
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 21 December 2021
Sec. Gastrointestinal Cancers: Gastric and Esophageal Cancers
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.777686
This article is part of the Research Topic Novel Immunotherapies to Treat Gastrointestinal Solid Tumor Cancers View all 13 articles
 Yi-Min Gu1†
Yi-Min Gu1† Qi-Xin Shang1†
Qi-Xin Shang1† Yue Zhuo2
Yue Zhuo2 Jian-Feng Zhou1
Jian-Feng Zhou1 Bo-Wei Liu1
Bo-Wei Liu1 Wen-Ping Wang1
Wen-Ping Wang1 Guo-Wei Che1
Guo-Wei Che1 Long-Qi Chen1*
Long-Qi Chen1*Background: The published evidence from several randomized controlled clinical trials of immunotherapy for advanced esophageal squamous cell carcinoma has shown promising results. This study aimed to investigate the efficacy and safety of immune checkpoint inhibitor treatment in esophageal squamous cell carcinoma.
Methods: PubMed, Web of Science, Cochrane Library, and Embase databases were searched for relevant articles published before December 30, 2020. The data for efficacy and safety of immune checkpoint inhibitor treatment were subjected to meta-analysis.
Results: Seven clinical trials comprising 1733 patients were included. The results showed that immune checkpoint inhibitor treatment as second- or later-line treatment was associated with an increased risk of the objective response rate (relative risk: 1.82, 95% confidence interval: 0.82–4.04; P=0.002) and median overall survival (hazard ratio: 0.75, 95% confidence interval: 0.67–0.85; P<0.001) compared with chemotherapy in locally advanced or metastatic esophageal squamous cell carcinoma. Moreover, immune checkpoint inhibitor treatment was associated with significant improvement in median overall survival (hazard ratio: 0.61, 95% confidence interval: 0.48–0.77, P<0.001) compared with chemotherapy in the programmed death-ligand 1 (PD-L1)-positive population. However, immune checkpoint inhibitor treatment was also effective in all patients independent of PD-L1 expression. The most common grade ≥3 treatment-related adverse events with immune checkpoint inhibitor therapy were anemia, asthenia, rash, fatigue, decreased appetite, diarrhea, pneumonia, decreased neutrophil count, and vomiting. Patients undergoing immune checkpoint inhibitor therapy was associated with a decreased risk of treatment-related adverse events (relative risk: 0.82, 95% confidence interval: 0.62–1.08; P<0.001) and grade ≥3 treatment-related adverse events (relative risk: 0.50, 95% confidence interval: 0.42–0.60; P<0.001) compared with those undergoing chemotherapy.
Conclusions: Immune checkpoint inhibitors as second- or later-line therapy may improve overall response rate and overall survival but not all oncological outcomes for patients with locally advanced or metastatic esophageal squamous cell carcinoma. Patients treated with immune checkpoint inhibitors might experience fewer treatment-related adverse events of any grade, but specifically grade ≥3, compared with those treated with chemotherapy.
Esophageal cancer is the seventh most common malignant tumor and the sixth leading cause of cancer death worldwide (1). To date, the optimal therapy for local advanced esophageal cancer has consisted of multidisciplinary therapy involving neoadjuvant chemotherapy or chemoradiotherapy plus surgery. Despite improvements in treatment, the long-term survival for patients with advanced esophageal cancer is still unsatisfactory (2, 3).
Immunotherapy, with agents such as immune checkpoint inhibitors (ICIs), cancer vaccines, and adoptive T-cell therapy, has recently increased hope for improved survival outcomes in patients with esophageal cancer (4–7). ICI therapy has dramatically changed the treatment of melanoma and advanced non-small cell lung cancer (8–12). In the past few years, published evidence from randomized controlled clinical trials (RCTs) has shown promise for treatment of esophageal squamous cell carcinoma (ESCC) (13–15). The two most common types of esophageal cancer are ESCC and esophageal adenocarcinoma (EAC), the incidence of which can vary by region, with the highest rate of EAC occurring in Western countries and of ESCC occurring in East Asian countries. There are clear differences between the etiology, molecular biological features, and prognosis of ESCC and EAC (16, 17). ESCC with a high level of tumor mutations appeared to be more sensitive to treatment than EAC (18). The randomized phase 3 trial KEYNOTE-181 showed that patients with ESCC treated with anti-programmed death-ligand 1 (PD-L1) antibody therapy tended to survive longer than the overall patient population but did not make a direct comparison between treatments (13). Given the high prevalence of ESCC in East Asia and the shortage of effective treatment options for advanced ESCC, conventional chemotherapy is far from satisfactory. Thus, there is an urgent need for the development of novel and effective treatments for advanced ESCC.
This study aimed to perform a meta-analysis to assess the efficacy and safety of ICI treatments for patients with advanced ESCC. Findings from this meta-analysis may be helpful in guiding ICI treatment for patients with ESCC.
We conducted a systematic literature review according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009 guidelines (19). Two authors independently searched PubMed, Web of Science, Cochrane Library, and Embase for relevant clinical trials published before December 31, 2020. The search keyword terms were as follows: ((esophageal neoplasm [MeSH Terms]) OR ((((((esophageal squamous cell carcinoma) OR (oesophageal squamous cell carcinoma)) OR (squamous cell carcinoma of esophagus)) OR (squamous cell carcinoma of oesophagus)) OR (esophageal cancer)) OR (oesophageal cancer))) AND (immunotherapy [MeSH Terms]) OR ((((((((((((immune checkpoint inhibitor) OR (PD-1)) OR (PD-L1)) OR (Nivolumab) OR (Pembrolizumab)) OR (Camrelizumab)) OR (SHR-1210) OR (Toripalimab)) OR (Ipilimumab)) OR (Avelumab) OR (Atezolizumab)) OR (Durvalumab))).
The inclusion criteria were clinical trials that included ICI monotherapy as second- or later-line treatment for patients with advanced or metastatic ESCC. Hazard ratio (HR) and relative risk (RR) for antitumor activity, survival outcomes, and safety indicators were available. Two researchers independently selected studies and extracted data; if there were any questions, another senior researcher was invited to discuss these. The following information was extracted from the selected articles: author, year, study name, study design, participant characteristics, sample size, and interventions. A quality appraisal of three randomized trials was performed using the Cochrane Risk of Bias tool (20).
HR, RR, and their associated 95% confidence interval (CI) were extracted from each article and combined to estimate the prognostic value. HR or RR <1 indicated a better oncologic outcome in patients with esophageal cancer treated with ICI than in those treated with chemotherapy. The Q test and I-squared statistic were used to assess the heterogeneity of the included studies. Pooled estimates of HR or RR were calculated initially using a fixed-effect model. If significant heterogeneity existed (P<0.10 or I2>50%), a random-effect model was used. Publication bias was evaluated by both Begg’s and Egger’s tests. All P-values were two-sided and significant publication bias was defined as P<0.05. Subgroup analyses were performed on the basis of which anti-PD-L1 antibody was used. All statistical analyses were performed using Stata/SE 12.0 software (StataCorp. LLC, version 12.0, College Station, TX, USA).
Our search screened 1452 eligible studies and identified nine clinical trials; one study by Kato et al., 2020 (21) used the same dataset as that reported by Kudo et al., 2017 (22). In another trial by Zhang et al., 2020 (23) the study arm applied ICI combined with chemotherapy, which did not meet the inclusion criteria. Finally, a total of seven articles (13–15, 22, 24–26) were included in this meta-analysis. The flow diagram for identifying relevant studies is shown in Figure 1A.
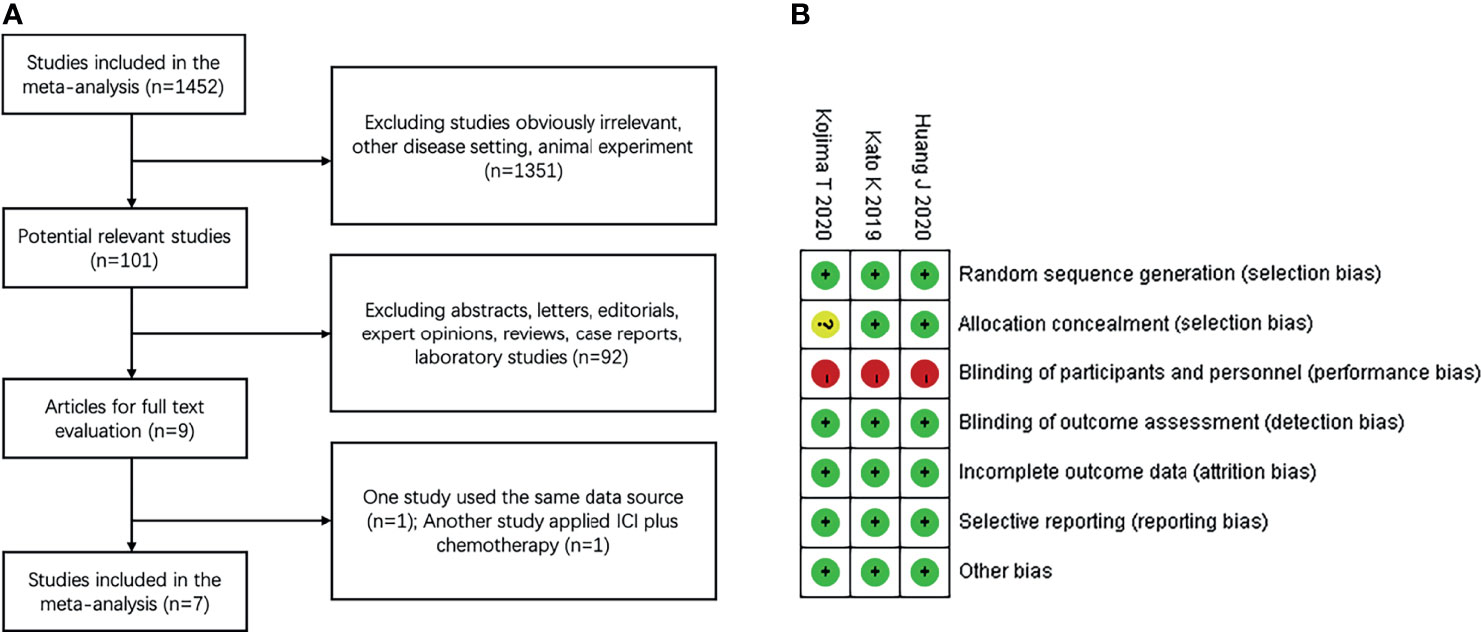
Figure 1 Study identification and risk of bias (A) Flow diagram of identification of relevant studies; (B) Summary of risk of bias summary of randomized controlled trials. + low risk,? unclear risk, – high risk.
All the included studies were published in peer-reviewed journals between 2010 and 2019 and were performed in eight countries (Japan, China, France, South Korea, USA, France, United Kingdom, and Germany). Of these clinical trials, three were multicenter, open-label, phase 3 RCTs (13–15) comparing ICI monotherapy vs. chemotherapy, and four were single-arm, prospective, phase 1–2 trials (22, 24–26) applying ICI monotherapy. Four trials enrolled patients with ESCC, three enrolled patients with both ESCC and EAC. All trials investigated anti-PD-L1 antibody therapy (three with pembrolizumab, two with camrelizumab, and two with nivolumab). A comprehensive outline of the characteristics of the included clinical trials are presented in Table 1. Three randomized trials reported the sample size assessment and follow-up time, but the method used for study allocation concealment in one study was unclear (Figure 1B). Because of a lack of appropriate evaluation tools, the risk of bias in the four single-arm trials was not estimated.
The pooled ORR and DCR of ICI treatment and a subgroup analysis are summarized in Table 2. The pooled ORR of ICI treatment was 18.3%. The ORRs of the pembrolizumab, camrelizumab, and nivolumab ICI subgroups were 16.3%, 24.2%, and 18.5%, respectively. The pooled DCR of ICI treatment was 38.4%. The DCRs of the pembrolizumab, camrelizumab, and nivolumab subgroups were 28.0%, 46.1%, and 33.0%, respectively.
Three RCTs including 1268 patients demonstrated that ICI treatment was significantly associated with improvement of ORR compared with chemotherapy, with an estimated RR of 1.82 (95% CI: 0.82–4.04, P=0.002) with significant heterogeneity (I2 = 85.7%, P=0.001) (Figure 2A). These results suggested that ICI as second- or later-line treatment for patients with locally advanced or metastatic ESCC was associated with an increased risk of response compared with chemotherapy.
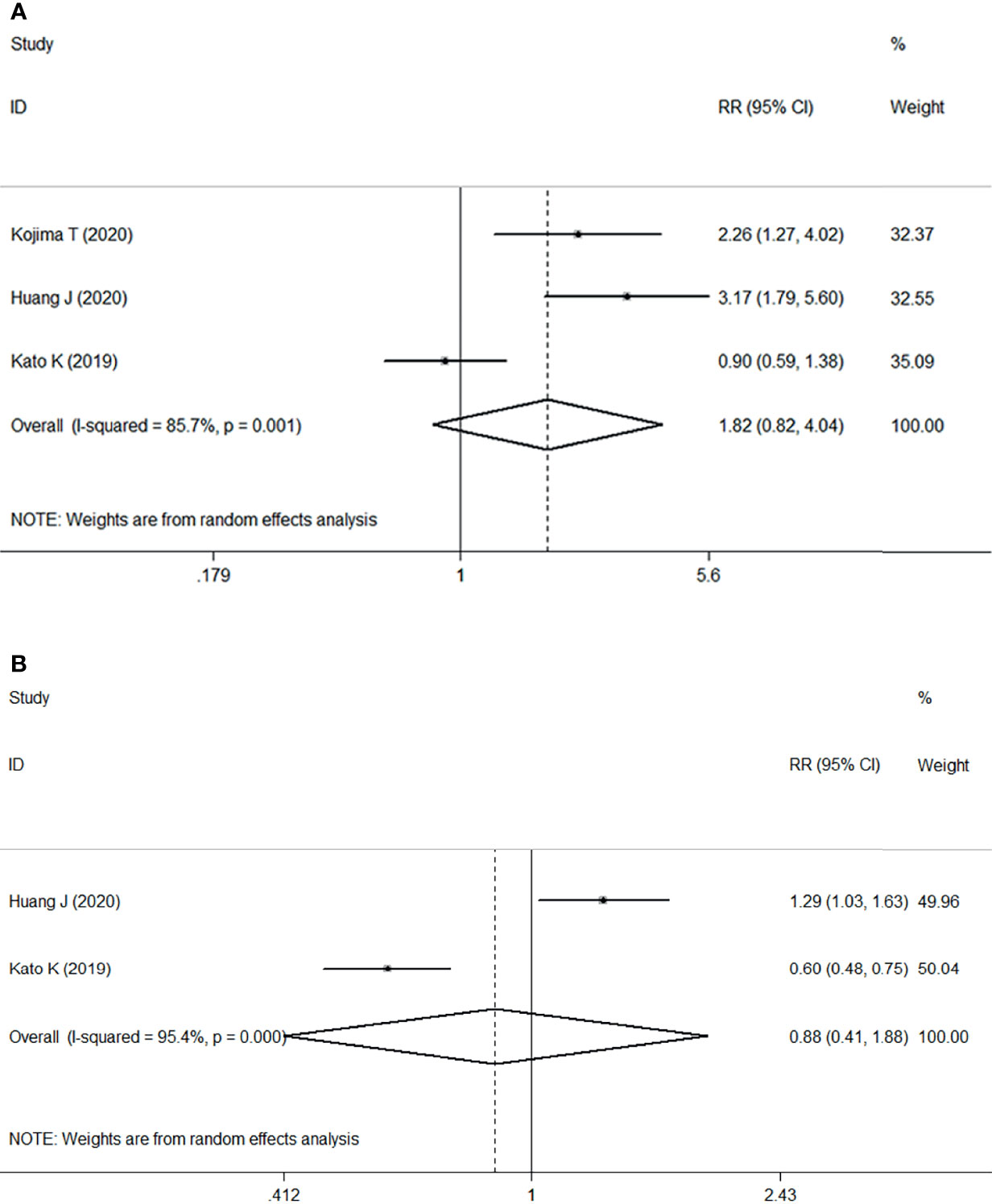
Figure 2 Forest plots. (A) Forest plots of RR comparing the objective response rate between patients treated with ICI and chemotherapy; (B) Forest plots of RR comparing disease control rate between patients treated with ICI and chemotherapy. RR, relative risk; CI, confidence interval; ICI, immune checkpoint inhibitor.
However, two studies comprising 867 patients compared the DCR between two groups, ICI versus chemotherapy. Pooled data from the two studies showed no significant difference between ICI treatment and chemotherapy, with an estimated RR of 0.88 (95% CI: 0.41–1.88, P=0.739) without apparent heterogeneity (I2 = 95.4%, P<0.001) (Figure 2B).
The results of analysis of pooled 6-month and 12-month OS rate of ICI treatment and the associated subgroup analysis are also summarized in Table 2. The pooled 6-month OS rate of ICI treatment was 57.1%. The 6-month OS rate of the pembrolizumab and camrelizumab ICI subgroups was 50.8% and 63.0%, respectively. The pooled 12-month OS rate of ICI treatment was 37.5%. The 12-month OS rate of the pembrolizumab, camrelizumab, and nivolumab ICI subgroups was 34.9%, 34.0%, and 47.0%, respectively. The highest 6-month OS rate (63.0%) was observed in the camrelizumab subgroup and the highest 12-month OS rate (47%) in the nivolumab subgroup.
Meta-analysis of three RCTs comprising 1268 patients revealed that ICI treatment improved median OS compared with chemotherapy when used as second- or later-line treatment of locally advanced or metastatic ESCC. This corresponded to a pooled HR of 0.75 (95% CI: 0.67–0.85; P<0.001) without obvious heterogeneity (I2 = 0.0%, P=0.801) (Figure 3A).
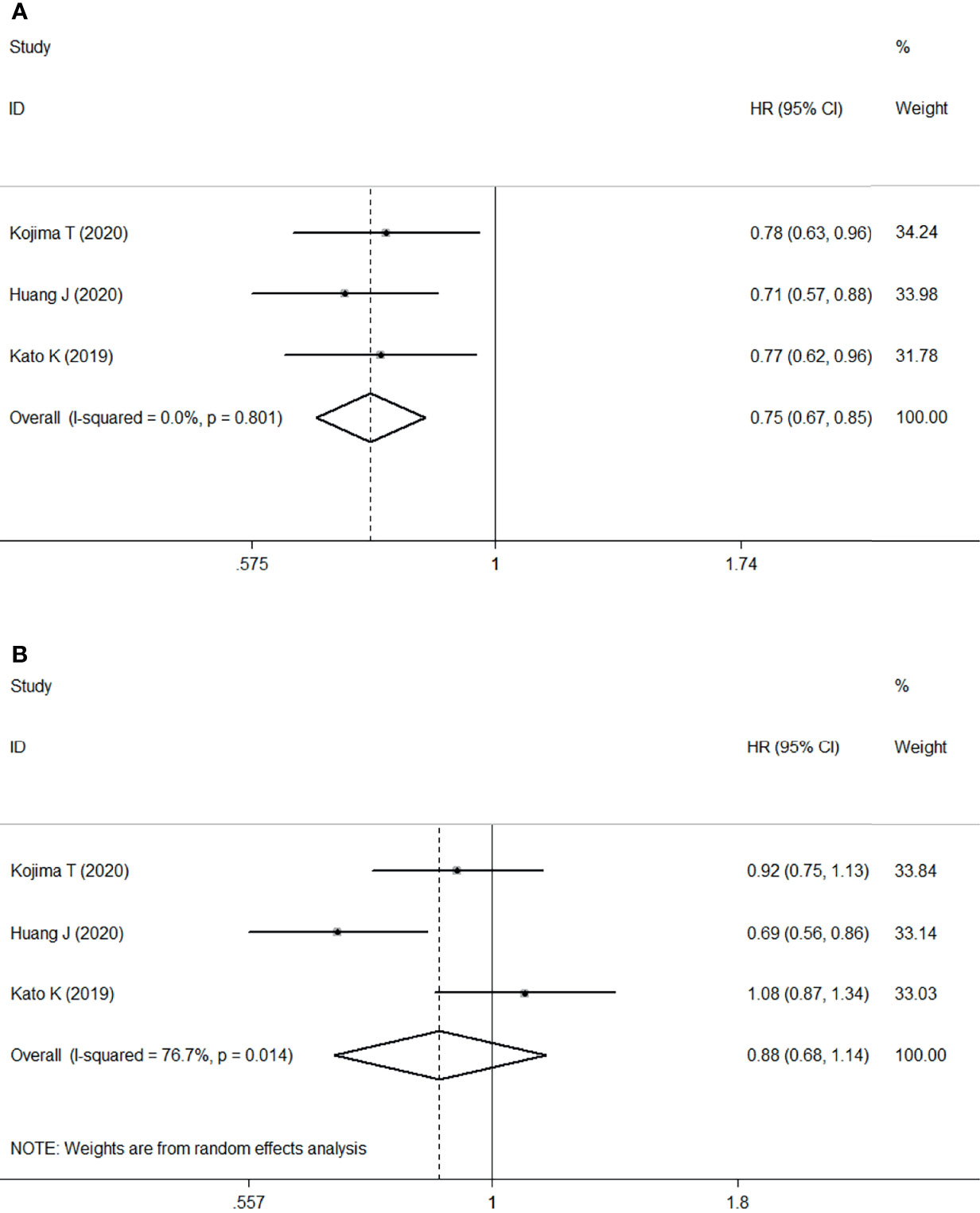
Figure 3 Forest plots. (A) Forest plots of HR comparing overall survival between patients treated with ICI and chemotherapy; (B) Forest plots of HR comparing progression-free survival between patients treated with ICI and chemotherapy. HR, hazard ratio; CI, confidence interval; ICI, immune checkpoint inhibitor.
However, no significant difference was found in the median PFS of patients treated with ICI or chemotherapy (HR: 0.88, 95% CI: 0.68–1.14, P=0.330) with significant heterogeneity (I2 = 76.7%, P=0.014) (Figure 3B).
Three studies compared the antitumor activity of treatment in patients with PD-L1 positive (≥1%) and negative (<1%) tumors. The ORR and DCR of patients with PD-L1 positive tumors were 22.2% and 48.0%, while those of patients with PD-L1 negative tumors were 6.7% and 26.9% (Table 2). Meta-analysis of three RCTs comprising 561 patients revealed that in patients with high PD-L1 expression, median OS was improved with ICI treatment versus chemotherapy. This corresponded to a pooled HR of 0.61 (95% CI: 0.48–0.77; P<0.001) without obvious heterogeneity (I2 = 0.0%, P=0.681) (Figure 4).
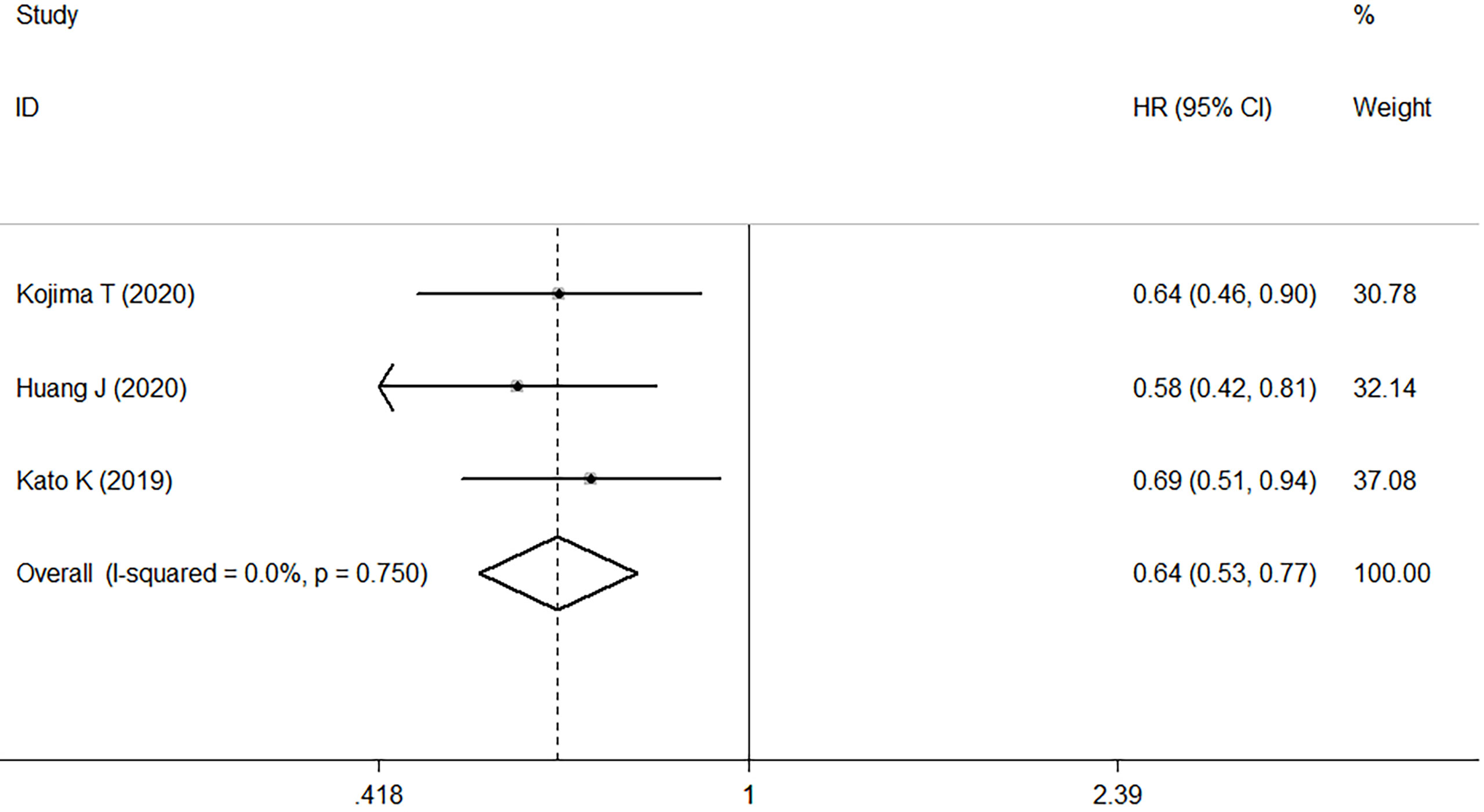
Figure 4 Forest plots of HR comparing overall survival between patients with PD-L1-positive tumors treated with ICI treatment and chemotherapy. HR, hazard ratio; CI, confidence interval; ICI, immune checkpoint inhibitor.
We investigated the pooled incidence of any-grade TRAEs and grade ≥3 TRAEs (both total and specific events) (Table 3). The overall incidence of TRAEs in patients treated with ICI was 61.9%. The incidence of TRAEs in the pembrolizumab and nivolumab subgroups was 50.7% and 85.0%, respectively. However, the incidence of grade ≥3 TRAE in patients treated with ICI was 16.7%. The incidence of grade ≥3 TRAE in the pembrolizumab, nivolumab, and camrelizumab subgroups was 16.2%, 19.5%, and 15.0%, respectively. The patients in camrelizumab subgroup had the least incidence (15%).
The most common TRAEs with ICI therapy of locally advanced or metastatic ESCC were rash (10.8%), hypothyroidism (10.1%), fatigue (9.3%), asthenia (7.0%), decreased appetite (7.0%), diarrhea (6.0%), anemia (4.7%), nausea (3.0%), pneumonia (2.7%), vomiting (2.0%), decreased neutrophil count (1.1%), and alopecia (0.7%). The most common grade ≥3 TRAEs with ICI therapy were anemia (1.8%), asthenia (1.2%), rash (1.1%), fatigue (0.8%), decreased appetite (0.8%), diarrhea (0.8%), pneumonia (0.5%), decreased neutrophil count (0.5%), and vomiting (0.3%).
The meta-analysis of the three RCTs indicated that patients undergoing ICI therapy was associated with a decreased risk of overall TRAEs (RR: 0.82, 95% CI 0.62–1.08; P<0.001) (Figure 5A) and grade ≥3 TRAEs (RR=0.50, 95% CI 0.42–0.60; P<0.001) (Figure 5B) compared with those undergoing chemotherapy.
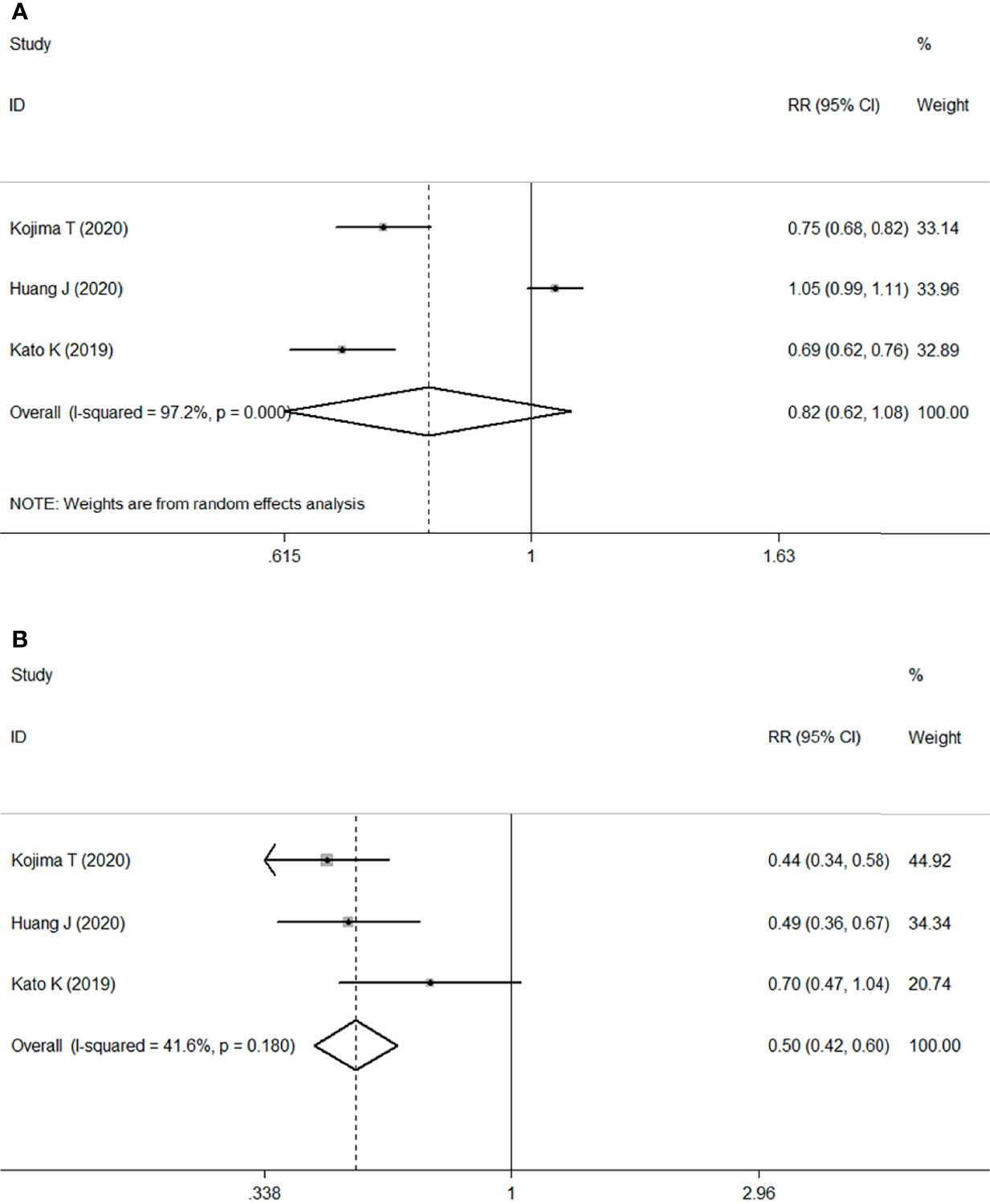
Figure 5 Forest plots. (A) Forest plots of RR comparing TRAEs between patients treated with ICI and chemotherapy; (B) Forest plots of RR comparing grade ≥3 TRAEs between patients treated with ICI and chemotherapy. RR, relative risk; CI, confidence interval; ICI, immune checkpoint inhibitor; TRAEs, treatment-related adverse events.
Publication bias was evaluated by both Begg’s and Egger’s tests (Table 4). All outcomes had P>0.05, except for the Egger’s test of ICI vs. chemotherapy TRAEs (P<0.05). Overall, no obvious publication bias was observed.
To our knowledge, this is the first meta-analysis to evaluate the efficacy and safety of ICIs (anti-PD-L1 antibody) as second- or later-line treatment for unresectable locally advanced or recurrent/metastatic ESCC. This study included seven published clinical trials including three RCTs and four single-arm trials published before December 31, 2020. The main outcomes showed that ICI therapy used as second- or later-line treatment for advanced or metastatic ESCC could increase ORR, improve OS, and decrease the incidence of any-grade TRAEs and grade ≥3 TRAEs compared with chemotherapy.
Several RCTs of ICIs have reported the clinical outcomes in patients with ESCC. The randomized phase 3 trial KEYNOTE-181 (13) reported that patients with ESCC treated with anti-PD-L1 antibody therapy showed a trend toward longer survival compared with the overall population of patients with ESCC but did not make a direct comparison. The randomized trial ATTRACTION-3 reported by Kato et al. (15) also demonstrated that median OS was significantly improved in patients treated with nivolumab compared with those treated with chemotherapy (10.9 months vs 8.4 months; HR: 0.77, 95% CI: 0.62–0.96; P=0.019). However, the effects of ICI on PFS and its antitumor activity differ between studies. The ESCORT randomized phase 3 study (14) showed that camrelizumab improved OS compared with chemotherapy as second-line therapy in Chinese patients with advanced ESCC.
Patients with PD-L1-positive tumors may derive more survival benefit with ICI therapy than with chemotherapy. However, ICI therapy was also reported to be effective in all patients independent of PD-L1 expression (14). The RCT reported by Kato et al. also observed no significant interaction between the effectiveness of ICI therapy and PD-L1 status (15). This suggests that PD-L1 might not be sufficiently specific to serve as the optimal biomarker for ICI treatment of ESCC. In advanced gastric or gastroesophageal junction cancer, high microsatellite instability and tumor mutational burden has been shown to be associated with the ORR of patients (27, 28). Studies have found that the tumor mutation burden is usually higher in ESCC than in EAC (18, 29). Further investigation of candidate biomarkers for ICI treatment is warranted.
The three randomized trials included in this meta-analysis all involved monotherapy with ICI vs. chemotherapy as a second- or later-line treatment. There are a number of unpublished clinical trials with an accessible conference abstract that evaluated the efficacy of ICI as first-line therapy and adjuvant therapy, such as the KEYNOTE-590 randomized phase 3 study (30); this showed that the median OS of patients with ESCC was longer with first-line treatment with pembrolizumab plus chemotherapy than with chemotherapy alone (12.6 months vs. 9.8 months; HR: 0.72, 95% CI: 0.60–0.88, P=0.0006). In the CheckMate 577 randomized phase 3 study (31), median DFS in patients treated with nivolumab after surgery was twice that in the placebo population (22.4 months vs. 11.0 months; HR: 0.69; 96.4% CI: 0.56–0.86; P=0.0003). The optimal timing, dosing, and combination of ICI regimens for treatment of esophageal cancer require further study.
The safety profile of ICIs showed a lower incidence of any-grade TRAEs and grade ≥3 TRAEs compared with chemotherapy. In this meta-analysis, 61.9% patients receiving ICI treatment reported TRAEs, but the probability of developing grade ≥3 TRAEs was 16.7%. Notably, the incidence of reactive cutaneous capillary endothelial proliferation after receiving camrelizumab was high (14, 23). However, no similar adverse event was noted in patients receiving pembrolizumab or nivolumab. Moreover, the incidence of treatment leading to death was almost zero. Accordingly, ICI treatment can be considered a relatively safe option.
This meta-analysis has some limitations that should be acknowledged. First, it included only seven studies comprising three RCTs and four single-arm trials. Even though no obvious publication bias was detected in the included RCTs by Begg’s or Egger’s test, the single-arm studies without a control group might introduce a potential bias. The low number of trials may limit the accuracy of the test. However, the number of 1733 included patients is relatively high, indicating reliability. Second, three studies included both patients with ESCC and those with EAC. Specific information about squamous cell carcinoma patients was provided by Kojima et al. (13), but was not available for two single-arm studies (24, 26) that included 81 ESCC patients and 63 EAC patients. Unfortunately, we do not have access to their raw data. These confounding factors may limit the interpretation of our results.
In conclusion, ICI treatment in patients with locally advanced or metastatic ESCC may improve the ORR and median OS but not all oncological outcomes, and result in a lower incidence of TRAEs compared with chemotherapy. Although ICI treatment was more effective in patients with PD-L1-positive tumors, it was also effective in all patients with ESCC independent of their PD-L1 expression. Further investigation of the optimal timing, dosing, combination of drug regimens, and candidate biomarkers for ICI treatment of esophageal cancer is warranted.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
L-QC and G-WC conceptualized the study, revised the manuscript and supervised the study. Y-MG and Q-XS conceptualized the study, drafted the manuscript and made the figures. Y-MG, YZ, J-FZ, B-WL and W-PW collected the literature and revised the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global Cancer Statistics, 2012. CA Cancer J Clin (2015) 65(2):87–108. doi: 10.3322/caac.21262
2. van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative Chemoradiotherapy for Esophageal or Junctional Cancer. N Engl J Med (2012) 366(22):2074–84. doi: 10.1056/NEJMoa1112088
3. Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, et al. Neoadjuvant Chemoradiotherapy Followed by Surgery Versus Surgery Alone for Locally Advanced Squamous Cell Carcinoma of the Esophagus (NEOCRTEC5010): A Phase III Multicenter, Randomized, Open-Label Clinical Trial. J Clin Oncol (2018) 36(27):2796–803. doi: 10.1200/jco.2018.79.1483
4. Baba Y, Nomoto D, Okadome K, Ishimoto T, Iwatsuki M, Miyamoto Y, et al. Tumor Immune Microenvironment and Immune Checkpoint Inhibitors in Esophageal Squamous Cell Carcinoma. Cancer Sci (2020) 111(9):3132–41. doi: 10.1111/cas.14541
5. Akin Telli T, Bregni G, Camera S, Deleporte A, Hendlisz A, Sclafani F. PD-1 and PD-L1 Inhibitors in Oesophago-Gastric Cancers. Cancer Lett (2020) 469:142–50. doi: 10.1016/j.canlet.2019.10.036
6. Iwahashi M, Katsuda M, Nakamori M, Nakamura M, Naka T, Ojima T, et al. Vaccination With Peptides Derived From Cancer-Testis Antigens in Combination With CpG-7909 Elicits Strong Specific CD8+ T Cell Response in Patients With Metastatic Esophageal Squamous Cell Carcinoma. Cancer Sci (2010) 101(12):2510–7. doi: 10.1111/j.1349-7006.2010.01732.x
7. Yamamoto TN, Kishton RJ, Restifo NP. Developing Neoantigen-Targeted T Cell-Based Treatments for Solid Tumors. Nat Med (2019) 25(10):1488–99. doi: 10.1038/s41591-019-0596-y
8. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, et al. Five-Year Survival With Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med (2019) 381(16):1535–46. doi: 10.1056/NEJMoa1910836
9. Chesney J, Puzanov I, Collichio F, Singh P, Milhem MM, Glaspy J, et al. Randomized, Open-Label Phase II Study Evaluating the Efficacy and Safety of Talimogene Laherparepvec in Combination With Ipilimumab Versus Ipilimumab Alone in Patients With Advanced, Unresectable Melanoma. J Clin Oncol (2018) 36(17):1658–67. doi: 10.1200/jco.2017.73.7379
10. Ribas A, Hamid O, Daud A, Hodi FS, Wolchok JD, Kefford R, et al. Association of Pembrolizumab With Tumor Response and Survival Among Patients With Advanced Melanoma. Jama (2016) 315(15):1600–9. doi: 10.1001/jama.2016.4059
11. Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim SW, Carcereny Costa E, et al. Nivolumab Plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N Engl J Med (2019) 381(21):2020–31. doi: 10.1056/NEJMoa1910231
12. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab Versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med (2015) 373(17):1627–39. doi: 10.1056/NEJMoa1507643
13. Kojima T, Shah MA, Muro K, Francois E, Adenis A, Hsu CH, et al. Randomized Phase III KEYNOTE-181 Study of Pembrolizumab Versus Chemotherapy in Advanced Esophageal Cancer. J Clin Oncol (2020) 38(35):4138–48. doi: 10.1200/jco.20.01888
14. Huang J, Xu J, Chen Y, Zhuang W, Zhang Y, Chen Z, et al. Camrelizumab Versus Investigator's Choice of Chemotherapy as Second-Line Therapy for Advanced or Metastatic Oesophageal Squamous Cell Carcinoma (ESCORT): A Multicentre, Randomised, Open-Label, Phase 3 Study. Lancet Oncol (2020) 21(6):832–42. doi: 10.1016/s1470-2045(20)30110-8
15. Kato K, Cho BC, Takahashi M, Okada M, Lin CY, Chin K, et al. Nivolumab Versus Chemotherapy in Patients With Advanced Oesophageal Squamous Cell Carcinoma Refractory or Intolerant to Previous Chemotherapy (ATTRACTION-3): A Multicentre, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol (2019) 20(11):1506–17. doi: 10.1016/s1470-2045(19)30626-6
16. Jones JO, Smyth EC. Gastroesophageal Cancer: Navigating the Immune and Genetic Terrain to Improve Clinical Outcomes. Cancer Treat Rev (2020) 84:101950. doi: 10.1016/j.ctrv.2019.101950
17. Ku GY. The Current Status of Immunotherapies in Esophagogastric Cancer. Surg Oncol Clin N Am (2017) 26(2):277–92. doi: 10.1016/j.soc.2016.10.012
18. Kelly RJ. The Emerging Role of Immunotherapy for Esophageal Cancer. Curr Opin Gastroenterol (2019) 35(4):337–43. doi: 10.1097/mog.0000000000000542
19. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Bmj (2009) 339:b2535. doi: 10.1136/bmj.b2535
20. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's Tool for Assessing Risk of Bias in Randomised Trials. Bmj (2011) 343:d5928. doi: 10.1136/bmj.d5928
21. Kato K, Doki Y, Ura T, Hamamoto Y, Kojima T, Tsushima T, et al. Long-Term Efficacy and Predictive Correlates of Response to Nivolumab in Japanese Patients With Esophageal Cancer. Cancer Sci (2020) 111(5):1676–84. doi: 10.1111/cas.14380
22. Kudo T, Hamamoto Y, Kato K, Ura T, Kojima T, Tsushima T, et al. Nivolumab Treatment for Oesophageal Squamous-Cell Carcinoma: An Open-Label, Multicentre, Phase 2 Trial. Lancet Oncol (2017) 18(5):631–9. doi: 10.1016/s1470-2045(17)30181-x
23. Zhang B, Qi L, Wang X, Xu J, Liu Y, Mu L, et al. Phase II Clinical Trial Using Camrelizumab Combined With Apatinib and Chemotherapy as the First-Line Treatment of Advanced Esophageal Squamous Cell Carcinoma. Cancer Commun (Lond) (2020) 40(12):711–20. doi: 10.1002/cac2.12119
24. Shah MA, Kojima T, Hochhauser D, Enzinger P, Raimbourg J, Hollebecque A, et al. Efficacy and Safety of Pembrolizumab for Heavily Pretreated Patients With Advanced, Metastatic Adenocarcinoma or Squamous Cell Carcinoma of the Esophagus: The Phase 2 KEYNOTE-180 Study. JAMA Oncol (2019) 5(4):546–50. doi: 10.1001/jamaoncol.2018.5441
25. Huang J, Xu B, Mo H, Zhang W, Chen X, Wu D, et al. Safety, Activity, and Biomarkers of SHR-1210, an Anti-PD-1 Antibody, for Patients With Advanced Esophageal Carcinoma. Clin Cancer Res (2018) 24(6):1296–304. doi: 10.1158/1078-0432.Ccr-17-2439
26. Doi T, Piha-Paul SA, Jalal SI, Saraf S, Lunceford J, Koshiji M, et al. Safety and Antitumor Activity of the Anti-Programmed Death-1 Antibody Pembrolizumab in Patients With Advanced Esophageal Carcinoma. J Clin Oncol (2018) 36(1):61–7. doi: 10.1200/jco.2017.74.9846
27. Tsao MS, Kerr KM, Kockx M, Beasley MB, Borczuk AC, Botling J, et al. PD-L1 Immunohistochemistry Comparability Study in Real-Life Clinical Samples: Results of Blueprint Phase 2 Project. J Thorac Oncol (2018) 13(9):1302–11. doi: 10.1016/j.jtho.2018.05.013
28. Ma C, Patel K, Singhi AD, Ren B, Zhu B, Shaikh F, et al. Programmed Death-Ligand 1 Expression Is Common in Gastric Cancer Associated With Epstein-Barr Virus or Microsatellite Instability. Am J Surg Pathol (2016) 40(11):1496–506. doi: 10.1097/pas.0000000000000698
29. Bockorny B, Pectasides E. The Emerging Role of Immunotherapy in Gastric and Esophageal Adenocarcinoma. Future Oncol (2016) 12(15):1833–46. doi: 10.2217/fon-2016-0103
30. Kato K SJ, Shah MA. Pembrolizumab Plus Chemotherapy Versus Chemotherapy as First-Line Therapy in Patients With Advanced Esophageal Cancer: The Phase 3 KEYNOTE-590 Study. Ann Oncol (2020) 31(Suppl 4):S1192–S3. doi: 10.1016/j.annonc.2020.08.2298
Keywords: esophageal squamous cell carcinoma, immune checkpoint inhibitor, anti-tumor activity, survival, adverse event
Citation: Gu Y-M, Shang Q-X, Zhuo Y, Zhou J-F, Liu B-W, Wang W-P, Che G-W and Chen L-Q (2021) Efficacy and Safety of Immune Checkpoint Inhibitor in Advanced Esophageal Squamous Cell Carcinoma: A Meta-Analysis. Front. Oncol. 11:777686. doi: 10.3389/fonc.2021.777686
Received: 15 September 2021; Accepted: 06 December 2021;
Published: 21 December 2021.
Edited by:
Sripathi Sureban, University of Oklahoma Health Sciences Center, United StatesReviewed by:
Takahiro Tsushima, Shizuoka Cancer Center, JapanCopyright © 2021 Gu, Shang, Zhuo, Zhou, Liu, Wang, Che and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Long-Qi Chen, ZHJjaGVubHFAc2N1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.