- Xiangya School of Pharmaceutical Sciences, Central South University, Changsha, China
Chemotherapy is one of the important means of tumor therapy. However, most of the anti-tumor drugs that currently used in clinic are hydrophobic non-specific drugs, which seriously affect the efficacy of drugs. With the development of nanotechnology, drug efficacy can be improved by selecting appropriate biodegradable nanocarriers for achieving the controlled release, targeting and higher bioavailability of drugs. This paper reviewed the research progress of anti-tumor drug nanoparticle carriers, which mainly summarized the materials used for anti-tumor drug nanoparticle carriers and their effects in anti-tumor drugs, as well as the targeted drug delivery methods of anti-tumor drugs based on nanocarriers.
Introduction
Tumor is still one of the main fatal diseases of human beings, and chemotherapy is one of the main methods to treat tumor in clinic (1). However, most anti-tumor drugs have poor water solubility and low bioavailability (2), which leads to the limited therapeutic effect of drugs on tumor tissues (3). In addition, chemotherapy will have serious toxic and side effects on other normal tissues and cells (4), which not only damages patients’ body function, but also causes patients to develop drug resistance (4), which seriously affects the curative effect. In 1978, Marty first used nanoparticles as drug carriers (5). Nanocarriers are nano-sized carriers based on the concept of targeted drug delivery system (TDDS) (4). At present, it has become a research hotspot due to its advantages of controlled release, targeting, high efficiency, low toxicity and high stability (6). With the development of nanotechnology, the anti-tumor drug with nanoparticles as carriers can achieve controlled release and targeted drug delivery through using special materials and surface modification (4). It can also improve the stability and bioavailability of anti-tumor drugs, overcome the limitations of traditional anti-tumor drugs, which has been widely used. In this review, we introduced main types of nano-drug carrier materials and their effects and discussed the targeted transport modes of nano-drug carriers.
Nano Carriers for Anti-Tumor Drug
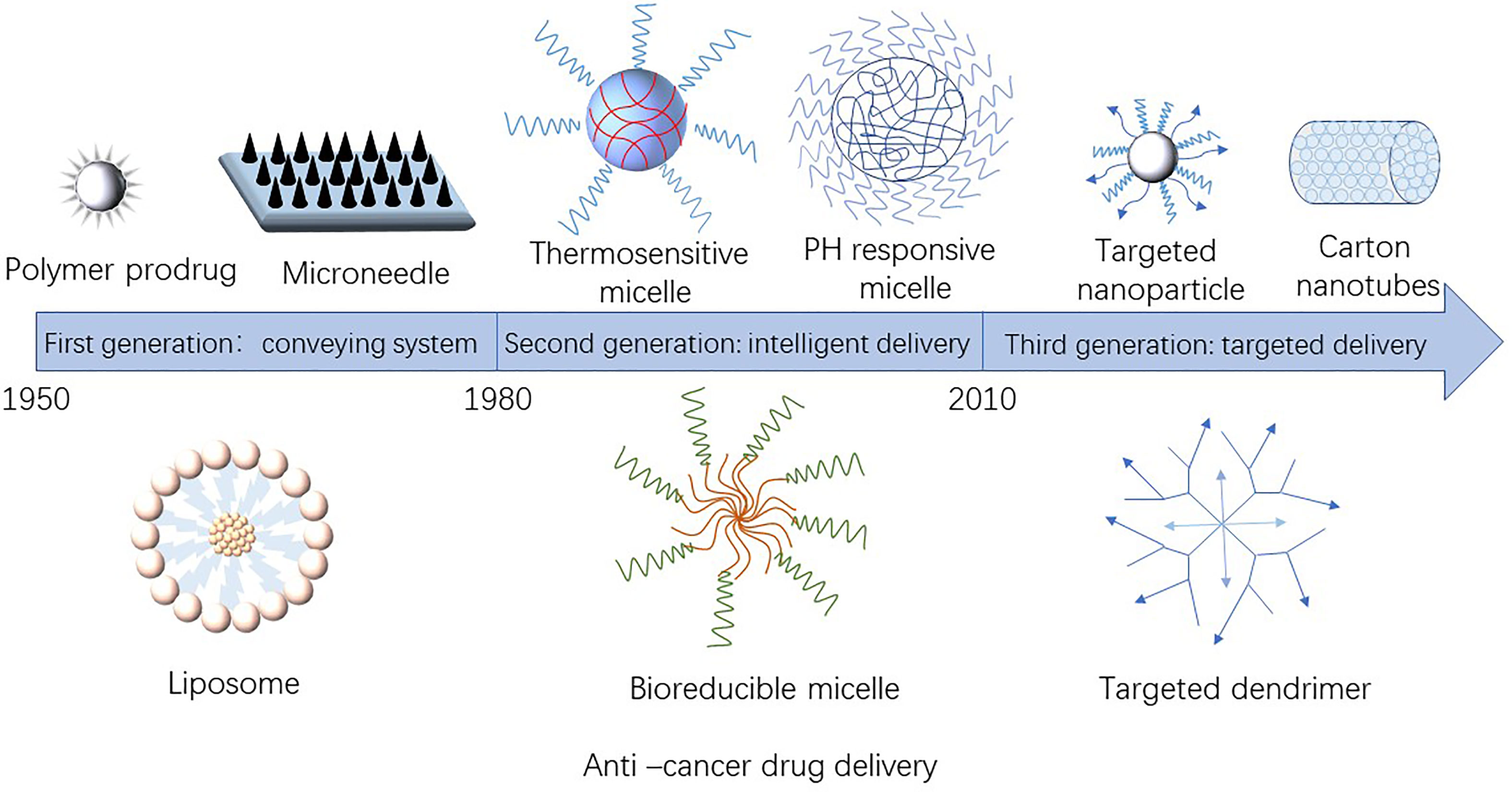
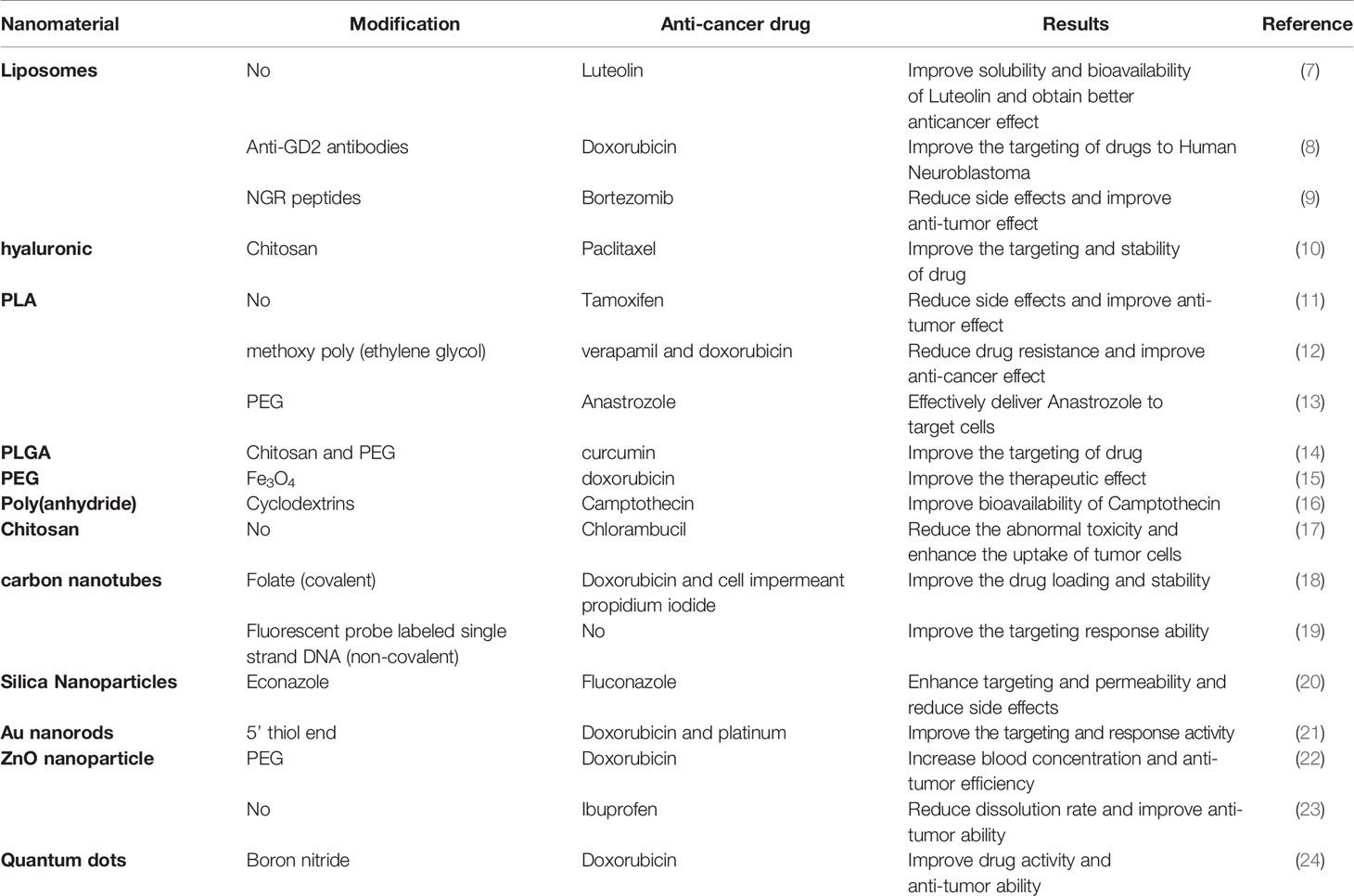
The nanoparticles used as anti-tumor drug loading system have a size of 1–100 nm (4) and mainly include nano-liposomes, nano-polymers, nano-gene carriers, nano-inorganic materials and other drug carriers. Figure 1 showed the development of nano-sized anti-tumor drug carriers. Table 1 presented the materials used as anti-tumor drugs delivery carriers.
Nano-Liposomes
Liposomes are structures, similar to biofilms, composed of hydrophilic head and lipophilic tail, which can encapsulate drugs in aqueous solution to form monolayer or multilayer vesicles (25). Liposomes are considered as promising drug carriers due to their low toxicity, high safety, high biocompatibility, strong drug loading capacity and more flexible regulation of drug release (25–27). Luteolin (LUT) is a kind of natural flavonoids widely distributed in a variety of plants and has anti-tumor activity. Due to the poor water solubility and low bioavailability of luteolin, so Wu et al. (7) prepared liposome LUT with encapsulation efficiency of up to 90%. In vitro studies show that LUT encapsulated by liposome can inhibit tumor growth by inducing apoptosis of tumor cells and has superior anti-tumor effect on mouse colon cancer cell CT26 compared with LUT without liposome.
Nano-Polymers
Nano-polymer carriers have good biocompatibility and biodegradability (28), and have been widely studied in the medical field. Currently, nano-polymer carriers used for anti-tumor drugs mainly divided into natural and synthetic nano-polymer carriers. Natural nano-polymer carriers mainly include hyaluronic acid-based polymers, agarose, collagen and chitosan while synthetic nano-polymer carriers include poly anhydrides, poly (ϵ-caprolactone) (PCL), polylactic acid (PLA), polyethylene glycol (PEG), polyglutamic acid(PGA),and poly D,L-lactide-co-glocolide(PLGA),etc. Among them, PEG with low toxicity have been the focus of research in recent years. PEG is a polymer material obtained by ring-opening polymerization of ethylene oxide. Its main characteristics are controllable polymerization degree, stable structure (29), and can avoid the recognition of human immune system, which has the property of “stealth” in vivo. Genexol-PM® in Korea and Paclical® in Russia are polymer nanodrugs that have been approved clinically, and both of them are polymer nanomedicine formulations of paclitaxel. Furthermore, there are many polymer nano-drugs that have entered preclinical research (30), such as Opaxio and Xyotax.
Nano-Gene Carriers
Gene therapy played an important role in cancer treatment (31). The carrier of gene therapy was the key to the success of gene therapy (32). At present, the commonly used gene vectors mainly include viral vectors and non-viral vectors. The difficulty and high cost in preparing viral vectors and the potential carcinogenicity limit their application in gene therapy (33). As a potential substitute for viral vectors, non-viral vectors are simple to prepare, high in portability and low in toxicity, and most of them are nano-vectors including peptides, liposome, polymers and so on (34–36). Wang et al. (37) prepared a multifunctional tumor therapeutic carrier transport plasmid Cas9-sgPlk-1 by electrostatic interaction with lipid encapsulated gold nanoparticles, which provides a multifunctional method for efficient targeted gene editing and makes nano-gene carriers more widely used in vivo and in vitro experiments.
Inorganic Nanoparticles
In recent years, inorganic nanoparticles have been widely used in tumor imaging and treatment (38) mainly including metals (e.g., gold, zinc, silver and iron nanoparticles), metal oxides (iron and titanium oxide nanoparticles), carbon dots, carbon nanotubes and semiconductors etc. (39, 40). Due to the unique physical and chemical properties and good stability of inorganic nanoparticles, especially optical, magnetic and other physical properties, inorganic nanoparticles may be more suitable for cancer treatment than traditional organic nanocarriers (38). Chen et al. (41) modify Fe3O4 nanoparticles onto carbon nanotubes to provide a double-targeted drug delivery system with about 110% excellent drug-loading capacity for tumor-targeted optical imaging and magnetic-targeted drug delivery. However, the toxicity of inorganic nanoparticles greatly limits its clinical application (42).
Targeted Delivery of Nano-Drug Carriers
Although traditional chemotherapy drugs can kill tumor cells with high efficiency, they also have toxic and side effects on normal tissues due to their lack of specificity while nanotechnology provides a new opportunity for tumor targeted therapy (43). At present, anti-tumor drugs based on nanoparticles can be targeted to transport drugs through three ways: passive transport, active transport and physical and chemical transport, which can identify cancerous tissues more accurately in complex organisms and release them at cancerous tissues to reduce toxic and side effects on normal cells.
Passive Targeted Transport
Passive targeting, mainly through permeation and retention effect(EPR), enables the drug to be swallowed by macrophages as a foreign body immediately after entering the human body, so as to reduce non-specific binding with non-target sites and reach the targeted sites for selective binding (44). Drug carriers, such as liposomes, mainly transported drugs through passive targeting (45). Mitra et al. (46) embedded adriamycin glucan complex in long-circulating nanoparticles, and enriched the drug targeting to the tumor site of mice by EPR effect, so as to achieve the purpose of slow targeting, high efficiency and low toxicity of drugs. At present, many passive targeting nanoparticles have shown promising therapeutic effects in clinical trials, such as Marqibo, Myocet and lysosomes (47).
Active Targeted Transport
The limitation of passive targeting is that it has lower specificity to tumor site, while active targeting has higher targeting. It is found that some antigens or receptors are over-expressed on the surface of tumor cells, while normal cells express them normally (48), such as folate receptor (49), prostate-specific membrane antigen (50), biotin receptors (51), transferrin receptor (52), peptide (53) and the carbonic anhydrase IX (54). Active targeting is based on the specific recognition between receptor and ligand or the covalent modification of targeting groups on the surface. Mackiewicz et al. (55) designed multifunctional poly(ethylene glycol)-block-poly(lactic acid)) nanoparticles modified by folic acid and fluorescent probes, which can achieve the purposes of cell imaging and targeted delivery of anti-tumor drugs at the same time.
Physical and Chemical Targeted Transport
The microenvironment of tumor cells is different from that of normal cells. Based on the unique physical and chemical environment of tumor site, researchers have developed a series of nano-drug carriers with stimulus response, which can achieve targeted release by controlling exogenous stimulus (change of temperature, magnetic field, light or electric pulse) or endogenous stimulus (change of PH value or redox), thereby improving drug efficacy and reducing side effects (56–59).
Magnetic-Responsive Nanocarriers
Since Widder et al. (60) proposed the targeted therapy of magnetic drugs in the 1970s, the research on magnetic targeted drug delivery system (MTDS) has become an important part of the current research on tumor diagnosis and treatment. Magnetic nanoparticles were fixed by external magnetic field, and then heated by alternating magnetic field to kill tumor cells (61). MTDS usually used core-shell nanoparticles (62), magnetic liposomes (63) and nanoporous metal capsules (64) as magnetic responsive nanocarriers. Among them, superparamagnetic iron oxide nano drug-loaded particles have become the current research focus due to their low cytotoxicity, chemical and magnetic stability. Shalaby et al. (65) combined magnetic nanoparticles with adenovirus to transfect them into human fibroma cells under the action of external magnetic field. The results showed that magnetic transfection could significantly inhibit cell proliferation and induce apoptosis.
PH-Responsive Nanocarriers
pH value in tumor cells is generally lower than that in normal tissues. The pH value of normal tissue is about 7.4, while the pH value of tumor extracellular microenvironment is about 6.5~7.2 (66). Researchers have developed pH-responsive drug carrier systems by introducing alkaline polymers containing multiple amino groups into polymers, or acetals, orthoesters, hydrazine bonds that can be broken in acidic environments (67–69). This carrier systems were often used to control drug release in specific organs (such as the gastrointestinal tract or vagina) or organelles (such as nucleosomes or lysosomes) and trigger drug release when microenvironment changes are associated with pathological conditions (70). Deng et al. (71) found that the amino protonation caused by chitosan swelling would lead to the release of tumor necrosis factor-α (TNF-α) encapsulated in chitosan in tumor tissues in local acidic environment.
Temperature-Responsive Nanocarriers
Temperature-responsive nanocarriers maintain structural integrity in normal tissues (37°C), and the drug is well encapsulated in nanomaterials, but the tumor tissue temperature of the patients treated by hyperthermia therapy is as high as 39.5°C, therefore, when the nano-carrier reaches the tumor site, at least one component of the nano-material responds to the nonlinear rapid change of temperature, the structure of the system is destroyed and the drug is released, thus realizing targeted drug delivery at the tumor site (72). Temperature-responsive nanocarriers usually included liposomes and polymer micelles (n-isopropylacrylamide). Shah et al. (73) wrapped photosensitizer tetrakis(hydroxymethyl)phosphonium chloride and anticancer drug doxorubicin in hydrophobic lipid bilayer membrane, and wrapped magnetic nanoparticles in hydrophilic inner capsule, realizing simultaneous magnetocaloric therapy, photodynamic therapy and chemotherapy. Experimental results show that combined therapy can almost eliminate completely cancer cells, and the therapeutic effect is remarkable.
Photo-Responsive Nano-Carrier
The photo-responsive nano-carrier can respond to specific wavelength light to achieve targeted drug delivery (74). The photosensitive azobenzene group and its derivatives can be optically isomerized from trans to cis under the irradiation of 300-380 nm, and can also be optically isomerized from cis to trans in the visible region, which makes it possible to optically control drug release (75). Because soft tissue has strong scattering in the ultraviolet visible region less than 700 nm, the penetration depth of light-responsive nanocarriers is low (~ 10 mm), which is only suitable for body parts that can be directly irradiated (such as eyes and skin, etc.) (74). Today, near infrared lasers (NIR) with wavelengths ranging from 700 to 1000 nm have wide clinical applications due to their low scattering, deep tissue penetration, and micro-tissue damage (76). YOU et al. (77) designed and synthesized multifunctional doxorubicin hollow gold nanoparticles, which accelerated the release of drugs under the irradiation of near infrared light. Compared with traditional chemotherapy methods, the anti-cancer activity was increased, and the systemic toxicity was reduced, proving that the NIR technology has a broad prospect.
Redox-Responsive Nanocarriers
Concentration of Glutathione (GSH) in tumor cells was about several hundred times higher than that in extracellular cells. In general, redox-responsive nanocarriers can intelligently target drug release in tumor cells by introducing disulfide or diselenide bonds (78), which is of great significance to many drug molecules (e. g. camptothecin, doxorubicin, etc.) that exert their effects in the organelles of tumor cells. Disulfide bonds are very stable under normal physiological condition but will be reduced and broken in the presence of high concentration GSH in tumor cells (79). Based on this principle, Wang et al. (80) developed Camptothecin (CPT) conjugated core cross-linked micelles that can break down disulfide bonds by oxidation-reduction, thus, destroying the micelle structure and releasing CPT rapidly. In vitro cytotoxicity study showed that the anti-cancer activity of redox-responsive core cross-linked micelles was significantly higher than that of nonresponsive micelles.
Conclusion and Prospect
Great progress has been made in the research of nano anti-tumor drug carrier, which effectively overcomes the limitations of poor solubility, low bioavailability and non-specificity of traditional chemotherapy drugs, and obviously improves the curative effect of drugs. At present, nano-carriers for anti-tumor drugs mainly include nano-liposomes, nano-polymers and nano-gene carriers, which belong to nano-organic materials and nano-inorganic materials. The application of anti-tumor drug nanocarriers can truly achieve targeted drug delivery at the focus, and its targeted transportation modes mainly include passive transportation, active transportation and physical and chemical transportation (according to the changes of temperature, magnetic field, light or electric pulse, PH and redox). However, most of the research on nano anti-tumor drug carrier focuses on the basic theory, and few drugs can be used clinically, which limits the wide application of nano anti-tumor drug carriers (81–83). In the future, researchers need to continuously explore and design nanoporous carriers of anti-tumor drugs with high drug loading, high efficiency, low toxicity, low cost and clinical application value. The modification of nanoparticles is combined with single surface group modification and combined physicochemical modification so as to establish a new route of administration, which is conducive to the interdisciplinary comprehensive research and maximize its scientific value and market value.
Author Contributions
JX wrote the paper. ZR participated in the design and drafting of this manuscript. BW, XS and XG performed the literature search and revised the paper. WL and ST guided the writing of the paper and reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a shared affiliation, though no other collaboration with the authors at the time of the review.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
JX and RZ contributed to this work and should be considered co-first authors.
References
1. Nygren P. What Is Cancer Chemotherapy? Acta Oncol (2001) 40(2–3):166–74. doi: 10.1080/02841860151116204
2. Chen H, Zheng Y, Tian G, Tian Y, Zeng X, Liu G, et al. Oral Delivery of DMAB-Modified Docetaxel-Loaded PLGA-TPGS Nanoparticles for Cancer Chemotherapy. Nanoscale Res Lett (2011) 6(1):4. doi: 10.1007/s11671-010-9741-8
3. Jain KK. An Overview of Drug Delivery Systems. Methods Mol Biol (2020) 1–54. doi: 10.1007/978-1-4939-9798-5_1
4. Van Vlerken LE, Amiji MM. Multi-Functional Polymeric Nanoparticles for Tumour-Targeted Drug Delivery. Expert Opin Drug Deliv (2006) 3(2):205–16. doi: 10.1517/17425247.3.2.205
5. Kreuter J. Nanoparticles—A Historical Perspective. Int J Pharmaceut (2007) 331(1):1–10. doi: 10.doi:10.1016/j.ijpharm.2006.10.021
6. Saltzman WM, Fung LK. Polymeric Implants for Cancer Chemotherapy. Adv Drug Deliv Rev (1997) 26(2-3):209–30. doi: 10.1016/s0169-409x(97)00036-7
7. Wu G, Li J, Yue J, Zhang S, Yunusi K. Liposome Encapsulated Luteolin Showed Enhanced Antitumor Efficacy to Colorectal Carcinoma. Mol Med Rep (2018) 17(2):2456–64. doi: 10.3892/mmr.2017.8185
8. Pastorino F, Brignole C, Marimpietri D, Sapra P, Moase EH, Allen TM, et al. Doxorubicin-Loaded Fab’ Fragments of Anti-Disialoganglioside Immunoliposomes Selectively Inhibit the Growth and Dissemination of Human Neuroblastoma in Nude Mice. Cancer Res (2003) 63(1):86–92. doi: 10.1186/s13054-015-0752-9
9. Zuccari G, Milelli A, Pastorino F, Loi M, Petretto A, Parise A, et al. Tumor Vascular Targeted Liposomal-Bortezomib Minimizes Side Effects and Increases Therapeutic Activity in Human Neuroblastoma. J Control Release (2015) 211:44–52. doi: 10.1016/j.jconrel.2015.05.286
10. Li J, Huang P, Chang L, Long X, Dong A, Liu J, et al. Tumor Targeting and pH-Responsive Polyelectrolyte Complex Nanoparticles Based on Hyaluronic Acid-Paclitaxel Conjugates and Chitosan for Oral Delivery of Paclitaxel. Macromol Res (2013) 21(12):1331–7. doi: 10.1007/s13233-013-1171-x
11. Pandey SK, Ghosh S, Maiti P, Haldar C. Therapeutic Efficacy and Toxicity of Tamoxifen Loaded PLA Nanoparticles for Breast Cancer. Int J Biol Macromol (2015) 72:309–19. doi: 10.1016/j.ijbiomac.2014.08.012
12. Zheng W, Li M, Lin Y, Zhan X. Encapsulation of Verapamil and Doxorubicin by MPEG-PLA to Reverse Drug Resistance in Ovarian Cancer. Biomed Pharmacother (2018) 108:565–73. doi: 10.1016/j.biopha.2018.09.039
13. Goudarzi F, Asadi A, Afsharpour M, Jamadi RH. In Vitro Characterization and Evaluation of the Cytotoxicity Effects of Nisin and Nisin-Loaded PLA-PEG-PLA Nanoparticles on Gastrointestinal (AGS and KYSE-30), Hepatic (HepG2) and Blood (K562) Cancer Cell Lines. AAPS PharmSciTech (2018) 19(4):1554–66. doi: 10.1208/s12249-018-0969-4
14. Arya G, Das M, Sahoo SK. Evaluation of Curcumin Loaded Chitosan/PEG Blended PLGA Nanoparticles for Effective Treatment of Pancreatic Cancer. Biomed Pharmacother (2018) 102:555–66. doi: 10.1016/j.biopha.2018.03.101
15. Dutta B, Shetake NG, Gawali SL, Barick BK, Barick KC, Babu PD, et al. PEG Mediated Shape-Selective Synthesis of Cubic Fe 3 O 4 Nanoparticles for Cancer Therapeutics. J Alloy Compd (2018) 737:347–55. doi: 10.1016/j.jallcom.2017.12.028
16. Huarte J, Espuelas S, Lai Y, He B, Tang J, Irache JM. Oral Delivery of Camptothecin Using Cyclodextrin/Poly(Anhydride) Nanoparticles. Int J Pharm (2016) 506(1-2):116–28. doi: 10.1016/j.ijpharm.2016.04.045
17. Shayegh A, Khalatbari F, Zonoubi N, Zarazvand F, Monavvari F, Hejazinia H, et al. Chlorambucil-Chitosan Nano-Conjugate: An Efficient Agent Against Breast Cancer Targeted Therapy. Curr Drug Deliv (2021). doi: 10.2174/1567201817666201027122620
18. Kapri S, Maiti S, Bhattacharyya S. Lemon Grass Derived Porous Carbon Nanospheres Functionalized for Controlled and Targeted Drug Delivery. Carbon (2016) 100:223–35. doi: 10.1016/j.carbon.2016.01.017
19. Li C, Meng Y, Wang S, Qian M, Wang J, Lu W, et al. Mesoporous Carbon Nanospheres Featured Fluorescent Aptasensor for Multiple Diagnosis of Cancer In Vitro and In Vivo. ACS Nano (2015) 9(12):12096–103. doi: 10.1021/acsnano.5b05137
20. Firooz A, Nafisi S, Maibach HI. Novel Drug Delivery Strategies for Improving Econazole Antifungal Action. Int J Pharm (2015) 495(1):599–607. doi: 10.1016/j.ijpharm.2015.09.015
21. Shanmugam V, Chien YH, Cheng YS, Liu TY, Huang CC, Su CH, et al. Oligonucleotides–assembled Au Nanorod-Assisted Cancer Photothermal Ablation and Combination Chemotherapy With Targeted Dual-Drug Delivery of Doxorubicin and Cisplatin Prodrug. ACS Appl Mater Interfaces (2014) 6(6):4382–93. doi: 10.1021/am5000905
22. Alarifi S, Ali D, Alkahtani S, Verma A, Ahamed M, Ahmed M, et al. Induction of Oxidative Stress, DNA Damage, and Apoptosis in a Malignant Human Skin Melanoma Cell Line After Exposure to Zinc Oxide Nanoparticles. Int J Nanomedicine (2013) 8:983–93. doi: 10.2147/ijn.S42028
23. Condello M, De Berardis B, Ammendolia MG, Barone F, Condello G, Degan P, et al. ZnO Nanoparticle Tracking From Uptake to Genotoxic Damage in Human Colon Carcinoma Cells. Toxicol In Vitro (2016) 35:169–79. doi: 10.1016/j.tiv.2016.06.005
24. Umrao S, Maurya AK, Shukla V, Grigoriev A, Ahuja R, Vinayak M, et al. Anticarcinogenic Activity of Blue Fluorescent Hexagonal Boron Nitride Quantum Dots: As an Effective Enhancer for DNA Cleavage Activity of Anticancer Drug Doxorubicin. Mater Today Bio (2019) 1:100001. doi: 10.1016/j.mtbio.2019.01.001
25. Tapeinos C, Battaglini M, Ciofani ,G. Advances in the Design of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Targeting Brain Diseases. J Control Release (2017) 264:306–32. doi: 10.1016/j.jconrel.2017.08.033
26. Zhao S, Minh LV, Li N, Garamus VM, Handge UA, Liu J, et al. Doxorubicin Hydrochloride-Oleic Acid Conjugate Loaded Nanostructured Lipid Carriers for Tumor Specific Drug Release. Colloids Surf B Biointerfaces (2016) 145:95–103. doi: 10.1016/j.colsurfb.2016.04.027
27. Patil A, Lakhani P, Taskar P, Wu KW, Sweeney C, Avula B, et al. Formulation Development, Optimization, and In Vitro-In Vivo Characterization of Natamycin-Loaded PEGylated Nano-Lipid Carriers for Ocular Applications. J Pharm Sci (2018) 107(8):2160–71. doi: 10.1016/j.xphs.2018.04.014
28. Astete CE, Sabliov CM. Synthesis and Characterization of PLGA Nanoparticles. Biomat Sci-Polym E (2006) 17(3):247–89. doi: 10.1163/156856206775997322
29. Barratt G. Colloidal Drug Carriers: Achievements and Perspectives. Cell Mol Life Sci (2003) 60(1):21–37. doi: 10.1007/s000180300002
30. Palazzolo S, Bayda S, Hadla M, Caligiuri I, Corona G, Toffoli G, et al. The Clinical Translation of Organic Nanomaterials for Cancer Therapy: A Focus on Polymeric Nanoparticles, Micelles, Liposomes and Exosomes. Curr Med Chem (2018) 25(34):4224–68. doi: 10.2174/0929867324666170830113755
31. Krishnagopal A, Reddy A, Sen D. Stent-Mediated Gene and Drug Delivery for Cardiovascular Disease and Cancer: A Brief Insight. J Gene Med (2017) 19(5):e2954. doi: 10.1002/jgm.2954
32. Kohn DB, Sadelain M, Glorioso JC. Occurrence of Leukaemia Following Gene Therapy of X-Linked SCID. Nat Rev Cancer (2003) 3(7):477–88. doi: 10.1038/nrc1122
33. Robbins PD, Ghivizzani SC. Viral Vectors for Gene Therapy. Pharmacol Therapeut (1998) 80(1):35–47. doi: 10.1016/S0163-7258(98)00020-5
34. Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG. Non-viral vectors for gene-based therapy. Nat Rev Genet (2014) 15(8):541–55. doi: 10.1038/nrg3763
35. Zhang Y, Satterlee A, Huang L. In Vivo Gene Delivery by Nonviral Vectors: Overcoming Hurdles? Mol Ther (2012) 20(7):1298–304. doi: 10.1038/mt.2012.79
36. Fang H, Feng Y, Chen J, Tian H, Chen X. Constructing Efficient Polycationic Gene Carriers Through Regulating the Physicochemical Properties. Mater Today Chem (2019) 11:269–82. doi: 10.1016/j.mtchem.2018.11.007
37. Wang P, Zhang L, Zheng W, Cong L, Guo Z, Xie Y, et al. Thermo-Triggered Release of CRISPR-Cas9 System by Lipid-Encapsulated Gold Nanoparticles for Tumor Therapy. Angew Chem Int Ed Engl (2018) 57(6):1491–6. doi: 10.1002/anie.201708689
38. Huang HC, Barua S, Sharma G, Dey SK, Rege K. Inorganic Nanoparticles for Cancer Imaging and Therapy. J Control Release (2011) 155(3):344–57. doi: 10.1016/j.jconrel.2011.06.004
39. Bayda S, Hadla M, Palazzolo S, Riello P, Corona G, Toffoli G, et al. Inorganic Nanoparticles for Cancer Therapy: A Transition From Lab to Clinic. Curr Med Chem (2018) 25(34):4269–303. doi: 10.2174/0929867325666171229141156
40. Pugazhendhi A, Edison T, Karuppusamy I, Kathirvel B. Inorganic Nanoparticles: A Potential Cancer Therapy for Human Welfare. Int J Pharm (2018) 539(1-2):104–11. doi: 10.1016/j.ijpharm.2018.01.034
41. Chen ML, He YJ, Chen XW, Wang JH. Quantum Dots Conjugated With Fe3O4-Filled Carbon Nanotubes for Cancer-Targeted Imaging and Magnetically Guided Drug Delivery. Langmuir (2012) 28(47):16469–76. doi: 10.1021/la303957y
42. Winnik FM, Maysinger D. Quantum Dot Cytotoxicity and Ways to Reduce it. Acc Chem Res (2013) 46(3):672–80. doi: 10.1021/ar3000585
43. You C, Wu H, Wang M, Wang S, Shi T, Luo Y, et al. A Strategy for Photothermal Conversion of Polymeric Nanoparticles by Polyaniline for Smart Control of Targeted Drug Delivery. Nanotechnology (2017) 28(16):165102. doi: 10.1088/1361-6528/aa645f
44. Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor Vascular Permeability and the EPR Effect in Macromolecular Therapeutics: A Review. J Control Release (2000) 65(1-2):271–84. doi: 10.1016/S0168-3659(99)00248-5
45. Nichols JW, Bae YH. EPR: Evidence and Fallacy. J Control Release (2014) 190:451–64. doi: 10.1016/j.jconrel.2014.03.057
46. Mitra S, Gaur U, Ghosh PC, Maitra AN. Tumour Targeted Delivery of Encapsulated Dextran-Doxorubicin Conjugate Using Chitosan Nanoparticles as Carrier. J Control Release (2001) 74(1-3):317–23. doi: 10.1016/s0168-3659(01)00342-x
47. Subhan MA, Yalamarty SSK, Filipczak N, Parveen F, Torchilin VP. Recent Advances in Tumor Targeting via EPR Effect for Cancer Treatment. J Pers Med (2021) 11(6):571. doi: 10.3390/jpm11060571
48. Lu Y, Low PS. Folate-Mediated Delivery of Macromolecular Anticancer Therapeutic Agents. Adv Drug Deliv Rev (2002) 54(5):675–93. doi: 10.1016/s0169-409x(02)00042-x
49. Elnakat H, Ratnam M. Role of Folate Receptor Genes in Reproduction and Related Cancers. Front Biosci (2006) 11:506–19. doi: 10.2741/1815
50. Kularatne SA, Zhou Z, Yang J, Post CB, Low PS. Design, Synthesis, and Preclinical Evaluation of Prostate-Specific Membrane Antigen Targeted (99m)Tc-Radioimaging Agents. Mol Pharm (2009) 6(3):790–800. doi: 10.1021/mp9000712
51. Bhuniya S, Maiti S, Kim EJ, Lee H, Sessler JL, Hong KS, et al. An Activatable Theranostic for Targeted Cancer Therapy and Imaging. Angew Chem Int Ed Engl (2014) 53(17):4469–74. doi: 10.1002/anie.201311133
52. Högemann-Savellano D, Bos E, Blondet C, Sato F, Abe T, Josephson L, et al. The Transferrin Receptor: A Potential Molecular Imaging Marker for Human Cancer. Neoplasia (2003) 5(6):495–506. doi: 10.1016/s1476-5586(03)80034-9
53. Reubi JC. Peptide Receptors as Molecular Targets for Cancer Diagnosis and Therapy. Endocr Rev (2003) 24(4):389–427. doi: 10.1210/er.2002-0007
54. Krall N, Pretto F, Decurtins W, Bernardes GJ, Supuran CT, Neri D. A Small-Molecule Drug Conjugate for the Treatment of Carbonic Anhydrase IX Expressing Tumors. Angew Chem Int Ed Engl (2014) 53(16):4231–5. doi: 10.1002/anie.201310709
55. Mackiewicz N, Nicolas J, Handke N, Noiray M, Mougin J, Daveu C, et al. Precise Engineering of Multifunctional PEGylated Polyester Nanoparticles for Cancer Cell Targeting and Imaging. Chem Mater (2014) 26(5):1834–47. doi: 10.1021/cm403822w
56. Thambi T, Son S, Lee DS, Park JH. Poly(ethylene Glycol)-B-Poly(Lysine) Copolymer Bearing Nitroaromatics for Hypoxia-Sensitive Drug Delivery. Acta Biomater (2016) 29:261–70. doi: 10.1016/j.actbio.2015.10.011
57. Thambi T, Deepagan VG, Yoon HY, Han HS, Kim SH, Son S, et al. Hypoxia-Responsive Polymeric Nanoparticles for Tumor-Targeted Drug Delivery. Biomaterials (2014) 35(5):1735–43. doi: 10.1016/j.biomaterials.2013.11.022
58. Zhang YJ, Gallis B, Taya M, Wang S, Ho RJ, Sasaki T. pH-Responsive Artemisinin Derivatives and Lipid Nanoparticle Formulations Inhibit Growth of Breast Cancer Cells In Vitro and Induce Down-Regulation of HER Family Members. PLoS One (2013) 8(3):e59086. doi: 10.1371/journal.pone.0059086
59. Zelzer M, Todd SJ, Hirst AR, Mcdonald TO, Ulijn RV. Enzyme Responsive Materials: Design Strategies and Future Developments. Biomater Sci (2013) 1(1):11–39. doi: 10.1039/c2bm00041e
60. Widder KJ, Senyel AE, Scarpelli GD. Magnetic Microspheres: A Model System of Site Specific Drug Delivery In Vivo. Proc Soc Exp Biol Med (1978) 158(2):141–6. doi: 10.3181/00379727-158-40158
61. Yang HW, Hua MY, Liu HL, Huang CY, Tsai RY, Lu YJ, et al. Self-Protecting Core-Shell Magnetic Nanoparticles for Targeted, Traceable, Long Half-Life Delivery of BCNU to Gliomas. Biomaterials (2011) 32(27):6523–32. doi: 10.1016/j.biomaterials.2011.05.047
62. Zhang L, Wang T, Yang L, Liu C, Wang C, Liu H, et al. General Route to Multifunctional Uniform Yolk/Mesoporous Silica Shell Nanocapsules: A Platform for Simultaneous Cancer-Targeted Imaging and Magnetically Guided Drug Delivery. Chemistry (2012) 18(39):12512–21. doi: 10.1002/chem.201200030
63. Yang C, Rait A, Pirollo KF, Dagata JA, Farkas N, Chang EH. Nanoimmunoliposome Delivery of Superparamagnetic Iron Oxide Markedly Enhances Targeting and Uptake in Human Cancer Cells In Vitro and In Vivo. Nanomedicine (2008) 4(4):318–29. doi: 10.1016/j.nano.2008.05.004
64. Zhang F, Braun GB, Pallaoro A, Zhang Y, Shi Y, Cui D, et al. Mesoporous Multifunctional Upconversion Luminescent and Magnetic “Nanorattle” Materials for Targeted Chemotherapy. Nano Lett (2012) 12(1):61–7. doi: 10.1021/nl202949y
65. Shalaby SM, Khater MK, Perucho AM, Mohamed SA, Helwa I, Laknaur A, et al. Magnetic Nanoparticles as a New Approach to Improve the Efficacy of Gene Therapy Against Differentiated Human Uterine Fibroid Cells and Tumor-Initiating Stem Cells. Fertil Steril (2016) 105(6):1638–1648.e1638. doi: 10.1016/j.fertnstert.2016.03.001
66. Stubbs M, Mcsheehy PM, Griffiths JR, Bashford CL. Causes and Consequences of Tumour Acidity and Implications for Treatment. Mol Med Today (2000) 6(1):15–9. doi: 10.1016/s1357-4310(99)01615-9
67. Du JZ, Du XJ, Mao CQ, Wang J. Tailor-Made Dual pH-Sensitive Polymer-Doxorubicin Nanoparticles for Efficient Anticancer Drug Delivery. J Am Chem Soc (2011) 133(44):17560–3. doi: 10.1021/ja207150n
68. Qiu L, Zhu M, Gong K, Peng H, Ge L, Zhao L, et al. pH-Triggered Degradable Polymeric Micelles for Targeted Anti-Tumor Drug Delivery. Mater Sci Eng C Mater Biol Appl (2017) 78:912–22. doi: 10.1016/j.msec.2017.04.137
69. Zheng L, Zhang X, Wang Y, Liu F, Peng J, Zhao X, et al. Fabrication of Acidic pH-Cleavable Polymer for Anticancer Drug Delivery Using a Dual Functional Monomer. Biomacromolecules (2018) 19(9):3874–82. doi: 10.1021/acs.biomac.8b01001
70. Sawant RM, Hurley JP, Salmaso S, Kale A, Tolcheva E, Levchenko TS, et al. "SMART" Drug Delivery Systems: Double-Targeted pH-Responsive Pharmaceutical Nanocarriers. Bioconjug Chem (2006) 17(4):943–9. doi: 10.1021/bc060080h
71. Deng Z, Zhen Z, Hu X, Wu S, Xu Z, Chu PK. Hollow Chitosan-Silica Nanospheres as pH-Sensitive Targeted Delivery Carriers in Breast Cancer Therapy. Biomaterials (2011) 32(21):4976–86. doi: 10.1016/j.biomaterials.2011.03.050
72. Fan T, Li M, Wu X, Li M, Wu Y. Preparation of Thermoresponsive and pH-Sensitivity Polymer Magnetic Hydrogel Nanospheres as Anticancer Drug Carriers. Colloids Surf B Biointerfaces (2011) 88(2):593–600. doi: 10.1016/j.colsurfb.2011.07.048
73. Shah SA, Aslam Khan MU, Arshad M, Awan SU, Hashmi MU, Ahmad N. Doxorubicin-Loaded Photosensitive Magnetic Liposomes for Multi-Modal Cancer Therapy. Colloids Surf B Biointerfaces (2016) 148:157–64. doi: 10.1016/j.colsurfb.2016.08.055
74. Fomina N, Sankaranarayanan J, Almutairi A. Photochemical Mechanisms of Light-Triggered Release From Nanocarriers. Adv Drug Deliv Rev (2012) 64(11):1005–20. doi: 10.1016/j.addr.2012.02.006
75. Schulze-Lefert P, Becker-André M, Schulz W, Hahlbrock K, Dangl JL. Functional Architecture of the Light-Responsive Chalcone Synthase Promoter From Parsley. Plant Cell (1989) 1(7):707–14. doi: 10.1105/tpc.1.7.707
76. Yang J, Lee J, Kang J, Oh SJ, Ko HJ, Son JH, et al. Smart Drug-Loaded Polymer Gold Nanoshells for Systemic and Localized Therapy of Human Epithelial Cancer. Adv Mater (2009) 21(43):4339–42. doi: 10.1002/adma.200900334
77. You J, Zhang R, Xiong C, Zhong M, Melancon M, Gupta S, et al. Effective Photothermal Chemotherapy Using Doxorubicin-Loaded Gold Nanospheres That Target EphB4 Receptors in Tumors. Cancer Res (2012) 72(18):4777–86. doi: 10.1158/0008-5472.Can-12-1003
78. Xia J, Du Y, Huang L, Chaurasiya B, Tu J, Webster TJ, et al. Redox-Responsive Micelles From Disulfide Bond-Bridged Hyaluronic Acid-Tocopherol Succinate for the Treatment of Melanoma. Nanomedicine (2018) 14(3):713–23. doi: 10.1016/j.nano.2017.12.017
79. Li Y, Liu T, Zhang G, Ge Z, Liu S. Tumor-Targeted Redox-Responsive Nonviral Gene Delivery Nanocarriers Based on Neutral-Cationic Brush Block Copolymers. Macromol Rapid Commun (2014) 35(4):466–73. doi: 10.1002/marc.201300719
80. Wang H, Tang L, Tu C, Song Z, Yin Q, Yin L, et al. Redox-Responsive, Core-Cross-Linked Micelles Capable of on-Demand, Concurrent Drug Release and Structure Disassembly. Biomacromolecules (2013) 14(10):3706–12. doi: 10.1021/bm401086d
81. Lammers T, Kiessling F, Hennink WE, Storm G. Drug Targeting to Tumors: Principles, Pitfalls and (Pre-) Clinical Progress. J Control Release (2012) 161(2):175–87. doi: 10.1016/j.jconrel.2011.09.063
82. Bae Y, Kataoka K. Intelligent Polymeric Micelles From Functional Poly(Ethylene Glycol)-Poly(Amino Acid) Block Copolymers. Adv Drug Deliv (2009) 61(10):768–84. doi: 10.1016/j.addr.2009.04.016
Keywords: cancer, chemotherapy, antitumor drug, nano drug carrier, targeted transportation
Citation: Xiang J, Zhao R, Wang B, Sun X, Guo X, Tan S and Liu W (2021) Advanced Nano-Carriers for Anti-Tumor Drug Loading. Front. Oncol. 11:758143. doi: 10.3389/fonc.2021.758143
Received: 13 August 2021; Accepted: 30 August 2021;
Published: 16 September 2021.
Edited by:
Kelong Ai, Central South University, ChinaCopyright © 2021 Xiang, Zhao, Wang, Sun, Guo, Tan and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Songwen Tan, c29uZ3dlbi50YW5AY3N1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Jia Xiang†
Jia Xiang† Songwen Tan
Songwen Tan