
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 14 December 2021
Sec. Pharmacology of Anti-Cancer Drugs
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.755439
 Dennis Christoph Harrer1*
Dennis Christoph Harrer1* Sebastian Buschauer1
Sebastian Buschauer1 Ulrich Sterz1
Ulrich Sterz1 Karin Menhart2
Karin Menhart2 Christina Wendl3
Christina Wendl3 Daniel Heudobler1
Daniel Heudobler1 Matthias Grube1
Matthias Grube1 Tobias Pukrop1
Tobias Pukrop1 Wolfgang Herr1
Wolfgang Herr1 Martin Vogelhuber1
Martin Vogelhuber1Background: Metastasized soft-tissue sarcomas still pose a significant therapeutic challenge given the limited efficacy of currently available multimodal treatment strategies. Recent progress in molecular characterization of sarcoma subtypes has enabled successful personalized therapy approaches in a minority of selected patients with targetable mutations. However, in the majority of patients with refractory soft tissue sarcomas, long-term survival remains poor.
Methods: We report on three adult patients with various soft tissue sarcomas subjected to Gemcitabine maintenance therapy. Tumor entities included leiomyosarcoma of the pancreas (patient 1), undifferentiated pleomorphic sarcoma of the right femur (patient 2), and peri-aortic leiomyosarcoma (patient 3). Metastatic sites encompassed liver, lung, and bones. All patients received Gemcitabine maintenance therapy until disease progression following prior salvage chemotherapy with Docetaxel and Gemcitabine. Patients were treated outside of clinical trials. Response assessment was based on radiological imaging.
Results: In response to salvage chemotherapy with Docetaxel and Gemcitabine, one patient exhibited a partial remission, and two patients showed stable disease. Patient 1 exhibited stable disease for 6 months during Gemcitabine maintenance therapy before suffering rapid progression of hepatic metastases. Patient 2 underwent 21 months of Gemcitabine maintenance therapy, which was discontinued after progressive pulmonary metastases were detected. Patient 3 is still being treated with Gemcitabine maintenance therapy. Remarkably, owing to significant chemotherapy-associated hematotoxicity, the dose of Gemcitabine dose was reduced by two-thirds. Nevertheless, stable disease with constant pulmonary metastases has been maintained in this patient for 14 months.
Conclusions: Gemcitabine maintenance therapy following prior Docetaxel and Gemcitabine chemotherapy is manageable and reveals potential benefits for patients with aggressive metastasized soft tissue sarcomas. Prospective trials evaluating Gemcitabine maintenance therapy are encouraged.
Despite multiple new anticancer agents available, the long-term survival of patients with advanced sarcomas remains dismal (1, 2). Soft tissue sarcomas (STS) represent a heterogeneous class of rare tumors originating from different soft connective tissues, such as smooth muscle or fat (3). Overall, STS comprise <1% of all adult malignancies amounting to 12,000 new cases per year in the US (2). While the majority of STS is located in the extremities, they can arise from virtually any site of the body (3). Metastatic spread occurs frequently into the lungs followed by the liver and bones (3). Distant metastases are associated with a bad prognosis reflected by 5-year survival rates of about 15%, whereas patients with local STS exhibit 5-year survival of approximately 80% (2). The paramount prognostic factors are the histological subtype, tumor–node–metastasis (TNM) stage, and surgical resection margins (2).
Current treatment strategies adopt a multimodal approach combining surgery, radiotherapy, chemotherapy, and targeted therapy (2, 4). Chemotherapy agents with efficacy in soft tissue sarcomas encompass Doxorubicin (5), Ifosfamide (6), Gemcitabine (7), Docetaxel (8), Dacarbazine (9), Eribulin (10), and Trabectedin (11). Targeted therapy approaches include tyrosine kinase inhibitors, such as the Pazopanib and the multikinase inhibitor Regorafenib (12, 13). In case of detectable fusion proteins involving neurotrophic receptor tyrosine kinase (NTRK) 1–3 present in <1% of all STS (14), second-line treatment with the NTRK inhibitor Larotrectinib could mediate tumor regression in the majority of patients with 16% of complete responders (15). The monoclonal antibody Olaratumab directed against the platelet-derived growth factor alpha (PDGFR-alpha) was initially approved for STS in combination with Doxorubicin but was later withdrawn from the market due to lacking efficacy in a phase III clinical trial (16). In aggregate, specific treatment strategies are devised based on histological subtypes, disease stage, and patient condition. Currently, a multitude of histologic subtypes of STS has been described (17).
Malignant tumors arising from smooth muscle tissue are denoted as leiomyosarcomas and account for 10% of all STS, making them one of the most common subtype in adults (18). Predilection sites for leiomyosarcomas are the extremities followed by the uterus, small intestine, and retroperitoneal space (19). Given an insufficient sensitivity to chemotherapy and radiation, surgical excision with wide resections margins constitutes the standard of care in non-uterine leiomyosarcoma treatment (20, 21). Relapse after resection or primary irresectable disease bodes prognostically ill, with 5-year survival rates approximately 10–15% (2, 22). Chemotherapeutic agents with activity in advanced leiomyosarcoma include Doxorubicin (5), Ifosfamide (6), Dacarbazine (9), and Trabectedin (23). Moreover, monotherapy with Pazopanib demonstrated clinically relevant efficacy and tolerability in patients with leiomyosarcoma (12).
In patients with metastatic/advanced STS, systemic therapy aims at reducing tumor burden, thereby alleviating symptoms and improving quality of life (24). However, many STS exhibit primary or acquired resistance to chemotherapy resulting in only short periods of tumor control achieved by systemic treatment (25). Moreover, the toxicity/efficacy ratio of potentially aggressive systemic treatments should be considered with regards to maintaining quality of life during this palliative treatment. Thus, continuation of systemic therapy with dose-reduced maintenance therapy intended to preserve beneficial responses (complete or partial response or stable disease) seems worth exploring in patients with STS. In general, maintenance therapy aims at slowing down disease progression and at improving quality of life. In clinical practice, maintenance therapy could be administered either until disease progression or until the occurrence of major toxicity. As for patients with metastatic/advanced soft tissue sarcomas, there is currently no recommended maintenance therapy, apart from combination maintenance therapy with Cyclophosphamide and Vinorelbine in localized high-risk rhabdomyosarcoma (24, 26). Moreover, only few studies evaluating the efficacy of maintenance therapy in patients with STS have been conducted (24).
In this study, we present a retrospective analysis of three patients with metastasized STS who underwent maintenance therapy with Gemcitabine on an off-label basis at the University Hospital of Regensburg. Gemcitabine maintenance therapy administered after prior Docetaxel and Gemcitabine chemotherapy showed potential to slow down tumor progression in all three patients without compromising quality of life to a significant extent.
Sarcoma diagnosis was confirmed in all three patients by histological examination [hematoxylin and eosin (H&E) staining and immunohistochemistry] and molecular analysis (fluorescence in situ hybridization) of biopsies obtained via excision or imaging-guided puncture. Tumor response was assessed by computed tomography (CT), magnetic resonance imaging (MRI), and F-18-fluorodeoxyglucose–positron emission tomography (PET)/computed tomography (CT). Patients were treated at the Sarcoma Center of the University Hospital of Regensburg between 2014 and 2021. All patients received treatment independent of clinical trials. Data analysis was carried out retrospectively. The study was approved by the Ethics Committee of the University Regensburg (ethics statement no. 21-2532-104). Initial Gemcitabine dosing varied from 900 mg/m2 (d1 and d8 with repetition d22) to 500 mg/m2 (d1, d15 with repetition d29) and was individually adapted during the course of treatment (Table 1). Clinical outcomes were tracked until June of 2021. Written, informed consent to publishing was obtained.
Patient 1. A 73-year-old male patient diagnosed with moderately differentiated leiomyosarcoma of the pancreas and multiple hepatic metastases was referred to our Department of Hematology and Oncology at the University Hospital of Regensburg (Table 2 and Figure 1). The patient received two cycles of Doxorubicin in combination with the PDGFR alpha blocking monoclonal antibody Olaratumab. However, magnetic resonance imaging (MRI) of the liver performed after 3 months of therapy showed rapid tumor progression in both the liver and the pancreas (Figure 1). Therefore, chemotherapy was switched to Gemcitabine combined with Docetaxel. In response to four cycles of treatment with Gemcitabine/Docetaxel remission control via liver MRI revealed partial remission with shrinking hepatic metastases and reduction in tumor size in the pancreas (Figure 1). Owing to considerable residual tumor volume and initial aggressive tumor growth refractory to first-line chemotherapy, the patient was individually selected for maintenance therapy with Gemcitabine, which was started 6 months after the first cycle of Gemcitabine/Docetaxel. Originally, dosing of Gemcitabine during maintenance therapy was supposed to be 1,000 mg/m2 on days 1, 8, and 15 with cycle repetition every 3 weeks. Nevertheless, due to previous hematotoxicity (prolonged neutropenia) experienced during Gemcitabine/Docetaxel treatment, the Gemcitabine dose was reduced to 900 mg/m2 in this patient administered on days 1 and 8 with repetition every 3 weeks (Table 1). No serious complications occurred during Gemcitabine monotherapy. Moreover, based on medical history records regularly updated on gemcitabine infusion appointments, quality of life was not severely impaired. Six months after the start of gemcitabine maintenance therapy, progressive disease was detected by liver MRI. Apart from an increase in size of known metastases, several new hepatic lesions appeared. Salvage therapy was first attempted with Dacarbazine monotherapy and, due to lacking efficacy, was switched to Pazopanib 3 months later. Unfortunately, disease progression could not be hampered by Pazopanib either, and in the face of beginning acute liver failure, palliative care was initiated, and the patient died in the following month, 2 1/2 years after the initial diagnosis of leiomyosarcoma. In aggregate, Gemcitabine/Docetaxel combination therapy with subsequent Gemcitabine maintenance therapy could induce partial tumor regression and slow tumor growth for several months in this patient, who suffered from rapid progressive leiomyosarcoma refractory to Doxorubicin, Dacarbazine, and Pazopanib (Figure 2).
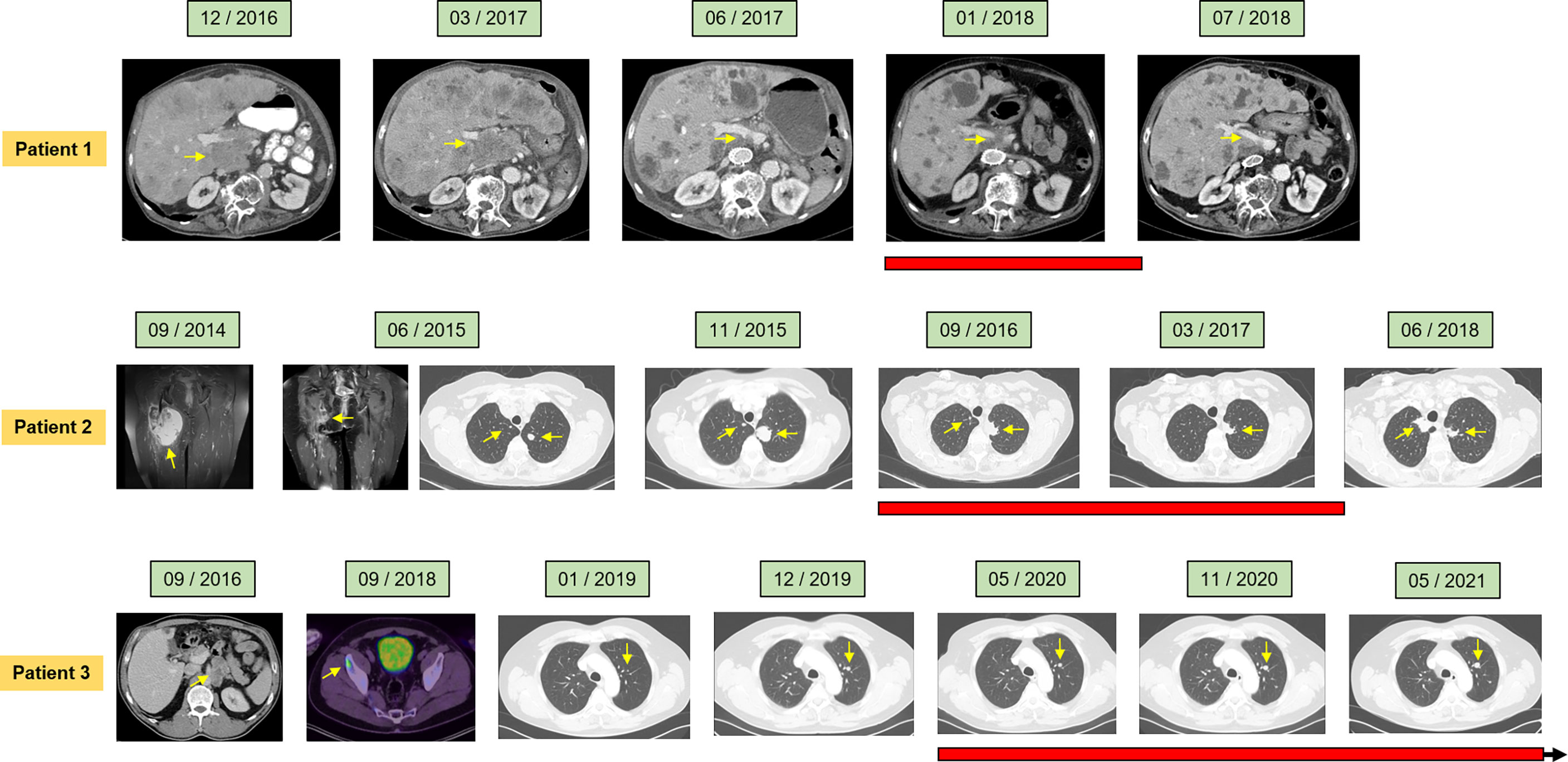
Figure 1 Clinical course of the individual patients assessed by radiological imaging depicting treatment response at the indicated time points. Computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography were utilized (PET). Red bars denote duration of Gemcitabine maintenance therapy. Arrows indicate ongoing Gemcitabine maintenance therapy.
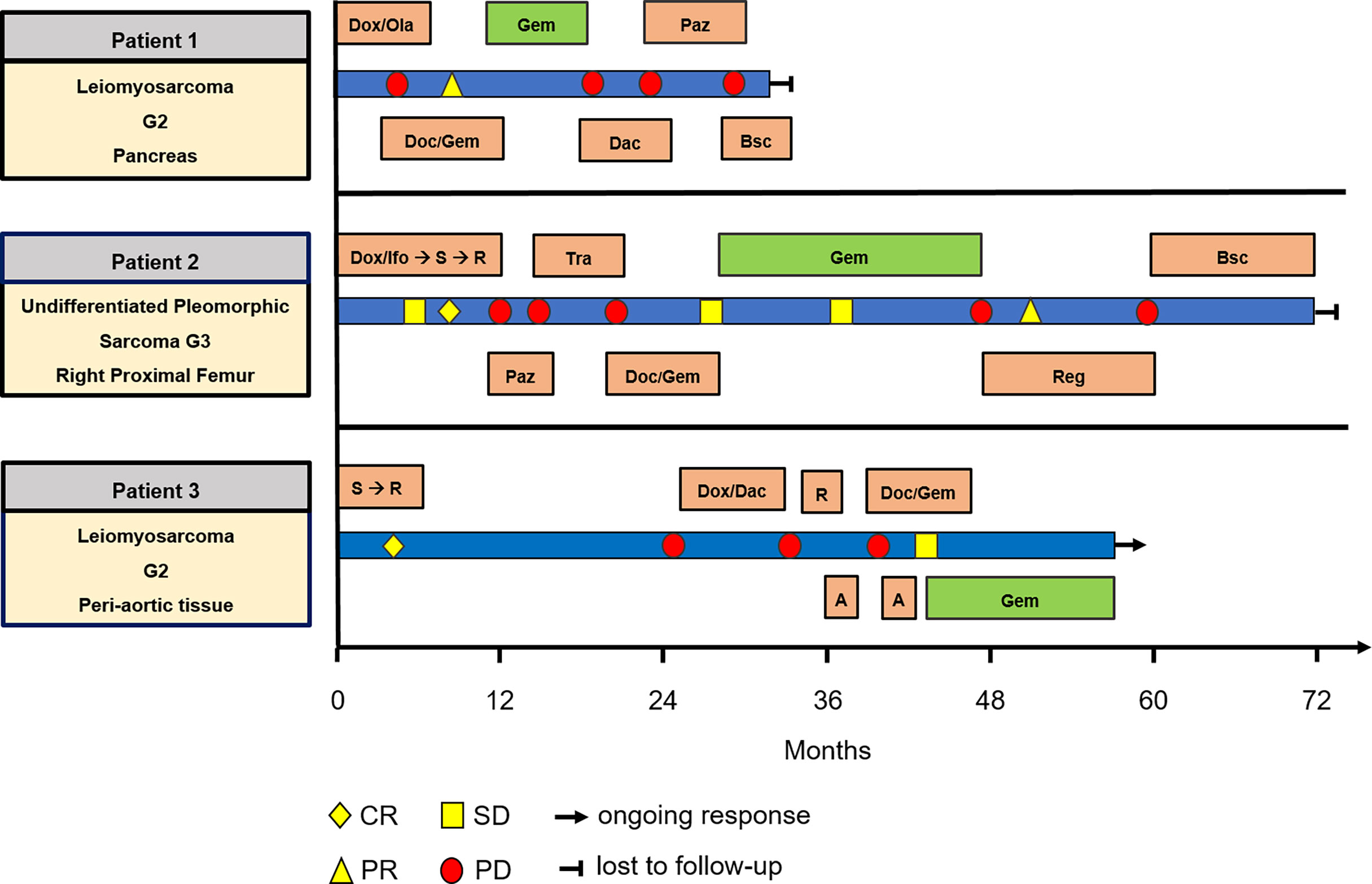
Figure 2 Swimmer plot including all patients, showing treatment and response. A, ablation; Bsc, best supportive care; Dac, Dacarbazine; Doc, Docetaxel; Dox, Doxorubicin; Gem, Gemcitabine; Ifo, Ifosfamide; Ola, Olaratumab; Paz, Pazopanib; R, radiation; Reg, Regorafenib; S, surgery; Tra, Trabectedin.
Patient 2. A 61-year-old female patient presented with an undifferentiated pleomorphic sarcoma of the right femur (Table 2). Radiological examination via MRI revealed a pseudoaneurysmatic tumor protruding from the back of the right femur (Figure 1). The original treatment strategy included surgical removal after prior chemotherapy. Accordingly, the patient received neoadjuvant chemotherapy with Doxorubicin and Ifosfamide. After two cycles of chemotherapy, however, no significant tumor regression could be achieved. Hence, a decision to surgically remove the tumor with subsequent radiation of the resection site was made. Sarcoma removal was performed at the Sarcoma Center of the University Hospital of Regensburg. After complete excision with no residual tumor cells on the resection margin (R0), the patient underwent adjuvant radiation therapy. Thereafter, regular follow-up was started. Unfortunately, 3 months after regular follow-up, MRI imaging showed a contrast-enhancing lesion at the upper resection margin (Figure 1). Besides, pulmonary CT imaging showed several lung metastases (Figure 1). Relapse therapy with Pazopanib was started, but no significant inhibition of growth of the pulmonary metastases could be attained necessitating third-line therapy with Trabectedin, which did not achieve any remission either (Figure 1). Analogous to patient 1, salvage therapy was attempted with the combination of Gemcitabine and Docetaxel. In response to eight cycles of Gemcitabine/Docetaxel treatment, the patient showed stable pulmonary metastases without any new or growing lesions. Equal to patient 1, residual tumor masses and the aggressive tumor biology with insensitivity to several different anti-sarcoma drugs prompted the start of Gemcitabine maintenance. Similar to patient 1, prior hematotoxicity (neutropenia) suffered during Gemcitabine/Docetaxel treatment rationalized a reduced dose of 900 mg/m2 in this patient, administered on days 1 and 8 with repetition every 3 weeks (Table 2). Six months after Gemcitabine maintenance therapy, pulmonary CT imaging showed stable disease with constant bipulmonary metastases (Figure 1). During Gemcitabine maintenance therapy, no serious complications were documented, and quality of life was not impaired in this patient, as assessed at doctor’s appointments on chemotherapy infusion days. After 21 months of Gemcitabine therapy, the patient showed increasing pulmonary metastases resulting in cessation of Gemcitabine treatment (Figure 1). Salvage therapy with Regorafenib was started, which could induce a partial remission after 2 months of treatment, which lasted for 1 year. Given no further treatment options available, best supportive care was commenced. The patient died 1 year later, nearly 5 years after the initial diagnosis of leiomyosarcoma. In aggregate, Gemcitabine/Docetaxel combination therapy with subsequent Gemcitabine maintenance therapy appeared to slow down tumor growth for more than 1 year in this patient, who displayed aggressive undifferentiated pleomorphic sarcoma refractory to Doxorubicin, Ifosfamide, Pazopanib, and Trabectedin (Figure 2).
Patient 3. A 54-year-old man presented with moderately differentiated peri-aortic leiomyosarcoma (Table 2). No metastases were present at initial diagnosis. The treatment strategy encompassed complete surgical excision followed by adjuvant radiotherapy. After successful surgical excision (R0 resection), adjuvant radiotherapy was started. Afterwards, regular follow-up examinations were initiated. Two years later, a metabolically active lesion was detected in the right ilium via PET-CT (Figure 1). Biopsy and histological examination revealed leiomyosarcoma relapse. Chemotherapy with Dacarbazine and Doxorubicin was administered. Under this therapy, increasing pulmonary metastases and new liver lesions were observed, indicating refractory disease. Subsequently, the osseous metastasis was irradiated, liver lesions were locally ablated, and analogous to patients 1 and 2, salvage therapy with Gemcitabine and Docetaxel was started. After two cycles of Gemcitabine/Docetaxel treatment, the patient exhibited stable disease and subsequently was put on Gemcitabine maintenance therapy to slow down further tumor progression. Patient 3 initially received three fully dosed cycles with 1,000 mg/m2 Gemcitabine on days 1, 8, and 15 (Table 1). However, severe prolonged neutropenia prompted first omission of day 8 and pegfilgrastim support on days 2 and 16 with cycle repetition on day 29. Later during the sixteenth cycle of Gemcitabine maintenance therapy, persistent neutropenia necessitated dose reduction in Gemcitabine to 500 mg/m2 administered every 2 weeks (Table 1). At 6 months after start of Gemcitabine monotherapy, the patient maintained stable disease with size constant pulmonary metastases (Figure 1). Until now, the patient has completed 14 months of Gemcitabine maintenance therapy without contracting any serious complications or reduction in quality of life. Just recently (May 2021), stable disease was reconfirmed via CT imaging (Figure 1). The patient is scheduled to continue Gemcitabine maintenance therapy until tumor progression or the occurrence of unbearable side effects. In aggregate, Gemcitabine/Docetaxel combination therapy with subsequent Gemcitabine maintenance therapy appeared to slow down tumor growth for more than 1 year in this patient, who displayed aggressive leiomyosarcoma refractory to Doxorubicin and Dacarbazine (Figure 2). Interestingly, this patient remains stable even with a significantly reduced Gemcitabine dosing, which allows for continuation of Gemcitabine maintenance therapy.
In patients with metastasized soft tissue sarcomas, long-term survival is rare, and effective treatment strategies are lacking (15). Particularly for patients with refractory or relapsed soft tissue sarcomas, there is an urgent need to enhance the efficacy of salvage chemotherapy. The concept of maintenance therapy aims at delaying disease progression and improving quality of life. In this case series, we retrospectively analyzed the concept of maintenance therapy in patients with different soft tissue sarcomas including leiomyosarcoma of the pancreas, undifferentiated pleomorphic sarcoma of the femur, and peri-aortic leiomyosarcoma. After salvage therapy with Gemcitabine and Docetaxel, Gemcitabine maintenance therapy in an off-label fashion was given, aiming to slow down disease progression in patients with aggressive soft tissue sarcomas.
We report promising therapeutic potential for Gemcitabine maintenance therapy even after significant dose reductions in Gemcitabine due to hematotoxicity. All patients in this case series suffered from aggressive metastasized STS refractory to at least one line of chemotherapy.
During Gemcitabine maintenance therapy, two patients showed stable disease lasting several months, and one patient exhibited partial disease regression maintained for 21 months. These favorable results could be achieved despite individual adaption of Gemcitabine dose according to comorbidities and pretreatment.
All patients included in this study were treated individually and independently from one another. Hence, individual treatment regimens differ; thus, direct comparability of patients is compromised, also the generation of pooled analyses for usual outcome parameters such as overall survival and progression-free survival. Further limitations to this study include the non-interventional retrospective design of this analysis and the limited number of patients allowing only a narrative description of the individual courses.
In general, data about the clinical benefit of maintenance therapy in sarcoma are scant (24). Only in patients with localized high-risk rhabdomyosarcoma combination maintenance therapy with Cyclophosphamide and Vinorelbine administered over 6 months could significantly improve overall survival (26). In contrast, maintenance therapy with both anti-PDGFR alpha blocking monoclonal antibody Olaratumab and the vascular endothelial growth factor antagonist Bevacizumab failed to significantly slow down disease progression (16, 27). In addition, the mammalian target of rapamycin (mTOR) inhibitor ridaforolimus (RIDA) significantly delayed disease progression in patients with advanced soft tissue sarcoma but did not significantly improve overall survival (28). In conclusion, the sole clinical setting in which maintenance therapy in soft tissue sarcoma is supported by positive clinical data is represented by high-risk localized rhabdomyosarcoma.
Furthermore, the toxicity profile of maintenance therapy must be contemplated carefully. In case of advanced disease, maintenance therapy regimens administered to delay disease progression must be well tolerated without significant impairments of quality of life. In all three patients presented, quality of life was not impaired by Gemcitabine maintenance therapy. Moreover, Gemcitabine is a generally well-tolerated chemotherapeutic agent, with hematotoxicity being the most important major side effect (29). Hence, especially with regards to the Gemcitabine/Docetaxel pretreatment, Gemcitabine maintenance therapy was individually adjusted based on condition and bone marrow function of the individual patients. Therefore, the targeted dose of 1,000 mg/m2 was slightly reduced in two patients to 900 mg/m2 and in one patient to 500 mg/m2. Despite the significant dose reduction in Gemcitabine in patient 3, the efficacy in terms of tumor progression was not affected. Thus, Gemcitabine maintenance therapy might also be suitable for elderly patients and patients with heavy pretreatment, as it can be used in reduced dose in case of marrow dysfunction.
Only a few effective chemotherapy regimens are currently available for treatment of advanced soft tissue sarcoma, and there have been very few phase III clinical trials of second-line chemotherapy. All patients in this study were progressive to at least one line of systemic therapy. Before initiation of Gemcitabine maintenance therapy, all patients received salvage therapy with Gemcitabine and Docetaxel. In a large retrospective study reported in 2006 analyzing 133 patients with soft tissue sarcoma from 10 institutions in France, the overall response rate to Gemcitabine and Docetaxel combination therapy was 18.4%, and the median overall survival was 12.1 months (30). Similarly, in a Korean study covering 281 patients, the objective response rate was 15.6%, while the median overall survival was 10.3 months (31). In contrast, a phase 2 clinical trial evaluating second-line therapy with gemcitabine monotherapy in patients with various sarcomas showed an objective response rate of only 5.5% (32). Based on the data of those studies, Gemcitabine/Docetaxel therapy was selected as salvage treatment. Finally, we suggest using Gemcitabine/Docetaxel prior to Gemcitabine maintenance therapy because an objective response achieved by Gemcitabine/Docetaxel might indicate increased chances of sensitivity to Gemcitabine chemotherapy.
In summary, we would like to raise awareness for the concept of Gemcitabine maintenance therapy as novel therapeutic modality in patients suffering from soft tissue sarcoma with special emphasis on patients with aggressive disease refractory to prior chemotherapy. In three patients with soft tissue sarcoma, aggressive disease progression could be slowed down with Gemcitabine maintenance therapy. Based on those remarkable results obtained from individual off-label treatments, we encourage the initiation of early phase clinical trials to prospectively evaluate Gemcitabine maintenance therapy in patients with soft tissue sarcomas.
The data analyzed in this study is subject to the following licenses/restrictions: the datasets underlying this study are available on request to the corresponding author. Requests to access these datasets should be directed to ZGVubmlzLmhhcnJlckB1a3IuZGU=.
The studies involving human participants were reviewed and approved by Ethics Committee of the University Regensburg. The patients/participants provided their written informed consent to participate in this study.
MV conceptualized the study. MV, US, DH, MG, and SB treated the patients. DCH drafted the manuscript. KM provided the positron emission imaging material. CW provided the CT and MRI material. WH, TP, and MG interpreted data and critically revised the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Hashimoto K, Nishimura S, Oka N, Akagi M. Clinical Features and Outcomes of Primary Bone and Soft Tissue Sarcomas in Adolescents and Young Adults. Mol Clin Oncol (2020) 12:358–64. doi: 10.3892/mco.2020.1994
2. Hoang NT, Acevedo LA, Mann MJ, Tolani B. A Review of Soft-Tissue Sarcomas: Translation of Biological Advances Into Treatment Measures. Cancer Manag Res (2018) 10:1089–114. doi: 10.2147/CMAR.S159641
3. Grünewald TG, Alonso M, Avnet S, Banito A, Burdach S, Cidre-Aranaz F, et al. Sarcoma Treatment in the Era of Molecular Medicine. EMBO Mol Med (2020) 12:e11131. doi: 10.15252/emmm.201911131
4. Linch M, Miah AB, Thway K, Judson IR, Benson C. Systemic Treatment of Soft-Tissue Sarcoma-Gold Standard and Novel Therapies. Nat Rev Clin Oncol (2014) 11:187–202. doi: 10.1038/nrclinonc.2014.26
5. Verschoor AJ, Litière S, Marréaud S, Judson I, Toulmonde M, Wardelmann E, et al. Survival of Soft Tissue Sarcoma Patients After Completing Six Cycles of First-Line Anthracycline Containing Treatment: An EORTC-STBSG Database Study. Clin Sarcoma Res (2020) 10:18. doi: 10.1186/s13569-020-00137-5
6. Noujaim J, Constantinidou A, Messiou C, Thway K, Miah A, Benson C, et al. Successful Ifosfamide Rechallenge in Soft-Tissue Sarcoma. Am J Clin Oncol (2018) 41:147–51. doi: 10.1097/COC.0000000000000243
7. Ferraresi V, Ciccarese M, Cercato MC, Nuzzo C, Zeuli M, Di Filippo F, et al. Gemcitabine at Fixed Dose-Rate in Patients With Advanced Soft-Tissue Sarcomas: A Mono-Institutional Phase II Study. Cancer Chemother Pharmacol (2008) 63:149–55. doi: 10.1007/s00280-008-0723-9
8. Maki RG, Wathen JK, Patel SR, Priebat DA, Okuno SH, Samuels B, et al. Randomized Phase II Study of Gemcitabine and Docetaxel Compared With Gemcitabine Alone in Patients With Metastatic Soft Tissue Sarcomas: Results of Sarcoma Alliance for Research Through Collaboration Study 002 Corrected. J Clin Oncol (2007) 25:2755–63. doi: 10.1200/JCO.2006.10.4117
9. Blay J-Y, Schöffski P, Bauer S, Krarup-Hansen A, Benson C, D'Adamo DR, et al. Eribulin Versus Dacarbazine in Patients With Leiomyosarcoma: Subgroup Analysis From a Phase 3, Open-Label, Randomised Study. Br J Cancer (2019) 120:1026–32. doi: 10.1038/s41416-019-0462-1
10. Schöffski P, Ray-Coquard IL, Cioffi A, Bui NB, Bauer S, Hartmann JT, et al. Activity of Eribulin Mesylate in Patients With Soft-Tissue Sarcoma: A Phase 2 Study in Four Independent Histological Subtypes. Lancet Oncol (2011) 12:1045–52. doi: 10.1016/S1470-2045(11)70230-3
11. Gordon EM, Sankhala KK, Chawla N, Chawla SP. Trabectedin for Soft Tissue Sarcoma: Current Status and Future Perspectives. Adv Ther (2016) 33:1055–71. doi: 10.1007/s12325-016-0344-3
12. Lee AT, Jones RL, Huang PH. Pazopanib in Advanced Soft Tissue Sarcomas. Signal Transduct Target Ther (2019) 4:16. doi: 10.1038/s41392-019-0049-6
13. Marrari A, Bertuzzi A, Bozzarelli S, Gennaro N, Giordano L, Quagliuolo V, et al. Activity of Regorafenib in Advanced Pretreated Soft Tissue Sarcoma: Results of a Single-Center Phase II Study. Med (Baltimore) (2020) 99:e20719. doi: 10.1097/MD.0000000000020719
14. Amatu A, Sartore-Bianchi A, Siena S. NTRK Gene Fusions as Novel Targets of Cancer Therapy Across Multiple Tumour Types. ESMO Open (2016) 1:e000023. doi: 10.1136/esmoopen-2015-000023
15. Hong DS, DuBois SG, Kummar S, Farago AF, Albert CM, Rohrberg KS, et al. Larotrectinib in Patients With TRK Fusion-Positive Solid Tumours: A Pooled Analysis of Three Phase 1/2 Clinical Trials. Lancet Oncol (2020) 21:531–40. doi: 10.1016/S1470-2045(19)30856-3
16. Tap WD, Wagner AJ, Schöffski P, Martin-Broto J, Krarup-Hansen A, Ganjoo KN, et al. Effect of Doxorubicin Plus Olaratumab vs Doxorubicin Plus Placebo on Survival in Patients With Advanced Soft Tissue Sarcomas: The ANNOUNCE Randomized Clinical Trial. JAMA (2020) 323:1266–76. doi: 10.1001/jama.2020.1707
17. van Vliet M, Kliffen M, Krestin GP, van Dijke CF. Soft Tissue Sarcomas at a Glance: Clinical, Histological, and MR Imaging Features of Malignant Extremity Soft Tissue Tumors. Eur Radiol (2009) 19:1499–511. doi: 10.1007/s00330-008-1292-3
18. Ferrari A, Sultan I, Huang TT, Rodriguez-Galindo C, Shehadeh A, Meazza C, et al. Soft Tissue Sarcoma Across the Age Spectrum: A Population-Based Study From the Surveillance Epidemiology and End Results Database. Pediatr Blood Cancer (2011) 57:943–9. doi: 10.1002/pbc.23252
19. Farid M, Ong WS, Tan MH, Foo LS, Lim YK, Chia WK, et al. The Influence of Primary Site on Outcomes in Leiomyosarcoma: A Review of Clinicopathologic Differences Between Uterine and Extrauterine Disease. Am J Clin Oncol (2013) 36:368–74. doi: 10.1097/COC.0b013e318248dbf4
20. Martin-Liberal J. Leiomyosarcoma: Principles of Management. Intractable Rare Dis Res (2013) 2:127–9. doi: 10.5582/irdr.2013.v2.4.127
21. Scurr M. Histology-Driven Chemotherapy in Soft Tissue Sarcomas. Curr Treat Options Oncol (2011) 12:32–45. doi: 10.1007/s11864-011-0140-x
22. Gladdy RA, Qin L-X, Moraco N, Agaram NP, Brennan MF, Singer S. Predictors of Survival and Recurrence in Primary Leiomyosarcoma. Ann Surg Oncol (2013) 20:1851–7. doi: 10.1245/s10434-013-2876-y
23. Schuetze S. Trabectedin: Useful in Leiomyosarcoma and Liposarcoma, But Less So in Other Soft Tissue Sarcomas. Ann Oncol (2021) 32(8):957–58. doi: 10.1016/j.annonc.2021.04.025
24. Lebellec L, Defachelles A-S, Cren P-Y, Penel N. Maintenance Therapy and Drug Holiday in Sarcoma Patients: Systematic Review. Acta Oncol (2020) 59:1084–90. doi: 10.1080/0284186X.2020.1759825
25. Sette G, Salvati V, Memeo L, Fecchi K, Colarossi C, Di Matteo P, et al. EGFR Inhibition Abrogates Leiomyosarcoma Cell Chemoresistance Through Inactivation of Survival Pathways and Impairment of CSC Potential. PloS One (2012) 7:e46891. doi: 10.1371/journal.pone.0046891
26. Casanova M, Ferrari A, Bisogno G, Merks JH, de Salvo GL, Meazza C, et al. Vinorelbine and Low-Dose Cyclophosphamide in the Treatment of Pediatric Sarcomas: Pilot Study for the Upcoming European Rhabdomyosarcoma Protocol. Cancer (2004) 101:1664–71. doi: 10.1002/cncr.20544
27. Chisholm JC, Merks JH, Casanova M, Bisogno G, Orbach D, Gentet J-C, et al. Open-Label, Multicentre, Randomised, Phase II Study of the EpSSG and the ITCC Evaluating the Addition of Bevacizumab to Chemotherapy in Childhood and Adolescent Patients With Metastatic Soft Tissue Sarcoma (the BERNIE Study). Eur J Cancer (2017) 83:177–84. doi: 10.1016/j.ejca.2017.06.015
28. Chawla SP, Staddon AP, Baker LH, Schuetze SM, Tolcher AW, D'Amato GZ, et al. Phase II Study of the Mammalian Target of Rapamycin Inhibitor Ridaforolimus in Patients With Advanced Bone and Soft Tissue Sarcomas. J Clin Oncol (2012) 30:78–84. doi: 10.1200/JCO.2011.35.6329
29. Tonato M, Mosconi AM, Martin C. Safety Profile of Gemcitabine. Anticancer Drugs (1995) 6(Suppl 6):27–32. doi: 10.1097/00001813-199512006-00005
30. Bay J-O, Ray-Coquard I, Fayette J, Leyvraz S, Cherix S, Piperno-Neumann S, et al. Docetaxel and Gemcitabine Combination in 133 Advanced Soft-Tissue Sarcomas: A Retrospective Analysis. Int J Cancer (2006) 119:706–11. doi: 10.1002/ijc.21867
31. Choi Y, Yun MS, Lim SH, Lee J, Ahn J-H, Kim YJ, et al. Gemcitabine and Docetaxel Combination for Advanced Soft Tissue Sarcoma: A Nationwide Retrospective Study. Cancer Res Treat (2018) 50:175–82. doi: 10.4143/crt.2016.535
Keywords: sarcoma, maintenance therapy, solid tumor, chemotherapy, stroma tumor
Citation: Harrer DC, Buschauer S, Sterz U, Menhart K, Wendl C, Heudobler D, Grube M, Pukrop T, Herr W and Vogelhuber M (2021) Gemcitabine Maintenance Therapy in Patients With Metastasized Soft Tissue Sarcomas. Front. Oncol. 11:755439. doi: 10.3389/fonc.2021.755439
Received: 18 August 2021; Accepted: 18 November 2021;
Published: 14 December 2021.
Edited by:
Cyril Corbet, Fonds National de la Recherche Scientifique (FNRS), BelgiumReviewed by:
Silvia Lemma, Università di Bologna, ItalyCopyright © 2021 Harrer, Buschauer, Sterz, Menhart, Wendl, Heudobler, Grube, Pukrop, Herr and Vogelhuber. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dennis Christoph Harrer, ZGVubmlzLmhhcnJlckB1a3IuZGU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.