- Department of Oncology, Beijing Shijitan Hospital, Capital Medical University, Beijing, China
Objective: Metastatic spinal dissemination (MSD) of supratentorial glioma is very rare and there is no established standard of care. The current study investigates the clinical characteristics and course of spinal dissemination of supratentorial glioma.
Methods: A retrospective analysis of adult patients with MSD of supratentorial glioma treated in the Department of Oncology in Beijing Shijitan Hospital, Capital Medical University from June 2012 until August 2021 was performed. The time to event was estimated using Kaplan–Meier analysis. Univariate analyses were performed using log-rank test and multivariate analysis was performed using the Cox proportional hazards model.
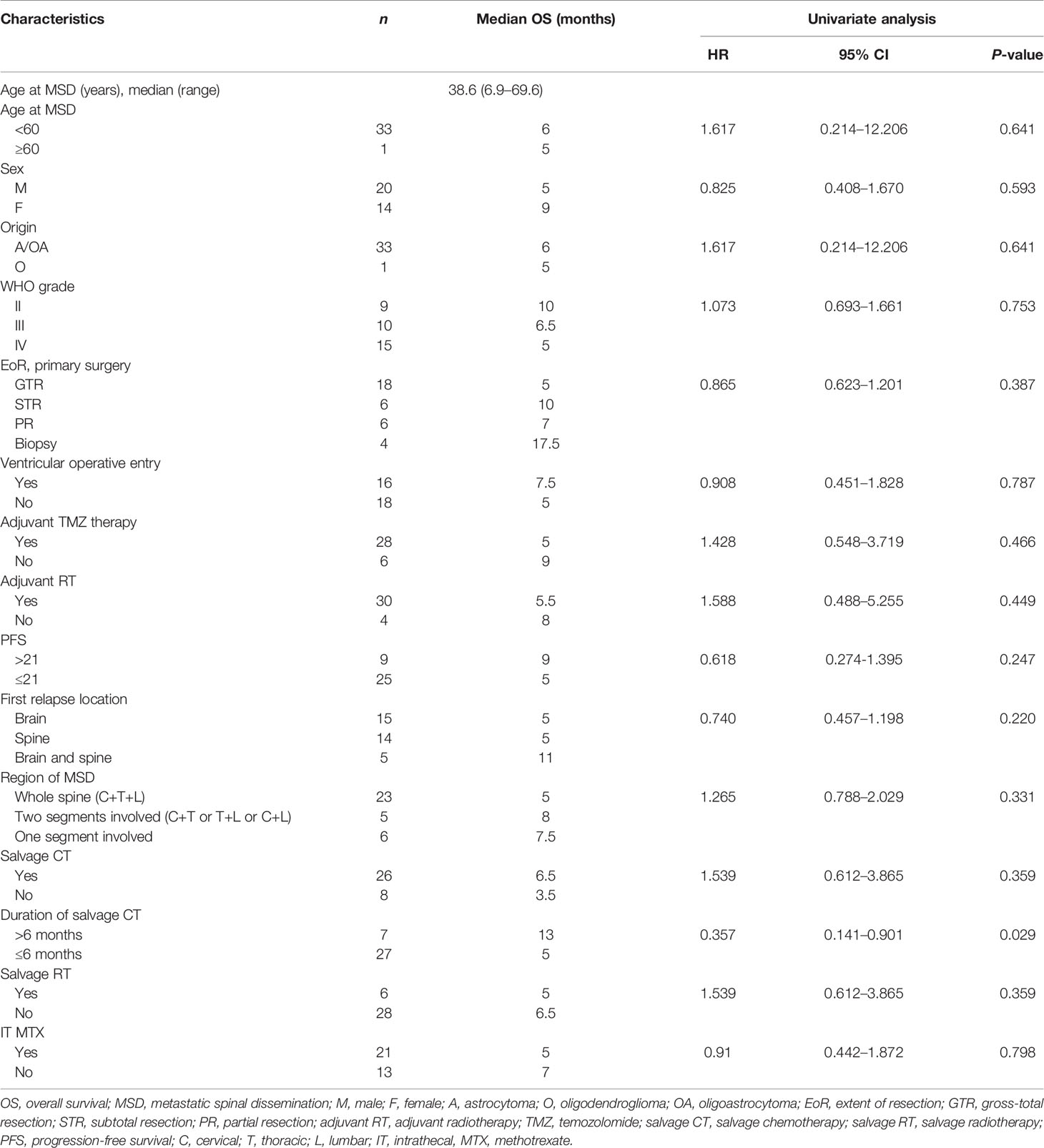
Results: Thirty-four adult patients with MSD of supratentorial glioma were enrolled in this retrospective study. The median time to MSD (TTMSD) and overall survival (OS) were 5 months (range: 0–78 months) and 15 months (range: 0.7–85 months), respectively, in the entire cohort. Univariate analysis demonstrated that the patients who had received TMZ therapy had a longer TTMSD than those who did not (mTTMSD: 15 vs. 3 months, log-rank P = 0.0004). Furthermore, a protracted duration of salvage chemotherapy of >6 months after MSD was associated with longer OS of the patients with MSD of supratentorial glioma (mOS: 13 vs. 5 months, log-rank P = 0.0163) and reduced the death risk by 64.3% (hazard ratio: 0.357, 95% CI: 0.141–0.901, P = 0.029) compared with a duration ≤6 months.
Conclusion: Patients with MSD of supratentorial glioma experienced poor prognosis and adjuvant chemotherapy may delay the occurrence of MSD. The protracted duration of systemic salvage chemotherapy may favor survival after spinal dissemination.
Introduction
Gliomas are the most common primary tumor in the brain and lead to most brain cancer-related deaths (1, 2). Although the relapse or progression of glioma occurs intracranially in most cases, the metastatic spread of glioma has been described increasingly with prolonged survival from the modest improvements in multimodality therapy of glioma (3–7).
The metastatic spread of glioma is uncommon and typically occurs within the neuroaxis. Among the types of the rare metastatic spread in the neuroaxis, spinal dissemination defined as the involvement of the spinal cord is a less common type of extracranially metastatic spread compared with the spread in intracranial cavity (8, 9).
Considering the low incidence of spinal dissemination of glioma, there is currently no standard of care established and the prognosis remains very poor (10, 11).
Here, we present a retrospective study of over 9 years of experience in our clinic to better understand the clinical course and characteristics of spinal dissemination of supratentorial glioma.
Methods and Subjects
Patients and Study Design
Patients with metastatic spinal dissemination (MSD) of supratentorial glioma treated in Beijing Shijitan Hospital between June 2011 and August 2021 were eligible for in this retrospective study, and the demographic, treatment, and survival data were collected for analysis. The pathologic diagnosis of glioma should be histologically confirmed. Spinal dissemination was radiologically diagnosed using enhanced spine magnetic resonance imaging (MRI) by at least two radiologists regardless of whether cerebrospinal fluid (CFS) was positive for tumor cells. Patients with initial glioma of WHO grade I were excluded because of their distinct molecular pathogenesis (12). The other exclusion criteria were age <18 years old, non-supratentorial location of primary glioma, and extracranial dissemination other than spine dissemination after the initial diagnosis of glioma.
The criteria of pathological diagnosis and response assessment were adopted from the 2007 WHO classification and RANO criteria (13–15).
This study was approved by the Institutional Ethical Committee, Beijing Shijitan Hospital. Written informed consent for the research was waived considering the retrospective nature of the study.
Statistical Analyses
Overall survival (OS) was defined as the interval from the time when spinal dissemination was diagnosed for the first time to death or the latest follow-up. The time to metastatic spinal dissemination (TTMSD) was defined as the time from initial surgery to the point at which spinal dissemination was diagnosed by means of enhanced MRI or CSF examination.
The time to event was estimated using Kaplan–Meier plotting. Univariate analyses were performed using the log-rank test and Cox proportional hazards model. All of the analyses were performed with SPSS (BMI, version 21). P-values <0.05 were considered statistically significant.
Results
Patient Characteristics
Among the 34 patients included in this analysis, 20 (58.8%) were male and 14 (41.2%) were female, with a median age of 42 years. The histology of the initial tumor was astrocytoma in 28 (82.4%), oligodendroglia in 1 (2.9%), and oligoastrocytoma in 5 (14.7%). At diagnosis, 30 (88.2%) patients underwent surgical resection of the primary tumor (18 GTR, 6 STR, and 6 PR) and 4 patients had biopsy. Following surgery, adjuvant radiotherapy was received in 30 (88.2%) patients, and adjuvant chemotherapy was received in 28 (82.35%) patients [temozolomide (TMZ), 150–200 mg/m2/day for the first 5 days of every 28 days] (Table 1).
Time and Location of MSD
Two patients (one patient with astrocytoma of grade II and one patient with GBM) had primarily a spinal dissemination at the diagnosis of initial glioma and 32 had disseminated relapse on the spine after surgery of the primary tumor. The predominant location of the first relapse was intracranial (15, 44.1%), followed by the spine (14, 41.2 %), and intracranial cavity and the spine dissemination simultaneously (5, 14.7%) (Table 1).
Non-surgical therapy was received by 10 patients before diagnosis of metastatic spread. Among these patients, six patients received platinum-based chemotherapy and one patient received semustine treatment after disease relapsed intracranially before spinal dissemination. Spinal dissemination occurred in three patients during the adjuvant therapy of TMZ. The remaining 21 patients have not received non-surgical therapy before diagnosis of metastatic spread except for adjuvant therapy with TMZ.
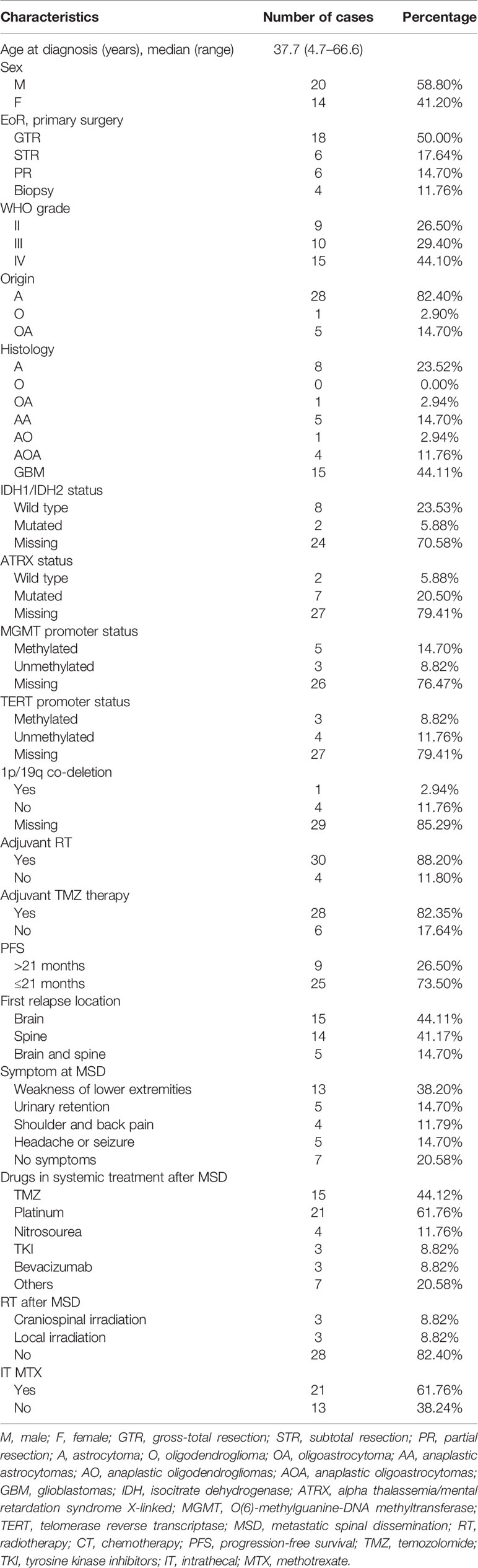
In this study, spinal dissemination was present in any region of the spine and the most common location (67.6%) was the entire spine involvement (Figure 1). Six (17.6%) patients showed only cervical lesion, and the remaining five patients had spinal dissemination covering two segments of the spine. Clinical presentation included weakness of lower extremities (for 13 patients), shoulder and back pain (for 4 patients), urinary retention (for 5 patients), and headache or seizure (for 5 patients) which was dependent on the site of disease dissemination. Of note, seven patients had no symptom when spinal dissemination was diagnosed by enhanced MRI.
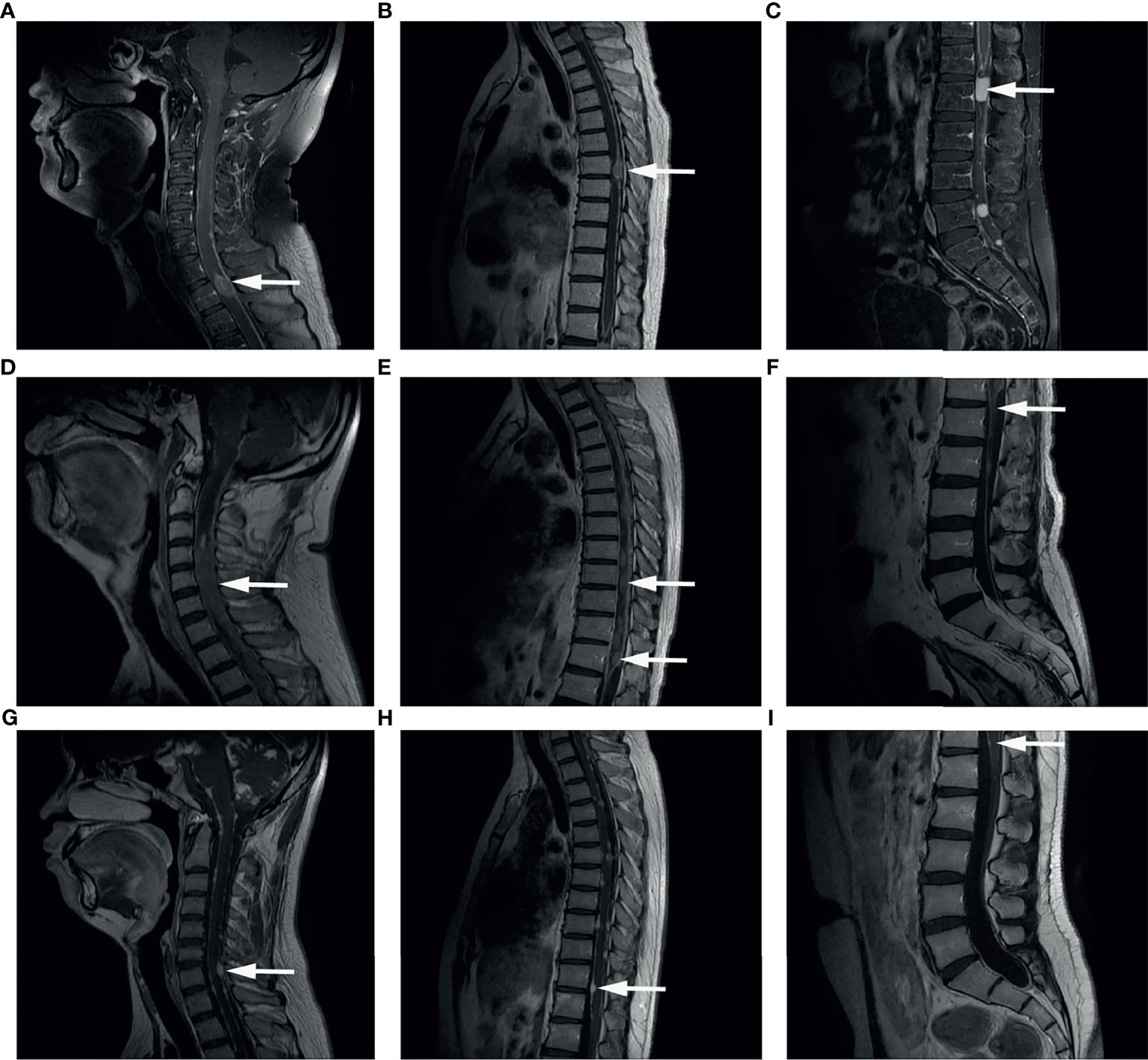
Figure 1 Representative images of Gd-enhanced MRI. Spinal dissemination presented as a hyperintense signal showing as mass or line (arrows) in the spinal canal in T1-weighted MR images. (A–C) Spinal Gd-MRI revealed multiple contrast-enhanced mass at the spine in a male aged 34 years old at MSD, who was initially diagnosed as GBM at the left occipital lobe. (D–F) Spinal Gd-MRI demonstrated thick linear and clumpy enhanced lesions along the whole spine in a male aged 40 years old at MSD, who was initially diagnosed as anaplastic oligodendroglioma (WHO grade III) at the left frontal lobe. (G–I) Spinal Gd-MRI demonstrated small enhanced lesion at the spine in a male aged 17 years old at MSD, who was initially diagnosed as astrocytoma (WHO grade II) at both cerebral hemispheres, cerebellar hemispheres, brainstem surface, ventricles, cisterns, and meninges.
The TTMSD was estimated using the Kaplan–Meier method. The result indicated that the median TTMSD was 5 months (range: 0–78 months) in the entire cohort (Figure 2). When the patients were stratified according to adjuvant chemotherapy, the patients who had received adjuvant TMZ therapy showed longer TTMSD than those who did not (mTTMSD: 15 vs. 3 months, log-rank P = 0.0004) (Figure 3). In terms of the origin of the tumor, patients with oligodendroglioma appeared to have a longer TTMSD than those with astrocytoma or oligoastrocytoma [mTTMSD: 78 vs. 14 months, log-rank P = 0.048, hazard ratio (HR): 0.034, P = 0.215] (Figure 3 and Table 2). We observed no significant association between TTMSD and sex, WHO grade, surgery, and adjuvant radiotherapy.
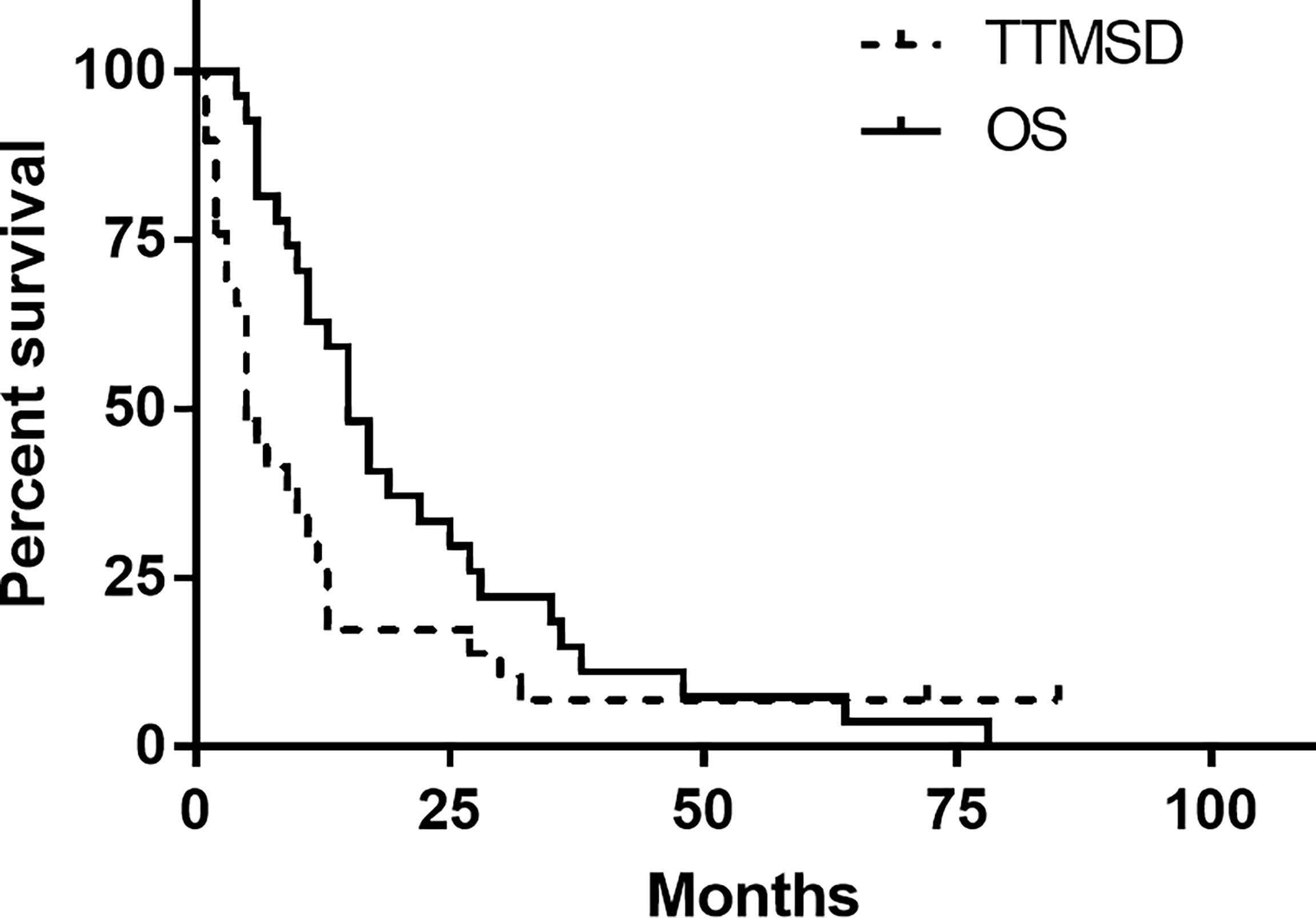
Figure 2 Kaplan–Meier estimated of time to metastatic spinal dissemination (TTMSD) and overall survival (OS).
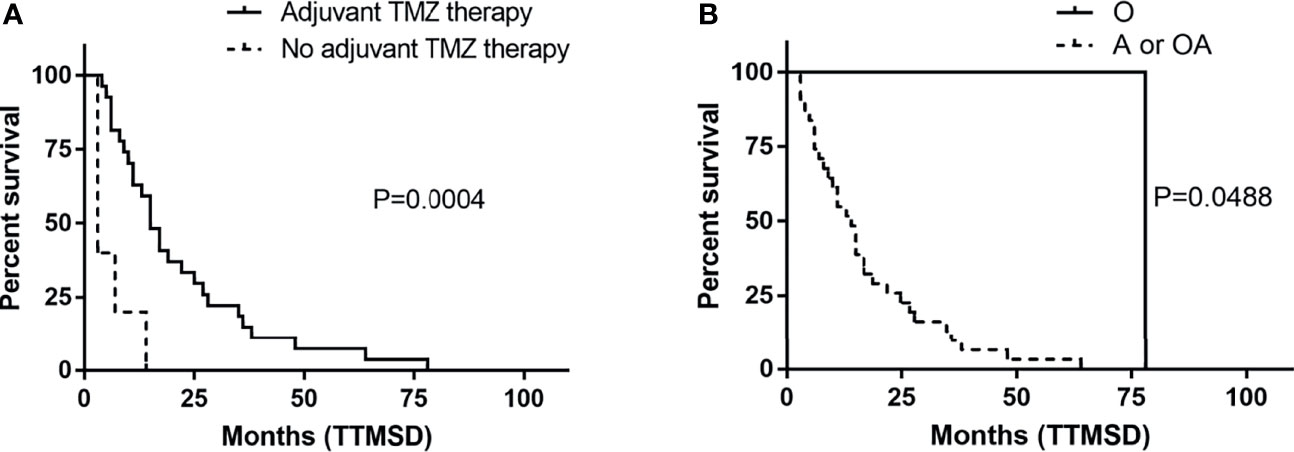
Figure 3 Kaplan–Meier estimated of time to metastatic spinal dissemination (TTMSD) in patients stratified by adjuvant TMZ therapy (A) and histologic subtype (B). A, astrocytoma; O, oligodendroglioma; OA, oligoastrocytoma; TMZ, temozolomide.
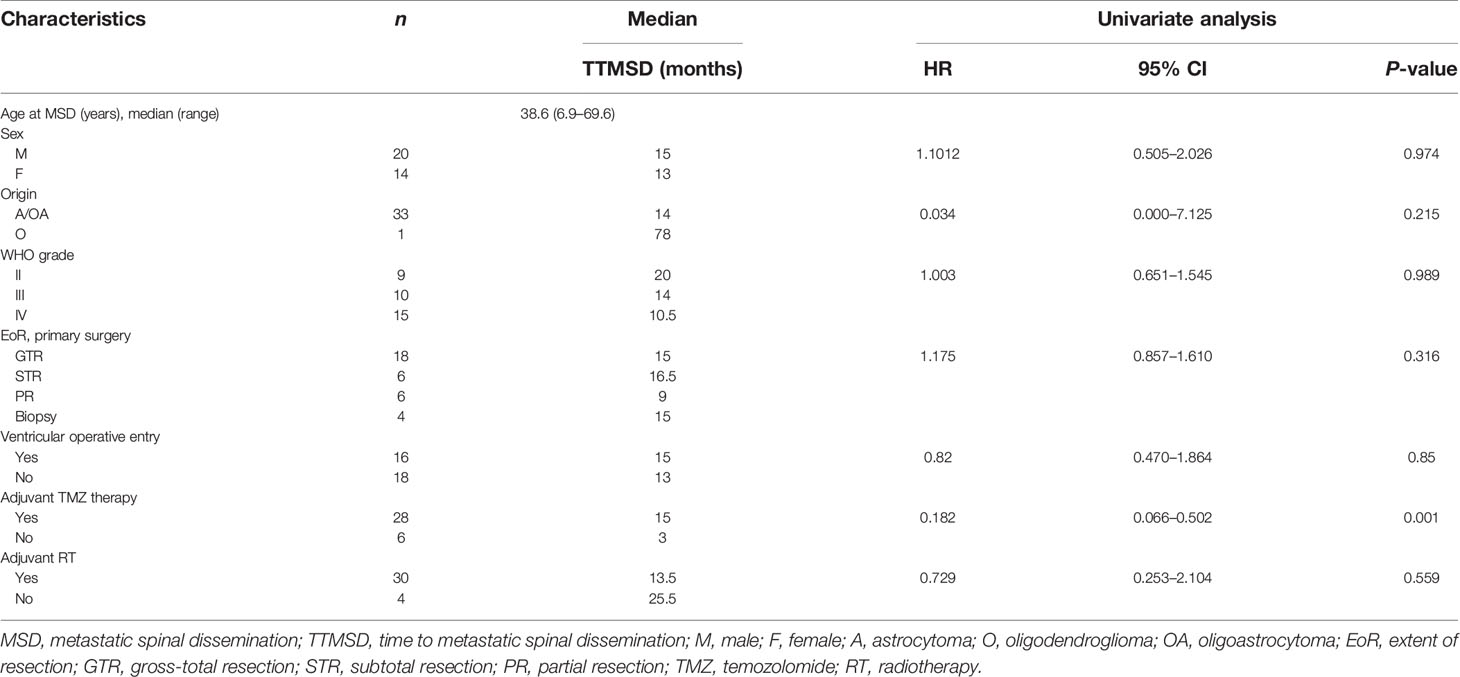
Table 2 Univariate analysis of the clinical parameters of the patients with time to metastatic spinal dissemination (TTMSD).
Treatment After MSD
The majority (76.5%) of the patients had received salvage treatment after spinal dissemination, while four patients received only best supportive care. The drugs utilized in salvage therapy mainly included TMZ (15 patients, 150 mg/m2/day, 1 week on and 1 week off), carboplatin (10 patients, AUC = 5, every 4 weeks), and cisplatin (9 patients,75 mg/m2 every 4 weeks). Systemic chemotherapy was delivered to the patients with Karnofsky performance status >60% and adequate hematologic, hepatic, and renal function. Six patients received radiotherapy (three for craniospinal irradiation and three for local radiotherapy) after spinal dissemination. Besides systemic chemotherapy, methotrexate (MTX) intrathecal injection was received in 21 patients (Table 1).
The duration of systemic salvage therapy was calculated regardless of the various regimens. The median duration of chemotherapy was 5.5 months (1–23 months) for the patients who received at least one cycle of salvage chemotherapy after spinal dissemination. We found that the duration >6 months was observed in seven patients, while five patients received systemic therapy for >10 months after spinal dissemination.
Overall Survival
At the time of this report, 32 (94.1%) of the 34 patients had died. The follow-up time for the 2 remaining patients still alive is 72 and 85 months, respectively.
The estimated median OS by Kaplan–Meier plotting was 15 months (range: 0.7–85months) in this study (Figure 2). On univariate analysis, a protracted duration of salvage chemotherapy of >6 months after MSD was associated with longer OS of the patients with MSD of supratentorial glioma (mOS: 13 vs. 5 months) and reduced the death risk by 64.3% (HR 0.357, 95% CI: 0.141–0.901, P = 0.029) compared to a duration of <6 months (Table 3 and Figure 4). However, no statistical significance was found between the groups concerning sex, histological classification, surgery and postoperative therapy, age of dissemination, whether there was dissemination for first recurrence, whether therapy was received after dissemination, the range of dissemination, or progression-free survival.
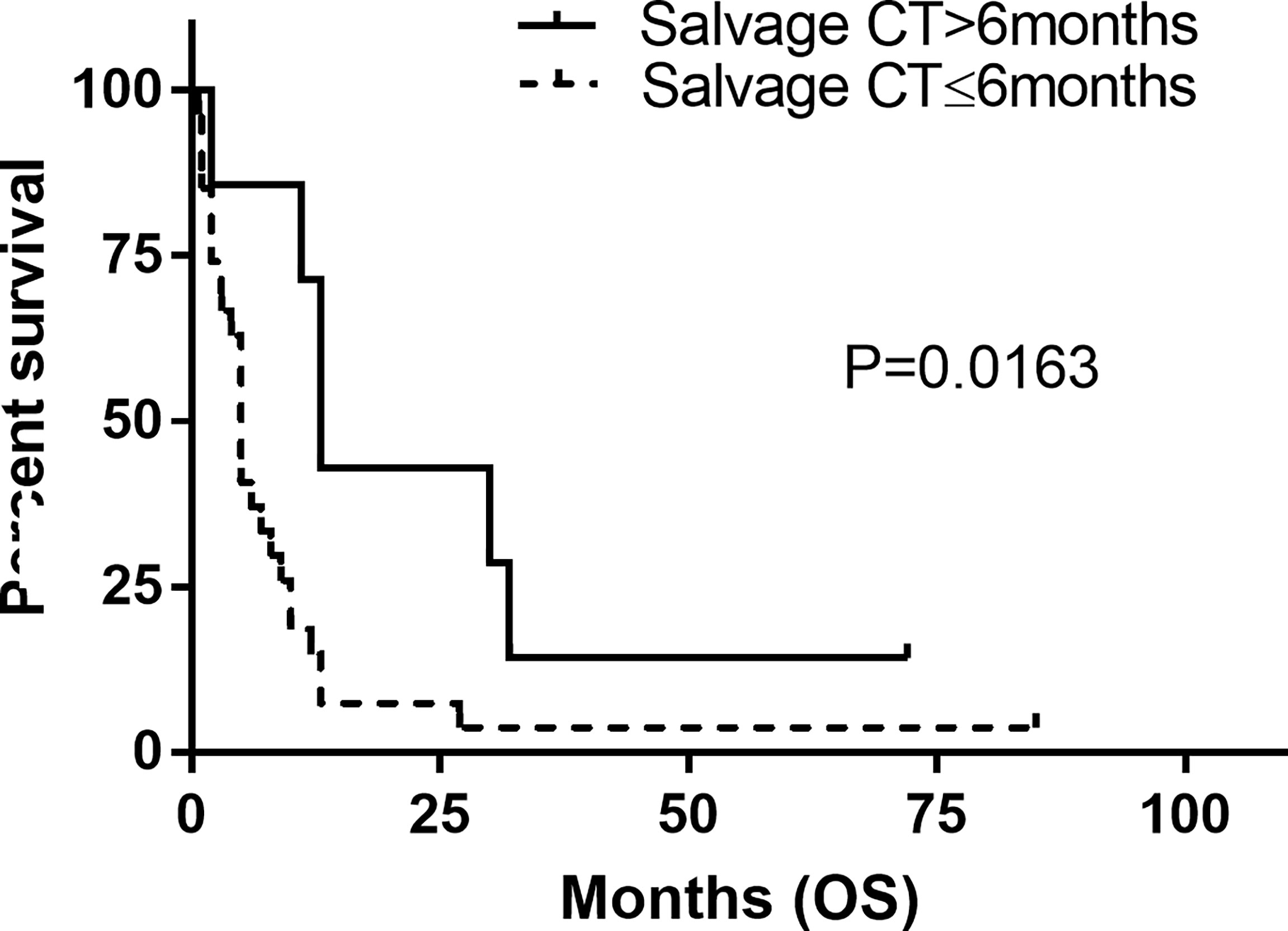
Figure 4 Kaplan–Meier estimated of overall survival (OS) in patients stratified by duration of systemic salvage therapy. Salvage CT, salvage chemotherapy.
Discussion
The spinal dissemination of supratentorial glioma is rare and represents a late event of the disease course (16, 17). The mechanism underlying the metastatic dissemination and prognostic factors is unclear. In this study, we found that adjuvant chemotherapy may delay the occurrence of MDS and that a protracted duration of salvage chemotherapy after MSD significantly prolonged the OS of the patients with MSD of supratentorial glioma.
Spinal dissemination was first reported and is more common in glioblastoma multiforme (GBM), a grade IV tumor with the most aggressive characteristics (18, 19). However, according to our own clinical experience and series of reports, spinal dissemination may also occur in patients with low-grade glioma (20, 21). In this study, on univariate analysis, adjuvant chemotherapy was identified to be associated with longer TTMSD indicating that MSD occurred later in patients with adjuvant chemotherapy than those without. The results suggest that adjuvant therapy is important and should be performed considering the WHO grade and other risk factors according to the NCCN guidelines.
In this study, patients with MSD of supratentorial glioma of WHO grades II (9 patients), III (10 patients), and IV (15 patients) were included, and the median overall survival after spinal dissemination was 15 months for all the patients. This rather long survival time for patients with MSD possibly contributes to a better prognosis of patients with low-grade glioma (LGG) in this cohort as well as clinical benefit from salvage treatment after MSD. It is worth noting that the span of survival range was rather wide suggesting that survival time differed between individuals, although the underlying mechanism remains unclear.
When the outcomes of patients with different WHO grades of initial tumor were compared, we found that the median survival time after MSD in patients with tumor of grade II (10 months) was longer than that in patients with tumor of grade III (6.5 months) and grade IV (5 months) in value, although no significant difference was found by statistical analysis. The limited sample size maybe the possible reason. Nevertheless, the results suggested that, on the one hand, the prognosis of patients with MSD of supratentorial glioma is poor as soon as the dissemination occurs regardless of the initial historical grade, and on the other hand, the prognosis of patients with MSD seemed to be better in patients with primary LGG than in patients with primary HGG. The median survival after MSD for patients with primary malignant glioma in this study is consistent with previous literature (22).
We have to address the issue that adults with low-grade astrocytomas sometimes progress to high-grade diseases (23). Among the nine patients with MSD after primary astrocytoma of grade II enrolled in this study, one patient received resection when disease relapsed and HGG was confirmed histologically which reflected the fact of evolution of LGG. However, it is difficult to evaluate the contribution of evolution or transformation of LGG in the process of MSD occurrence and development due to so limited data in this study.
As far as we know, there is no standard of care established for MSD of glioma. In this study, multiple regimens or drugs were used in the salvage systemic treatment of MSD. The specified regimen or drug failed to bring clinical benefit on the overall survival of patients considering the limited sample size and unbalance distribution in each group of treatment. However, we found that a longer duration of systemic chemotherapy favored survival after spinal dissemination. This result indicated that clinical benefit could be conferred by systemic chemotherapy after spinal dissemination for partial patients.
Among the patients who had received systemic treatment after spinal dissemination, most of them received TMZ or platinum-based chemotherapy. Generally, patients who received chemotherapy showed better clinical condition compared with patients who did not. We could not exclude the possibility that patients who received chemotherapy had longer survival might be the cause or consequence of clinical conditions. Thus, how to screen specific patients who may gain benefit from salvage chemotherapy should be addressed in a prospective study with a larger sample size.
It has been reported that male, age, and ventricular operative entry are risk factors for spinal dissemination of glioma (6, 24). Spinal dissemination was reported to more frequently occur in young patients (25) because younger patients tend to have longer survival time. Meanwhile, patients with high-grade glioma who are >65 years old only survive 7–9 months (26). In this study series, the average age at dissemination was 38.9 years old and 16 patients had experienced possible opening of the ventricular system.
This study has several limitations. First, the retrospective nature of this study may be a source of potential bias and may exclude meaningful comparison and conclusion to some extent. Second, the limited sample may also contribute to the bias. Third, the lack of molecular characteristic information for a considerable number of patients and multiple modality or drugs used in salvage treatment after MSD make it hard to compare and figure out factors related to the prognosis of patients with MSD.
Considering the preliminary result from this retrospective study with limited sample size, well-designed prospective studies with an expanded sample will be performed in the future.
Conclusion
In conclusion, the prognosis of patients with MSD of supratentorial glioma is considered poor as soon as dissemination occurs regardless of the initial historical grade, and adjuvant chemotherapy may delay the occurrence of MSD. Moreover, a protracted duration of systemic salvage chemotherapy may favor survival after spinal dissemination.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Ethics Committee of Beijing Shijitan Hospital of Capital Medical University [No. sjtkyll-lx-2021(64)]. Written informed consent to participate in this study was provided by the legal guardian/next of kin of the participants.
Author Contributions
Conception and design: JC and HH. Provision of study materials and patients: JC and YZ. Collection and assembly of data: QS, FY, and JC. Data analysis and interpretation: FY and HH. Manuscript writing: all authors. Final approval of the manuscript: all authors.
Funding
This work was funded by the National Natural Science Foundation of China (grant number 81572799).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
References
1. Bush NA, Chang SM, Berger MS. Current and Future Strategies for Treatment of Glioma. Neurosurg Rev (2017) 40(1):1–14. doi: 10.1007/s10143-016-0709-8
2. Ostrom QT, Patil N, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. Corrigendum to: CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2013-2017. Neuro Oncol (2020) 22(S1):1–96. doi: 10.1093/neuonc/noaa269
3. Sun Q, Xu R, Xu H, Wang G, Shen X, Jiang H. Extracranial Metastases of High-Grade Glioma: The Clinical Characteristics and Mechanism. World J Surg Oncol (2017) 15(1):181. doi: 10.1186/s12957-017-1249-6
4. Liu J, Shen L, Tang G, Tang S, Kuang W, Li H, et al. Multiple Extracranial Metastases From Glioblastoma Multiforme: A Case Report and Literature Review. J Int Med Res (2020) 48(6):300060520930459. doi: 10.1177/0300060520930459
5. Ginat DT, Schaefer PW. Imaging Guidelines and Findings of Extracranial Glioblastoma. J Neurooncol (2014) 118(1):9–18. doi: 10.1007/s11060-014-1404-7
6. Saito R, Kumabe T, Jokura H, Shirane R, Yoshimoto T. Symptomatic Spinal Dissemination of Malignant Astrocytoma. J Neurooncol (2003) 61(3):227–35. doi: 10.1023/a:1022536828345
7. Jellinger K. Metastatic Oligodendrogliomas: A Review of the Literature and Case Report. Acta Neurochir (Wien) (2009) 151(8):987. doi: 10.1007/s00701-009-0342-1
8. Shibahara I, Saito R, Osada Y, Kanamori M, Sonoda Y, Kumabe T, et al. Incidence of Initial Spinal Metastasis in Glioblastoma Patients and the Importance of Spinal Screening Using MRI. J Neurooncol (2019) 141(2):337–45. doi: 10.1007/s11060-018-03036-4
9. Chen-Zhao X, Aznar-Garcia L. Diagnosis and Management of Spinal Metastasis of Primary Brain Tumours. AME Case Rep (2018) 2:26. doi: 10.21037/acr.2018.03.02
10. Bae JS, Yang SH, Yoon WS, Kang SG, Hong YK, Jeun SS. The Clinical Features of Spinal Leptomeningeal Dissemination From Malignant Gliomas. J Korean Neurosurg Soc (2011) 49(6):334–8. doi: 10.3340/jkns.2011.49.6.334
11. Bae JW, Hong EK, Gwak HS. Response of Leptomeningeal Dissemination of Anaplastic Glioma to Temozolomide: Experience of Two Cases. Brain Tumor Res Treat (2017) 5(2):99–104. doi: 10.14791/btrt.2017.5.2.99
12. Ryall S, Tabori U, Hawkins C. A Comprehensive Review of Paediatric Low-Grade Diffuse Glioma: Pathology, Molecular Genetics and Treatment. Brain Tumor Pathol (2017) 34(2):51–61. doi: 10.1007/s10014-017-0282-z
13. Chukwueke UN, Wen PY. Use of the Response Assessment in Neuro-Oncology (RANO) Criteria in Clinical Trials and Clinical Practice. CNS Oncol (2019) 8(1):CNS28. doi: 10.2217/cns-2018-0007
14. Wen PY, Chang SM, Van den Bent MJ, Vogelbaum MA, Macdonald DR, Lee EQ. Response Assessment in Neuro-Oncology Clinical Trials. J Clin Oncol (2017) 35(21):2439–49. doi: 10.1200/JCO.2017.72.7511
15. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO Classification of Tumours of the Central Nervous System. Acta Neuropathol (2007) 114(2):97–109. doi: 10.1007/s00401-007-0243-4
16. Schwaninger M, Patt S, Henningsen P, Schmidt D. Spinal Canal Metastases: A Late Complication of Glioblastoma. J Neurooncol (1992) 12(1):93–8. doi: 10.1007/BF00172461
17. Sibanda Z, Farahani N, Ogbonnaya E, Albanese E. Glioblastoma Multiforme: A Rare Case of Spinal Drop Metastasis. World Neurosurg (2020) 144:24–7. doi: 10.1016/j.wneu.2020.08.086
18. Wright CH, Wright J, Onyewadume L, Raghavan A, Lapite I, Casco-Zuleta A, et al. Diagnosis, Treatment, and Survival in Spinal Dissemination of Primary Intracranial Glioblastoma: Systematic Literature Review. J Neurosurg Spine (2019) 31(5):723–32. doi: 10.3171/2019.5.SPINE19164
19. Saito T, Sugiyama K, Hama S, Yamasaki F, Takayasu T, Nosaka R, et al. High Expression of Glypican-1 Predicts Dissemination and Poor Prognosis in Glioblastomas. World Neurosurg (2017) 105:282–8. doi: 10.1016/j.wneu.2017.05.165
20. D'Haene N, Coen N, Neugroschl C, Baleriaux D, Salmon I. Leptomeningeal Dissemination of Low-Grade Intramedullary Gliomas: About One Case and Review. Clin Neurol Neurosurg (2009) 111(4):390–4. doi: 10.1016/j.clineuro.2008.11.013
21. Perilongo G, Garre ML, Giangaspero F. Low-Grade Gliomas and Leptomeningeal Dissemination: A Poorly Understood Phenomenon. Childs Nerv Syst (2003) 19(4):197–203. doi: 10.1007/s00381-003-0733-1
22. Ng WH, Yeo TT, Kaye AH. Spinal and Extracranial Metastatic Dissemination of Malignant Glioma. J Clin Neurosci (2005) 12(4):379–82. doi: 10.1016/j.jocn.2004.11.004
23. Babu R, Bagley JH, Park JG, Friedman AH, Adamson C. Low-Grade Astrocytomas: The Prognostic Value of Fibrillary, Gemistocytic, and Protoplasmic Tumor Histology. J Neurosurg (2013) 119(2):434–41. doi: 10.3171/2013.4.JNS122329
24. Mistry AM, Kelly PD, Thompson RC, Chambless LB. Cancer Dissemination, Hydrocephalus, and Survival After Cerebral Ventricular Entry During High-Grade Glioma Surgery: A Meta-Analysis. Neurosurgery (2018) 83(6):1119–27. doi: 10.1093/neuros/nyy202
25. Arita N, Taneda M, Hayakawa T. Leptomeningeal Dissemination of Malignant Gliomas. Incidence, Diagnosis and Outcome. Acta Neurochir (Wien) (1994) 126(2-4):84–92. doi: 10.1007/BF01476415
Keywords: supratentorial glioma, metastatic spinal dissemination (MSD), overall survival (OS), prognosis, chemotherapy
Citation: Chen J, Yang F, Shi Q, Zhao Y and Huang H (2021) A Retrospective Study on Spinal Dissemination of Supratentorial Glioma. Front. Oncol. 11:765399. doi: 10.3389/fonc.2021.765399
Received: 27 August 2021; Accepted: 03 December 2021;
Published: 22 December 2021.
Edited by:
Matthew Tate, Northwestern University, United StatesReviewed by:
Antonio Silvani, Carlo Besta Neurological Institute (IRCCS), ItalyCarl Friedrich Classen, University Hospital Rostock, Germany
Copyright © 2021 Chen, Yang, Shi, Zhao and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongyan Huang, aGhvbmd5MTk5OUAxMjYuY29t
†These authors have contributed equally to this work and share first authorship
 Jianxin Chen†
Jianxin Chen† Fan Yang
Fan Yang Qi Shi
Qi Shi Hongyan Huang
Hongyan Huang