- 1Department of Urology, Affiliated Drum Tower Hospital, Medical School of Nanjing University, Nanjing, China
- 2Institute of Urology, Nanjing University, Nanjing, China
- 3Department of Pathology, Affiliated Drum Tower Hospital, Medical School of Nanjing University, Nanjing, China
- 4Department of Nuclear Medicine, Nanjing First Hospital, Nanjing Medical University, Nanjing, China
- 5GloriousMed Clinical Laboratory (Shanghai) Co., Ltd., Shanghai, China
More emerging evidence showed that homologous recombination (HR) defect (HRD) may predict sensitivity to platinum agents in metastatic prostate cancer (PCa). Platinum-based neoadjuvant chemotherapy for PCa with HRD has not been reported. Here, we reported a man diagnosed as locally advanced PCa with high Gleason Score (5 + 5) and low PSA level (5.2 ng/ml). Next-generation sequencing (NGS) demonstrated HRD. He received six cycles of platinum-based neoadjuvant chemotherapy before radical prostatectomy (RP). Fifteen months after RP, his PSA level was still undetectable, and no imaging progression was found, indicating a potential role for platinum-based neoadjuvant chemotherapy in locally advanced PCa with HRD.
Introduction
The incidence of prostate cancer (PCa) has been increasing and shows younger trend over the years in China, especially in the urban areas, possibly because of more active prostate-specific antigen (PSA) screening, improved biopsy techniques, or popular Westernized lifestyle (1). Most Chinese patients are diagnosed with high-risk disease, and they have higher rate of relapse and death after radical prostatectomy (RP) (2). Neoadjuvant therapy performed before RP intends to improve the surgery efficacy by reducing the tumor burden and minimal residual disease. However, the effectiveness of neoadjuvant therapy on PCa remains controversial.
Previous studies have identified that about 20%–30% of patients with primary PCa harbor alterations in genes involved in homologous recombination (HR) pathway, including BRCA2 (3, 4). Deleterious alterations of genes in HR are associated with aggressive disease and poor clinical outcomes (5, 6). More emerging studies also suggested that HR defect (HRD) may predict sensitivity to platinum agents or poly(ADP-ribose) polymerase (PARP) inhibitors in metastatic PCa (7–12). Here, we reported a patient with locally advanced PCa with HRD who responded outstandingly to platinum-based neoadjuvant chemotherapy.
Case Presentation
A 67-year-old man was referred to the hospital due to frequency and urgency of urination, and difficulty of defecation. Cystoscopy reexamination showed no obvious abnormality in the bladder but irregular mass at the urethral orifice, indicating the possibility of PCa. The PSA level was 5.2 ng/ml. Transrectal ultrasound (TRUS) showed that the prostate was in irregular shape with an estimated volume of 84.6 ml. The boundary of the left lobe of the prostate with the rectum and the bilateral seminal vesicles was not clear. Pathological examination and immunohistochemistry of the tumor tissue from transurethral resection demonstrated that it was PCa adenocarcinoma with a Gleason score 10 (5 + 5) (Figures 1A, B). Results of prostate-specific membrane antigen (PSMA)-PET/CT scan showed a high level of asymmetrical PSMA uptake in the prostate, confirming invasion of the seminal vesicle and rectum (Figure 2A). The final clinical American Joint Committee on Cancer (8th edition) staging was IIIC (cT4, N0, cM0).
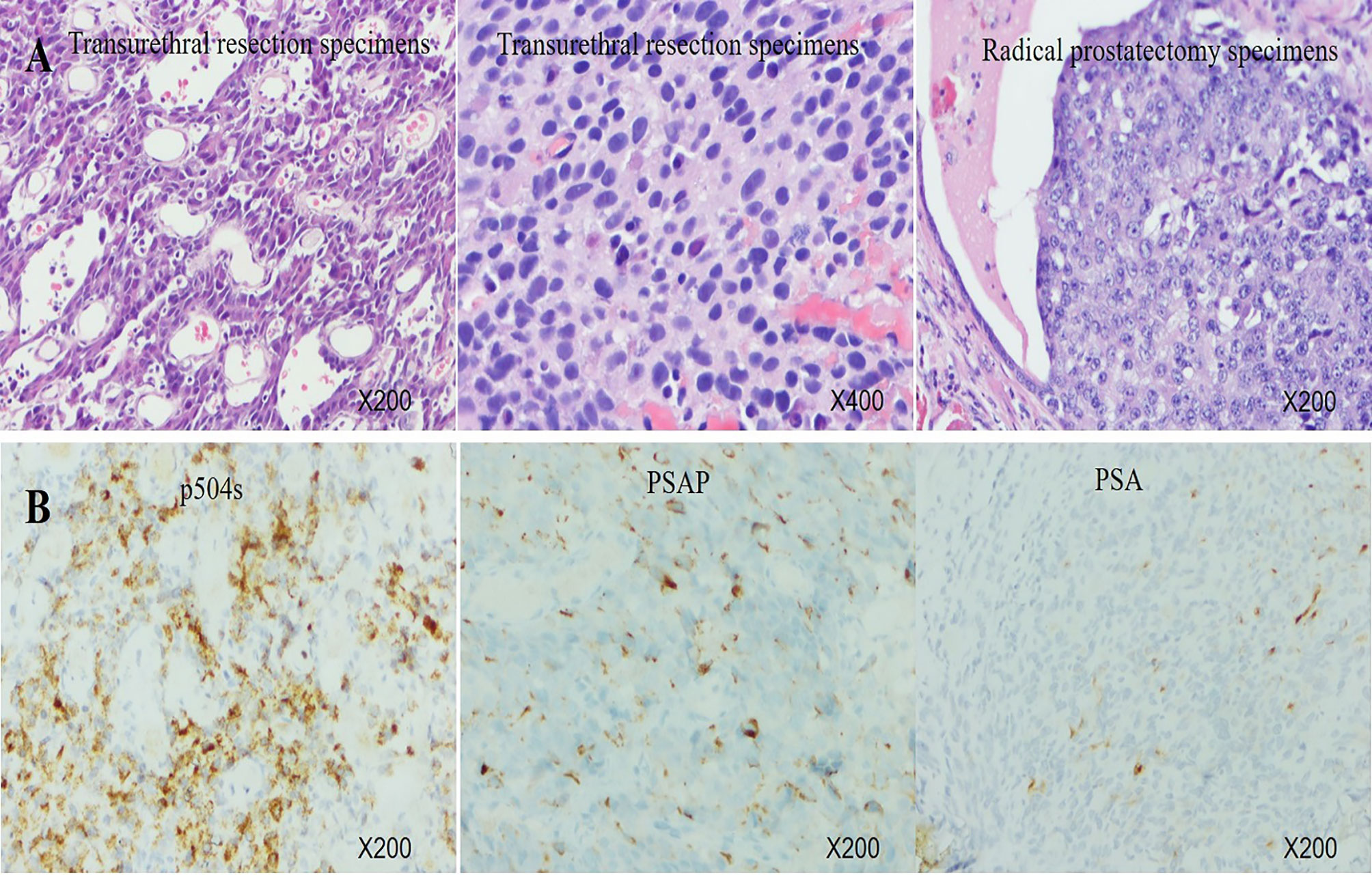
Figure 1 (A) Histology of transurethral resection specimens and radical prostatectomy specimens. (B) Immunohistochemistry of transurethral resection specimens showed very strong expression of P504S, middle expression of PSAP, and focal positivity for PSA.
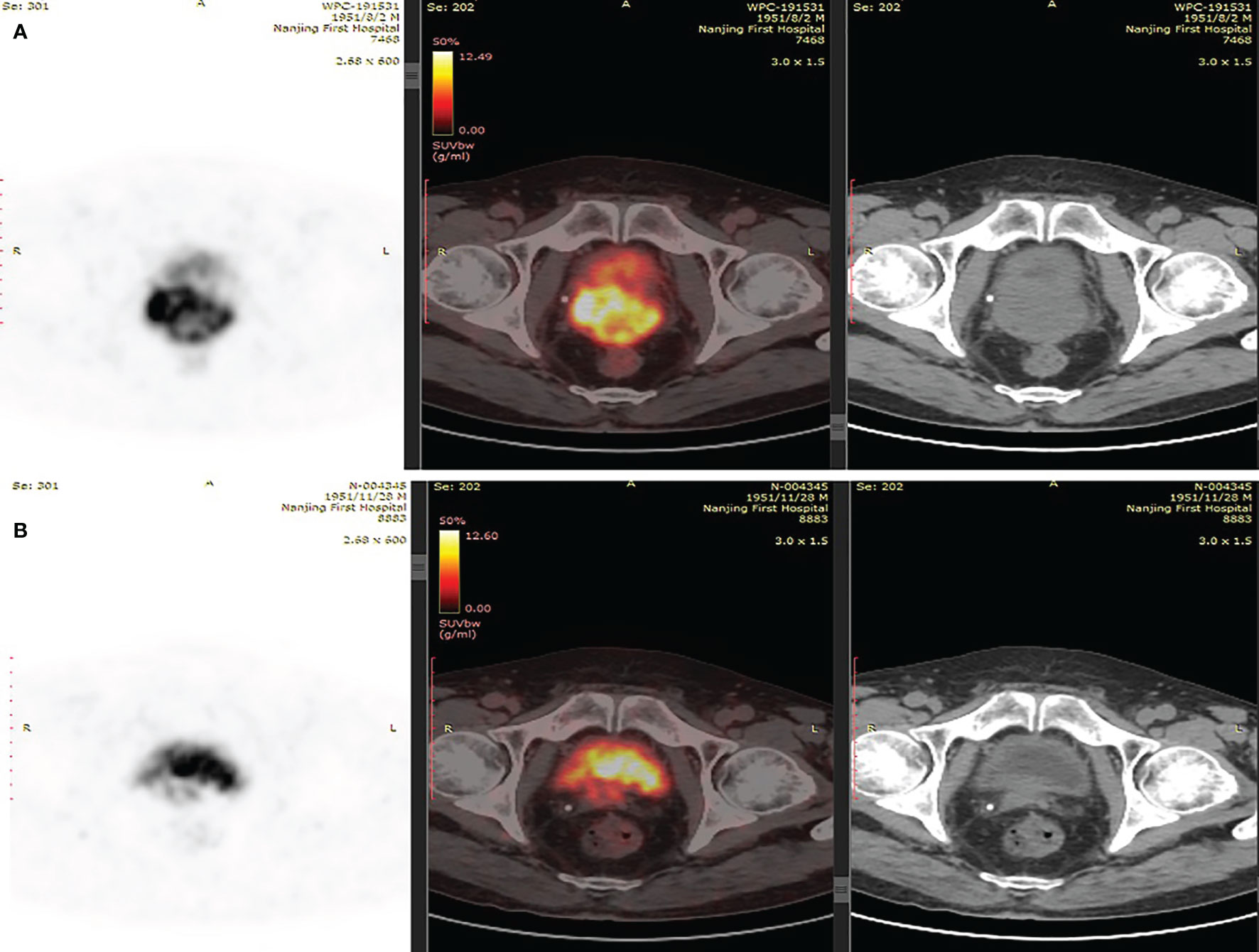
Figure 2 (A) PSMA-PET/CT scans suggested of uneven increase in PSMA expression of prostate. (B) PSMA-PET/CT scans showed shrunken lesions after platinum-based neoadjuvant chemotherapy.
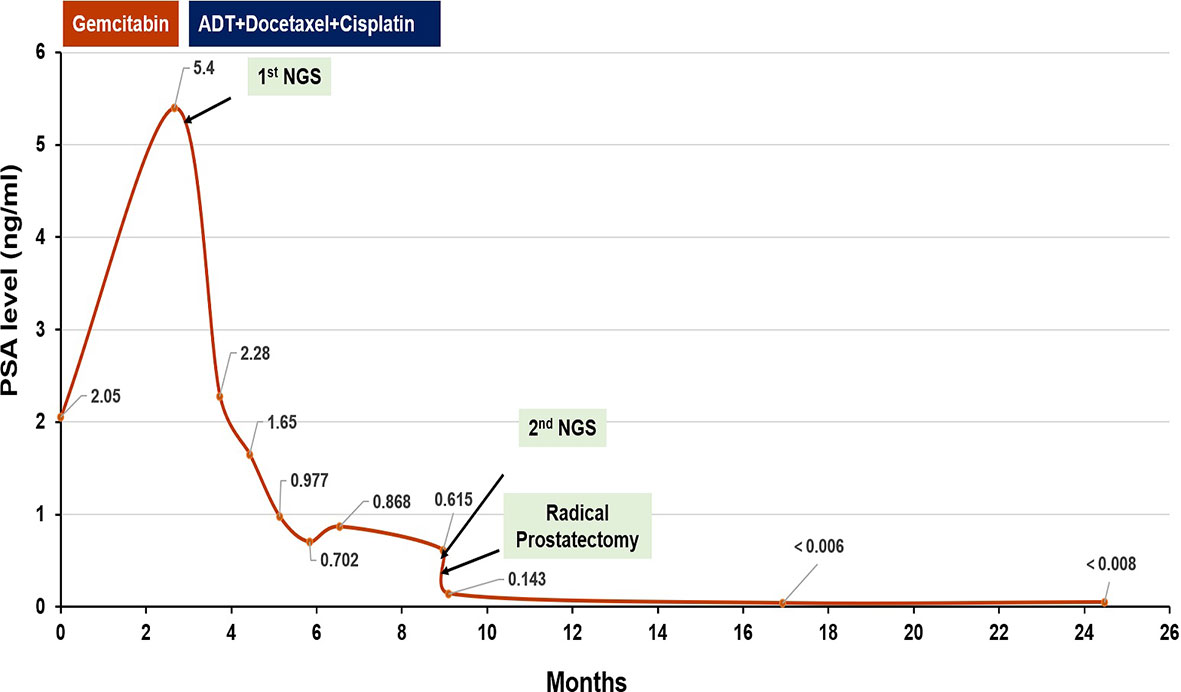
As the patient had high Gleason Score and low PSA level, we performed next-generation sequencing (NGS) on his blood sample. The results revealed several pathogenic mutations in the HR pathway including BRCA2 p.F2234fs with a mutant allele frequency (MAF) of 1.2%, RAD51C c.905-2A>G (MAF, 1%). As the patient refused radiotherapy, neoadjuvant therapy combined with RP was considered. Due to NGS detection of several somatic pathogenic mutations in the HR pathway, he participated in a clinical trial (NCT04869371) and commenced on androgen deprivation therapy (ADT) plus platinum-based neoadjuvant chemotherapy (docetaxel 75 mg/m2 plus cisplatin 130 mg/m2) on October 11, 2019 (Figure 3). After six courses of treatment, his PSA level decreased to 0.615 ng/ml, and his symptoms of frequency and urgency of urination were relieved. The adverse reactions during neoadjuvant therapy mainly include fatigue, poor appetite, and diarrhea. In addition, TRUS indicated that the size of the prostate was smaller with estimated volume of 34.3 ml. Furthermore, PSMA-PET/CT scan found that the lesions obviously shrank (Figure 2B). Meanwhile, a second NGS was performed on the patient’s blood sample. Compared with the result of the first NGS, all the pathogenic mutations in the HR pathway were undetectable, and no new pathogenic mutations emerged.
On February 26, 2020, the patient underwent robotic-assisted RP and enlarged pelvic lymph node dissection. The surgical pathology revealed that it was prostate adenocarcinoma, with reduced tumor stage of IIIB (ypT3b, N0, cM0) (Figure 1A). The surgical margin and pelvic lymph node were negative. No adjuvant therapy was used after RP. At final follow-up (June 3, 2021), his PSA was still undetectable (<0.008 ng/ml) with normal level of testosterone, and no imaging progression was found by electroconvulsive therapy (ECT) and MRI scan (Figure 3).
Discussion
High-risk PCa treated with curative intent is at increased risk of experiencing PSA failure, metastatic progression, and cancer-related death. The existence of micro-metastases disease is one of the main causes of this phenomenon. Neoadjuvant treatment not merely improves the function of traditional radical therapies but eliminates minimal residual disease to avoid tumor recurrence and metastatic progression. This has been proven by other solid cancers (13–15).
To improve surgical efficacy, neoadjuvant treatment strategies also have been employed in patients with high risk PCa. Shelley et al. performed meta-analysis of randomized trials of neoadjuvant hormone therapy (NHT) for localized or locally advanced PCa during 1966–2007. The results showed that NHT prior to RP significantly reduced positive margin rates, grade of tumor, and lymph node invasion but failed to improve overall disease-free survival (16). Nowadays, docetaxel or second-generation androgen receptor inhibitors combined with ADT have been investigated in neoadjuvant settings, as they result in significant improvement of overall survival in metastatic hormone-sensitive PCa (17–20). So far, several studies reported that neoadjuvant chemotherapy with docetaxel plus ADT was feasible and showed positive results for survival in high-risk PCa (21–23). However, the final outcomes for neoadjuvant chemotherapy with docetaxel have been inconclusive, which need more powerful evidence. Recently, neoadjuvant abiraterone or enzalutamide was reported to result in favorable pathological responses in some patients with high-risk disease (24, 25). Some pooled studies had indicated the better clinical oncological outcomes transferred from favorable pathological responses under neoadjuvant therapy of intense hormone therapy (26, 27). Although the efficacy of neoadjuvant therapies have not been determined in high-risk PCa patients, it is hopeful to witness final oncological benefits. In this regard, the identification of those patients who can benefit the most from such treatments is crucial.
PCa is a highly heterogeneous tumor even at its local stage. Neoadjuvant treatment of PCa is still focusing on intensive hormone therapy and thus lack of individualization, which may result in the limitation of complete pathological response (27). Precision and individualized treatment has been practiced in advanced PCa, but it can also be used for localized diseases. HRD is an important biomarker for precision therapy, which also leads to poor efficacy of traditional intensive hormone therapy (5, 6). Recent studies have reported that HRD are associated with platinum sensitivity in metastatic PCa. A retrospective study analyzed the association between germline BRCA2 variants and PSA response to carboplatin-based chemotherapy in a cohort of 141 men with metastatic castration-resistant PCa (mCRPC). Results suggested that pathogenic germline BRCA2 carriers had a higher response rate to carboplatin-based chemotherapy than non-BRCA2 carriers (10). Zafeiris Zafeiriou et al. presented three mCRPC cases with HRD who experienced impressive and durable responses to carboplatin (7). Another study presented that mCRPC patients with deleterious alterations in BRCA2, BRCA1, ATM, PALB2, FANCA, or CDK12 increased likelihood of achieving a PSA50 response and had longer time on platinum chemotherapy (8). More recently, Liancheng Fan et al. revealed that mCRPC harboring alterations in different genes of HR pathway displayed distinct response to platinum-based chemotherapy, and patients with BRCA2 and ATM alterations might experience more superior outcomes to platinum-based chemotherapy compared with other HR genes carriers (9).
In our case, the patient was with low PSA level and high Gleason Score at diagnosis. PSMA-PET/CT scan confirmed invasion of his seminal vesicle and rectum. Results of NGS analysis revealed pathogenic somatic mutations in the HR pathway. All of the above features suggested that the patient seemed to be more prone to micro-metastases and produce rapid clinical progression. Thus, a more aggressive treatment, ADT plus platinum-based neoadjuvant chemotherapy combined with RP, was finally chosen for him. After six cycles of treatment, the patient got better with decreased PSA level (0.615 ng/ml), reduced prostate size (from 84.6 to 34.3 ml), and diminished clinical symptoms. In addition, his second NGS results on blood sample collected after neoadjuvant therapy demonstrated that pathogenic mutations in the HR pathway disappeared, indicating tumor cell destroyed by platinum-based chemotherapy. Furthermore, 15 months after RP, the patient remains in clinical benefit and stable disease without increase in PSA and imaging progression. To our knowledge, this is the first case to report locally advanced PCa with HRD exhibiting outstanding response to platinum-based chemotherapy in neoadjuvant setting.
Conclusion
We reported a patient with locally advanced PCa with HRD presenting pathological response and long duration of stable disease with no biochemical recurrence or imaging appearance of metastasis to platinum-based neoadjuvant chemotherapy. This work indicates a potential role for platinum-based neoadjuvant chemotherapy in the treatment of locally advanced PCa with HRD and advocate that treatment decisions can be individually tailored, possibly improving patient outcomes.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
Conception and design of the work: JZ, SZ, XQ, YF, SA, TZ, YY, and HG. Supervision and writing of the paper: JZ. All authors contributed to the article and approved the submitted version.
Conflict of Interest
TZ and YY are employed by GloriousMed Clinical Laboratory (Shanghai) Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
PCa, prostate cancer; PSA, prostate-specific antigen; RP, radical prostatectomy; HR, homologous recombination; HRD, homologous recombination defect; TRUS, transrectal ultrasound; NGS, next-generation sequencing; MAF, mutant allele frequency; ADT, androgen deprivation therapy; NHT, neoadjuvant hormone therapy; OS, overall survival; mCRPC, metastatic castration-resistant prostate cancer.
References
1. Zhai Z, Zheng Y, Li N, Deng Y, Zhou L, Tian T, et al. Incidence and Disease Burden of Prostate Cancer From 1990 to 2017: Results From the Global Burden of Disease Study 2017. Cancer (2020) 126(9):1969–78. doi: 10.1002/cncr.32733
2. Stephenson AJ, Kattan MW, Eastham JA, Bianco FJ Jr., Yossepowitch O, Vickers AJ, et al. Prostate Cancer-Specific Mortality After Radical Prostatectomy for Patients Treated in the Prostate-Specific Antigen Era. J Clin Oncol (2009) 27(26):4300–5. doi: 10.1200/jco.2008.18.2501
3. Cancer Genome Atlas Research Network. The Molecular Taxonomy of Primary Prostate Cancer. Cell (2015) 163(4):1011–25. doi: 10.1016/j.cell.2015.10.025
4. Dall'Era MA, McPherson JD, Gao AC, DeVere White RW, Gregg JP, Lara PN Jr. Germline and Somatic DNA Repair Gene Alterations in Prostate Cancer. Cancer (2020) 126(13):2980–5. doi: 10.1002/cncr.32908
5. Castro E, Goh C, Olmos D, Saunders E, Leongamornlert D, Tymrakiewicz M, et al. Germline BRCA Mutations Are Associated With Higher Risk of Nodal Involvement, Distant Metastasis, and Poor Survival Outcomes in Prostate Cancer. J Clin Oncol (2013) 31(14):1748–57. doi: 10.1200/jco.2012.43.1882
6. Castro E, Goh C, Leongamornlert D, Saunders E, Tymrakiewicz M, Dadaev T, et al. Effect of BRCA Mutations on Metastatic Relapse and Cause-Specific Survival After Radical Treatment for Localised Prostate Cancer. Eur Urol (2015) 68(2):186–93. doi: 10.1016/j.eururo.2014.10.022
7. Zafeiriou Z, Bianchini D, Chandler R, Rescigno P, Yuan W, Carreira S, et al. Genomic Analysis of Three Metastatic Prostate Cancer Patients With Exceptional Responses to Carboplatin Indicating Different Types of DNA Repair Deficiency. Eur Urol (2019) 75(1):184–92. doi: 10.1016/j.eururo.2018.09.048
8. Mota JM, Barnett E, Nauseef JT, Nguyen B, Stopsack KH, Wibmer A, et al. Platinum-Based Chemotherapy in Metastatic Prostate Cancer With DNA Repair Gene Alterations. JCO Precis Oncol (2020) 4:355–66. doi: 10.1200/po.19.00346
9. Fan L, Fei X, Zhu Y, Chi C, Pan J, Sha J, et al. Distinct Response to Platinum-Based Chemotherapy Among Patients With Metastatic Castration-Resistant Prostate Cancer Harboring Alterations in Genes Involved in Homologous Recombination. J Urol (2021) 206(3):630–7. doi: 10.1097/ju.0000000000001819
10. Pomerantz MM, Spisák S, Jia L, Cronin AM, Csabai I, Ledet E, et al. The Association Between Germline BRCA2 Variants and Sensitivity to Platinum-Based Chemotherapy Among Men With Metastatic Prostate Cancer. Cancer (2017) 123(18):3532–9. doi: 10.1002/cncr.30808
11. Mateo J, Carreira S, Sandhu S, Miranda S, Mossop H, Perez-Lopez R, et al. DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. N Engl J Med (2015) 373(18):1697–708. doi: 10.1056/NEJMoa1506859
12. Abida W, Patnaik A, Campbell D, Shapiro J, Bryce AH, McDermott R, et al. Rucaparib in Men With Metastatic Castration-Resistant Prostate Cancer Harboring a BRCA1 or BRCA2 Gene Alteration. J Clin Oncol (2020) 38(32):3763–72. doi: 10.1200/jco.20.01035
13. Amiri-Kordestani L, Wedam S, Zhang L, Tang S, Tilley A, Ibrahim A, et al. First FDA Approval of Neoadjuvant Therapy for Breast Cancer: Pertuzumab for the Treatment of Patients With HER2-Positive Breast Cancer. Clin Cancer Res (2014) 20(21):5359–64. doi: 10.1158/1078-0432.Ccr-14-1268
14. Donahue JM, Nichols FC, Li Z, Schomas DA, Allen MS, Cassivi SD, et al. Complete Pathologic Response After Neoadjuvant Chemoradiotherapy for Esophageal Cancer Is Associated With Enhanced Survival. Ann Thorac Surg (2009) 87(2):392–8; discussion 398-9. doi: 10.1016/j.athoracsur.2008.11.001
15. Petrelli F, Coinu A, Cabiddu M, Ghilardi M, Vavassori I, Barni S. Correlation of Pathologic Complete Response With Survival After Neoadjuvant Chemotherapy in Bladder Cancer Treated With Cystectomy: A Meta-Analysis. Eur Urol (2014) 65(2):350–7. doi: 10.1016/j.eururo.2013.06.049
16. Shelley MD, Kumar S, Wilt T, Staffurth J, Coles B, Mason MD. A Systematic Review and Meta-Analysis of Randomised Trials of Neo-Adjuvant Hormone Therapy for Localised and Locally Advanced Prostate Carcinoma. Cancer Treat Rev (2009) 35(1):9–17. doi: 10.1016/j.ctrv.2008.08.002
17. Sweeney CJ, Chen YH, Carducci M, Liu G, Jarrard DF, Eisenberger M, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N Engl J Med (2015) 373(8):737–46. doi: 10.1056/NEJMoa1503747
18. James ND, Sydes MR, Clarke NW, Mason MD, Dearnaley DP, Spears MR, et al. Addition of Docetaxel, Zoledronic Acid, or Both to First-Line Long-Term Hormone Therapy in Prostate Cancer (STAMPEDE): Survival Results From an Adaptive, Multiarm, Multistage, Platform Randomised Controlled Trial. Lancet (2016) 387(10024):1163–77. doi: 10.1016/s0140-6736(15)01037-5
19. Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, et al. Abiraterone Acetate Plus Prednisone in Patients With Newly Diagnosed High-Risk Metastatic Castration-Sensitive Prostate Cancer (LATITUDE): Final Overall Survival Analysis of a Randomised, Double-Blind, Phase 3 Trial. Lancet Oncol (2019) 20(5):686–700. doi: 10.1016/s1470-2045(19)30082-8
20. Armstrong AJ, Szmulewitz RZ, Petrylak DP, Holzbeierlein J, Villers A, Azad A, et al. ARCHES: A Randomized, Phase III Study of Androgen Deprivation Therapy With Enzalutamide or Placebo in Men With Metastatic Hormone-Sensitive Prostate Cancer. J Clin Oncol (2019) 37(32):2974–86. doi: 10.1200/jco.19.00799
21. Sella A, Zisman A, Kovel S, Yarom N, Leibovici D, Lindner A. Neoadjuvant Chemohormonal Therapy in Poor-Prognosis Localized Prostate Cancer. Urology (2008) 71(2):323–7. doi: 10.1016/j.urology.2007.08.060
22. Fizazi K, Faivre L, Lesaunier F, Delva R, Gravis G, Rolland F, et al. Androgen Deprivation Therapy Plus Docetaxel and Estramustine Versus Androgen Deprivation Therapy Alone for High-Risk Localised Prostate Cancer (GETUG 12): A Phase 3 Randomised Controlled Trial. Lancet Oncol (2015) 16(7):787–94. doi: 10.1016/s1470-2045(15)00011-x
23. Pan J, Chi C, Qian H, Zhu Y, Shao X, Sha J, et al. Neoadjuvant Chemohormonal Therapy Combined With Radical Prostatectomy and Extended PLND for Very High Risk Locally Advanced Prostate Cancer: A Retrospective Comparative Study. Urol Oncol (2019) 37(12):991–8. doi: 10.1016/j.urolonc.2019.07.009
24. Taplin ME, Montgomery B, Logothetis CJ, Bubley GJ, Richie JP, Dalkin BL, et al. Intense Androgen-Deprivation Therapy With Abiraterone Acetate Plus Leuprolide Acetate in Patients With Localized High-Risk Prostate Cancer: Results of a Randomized Phase II Neoadjuvant Study. J Clin Oncol (2014) 32(33):3705–15. doi: 10.1200/jco.2013.53.4578
25. McKay RR, Ye H, Xie W, Lis R, Calagua C, Zhang Z, et al. Evaluation of Intense Androgen Deprivation Before Prostatectomy: A Randomized Phase II Trial of Enzalutamide and Leuprolide With or Without Abiraterone. J Clin Oncol (2019) 37(11):923–31. doi: 10.1200/jco.18.01777
26. McKay RR, Berchuck J, Kwak L, Xie W, Silver R, Bubley GJ, et al. Outcomes of Post-Neoadjuvant Intense Hormone Therapy and Surgery for High Risk Localized Prostate Cancer: Results of a Pooled Analysis of Contemporary Clinical Trials. J Urol (2021) 205(6):1689–97. doi: 10.1097/ju.0000000000001632
Keywords: prostate cancer, neoadjuvant chemotherapy, platinum, next-generation sequencing, homologous recombination defect
Citation: Zhuang J, Zhang S, Qiu X, Fu Y, Ai S, Zhao T, Yang Y and Guo H (2022) Platinum-Based Neoadjuvant Chemotherapy Before Radical Prostatectomy for Locally Advanced Prostate Cancer With Homologous Recombination Deficiency: A Case Report. Front. Oncol. 11:777318. doi: 10.3389/fonc.2021.777318
Received: 15 September 2021; Accepted: 08 December 2021;
Published: 05 January 2022.
Edited by:
Mohamed Saad Zaghloul, Cairo University, EgyptReviewed by:
Francesco Del Giudice, Sapienza University of Rome, ItalyKazutaka Narimoto, St. Luke’s International Hospital, Japan
Copyright © 2022 Zhuang, Zhang, Qiu, Fu, Ai, Zhao, Yang and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongqian Guo, ZHIuZ2hxQG5qdS5lZHUuY24=
†These authors have contributed equally to this work
 Junlong Zhuang
Junlong Zhuang Shun Zhang
Shun Zhang Xuefeng Qiu1,2
Xuefeng Qiu1,2 Tingting Zhao
Tingting Zhao Hongqian Guo
Hongqian Guo