suppressor protein p53, which plays a key role in cell division, apoptosis and genomic stability. TP53 signaling can be impaired through deletion of the TP53 gene in the chromosomal locus 17p13.1, i.e. del(17p), or through genetic lesions, including missense and nonsense mutations, deletions, insertions or splice-site mutations. Cumulatively, these aberrations are present in 4-8% of all CLL patients at diagnosis, 10% at start of first-line therapy and 30-40% in relapsed or refractory (R/R) CLL patients who were previously treated with chemoimmunotherapy (10). In CLL patients, impaired TP53 signaling is associated with a poor response to chemotherapy and chemoimmunotherapy. Chemotherapeutic agents exert their cytotoxic effects by causing DNA damage. In TP53 wildtype cells, such irreparable damage results in apoptotic cell death. However, if TP53 is not expressed or not functional, chemotherapy-induced DNA damage does not lead to apoptosis and as consequence, administration of these drugs leads to accumulation of detrimental genomic mutations while failing to induce cell death, possibly worsening disease prognosis.
First-Line Setting
Indeed, after first-line treatment with fludarabine monotherapy (F) or fludarabine and cyclophosphamide (FC) in the CLL4 trial, conducted by the German CLL study group (GCLLSG), the overall survival (OS) of CLL patients was markedly shorter in patients harboring TP53 mutations, compared with those without (median OS, 23.3 versus 62.2 months) (11, 12). The comparable E2997 (US) and LRF CLL4 (UK) trials yielded similar results (13, 14). In the phase III GCLLSG CLL8 trial, first-line treatment with FC was compared with fludarabine, cyclophosphamide and rituximab (FCR) (15, 16). While patients with del(17p) had relatively short PFS in both arms, median PFS in the FCR-arm was superior (FCR, 11.2 months versus FC, 9.1 months; hazard ratio [HR], 0.49; 95% CI, 0.25-0.93; P=0.02). Although FCR was the first treatment regimen that prolonged OS in CLL patients, the OS of patients with del(17p) was not significantly different between both arms (HR 0.66; 95%CI 0.33-1.31), underscoring the poor response in the TP53 aberrant group. Indeed, multivariable analysis identified TP53 aberrations as the strongest predictor of inferior PFS (HR 2.92; 1.78-4.78) and OS (HR 2.72, 1.60-4.60). Similarly, in a large Italian retrospective cohort study, patients with del(17p) had worse prognosis after FCR (median PFS, 22.5 months, compared with 58.9 months for patients without del(17p), HR 3.72; 95%CI, 2.42-5.72) and a high probability (19%) of developing secondary malignancies (17).
Based on the evidence from the trials discussed above, chemoimmunotherapy is no longer considered an acceptable first-line treatment for patients with TP53 aberrations. Initially, the anti-CD52 monoclonal antibody (mAb) alemtuzumab, with or without methylprednisolone, was considered the only effective pharmaceutical treatment in patients with TP53 aberrations, despite limited efficacy (overall response rate [ORR], 82%, median PFS 11.8 months, median OS 23 months) and an unfavorable toxicity profile (18). In addition, an allogeneic hematopoietic stem cell transplantation (alloHSCT) was considered as a first-line consolidation treatment for patients with TP53 aberrations (19–21). The GCLLSG CLL3X trial demonstrated that the 6-year OS rate after alloHSCT was approximately 60%, irrespective of TP53 aberrations (19). Although potentially curative, alloHSCT is only available for a highly selected young and fit CLL patient population, given the high risk of transplant-related morbidity and mortality. Thus, historically, treatment of patient with TP53 aberrations has been challenging. Fortunately, alemtuzumab and alloHSCT have been replaced as treatment of choice for these patients by newer agents with greatly improved efficacy.
Advanced insight in the pathophysiology of CLL have led to the development of small molecule inhibitors that have revolutionized the treatment of patients with TP53 aberrations. Ibrutinib is an irreversible inhibitor of Bruton’s tyrosine kinase (Btk), a signaling molecule downstream of the B cell receptor (BCR) (22–24). In the phase III RESONATE-2 trial, the efficacy of ibrutinib was compared with chlorambucil monotherapy (Clb) in unfit, treatment-naïve CLL patients (25–27). Although CLL patients with del(17p) were excluded from this trial, twelve ibrutinib-treated patients had mutated TP53. Their outcome following ibrutinib treatment was comparable to patients without TP53 mutations (5-year PFS rate 56% versus 73%, HR 0.87; 95%CI, 0.26-2.85). Furthermore, in a phase II trial conducted by Farooqui et al., median PFS was not reached in TP53 mutated CLL patients treated with ibrutinib monotherapy after a median follow-up of 35 months (28, 29). In the phase III ALLIANCE trial, previously untreated unfit CLL patients were treated with bendamustine rituximab (BR), ibrutinib alone or ibrutinib plus rituximab (R-ibrutinib). PFS was superior in all patients in both ibrutinib-based arms, compared with the BR arm, but this difference was most pronounced in patients with del(17p) (median PFS not reached [NR] after 38 months for both ibrutinib and R-ibrutinib versus 7 months for BR) (30). There was no difference in PFS for patients with del(17p) between the ibrutinib and R-ibrutinib arms (HR 1.57; 95%CI, 0.80-3.09). In the first-line phase III iLLUMINATE CLL trial, obinutuzumab plus ibrutinib (O-ibrutinib) was compared with obinutuzumab plus chlorambucil (O-Clb). In CLL patients with TP53 aberrations, median PFS after O-ibrutinib was superior, compared with O-Clb (NR after 31 months versus 15.2 months, HR 0.14; 95%CI, 0.04-0.51) (31). Based on the evidence presented above, ibrutinib has thus become the gold standard for first-line therapy in patients with TP53 aberrations. Interestingly, Brieghel et al. demonstrated that in ibrutinib-treated patients, the co-occurrence of multiple TP53 aberrations (multi-hit) was associated with an inferior PFS and time-to-progression as compared with those with a single-hit (HR for PFS 14.1; 95%CI, 1.60-1849) (32). This observation has not yet been validated in larger cohorts, or in different treatment settings.
Importantly, ibrutinib monotherapy needs continuation until progression and is associated with adverse events such as fatigue, diarrhea, nausea, bleeding complications, cardiac arrhythmias and, rarely, sudden death (in 1% of treated patients) (33). In addition, in some population-based studies, adverse events were more severe resulting in a higher discontinuation rate, compared with those in clinical trials (34–36). The second-generation Btk-inhibitor acalabrutinib has a more selective binding profile and could therefore potentially overcome ibrutinib-associated toxicities. In the phase III ELEVATE-TN study, acalabrutinib and acalabrutinib plus obinutuzumab (O-acalabrutinib) were compared with O-Clb in unfit, treatment-naïve CLL patients (37). The estimated median 24-month PFS rate in patients with del(17p) was longer following treatment with O-acalabrutinib, compared with O-Clb (88%; 95%CI, 61–97% versus 22%; 95%CI, 5–45%). Similar results were obtained in patients with mutated TP53 (24-month PFS rate 95%; 95%CI 70-99% versus 19%; 95%CI 5-41%).
Another small molecule inhibitor is the Bcl2-inhibitor venetoclax. Although this compound mainly acts through induction of apoptosis, it does so in a p53-independent manner (38). In the phase III GCLLSG CLL14 study, time-limited venetoclax plus obinutuzumab (Ven-O) was compared with O-Clb in previously untreated, unfit CLL patients. In patients with TP53 aberrations, response and survival outcomes were better for Ven-O treated patients, compared with O-Clb treated patients (ORR 81% versus 36%, median PFS approximately 35 months versus 17 months, 24-month PFS rate approximately 96% versus 77%) (39–41). However, in the Ven-O arm, PFS for patients with TP53 aberrancies was still inferior, compared with patients with intact TP53 (HR 1.96; 95%CI, 0.92-4.17). Of note, none of the patients with TP53 aberrations had progressive disease while receiving a therapeutic dose of venetoclax (39).
R/R Setting
In the R/R setting, not surprisingly, chemoimmunotherapy yields disappointing results in patients with TP53 aberrations. Badoux et al. reported poor response (ORR, 35%) and survival (median PFS 5 months and median OS 10.5 months) in R/R CLL patients with TP53 aberrations after treatment with FCR (42). Consequently, R/R CLL patients with TP53 aberrations require treatment with novel agent-based regimens.
In the R/R setting, Zelenetz et al. evaluated the efficacy of idelalisib (IDELA), a small molecule inhibitor of phosphoinositide 3-kinase (PI3K) in combination with BR (BR-IDELA) to treatment with BR alone. In patients with aberrant TP53 signaling, ORR and PFS were superior after treatment with BR-IDELA, compared with BR alone (ORR: 58% vs 23%, median PFS 11.3 months versus 8.3 months, HR 0.47; 95%CI, 0.31-0.72) (43). However, when comparing patients with either del(17p) and/or TP53 to patients with neither, median PFS after BR-IDELA remained poorer in patients with TP53 aberrations (11.3 months versus 24.5 months) (43). Notably, in a R/R CLL cohort treated with rituximab and IDELA, followed by open-label, single-agent IDELA, the presence of TP53 aberrations did not influence PFS (TP53 aberrations: median PFS 20.8 months versus wildtype: 18.8 months, HR 1.03; 95%CI, 0.62-1.72) (44).
Several trials have demonstrated the impressive efficacy of ibrutinib in R/R CLL with TP53 aberrations. In the phase III RESONATE trial, ibrutinib was compared with ofatumumab monotherapy in patients with R/R CLL (45–47). The 6-months PFS rate was 83% and 49% for patients with TP53 aberrations treated with ibrutinib and ofatumumab, respectively (45). After six years of follow-up, ibrutinib-treated patients without TP53 aberrations had a median PFS of 56.9 months (95%CI, 36.4-NR), compared to 40.7 months (95%CI, 25.4-57.3) for patients with either del(17p) or TP53 mutations (48). Similarly, O’Brien et al. evaluated the efficacy of ibrutinib in R/R CLL patients with del(17p) in the RESONATE-17 trial (49). At 24 months, the ORR was 83% (95%CI, 76%-88%), with 63% of patients remaining progression-free (95%CI, 54%-70%). More recently, in the phase III ASCEND trial, acalabrutinib was compared with BR or R-IDELA in fit, R/R CLL patients (50). After a median follow-up period of 16.1 months, median PFS was NR and 16.5 months (95%CI, 14.0-17.1) for acalabrutinib and the investigators choice, respectively (HR 0.31; 95%CI, 0.20-0.49). Interestingly, very recently, the first head-to-head comparison of ibrutinib and acalabrutinib was performed (51). In this trial, Byrd et al. compared the efficacy of ibrutinib and acalabrutinib in R/R CLL patients. Acalabrutinib was non-inferior compared with ibrutinib in patients with del(17p): median PFS was 32.9 months (95%CI, 25.2-38.4) after treatment with acalabrutinib, compared with 27.6 months (95%CI, 21.8-28.5) for ibrutinib (HR 1.00; 95%CI, 0.73-1.38) (51).
Venetoclax has demonstrated comparable efficacy in R/R CLL with TP53 aberrations. In the phase II Pivotal trial, venetoclax monotherapy was evaluated in R/R CLL patients with del(17p). The ORR was 77% for the overall cohort. The estimated 24-months PFS and OS were 66% (95%CI, 55%-74%) and 73% (95%CI, 65%-79%). In the phase III MURANO trial, venetoclax plus rituximab (Ven-R) was compared with BR in physically fit, R/R CLL patients (52–54). Outcome with Ven-R was superior in terms of median PFS as compared with BR in patients with del(17p) (NR after 48 months versus 15.4 months, respectively). However, in a pooled analysis of four early-stage trials, patients with either del(17p) or TP53 mutations remained at higher hazard of relapse, following venetoclax-based treatment (HR 1.7; 95%CI, 1.2-2.4) (55).
Taken together, given the availability of more efficacious drugs, chemoimmunotherapy is no longer a suitable treatment option for CLL patients with TP53 aberrations, both in first-line and in R/R settings. As a consequence, ideally every CLL patient with an indication for treatment should undergo cytogenetic and molecular testing for TP53 disruption. At present, patients with TP53 aberrations qualify for treatment with novel agents such as ibrutinib, acalabrutinib or venetoclax. Still, even after novel agent-based treatment, patients with TP53 disruption seem to have inferior outcome, compared with patients with intact TP53, although these differences are not statistically significant in every trial. At present, there is no preference for either ibrutinib, acalabrutinib or venetoclax if considering treatment for patients with TP53 aberrations. As such, the choice should be determined by the physician and the patient jointly, taking into account treatment duration, side effects, comorbidities and previous lines of therapy. A complete overview of clinical trials comparing treatment regimens in previously untreated or R/R CLL patients with TP53 aberrations is given in Table 1 and Table 2, respectively.
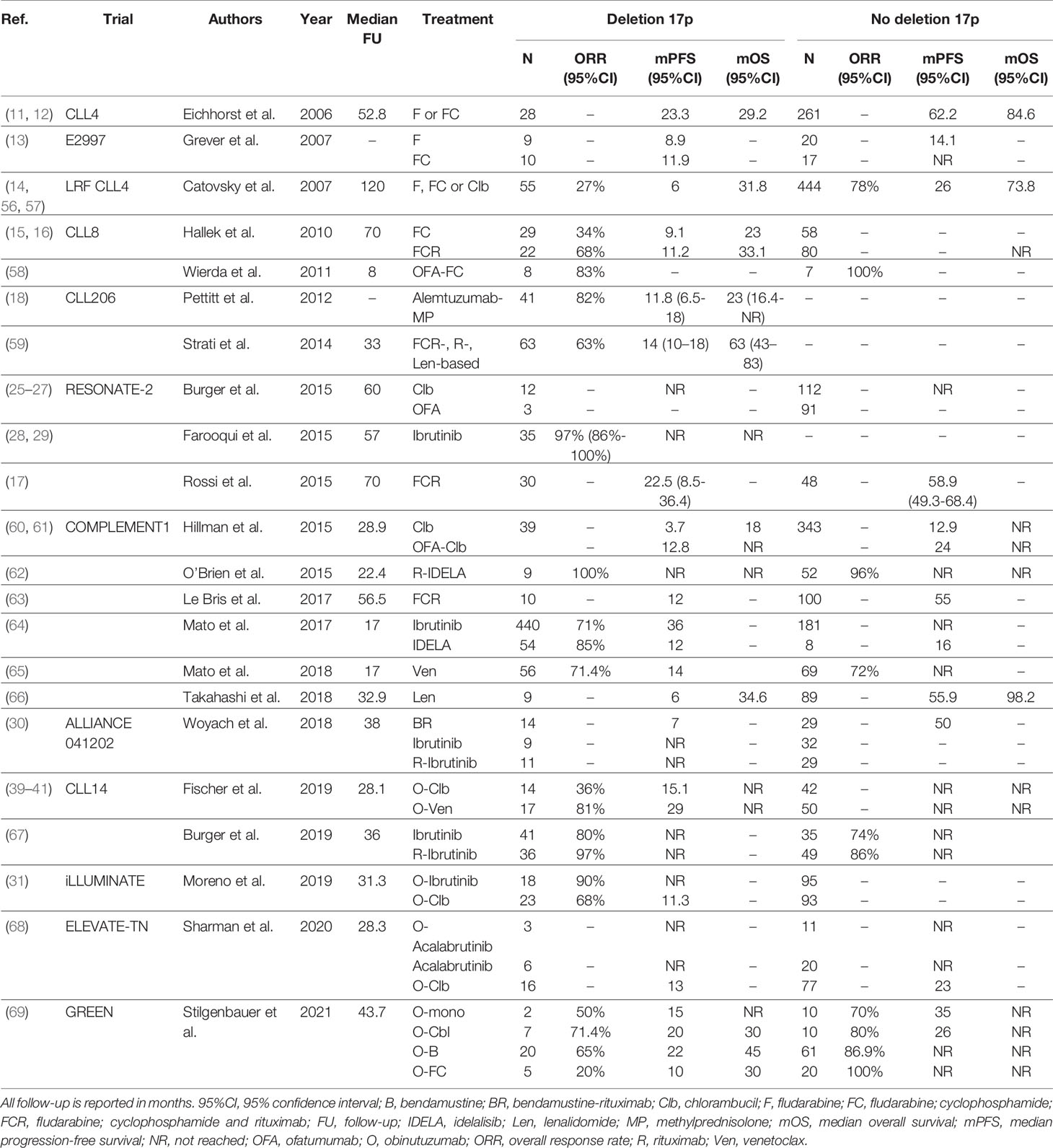
Table 1 An overview of clinical trials comparing first-line treatment regimens for patients with TP53 aberrations.
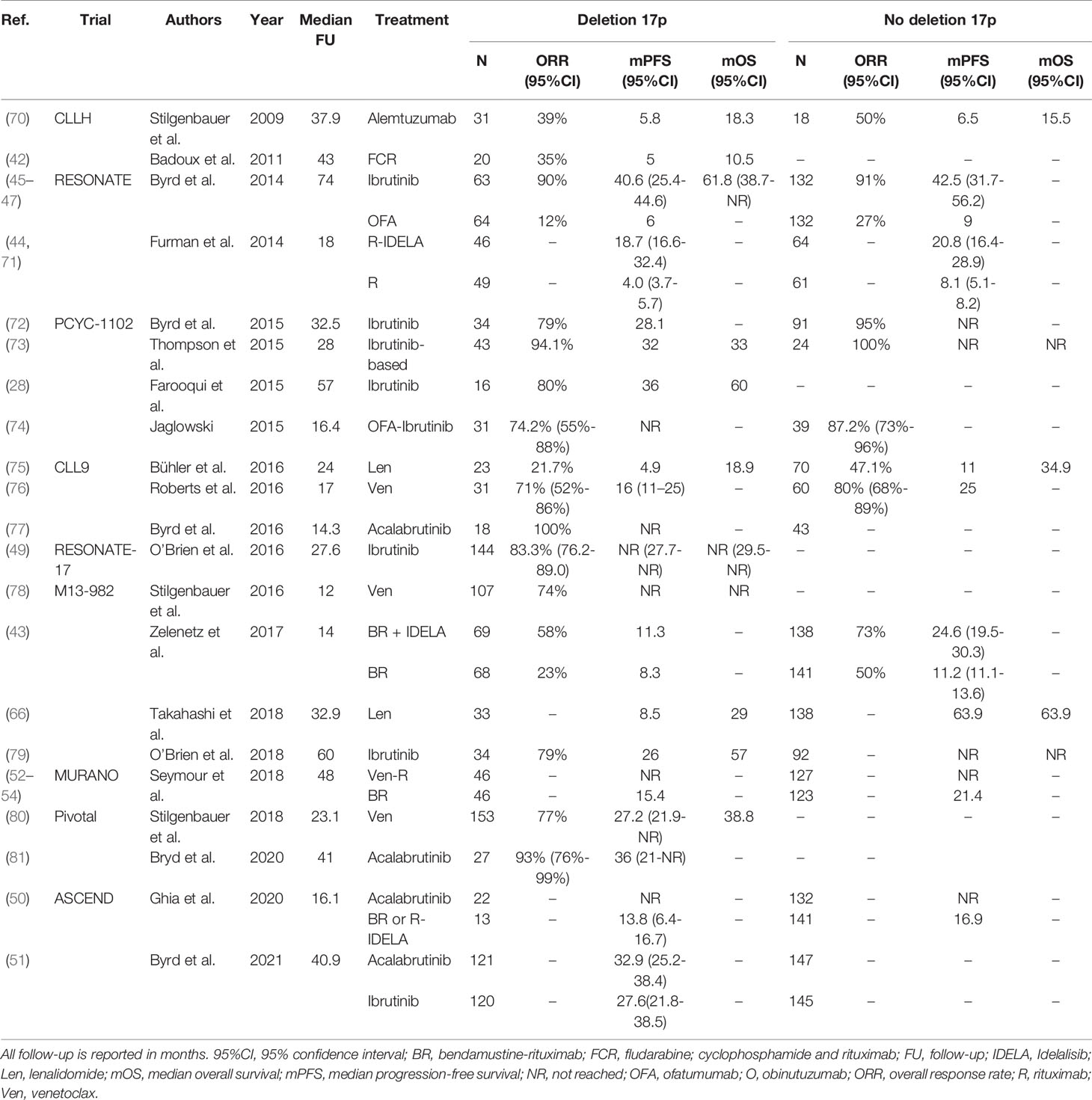
Table 2 An overview of clinical trials comparing treatment regimens for relapsed or refractory CLL patients with TP53 aberrations.
Deletion of the Long Arm of Chromosome 11
Deletion of the long arm of chromosome 11, i.e. del(11q), is one of the most common structural chromosomal aberrations in CLL. At diagnosis, del(11q) is present in 10% of patients with early-stage and 25% of patients with advanced stage, treatment-naïve CLL (82, 83). Patients carrying a del(11q) characteristically have bulky disease, rapid progression and a shorter OS (82). The minimally deleted region in del(11q) encompasses several tumor suppressor genes such as ATM, FDX, MLL and RDX. The tumor suppressor gene ATM encodes the serine-threonine kinase ATM, which is important in the repair of double-strand DNA breaks. Deleterious ATM mutations in the residual allele can be found in 36% of the CLL patients with a del(11q) and are associated with a poorer prognosis, compared with patients with del(11q) alone (84–86). Since ATM functions as a positive upstream regulator of p53, loss of ATM could interfere with chemotherapy-induced apoptosis.
First-Line Setting
Concordantly, in the chemotherapy era, prognosis for CLL patients with del(11q) was poor (83). In the US E2997 trial, first-line treatment with FC did not improve PFS in patients with del(11q), compared with F alone (median PFS 25.2 and 16 months, respectively). In the FC arm, PFS was 25.2 months and NR after a follow-up of 60 months for patients with del(11q) and those without, respectively. Moreover, in a multivariable analysis, del(11q) was strongly associated with reduced PFS (HR 1.904; P=0.006) (13, 87). Similarly, in the CLL8 trial, first-line treatment with FC resulted in a complete response (CR) rate of only 19% in del(11q) patients (15, 16). In contrast, in this trial, patients with del(11q) responded very well to treatment with FCR (CR rate 71%). In addition, the 5-year PFS rate for patients with del(11q) was 11.4% after FC, compared with 31.4% after FCR (HR 0.47; 95%CI, 0.32-0.68). Similarly, the 5-year OS rate of patients with del(11q) was superior after treatment with FCR, compared with FC (HR 0.35; 95%CI, 0.20-0.61). Indeed, subgroup analysis revealed that patients with del(11q) and mutated IGHV genes responded very well to FCR, with outcomes similar to patients without del(17p) and del(11q) (88). In a retrospective observational cohort study by Rossi et al., physically fit, treatment-naïve CLL patients were treated with FCR (17). After a median follow-up of 70 months, median PFS was 43.5 months (95%CI, 32.2-54.7) for patients with del(11q) and 56.9 months (95%CI, 47.1-66.6) for patients without del(11q) (P=0.01). In a multivariable analysis, del(11q) was identified as an independent predictor of PFS (HR 1.67; 95%CI, 1.13-2.46). Furthermore, in the GCLLSG CLL10 trial, a phase III non-inferiority trial comparing FCR to BR in physically fit, treatment-naïve CLL, patients with del(11q) had shorter PFS in the BR arm (HR 2.33; 95%CI, 1.47-3.67) (89). In summary, although the introduction of chemoimmunotherapy, especially FCR, has significantly improved the outcome of patients with del(11q), their prognosis after chemoimmunotherapy is still inferior, compared with patients without del(11q).
Treatment with ibrutinib has proven very effective in patients with del(11q), yielding outcomes similar to patients without del(11q) (25–27, 30, 90). For example, after first-line treatment with ibrutinib alone in the RESONATE-2 trial, the 60-month PFS rate was 79% in patients with del(11q), compared with 67% for patients without del(11q) (25–27). Surprisingly, pooled data from three phase III ibrutinib trials (RESONATE, RESONATE-2 and HELIOS) demonstrated that ibrutinib-treated patients with del(11q) had slightly longer PFS, compared with ibrutinib-treated patients without del(11q) (42-month PFS rate, 70% versus 65%, P=0.02) (91). In the trial by Shanafelt et al., PFS was superior for ibrutinib-R compared with FCR in treatment-naïve CLL patients with del(11q) (HR 0.24; 95%CI, 0.10-0.62) (90). Likewise, Woyach et al. demonstrated that in patients with del(11q) PFS was comparable after treatment with ibrutinib (median PFS NR) or ibrutinib-R (median PFS NR), but inferior after treatment with BR (median PFS 41 months; 95%CI, 36-NR) (30). In the ILLUMINATE trial, treatment-naïve CLL patients with del(11q) had significantly better PFS after treatment with ibrutinib (median PFS NR (95%CI, 17.4-NR), compared with O-Clb (median PFS 15.2 months (95%CI, 14.1-20.8). The ELEVATE-TN trial has demonstrated that after first-line treatment with acalabrutinib, PFS is comparable for patients with and without del(11q) (68). A complete overview of clinical trials comparing first-line treatment regimens for patients with del(11q) is provided in Table 3.
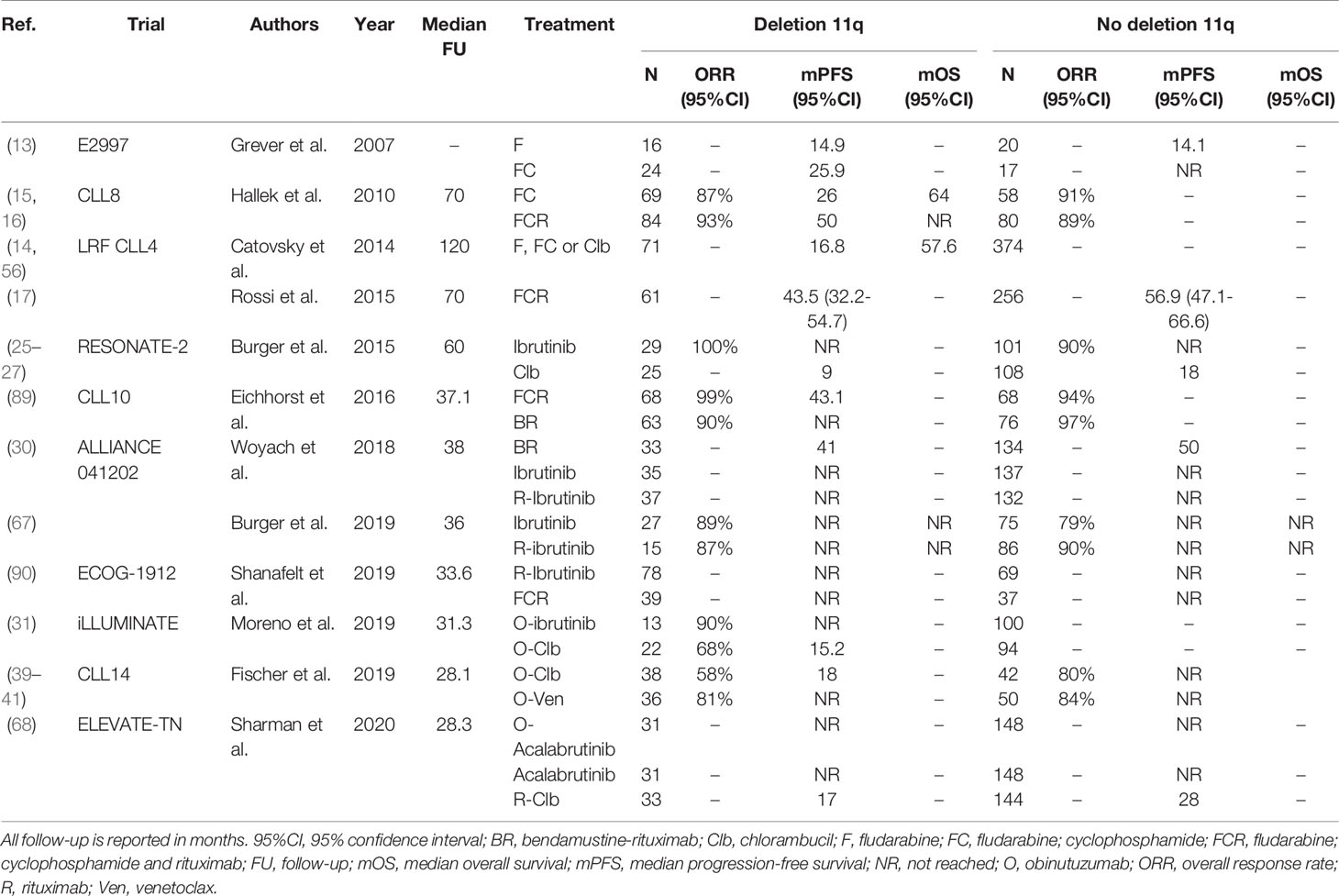
Table 3 An overview of clinical trials comparing first-line treatment regimens for patients with deletion 11q.
R/R Setting
Treatment with chemoimmunotherapy in patients with R/R CLL with del(11q) is generally ineffective: in a study by Badoux et al. in the R/R setting, patients with del(11q) treated with FCR had a median PFS of only 12 months (42).Similar to the first-line setting, in R/R CLL with del(11q) treatment with novel agents has impressive efficacy. In the ASCEND trial, the 12-month PFS rate of patients with del(11q) after acalabrutinib was approximately 90%, compared with approximately 60% in patients with del(11q) after treatment with BR or R-IDELA (50). In the recent head-to-head trial in the R/R setting by Byrd et al., acalabrutinib was demonstrated to be non-inferior to ibrutinib in patients with del(11q) (51). In the CLL14 study, the presence of a del(11q) was associated with adverse PFS in the context of O-Clb (HR 3.44; 95%CI, 1.80-6.60), but not in the context of Ven-O (HR 0.94; 95%CI, 0.29-3.05) (39–41). Likewise, treatment with Ven-R in the MURANO trial resulted in a comparable PFS for R/R CLL patients with del(11q) (median PFS 48 months) and without del(11q) (median PFS 49 months) (52–54). A complete overview of all trials evaluating regimens for R/R CLL patients with del(11q) is provided in Table 4.
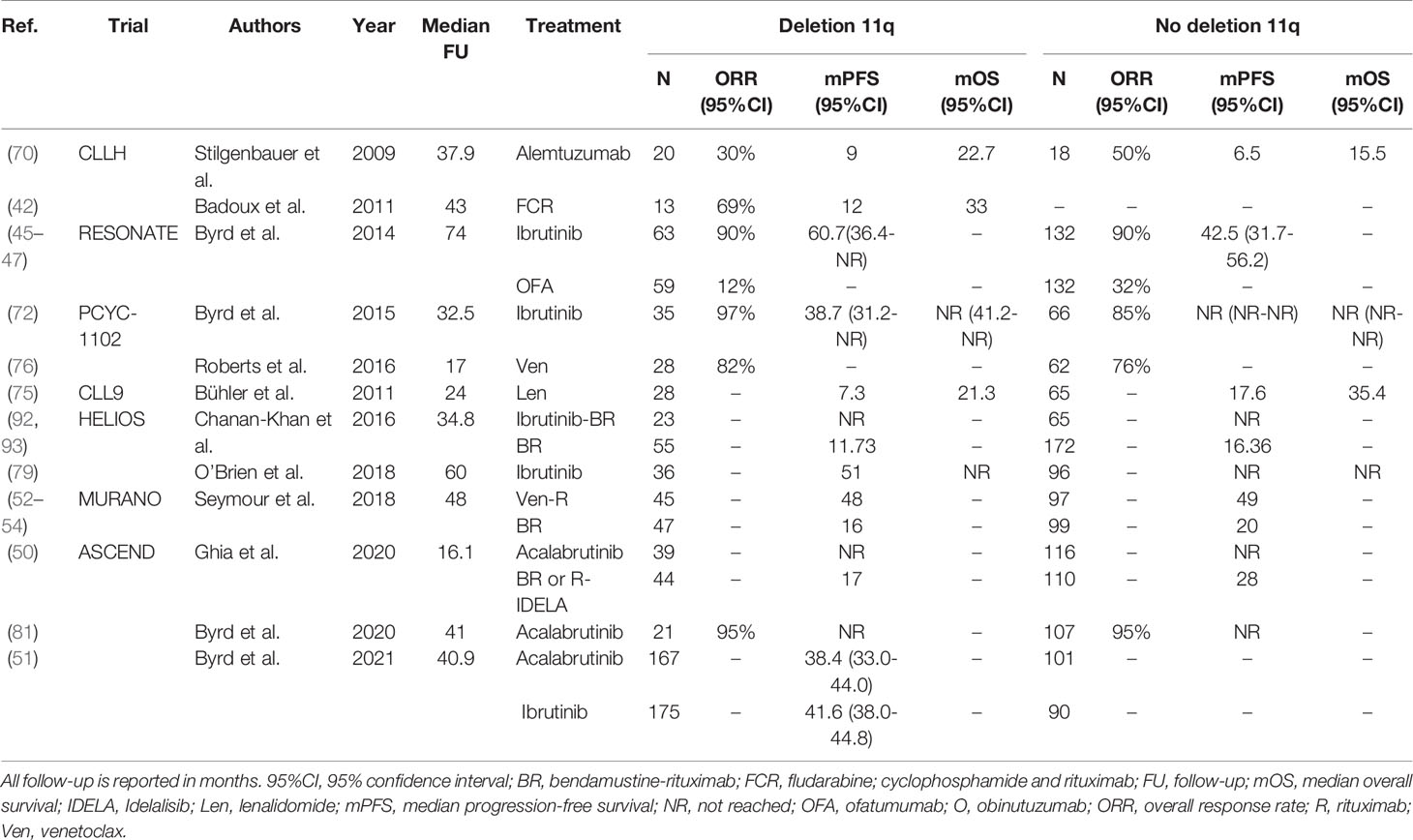
Table 4 An overview of clinical trials comparing treatment regimens for relapsed or refractory CLL patients with deletion 11q.
In summary, in the historical context of chemotherapy, del(11q) was considered a marker of poor prognosis. Treatment with chemoimmunotherapy, specifically FCR, largely mitigates the deleterious outcome associated with del(11q), although survival of patients without del(11q) after FCR remains somewhat superior. Treatment with ibrutinib, acalabrutinib or venetoclax yields equal outcomes in patients with and without del(11q).
Complex Karyotype/Genomic Complexity
The prognostic and predictive value of complex karyotype (CK) or genomic complexity (GC) in CLL has been reported in an increasing number of studies over the past decade. CK is traditionally defined as CLL with 3 or more cytogenetic aberrations, as measured by chromosomal banding analysis (CBA) or interphase FISH. When chromosomal aberrations are detected using chromosomal microarray analysis (CMA), the presence of 3 or more aberrations it is generally referred to as genomic complexity (GC). CBA and interphase FISH are complementary techniques (94). More specifically, CBA can detect abnormalities that cannot be found by a limited panel of FISH probes and FISH can detect abnormalities that cannot be found by CBA due to an inherently lower resolution (95–98). As such, performing CBA in combination with interphase FISH results in a more sensitive measurement of CK. Alternatively, CMA, often referred to as digital karyotyping, allows for screening the entire genome, and therefore has a higher detection rate of cytogenetic abnormalities, but may fail to detect cytogenetic aberrations without copy number aberrations, such as balanced translocations. Another advantage of CMA over CBA is that it is a DNA-based analysis and therefore culturing of B-cells in combination with mitogen stimulation is not needed to generate chromosome metaphases (99–101).
CK is associated with poor OS in CLL patients (98, 102–113). Indeed, in a large multicenter cohort study, Baliakas et al. confirmed that the presence of CK was associated with a shorter time-to-first-treatment (TTFT), compared with CLL patients with a normal karyotype (P=0.01) (114). Furthermore, TTFT was even shorter in patients harboring ≥5 chromosomal aberrations (P<0.001). The latter was confirmed in 2019, when the same research group proposed a hierarchical model for CK in which high CK (≥5 aberrations) exhibits the worst prognosis (median OS 3.1 years), compared with intermediate CK (4 aberrations, median OS 7.25 years) and low CK (3 chromosomal aberrations, median OS 12.3 years) (115). In that study, high CK emerged as a powerful prognostically adverse biomarker, independent of well-established prognostic indices. An exception to the former is posed by CLL patients that either have two translocations or trisomy 12 and trisomy 19, in combination with another cytogenetic lesion, which have a remarkably indolent disease course. The median survival of these patients was not reached after a median follow-up time of 7.1 years, and was superior compared with patients with no CK (median OS, 6.2 years, P<0.001) and patients with normal karyotype (median OS, 11.1, P<0.001). Although the impact of CK in CLL has been examined in many trials, there is no official consensus on the definition of CK. Consequently, it is challenging to evaluate the clinical significance of CK in the context of treatment. For example, several trials do not report on the minimum number of cytogenetic abnormalities required to define CK. Moreover, measurement techniques have not been standardized and it is often unclear whether only abnormalities that are present in a single clone are counted, or whether cytogenetic abnormalities in unrelated clones can also contribute to CK definition (116). Finally, patients with two translocations or trisomy 12 and 19 and another aberration, which have a more favorable prognosis, are rarely classified separately.
First-Line Setting
Several trials indicate that CLL with CK (defined as ≥3 chromosomal aberrations) respond poorly to chemo-immunotherapy. In a study by Le Bris et al., in patients with previously untreated CLL treated with FCR, the presence of CK was associated with a significantly shorter median PFS (21 versus 55 months) and an inferior 5-year OS rate (72.4% vs 85.8%), compared with CLL patients without CK (63). In the R/R setting, Badoux et al. reported an ORR of 64% in patients with CK after treatment with FCR, with significantly shorter median PFS and OS compared with patients without CK (median PFS: 9 months versus 20.9 months, P<0.001, median OS: 26 months versus 46.7 months, P<0.001) (42). In a multivariable analysis, CK was associated with poorer PFS and OS independently of the presence of del(17p) (PFS: HR 2.6; 95%CI, 1.5-2.4 and OS: HR 1.9; 95%CI, 1.1-3.2). In the phase III GCLLSG CLL11 trial, the efficacy of Clb alone was compared with treatment with rituximab and chlorambucil (R-Clb) and O-Clb (117). In a subgroup analysis, Herling et al. reported on the impact of CK and coexisting mutations on the survival outcome of patients treated in this trial (118). Patients with CK pooled over all treatment arms, here defined as ≥3 aberrations by karyotyping, had inferior median OS, compared with patients without CK (37 months versus 60 months, P<0.001). This deleterious effect was largely dependent on patients with both CK and TP53 aberrations, as their median OS was markedly poorer, compared with patients with CK and intact TP53 (26 months versus 50 months). In a multivariable analysis, CK was a predictor for inferior OS, independent of well-established prognostic markers such as advanced clinical stage, unmutated IGHV and elevated β-2-microglobulin (HR 2.7; 95%CI, 1.4-5.3). The adverse impact of CK was retained when the model was limited to patients treated with chemoimmunotherapy (HR 2.6; 95%CI, 1.2-5.7) (118). In the CLL14 trial, first-line treatment with O-Clb resulted in significantly shorter PFS and OS in patients with CK, compared with those without (2-year PFS rate, 36.6% versus 69.6%, OS: HR 3.76; 95%CI, 1.36;10.29) (40). Contrastingly, in this trial PFS and OS were similar between patients with and without CK after treatment with Ven-O (2-year PFS rate: 78.9% versus 91.1%, HR. 1.91; 95%CI, 0.81-4.52 and 2-year OS rate: 88.2% and 93.2%; HR 1.51, 95%CI, 0.50-4.60) (40). Moreover, the presence of del(17p) and/or TP53 mutation in patients with CK was associated with inferior PFS in the O-Clb arm (HR 2.10; 95%CI, 0.80-5.57), but not in the Ven-O arm (HR 1.42; 95%CI, 0.32-6.35).
R/R Setting
Kreuzer et al. assessed the prognostic value of CK in R/R patients treated with R-IDELA (119). Interestingly, there was no impact of the presence of CK on ORR (80.8% versus 89.2%) and median PFS (20.9 versus 19.4 months; HR 1.22, 95%CI; 0.60-2.47). Similarly, Mato et al. found that R/R CLL patients with CK treated with IDELA had similar PFS to patients without CK (median PFS 9 months versus 12 months) (64).
Thompson et al. were the first to report on the impact of CK in the context of ibrutinib-based therapy (73). Although R/R CLL patients with and without CK had high ORR (90.5% versus 97.1%), median event-free survival (EFS) was significantly worse in patients with CK (19 versus 38 months; P<0.001). Importantly, in this trial, almost all patients with CK had an additional del(17p) (81%). Comparing patients with CK including or excluding del(17p) yielded a strong trend towards inferior median EFS in those with an additional del(17p) (22 months versus 34 months; P=0.056). OS was inferior in patients with CK, independently of the presence of del(17p) (HR 5.9; 95%CI,1.6-22.2). Likewise, in the RESONATE trial in R/R CLL, median PFS after treatment with ibrutinib was shorter in patients with CK, compared with those without (40.8 months versus 44.6 months, HR 1.292; 95%CI, 0.770-2.168) (45). Furthermore, with a median follow-up of 60 months, O’Brien et al. also demonstrated that R/R CLL patients with CK have inferior PFS and OS after treatment with ibrutinib, compared with patients without CK (median PFS: 31 months versus NR, median OS: 54 months versus NR) (49). In contrast, in a pooled analysis of three phase III ibrutinib trials for both treatment-naïve and previously treated CLL patients (the RESONATE-2, RESONATE and HELIOS trials), no effect of the presence of CK on PFS could be demonstrated (91). Specifically, the 42-month PFS rate was 63% in patients with CK, compared with 69% in those without (HR 1.02; P=0.95). Of note, the RESONATE-2 and HELIOS trials excluded patients with del(17p) (25, 92). Likewise, the ALLIANCE trial did not substantiate inferior survival in patients with CK after first line treatment with BR, ibrutinib and R-ibrutinib, compared with those without CK (HR 1.01; 95%CI, 0.68-1.51) (30). The impact of CK on treatment with acalabrutinib has only been evaluated in a single phase II trial in R/R CLL patients (81). PFS was shorter for patients with CK, compared with the overall cohort (33 months versus NR after 41 months).
The MURANO trial analyzed the impact of GC (≥3 cytogenetic aberrations, as measured by CMA) after treatment with Ven-R (53). In this trial, GC was stratified into low GC (3-4 aberrations) and high GC (≥5 aberrations). After treatment with Ven-R, patients without GC had superior PFS, compared with patients with low- and high GC (HR 2.9; 95%CI, 1.1-3.6). In addition, the co-occurrence of TP53 aberrations only negatively affected PFS in patients with high CK, but not low CK. In concordance, in a pooled analysis of three phase I trials, Anderson et al. showed that the presence of CK was associated with shorter PFS in R/R CLL patients treated with venetoclax monotherapy (HR 6.61; 95%CI, 1.47-29.75) (120).
Altogether, the predictive impact of CK is challenging to disentangle, mostly due to inconsistent reporting and co-occurrence of TP53 aberrations. While patients with CK seem to have a high chance of relapse, even in the absence of TP53 aberrations, after treatment with chemoimmunotherapy, evidence of the impact of CK on the efficacy of novel agents such as ibrutinib and venetoclax is contradictory. Consequently, further research into the impact of CK is warranted, including clear reporting on the definition of CK and multivariable analysis to correct for the co-occurrence of TP53 aberrations. For an overview of clinical trials comparing treatment regimens in CLL patients with and without CK, see Table 5.
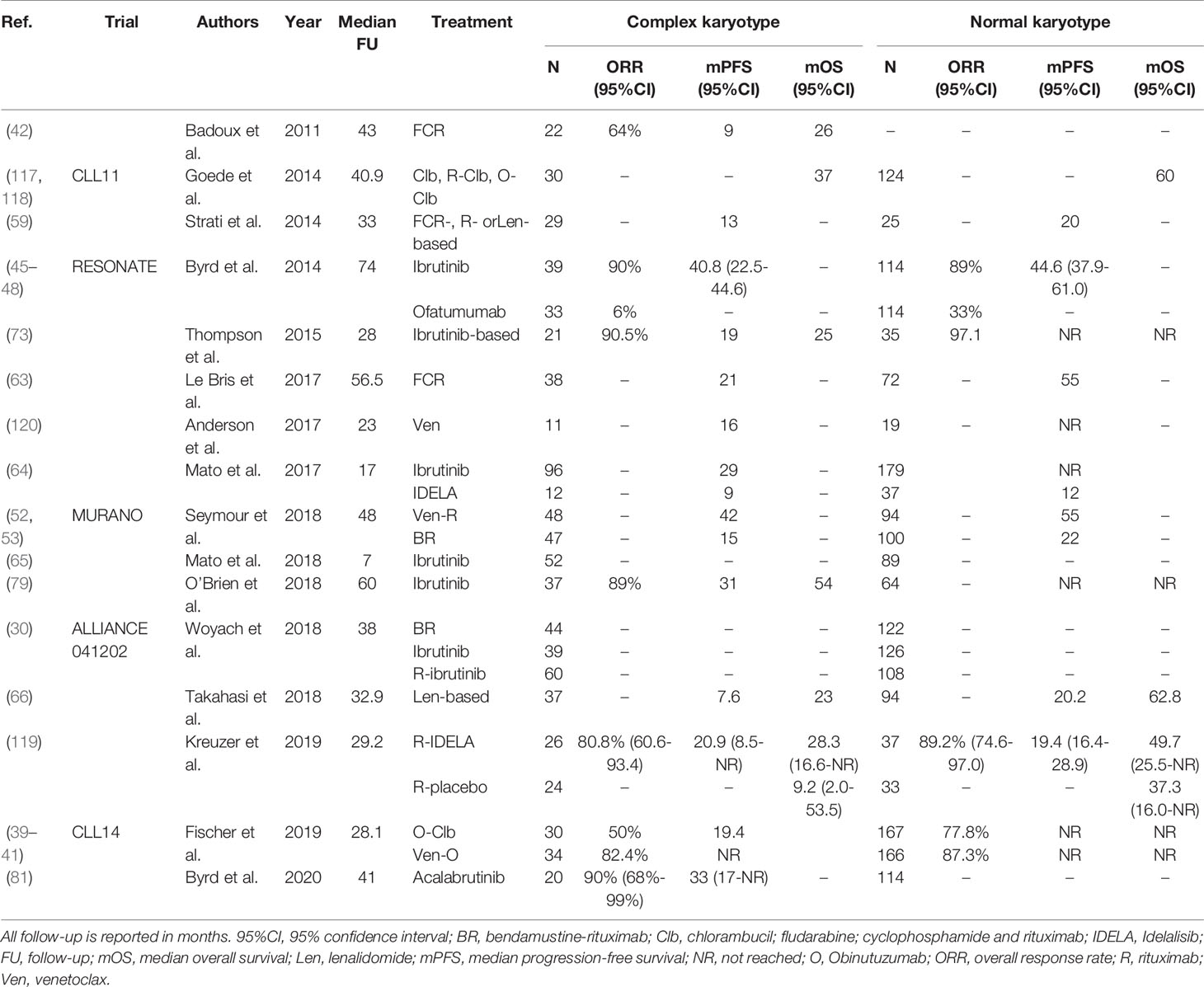
Table 5 An overview of clinical trials comparing treatment regimens for patients with complex karyotype defined as ≥3 chromosomal aberrations.
Unmutated IGHV
The prognostic and predictive impact of the somatic hypermutation (SHM) imprint on the IGHV gene of the leukemia-specific BCR rearrangement has been recognized over the past two decades (121, 122). Whereas CLL with abundant SHM (IGHV germline identity <98%, ‘IGHV mutated’, or M-CLL) generally has an indolent disease course, a paucity of SHM (IGHV germline identity ≥98%, ‘IGHV unmutated’, or U-CLL) is a biomarker of high-risk disease and is associated with a shorter TTFT and OS, compared to M-CLL patients (121–123). Consequently, in early stage CLL, the proportion of patients with unmutated IGHV is around 50%, whereas this prevalence enriches to approximately 60% at first-line therapy and up to 80% in R/R CLL.
First-Line Setting
After first-line treatment with FCR, a significant proportion of patients with M-CLL experience durable remission, whereas almost all patients with U-CLL eventually relapse (88, 124). For example, in the CLL8 trial U-CLL patients had significantly shorter PFS and OS following first-line treatment with FCR, compared with M-CLL patients (PFS: 5-year rate 33.1% versus 66.6%, OS: 5-year rate ~73% versus 83.6%, both P<0.001) (15, 16, 89). Similarly, in a trial by the MD Anderson Cancer Center the median PFS and OS of U-CLL patients after first-line FCR was 50.4 and 112.8 months, respectively, whereas median PFS and OS of M-CLL patients were NR after 12.8 years of follow-up (125). Additionally, the CLL10 trial demonstrated that the median PFS of U-CLL patients after treatment with FCR or BR was markedly shorter, compared with the median PFS of M-CLL patients (FCR: 42.7 months (95%CI,36.2-55.2) and BR: (95%CI, 33.6 months (30.3-38.4) in U-CLL, versus NR for both in M-CLL, P<0.001) (89).
Controversy remains as to whether chemoimmunotherapy or novel agent-based regimens are more appropriate as first-line treatment for patients with U-CLL. Head-to-head comparisons in U-CLL patients have demonstrated that, compared with chemoimmunotherapy, treatment with ibrutinib results in longer PFS. In the ALLIANCE trial, while U-CLL patients in both ibrutinib-based arms had similar median PFS (NR after 38 months), median PFS in the BR arm was markedly shorter (39 months, 95%CI,32-NR) (30). Likewise, the ECOG-1912 phase III trial compared the efficacy of R-ibrutinib to FCR in previously untreated CLL (90). In this trial, the 3-year PFS rate after FCR was 62.5% in patients with U-CLL, compared with 90.7% after R-ibrutinib (HR 0.26; 95%CI, 0.14-0.50). However, an OS benefit for patients with U-CLL after first-line treatment with ibrutinib-based regimens, compared with chemoimmunotherapy, has not been conclusively demonstrated. Consequently, guidelines differ on the most appropriate first-line regimen for U-CLL.
There is similar ambiguity regarding the most appropriate first-line treatment for unfit U-CLL patients, in which treatment with FCR or BR is contraindicated. In the CLL11 trial, first-line treatment with O-Clb resulted in significantly longer PFS, both compared with Clb alone (HR 0.23; 95%CI, 0.13-0.42) and R-Clb (HR 0.39; 95%CI, 0.29-0.53) in U-CLL (117). In the RESONATE-2 trial, the 18-month PFS of U-CLL patients was 89% after treatment with ibrutinib, compared with 47% after treatment with Clb (25). As both these trials demonstrated an OS benefit over Clb alone, treatment with O-Clb, R-Clb or ibrutinib has become the cornerstone of first-line therapy in unfit CLL patients. In a similar setting, U-CLL patients in the iLLUMINATE trial treated with O-ibrutinib had longer median PFS, compared with O-Clb (NR after 31.3 months versus 14.6 months, HR 0.15, 95%CI,0.08-0.27) (31). Newer agents have also been evaluated as first-line treatment for unfit CLL patients. In the CLL14 trial, treatment with Ven-O resulted in a superior 24-months PFS rate in U-CLL patients, compared with O-Clb (89.4% versus 51%, HR 0.22, 95%CI, 0.12-0.38) (40). Additionally, in the ELEVATE-TN trial, first-line treatment with O-acalabrutinib resulted in a 24-month PFS rate in U-CLL patients of 91% (95%CI, 83%-95%), compared with 31% (95%CI, 22%-40%) after O-Clb (68). However, none of these trials have so far demonstrated an OS benefit over O-Clb (51). Moreover, ibrutinib, venetoclax and acalabrutinib have not been compared in a head-to-head fashion in first-line setting. For these reasons, similarly to fit U-CLL patients, the most appropriate first-line treatment for unfit CLL patients remains controversial. In any case, all available options should be carefully discussed with the patient, taking into account the efficacy, contraindications, treatment duration and any side effects. For an overview of clinical trials comparing first-line treatment regimens in U-CLL, see Table 6.
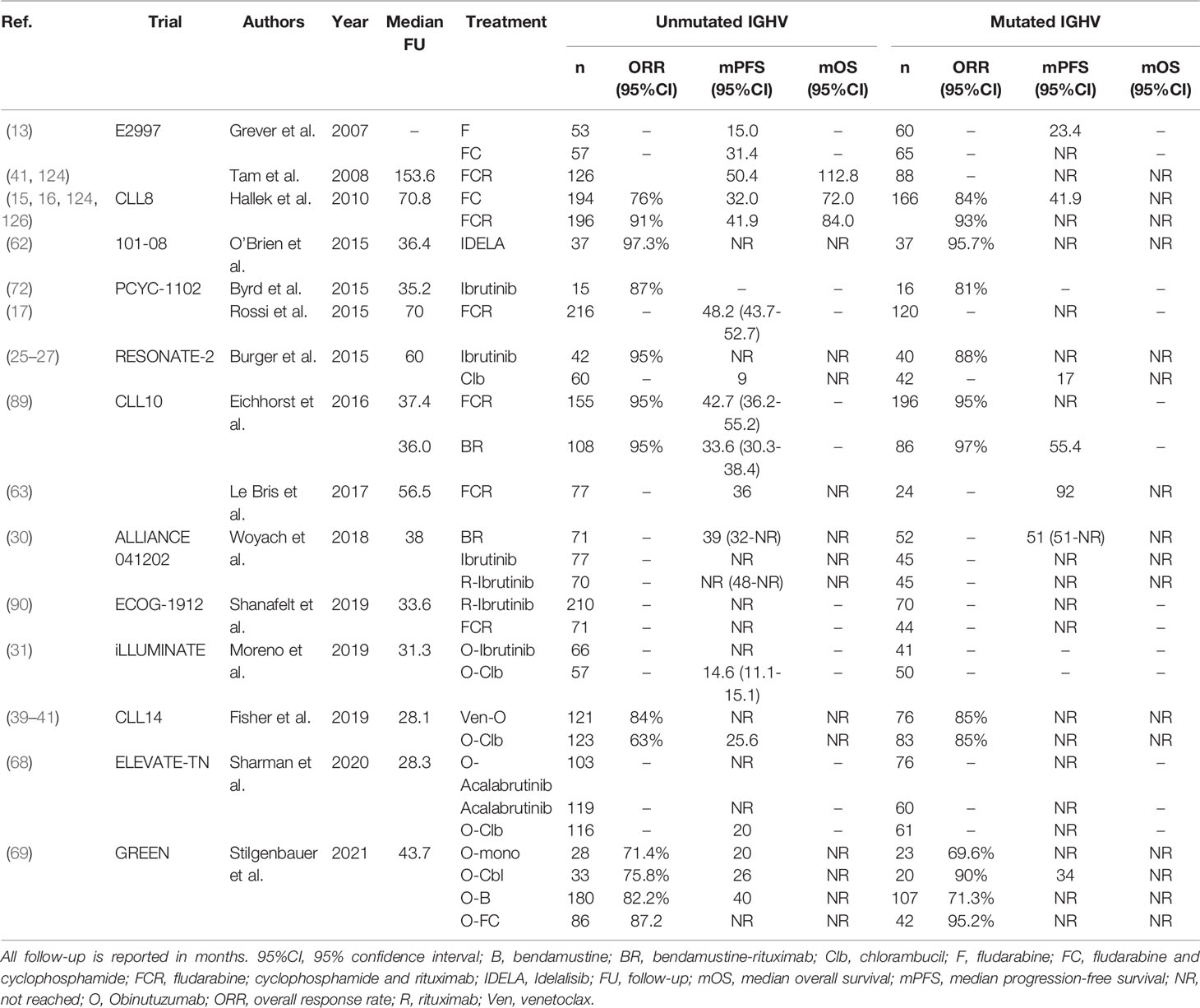
Table 6 An overview of clinical trials comparing first-line treatment regimens for patients with U-CLL.
R/R Setting
In U-CLL patients with early relapse or refractory disease, chemoimmunotherapy usually yields disappointing results. In a trial conducted by Fischer et al., median PFS and OS in R/R U-CLL patients after treatment with BR were 13.8 and 25.6 months, respectively (127). Consequently, patients with R/R U-CLL usually require treatment with novel agent-based regimens. Two trials have demonstrated the efficacy of IDELA-based regimens in R/R U-CLL. In the trial operated by Furman et al., treatment with R-IDELA achieved a median PFS of 19.4 months in R/R U-CLL patients, compared with 5.6 months after rituximab alone (44, 71). In a similar setting, Zelenetz et al. demonstrated that R/R U-CLL patients have superior ORR, PFS and OS after treatment with BR-IDELA, compared with BR alone (ORR: 71% (95%CI, 63%-77%) versus 43% (95%CI, 35%-51%), median PFS: 19.5 months (95%CI, 16.1-24.6) versus 10.9 months (95%CI, 8.6-11.1), median OS: NR versus 31.6 months (95%CI, 22.2-NR) (43). However, due to the introduction of newer agents with better tolerability, IDELA is now used less frequently.
Several trials have demonstrated the impressive efficacy of ibrutinib in R/R U-CLL. In the RESONATE trial, the median PFS of U-CLL patients in the ibrutinib arm was 49.7 months (45–47). In the HELIOS trial, treatment with ibrutinib-BR for R/R U-CLL resulted in longer PFS, compared with BR (median PFS NR versus 13.8 months, HR 0.16; 95%CI, 0.11-0.21) (92, 93). Similarly, in the ASCEND trial, treatment with acalabrutinib alone achieved longer PFS in R/R U-CLL patients (median NR after 16 months), compared with BR (median 16.9 months; 95%CI, 11.6-NR) or R-IDELA (median 15.8 months; 95%CI, 13.9-17.1 months) (50). Recently, a head-to-head trial demonstrated that acalabrutinib and ibrutinib achieved similar PFS in R/R U-CLL patients (HR 1.09; 95%CI, 0.85-1.40) (51). Finally, venetoclax-based regimens are an efficacious option for R/R U-CLL. In the MURANO trial, R/R U-CLL patients achieved longer PFS after treatment with Ven-R, compared with BR (median PFS NR after 48 months versus 15.7 months, HR 0.16 (95%CI, 0.10-0.26)) (52). In R/R CLL, venetoclax, ibrutinib and acalabrutinib have not been compared in a head-to-head fashion.
In summary, for refractory or early relapsed U-CLL, novel agent-based regimens, either ibrutinib-, acalabrutinib- or venetoclax-based, are appropriate treatment options. For an overview of clinical trials comparing treatment regimens in R/R U-CLL, see Table 7.
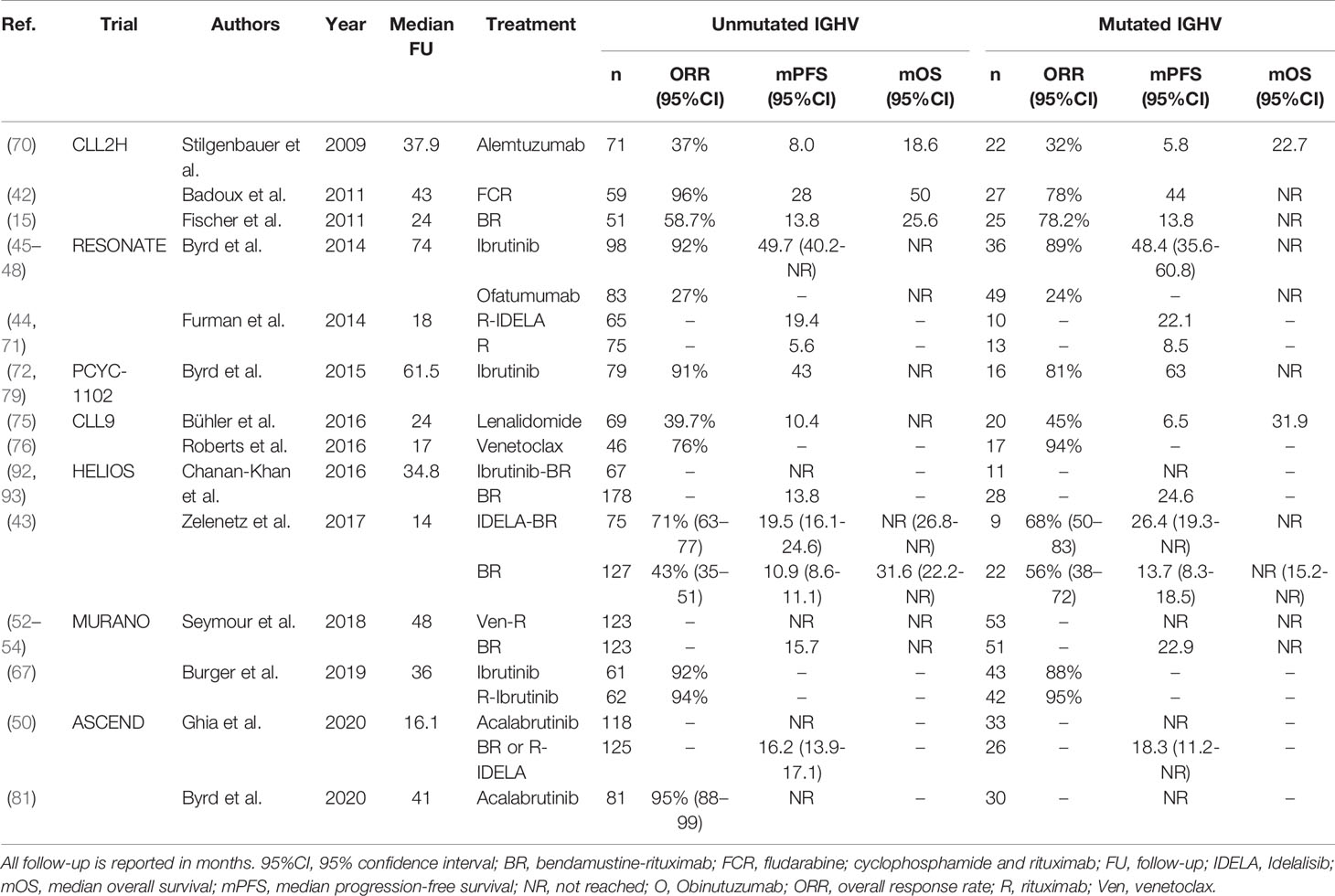
Table 7 An overview of clinical trials comparing treatment regimens for patients with relapsed or refractory U-CLL.
BCR Stereotyped Subsets
The molecular composition of the leukemia-specific BCR rearrangement has importance beyond its mutational status. Despite the immense theoretical variety of the BCR repertoire, subgroups of unrelated CLL patients express (quasi)identical leukemia-specific BCRs, a phenomenon known as BCR stereotypy. Although cumulatively, BCR stereotyped subsets are common, encompassing up to 41% of all CLL, the prevalence of each individual subset is low: the largest subset, subset #2 (defined as patients with a BCR comprised of IGHV3-21, IGLV3-21, with a short, stereotypic heavy-chain complementarity-determining region of 9 amino acids), represents around 2.5% of all patients (128). Certain stereotyped subsets have been associated with distinct clinico-biological profiles. For example, expression of a subset #2 stereotyped BCR is associated with poor prognosis, irrespective of IGHV mutational status, whereas patients from subset #8 have a very high risk of developing Richter’s syndrome (5-year risk: 68.7%) (129, 130).
Due to their low individual prevalence, little is known about the predictive impact of BCR stereotyped subsets. Jaramillo et al. analyzed the pooled results from the CLL8, CLL10 and CLL11 trials, which evaluated the efficacy of chemoimmunotherapy as first-line treatment for CLL (129). In these trials, compared with all other patients with mutated IGHV, patients with subset #2 and mutated IGHV had significantly shorter time to next treatment (TTNT) (HR 2.01; 95%CI, 1.23-3.28), numerically comparable to U-CLL patients. As of yet, there is no available data regarding the predictive impact of BCR stereotypy in the context of novel agent-based treatment regimens. Consequently, the therapeutic consequences of BCR stereotypy remain undefined, and testing for stereotypy has thus far not been embedded in regular CLL care.
NOTCH1 Mutated CLL
Activating mutations in NOTCH1, most often located in the PEST-domain or 3’-untranslated region (3’-UTR), are present in around 6-12% CLL patients at diagnosis and in 15-20% of patients with relapsed or refractory disease (131). CLL patients harboring a mutation in NOTCH1 (NOTCH1-mut) have shorter OS, compared with their NOTCH1-wildtype (-wt) peers (132–134). Interestingly, some trials have provided evidence that NOTCH1-mut CLL patients may not benefit from treatment with an anti-CD20 mAb. In the CLL8 trial, NOTCH1-wt patients benefited significantly from inclusion of rituximab in their treatment regimen (FC: median PFS 32.8 months versus FCR: median PFS 57.3 months, P<0.001) (16). Contrastingly, the median PFS of NOTCH1-mut patients did not improve significantly upon the addition of rituximab (FC: 33.9 months versus FCR: 34.2 months, p=0.9). Multivariable survival analysis yielded a statistically significant regression coefficient for the interaction term between NOTCH1 status and treatment arm (HR 1.65; 95%CI, 1.076-2.535), thereby satisfying the formal criterion for a predictive variable (16). Concordantly, Dal Bo et al. demonstrated that NOTCH1-mut patients, in contrast to NOTCH1-wt patients, do not benefit from rituximab consolidation following treatment with fludarabine and rituximab (135). Finally, in the phase III COMPLEMENT1 study, which evaluated the efficacy of ofatumumab-Clb compared with Clb alone in unfit, treatment-naïve CLL patients, NOTCH1-mut patients did not benefit from the incorporation of ofatumumab in their treatment regimen (60). More specifically, the median PFS of NOTCH-wt patients treated with ofatumumab-Clb was longer, compared with those treated with Clb alone (23.8 months versus 13.3 months, HR 0.50; 95%CI, 0.39-0.63), whereas NOTCH1-mut patients had similar PFS, regardless of the treatment arm (17.2 months versus 13.1 months, HR 0.81; 95%CI,0.50-1.31). Again, a statistically significant interaction term between NOTCH1 status and treatment arm provided evidence of the predictive impact (P=0.05) (60). Similar research for obinutuzumab, a type II anti-CD20 mAb, has not been performed and is warranted. PFS of NOTCH1-mut patients was similar to that of NOTCH1-wt patients in the RESONATE, CLL14 and MURANO trials, suggesting that NOTCH1 status is not predictive in the context of novel agents (39, 46, 53). An overview of all clinical trials evaluating NOTCH1 mutations in the context of therapy is given in Table 8.
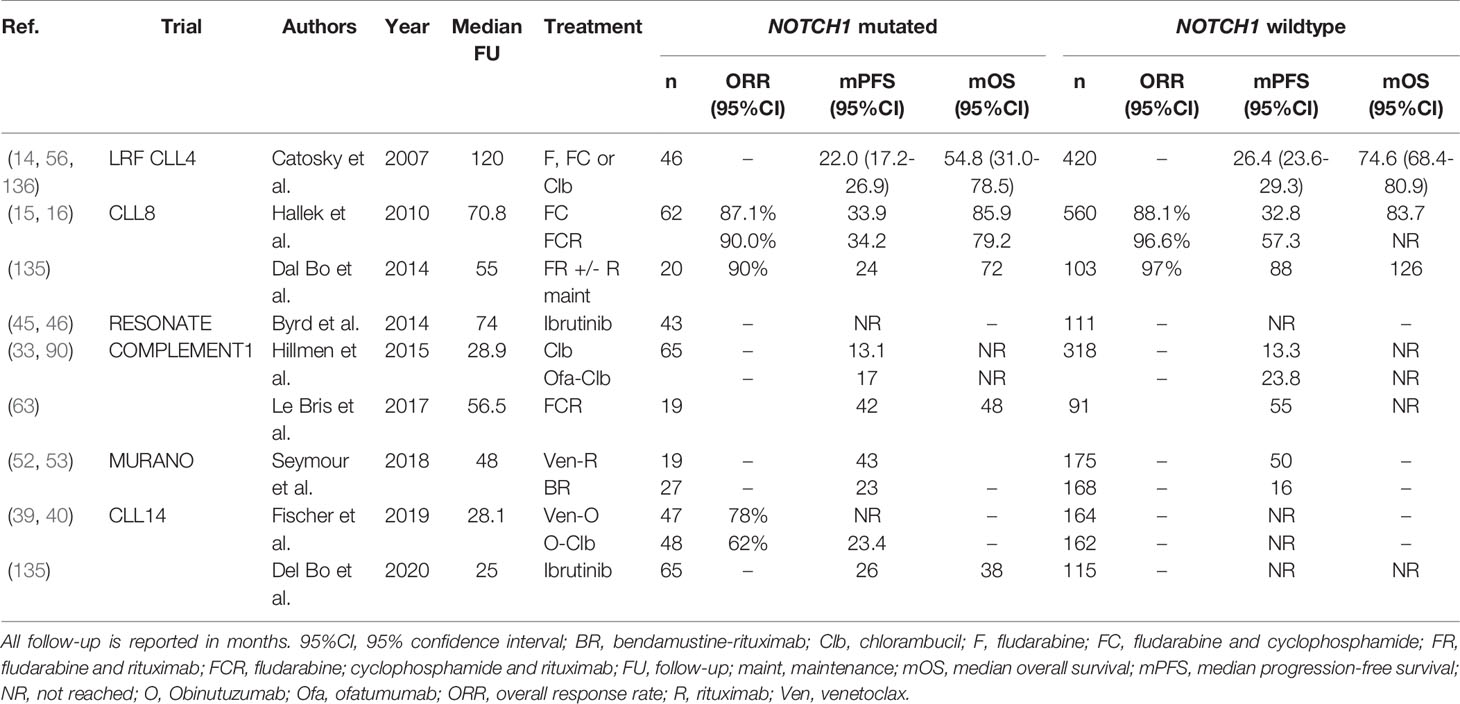
Table 8 An overview of clinical trials comparing treatment regimens in patients with NOTCH1 mutated CLL.
BIRC3 Mutated CLL
Deleterious mutations in BIRC3, a negative regulator of non-canonical NF-κB signaling, are present in 3-5% of newly diagnosed CLL patients (84). Though relatively rare, BIRC3 mutations are associated with poor outcome, with a 10-year OS rate in the chemoimmunotherapy era of just 29%, which was comparable to the OS of patients with TP53 aberrations (137). Evidence from several trials suggests that patients with a BIRC3 mutation (BIRC3-mut) respond poorly to chemoimmunotherapy. Firstly, BIRC3 mutations are enriched in patients with fludarabine-refractory disease, with a prevalence of up to 24% (84). Furthermore, Diop et al. demonstrated that after treatment with FCR, BIRC3-mut patients had significantly shorter PFS, compared with patients without BIRC3 mutations (BIRC3-wt) (median PFS 26.4 months versus ~54 months, P<0.001) (138). Moreover, in the CLL14 trial, the 24-month PFS rate of BIRC3-mut patients after treatment with O-Clb was considerably lower compared with BIRC3-wt patients, and similar to that of patients with del(17p) (BIRC3-mut: 14.3%, del(17p): 23.1%, all patients: 64.1%) (39, 40). Indeed, the ORR of BIRC3-mut patients after O-Clb was 38%, considerably lower than the ORR of the overall O-Clb arm (71%, P<0.05). In contrast, the presence of a BIRC3 mutation does not seem to associate with inferior response to novel agents. In the Ven-O arm of the CLL14 trial, the 24-month PFS rate of BIRC3-mut patients was similar to the overall 24-month PFS rate in that arm (85.7% versus 88.2%). Similarly, in the RESONATE trial, PFS after treatment with ibrutinib for BIRC3-mut and BIRC3-wt patients was not significantly different (46).
Interestingly, BIRC3 is located on chromosome 11q22, and is co-deleted together with ATM in 80% of CLL cases with del(11q) (85). In addition, genomic BIRC3 defects mainly cluster in del(11q) patients, leading to biallelic loss of BIRC3 (85). As such, the clinical significance of trials that report on the impact of mutated BIRC3 need to be interpreted with caution, and ideally stratified for the presence of del(11q) to avoid confounding effects. Monoallelic loss of BIRC3 did not influence survival after chemotherapy in the LRF CLL4 trial, whereas biallelic loss of BIRC3 was associated with shorter OS (median OS 3.3 years versus 4.8 years, P=0.03) (56). Additional research on the predictive impact of monoallelic versus biallelic defects in BIRC3 is warranted. An overview of all clinical trials evaluating BIRC3 in the context of therapy is given in Table 9.
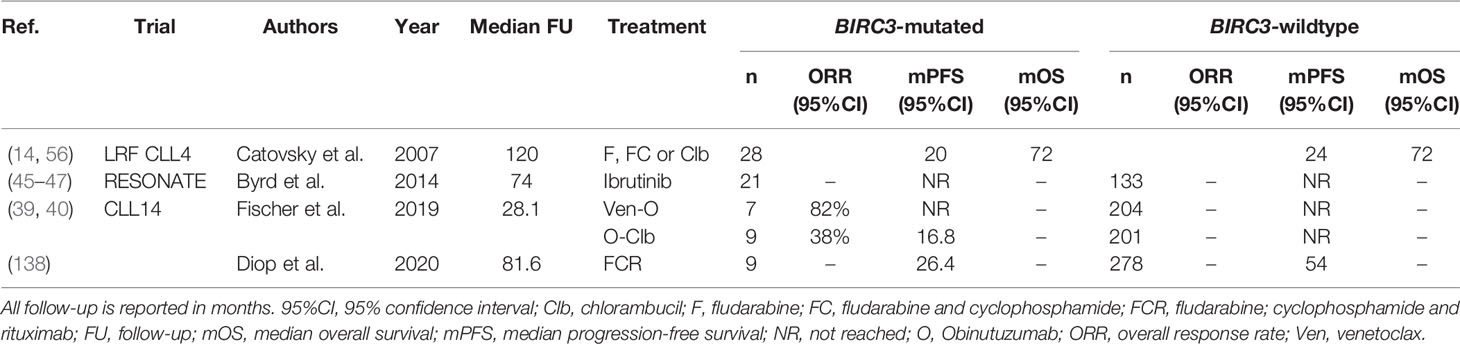
Table 9 An overview of clinical trials comparing treatment regimens in patients with BIRC3 mutated CLL.
New Pharmaceutical Perspectives for CLL Patients With High-Risk Molecular Features
Notwithstanding the significant advances in the field of CLL therapy, as detailed above, a proportion of CLL patients will exhaust all currently approved treatment options. In general, these are patients suffering from CLL with high-risk molecular features, such as aberrant TP53 signaling, presence of CK/GC, or both. For these patients, a number of experimental treatments are currently under development (see Table 10).
Zanubrutinib is a second-generation Btk inhibitor, which, compared with ibrutinib and acalabrutinib, has fewer off-target effects and a longer half-life. In a phase I trial in previously untreated or R/R CLL patients, zanubrutinib monotherapy resulted in an ORR in the overall cohort of 96.2%, and an ORR of 100% in patients with del(17p) (140). Moreover, in another phase I trial, the efficacy and safety of zanubrutinib plus obinutuzumab (O-zanubrutinib) was evaluated (143). This combination yielded an ORR of 100% in previously untreated CLL patients and 92% in R/R CLL patients. The ORR was 100% and 80% in treatment-naïve and R/R CLL patients with del(17p), respectively. The currently ongoing phase III SEQUOIA trial will evaluate the efficacy of zanubrutinib compared with BR in treatment-naïve CLL patients (148). Considering the poor outcome associated with any chemoimmunotherapy in patients with del(17p), those patients were not randomized in this trial but assigned to receive single-agent zanubrutinib, analyzed separately. Tam et al. published the primary report on safety and efficacy of the latter cohort (141). The ORR was 94.5%, with estimated 18-months PFS and OS of 88.6% (95%CI, 79%-94%) and 95.1% (95%CI, 88%-94%), respectively. The currently ongoing, phase III ALPINE trial will evaluate whether in R/R CLL, the efficacy of zanubrutinib is non-inferior, compared with ibrutinib (149).
To overcome treatment resistance against covalent Btk inhibitors, pirtobrutinib, previously known as LOXO-305, a non-covalent Btk inhibitor, has been developed. In the phase I/II BRUIN study, the safety and efficacy of pirtobrutinib were evaluated in R/R B-cell malignancies, including CLL (147). The ORR was 63% (95%CI, 55%-71%) for patients with CLL or SLL, irrespective of whether patients previously discontinued a covalent BTK inhibitor for progression, toxicity or other reasons. In patients with del(17p) and/or TP53 mutation the ORR was 79%, compared with 60% in patients with del(11q) and 68% in patients with U-CLL. The currently ongoing phase III BRUIN CLL-321 trial will compare the efficacy of pirtobrutinib to either R-IDELA or BR in R/R-CLL patients that have been previously treated with a covalent Btk inhibitor (150).
Whereas IDELA only targets the delta subunit of PI3K, the novel agent duvelisib is a dual inhibitor of both the delta and gamma isoforms of PI3K. In the phase III DUO trial, duvelisib treatment was evaluated in patients who were progressive on ofatumumab (144, 145). Duvelisib treatment resulted in an ORR of 77%, with and median PFS of 15.7 months. Comparable responses were seen in patients with del(17p) and/or TP53 mutation (ORR, 77%, median PFS 14.7 months). Based on these results, the Food and Drug Administration (FDA) approved duvelisib for the treatment of R/R CLL and SLL in September 2018 (151). Duvelisib in combination with FCR was recently evaluated in young treatment-naïve CLL patients. The overall response was 88%. Hematological toxicity and infectious complications were common. Only three patients with TP53 aberrations were identified, of whom two responded to the treatment (146).
Ublituximab is a next-generation, glyco-engineered, type I, anti-CD20 mAb that binds to a unique CD20 epitope which is different from the target site of rituximab, obinutuzumab and ofatumumab. In a phase II trial, ublituximab plus ibrutinib yielded an ORR of 88% in all R/R patients and 95% of patients with either TP53 aberrations or del(11q) (139). In a recent, multicenter, phase III trial, ublituximab plus ibrutinib was compared with ibrutinib alone in R/R CLL patients (139). In the overall cohort, the ORR was 83% for ublituximab plus ibrutinib and 65% for ibrutinib alone (P=0.02). PFS was significantly longer in patients treated with ublituximab plus ibrutinib (HR 0.46; 95%CI, 0.24-0.87). In a subgroup analysis, PFS benefit was retained in patients with aberrant TP53 signaling (HR 0.25; 95%CI, 0.10-0.65), but not in patients with del(11q) (HR 0.97; 95%CI, 0.36-2.61).
Cellular Immunotherapy as New Perspective for CLL Patients With High-Risk Molecular Features
Although novel-based agents have revolutionized CLL therapy, they remain incapable of complete disease eradication. To this day, alloHSCT remains, in the context of CLL, the sole treatment with curative intent. Notwithstanding the availability of highly effective, highly tolerable agents, alloHSCT remains a relevant treatment option in several specified situations (152, 153). Currently, alloHSCT can be considered for patients with a relapse after chemoimmunotherapy, either in the presence of TP53 aberrations (high-risk category 1) or with additional failure to BTK inhibitors and/or BCL2 inhibitors, irrespective of the presence of TP53 aberrations (high-risk category 2) (153–155). In the high-risk category 1, the long-term benefits and risks of alloHSCT should be carefully balanced on an individualized basis. Specifically, a younger patient age (<65 years), absence of comorbidities and the availability of a suitable stem cell donor would argue in favor of an alloHSCT, whereas in the converse situation, novel-based agents would be more suitable. Contrastingly, alloHSCT is a more proportional treatment option for patients in high-risk category 2, considering their markedly poor prognosis, due to the limited availability of alternative options (154).
However, the field of immunotherapy has in recent years been revolutionized by the generation of chimeric antigen receptor (CAR) cytotoxic cells. These cells, most often T cells, are molecularly modified to express a single-chain antibody-variable fragment (scFab) with specificity for a marker that is ubiquitously expressed on the malignant target cells, fused to an intracellular CD3ζ domain. CAR efficacy has been further enhanced by the inclusion of a costimulatory domain, most often either CD28 or 4-1BB. CAR-T cells have been approved for the treatment of acute lymphoblastic leukemia, certain types of large B-cell lymphoma and multiple myeloma (156–158). Several investigators have evaluated the efficacy of CAR-T cells, most often directed against CD19, in the setting of R/R CLL (see Table 11). While the reported efficacy differs from study to study, the ORR, CR and median PFS reported in the larger studies (n≥10) are markedly lower (ORR: weighted mean 53%, range 38-71%, CR: weighted mean 26%, range 21%-29%, median PFS 3.1-7 months), compared with the impressive efficacy of CAR-T cell treatment in other lymphatic cancers (165, 169, 171, 174). One possible explanation for the unexpectedly low efficacy or CAR-T cell treatment in CLL is T cell exhaustion. In CLL patients, T cells express markers associated with T cell exhaustion, and the CAR-T cells generated from these source cells may have an impaired ability to kill malignant cells (177). Interestingly, ibrutinib has been found to boost T cell numbers and function in CLL, possibly through off-target effects on interleukin-2 inducible T cell kinase (ITK) or ZAP70, providing a rationale for concurrent treatment with CAR-T cells and ibrutinib (178). Indeed, Gauthier et al. treated 19 heavily-pretreated R/R CLL patients with anti-CD19 CAR-T cells and ibrutinib, achieving an ORR and CR of 83% and 22%, resulting in 1-year PFS and OS rate of 59% and 86%, respectively (172). An alternative approach to circumvent the problem posed by T cell exhaustion is using CAR-transduced natural killer (NK) cells. Allogeneic NK cells can be safely transfused irrespective of a full human leukocyte antigen (HLA) match, allowing the generation of an off-the-shelf, non-patient-specific CAR-construct generated from healthy donor cord blood. In a pilot study, Liu et al. treated 5 R/R CLL patients with anti-CD19 CAR-NK cells from HLA-mismatched donors (173). Of these five patients, three had a complete response, one had a partial response, and one patient did not respond and went on to receive a stem-cell transplantation. Although these results seem promising, both CAR-T cell therapy combined with ibrutinib treatment and CAR-NK cell therapy require more comprehensive evaluation in a larger cohort.
Measuring Predictive Biomarkers in Clinical Care and Research: Author Recommendations
In this paragraph, based on the evidence presented above and summarized in the provided tables, we outline recommendations regarding when to measure the previously discussed predictive biomarkers. The powerful predictive impact of TP53 aberrations is universally recognized and is presently incorporated in treatment decision algorithms in routine care. Consequently, TP53 status should be both cytogenetically and molecularly assessed in all clinical trials and in all CLL patients with an indication for treatment. Although ambiguity remains whether the IGHV mutational status should influence the choice of first-line therapy, it can differentiate between patients with potential long-term remission and patients with a risk of earlier relapse after a time-limited highly effective first-line treatment regimen such as FCR. As such, we recommend assessment of the IGHV mutational status in all clinical trials and in all CLL patients with active disease. Accumulating evidence suggests that CK/GC could function as a predictive marker and is associated with poorer prognosis after chemoimmunotherapy. Although routine measurement of CK/GC may be desirable in clinical care, more evidence is required of the impact of CK in the context of novel agents before it can be incorporated in therapeutic decision-making. Consequently, we strongly recommend to perform CBA or CMA in all clinical trials, especially in trials evaluating novel agents. Additionally, measurement by CBA/CMA will detect the presence of more classical cytogenetic abnormalities, including del(17p) and del(11q), although the predictive relevance of the latter has significantly diminished since the introduction of chemoimmunotherapy and novel agents. The proposed resistance of NOTCH1-mutated CLL to treatment with rituximab and ofatumumab is intriguing and requires further research, especially focusing on the predictive impact of NOTCH1 mutations in the context of regimens containing obininuzumab or ublituximab. Although the incorporation of NOTCH1-mutations in clinical care is not yet warranted, we recommend the assessment of NOTCH1 mutational status in all trials evaluating regimens that include anti-CD20 mAbs. The predictive impact of BCR stereotypy and BIRC3 mutations is currently unclear. As such, we do not recommend their routine assessment in clinical research, nor in patient care. As the prevalence of individual stereotyped subsets and BIRC3 mutations is relatively low, the predictive impact of these biomarkers should be assessed either retrospectively in several pooled trials, or prospectively in specialized trials that specifically recruit patients with the molecular features of interest.
Concluding Remarks
In this review, we have discussed treatment approaches to CLL with high-risk molecular features, providing a comprehensive overview of trials on this topic. Catalyzed by the advent of more advanced molecular techniques, our understanding of the pathophysiology of CLL has deepened over the years, leading to the identification of several cytogenetic, immunogenetic and molecular features that can differentiate between patients with low- and high-risk disease. Some of these, most notably TP53 aberrations, have clear predictive impact and are presently incorporated in decision algorithms in routine care. Their presence is strongly associated with inferior response to chemoimmunotherapy and necessitates the use of novel agents. The predictive capability of other molecular features is less clear, especially in the context of novel-based treatment. The predictive importance of del(11q) has significantly diminished since the advent of chemoimmunotherapy and novel agents and is redundant in therapeutic decision-making. Despite accumulating evidence of a predictive impact from clinical trials, the lack of consistent reporting and standardization prohibits the current use of CK/GC in therapeutic decision-making. In fit patients, stratification by IGHV mutational status identifies patients who benefit markedly from treatment with first-line FCR, but whether U-CLL always warrants first-line treatment with novel agents remains controversial. Interestingly, some aberrations may be predictive in certain specific contexts only, such as the proposed resistance of NOTCH1-mutated CLL to treatment with rituximab and ofatumumab, but this observation requires further validation before NOTCH1 status should be used to guide treatment choice. The data concerning the predictive impact of BCR stereotypy and BIRC3 mutations are currently immature, and treatment choice should not dependent on the presence of these features. The place of second-generation novel agents and cellular immunotherapy in the treatment of CLL with high risk features is still elusive, but forthcoming data from early-stage trials is promising, necessitating further study. Of note, the vast majority of data concerning the predictive impact of the biomarkers discussed above has been obtained through prespecified or post-hoc subgroup analysis. While informative, these trials have not necessarily been powered to answer such questions, especially in the case of rare features. As such, there is an unmet need for randomized trials that evaluate the efficacy of treatments, especially of novel agent-based regimens, in cohorts of patients with pre-specified high-risk molecular features, to move further towards patient-tailored treatment strategies.
Author Contributions
LvdS and PJH performed the collection and interpretations of all relevant literature. LvdS and PJH write the manuscript. APK, AWL and M-DL critically read and revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kristinsson SY, Dickman PW, Wilson WH, Caporaso N, Björkholm M, Landgren O. Improved Survival in Chronic Lymphocytic Leukemia in the Past Decade: A Population-Based Study Including 11,179 Patients Diagnosed Between 1973–2003 in Sweden. Haematologica (2009) 94(9):1259–65. doi: 10.3324/haematol.2009.007849
2. Van den Broek E, Kater A, van de Schans S, Karim-Kos H, Janssen-Heijnen M, Peters W, et al. Chronic Lymphocytic Leukaemia in the Netherlands: Trends in Incidence, Treatment and Survival, 1989–2008. Eur J Cancer (2012) 48(6):889–95. doi: 10.1016/j.ejca.2011.06.053
3. Thygesen LC, Nielsen OJ, Johansen C. Trends in Adult Leukemia Incidence and Survival in Denmark, 1943–2003. Cancer Causes Control (2009) 20(9):1671. doi: 10.1007/s10552-009-9417-9
4. Smith A, Howell D, Patmore R, Jack A, Roman E. Incidence of Haematological Malignancy by Sub-Type: A Report From the Haematological Malignancy Research Network. Br J Cancer (2011) 105(11):1684–92. doi: 10.1038/bjc.2011.450
5. van der Straten L, Levin M-D, Visser O, Posthuma EFM, Doorduijn JK, Kater AP, et al. Survival Continues to Increase in Chronic Lymphocytic Leukaemia: A Population-Based Analysis Among 20 468 Patients Diagnosed in the Netherlands Between 1989 and 2016. Br J Haematol (2020) 189(3):574–7. doi: 10.1111/bjh.16397
6. Rai K, Sawitsky A, Cronkite E, Chanana A, Levy R, Pasternack B. Clinical Staging of Chronic Lymphocytic Leukemia. Blood (1975) 46(2):219–34. doi: 10.1182/blood.V46.2.219.bloodjournal462219
7. Binet J, Auquier A, Dighiero G, Chastang C, Piguet H, Goasguen J, et al. A New Prognostic Classification of Chronic Lymphocytic Leukemia Derived From a Multivariate Survival Analysis. Cancer (1981) 48(1):198–206. doi: 10.1002/1097-0142(19810701)48:1<198::AID-CNCR2820480131>3.0.CO;2-V
8. Group IC-IW. An International Prognostic Index for Patients With Chronic Lymphocytic Leukaemia (CLL-IPI): A Meta-Analysis of Individual Patient Data. Lancet Oncol (2016) 17(6):779–90. doi: 10.1016/S1470-2045(16)30029-8
9. Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Döhner H, et al. Guidelines for Diagnosis, Indications for Treatment, Response Assessment and Supportive Management of Chronic Lymphocytic Leukemia. Blood (2018) 131(25):2745–60. doi: 10.1182/blood-2017-09-806398
10. Gaidano G, Rossi D. The Mutational Landscape of Chronic Lymphocytic Leukemia and Its Impact on Prognosis and Treatment. Hematol Am Soc Hematol Educ Program (2017) 2017(1):329–37. doi: 10.1182/asheducation-2017.1.329
11. Zenz T, Eichhorst B, Busch R, Denzel T, Häbe S, Winkler D, et al. TP53 Mutation and Survival in Chronic Lymphocytic Leukemia. J Clin Oncol (2010) 28(29):4473–9. doi: 10.1200/JCO.2009.27.8762
12. Eichhorst BF, Busch R, Hopfinger G, Pasold R, Hensel M, Steinbrecher C, et al. Fludarabine Plus Cyclophosphamide Versus Fludarabine Alone in First-Line Therapy of Younger Patients With Chronic Lymphocytic Leukemia. Blood (2006) 107(3):885–91. doi: 10.1182/blood-2005-06-2395
13. Grever MR, Lucas DM, Dewald GW, Neuberg DS, Reed JC, Kitada S, et al. Comprehensive Assessment of Genetic and Molecular Features Predicting Outcome in Patients With Chronic Lymphocytic Leukemia: Results From the US Intergroup Phase III Trial E2997. J Clin Oncol (2007) 25(7):799–804. doi: 10.1200/JCO.2006.08.3089
14. Catovsky D, Richards S, Matutes E, Oscier D, Dyer M, Bezares R, et al. Assessment of Fludarabine Plus Cyclophosphamide for Patients With Chronic Lymphocytic Leukaemia (the LRF CLL4 Trial): A Randomised Controlled Trial. Lancet (2007) 370(9583):230–9. doi: 10.1016/S0140-6736(07)61125-8
15. Hallek M, Fischer K, Fingerle-Rowson G, Fink A, Busch R, Mayer J, et al. Addition of Rituximab to Fludarabine and Cyclophosphamide in Patients With Chronic Lymphocytic Leukaemia: A Randomised, Open-Label, Phase 3 Trial. Lancet (2010) 376(9747):1164–74. doi: 10.1016/S0140-6736(10)61381-5
16. Stilgenbauer S, Schnaiter A, Paschka P, Zenz T, Rossi M, Döhner K, et al. Gene Mutations and Treatment Outcome in Chronic Lymphocytic Leukemia: Results From the CLL8 Trial. Blood J Am Soc Hematol (2014) 123(21):3247–54. doi: 10.1182/blood-2014-01-546150
17. Rossi D, Terzi-di-Bergamo L, De Paoli L, Cerri M, Ghilardi G, Chiarenza A, et al. Molecular Prediction of Durable Remission After First-Line Fludarabine-Cyclophosphamide-Rituximab in Chronic Lymphocytic Leukemia. Blood (2015) 126(16):1921–4. doi: 10.1182/blood-2015-05-647925
18. Pettitt AR, Jackson R, Carruthers S, Dodd J, Dodd S, Oates M, et al. Alemtuzumab in Combination With Methylprednisolone Is a Highly Effective Induction Regimen for Patients With Chronic Lymphocytic Leukemia and Deletion of TP53: Final Results of the National Cancer Research Institute CLL206 Trial. J Clin Oncol (2012) 30(14):1647–55. doi: 10.1200/JCO.2011.35.9695
19. Dreger P, Schnaiter A, Zenz T, Böttcher S, Rossi M, Paschka P, et al. TP53, SF3B1, and NOTCH1 Mutations and Outcome of Allotransplantation for Chronic Lymphocytic Leukemia: Six-Year Follow-Up of the GCLLSG CLL3X Trial. Blood J Am Soc Hematol (2013) 121(16):3284–8. doi: 10.1182/blood-2012-11-469627
20. Dreger P, Döhner H, Ritgen M, Böttcher S, Busch R, Dietrich S, et al. Allogeneic Stem Cell Transplantation Provides Durable Disease Control in Poor-Risk Chronic Lymphocytic Leukemia: Long-Term Clinical and MRD Results of the German CLL Study Group CLL3X Trial. Blood (2010) 116(14):2438–47. doi: 10.1182/blood-2010-03-275420
21. Sorror ML, Storer BE, Sandmaier BM, Maris M, Shizuru J, Maziarz R, et al. Five-Year Follow-Up of Patients With Advanced Chronic Lymphocytic Leukemia Treated With Allogeneic Hematopoietic Cell Transplantation After Nonmyeloablative Conditioning. J Clin Oncol (2008) 26(30):4912. doi: 10.1200/JCO.2007.15.4757
22. de Rooij MF, Kuil A, Geest CR, Eldering E, Chang BY, Buggy JJ, et al. The Clinically Active BTK Inhibitor PCI-32765 Targets B-Cell Receptor–and Chemokine-Controlled Adhesion and Migration in Chronic Lymphocytic Leukemia. Blood J Am Soc Hematol (2012) 119(11):2590–4. doi: 10.1182/blood-2011-11-390989
23. Herman SE, Gordon AL, Hertlein E, Ramanunni A, Zhang X, Jaglowski S, et al. Bruton Tyrosine Kinase Represents a Promising Therapeutic Target for Treatment of Chronic Lymphocytic Leukemia and Is Effectively Targeted by PCI-32765. Blood (2011) 117(23):6287–96. doi: 10.1182/blood-2011-01-328484
24. de Gorter DJ, Beuling EA, Kersseboom R, Middendorp S, van Gils JM, Hendriks RW, et al. Bruton's Tyrosine Kinase and Phospholipase Cγ2 Mediate Chemokine-Controlled B Cell Migration and Homing. Immunity (2007) 26(1):93–104. doi: 10.1016/j.immuni.2006.11.012
25. Burger JA, Tedeschi A, Barr PM, Robak T, Owen C, Ghia P, et al. Ibrutinib as Initial Therapy for Patients With Chronic Lymphocytic Leukemia. N Engl J Med (2015) 373(25):2425–37. doi: 10.1056/NEJMoa1509388
26. Barr PM, Robak T, Owen C, Tedeschi A, Bairey O, Bartlett NL, et al. Sustained Efficacy and Detailed Clinical Follow-Up of First-Line Ibrutinib Treatment in Older Patients With Chronic Lymphocytic Leukemia: Extended Phase 3 Results From RESONATE-2. Haematologica (2018) 103(9):1502–10. doi: 10.3324/haematol.2018.192328
27. Burger JA, Barr PM, Robak T, Owen C, Ghia P, Tedeschi A, et al. Long-Term Efficacy and Safety of First-Line Ibrutinib Treatment for Patients With CLL/SLL: 5 Years of Follow-Up From the Phase 3 RESONATE-2 Study. Leukemia (2020) 34(3):787–98. doi: 10.1038/s41375-019-0602-x
28. Farooqui MZ, Valdez J, Martyr S, Aue G, Saba N, Niemann CU, et al. Ibrutinib for Previously Untreated and Relapsed or Refractory Chronic Lymphocytic Leukaemia With TP53 Aberrations: A Phase 2, Single-Arm Trial. Lancet Oncol (2015) 16(2):169–76. doi: 10.1016/S1470-2045(14)71182-9
29. Ahn IE, Farooqui MZH, Tian X, Valdez J, Sun C, Soto S, et al. Depth and Durability of Response to Ibrutinib in CLL: 5-Year Follow-Up of a Phase 2 Study. Blood (2018) 131(21):2357–66. doi: 10.1182/blood-2017-12-820910
30. Woyach JA, Ruppert AS, Heerema NA, Zhao W, Booth AM, Ding W, et al. Ibrutinib Regimens Versus Chemoimmunotherapy in Older Patients With Untreated CLL. N Engl J Med (2018) 379(26):2517–28. doi: 10.1056/NEJMoa1812836
31. Moreno C, Greil R, Demirkan F, Tedeschi A, Anz B, Larratt L, et al. Ibrutinib Plus Obinutuzumab Versus Chlorambucil Plus Obinutuzumab in First-Line Treatment of Chronic Lymphocytic Leukaemia (iLLUMINATE): A Multicentre, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol (2019) 20(1):43–56. doi: 10.1016/S1470-2045(18)30788-5
32. Brieghel C, Aarup K, Torp MH, Andersen MA, Yde CW, Tian X, et al. Clinical Outcomes in Patients With Multi-Hit TP53 Chronic Lymphocytic Leukemia Treated With Ibrutinib. Clin Cancer Res (2021) 27(16):4531–8. doi: 10.1158/1078-0432.CCR-20-4890
33. Lampson BL, Yu L, Glynn RJ, Barrientos JC, Jacobsen ED, Banerji V, et al. Ventricular Arrhythmias and Sudden Death in Patients Taking Ibrutinib. Blood (2017) 129(18):2581–4. doi: 10.1182/blood-2016-10-742437
34. Ysebaert L, Aurran-Schleinitz T, Dartigeas C, Dilhuydy MS, Feugier P, Michallet AS, et al. Real-World Results of Ibrutinib in Relapsed/Refractory CLL in France: Early Results on a Large Series of 428 Patients. Am J Hematol (2017) 92(8):E166–8. doi: 10.1002/ajh.24773
35. van der Straten L, Levin MD, Visser O, B NMA, Cornelissen JJ, Doorduijn JK, et al. The Effectiveness of Ibrutinib in Chronic Lymphocytic Leukaemia: A Nationwide, Population-Based Study in the Netherlands. Br J Haematol (2020) 188:e80–e112. doi: 10.1111/bjh.16391
36. Mato AR, Nabhan C, Thompson MC, Lamanna N, Brander DM, Hill B, et al. Toxicities and Outcomes of 621 Ibrutinib-Treated Chronic Lymphocytic Leukemia Patients in the United States: A Real-World Analysis. Haematologica (2018) 103(5):874–9. doi: 10.3324/haematol.2017.182907
37. Sharman JP, Banerji V, Fogliatto LM, Herishanu Y, Munir T, Walewska R, et al. ELEVATE TN: Phase 3 Study of Acalabrutinib Combined With Obinutuzumab (O) or Alone Vs O Plus Chlorambucil (Clb) in Patients (Pts) With Treatment-Naive Chronic Lymphocytic Leukemia (CLL). Blood (2019) 134(Supplement_1):31. doi: 10.1182/blood-2019-128404
38. Anderson MA, Deng J, Seymour JF, Tam C, Kim SY, Fein J, et al. The BCL2 Selective Inhibitor Venetoclax Induces Rapid Onset Apoptosis of CLL Cells in Patients via a TP53-Independent Mechanism. Blood (2016) 127(25):3215–24. doi: 10.1182/blood-2016-01-688796
39. Tausch E, Schneider C, Robrecht S, Zhang C, Dolnik A, Bloehdorn J, et al. Prognostic and Predictive Impact of Genetic Markers in Patients With CLL Treated With Obinutuzumab and Venetoclax. Blood (2020) 135(26):2402–12. doi: 10.1182/blood.2019004492
40. Fischer K, Al-Sawaf O, Bahlo J, Fink A-M, Tandon M, Dixon M, et al. Venetoclax and Obinutuzumab in Patients With CLL and Coexisting Conditions. N Engl J Med (2019) 380(23):2225–36. doi: 10.1056/NEJMoa1815281
41. Al-Sawaf O, Zhang C, Tandon M, Sinha A, Fink AM, Robrecht S, et al. Venetoclax Plus Obinutuzumab Versus Chlorambucil Plus Obinutuzumab for Previously Untreated Chronic Lymphocytic Leukaemia (CLL14): Follow-Up Results From a Multicentre, Open-Label, Randomised, Phase 3 Trial. Lancet Oncol (2020) 21(9):1188–200. doi: 10.1016/S1470-2045(20)30443-5
42. Badoux XC, Keating MJ, Wang X, O'Brien SM, Ferrajoli A, Faderl S, et al. Fludarabine, Cyclophosphamide, and Rituximab Chemoimmunotherapy Is Highly Effective Treatment for Relapsed Patients With CLL. Blood (2011) 117(11):3016–24. doi: 10.1182/blood-2010-08-304683
43. Zelenetz AD, Barrientos JC, Brown JR, Coiffier B, Delgado J, Egyed M, et al. Idelalisib or Placebo in Combination With Bendamustine and Rituximab in Patients With Relapsed or Refractory Chronic Lymphocytic Leukaemia: Interim Results From a Phase 3, Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Oncol (2017) 18(3):297–311. doi: 10.1016/S1470-2045(16)30671-4
44. Sharman JP, Coutre SE, Furman RR, Cheson BD, Pagel JM, Hillmen P, et al. Final Results of a Randomized, Phase III Study of Rituximab With or Without Idelalisib Followed by Open-Label Idelalisib in Patients With Relapsed Chronic Lymphocytic Leukemia. J Clin Oncol (2019) 37(16):1391–402. doi: 10.1200/JCO.18.01460
45. Byrd JC, Brown JR, O'brien S, Barrientos JC, Kay NE, Reddy NM, et al. Ibrutinib Versus Ofatumumab in Previously Treated Chronic Lymphoid Leukemia. N Engl J Med (2014) 371(3):213–23. doi: 10.1056/NEJMoa1400376
46. Byrd JC, Hillmen P, O'Brien S, Barrientos JC, Reddy NM, Coutre S, et al. Long-Term Follow-Up of the RESONATE Phase 3 Trial of Ibrutinib vs Ofatumumab. Blood (2019) 133(19):2031–42. doi: 10.1182/blood-2018-08-870238
47. Brown JR, Hillmen P, O'Brien S, Barrientos JC, Reddy NM, Coutre SE, et al. Extended Follow-Up and Impact of High-Risk Prognostic Factors From the Phase 3 RESONATE Study in Patients With Previously Treated CLL/SLL. Leukemia (2018) 32(1):83–91. doi: 10.1038/leu.2017.175
48. Munir T, Brown JR, O'Brien S, Barrientos JC, Barr PM, Reddy NM, et al. Final Analysis From RESONATE: Up to Six Years of Follow-Up on Ibrutinib in Patients With Previously Treated Chronic Lymphocytic Leukemia or Small Lymphocytic Lymphoma. Am J Hematol (2019) 94(12):1353–63. doi: 10.1002/ajh.25638
49. O'Brien S, Jones JA, Coutre SE, Mato AR, Hillmen P, Tam C, et al. Ibrutinib for Patients With Relapsed or Refractory Chronic Lymphocytic Leukaemia With 17p Deletion (RESONATE-17): A Phase 2, Open-Label, Multicentre Study. Lancet Oncol (2016) 17(10):1409–18. doi: 10.1016/S1470-2045(16)30212-1
50. Ghia P, Pluta A, Wach M, Lysak D, Kozak T, Simkovic M, et al. ASCEND: Phase III, Randomized Trial of Acalabrutinib Versus Idelalisib Plus Rituximab or Bendamustine Plus Rituximab in Relapsed or Refractory Chronic Lymphocytic Leukemia. J Clin Oncol (2020) 38(35):2849–61. doi: 10.1200/JCO.19.03355
51. Byrd JC, Hillmen P, Ghia P, Kater AP, Chanan-Khan A, Furman RR, et al. Acalabrutinib Versus Ibrutinib in Previously Treated Chronic Lymphocytic Leukemia: Results of the First Randomized Phase III Trial. J Clin Oncol (2021) 39(31):3441–52. doi: 10.1002/hon.33_2879
52. Seymour JF, Kipps TJ, Eichhorst B, Hillmen P, D’Rozario J, Assouline S, et al. Venetoclax–rituximab in Relapsed or Refractory Chronic Lymphocytic Leukemia. N Engl J Med (2018) 378(12):1107–20. doi: 10.1056/NEJMoa1713976
53. Kater AP, Wu JQ, Kipps T, Eichhorst B, Hillmen P, D'Rozario J, et al. Venetoclax Plus Rituximab in Relapsed Chronic Lymphocytic Leukemia: 4-Year Results and Evaluation of Impact of Genomic Complexity and Gene Mutations From the MURANO Phase III Study. J Clin Oncol (2020) 38(34):4042–54. doi: 10.1200/JCO.20.00948
54. Kater AP, Seymour JF, Hillmen P, Eichhorst B, Langerak AW, Owen C, et al. Fixed Duration of Venetoclax-Rituximab in Relapsed/Refractory Chronic Lymphocytic Leukemia Eradicates Minimal Residual Disease and Prolongs Survival: Post-Treatment Follow-Up of the MURANO Phase III Study. J Clin Oncol (2019) 34(4):269–77. doi: 10.1200/JCO.18.01580
55. Roberts AW, Ma S, Kipps TJ, Coutre SE, Davids MS, Eichhorst B, et al. Efficacy of Venetoclax in Relapsed Chronic Lymphocytic Leukemia Is Influenced by Disease and Response Variables. Blood (2019) 134(2):111–22. doi: 10.1182/blood.2018882555
56. Blakemore SJ, Clifford R, Parker H, Antoniou P, Stec-Dziedzic E, Larrayoz M, et al. Clinical Significance of TP53, BIRC3, ATM and MAPK-ERK Genes in Chronic Lymphocytic Leukaemia: Data From the Randomised UK LRF CLL4 Trial. Leukemia (2020) 34(7):1760–74. doi: 10.1038/s41375-020-0723-2
57. Gonzalez D, Martinez P, Wade R, Hockley S, Oscier D, Matutes E, et al. Mutational Status of the TP53 Gene as a Predictor of Response and Survival in Patients With Chronic Lymphocytic Leukemia: Results From the LRF CLL4 Trial. J Clin Oncol (2011) 29(16):2223–9. doi: 10.1200/JCO.2010.32.0838
58. Wierda WG, Kipps TJ, Dürig J, Griskevicius L, Stilgenbauer S, Mayer J, et al. Chemoimmunotherapy With O-FC in Previously Untreated Patients With Chronic Lymphocytic Leukemia. Blood (2011) 117(24):6450–8. doi: 10.1182/blood-2010-12-323980
59. Strati P, Keating MJ, O’Brien SM, Ferrajoli A, Burger J, Faderl S, et al. Outcomes of First-Line Treatment for Chronic Lymphocytic Leukemia With 17p Deletion. Haematologica (2014) 99(8):1350. doi: 10.3324/haematol.2014.104661
60. Tausch E, Beck P, Schlenk RF, Jebaraj BM, Dolnik A, Yosifov DY, et al. Prognostic and Predictive Role of Gene Mutations in Chronic Lymphocytic Leukemia: Results From the Pivotal Phase III Study COMPLEMENT1. Haematologica (2020) 105(10):2440–7. doi: 10.3324/haematol.2019.229161
61. Hillmen P, Robak T, Janssens A, Babu KG, Kloczko J, Grosicki S, et al. Chlorambucil Plus Ofatumumab Versus Chlorambucil Alone in Previously Untreated Patients With Chronic Lymphocytic Leukaemia (COMPLEMENT 1): A Randomised, Multicentre, Open-Label Phase 3 Trial. Lancet (2015) 385(9980):1873–83. doi: 10.1016/S0140-6736(15)60027-7
62. O'Brien SM, Lamanna N, Kipps TJ, Flinn I, Zelenetz AD, Burger JA, et al. A Phase 2 Study of Idelalisib Plus Rituximab in Treatment-Naïve Older Patients With Chronic Lymphocytic Leukemia. Blood (2015) 126(25):2686–94. doi: 10.1182/blood-2015-03-630947
63. Le Bris Y, Struski S, Guièze R, Rouvellat C, Prade N, Troussard X, et al. Major Prognostic Value of Complex Karyotype in Addition to TP53 and IGHV Mutational Status in First-Line Chronic Lymphocytic Leukemia. Hematol Oncol (2017) 35(4):664–70. doi: 10.1002/hon.2349
64. Mato A, Hill B, Lamanna N, Barr P, Ujjani C, Brander D, et al. Optimal Sequencing of Ibrutinib, Idelalisib, and Venetoclax in Chronic Lymphocytic Leukemia: Results From a Multicenter Study of 683 Patients. Ann Oncol (2017) 28(5):1050–6. doi: 10.1093/annonc/mdx031
65. Mato AR, Thompson M, Allan JN, Brander DM, Pagel JM, Ujjani CS, et al. Real-World Outcomes and Management Strategies for Venetoclax-Treated Chronic Lymphocytic Leukemia Patients in the United States. Haematologica (2018) 103(9):1511–7. doi: 10.3324/haematol.2018.193615
66. Takahashi K, Hu B, Wang F, Yan Y, Kim E, Vitale C, et al. Clinical Implications of Cancer Gene Mutations in Patients With Chronic Lymphocytic Leukemia Treated With Lenalidomide. Blood (2018) 131(16):1820–32. doi: 10.1182/blood-2017-11-817296
67. Burger JA, Sivina M, Jain N, Kim E, Kadia T, Estrov Z, et al. Randomized Trial of Ibrutinib vs Ibrutinib Plus Rituximab in Patients With Chronic Lymphocytic Leukemia. Blood (2019) 133(10):1011–9. doi: 10.1182/blood-2018-10-879429
68. Sharman JP, Egyed M, Jurczak W, Skarbnik A, Pagel JM, Flinn IW, et al. Acalabrutinib With or Without Obinutuzumab Versus Chlorambucil and Obinutuzmab for Treatment-Naive Chronic Lymphocytic Leukaemia (ELEVATE TN): A Randomised, Controlled, Phase 3 Trial. Lancet (2020) 395(10232):1278–91. doi: 10.1016/S0140-6736(20)30262-2
69. Stilgenbauer S, Bosch F, Ilhan O, Kisro J, Mahé B, Mikuskova E, et al. Safety and Efficacy of Obinutuzumab Alone or With Chemotherapy in Previously Untreated or Relapsed/Refractory Chronic Lymphocytic Leukaemia Patients: Final Analysis of the Phase IIIb GREEN Study. Br J Haematol (2021) 193(2):325–38. doi: 10.1111/bjh.17326
70. Stilgenbauer S, Zenz T, Winkler D, Bühler A, Schlenk RF, Groner S, et al. Subcutaneous Alemtuzumab in Fludarabine-Refractory Chronic Lymphocytic Leukemia: Clinical Results and Prognostic Marker Analyses From the CLL2H Study of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol (2009) 27(24):3994–4001. doi: 10.1200/JCO.2008.21.1128
71. Furman RR, Sharman JP, Coutre SE, Cheson BD, Pagel JM, Hillmen P, et al. Idelalisib and Rituximab in Relapsed Chronic Lymphocytic Leukemia. N Engl J Med (2014) 370(11):997–1007. doi: 10.1056/NEJMoa1315226
72. Byrd JC, Furman RR, Coutre SE, Burger JA, Blum KA, Coleman M, et al. Three-Year Follow-Up of Treatment-Naïve and Previously Treated Patients With CLL and SLL Receiving Single-Agent Ibrutinib. Blood (2015) 125(16):2497–506. doi: 10.1182/blood-2014-10-606038
73. Thompson PA, O'Brien SM, Wierda WG, Ferrajoli A, Stingo F, Smith SC, et al. Complex Karyotype Is a Stronger Predictor Than Del(17p) for an Inferior Outcome in Relapsed or Refractory Chronic Lymphocytic Leukemia Patients Treated With Ibrutinib-Based Regimens. Cancer (2015) 121(20):3612–21. doi: 10.1002/cncr.29566
74. Jaglowski SM, Jones JA, Nagar V, Flynn JM, Andritsos LA, Maddocks KJ, et al. Safety and Activity of BTK Inhibitor Ibrutinib Combined With Ofatumumab in Chronic Lymphocytic Leukemia: A Phase 1b/2 Study. Blood (2015) 126(7):842–50. doi: 10.1182/blood-2014-12-617522
75. Bühler A, Wendtner CM, Kipps TJ, Rassenti L, Fraser GAM, Michallet AS, et al. Lenalidomide Treatment and Prognostic Markers in Relapsed or Refractory Chronic Lymphocytic Leukemia: Data From the Prospective, Multicenter Phase-II CLL-009 Trial. Blood Cancer J (2016) 6(3):e404. doi: 10.1038/bcj.2016.9
76. Roberts AW, Davids MS, Pagel JM, Kahl BS, Puvvada SD, Gerecitano JF, et al. Targeting BCL2 With Venetoclax in Relapsed Chronic Lymphocytic Leukemia. N Engl J Med (2016) 374(4):311–22. doi: 10.1056/NEJMoa1513257
77. Byrd JC, Harrington B, O'Brien S, Jones JA, Schuh A, Devereux S, et al. Acalabrutinib (ACP-196) in Relapsed Chronic Lymphocytic Leukemia. N Engl J Med (2016) 374(4):323–32. doi: 10.1056/NEJMoa1509981
78. Stilgenbauer S, Eichhorst B, Schetelig J, Coutre S, Seymour JF, Munir T, et al. Venetoclax in Relapsed or Refractory Chronic Lymphocytic Leukaemia With 17p Deletion: A Multicentre, Open-Label, Phase 2 Study. Lancet Oncol (2016) 17(6):768–78. doi: 10.1016/S1470-2045(16)30019-5
79. O’Brien S, Furman RR, Coutre S, Flinn IW, Burger JA, Blum K, et al. Single-Agent Ibrutinib in Treatment-Naïve and Relapsed/Refractory Chronic Lymphocytic Leukemia: A 5-Year Experience. Blood (2018) 131(17):1910–9. doi: 10.1182/blood-2017-10-810044
80. Stilgenbauer S, Eichhorst B, Schetelig J, Hillmen P, Seymour JF, Coutre S, et al. Venetoclax for Patients With Chronic Lymphocytic Leukemia With 17p Deletion: Results From the Full Population of a Phase II Pivotal Trial. J Clin Oncol (2018) 36(19):1973–80. doi: 10.1200/JCO.2017.76.6840
81. Byrd JC, Wierda WG, Schuh A, Devereux S, Chaves JM, Brown JR, et al. Acalabrutinib Monotherapy in Patients With Relapsed/Refractory Chronic Lymphocytic Leukemia: Updated Phase 2 Results. Blood (2020) 135(15):1204–13. doi: 10.1182/blood.2018884940
82. Döhner H, Stilgenbauer S, James MR, Benner A, Weilguni T, Bentz M, et al. 11q Deletions Identify a New Subset of B-Cell Chronic Lymphocytic Leukemia Characterized by Extensive Nodal Involvement and Inferior Prognosis. Blood (1997) 89(7):2516–22. doi: 10.1182/blood.V89.7.2516
83. Döhner H, Stilgenbauer S, Benner A, Leupolt E, Kröber A, Bullinger L, et al. Genomic Aberrations and Survival in Chronic Lymphocytic Leukemia. N Engl J Med (2000) 343(26):1910–6. doi: 10.1056/NEJM200012283432602
84. Rossi D, Fangazio M, Rasi S, Vaisitti T, Monti S, Cresta S, et al. Disruption of BIRC3 Associates With Fludarabine Chemorefractoriness in TP53 Wild-Type Chronic Lymphocytic Leukemia. Blood (2012) 119(12):2854–62. doi: 10.1182/blood-2011-12-395673
85. Rose-Zerilli MJ, Forster J, Parker H, Parker A, Rodríguez AE, Chaplin T, et al. ATM Mutation Rather Than BIRC3 Deletion and/or Mutation Predicts Reduced Survival in 11q-Deleted Chronic Lymphocytic Leukemia: Data From the UK LRF CLL4 Trial. Haematologica (2014) 99(4):736. doi: 10.3324/haematol.2013.098574
86. Austen B, Skowronska A, Baker C, Powell JE, Gardiner A, Oscier D, et al. Mutation Status of the Residual ATM Allele Is an Important Determinant of the Cellular Response to Chemotherapy and Survival in Patients With Chronic Lymphocytic Leukemia Containing an 11q Deletion. J Clin Oncol (2007) 25(34):5448–57. doi: 10.1200/JCO.2007.11.2649
87. Flinn IW, Neuberg DS, Grever MR, Dewald GW, Bennett JM, Paietta EM, et al. Phase III Trial of Fludarabine Plus Cyclophosphamide Compared With Fludarabine for Patients With Previously Untreated Chronic Lymphocytic Leukemia: US Intergroup Trial E2997. J Clin Oncol (2007) 25(7):793–8. doi: 10.1200/JCO.2006.08.0762
88. Fischer K, Bahlo J, Fink AM, Goede V, Herling CD, Cramer P, et al. Long-Term Remissions After FCR Chemoimmunotherapy in Previously Untreated Patients With CLL: Updated Results of the CLL8 Trial. Blood (2016) 127(2):208–15. doi: 10.1182/blood-2015-06-651125
89. Eichhorst B, Fink A-M, Bahlo J, Busch R, Kovacs G, Maurer C, et al. First-Line Chemoimmunotherapy With Bendamustine and Rituximab Versus Fludarabine, Cyclophosphamide, and Rituximab in Patients With Advanced Chronic Lymphocytic Leukaemia (CLL10): An International, Open-Label, Randomised, Phase 3, Non-Inferiority Trial. Lancet Oncol (2016) 17(7):928–42. doi: 10.1016/S1470-2045(16)30051-1
90. Shanafelt TD, Wang XV, Kay NE, Hanson CA, O’Brien S, Barrientos J, et al. Ibrutinib–Rituximab or Chemoimmunotherapy for Chronic Lymphocytic Leukemia. N Engl J Med (2019) 381(5):432–43. doi: 10.1056/NEJMoa1817073
91. Kipps TJ, Fraser G, Coutre SE, Brown JR, Barrientos JC, Barr PM, et al. Long-Term Studies Assessing Outcomes of Ibrutinib Therapy in Patients With Del (11q) Chronic Lymphocytic Leukemia. Clin Lymphoma Myeloma Leukemia (2019) 19(11):715–22.e6. doi: 10.1016/j.clml.2019.07.004
92. Fraser G, Cramer P, Demirkan F, Silva RS, Grosicki S, Pristupa A, et al. Updated Results From the Phase 3 HELIOS Study of Ibrutinib, Bendamustine, and Rituximab in Relapsed Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma. Leukemia (2019) 33(4):969–80. doi: 10.1038/s41375-018-0276-9
93. Chanan-Khan A, Cramer P, Demirkan F, Fraser G, Silva RS, Grosicki S, et al. Ibrutinib Combined With Bendamustine and Rituximab Compared With Placebo, Bendamustine, and Rituximab for Previously Treated Chronic Lymphocytic Leukaemia or Small Lymphocytic Lymphoma (HELIOS): A Randomised, Double-Blind, Phase 3 Study. Lancet Oncol (2016) 17(2):200–11. doi: 10.1016/S1470-2045(15)00465-9
94. Rack KA, van den Berg E, Haferlach C, Beverloo HB, Costa D, Espinet B, et al. European Recommendations and Quality Assurance for Cytogenomic Analysis of Haematological Neoplasms. Leukemia (2019) 33(8):1851–67. doi: 10.1038/s41375-019-0378-z
95. Cuneo A, Rigolin GM, Bigoni R, De Angeli C, Veronese A, Cavazzini F, et al. Chronic Lymphocytic Leukemia With 6q- Shows Distinct Hematological Features and Intermediate Prognosis. Leukemia (2004) 18(3):476–83. doi: 10.1038/sj.leu.2403242
96. Kostopoulou F, Gabillaud C, Chapiro E, Grange B, Tran J, Bouzy S, et al. Gain of the Short Arm of Chromosome 2 (2p Gain) has a Significant Role in Drug-Resistant Chronic Lymphocytic Leukemia. Cancer Med (2019) 8(6):3131–41. doi: 10.1002/cam4.2123
97. Chapiro E, Radford-Weiss I, Bastard C, Luquet I, Lefebvre C, Callet-Bauchu E, et al. The Most Frequent T ((Q32;Q13)-Positive B-Cell Malignancy Corresponds to an Aggressive Subgroup of Atypical Chronic Lymphocytic Leukemia. Leukemia (2008) 22(11):2123–7. doi: 10.1038/leu.2008.102
98. Rigolin GM, Cibien F, Martinelli S, Formigaro L, Rizzotto L, Tammiso E, et al. Chromosome Aberrations Detected by Conventional Karyotyping Using Novel Mitogens in Chronic Lymphocytic Leukemia With “Normal” FISH: Correlations With Clinicobiologic Parameters. Blood (2012) 119(10):2310–3. doi: 10.1182/blood-2011-11-395269
99. Sargent R, Jones D, Abruzzo LV, Yao H, Bonderover J, Cisneros M, et al. Customized Oligonucleotide Array-Based Comparative Genomic Hybridization as a Clinical Assay for Genomic Profiling of Chronic Lymphocytic Leukemia. J Mol Diagnostics JMD (2009) 11(1):25–34. doi: 10.2353/jmoldx.2009.080037
100. Simons A, Sikkema-Raddatz B, de Leeuw N, Konrad NC, Hastings RJ, Schoumans J. Genome-Wide Arrays in Routine Diagnostics of Hematological Malignancies. Hum Mutat (2012) 33(6):941–8. doi: 10.1002/humu.22057
101. Schoumans J, Suela J, Hastings R, Muehlematter D, Rack K, van den Berg E, et al. Guidelines for Genomic Array Analysis in Acquired Haematological Neoplastic Disorders. Genes Chromosomes Cancer (2016) 55(5):480–91. doi: 10.1002/gcc.22350
102. Juliusson G, Oscier DG, Fitchett M, Ross FM, Stockdill G, Mackie MJ, et al. Prognostic Subgroups in B-Cell Chronic Lymphocytic Leukemia Defined by Specific Chromosomal Abnormalities. N Engl J Med (1990) 323(11):720–4. doi: 10.1056/NEJM199009133231105
103. Haferlach C, Dicker F, Schnittger S, Kern W, Haferlach T. Comprehensive Genetic Characterization of CLL: A Study on 506 Cases Analysed With Chromosome Banding Analysis, Interphase FISH, IgV(H) Status and Immunophenotyping. Leukemia (2007) 21(12):2442–51. doi: 10.1038/sj.leu.2404935
104. Mayr C, Speicher MR, Kofler DM, Buhmann R, Strehl J, Busch R, et al. Chromosomal Translocations Are Associated With Poor Prognosis in Chronic Lymphocytic Leukemia. Blood (2006) 107(2):742–51. doi: 10.1182/blood-2005-05-2093
105. Dierlamm J, Michaux L, Criel A, Wlodarska I, Van den Berghe H, Hossfeld DK. Genetic Abnormalities in Chronic Lymphocytic Leukemia and Their Clinical and Prognostic Implications. Cancer Genet Cytogenet (1997) 94(1):27–35. doi: 10.1016/S0165-4608(96)00246-4
106. Gahrton G, Robèrt KH, Friberg K, Zech L, Bird AG. Nonrandom Chromosomal Aberrations in Chronic Lymphocytic Leukemia Revealed by Polyclonal B-Cell-Mitogen Stimulation. Blood (1980) 56(4):640–7. doi: 10.1182/blood.V56.4.640.640
107. Haferlach C, Dicker F, Weiss T, Schnittger S, Beck C, Grote-Metke A, et al. Toward a Comprehensive Prognostic Scoring System in Chronic Lymphocytic Leukemia Based on a Combination of Genetic Parameters. Genes Chromosomes Cancer (2010) 49(9):851–9. doi: 10.1002/gcc.20794
108. Puiggros A, Collado R, Calasanz MJ, Ortega M, Ruiz-Xivillé N, Rivas-Delgado A, et al. Patients With Chronic Lymphocytic Leukemia and Complex Karyotype Show an Adverse Outcome Even in Absence of TP53/ATM FISH Deletions. Oncotarget (2017) 8(33):54297–303. doi: 10.18632/oncotarget.17350
109. Blanco G, Puiggros A, Baliakas P, Athanasiadou A, García-Malo M, Collado R, et al. Karyotypic Complexity Rather Than Chromosome 8 Abnormalities Aggravates the Outcome of Chronic Lymphocytic Leukemia Patients With TP53 Aberrations. Oncotarget (2016) 7(49):80916–24. doi: 10.18632/oncotarget.13106
110. Oscier D, Stevens J, Hamblin T, Pickering R, Lambert R, Fitchett M. Correlation of Chromosome Abnormalities With Laboratory Features and Clinical Course in B-Cell Chronic Lymphocytic Leukaemia. Br J haematol (1990) 76(3):352–8. doi: 10.1111/j.1365-2141.1990.tb06367.x
111. Oscier D, Fitchett M, Herbert T, Lambert R. Karyotypic Evolution in B-Cell Chronic Lymphocytic Leukaemia. Genes Chromosomes Cancer (1991) 3(1):16–20. doi: 10.1002/gcc.2870030104
112. Datta T, Bauchinger M, Emmerich B, Reichle A. Chromosome Analyses in Chronic Lymphocytic Leukemia and Related B-Cell Neoplasms. Cancer Genet Cytogenet (1991) 55(1):49–56. doi: 10.1016/0165-4608(91)90234-L
113. Juliusson G, Robèrt KH, Öst Å, Friberg K, Biberfeld P, Nilsson B, et al. Prognostic Information From Cytogenetic Analysis in Chronic B-Lymphocytic Leukemia and Leukemic Immunocytoma. Blood (1985) 65(1):134–41. doi: 10.1182/blood.V65.1.134.134
114. Baliakas P, Iskas M, Gardiner A, Davis Z, Plevova K, Nguyen-Khac F, et al. Chromosomal Translocations and Karyotype Complexity in Chronic Lymphocytic Leukemia: A Systematic Reappraisal of Classic Cytogenetic Data. Am J Hematol (2014) 89(3):249–55. doi: 10.1002/ajh.23618
115. Baliakas P, Jeromin S, Iskas M, Puiggros A, Plevova K, Nguyen-Khac F, et al. Cytogenetic Complexity in Chronic Lymphocytic Leukemia: Definitions, Associations, and Clinical Impact. Blood (2019) 133(11):1205–16. doi: 10.1182/blood-2018-09-873083
116. Jondreville L, Krzisch D, Chapiro E, Nguyen-Khac F. The Complex Karyotype and Chronic Lymphocytic Leukemia: Prognostic Value and Diagnostic Recommendations. Am J Hematol (2020) 95(11):1361–7. doi: 10.1002/ajh.25956
117. Goede V, Fischer K, Busch R, Engelke A, Eichhorst B, Wendtner CM, et al. Obinutuzumab Plus Chlorambucil in Patients With CLL and Coexisting Conditions. N Engl J Med (2014) 370(12):1101–10. doi: 10.1056/NEJMoa1313984
118. Herling CD, Klaumünzer M, Rocha CK, Altmüller J, Thiele H, Bahlo J, et al. Complex Karyotypes and KRAS and POT1 Mutations Impact Outcome in CLL After Chlorambucil-Based Chemotherapy or Chemoimmunotherapy. Blood (2016) 128(3):395–404. doi: 10.1182/blood-2016-01-691550
119. Kreuzer KA, Furman RR, Stilgenbauer S, Dubowy RL, Kim Y, Munugalavadla V, et al. The Impact of Complex Karyotype on the Overall Survival of Patients With Relapsed Chronic Lymphocytic Leukemia Treated With Idelalisib Plus Rituximab. Leukemia (2020) 34(1):296–300. doi: 10.1038/s41375-019-0533-6
120. Anderson MA, Tam C, Lew TE, Juneja S, Juneja M, Westerman D, et al. Clinicopathological Features and Outcomes of Progression of CLL on the BCL2 Inhibitor Venetoclax. Blood (2017) 129(25):3362–70. doi: 10.1182/blood-2017-01-763003
121. Damle RN, Wasil T, Fais F, Ghiotto F, Valetto A, Allen SL, et al. Ig V Gene Mutation Status and CD38 Expression as Novel Prognostic Indicators in Chronic Lymphocytic Leukemia. Blood (1999) 94(6):1840–7. doi: 10.1182/blood.V94.6.1840.418k06_1840_1847
122. Hamblin TJ, Davis Z, Gardiner A, Oscier DG, Stevenson FK. Unmutated Ig V(H) Genes Are Associated With a More Aggressive Form of Chronic Lymphocytic Leukemia. Blood (1999) 94(6):1848–54. doi: 10.1182/blood.V94.6.1848.418k05_1848_1854
123. Del Giudice I, Mauro FR, De Propris MS, Santangelo S, Marinelli M, Peragine N, et al. White Blood Cell Count at Diagnosis and Immunoglobulin Variable Region Gene Mutations Are Independent Predictors of Treatment-Free Survival in Young Patients With Stage A Chronic Lymphocytic Leukemia. Haematologica (2011) 96(4):626. doi: 10.3324/haematol.2010.028779
124. Thompson PA, Tam CS, O’Brien SM, Wierda WG, Stingo F, Plunkett W, et al. Fludarabine, Cyclophosphamide, and Rituximab Treatment Achieves Long-Term Disease-Free Survival in IGHV-Mutated Chronic Lymphocytic Leukemia. Blood (2016) 127(3):303–9. doi: 10.1182/blood-2015-09-667675
125. Tam CS, O'Brien S, Wierda W, Kantarjian H, Wen S, Do KA, et al. Long-Term Results of the Fludarabine, Cyclophosphamide, and Rituximab Regimen as Initial Therapy of Chronic Lymphocytic Leukemia. Blood (2008) 112(4):975–80. doi: 10.1182/blood-2008-02-140582
126. Fink AM, Böttcher S, Ritgen M, Fischer K, Pflug N, Eichhorst B, et al. Prediction of Poor Outcome in CLL Patients Following First-Line Treatment With Fludarabine, Cyclophosphamide and Rituximab. Leukemia (2013) 27(9):1949–52. doi: 10.1038/leu.2013.190
127. Fischer K, Cramer P, Busch R, Böttcher S, Bahlo J, Schubert J, et al. Bendamustine in Combination With Rituximab for Previously Untreated Patients With Chronic Lymphocytic Leukemia: A Multicenter Phase II Trial of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol (2012) 30(26):3209–16. doi: 10.1200/JCO.2011.39.2688
128. Agathangelidis A, Chatzidimitriou A, Gemenetzi K, Giudicelli V, Karypidou M, Plevova K, et al. Higher-Order Connections Between Stereotyped Subsets: Implications for Improved Patient Classification in CLL. Blood (2021) 137(10):1365–76. doi: 10.1182/blood.2020007039
129. Jaramillo S, Agathangelidis A, Schneider C, Bahlo J, Robrecht S, Tausch E, et al. Prognostic Impact of Prevalent Chronic Lymphocytic Leukemia Stereotyped Subsets: Analysis Within Prospective Clinical Trials of the German CLL Study Group (GCLLSG). Haematologica (2020) 105(11):2598–607. doi: 10.3324/haematol.2019.231027
130. Rossi D, Spina V, Cerri M, Rasi S, Deambrogi C, De Paoli L, et al. Stereotyped B-Cell Receptor Is an Independent Risk Factor of Chronic Lymphocytic Leukemia Transformation to Richter Syndrome. Clin Cancer Res (2009) 15(13):4415–22. doi: 10.1158/1078-0432.CCR-08-3266
131. Rosati E, Baldoni S, De Falco F, Del Papa B, Dorillo E, Rompietti C, et al. NOTCH1 Aberrations in Chronic Lymphocytic Leukemia. Front Oncol (2018) 8:229–. doi: 10.3389/fonc.2018.00229
132. Fabbri G, Rasi S, Rossi D, Trifonov V, Khiabanian H, Ma J, et al. Analysis of the Chronic Lymphocytic Leukemia Coding Genome: Role of NOTCH1 Mutational Activation. J Exp Med (2011) 208(7):1389–401. doi: 10.1084/jem.20110921
133. Puente XS, Beà S, Valdés-Mas R, Villamor N, Gutiérrez-Abril J, Martín-Subero JI, et al. Non-Coding Recurrent Mutations in Chronic Lymphocytic Leukaemia. Nature (2015) 526(7574):519–24. doi: 10.1038/nature14666
134. Leeksma AC, Taylor J, Wu B, Gardner JR, He J, Nahas M, et al. Clonal Diversity Predicts Adverse Outcome in Chronic Lymphocytic Leukemia. Leukemia (2019) 33(2):390–402. doi: 10.1038/s41375-018-0215-9
135. Bo MD, Del Principe MI, Pozzo F, Ragusa D, Bulian P, Rossi D, et al. NOTCH1 Mutations Identify a Chronic Lymphocytic Leukemia Patient Subset With Worse Prognosis in the Setting of a Rituximab-Based Induction and Consolidation Treatment. Ann Hematol (2014) 93(10):1765–74. doi: 10.1007/s00277-014-2117-x
136. Oscier DG, Rose-Zerilli MJJ, Winkelmann N, Gonzalez de Castro D, Gomez B, Forster J, et al. The Clinical Significance of NOTCH1 and SF3B1 Mutations in the UK LRF CLL4 Trial. Blood (2013) 121(3):468–75. doi: 10.1182/blood-2012-05-429282
137. Rossi D, Rasi S, Spina V, Bruscaggin A, Monti S, Ciardullo C, et al. Integrated Mutational and Cytogenetic Analysis Identifies New Prognostic Subgroups in Chronic Lymphocytic Leukemia. Blood (2013) 121(8):1403–12. doi: 10.1182/blood-2012-09-458265
138. Diop F, Moia R, Favini C, Spaccarotella E, De Paoli L, Bruscaggin A, et al. Biological and Clinical Implications of BIRC3 Mutations in Chronic Lymphocytic Leukemia. Haematologica (2020) 105(2):448. doi: 10.3324/haematol.2019.219550
139. Sharman JP, Brander DM, Mato AR, Ghosh N, Schuster SJ, Kambhampati S, et al. Ublituximab Plus Ibrutinib Versus Ibrutinib Alone for Patients With Relapsed or Refractory High-Risk Chronic Lymphocytic Leukaemia (GENUINE): A Phase 3, Multicentre, Open-Label, Randomised Trial. Lancet Haematol (2021) 8(4):e254–e66. doi: 10.1016/S2352-3026(20)30433-6
140. Tam CS, Trotman J, Opat S, Burger JA, Cull G, Gottlieb D, et al. Phase 1 Study of the Selective BTK Inhibitor Zanubrutinib in B-Cell Malignancies and Safety and Efficacy Evaluation in CLL. Blood (2019) 134(11):851–9. doi: 10.1182/blood.2019001160
141. Tam CS, Robak T, Ghia P, Kahl BS, Walker P, Janowski W, et al. Zanubrutinib Monotherapy for Patients With Treatment Naïve Chronic Lymphocytic Leukemia and 17p Deletion. Haematologica (2020) 106(9):2363. doi: 10.3324/haematol.2020.259432
142. Xu W, Yang S, Zhou K, Pan L, Li Z, Zhou J, et al. Treatment of Relapsed/Refractory Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma With the BTK Inhibitor Zanubrutinib: Phase 2, Single-Arm, Multicenter Study. J Hematol Oncol (2020) 13:1–12. doi: 10.1186/s13045-020-00884-4
143. Tam CS, Quach H, Nicol A, Badoux X, Rose H, Prince HM, et al. Zanubrutinib (BGB-3111) Plus Obinutuzumab in Patients With Chronic Lymphocytic Leukemia and Follicular Lymphoma. Blood Adv (2020) 4(19):4802–11. doi: 10.1182/bloodadvances.2020002183
144. Davids MS, Kuss BJ, Hillmen P, Montillo M, Moreno C, Essell J, et al. Efficacy and Safety of Duvelisib Following Disease Progression on Ofatumumab in Patients With Relapsed/Refractory CLL or SLL in the DUO Crossover Extension Study. Clin Cancer Res (2020) 26(9):2096–103. doi: 10.1158/1078-0432.CCR-19-3061
145. Flinn IW, O’Brien S, Kahl B, Patel M, Oki Y, Foss FF, et al. Duvelisib, a Novel Oral Dual Inhibitor of PI3K-δ,γ, Is Clinically Active in Advanced Hematologic Malignancies. Blood (2018) 131(8):877–87. doi: 10.1182/blood-2017-05-786566
146. Davids MS, Fisher DC, Tyekucheva S, McDonough M, Hanna J, Lee B, et al. A Phase 1b/2 Study of Duvelisib in Combination With FCR (DFCR) for Frontline Therapy for Younger CLL Patients. Leukemia (2021) 35(4):1064–72. doi: 10.1038/s41375-020-01010-6
147. Mato AR, Shah NN, Jurczak W, Cheah CY, Pagel JM, Woyach JA, et al. Pirtobrutinib in Relapsed or Refractory B-Cell Malignancies (BRUIN): A Phase 1/2 Study. Lancet (2021) 397(10277):892–901. doi: 10.1016/S0140-6736(21)00224-5
148. ClinicalTrials.gov. A Study Comparing Zanubrutinib With Bendamustine Plus Rituximab in Participants With Previously Untreated CLL or SLL (SEQUOIA). (2017). Available at: https://clinicaltrials.gov/ct2/show/NCT03336333
149. ClinicalTrials.gov. A Study of Zanubrutinib (BGB-3111) Versus Ibrutinib in Participants With Relapsed/Refractory Chronic Lymphocytic Leukemia (ALPINE)(2018). Available at: https://clinicaltrials.gov/ct2/show/NCT03734016.
150. ClinicalTrials.gov. Study of LOXO-305 Versus Investigator's Choice (IdelaR or BR) in Patients With CLL or SLL(2020). Available at: https://clinicaltrials.gov/ct2/show/NCT04666038.
151. FDA. Duvelisib (COPIKTRA, Verastem, Inc.) for Adult Patients With Relapsed or Refractory Chronic Lymphocytic Leukemia (CLL) or Small Lymphocytic Lymphoma (SLL). (2018). Available at: https://www.fda.gov/drugs/resources-information-approved-drugs/duvelisib-copiktra-verastem-inc-adult-patients-relapsed-or-refractory-chronic-lymphocytic-leukemia
152. Kharfan-Dabaja MA, Kumar A, Hamadani M, Stilgenbauer S, Ghia P, Anasetti C, et al. Clinical Practice Recommendations for Use of Allogeneic Hematopoietic Cell Transplantation in Chronic Lymphocytic Leukemia on Behalf of the Guidelines Committee of the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant (2016) 22(12):2117–25. doi: 10.1016/j.bbmt.2016.09.013
153. Hoffmann A, Dietrich S, Hain S, Rieger M, Hegenbart U, Sellner L, et al. Allogeneic Transplantation in High-Risk Chronic Lymphocytic Leukemia: A Single-Center, Intent-to-Treat Analysis. Haematologica (2019) 104(7):e304–e6. doi: 10.3324/haematol.2018.209486
154. Dreger P, Ghia P, Schetelig J, van Gelder M, Kimby E, Michallet M, et al. High-Risk Chronic Lymphocytic Leukemia in the Era of Pathway Inhibitors: Integrating Molecular and Cellular Therapies. Blood (2018) 132(9):892–902. doi: 10.1182/blood-2018-01-826008
155. Gribben JG. How and When I do Allogeneic Transplant in CLL. Blood (2018) 132(1):31–9. doi: 10.1182/blood-2018-01-785998
156. FDA. FDA Approves Tisagenlecleucel for B-Cell ALL and Tocilizumab for Cytokine Release Syndrome Internet. Available at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-tisagenlecleucel-b-cell-all-and-tocilizumab-cytokine-release-syndrome.
157. FDA. FDA Approves CAR-T Cell Therapy to Treat Adults With Certain Types of Large B-Cell Lymphoma. (2017). Available at: https://www.fda.gov/news-events/press-announcements/fda-approves-car-t-cell-therapy-treat-adults-certain-types-large-b-cell-lymphoma#:~:text=The%20U.S.%20Food%20and%20Drug,two%20other%20kinds%20of%20treatment.
158. FDA. FDA Approves First Cell-Based Gene Therapy for Adult Patients With Multiple Myeloma (2021). Available at: https://www.fda.gov/news-events/press-announcements/fda-approves-first-cell-based-gene-therapy-adult-patients-multiple-myeloma
159. Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric Antigen Receptor–Modified T Cells in Chronic Lymphoid Leukemia. N Engl J Med (2011) 365:725–33. doi: 10.1056/NEJMoa1103849
160. Brentjens RJ, Riviere I, Park JH, Davila ML, Wang X, Stefanski J, et al. Safety and Persistence of Adoptively Transferred Autologous CD19-Targeted T Cells in Patients With Relapsed or Chemotherapy Refractory B-Cell Leukemias. Blood J Am Soc Hematol (2011) 118(18):4817–28. doi: 10.1182/blood-2011-04-348540
161. Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, et al. T Cells With Chimeric Antigen Receptors Have Potent Antitumor Effects and can Establish Memory in Patients With Advanced Leukemia. Sci Trans Med (2011) 3(95):95ra73–3. doi: 10.1126/scitranslmed.3002842
162. Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, et al. B-Cell Depletion and Remissions of Malignancy Along With Cytokine-Associated Toxicity in a Clinical Trial of Anti-CD19 Chimeric-Antigen-Receptor–Transduced T Cells. Blood J Am Soc Hematol (2012) 119(12):2709–20. doi: 10.1182/blood-2011-10-384388
163. Cruz CRY, Micklethwaite KP, Savoldo B, Ramos CA, Lam S, Ku S, et al. Infusion of Donor-Derived CD19-Redirected Virus-Specific T Cells for B-Cell Malignancies Relapsed After Allogeneic Stem Cell Transplant: A Phase 1 Study. Blood J Am Soc Hematol (2013) 122(17):2965–73. doi: 10.1182/blood-2013-06-506741
164. Kochenderfer JN, Dudley ME, Kassim SH, Somerville RP, Carpenter RO, Stetler-Stevenson M, et al. Chemotherapy-Refractory Diffuse Large B-Cell Lymphoma and Indolent B-Cell Malignancies can be Effectively Treated With Autologous T Cells Expressing an Anti-CD19 Chimeric Antigen Receptor. J Clin Oncol (2015) 33(6):540. doi: 10.1200/JCO.2014.56.2025
165. Porter DL, Hwang W-T, Frey NV, Lacey SF, Shaw PA, Loren AW, et al. Chimeric Antigen Receptor T Cells Persist and Induce Sustained Remissions in Relapsed Refractory Chronic Lymphocytic Leukemia. Sci Trans Med (2015) 7(303):303ra139–303ra139. doi: 10.1126/scitranslmed.aac5415
166. Fraietta JA, Beckwith KA, Patel PR, Ruella M, Zheng Z, Barrett DM, et al. Ibrutinib Enhances Chimeric Antigen Receptor T-Cell Engraftment and Efficacy in Leukemia. Blood J Am Soc Hematol (2016) 127(9):1117–27. doi: 10.1182/blood-2015-11-679134
167. Brudno JN, Somerville RP, Shi V, Rose JJ, Halverson DC, Fowler DH, et al. Allogeneic T Cells That Express an Anti-CD19 Chimeric Antigen Receptor Induce Remissions of B-Cell Malignancies That Progress After Allogeneic Hematopoietic Stem-Cell Transplantation Without Causing Graft-Versus-Host Disease. J Clin Oncol (2016) 34(10):1112. doi: 10.1200/JCO.2015.64.5929
168. Ramos CA, Savoldo B, Torrano V, Ballard B, Zhang H, Dakhova O, et al. Clinical Responses With T Lymphocytes Targeting Malignancy-Associated κ Light Chains. J Clin Invest (2016) 126(7):2588–96. doi: 10.1172/JCI86000
169. Turtle CJ, Hay KA, Hanafi L-A, Li D, Cherian S, Chen X, et al. Durable Molecular Remissions in Chronic Lymphocytic Leukemia Treated With CD19-Specific Chimeric Antigen Receptor–Modified T Cells After Failure of Ibrutinib. J Clin Oncol (2017) 35(26):3010. doi: 10.1200/JCO.2017.72.8519
170. Geyer MB, Rivière I, Sénéchal B, Wang X, Wang Y, Purdon TJ, et al. Autologous CD19-Targeted CAR T Cells in Patients With Residual CLL Following Initial Purine Analog-Based Therapy. Mol Ther (2018) 26(8):1896–905. doi: 10.1016/j.ymthe.2018.05.018
171. Geyer MB, Rivière I, Sénéchal B, Wang X, Wang Y, Purdon TJ, et al. Safety and Tolerability of Conditioning Chemotherapy Followed by CD19-Targeted CAR T Cells for Relapsed/Refractory CLL. JCI Insight (2019) 4(9):e122627. doi: 10.1172/jci.insight.122627
172. Gauthier J, Hirayama AV, Purushe J, Hay KA, Lymp J, Li DH, et al. Feasibility and Efficacy of CD19-Targeted CAR T Cells With Concurrent Ibrutinib for CLL After Ibrutinib Failure. Blood (2020) 135(19):1650–60. doi: 10.1182/blood.2019002936
173. Liu E, Marin D, Banerjee P, Macapinlac HA, Thompson P, Basar R, et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N Engl J Med (2020) 382(6):545–53. doi: 10.1056/NEJMoa1910607
174. Frey NV, Gill S, Hexner EO, Schuster S, Nasta S, Loren A, et al. Long-Term Outcomes From a Randomized Dose Optimization Study of Chimeric Antigen Receptor Modified T Cells in Relapsed Chronic Lymphocytic Leukemia. J Clin Oncol (2020) 38(25):2862–71. doi: 10.1200/JCO.19.03237
175. Shah NN, Johnson BD, Schneider D, Zhu F, Szabo A, Keever-Taylor CA, et al. Bispecific Anti-CD20, Anti-CD19 CAR T Cells for Relapsed B Cell Malignancies: A Phase 1 Dose Escalation and Expansion Trial. Nat Med (2020) 26(10):1569–75. doi: 10.1038/s41591-020-1081-3
176. Cappell KM, Sherry RM, Yang JC, Goff SL, Vanasse DA, McIntyre L, et al. Long-Term Follow-Up of Anti-CD19 Chimeric Antigen Receptor T-Cell Therapy. J Clin Oncol (2020) 38(32):3805–15. doi: 10.1200/JCO.20.01467
177. Riches JC, Davies JK, McClanahan F, Fatah R, Iqbal S, Agrawal S, et al. T Cells From CLL Patients Exhibit Features of T-Cell Exhaustion But Retain Capacity for Cytokine Production. Blood J Am Soc Hematol (2013) 121(9):1612–21. doi: 10.1182/blood-2012-09-457531
Keywords: chronic lymphocytic leukemia, high-risk, treatment, TP53, del(11q), complex karyotype, unmutated IGHV, NOTCH1
Citation: van der Straten L, Hengeveld PJ, Kater AP, Langerak AW and Levin M-D (2021) Treatment Approaches to Chronic Lymphocytic Leukemia With High-Risk Molecular Features. Front. Oncol. 11:780085. doi: 10.3389/fonc.2021.780085
Received: 20 September 2021; Accepted: 23 November 2021;
Published: 09 December 2021.
Edited by:
Eugen Tausch, University of Ulm, GermanyReviewed by:
Josep Nomdedeu, Hospital de la Santa Creu i Sant Pau, SpainGianluigi Reda, IRCCS Ca ‘Granda Foundation Maggiore Policlinico Hospital (IRCCS), Italy
Copyright © 2021 van der Straten, Hengeveld, Kater, Langerak and Levin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mark-David Levin, bS1kLmxldmluQGFzei5ubA==
†These authors have contributed equally to this work and share first authorship
 Lina van der Straten
Lina van der Straten Paul J. Hengeveld
Paul J. Hengeveld Arnon P. Kater
Arnon P. Kater Anton W. Langerak
Anton W. Langerak Mark-David Levin1*
Mark-David Levin1*