- 1The Second Clinical Medical College, Zhejiang Chinese Medical University, Hangzhou, China
- 2School of Basic Medical Sciences, Zhejiang Chinese Medical University, Hangzhou, China
- 3Department of Obstetrics and Gynecology, Ningbo Yinzhou No. 2 Hospital, Ningbo, China
Background: Although minimally invasive surgery (MIS) was commonly used to treat patients with early-stage cervical cancer, its efficacy remained controversial.
Methods: We systematically searched PubMed, Web of Science, and Cochrane Library databases until March 2021 to compare the prognosis of early-stage cervical cancer patients who underwent MIS (laparoscopic or robot-assisted radical hysterectomy) or ARH. The primary outcomes included rates of 3- and 5-year disease-free survival (DFS) and overall survival (OS). The study protocol was registered in PROSPERO: CRD42021258116.
Results: This meta-analysis included 48 studies involving 23346 patients (11220, MIS group; 12126, ARH group). The MIS group had a poorer medium-term (3-year) DFS (HR=1.08, 95% CI: 1.01-1.16, p=0.031) than the ARH group, without significant difference in medium-term OS as well as long-term (5-year) DFS and OS. Subgroup analysis of 3-year prognosis revealed that although patients in Western countries who underwent MIS had shorter DFS than those who underwent ARH (HR=1.10, p=0.024), no difference was observed in DFS among those in Asian countries. Moreover, MIS was linked to poorer 3-year DFS in patients with stage I cervical cancer (HR=1.07, p=0.020). Notably, subgroup analysis of 5-year prognosis revealed that patients with tumor size ≥2 cm undergoing MIS exhibited a shorter DFS than those who underwent ARH (HR=1.65, p=0.041).
Conclusion: Patients with early-stage cervical cancer undergoing MIS may have a poorer prognosis than those undergoing ARH. Therefore, applying MIS in early-stage cervical cancer patients should be conducted with caution.
Systematic Review Registration: The study protocol was registered in PROSPERO: CRD42021258116.
Introduction
Cervical cancer was ranked as the fourth most frequently diagnosed cancer and the fourth leading cause of cancer death in women. Most of these cases occurred in sub-Saharan Africa, Melanesia, South America, and South-Eastern Asia, with the highest morbidity and mortality rates in sub-Saharan African (1). Surgery was the primary treatment option for early-stage cervical cancer to treat stage IA, IB1, and selected IIA1 cases (2). Conization alone or simple hysterectomy was an appropriate treatment option for patients with stage IA disease, whereas radical hysterectomy was the preferred treatment modality for stage IB1 or IIA1 patients (3). Abdominal radical hysterectomy (ARH) was a standard and historical treatment for early-stage cervical cancer (4, 5). As the research progressed, minimally invasive surgery (MIS) became the preferred treatment option for early-stage cervical cancer over the past two decades (6, 7).
The feasibility and safety of MIS (laparoscopic or robot-assisted radical hysterectomy) were gradually widely accepted (7, 8). Several retrospective studies and reviews (9–11) highlighted MIS benefits in reducing blood loss, shortening hospital stay, accelerating recovery time, and reducing the risk of postoperative complications, with equal survival outcomes as ARH. Nevertheless, preliminary results from a phase 3 multicenter randomized controlled trial (RCT) (12), presented at the Society of Gynecological Oncology (SGO) meeting in March 2018, indicated that early-stage cervical cancer patients undergoing MIS had a lower disease-free survival (DFS) and overall survival (OS) than those undergoing ARH. The RCT results were unexpected and sparked a huge debate (2). Since then, the guidelines from National Comprehensive Cancer Network (NCCN) (2) and International Federation of Gynecology and Obstetrics (FIGO) (13) have been revised to indicate that ARH remains the gold standard for treating early-stage cervical cancer.
Consequently, the comparison of prognosis between MIS and ARH in patients with early-stage cervical cancer remains controversial. Then, some clinical trials and reviews (14–17) demonstrated that patients with early-stage cervical cancer who underwent MIS or ARH had similar OS, but those who underwent MIS exhibited shorter DFS. Therefore, we performed a meta-analysis of available evidence to compare and evaluate medium- (3-year) and long-term (5-year) survival outcomes in patients with early-stage cervical cancer who underwent MIS or ARH.
Methods
Search Strategy
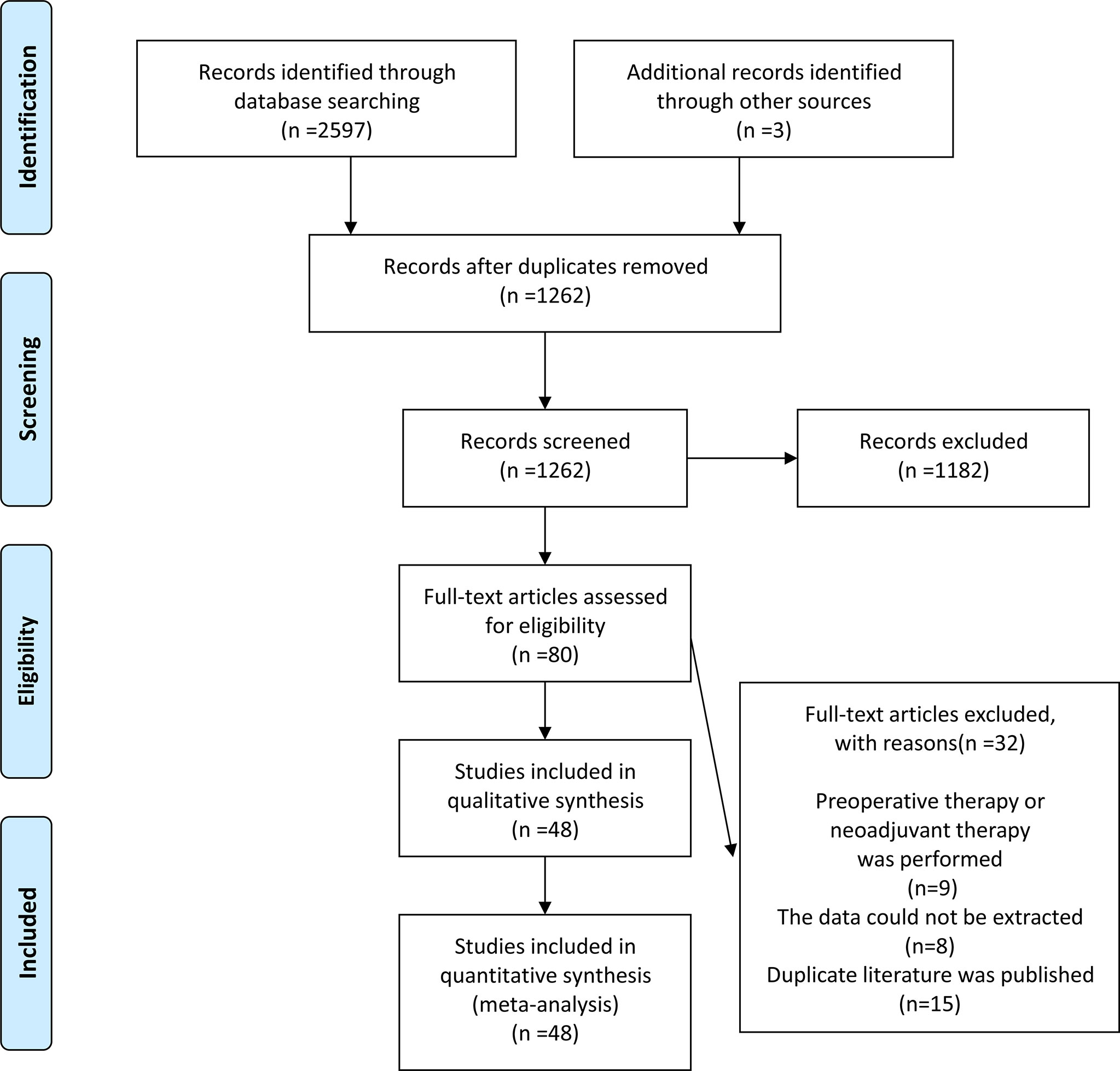
We conducted a systematic search of PubMed, Web of Science, and Cochrane Library to identify relevant reports published from inception until March 2021. The search terms included (uterine cervical neoplasms OR cervical cancer OR cervix cancer OR cervical carcinoma) AND (minimally invasive OR laparoscopic OR robotic OR robot-assisted OR Davinci) AND (open OR abdominal OR traditional) AND (radical hysterectomy OR surgery OR hysterectomy OR surgical procedure OR operation). Additionally, potential studies were identified by manually searching the references of included articles. From the initial search to the final selection of studies, the entire review process was mapped using the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram. The study protocol was registered in PROSPERO: CRD42021258116.
Eligible Criteria
The included studies met the following criteria: (1) the articles were observational studies or RCTs comparing patients with early-stage cervical cancer who underwent MIS (laparoscopic and/or robot-assisted radical hysterectomy) or ARH. (2) The studies contained detailed data on prognosis (DFS and OS) for patients with early-stage cervical cancer. (3) At least 3-year survival data was provided in the study. (4) The articles were published in English.
The exclusion criteria were as follows: (1) there was no extractable data for the study. (2) The studies included patients treated with preoperative neoadjuvant therapy or fertility-saving surgery (such as cervical resection). (3) Patients with distant metastases or those who underwent non-radical hysterectomy were investigated. (4) When a publication has been continuously updated or is duplicated, the highest-quality article is selected.
Data Extraction and Quality Assessment
Two independent researchers extracted data from each relevant article, and a third researcher arbitrated disagreements. We recorded information on author, year of publication, country, age, number of patients, study design, MIS type, primary FIGO tumor stage, tumor size, pathologic type, lymph node metastasis, lymph-vascular space invasion, tumor differentiation, and follow-up time. Additionally, this meta-analysis used medium- and long-term prognosis endpoints, including 3- and 5-year DFS and OS. Following that, the methodological quality of included RCTs and observational studies was assessed using Cochrane Collaboration’s tool and Newcastle-Ottawa Quality Assessment Scale, respectively.
Statistical Analysis
All statistical analyses were performed using Stata 12.0 (18). Medium- and long-term prognoses in MIS and ARH groups were analyzed using a hazard ratio (HR) with a 95% confidence interval (95% CI) (19). Moreover, heterogeneity of HR was assessed based on I2 statistics. Due to differences in study design and surgical treatment, a random-effects model was used to improve the credibility of results. Furthermore, Egger’s test used p<0.05 as the significance level to evaluate publication bias (20). Sensitivity analysis for the stability of results was performed. All statistical tests were two-sided, and p<0.05 was considered statistically significant.
Results
Characteristics of Eligible Research
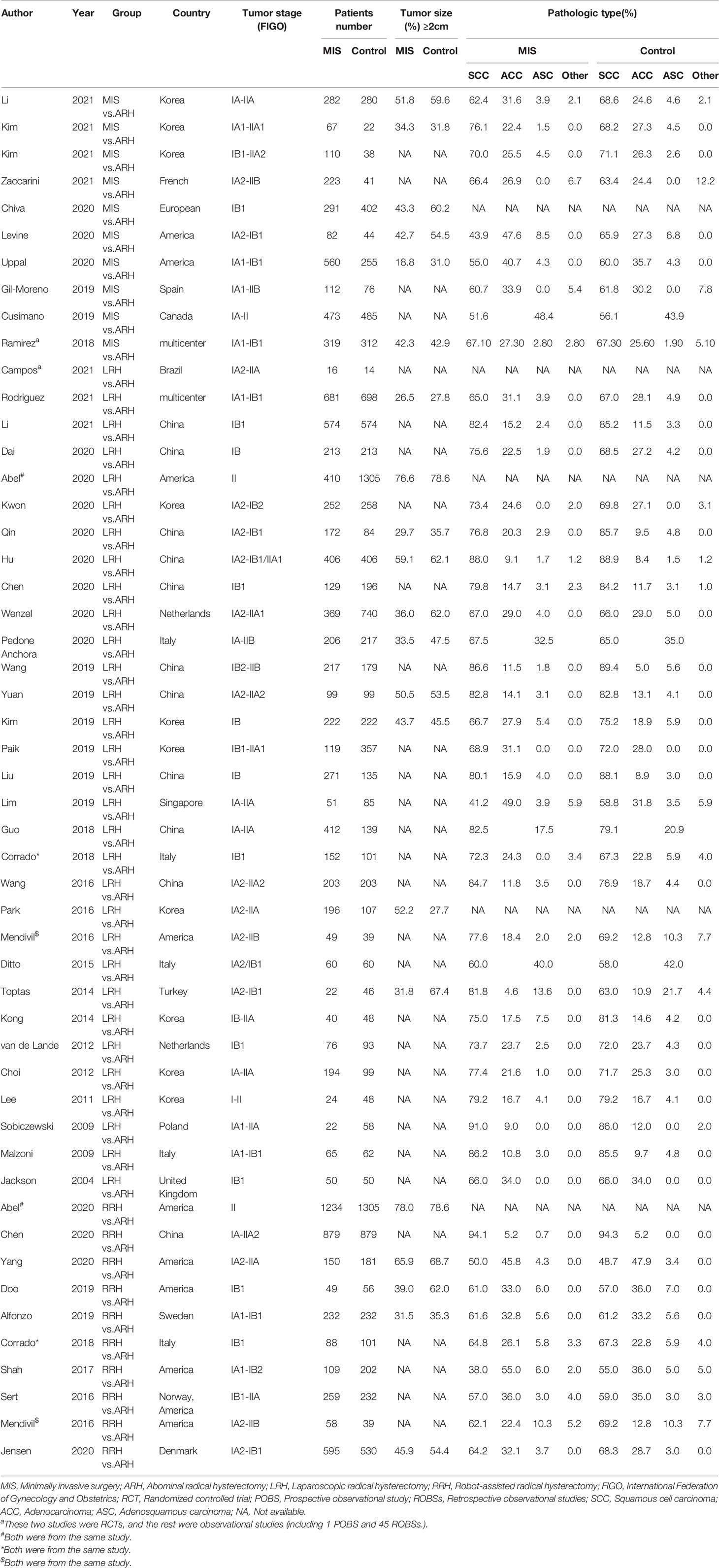
The initial search resulted in 2600 relevant studies from different electronic databases. Following screening, 48 studies (10–12, 14–17, 21–61) encompassing 23346 patients fulfilled the inclusion criteria. The detailed screening process of articles is summarized in Figure 1. The basic characteristics of selected studies are listed in Table 1. Two studies were RCTs (12, 29), and the remaining were observational studies (10, 11, 14–17, 21–28, 30–61) (n=46). Three treatment modalities were adopted by contrast: MIS (laparoscopic radical hysterectomy plus robot-assisted radical hysterectomy) versus ARH (n=10), laparoscopic radical hysterectomy versus ARH (involved 31 studies), and robot-assisted radical hysterectomy versus ARH (involved 10 studies). Of 48 studies, 23 were conducted in Asian countries and 24 in Western countries. The remaining study was conducted in both Asian and Western countries. Among the studies conducted in Asian countries, 11 were conducted in China, 10 in Korea, 1 in Singapore, and 1 in Turkey. Of the studies conducted in Western countries, 7 were conducted in America, 1 in Brazil, 1 in Canada, 1 in Denmark, 1 in France, 4 in Italy, 3 in multicenters, 2 in the Netherlands, 1 in Poland, 1 in Spain, 1 in Sweden, and 1 in the United Kingdom. All studies were published between 2004 and 2021. In these eligible studies, the number of patients was a minimum of 14 and a maximum of 1305. Almost all patients were diagnosed with FIGO stage IA-IIA cervical cancer. In addition, the proportion of patients with tumor size ≥2 cm ranged from a minimum of 18.8% to a maximum of 78.6%. Squamous cell carcinoma was the most common pathological type of cervical cancer in our study. Additional details of included studies are presented in Supplementary Table 1.
Quality Assessment
Cochrane collaboration’s tool was employed to assess the quality of RCT enrolled in the study. For observational studies, quality assessment was performed based on the Newcastle-Ottawa Quality Assessment Scale, and only high-quality studies with a total score ≥6 were included in the final analysis. Details on quality assessment are displayed in Supplementary Table 2.
Prognostic Analysis
Random effect analysis of 14 of 48 reports on 3-year DFS encompassing 7003 patients with early-stage cervical cancer revealed a statistically significant difference, suggesting that the MIS group had a shorter 3-year DFS than the ARH group (HR=1.08, 95% CI: 1.01-1.16, p=0.031; Figure 2). Besides, 14 studies involving 7118 patients with early-stage cervical cancer were assessed for 3-year OS, without observing a significant difference in 3-year OS between MIS and ARH groups (HR=1.09, 95% CI: 0.99-1.20, p=0.082; Figure 3).
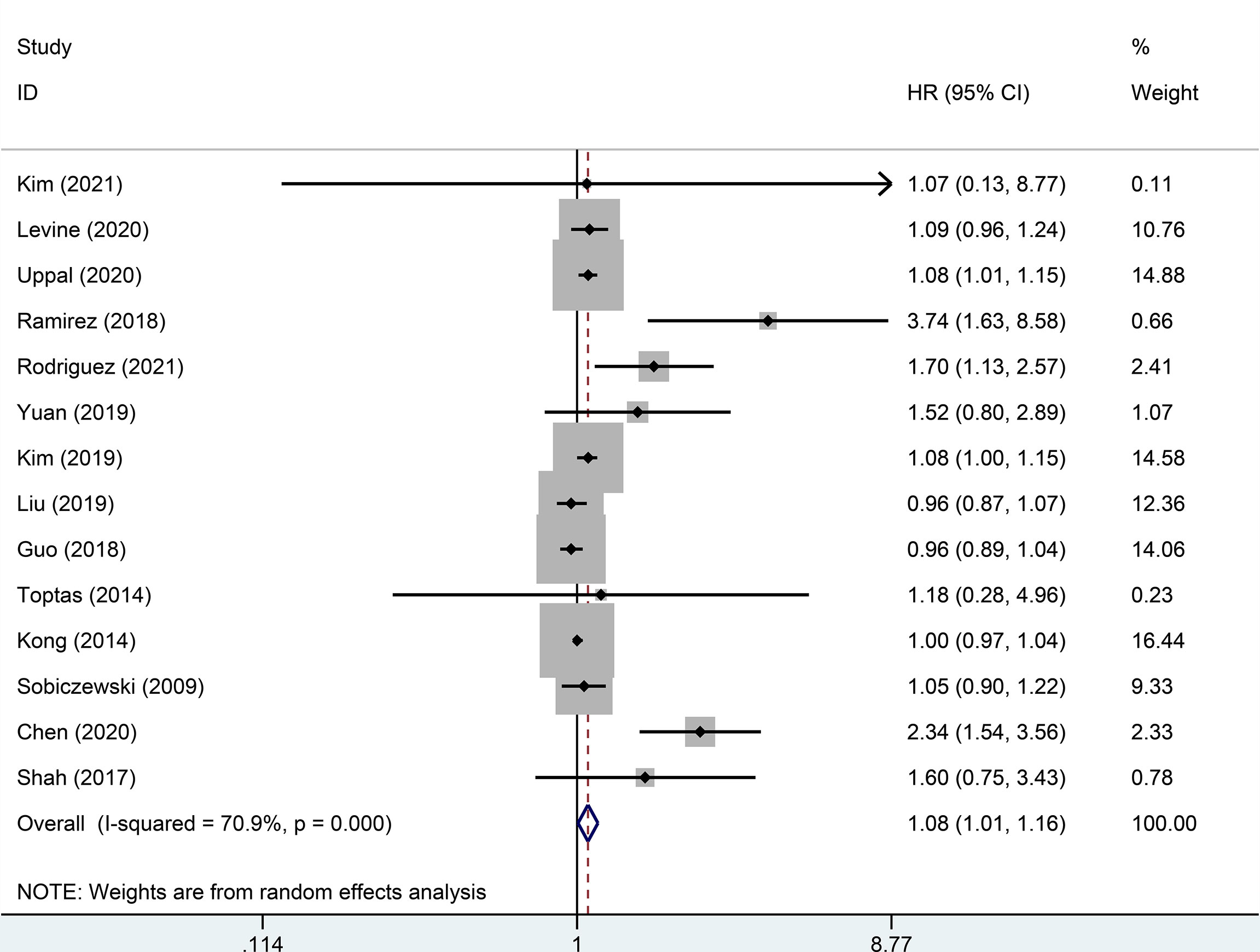
Figure 2 Forest plot of the 3-year disease-free survival (DFS) of patients with early-stage cervical cancer on minimally invasive surgery (MIS) (p=0.031).
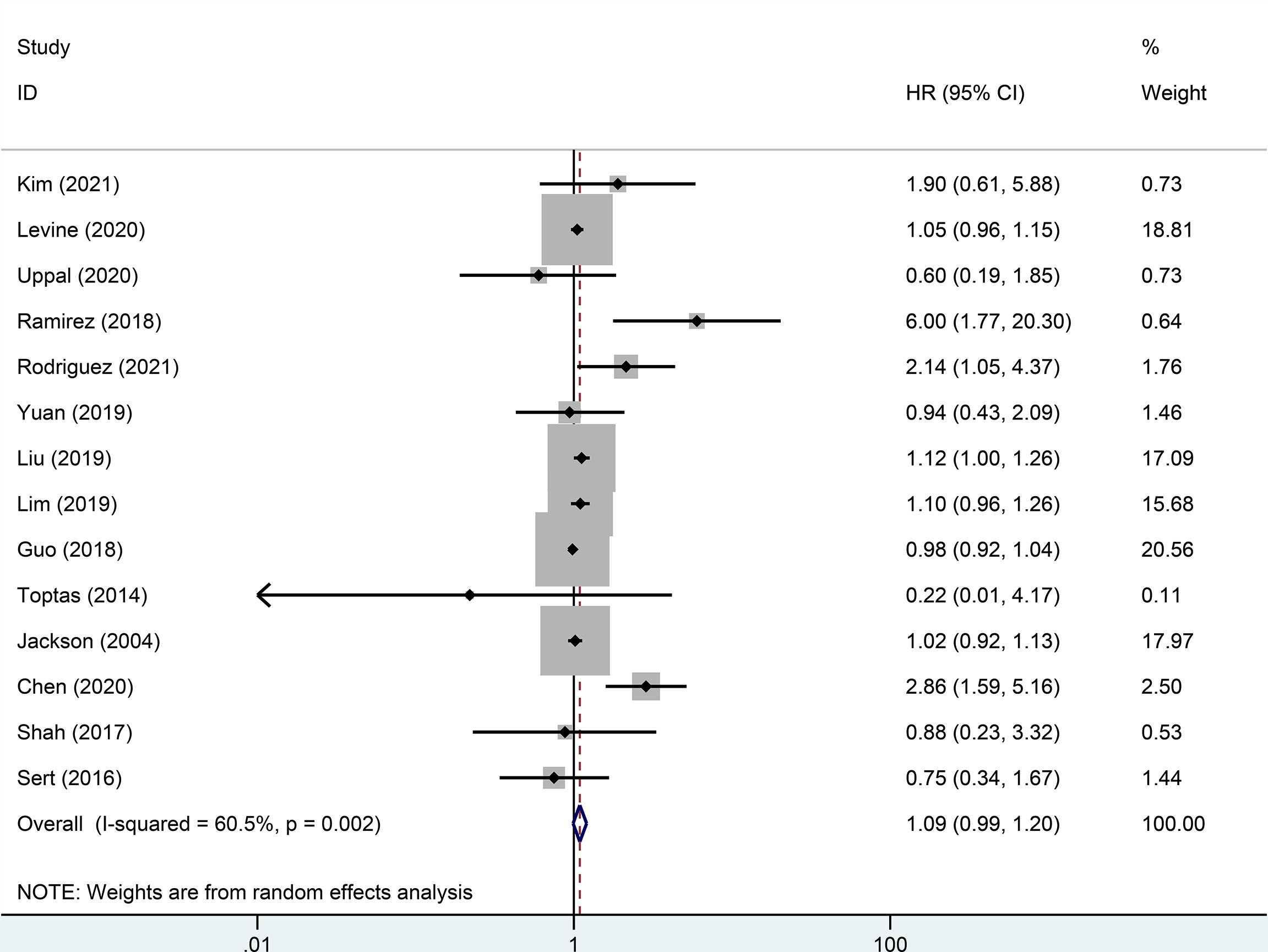
Figure 3 Forest plot of the 3-year overall survival (OS) of early-stage cervical cancer patients on minimally invasive surgery (MIS) (p=0.082).
There were 32 studies that provided 5-year DFS, including 13025 patients with early-stage cervical cancer. Among them, there were 6471 cases in the MIS group and 6312 cases in the ARH group. The results revealed that MIS had no significant effect on long-term DFS in patients with early-stage cervical cancer compared with ARH (HR=1.02, 95% CI: 0.98-1.07, p=0.266; Figure 4). Moreover, 15551 patients were evaluated for 5-year OS in 32 studies. The results illustrated that long-term OS of patients undergoing MIS was similar to that of patients undergoing ARH (HR=1.01, 95% CI: 0.97-1.05, p=0.795; Figure 5).
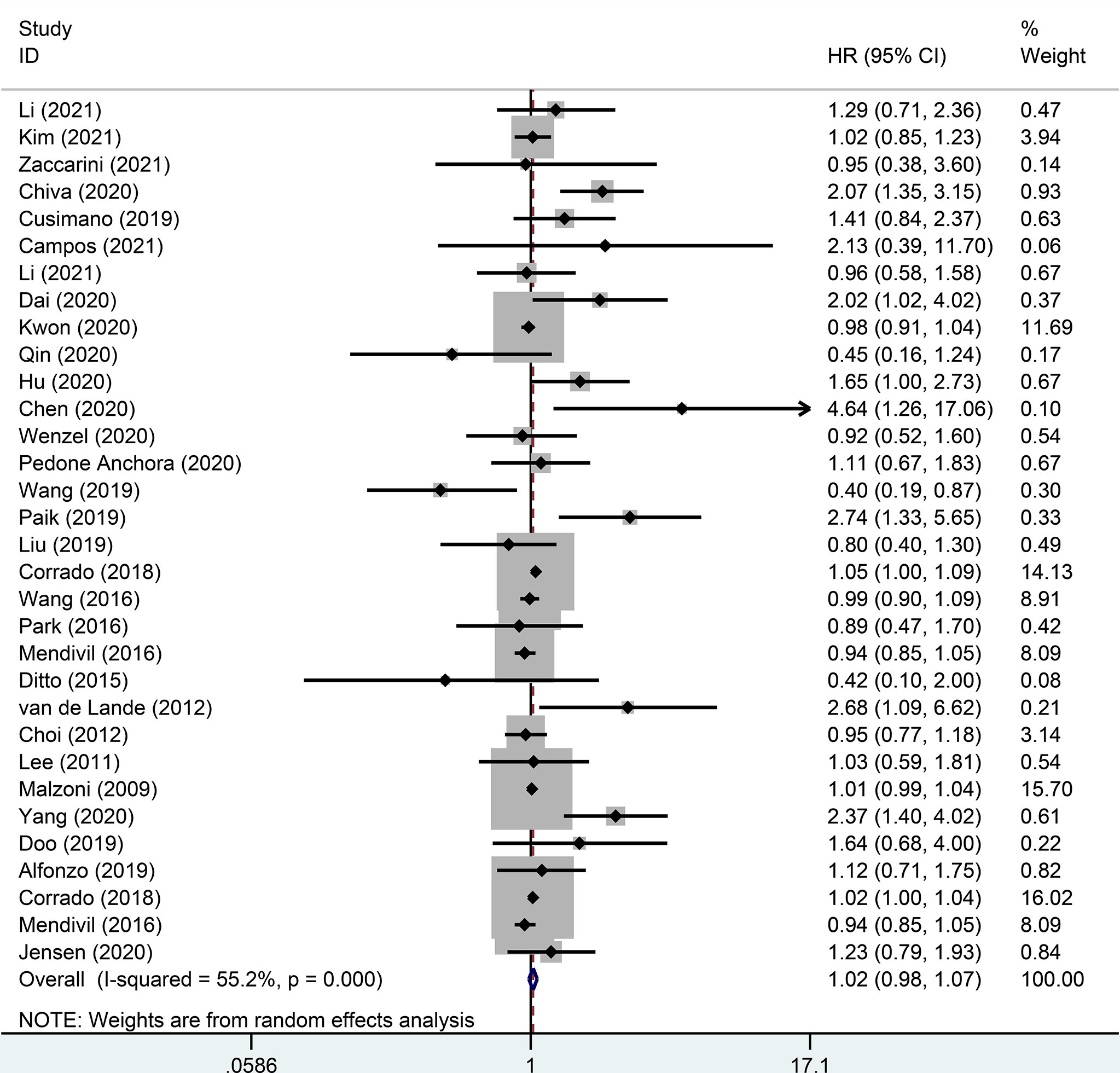
Figure 4 Forest plot for the 5-year disease-free survival (DFS) of early-stage cervical cancer patients on minimally invasive surgery (MIS) (p=0.266).
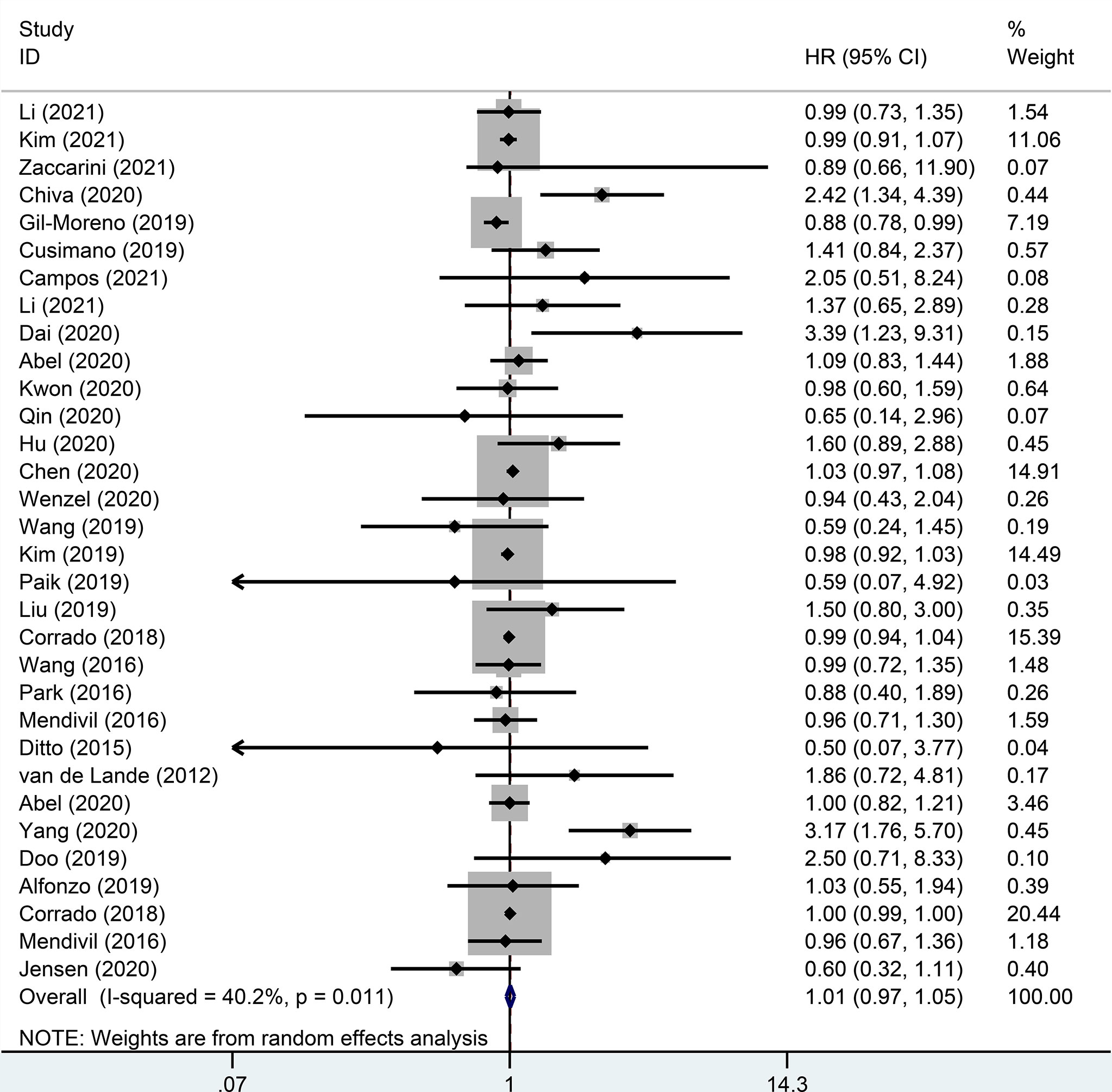
Figure 5 Forest plot for the 5-year overall survival (OS) of early-stage cervical cancer patients on minimally invasive surgery (MIS) (p=0.795).
Subgroup Analysis of 3- and 5-Year Survival
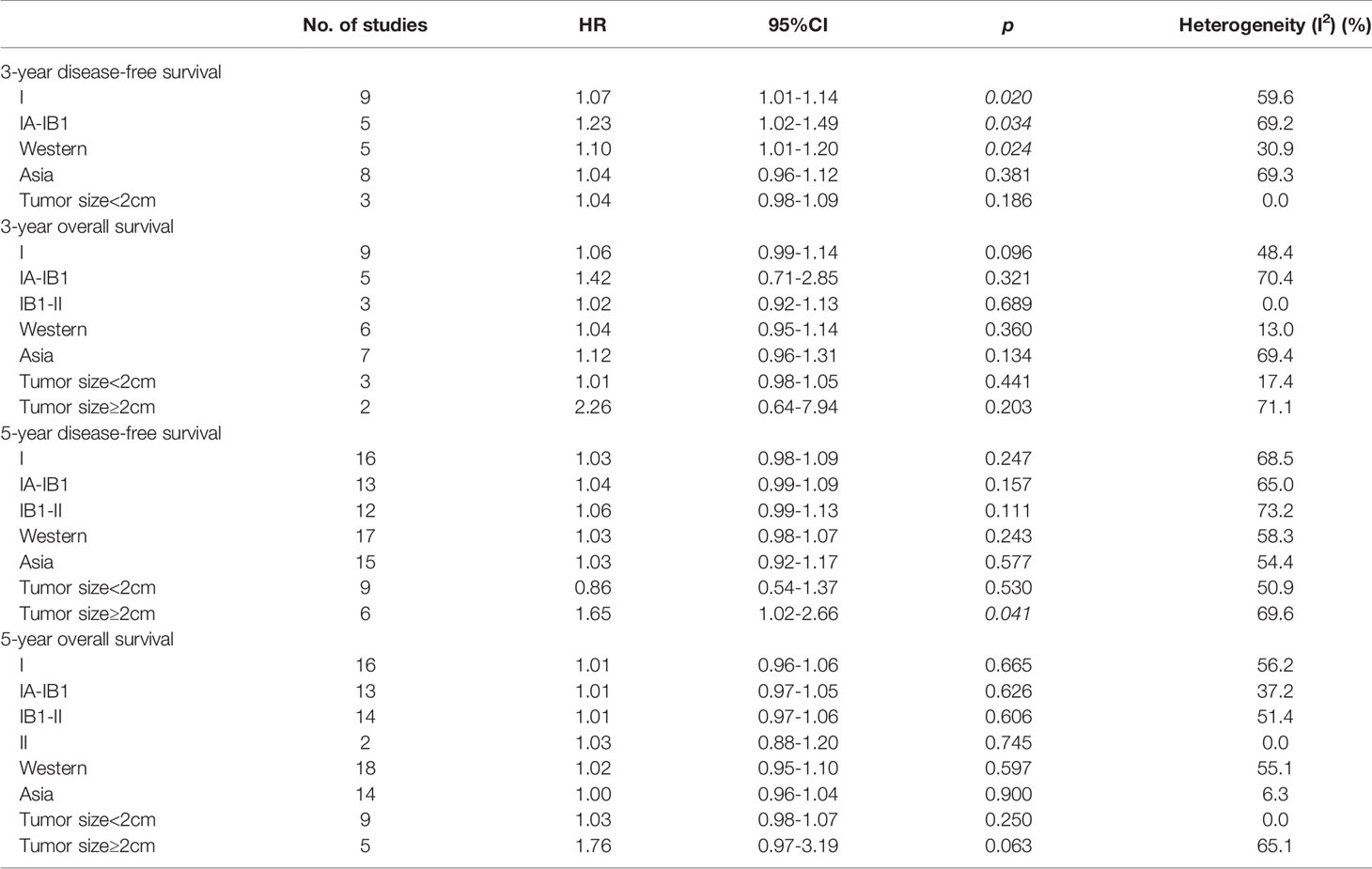
Of 14 studies that provided 3-year DFS, a subgroup analysis was performed on cervical cancer stage, including nine studies for stage I (HR=1.07, 95% CI: 1.01-1.14, p=0.020) and five studies for IA-IB1 (HR=1.23, 95% CI: 1.02-1.49, p=0.034). Patients with stages I and IA-IB1 who underwent MIS had a poorer 3-year DFS than those with ARH. Additionally, five studies were conducted in Western countries (HR=1.10, 95% CI: 1.01-1.20, p=0.024), whereas eight studies were conducted in Asian countries (HR=1.04, 95% CI: 0.96-1.12, p=0.381). Overall, the pooled subgroup results demonstrated that in Western countries, patients treated with MIS exhibited a significantly shorter 3-year DFS than those treated with ARH. Nevertheless, in studies of Asian countries, no difference in DFS was observed between MIS and ARH groups. Regarding patients with tumor size <2 cm, a subgroup analysis revealed no statistically significant difference in 3-year DFS between both groups (HR=1.04, 95% CI: 0.98-1.09, p=0.186) (Table 2).
Likewise, there were 14 studies that reported 3-year OS, with nine reporting stage I (HR=1.06, 95% CI: 0.99-1.14, p=0.096), five reporting stage IA-IB1 (HR=1.42, 95% CI: 0.71-2.85, p=0.321) and three reporting stage IB1-II (HR=1.02, 95% CI: 0.92-1.13, p=0.689). The subgroup analysis indicated that compared with patients undergoing ARH, no statistical difference was observed in 3-year OS in patients with stage I, IA-IB1, and IB1-II cervical cancer undergoing MIS. In addition, six studies were conducted in Western countries (HR=1.04, 95% CI: 0.95-1.14, p=0.36), while the remaining seven were conducted in Asian countries (HR=1.12, 95% CI: 0.96-1.31, p=0.134). Additionally, a subgroup analysis based on tumor size revealed that compared with patients undergoing ARH, those with early-stage cervical cancer undergoing MIS included tumor size <2 cm (HR=1.01, 95% CI: 0.98-1.05, p=0.441) and tumor size ≥2 cm (HR=2.26, 95% CI: 0.64-7.94, p=0.203), but no statistically significant difference was observed in 3-year OS (Table 2).
Of 32 studies on 5-year DFS, 16 were for stage I cervical cancer (HR=1.03, 95% CI: 0.98-1.09, p=0.247), 13 were for stage IA-IB1 (HR=1.04, 95% CI: 0.99-1.09, p=0.157), and 12 were for stage IB1-II (HR=1.06, 95% CI: 0.99-1.13, p=0.111). A total of 17 of 32 studies were conducted in Western countries (HR=1.03, 95% CI: 0.98-1.07, p=0.243), whereas 15 studies were conducted in Asian countries (HR=1.03, 95% CI: 0.92-1.17, p=0.577). Furthermore, nine studies evaluated 5-year DFS in patients with tumor size <2 cm (HR=0.86, 95% CI: 0.54-1.37, p=0.53). All above subgroup analyses revealed that MIS was not linked to long-term DFS in patients with early-stage cervical cancer. Notably, for early-stage cervical cancer patients with tumor size ≥2 cm, the pooled results disclosed that the MIS group may have a significantly shorter 5-year DFS than ARH group (HR=1.65, 95% CI: 1.02-2.66, p=0.041) (Table 2).
5-year OS was assessed in 32 studies, including 16 for stage I cervical cancer (HR=1.01, 95%CI: 0.96-1.06, p=0.665), 13 for stage IA-IB1 (HR=1.01, 95% CI: 0.97-1.05, p=0.626), 14 for stage IB1-II (HR=1.01, 95% CI: 0.97-1.06, p=0.606), and 2 for stage II (HR=1.03, 95% CI: 0.88-1.20, p=0.745). Moreover, 18 of 32 studies were performed in Western countries (HR=1.02, 95% CI: 0.95-1.10, p=0.597), while the remaining studies (n=14) were conducted in Asian countries (HR=1.00, 95% CI: 0.96-1.04, p=0.9). Briefly, in the above subgroup analyses, the results revealed that MIS was not correlated with 5-year OS in patients with early-stage cervical cancer. Additionally, subgroup analyses of 5-year OS were performed for patients with various tumor sizes. According to the results for patients with tumor size <2 cm, long-term OS was not statistically different between the MIS and ARH groups (HR=1.03, 95% CI: 0.98-1.07, p=0.25). Although patients with tumor size ≥2 cm undergoing MIS had a poorer long-term OS than those undergoing ARH, the difference was not statistically significant (HR=1.76, 95% CI: 0.97-3.19, p=0.063) (Table 2).
Publication Bias and Sensitivity Analysis of 3- and 5-Year Survival
Egger’s test revealed no significant publication bias in this meta-analysis (p>0.05). Additionally, sensitivity analysis of 3- and 5-year survival rates revealed that the results remained stable.
Discussion
Academics had cast doubt on previous surgical findings following the publication of 2018 Laparoscopic Approach to Cervical Cancer (LACC) trial. Additionally, the impact of MIS and ARH on the prognosis of patients with early-stage cervical cancer has been controversial. The RCT findings (12) revealed that MIS was associated with poorer DFS and OS than ARH, but some limitations were found. On the one hand, the trial of patients in the MIS group was terminated prematurely, and some patients received insufficient follow-up time. On the other hand, the results were inapplicable to assessing survival outcomes in “low-risk” cervical cancer patients. Moreover, the trial lacked specific preoperative imaging and central pathology (62). As a result, this meta-analysis systematically evaluated and compared prognosis (3- and 5-year DFS and OS) of patients with early-stage cervical cancer in MIS and ARH groups, and subgroup analyses of associated factors were conducted.
Our meta-analysis included 48 studies with 23346 patients. Based on evaluating 3-year prognosis, the results revealed that patients with early-stage cervical cancer undergoing MIS had a poorer 3-year DFS than those undergoing ARH, without observing a statistical difference in 3-year OS. In a multi-institution retrospective study, Uppal et al. (15) indicated that patients undergoing MIS had a poorer DFS, but no difference was observed in OS compared to those undergoing ARH, consistent with our findings. Meanwhile, subgroup analyses of tumor stage, region, and tumor size were performed on a 3-year prognosis. The pooled results of 3-year prognosis revealed that patients with stage I cervical cancer undergoing MIS exhibited poorer DFS than those undergoing ARH. Similarly, the pooled results in 3-year DFS demonstrated that patients with stage IA-IB1 and Western countries undergoing MIS indicated a shorter DFS than those undergoing ARH. Besides, no significant difference was observed in other subgroup analyses. For patients with stage I and IA-IB1, the poor results may be influenced by the frequency of the use of postoperative adjuvant therapy. In a Norwegian study (63), the incidence of postoperative radiotherapy was low in the MIS and ARH groups (6.1% vs. 12.5%). This study indicated that early-stage cervical cancer patients with stage IB1 and tumor size ≤2 cm who underwent MIS had significantly worse DFS than those with ARH. Nevertheless, a population-based study from Denmark (FIGO Stage IA2-IB1) (61) and a population-based study from Sweden (FIGO Stage IA1-IB1) (58) indicated a relatively high incidence of adjuvant therapy in the MIS and ARH groups (21.9% vs. 31.9%; 30.6% vs. 31.9%, respectively), with no difference in survival outcomes between the two groups. Since there was no difference in surgical techniques among the 3 closely related countries, it was tempting to speculate that the use of adjuvant radiotherapy had an impact on the survival outcomes. This speculation needs to be further confirmed. For patients from Western countries, DFS of MIS group was obviously inferior to that of ARH group. There was no clear explanation for this result. We suspected that it may be related to the different types of adjuvant therapy available in different geographic areas. Additionally, the frequency of use of adjuvant treatment in a study may influence the result. The relatively high number of patients receiving adjuvant therapy was likely to reduce the difference in survival between the two groups. NCCN guidelines (2) stated that for patients with stage IA2, IB1, or IIA1 who had negative lymph nodes after surgery but had other risk factors, pelvic external-beam radiation therapy was recommended with (or without) concurrent chemotherapy. A multicenter retrospective study from some Western countries (30) implicated that the incidence of postoperative adjuvant therapy was lower in the MIS group and the ARH group (29.2% vs. 30.7%), and the MIS group had worse prognosis after adjuvant therapy adjustment. By contrast, in a multi-center retrospective study from China (31), postoperative adjuvant therapy consisted of chemotherapy, radiotherapy or chemoradiotherapy. The study implicated that the incidence of postoperative adjuvant therapy was relatively high in the MIS and ARH groups (57.7% vs. 59.6%), with no significant difference between the two groups. Unfortunately, additional confirmation is required due to the scarcity of studies on adjuvant therapy and prognosis.
Likewise, 5-year prognosis was assessed in patients with early-stage cervical cancer. The results demonstrated no statistically significant difference between MIS and ARH groups. Brandt et al. (43) evaluated 196 cases and presented similar results that MIS had no association with a 5-year prognosis in patients with early-stage cervical cancer. Additionally, Abel et al. (33) revealed that stage II cervical cancer undergoing MIS or ARH revealed comparable 5-year survival rates. Nonetheless, some recent studies have demonstrated that early-stage patients undergoing MIS had poorer DFS and OS than those undergoing ARH. According to Dai et al. (32), patients with stage IB undergoing ARH had better DFS and OS than those undergoing MIS (5-year DFS rate, 94.1% vs. 87.5%; 5-year OS rate, 98.1% vs. 92.3%). Chiva et al. (25) reported in an international European cohort observational study that early-stage cervical cancer patients undergoing MIS increased the risk of recurrence and death compared with those undergoing ARH. In addition, we observed that in many retrospective studies (28, 30, 33, 41, 47, 51, 57, 59), the MIS group had a shorter follow-up time than that of the ARH group. In order to objectively evaluate the effect of MIS and ARH on the prognosis of patients with early-stage cervical cancer, more adequate follow-up time is needed. Furthermore, 5-year prognosis subgroups were analyzed based on tumor stage, region, and tumor size. Except for tumor size ≥2 cm, no statistical difference was observed in other subgroup analyses. The lack of discrepancy in stage II may be due to the relatively small number of studies.
Specifically, the pooled results revealed that patients with tumor size ≥2 cm treated with MIS had a poorer long-term prognosis than those treated with ARH. Consistent with the results of Li et al. (21) and Chen et al. (64), patients with tumor size ≥2 cm undergoing MIS had a shorter DFS than those treated with ARH. The following reasons may be responsible for poorer DFS in patients with tumor size ≥2 cm undergoing MIS: (1) Wagner et al. (65) pointed out that tumor size was an independent prognostic factor for each stage and greatly influenced the prognosis of cervical cancer patients. Larger tumors have a higher risk of lymphatic metastasis (66–69), requiring greater tumor resection (66). However, MIS might be less thoroughly resected than ARH. (2) Pressing the tumor while using a uterine manipulator may spread cancer or increase lymphatic vascular space infiltration (70–72). The SUCCOR study (25) indicated that the risk of recurrence was 2.76-fold higher in patients undergoing MIS with a uterine manipulator compared to those undergoing ARH. (3) When tumors are large, selection bias of surgical methods may affect the results (73). MIS probably brings some surgical difficulties to surgeons (21, 22, 66, 70, 74), reducing the surgical effect. (4) Pneumoperitoneum environment may be a prognostic factor in patients undergoing MIS. An in vitro study (75) demonstrated that when cervical cancer cells were stimulated in CO2 pneumoperitoneum environment in vitro, their proliferation ability was enhanced following a short period of inhibition. A retrospective analysis by Kong et al. (76) found that patients with early-stage cervical cancer undergoing MIS in pneumoperitoneal conditions increased the risk of recurrence and intraperitoneal tumor spread. In addition, the SUCCOR study (25) proposed that implementing a preoperative protective vaginal closure in patients undergoing MIS dramatically reduced the risk of recurrence and peritoneal metastasis compared to those undergoing ARH.
Compared with other studies, the strengths of this meta-analysis included the division of patients’ prognoses into medium- (3-year) and long-term (5-year) categories, as well as subgroup analyses for various factors such as tumor stage, region, and tumor size. Indeed, our meta-analysis had several limitations. First, only two of the included studies were RCTs, while the remaining were observational studies, resulting in inevitable risks such as selection bias. Second, the baseline characteristics of studies varied, such as tumor stage and surgical procedure. Besides, sentinel lymph node and adjuvant therapy assessments were not performed due to limited data. Furthermore, the sample size of our study was impacted by language restrictions associated with included literature. Finally, the retrieval time span was relatively long, allowing for MIS technology development, resulting in studies that may not accurately reflect changes in survival outcomes over time.
Conclusion
In patients with Western countries and stage I cervical cancer, MIS was linked to a shorter medium-term DFS, particularly in stage IA-IB1. Regarding long-term prognosis, patients with tumor size ≥2 cm were unsuitable for MIS and had shorter DFS than ARH. Accordingly, MIS should be chosen with caution in patients with early-stage cervical cancer. Nevertheless, more large-scale RCTs, including two ongoing trials (NCT03739944, NCT03719547), and clinical studies are required to provide relevant data.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
Each author contributed significantly to concept and development of the present paper. LYY and MZ designed the research process. WD and MZ searched the database for corresponding articles and extracted useful information from the articles above. YXS and YTS used statistical software for analysis. XL, KJ, and JS drafted the meta-analysis. All authors had read and approved the manuscript and ensured that this was the case.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.762921/full#supplementary-material
Supplementary Table 1 | Characteristics of all studies included in the meta-analysis.
Supplementary Table 2 | Quality assessment of included studies in this meta-analysis.
Abbreviations
MIS, minimally invasive surgery; ARH, Abdominal radical hysterectomy; OS, overall survival; DFS, disease-free survival; HR, hazard ratio; CI, confidence interval; FIGO, International Federation of Gynecology and Obstetrics; RCT, randomized controlled trial.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Koh WJ, Abu-Rustum NR, Bean S, Bradley K, Campos SM, Cho KR, et al. Cervical Cancer, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw (2019) 17(1):64–84. doi: 10.6004/jnccn.2019.0001
3. Cibula D, Pötter R, Planchamp F, Avall-Lundqvist E, Fischerova D, Haie Meder C, et al. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology Guidelines for the Management of Patients With Cervical Cancer. Radiother Oncol (2018) 127(3):404–16. doi: 10.1016/j.radonc.2018.03.003
4. Nezhat CR, Burrell MO, Nezhat FR, Benigno BB, Welander CE. Laparoscopic Radical Hysterectomy With Paraaortic and Pelvic Node Dissection. Am J Obstetrics Gynecology (1992) 166(3):864–5. doi: 10.1016/0002-9378(92)91351-A
5. Abu-Rustum NR, Hoskins WJ. Radical Abdominal Hysterectomy. Surg Clin North Am (2001) 81(4):815–28. doi: 10.1016/S0039-6109(05)70167-5
6. Spirtos NM, Eisenkop SM, Schlaerth JB, Ballon SC. Laparoscopic Radical Hysterectomy (Type III) With Aortic and Pelvic Lymphadenectomy in Patients With Stage I Cervical Cancer: Surgical Morbidity and Intermediate Follow-Up. Am J Obstet Gynecol (2002) 187(2):340–8. doi: 10.1067/mob.2002.123035
7. Stewart KI, Fader AN. New Developments in Minimally Invasive Gynecologic Oncology Surgery. Clin Obstet Gynecol (2017) 60(2):330–48. doi: 10.1097/GRF.0000000000000286
8. Koh WU, Greer BE, Abu-Rustum NR, Apte SM, Campos SM, Cho KR, et al. Cervical Cancer, Version 2.2015. J Natl Compr Cancer Network JNCCN (2015) 13(4):395–404. doi: 10.6004/jnccn.2015.0055
9. Clayton RD. Hysterectomy. Best Pract Res Clin Obstet Gynaecol (2006) 20(1):73–87. doi: 10.1016/j.bpobgyn.2005.09.007
10. Wenzel HHB, Smolders RGV, Beltman JJ, Lambrechts S, Trum HW, Yigit R, et al. Survival of Patients With Early-Stage Cervical Cancer After Abdominal or Laparoscopic Radical Hysterectomy: A Nationwide Cohort Study and Literature Review. Eur J Cancer (2020) 133:14–21. doi: 10.1016/j.ejca.2020.04.006
11. Yuan Z, Cao D, Yang J, Yu M, Shen K, Yang J, et al. Laparoscopic vs. Open Abdominal Radical Hysterectomy for Cervical Cancer: A Single-Institution, Propensity Score Matching Study in China. Front Oncol (2019) 9:1107. doi: 10.3389/fonc.2019.01107
12. Ramirez PT, Frumovitz M, Pareja R, Lopez A, Vieira M, Ribeiro R, et al. Minimally Invasive Versus Abdominal Radical Hysterectomy for Cervical Cancer. N Engl J Med (2018) 379(20):1895–904. doi: 10.1056/NEJMoa1806395
13. Committee FGO. FIGO Statement on Minimally Invasive Surgery in Cervical Cancer. Int J Gynaecol Obstet (2020) 149(3):264. doi: 10.1002/ijgo.13141
14. Wang W, Li L, Wu M, Ma S, Tan X, Zhong S. Laparoscopic vs. Abdominal Radical Hysterectomy for Locally Advanced Cervical Cancer. Front Oncol (2019) 9:1331. doi: 10.3389/fonc.2019.01331
15. Uppal S, Gehrig PA, Peng K, Bixel KL, Matsuo K, Vetter MH, et al. Recurrence Rates in Patients With Cervical Cancer Treated With Abdominal Versus Minimally Invasive Radical Hysterectomy: A Multi-Institutional Retrospective Review Study. J Clin Oncol Off J Am Soc Clin Oncol (2020) 38(10):1030–40. doi: 10.1200/JCO.19.03012
16. Kim SI, Lee M, Lee S, Suh DH, Kim HS, Kim K, et al. Impact of Laparoscopic Radical Hysterectomy on Survival Outcome in Patients With FIGO Stage IB Cervical Cancer: A Matching Study of Two Institutional Hospitals in Korea. Gynecol Oncol (2019) 155(1):75–82. doi: 10.1016/j.ygyno.2019.07.019
17. Paik ES, Lim MC, Kim MH, Kim YH, Song ES, Seong SJ, et al. Comparison of Laparoscopic and Abdominal Radical Hysterectomy in Early Stage Cervical Cancer Patients Without Adjuvant Treatment: Ancillary Analysis of a Korean Gynecologic Oncology Group Study (KGOG 1028). Gynecol Oncol (2019) 154(3):547–53. doi: 10.1016/j.ygyno.2019.06.023
18. Gu L, Huang X, Li S, Mao D, Shen Z, Khadaroo PA, et al. A Meta-Analysis of the Medium- and Long-Term Effects of Laparoscopic Sleeve Gastrectomy and Laparoscopic Roux-En-Y Gastric Bypass. BMC Surg (2020) 20(1):30. doi: 10.1186/s12893-020-00695-x
19. Gu L, Du N, Jin Q, Li S, Xie L, Mo J, et al. Magnitude of Benefit of the Addition of Poly ADP-Ribose Polymerase (PARP) Inhibitors to Therapy for Malignant Tumor: A Meta-Analysis. Crit Rev Oncol Hematol (2020) 147:102888. doi: 10.1016/j.critrevonc.2020.102888
20. Gu L, Chen M, Guo D, Zhu H, Zhang W, Pan J, et al. PD-L1 and Gastric Cancer Prognosis: A Systematic Review and Meta-Analysis. PloS One (2017) 12(8):e0182692. doi: 10.1371/journal.pone.0182692
21. Li LY, Wen LY, Park SH, Nam EJ, Lee JY, Kim S, et al. Impact of the Learning Curve on the Survival of Abdominal or Minimally Invasive Radical Hysterectomy for Early-Stage Cervical Cancer. Cancer Res Treat (2021) 53(1):243–51. doi: 10.4143/crt.2020.063
22. Kim S, Min KJ, Lee S, Hong JH, Song JY, Lee JK, et al. Learning Curve Could Affect Oncologic Outcome of Minimally Invasive Radical Hysterectomy for Cervical Cancer. Asian J Surg (2021) 44(1):174–80. doi: 10.1016/j.asjsur.2020.05.006
23. Kim SI, Lee J, Hong J, Lee SJ, Park DC, Yoon JH. Comparison of Abdominal and Minimally Invasive Radical Hysterectomy in Patients With Early Stage Cervical Cancer. Int J Med Sci (2021) 18(5):1312–7. doi: 10.7150/ijms.55017
24. Zaccarini F, Santy A, Dabi Y, Lavoue V, Carcopino X, Bendifallah S, et al. Comparison of Survival Outcomes Between Laparoscopic and Abdominal Radical Hysterectomy for Early-Stage Cervical Cancer: A French Multicentric Study. J Gynecol Obstet Hum Reprod (2021) 50(2):102046. doi: 10.1016/j.jogoh.2020.102046
25. Chiva L, Zanagnolo V, Querleu D, Martin-Calvo N, Arévalo-Serrano J, Căpîlna ME, et al. SUCCOR Study: An International European Cohort Observational Study Comparing Minimally Invasive Surgery Versus Open Abdominal Radical Hysterectomy in Patients With Stage IB1 Cervical Cancer. Int J Gynecol Cancer (2020) 30(9):1269–77. doi: 10.1136/ijgc-2020-001506
26. Levine MD, Brown J, Crane EK, Tait DL, Naumann RW. Outcomes of Minimally Invasive Versus Open Radical Hysterectomy for Early Stage Cervical Cancer Incorporating 2018 FIGO Staging. J Minim Invasive Gynecol (2021) 28(4):824–8. doi: 10.1016/j.jmig.2020.07.021
27. Gil-Moreno A, Carbonell-Socias M, Salicrú S, Centeno-Mediavilla C, Franco-Camps S, Colas E, et al. Radical Hysterectomy: Efficacy and Safety in the Dawn of Minimally Invasive Techniques. J Minim Invasive Gynecol (2019) 26(3):492–500. doi: 10.1016/j.jmig.2018.06.007
28. Cusimano MC, Baxter NN, Gien LT, Moineddin R, Liu N, Dossa F, et al. Impact of Surgical Approach on Oncologic Outcomes in Women Undergoing Radical Hysterectomy for Cervical Cancer. Am J Obstet Gynecol (2019) 221(6):619 e1–619 e24. doi: 10.1016/j.ajog.2019.07.009
29. Campos L, Limberger LF, Stein AT, Caldas JM. Survival After Laparoscopic Versus Abdominal Radical Hysterectomy in Early Cervical Cancer: A Randomized Controlled Trial. Asian Pacific J Cancer Prev (2021) 22(1):93–7. doi: 10.31557/APJCP.2021.22.1.93
30. Rodriguez J, Rauh-Hain JA, Saenz J, Isla DO, Rendon Pereira GJ, Odetto D, et al. Oncological Outcomes of Laparoscopic Radical Hysterectomy Versus Radical Abdominal Hysterectomy in Patients With Early-Stage Cervical Cancer: A Multicenter Analysis. Int J Gynecol Cancer (2021) 31(4):504–11. doi: 10.1136/ijgc-2020-002086
31. Li P, Chen L, Ni Y, Liu J, Li D, Guo J, et al. Comparison Between Laparoscopic and Abdominal Radical Hysterectomy for Stage IB1 and Tumor Size <2 Cm Cervical Cancer With Visible or Invisible Tumors: A Multicentre Retrospective Study. J Gynecol Oncol (2021) 32(2):e17. doi: 10.3802/jgo.2021.32.e17
32. Dai D, Huang H, Feng Y, Wan T, Liu Z, Tong C, et al. Minimally Invasive Surgery vs Laparotomy for Early Stage Cervical Cancer: A Propensity Score-Matched Cohort Study. Cancer Med (2020) 9(24):9236–45. doi: 10.1002/cam4.3527
33. Abel MK, Chan JK, Chow S, Darcy K, Tian C, Kapp DS, et al. Trends and Survival Outcomes of Robotic, Laparoscopic, and Open Surgery for Stage II Uterine Cancer. Int J Gynecol Cancer (2020) 30(9):1347–55. doi: 10.1136/ijgc-2020-001646
34. Kwon BS, Roh HJ, Lee S, Yang J, Song YJ, Lee SH, et al. Comparison of Long-Term Survival of Total Abdominal Radical Hysterectomy and Laparoscopy-Assisted Radical Vaginal Hysterectomy in Patients With Early Cervical Cancer: Korean Multicenter, Retrospective Analysis. Gynecol Oncol (2020) 159(3):642–8. doi: 10.1016/j.ygyno.2020.09.035
35. Qin M, Siyi L, Huang HF, Li Y, Gu Y, Wang W, et al. A Comparison of Laparoscopies and Laparotomies for Radical Hysterectomy in Stage IA1-IB1 Cervical Cancer Patients: A Single Team With 18 Years of Experience. Front Oncol (2020) 10:1738. doi: 10.3389/fonc.2020.01738
36. Hu TWY, Huang Y, Li N, Nie D, Li Z. Comparison of Laparoscopic Versus Open Radical Hysterectomy in Patients With Early-Stage Cervical Cancer: A Multicenter Study in China. Int J Gynecol Cancer (2020) 30(8):1143–50. doi: 10.1136/ijgc-2020-001340
37. Chen X, Zhao N, Ye P, Chen J, Nan X, Zhao H, et al. Comparison of Laparoscopic and Open Radical Hysterectomy in Cervical Cancer Patients With Tumor Size </=2 Cm. Int J Gynecol Cancer (2020) 30(5):564–71. doi: 10.1136/ijgc-2019-000994
38. Pedone Anchora L, Turco LC, Bizzarri N, Capozzi VA, Lombisani A, Chiantera V, et al. How to Select Early-Stage Cervical Cancer Patients Still Suitable for Laparoscopic Radical Hysterectomy: A Propensity-Matched Study. Ann Surg Oncol (2020) 27(6):1947–55. doi: 10.1245/s10434-019-08162-5
39. Liu Y, Li L, Wu M, Ma S, Tan X, Zhong S, et al. The Impact of the Surgical Routes and Learning Curve of Radical Hysterectomy on the Survival Outcomes in Stage IB Cervical Cancer: A Retrospective Cohort Study. Int J Surg (2019) 68:72–7. doi: 10.1016/j.ijsu.2019.06.009
40. Lim TYK, Lin KKM, Wong WL, Aggarwal IM, Yam PKL. Surgical and Oncological Outcome of Total Laparoscopic Radical Hysterectomy Versus Radical Abdominal Hysterectomy in Early Cervical Cancer in Singapore. Gynecol Minim Invasive Ther (2019) 8(2):53–8. doi: 10.4103/GMIT.GMIT_43_18
41. Guo J, Yang L, Cai J, Xu L, Min J, Shen Y, et al. Laparoscopic Procedure Compared With Open Radical Hysterectomy With Pelvic Lymphadenectomy in Early Cervical Cancer: A Retrospective Study. Onco Targets Ther (2018) 11:5903–8. doi: 10.2147/OTT.S156064
42. Corrado G, Vizza E, Legge F, Pedone Anchora L, Sperduti I, Fagotti A, et al. Comparison of Different Surgical Approaches for Stage IB1 Cervical Cancer Patients: A Multi-Institution Study and a Review of the Literature. Int J Gynecol Cancer (2018) 28(5):1020–8. doi: 10.1097/IGC.0000000000001254
43. Brandt B, Sioulas V, Basaran D, Kuhn T, LaVigne K, Gardner GJ, et al. Minimally Invasive Surgery Versus Laparotomy for Radical Hysterectomy in the Management of Early-Stage Cervical Cancer: Survival Outcomes. Gynecol Oncol (2020) 156(3):591–7. doi: 10.1016/j.ygyno.2019.12.038
44. Park JY, Kim D, Suh DS, Kim JH, Kim YM, Kim YT, et al. The Role of Laparoscopic Radical Hysterectomy in Early-Stage Adenocarcinoma of the Uterine Cervix. Ann Surg Oncol (2016) 23(Suppl 5):825–33. doi: 10.1245/s10434-016-5489-4
45. Mendivil AA, Rettenmaier MA, Abaid LN, Brown JV 3rd, Micha JP, Lopez KL, et al. Survival Rate Comparisons Amongst Cervical Cancer Patients Treated With an Open, Robotic-Assisted or Laparoscopic Radical Hysterectomy: A Five Year Experience. Surg Oncol (2016) 25(1):66–71. doi: 10.1016/j.suronc.2015.09.004
46. Ditto A, Martinelli F, Bogani G, Gasparri ML, Di Donato V, Zanaboni F, et al. Implementation of Laparoscopic Approach for Type B Radical Hysterectomy: A Comparison With Open Surgical Operations. Eur J Surg Oncol (2015) 41(1):34–9. doi: 10.1016/j.ejso.2014.10.058
47. Toptas T, Simsek T. Total Laparoscopic Versus Open Radical Hysterectomy in Stage IA2-IB1 Cervical Cancer: Disease Recurrence and Survival Comparison. J Laparoendosc Adv Surg Tech A (2014) 24(6):373–8. doi: 10.1089/lap.2013.0514
48. Kong TW, Chang SJ, Lee J, Paek J, Ryu HS, et al. Comparison of Laparoscopic Versus Abdominal Radical Hysterectomy for FIGO Stage IB and IIA Cervical Cancer With Tumor Diameter of 3 Cm or Greater. Int J Gynecol Cancer (2014) 24(2):280–8. doi: 10.1097/IGC.0000000000000052
49. van de Lande J, von Mensdorff-Pouilly S, Lettinga RG, Piek JM, Verheijen RH. Open Versus Laparoscopic Pelvic Lymph Node Dissection in Early Stage Cervical Cancer: No Difference in Surgical or Disease Outcome. Int J Gynecol Cancer (2012) 22(1):107–14. doi: 10.1097/IGC.0b013e31822c273d
50. Choi CH, Lee JW, Lee YY, Kim HJ, Song T, Kim MK, et al. Comparison of Laparoscopic-Assisted Radical Vaginal Hysterectomy and Laparoscopic Radical Hysterectomy in the Treatment of Cervical Cancer. Ann Surg Oncol (2012) 19(12):3839–48. doi: 10.1245/s10434-012-2406-3
51. Lee EJ, Kang H, Kim DH. A Comparative Study of Laparoscopic Radical Hysterectomy With Radical Abdominal Hysterectomy for Early-Stage Cervical Cancer: A Long-Term Follow-Up Study. Eur J Obstet Gynecol Reprod Biol (2011) 156(1):83–6. doi: 10.1016/j.ejogrb.2010.12.016
52. Sobiczewski P, Bidzinski M, Derlatka P, Panek G, Danska-Bidzinska A, Gmyrek L, et al. Early Cervical Cancer Managed by Laparoscopy and Conventional Surgery: Comparison of Treatment Results. Int J Gynecol Cancer (2009) 19(8):1390–5. doi: 10.1111/IGC.0b013e3181ba5e88
53. Malzoni M, Tinelli R, Cosentino F, Fusco A, Malzoni C. Total Laparoscopic Radical Hysterectomy Versus Abdominal Radical Hysterectomy With Lymphadenectomy in Patients With Early Cervical Cancer: Our Experience. Ann Surg Oncol (2009) 16(5):1316–23. doi: 10.1245/s10434-009-0342-7
54. Jackson KS, Das N, Naik R, Lopes AD, Godfrey KA, Hatem MH, et al. Laparoscopically Assisted Radical Vaginal Hysterectomy vs. Radical Abdominal Hysterectomy for Cervical Cancer: A Match Controlled Study. Gynecol Oncol (2004) 95(3):655–61. doi: 10.1016/j.ygyno.2004.07.055
55. Chen B, Ji M, Li P, Liu P, Zou W, Zhao Z, et al. Comparison Between Robot-Assisted Radical Hysterectomy and Abdominal Radical Hysterectomy for Cervical Cancer: A Multicentre Retrospective Study. Gynecol Oncol (2020) 157(2):429–36. doi: 10.1016/j.ygyno.2020.02.019
56. Yang J, Mead-Harvey C, Polen-De C, Magtibay P, Butler K, Cliby W, et al. Survival Outcomes in Patients With Cervical Cancer Treated With Open Versus Robotic Radical Hysterectomy: Our Surgical Pathology Interrogation. Gynecol Oncol (2020) 159(2):373–80. doi: 10.1016/j.ygyno.2020.08.031
57. Doo DW, Kirkland CT, Griswold LH, McGwin G, Huh WK, Leath CA 3rd, et al. Comparative Outcomes Between Robotic and Abdominal Radical Hysterectomy for IB1 Cervical Cancer: Results From a Single High Volume Institution. Gynecol Oncol (2019) 153(2):242–7. doi: 10.1016/j.ygyno.2019.03.001
58. Alfonzo E, Wallin E, Ekdahl L, Staf C, Rådestad AF, Reynisson P, et al. No Survival Difference Between Robotic and Open Radical Hysterectomy for Women With Early-Stage Cervical Cancer: Results From a Nationwide Population-Based Cohort Study. Eur J Cancer (2019) 116:169–77. doi: 10.1016/j.ejca.2019.05.016
59. Shah CA, Beck T, Liao JB, Giannakopoulos NV, Veljovich D, Paley P, et al. Surgical and Oncologic Outcomes After Robotic Radical Hysterectomy as Compared to Open Radical Hysterectomy in the Treatment of Early Cervical Cancer. J Gynecol Oncol (2017) 28(6):e82. doi: 10.3802/jgo.2017.28.e82
60. Sert BM, Boggess JF, Ahmad S, Jackson AL, Stavitzski NM, Dahl AA, et al. Robot-Assisted Versus Open Radical Hysterectomy: A Multi-Institutional Experience for Early-Stage Cervical Cancer. Eur J Surg Oncol (2016) 42(4):513–22. doi: 10.1016/j.ejso.2015.12.014
61. Jensen PT, Schnack TH, Frøding LP, Bjørn SF, Lajer H, Markauskas A, et al. Survival After a Nationwide Adoption of Robotic Minimally Invasive Surgery for Early-Stage Cervical Cancer – A Population-Based Study. Eur J Cancer (2020) 128:47–56. doi: 10.1016/j.ejca.2019.12.020
62. Naumann RW. Minimally Invasive Radical Hysterectomy Has Many Benefits Compared With Open Radical Hysterectomy: Will the LACC Trial Cause the Premature Demise of This Procedure? J Minim Invasive Gynecol (2019) 26(3):379–80. doi: 10.1016/j.jmig.2019.01.002
63. Sert BM, Kristensen GB, Kleppe A, Dørum A. Long-Term Oncological Outcomes and Recurrence Patterns in Early-Stage Cervical Cancer Treated With Minimally Invasive Versus Abdominal Radical Hysterectomy: The Norwegian Radium Hospital Experience. Gynecol Oncol (2021) 162(2):284–91. doi: 10.1016/j.ygyno.2021.05.028
64. Chen C, Fang Z, Wang Q, Li W, Li P, Wang L, et al. Comparative Study on the Oncological Prognosis of Laparoscopy and Laparotomy for Stage IIA1 Cervical Squamous Cell Carcinoma. Eur J Surg Oncol (2021) 47(2):346–52. doi: 10.1016/j.ejso.2020.07.016
65. Wagner AE, Pappas L, Ghia AJ, Gaffney DK. Impact of Tumor Size on Survival in Cancer of the Cervix and Validation of Stage IIA1 and IIA2 Subdivisions. Gynecol Oncol (2013) 129(3):517–21. doi: 10.1016/j.ygyno.2013.03.008
66. Armbrust R, Chen F, Richter R, Muallem MZ, Mustea A, Holthaus B, et al. Results of a German Wide Survey Towards Current Surgical Approach in Early Stage Cervical Cancer NOGGO MONITOR 11. Sci Rep (2021) 11(1):9774. doi: 10.1038/s41598-021-89071-0
67. Fagotti A, Pedone Anchora L, Conte C, Chiantera V, Vizza E, Tortorella L, et al. Beyond Sentinel Node Algorithm. Toward a More Tailored Surgery for Cervical Cancer Patients. Cancer Med (2016) 5(8):1725–30. doi: 10.1002/cam4.722
68. Gulseren V, Kocaer M, Gungorduk O, Ozdemir IA, Gokcu M, Mart EM, et al. Preoperative Predictors of Pelvic and Para-Aortic Lymph Node Metastases in Cervical Cancer. J Cancer Res Ther (2019) 15(6):1231–4. doi: 10.4103/jcrt.JCRT_467_17
69. Casarin J, Buda A, Bogani G, Fanfani F, Papadia A, Ceccaroni M, et al. Predictors of Recurrence Following Laparoscopic Radical Hysterectomy for Early-Stage Cervical Cancer: A Multi-Institutional Study. Gynecol Oncol (2020) 159(1):164–70. doi: 10.1016/j.ygyno.2020.06.508
70. Eoh KJ, Lee JY, Nam EJ, Kim S, Kim SW, Kim YT. The Institutional Learning Curve is Associated With Survival Outcomes of Robotic Radical Hysterectomy for Early-Stage Cervical Cancer-a Retrospective Study. BMC Cancer (2020) 20(1):152. doi: 10.1186/s12885-020-6660-7
71. Wang Y, Li B, Ren F, Song Z, Ouyang L, Liu K. Survival After Minimally Invasive vs. Open Radical Hysterectomy for Cervical Cancer: A Meta-Analysis. Front Oncol (2020) 10:1236. doi: 10.3389/fonc.2020.01236
72. Nica A, Kim SR, Gien LT, Covens A, Bernardini MQ, Bouchard-Fortier G, et al. Survival After Minimally Invasive Surgery in Early Cervical Cancer: Is the Intra-Uterine Manipulator to Blame? Int J Gynecol Cancer (2020) 30(12):1864–70. doi: 10.1136/ijgc-2020-001816
73. Kim JH, Kim K, Park SJ, Lee JY, Kim K, Lim MC, et al. Comparative Effectiveness of Abdominal Versus Laparoscopic Radical Hysterectomy for Cervical Cancer in the Postdissemination Era. Cancer Res Treat (2019) 51(2):788–96. doi: 10.4143/crt.2018.120
74. Schermerhorn SMV, Christman MS, Rocco NR, Abdul-Muhsin H, L'Esperance JO, Castle EP, et al. Learning Curve for Robotic-Assisted Laparoscopic Retroperitoneal Lymph Node Dissection. J Endourol (2021) 35:1483–9. doi: 10.1089/end.2020.0549
75. Lin F, Pan L, Li L, Li D, Mo L. Effects of a Simulated CO2 Pneumoperitoneum Environment on the Proliferation, Apoptosis, and Metastasis of Cervical Cancer Cells In Vitro. Med Sci Monit (2014) 20:2497–503. doi: 10.12659/MSM.891179
Keywords: abdominal radical hysterectomy, early-stage cervical cancer, prognosis, meta-analysis, minimally invasive surgery
Citation: Zhang M, Dai W, Si Y, Shi Y, Li X, Jiang K, Shen J and Ying L (2022) Comparison of Minimally Invasive Versus Abdominal Radical Hysterectomy for Early-Stage Cervical Cancer: An Updated Meta-Analysis. Front. Oncol. 11:762921. doi: 10.3389/fonc.2021.762921
Received: 23 August 2021; Accepted: 30 December 2021;
Published: 24 January 2022.
Edited by:
Stefano Restaino, Ospedale Santa Maria della Misericordia di Udine, ItalyReviewed by:
Luigi Pedone Anchora, Agostino Gemelli University Polyclinic (IRCCS), ItalyGunnar Kristensen, Oslo University Hospital, Norway
Copyright © 2022 Zhang, Dai, Si, Shi, Li, Jiang, Shen and Ying. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liying Ying, em10Y2NtaXRAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Mengting Zhang
Mengting Zhang Wei Dai1†
Wei Dai1† Yetan Shi
Yetan Shi