
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Oncol., 14 December 2021
Sec. Gastrointestinal Cancers: Colorectal Cancer
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.766500
This article is part of the Research TopicWomen in Colorectal Cancers: 2021View all 8 articles
 Angela Damato1,2*
Angela Damato1,2* Francesca Bergamo3
Francesca Bergamo3 Lorenzo Antonuzzo4
Lorenzo Antonuzzo4 Guglielmo Nasti5
Guglielmo Nasti5 Francesco Iachetta1
Francesco Iachetta1 Alessandra Romagnani1
Alessandra Romagnani1 Erika Gervasi1
Erika Gervasi1 Mario Larocca1
Mario Larocca1 Carmine Pinto1
Carmine Pinto1The NIVACOR trial is a phase II study assessing the efficacy and safety of nivolumab in combination with FOLFOXIRI/bevacizumab in first-line setting in patients affected by metastatic colorectal cancer (mCRC) RAS/BRAF mutated. We report safety run-in results in the first 10 patients enrolled. Patients received triplet chemotherapy with FOLFOXIRI scheme plus bevacizumab, in association with nivolumab every 2 weeks for 8 cycles (induction phase) followed by bevacizumab plus nivolumab every 2 weeks (maintenance phase), until progression of disease or unacceptable toxicities. The first ten patients were evaluated: 7 experienced at least one adverse event (AE) related to FOLFOXIRI/bevacizumab and 2 related to nivolumab. The most frequent grade 1–2 AEs related to FOLFOXIRI/bevacizumab were diarrhea and fatigue (71%), nausea and vomiting (57%); 3 (43%) had grade 3–4 neutropenia, and 2 (20%) patients developed grade 1–2 AEs nivolumab related: skin rash and salivary gland infection. Two patients delayed the dose because of serious AEs, proteinuria and salivary gland infection; one patient discontinued experimental treatment due to the ileo-urethral fistula and concurrent Clostridium infection diarrhea. No treatment- related death occurred. The safety run-in analysis of NIVACOR trial reassured using co-administration of FOLFOXIRI/bevacizumab and nivolumab was well tolerated with an acceptable toxicity profile.
Clinical Trial Registration: https://clinicaltrials.gov/, (NCT04072198).
Colorectal cancer is the fourth commonly diagnosed cancers and among the leading cause of cancer-related deaths globally (1). Around 40–50% of cases are diagnosed in advanced stages with a median overall survival (OS) increased over the 30 months and a 5-year survival rate of approximately 12% (1, 2). The backbone of treatment in first-line in mCRC comprises chemotherapy scheme combination (5-fluorouracil, oxaliplatin, irinotecan) combined with specific monoclonal antibody anti-epidermal growth factor receptor (EGFR), cetuximab or panitumumab, or anti-vascular endothelial growth factor (VEGF), such as bevacizumab (3, 4).
The antibodies’ choice depends on RAS mutation occurrence, which represent 50-55% of cases in mCRC. Therefore, BRAF V600E mutation represented the additional prognostic negative factor in mCRC occurring in 5–21% of cases (5–10), although recently there has been published a randomized, phase III trial of combination with an anti-BRAF inhibitor (encorafenib), cetuximab, and an anti-MEK inhibitor (binimetinib), in patients with mCRC BRAF V600E mutated, after one or two previous regimens, resulting in significantly longer OS and a higher response rate than standard therapy (irinotecan plus cetuximab) (11).
The TRIBE phase III trial demonstrated a superiority of FOLFOXIRI plus bevacizumab compared to FOLFIRI plus bevacizumab in the first-line setting in mCRC in terms of progression-free survival (PFS) (12.3 versus 9.7 months, hazard ratio 0.77, 95% CI 0.65–0.93; p = 0.006), also in BRAF mutated subgroup (12, 13). Selecting the best upfront therapy is crucial in the mCRC to warrant a better survival and response to treatment. For these reasons, the intensification of the chemotherapy backbone in previously untreated mCRC patients is recommended.
Immune checkpoint inhibitors (ICIs) have announced new opportunities in cancer therapy (14–17). Programmed death 1 (PD-1) blockade has clinical benefit in MSI-H or dMMR mCRC after previous therapies. Indeed, the FDA approved nivolumab and pembrolizumab for patients with MSI-H/dMMR mCRC evolved after treatments with fluoropyrimidine, oxaliplatin, and irinotecan (18–20). Recently, the KEYNOTE-177 phase III trial revealed that front-line pembrolizumab was superior to chemotherapy in terms of PFS (16.5 versus 8.2 months, hazard ratio, 0.60; 95% CI, 0.45 to 0.80; P=0.0002). The overall response (complete or partial response) was observed in 43.8% of patients (95% CI, 35.8 to 52.0) in the pembrolizumab arm compared to 33.1% (95% CI, 25.8 to 41.1) in the chemotherapy arm; the complete response of disease was observed in 11 and 4% of patients, respectively. Among these, 83% of patients had ongoing responses in the pembrolizumab arm (21).
The sensitivity to immune checkpoint inhibitors in MSI-H/dMMR mCRC is reasonably due to a more efficient immune background linked to a high burden of neoantigens arising from the hypermutated tumor cells, able to prompt a compelling immune response. Conversely, MSS/pMMR tumors are mainly immune deprived or immune desert, because of a low or deficient T-cell infiltration and reduced expression of checkpoint proteins (22). To recruit stimulated immune cells in the tumor microenvironment, combination strategies are investigated including immunotherapy and other drugs with immunomodulatory features, such as dual immune checkpoint blockade, radiotherapy, chemotherapy, or other targeted therapies. Furthermore, there is a rising effort to detect different biomarkers to select patients most likely responsive to immunotherapy and to recognize possible resistance mechanisms.
The NIVACOR study was designed to assess the efficacy and safety of nivolumab in combination with chemotherapy triplet scheme (FOLFOXIRI) plus bevacizumab in first-line treatment in patients affected by mCRC RAS/BRAF mutated, regardless of the microsatellite status. A preliminary safety analysis was planned after the 10th patient enrolled to detect early and acute toxicity. We present the safety run-in results of phase II NIVACOR trial.
The NIVACOR (NCT04072198) is a single arm, open-label, multicenter, phase II study with a safety run-in phase. Patients with mCRC RAS/BRAF mutated in the first-line setting received nivolumab in combination with a triplet of chemotherapy, FOLFOXIRI, plus bevacizumab for eight cycles (induction phase) and subsequently, in patients with partial response or control of disease, nivolumab and bevacizumab until progression of disease or unacceptable toxicities (maintenance phase) (Figure 1). Collateral studies are also in process; liquid biopsy will be performed at baseline, before cycle 5, and at disease progression.
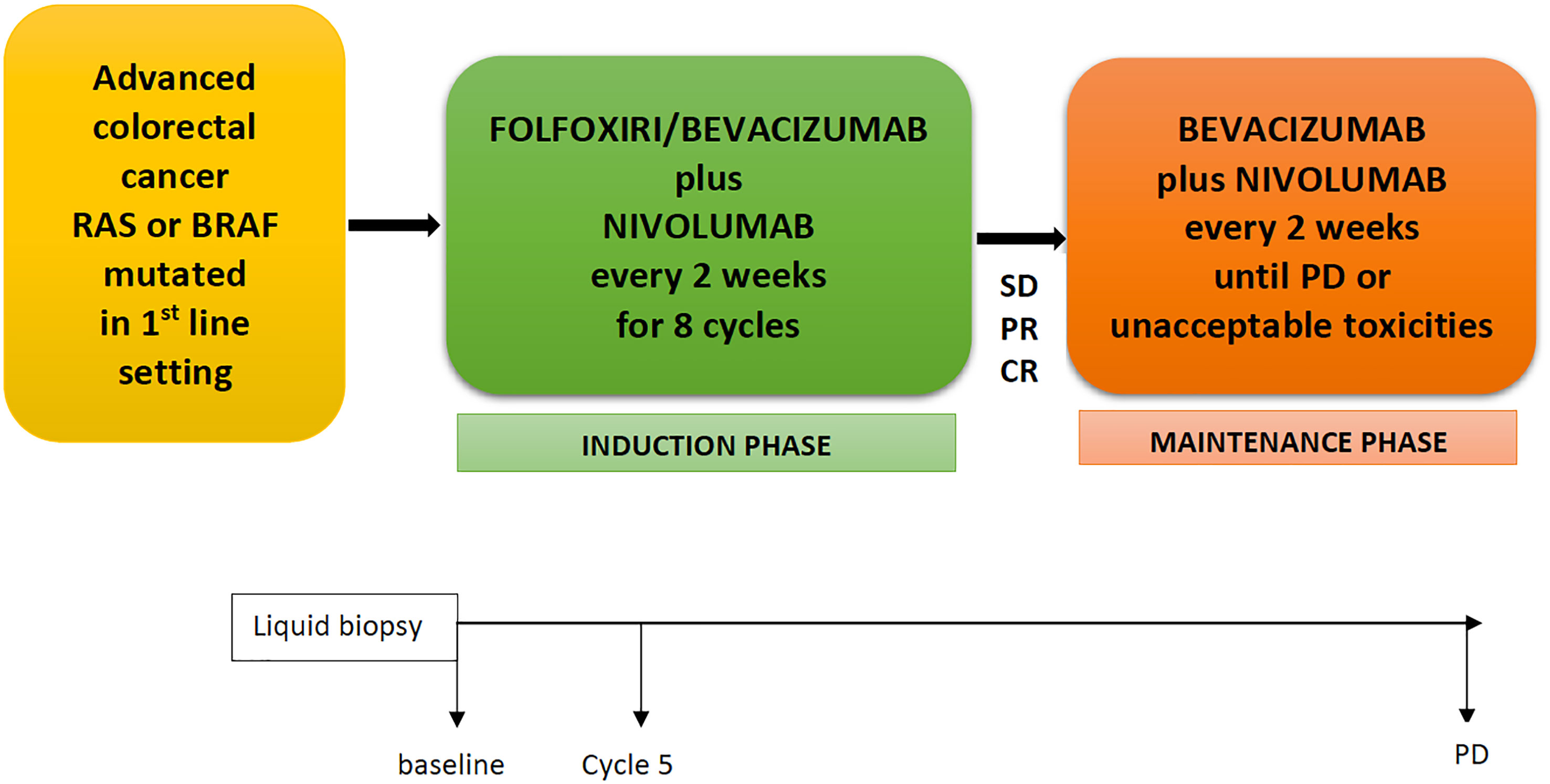
Figure 1 NIVACOR Study design. FOLFOXIRI, 5-fluorouracil, irinotecan, oxaliplatin; SD, stable disease; PR, partial response; CR, complete response; PD, progression disease.
Patients eligible for the enrolment require a histological diagnosis of adenocarcinoma, mutation of RAS or BRAF confirmed, advanced and unresectable disease untreated with previous chemotherapy for metastatic disease. All inclusion and exclusion criteria can be available in the study protocol (Table S1).
The trial was designed to assess the efficacy of nivolumab in addition to FOLFOXIRI plus bevacizumab in terms of response rate. The primary endpoint was the objective response rate (ORR) evaluated by the investigator assessment according to RECIST 1.1 criteria. Secondary objectives were safety profile according to Common Terminology Criteria for Adverse Events (CTCAE) v.4.03, overall survival (OS), time to progression (TTP), duration of response (DoR), and quality of life evaluated with the EORTC QLQ-C30.
All eligible patients receive 8 cycles of nivolumab in combination with FOLFOXIRI/bevacizumab every 2 weeks (induction phase), followed by nivolumab plus bevacizumab every 2 weeks in patients that achieved partial response, complete response, or stable disease. The treatment will be continued until unacceptable toxicity, disease progression, or patient/physician’s decision. All drugs are administered intravenously. Bevacizumab at a dose of 5 mg/kg every 2 weeks; nivolumab at flat dose of 240 mg every 2 weeks. FOLFOXIRI scheme will be administered as irinotecan infusion at 165 mg/m2 for 60 min (min), followed by oxaliplatin infusion at 85 mg/m2 simultaneously with leucovorin 200 mg/m2 for 120 min, and lastly continuous infusion for 48 h of 5-fluorouracil at dose of 3,200 mg/m2.
Treatment visits were performed on day 1 (+3 days) of each cycle with vital signs, a physical examination, hematology and biochemical laboratory analysis, AEs description, and concomitant treatments report. Treatment efficacy was evaluated by the investigator according to Response Evaluation Criteria in Solid Tumours (RECIST) 1.1 criteria every 8 weeks during investigational treatment, and subsequently every 3 months for 3 years.
Considering the safety profile of nivolumab in combination with FOLFOXIRI/bevacizumab has not been evaluated in mCRC patients so far, a safety run-in phase was achieved. This analysis was arranged to evaluate the feasibility of the investigational treatment by noticing the first ten patients enrolled. In order to be able to describe the safety profile, an Independent Monitoring Committee (IDMC) reviewed data 28 days after the 10th patient included. At least one cycle of experimental treatment had been administered. Grading of adverse events (AEs) was collected according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.03. The IDMC provided an endorsement as to whether the study may resume, whether amendments to the protocol should be implemented, or whether the study should be closed. If the experimental treatment study was judged feasible and no major safety concerns arose, the enrollment would be resumed and involved further participating sites.
The sample size was estimated using the A’Hern (23) variation of the original Fleming (24) one-stage design. The study needs 64 patients to decide whether P < 0,66 or > 0,80. An overall 70 patients should be enrolled supposing 10% of patients’ discontinuation due to unacceptable toxicity or non-compliance to the treatment. Our hypothesis is that nivolumab in addition to FOLFOXIRI/bevacizumab increases the overall response rate (ORR) from 66 to 80%, the latter considered sufficiently valuable to pursue this experimental combination in a phase III trial.
Between October 2019 and June 2020, a safety run-in phase was conducted at the study Coordinating Center. Ten patients were enrolled and received experimental study treatment. According to the study protocol, IDMC reviewed the safety data when 10 patients had received at least 1 cycle of the study treatment. The median age of patients was 58 (range 32–66), and 6 patients (60%) were male. The median cycles of treatment were 5.5 (range 1–9). Nine (90%) patients had Eastern Cooperative Oncology Group (ECOG) performance status equal to 0. All of patients (100%) were KRAS G12D mutated and BRAF wild type; 2 (2%) patients had MSI-H/dMMR. Patient characteristics and baseline demographics are summarized in Table 1.
Adverse events (AEs) are summarized in Table 2.
Among all (N=10, 100%), 70% (N=7) of patients experienced at least one AE related to FOLFOXIRI plus bevacizumab, and 20% (N=2) developed immune-related AEs (irAEs) to nivolumab. The most frequent grade 1–2 AEs related to FOLFOXIRI plus bevacizumab were diarrhea (71%, N=5), fatigue (71%, N=5), nausea (57%, N=4), and vomiting (57%, N=4). Instead, grade 1–2 skin rash (50%, N=1) and salivary gland inflammation (50%, N=1) occurred as irAEs. One patient (14%) developed recurrent infusion-related toxicity to oxaliplatin during the 1st and 2nd cycle of treatment, leading to permanent drug discontinuation. Concerning grade 3–4 AEs, 43% (N=3) of patients developed neutropenia, and N=1 (14%) patient had febrile neutropenia associated to FOLFOXIRI plus bevacizumab. Additionally, 1 (14%) patient developed a G3/4 skin rash, which was attributed to toxicity of chemotherapy and possibly to oxaliplatin but did not appear during intravenous infusion but approximately 6 h after administration of the 2nd and 3rd cycle of treatment. No one patient exhibited grade 3–4 irAEs. Regarding the serious AEs (SAEs), 4 of them were reported: 1 (25%) patient revealed proteinuria associated to bevacizumab, resulting in the treatment delay; 1 (25%) patient developed salivary gland infection possibly related to nivolumab, treated with non-steroidal anti-inflammatory drug (ketoprofen) and pain relievers; and 1 patient had ileo-urethral fistula (25%) and concurrent diarrhea secondary to Clostridium difficile infection (25%) after the first cycle of FOLFOXIRI/bevacizumab plus nivolumab, leading to colostomy packaging and permanent treatment discontinuation. Despite this, the IDMC judged the study treatment combination tolerable and feasible and worthy of further investigation. The enrollment resumed and currently is concluded at 73 patients enrolled in 11 Italian participating sites.
Overall, 1 patient discontinued treatment for SAEs. After a median follow-up of 17 months, 4 out of 10 patients still remained in the experimental treatment.
In the NIVACOR study, the most common toxicities reported for the experimental combination were those anticipated with FOLFOXIRI plus bevacizumab alone, as reported in the TRIBE study (12, 13).
Several clinical trials exploiting combination of chemotherapy with immune-checkpoint inhibitors have been published. Among these, we discuss studies using chemotherapy regimen with fluorouracil, oxaliplatin, and irinotecan (Table 3).
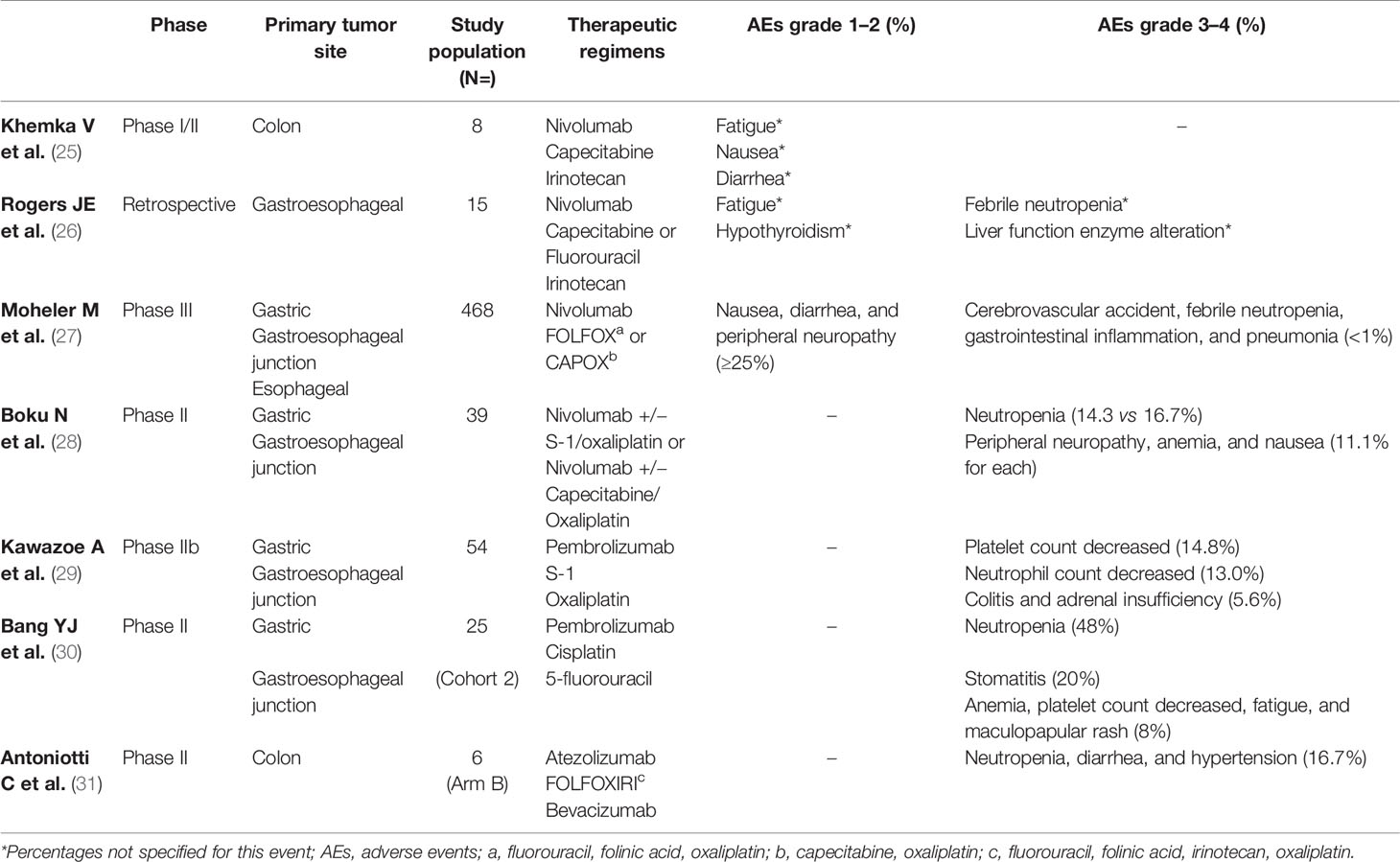
Table 3 Most common treatment-related adverse events with immunotherapy and chemotherapy combinations in Phase II and III trials.
Khemka et al. conducted a phase Ib/II study on colorectal cancer and pancreatic adenocarcinoma patients to evaluate safety and clinical activity of nivolumab plus an oral fluoropyrimidine, capecitabine, and irinotecan (CAPIRI scheme) (25). Patients were treated with nivolumab 3 mg/kg on day 1 and day 15 every 28 days cycle along with CAPIRI until disease progression or toxicity. The most common grade 1–2 AEs were fatigue, nausea, and diarrhea in 50% of patients; no infusion-related reactions were observed. There were no dose-limiting toxicities or SAEs reported. The authors suggested the following dosing scheme for the phase 2 section of the trial: nivolumab 3 mg/kg days 1 and 15, irinotecan 175 mg/m2 day 1 and 15, capecitabine 1,000 mg twice daily 5 days on and 2 days off every week.
In a retrospective analysis, 15 patients affected by advanced gastroesophageal cancers treated with nivolumab 240 mg flat dose, irinotecan 120 mg/m2, and 5-FU 2,000 mg/m2 over 46 h (or capecitabine 1,250 mg/m2/day for 7 days on/7 days off) given every 2 weeks. One patient had febrile neutropenia, and 1 patient grade 3 liver function enzyme alteration. Most patients had grade ≤2 AEs, especially fatigue (26).
The CheckMate 649, the largest phase III randomized trial, displayed the first results comparing two arms of treatment, nivolumab plus chemotherapy (FOLFOX or XELOX) versus chemotherapy alone, in patients affected by advanced gastric/gastroesophageal junction/esophageal cancer. The most common any grade TRAEs (≥25%) across both arms were nausea, diarrhea, and peripheral neuropathy. Regarding TRAEs with potential immunologic etiology, grade 3–4 TRAE events occurred in ≤5% of patients, and there were no grade 5 events. The incidence of TRAEs in patients whose tumors expressed PD-L1 CPS ≥ 5 was consistent with all treated patients across both arms (27).
The safety of nivolumab added to S-1 plus oxaliplatin (SOX) or capecitabine plus oxaliplatin (CapeOX) as first-line therapy for advanced or recurrent HER2-negative gastric or esophageal-gastric cancer was evaluated in the part 1 of the ATTRACTION-4 study. Patients were randomized to receive nivolumab flat dose of 360 mg every 3 weeks, S-1 40 mg/m2 orally twice daily for 14 days and 7 days off (or capecitabine 1,000 mg/m2 orally twice daily for 14 days and 7 days off) plus oxaliplatin 130 mg/m2 on day 1 every 3 weeks, until disease progression or unacceptable toxicity. Among 40 patients enrolled, 38 included the safety and efficacy analysis. Most of grade 3–4 AEs were neutropenia (14.3%) in the nivolumab plus SOX group, while in CapeOX group described neutropenia (16.7%), followed by anemia, nausea, and neurological toxicities in 11.1% of cases, respectively. No treatment-related death occurred. Discontinuations due to treatment-related adverse events (TRAEs) were reported in five patients (28).
The safety of pembrolizumab combined to S-1 and oxaliplatin (SOX) as the first-line therapy in Japanese patients affected by advanced gastric or gastroesophageal junction cancer was evaluated in a phase IIb KEYNOTE-659 study. Grade >3 TRAEs were reported in 57.4% of patients. The most common grade >3 TRAEs were platelet and neutrophil counts decreased (14.8 and 13.0%), colitis and adrenal insufficiency in 5.6% of cases. No treatment-related deaths occurred (29).
Furthermore, in the KEYNOTE-059 trial, 25 patients with advanced gastric or gastroesophageal junction cancer received pembrolizumab in combination with cisplatin and continuous infusion of 5-fluorouracil. Grade 3 TRAEs occurred in 15 patients (60%), and 4 (16%) experienced grade 4 neutropenia. Pembrolizumab-related AEs were observed in 12 (48.0%) patients; grade 3 stomatitis and maculopapular rash occurred in 2 (8%) patients and 1 (4%) patient, respectively (30).
Recently, the ongoing phase II AtezoTribe study was published. Patients with mCRC, regardless of microsatellite status, were randomized 1:2 in first-line therapy to receive FOLFOXIRI plus bevacizumab (arm A) or FOLFOXIRI plus bevacizumab and atezolizumab (arm B) up to 8 cycles, followed by maintenance with 5-FU/leucovorin plus bevacizumab with or without atezolizumab until disease progression or unacceptable toxicity. A safety run-in phase was conducted in the first 8 patients enrolled (2 in arm A and 6 in arm B). No grade 4 or SAEs were described. Grade 3 AEs that emerged were neutropenia for 1 patient in arm A and 1 patient in arm B; diarrhea and hypertension were experienced in 1 patient in arm A and 1 patient in arm B. This study was judged safe, and the enrollment has been resumed and currently completed (31).
In line with the literature data, the main grade 1–2 AEs related to chemotherapy and antiangiogenetic drug affect mainly the gastrointestinal and hematological toxicity, such as diarrhea and fatigue (71%), nausea and vomiting (57%), followed by grade 3–4 neutropenia (43%) and febrile neutropenia (14%). Grade 1–2 immune-related AEs were reported in two patients as described above (skin rash and salivary gland infection). Two SAEs have been reported but both related to FOLFOXIRI and bevacizumab. Overall, these toxicities appear to be manageable.
The safety run-in results of the NIVACOR trial do not raise concerns regarding the co-administration of chemotherapy (FOLFOXIRI), an anti-VEGF antibody (bevacizumab), and an anti-PD-L1 antibody (nivolumab). Although the analysis concerns only the first ten patients enrolled, the study resumed and the enrolment on March 1, 2021, was concluded. Data on the primary and secondary endpoints will be presented in the final analysis, including comprehensive safety analysis across overall population.
These preliminary results support the clinicians’ decision to continue the phase II NIVACOR trial to evaluate the efficacy of the association between immune checkpoint agent with a triplet chemotherapy and anti-VEGF inhibitor in mCRC RAS/BRAF mutated, regardless of microsatellite status.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
The studies involving human participants were reviewed and approved by Agenzia Italiana del Farmaco—AIFA) on February 8, 2019 (Protocol version 2.0, January 14th 2019), and an Independent Ethics Committee (Area Vasta Emilia Nord). The patients/participants provided their written informed consent to participate in this study.
Conceptualization: AD, CP, and FI. Methodology: AD and CP. Formal analysis: AD and CP. Investigation: AD, CP, FI, FB, LA, GN, and ML. Data curation: AD, CP, AR, and EG. Writing—original draft preparation: AD. Writing—review and editing: AD, CP, FI, LA, and AR. Visualization: AD, CP, FI, FB, LA, GN, ML, AR, and EG. Supervision: AD and CP. Project administration: AD. All authors contributed to the article and approved the submitted version.
This research received an unrestricted grant by Bristol Meyer Squibb Italy.
CP reports outside the submitted work personal fees for advisory role, speaker engagements, and travel and accommodation expenses from Amgen, Astellas, Astra-Zeneca, Bayer, Bristol Meyer Squibb, Clovis Oncology, Ipsen, Janssen, Incyte, Merck-Serono, Merck Sharp and Dohme, Novartis, Roche, and Sanofi.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to thank the patients who participated in the study and their families and study clinical site.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.766500/full#supplementary-material
1. National Cancer Institute Surveillance, Epidemiology, and End Results Program. In: SEER Stat Fact Sheets: Colon and Rectum Cancer. Available at: https://seer.cancer.gov/statfacts/html/colorect.html.
2. Hamers PAH, Elferink MAG. Informing Metastatic Colorectal Cancer Patients by Quantifying Multiple Scenarios for Survival Time Based on Real-Life Data. Int J Cancer (2021) 148:296–306. doi: 10.1002/ijc.33200
3. Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, et al. ESMO Consensus Guidelines for the Management of Patients With Metastatic Colorectal Cancer. Ann Oncol (2016) 27:1386–422. doi: 10.1093/annonc/mdw235
4. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Rectal Cancer, Version 3. (2020). doi: 10.1093/annonc/mdw235.
5. Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF Gene in Human Cancer. Nature (2002) 417:949–54. doi: 10.1038/nature00766
6. Sorbye H, Dragomir A, Sundstrom M, Pfeiffer P, Thunberg U, Bergfors M, et al. High BRAF Mutation Frequency and Marked Survival Differences in Subgroups According to KRAS/BRAF Mutation Status and Tumor Tissue Availability in a Prospective Population-Based Metastatic Colorectal Cancer Cohort. PloS One (2015) 10(6):e0131046. doi: 10.1371/journal.pone.0131046
7. Loupakis F, Ruzzo A, Cremolini C, Vincenzi B, Salvatore L, Santini D, et al. KRAS Codon 61, 146 and BRAF Mutations Predict Resistance to Cetuximab Plus Irinotecan in KRAS Codon 12 and 13 Wild-Type Metastatic Colorectal Cancer. Br J Cancer (2009) 101:715–21. doi: 10.1038/sj.bjc.6605177
8. Yaeger R, Chatila WK, Lipsyc MD, Hecthman JF, Cercek A, Sanchez-Vega F, et al. Clinical Sequencing Defines the Genomic Landscape of Metastatic Colorectal Cancer. Cancer Cell (2018) 33(1):125.e3–36.e3. doi: 10.1016/j.ccell.2017.12.004
9. Bylsma LC, Gillezeau C, Garawin T, Kelsh MA, Fryzek J, Sangarè L, et al. Prevalence of RAS and BRAF Mutations in Metastatic Colorectal Cancer (mCRC) Patients by Tumor Location. J Clin Oncol (2018) 36:Suppl:681–1. doi: 10.1200/JCO.2018.36.4_suppl.681
10. Clarke CN, Kopetz ES. BRAF Mutant Colorectal Cancer as a Distinct Subset of Colorectal Cancer: Clinical Characteristics, Clinical Behavior, and Response to Targeted Therapies. J Gastrointest Oncol (2015) 6:660–7. doi: 10.3978/j.issn.2078-6891.2015.077
11. Kopetz S, Grothey A. Encorafenib, Binimetinib, and Cetuximab in BRAF V600E-Mutated Colorectal Cancer. N Engl J Med (2019) 381:1632–43. doi: 10.1056/NEJMoa1908075
12. Loupakis F, Cremolini C, Masi G, Lonardi S, Zagonel V, Salvatore L, et al. Initial Therapy With FOLFOXIRI and Bevacizumab for Metastatic Colorectal Cancer. N Engl J Med (2014) 371(17):1609–18. doi: 10.1056/NEJMoa1403108
13. Cremolini C, Loupakis F, Antoniotti C, Lupi C, Sensi E, Lonardi S, et al. FOLFOXIRI Plus Bevacizumab Versus FOLFIRI Plus Bevacizumab as First-Line Treatment of Patients With Metastatic Colorectal Cancer: Updated Overall Survival and Molecular Subgroup Analyses of the Open-Label, Phase 3 TRIBE Study. Lancet Oncol (2015) 16(13):1306–15. doi: 10.1016/S1470-2045(15)00122-9
14. Pardoll DM. The Blockade of Immune Checkpoints in Cancer Immunotherapy. Nat Rev Canc (2012) 12:252e64. doi: 10.1038/nrc3239
15. Hodi FS, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Cowey CL, et al. Nivolumab Plus Ipilimumab or Nivolumab Alone Versus Ipilimumab Alone in Advanced Melanoma (CheckMate 067): 4-Year Outcomes of a Multicentre, Randomised, Phase 3 Trial. Lancet Oncol (2018) 19:1480e92. doi: 10.1016/S1470-2045(18)30700-9
16. Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J Clin Oncol (2019) 37(7):537–46. doi: 10.1200/JCO.18.00149
17. Motzer RJ, Rini BI, McDermott DF, Frontera OA, Hammers HJ, Carducci MA, et al. Nivolumab Plus Ipilimumab Versus Sunitinib in First-Line Treatment for Advanced Renal Cell Carcinoma: Extended Follow-Up of Efficacy and Safety Results From a Randomized, Controlled, Phase 3 Trial. Lancet Oncol (2019) 20(10):137. doi: 10.1016/S1470-2045(19)30413-9
18. Le DT, Uram JN, Wang H, Bartlett BS, Kemberling H, Eyring AD, et al. PD-1 Blockade in Tumors With Mismatch-Repair Deficiency. N Engl J Med (2015) 372:2509–20. doi: 10.1056/NEJMoa1500596
19. Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch Repair Deficiency Predicts Response of Solid Tumors to PD-1 Blockade. Science (2017) 357:409–13. doi: 10.1126/science.aan6733
20. Le DT, Kim TW, Van Cutsem E, Geva R, Jager D, Hara H, et al. Phase II Open-Label Study of Pembrolizumab in Treatment-Refractory, Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: KEYNOTE-164. J Clin Oncol (2020) 38:11–9. doi: 10.1200/JCO.19.02107
21. Andrè T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, et al. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N Engl J Med (2020) 383:2207–18. doi: 10.1056/NEJMoa2017699
22. Ganesh K, Stadler ZK, Cercek A, Mendelsohn RB, Shia J, Segal NH, et al. Immunotherapy in Colorectal Cancer: Rationale, Challenges and Potential. Nat Rev Gastroenterol Hepatol (2019) 16:361–75. doi: 10.1038/s41575-019-0126-x
23. A’Hern RP. Sample Size Tables for Exact Single-Stage Phase II Designs. Statist Med (2001) 20:859–66. doi: 10.1002/sim.721
24. Fleming TR. One Sample Multiple Testing Procedure for Phase II Clinical Trials. Biometrics (1982) 38:142–51. doi: 10.1038/bjc.2012.444
25. Khemka V, Haire P, Waypa J, Weiss G. Phase Ib/II Study of Nivolumab Plus CAPIRI: Initial Results of Metastatic Colon Cancer and Pancreatic Adenocarcinoma Patients. Ann Oncol (2016) 27(Suppl 2):ii80–1. doi: 10.1093/annonc/mdw199.268
26. Rogers JE, Xiao L, Trail A, Murphy M, Palmer M, Ajani JA, et al. Nivolumab in Combination With Irinotecan and 5-Fluorouracil (FOLFIRI) for Refractory Advanced Gastroesophageal Cancer. Oncology (2020) 98:289–94. doi: 10.1159/000505974
27. Moheler M, Shitara K, Garrido M, Salman P, Shen L, Wyrwicz L, et al. Nivolumab (Niv) Plus Chemotherapy (Chemo) Versus Chemo as First-Line (1L) Treatment for Gastric Cancer/Gastroesophageal Junction Cancer (GC/GEJC)/esophageal Adenocarcinoma (EAC): First Results of the CheckMate 649 Study. Abstract Lba6_PR. Ann Oncol (2020) 31(suppl_4):S1142–215. doi: 10.1016/annonc/annonc325
28. Boku N, Ryu MH, Chung HC, Minashi K, Lee KW, Cho H, et al. Safety and Efficacy of Nivolumab in Combination With S-1/Capecitabine Plus Oxaliplatin in Patients With Previously Untreated, Unresectable, Advanced, or Recurrent Gastric/Gastroesophageal Junction Cancer: Interim Results of a Randomized, Phase II Trial (ATTRACTION-4). Ann Oncol (2019) 30:250–8. doi: 10.1093/annonc/mdy540
29. Kawazoe A, Yamaguchi K, Yasui H, Negoro H, Azuma M, Amagai K, et al. Safety and Efficacy of Pembrolizumab in Combination With S-1 Plus Oxaliplatin as a First-Line Treatment in Patients With Advanced Gastric/Gastroesophageal Junction Cancer: Cohort 1 Data From the KEYNOTE-659 Phase IIb Study. Eur J Cancer (2020) 129:97–106. doi: 10.1016/j.ejca.2020.02.002
30. Bang YJ, Kang YK, Catenacci DV, Muro K, Fuchs CS, Geva E, et al. Pembrolizumab Alone or in Combination With Chemotherapy as First- Line Therapy for Patients With Advanced Gastric or Gastroesophageal Junction Adenocarcinoma: Results From the Phase II Nonrandomized KEYNOTE-059 Study. Gastric Cancer (2019) 22:828–37. doi: 10.1007/s10120-018-00909-5
31. Antoniotti C, Borelli B, Rossini D, Pietrantonio F, Morano F, Salvatore L, et al. AtezoTRIBE: A Randomized Phase II Study of FOLFOXIRI Plus Bevacizumab Alone or in Combination With Atezolizumab as Initial Therapy for Patients With Unresectable Metastatic Colorectal Cancer. BMC Cancer (2020) 20(1):683. doi: 10.1186/s12885-020-07169-6
Keywords: safety run-in, nivolumab, FOLFOXIRI, bevacizumab, colorectal cancer, RAS mutation, BRAF mutation
Citation: Damato A, Bergamo F, Antonuzzo L, Nasti G, Iachetta F, Romagnani A, Gervasi E, Larocca M and Pinto C (2021) FOLFOXIRI/Bevacizumab Plus Nivolumab as First-Line Treatment in Metastatic Colorectal Cancer RAS/BRAF Mutated: Safety Run-In of Phase II NIVACOR Trial. Front. Oncol. 11:766500. doi: 10.3389/fonc.2021.766500
Received: 29 August 2021; Accepted: 22 November 2021;
Published: 14 December 2021.
Edited by:
Hironaga Satake, Kansai Medical University Hospital, JapanReviewed by:
Sara De Dosso, Oncology Institute of Southern Switzerland (IOSI), SwitzerlandCopyright © 2021 Damato, Bergamo, Antonuzzo, Nasti, Iachetta, Romagnani, Gervasi, Larocca and Pinto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Angela Damato, YW5nZWxhLmRhbWF0b0BhdXNsLnJlLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.