- Medical Imaging Key Laboratory of Sichuan Province, and Department of Radiology, Affiliated Hospital of North Sichuan Medical College, Nanchong, China
Objective: To investigate relationship of tumor stage-based gross tumor volume (GTV) of esophageal squamous cell carcinoma (ESCC) measured on computed tomography (CT) with early recurrence (ER) after esophagectomy.
Materials and Methods: Two hundred and four consecutive patients with resectable ESCC including 159 patients enrolled in the training cohort (TC) and 45 patients in validation cohort (VC) underwent contrast-enhanced CT less than 2 weeks before esophagectomy. GTV was retrospectively measured by multiplying sums of all tumor areas by section thickness. For the TC, univariate and multivariate analyses were performed to determine factors associated with ER. Mann-Whitney U test was conducted to compare GTV in patients with and without ER. Receiver operating characteristic (ROC) analysis was performed to determine if tumor stage-based GTV could predict ER. For the VC, unweighted Cohen’s Kappa tests were used to evaluate the performances of the previous ROC predictive models.
Results: ER occurred in 63 of 159 patients (39.6%) in the TC. According to the univariate analysis, histologic differentiation, cT stage, cN stage, and GTV were associated with ER after esophagectomy (all P-values < 0.05). Multivariate analysis revealed that cT stage and GTV were independent risk factors with hazard ratios of 3.382 [95% confidence interval (CI): 1.533–7.459] and 1.222 (95% CI: 1.125–1.327), respectively (all P-values < 0.05). Mann-Whitney U tests showed that GTV could help differentiate between ESCC with and without ER in stages cT1-4a, cT2, and cT3 (all P-values < 0.001), and the ROC analysis demonstrated the corresponding cutoffs of 13.31, 17.22, and 17.83 cm3 with areas under the curve of more than 0.8, respectively. In the VC, the Kappa tests validated that the ROC predictive models had good performances for differentiating between ESCC with and without ER in stages cT1-4a, cT2, and cT3 with Cohen k of 0.696 (95% CI, 0.498–0.894), 0.733 (95% CI, 0.386–1.080), and 0.862 (95% CI, 0.603–1.121), respectively.
Conclusion: GTV and cT stage can be independent risk factors of ER in ESCC after esophagectomy, and tumor stage-based GTV measured on CT can help predict ER.
Introduction
Esophageal cancer is the seventh most common malignant tumor and the sixth leading cause of cancer-related deaths in the world (1, 2). Esophageal squamous cell carcinoma (ESCC) and adenocarcinoma are the two main histologic types, and in the high-risk areas, 90% of cases are ESCC (3, 4). Esophagectomy is considered the optimal treatment for resectable esophageal cancer (1, 5). But the long-term outcome remains poor, and even with radical resection, the 5-year postoperative survival rate does not exceed 30%, and postoperative recurrence is the main cause of treatment failure (6, 7). Some works have reported that the incidence of postoperative recurrence of esophageal cancer is the highest in the first year after esophagectomy with the incidence of more than 50%, and the median survival time after recurrence is less than 1 year (8–10). Hence, it is very important to predict the postoperative early recurrence (ER) of ESCC by integrating various factors and indicators.
Computed tomography (CT) plays a vital role in the management of esophageal cancer, such as diagnosis, treatment guidance, and follow-up (11, 12). With the continuous development of imaging technology, there are many alternative parameters that emerged, which are helpful for outcome prediction, and gross tumor volume (GTV) obtained on CT has been considered as one of these parameters (13). There are some researches about the relationship between GTV and the tumor stage, nodal disease, or treatment response based on imaging technologies to date. Lagarde et al. reported that the larger the tumor volume of esophageal cancer, the more likely lymph node metastasis and distant organ metastasis, and the greater possibility of incomplete surgical resection (14). Blom et al. reported that tumor volume of adenocarcinoma of the esophagus and gastroesophageal junction had an impact on tumor response to chemotherapy (15). Wu et al. suggested that GTV of resectable ESCC measured with MRI correlated well with T category and lymphatic metastasis (16). To the best of our knowledge, there are no reports to demonstrate if GTV of ESCC could predict the postoperative ER after esophagectomy. This study was devoted to investigating the factors associated with postoperative ER of resectable ESCC after esophagectomy, and the relationship of tumor stage-based GTV with ER.
Materials and Methods
Patients
This study was approved by the institutional ethics committee of our hospital, and each patient signed informed consent before participating in this study.
From March 2016 to June 2018, patients with ESCC proved by endoscopic biopsy were recruited into our study according to the following inclusion criteria: (a) patients did not receive any tumor-related treatments (e.g., chemotherapy or radiotherapy) before undergoing CT; (b) the tumors were resectable according to the National Comprehensive Cancer Network (NCCN) guidelines based on the CT manifestations (11); and (c) patients underwent esophagectomy with no residual disease at surgical margins, and were regularly followed up after surgery following the previous guidelines. There were 218 consecutive cases according to the inclusion criteria. The exclusion criteria were as follows: (a) patients had other thoracic surgery history (n = 6); (b) patients had other serious illnesses that made the patient cannot tolerate the surgery (n = 5); or (c) the quality of the images is poor (n = 3). The previous CT image quality was subjectively analyzed on a five-point scale (1, worst; 2, suboptimal; 3, adequate; 4, very good; 5, excellent) by two senior radiologists according to the image-quality scoring system (17). Therefore, 14 cases were excluded from our study, and our study ultimately involved 204 patients. All patients were randomly divided into a training cohort (TC, 159 patients) and a validation cohort (VC, 45 patients). The clinical, surgical, and pathological data in both cohorts were collected from the clinical database. All involved patients underwent thoracic contrast-enhanced CT examination within 2 weeks before the esophagectomy.
Tumor Lesion Characteristics
According to the postoperative histopathology and American Joint Committee on Cancer (AJCC) staging system of esophageal cancer (18), the tumor anatomic location, the differentiation, T stage, N stage, and postoperative chemoradiation are listed in Table 1.
Follow-Up and Definition of ER
During the follow-up period, all patients underwent postoperative medical and blood examinations and thoracic CT imaging or barium-swallow every 3–6 months in the first year after surgery. If recurrence was suspected based on the above examinations or patients had clinical symptoms associated with recurrence after surgery, patients underwent further examinations like cervical ultrasonography, thoracoabdominal CT, and endoscopic examination with biopsy. More selective investigations such as bone scintigraphy and cerebral CT were carried out based on specific symptomatology, clinical examination, and biochemical profile.
Based on the published literatures (9, 19, 20), we defined the recurrence within 12 months after surgery as ER. Subsequently, we divided our involved patients into the ER group and the non-ER group in the TC and VC. The patterns of the ER were classified into three types including local recurrence (at the site of the primary tumor or anastomotic and residual tissue recurrence), regional recurrence (regional lymph node recurrence), and distant recurrence (distant organ recurrence) according to the previous studies (6, 9).
Contrast-Enhanced CT Scans
The CT data were obtained with enhanced 64-section multidetector computed tomography (MDCT) (LightSpeed VCT, GE Medical systems, USA). One hundred to 200 ml of water was given orally as negative contrast material before CT data acquisitions. All examinations were carried out in the supine position. After routine unenhanced CT scans, the contrast-enhanced CT data were obtained 25–30 s after the initiation of contrast agent (Omnipaque, Iohexol, GE Healthcare, USA) injection via a 20-G needle into an antecubital vein at a rate of 3.0 ml/s for a total of 70–100 ml tailored to body weight at the ratio of 1.5 ml/kg weight, followed by a 20 ml saline flush with a pump injector (Vistron CT Injection System, Medrad, USA). The CT scanning parameters were as follows: 120 kV of peak voltage, 200 mA of tube current (automatic exposure control employed), rotation time of 0.5 s, collimation of 64 × 0.6 mm, pitch of 0.9, slice thickness of 5 mm, and matrix of 512 × 512 mm. Examinations were performed during one breath-hold at full suspended inspiration for 10–15 s. The coverage of CT scan was from the neck to the middle of the left kidney. Subsequently, CT data were directly transferred to the General Electric Advantage Workstation 4.4 at the mediastinal window settings with window width of 400 HU and window level of 40 HU.
Gross Tumor Volume Measurement
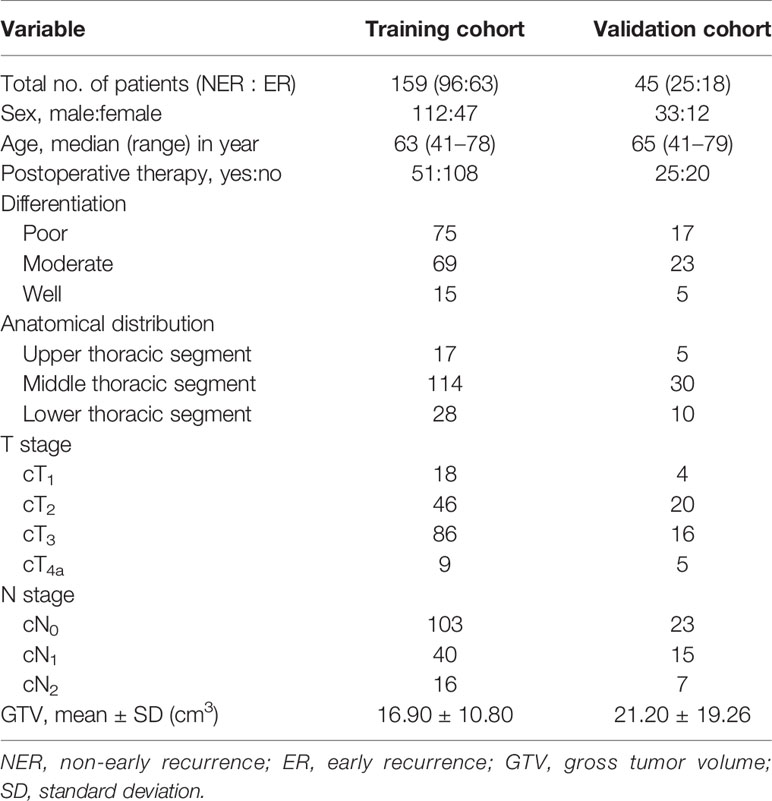
The GTV of resectable ESCC was measured on the abovementioned workstation, obtained by multiplying the sums of all tumor areas by the section thickness according to the method used in the published studies (21, 22). When the thickness of the esophageal wall was more than 5 mm on the axial contrast-enhanced CT scan, it was considered as the abnormal thickness caused by the tumor (23). The shape of the tumor was manually delineated along the margin of the abnormal esophageal wall (Figures 1A, 2), and then the software automatically calculated the tumor area on each contiguous tumor section. Ultimately, the tumor areas were summed and subsequently multiplied by the layer thickness to obtain the GTV. In order to minimize measurement errors, we tried to avoid air and liquid within the esophageal lumen as much as possible when we sketched the tumor.
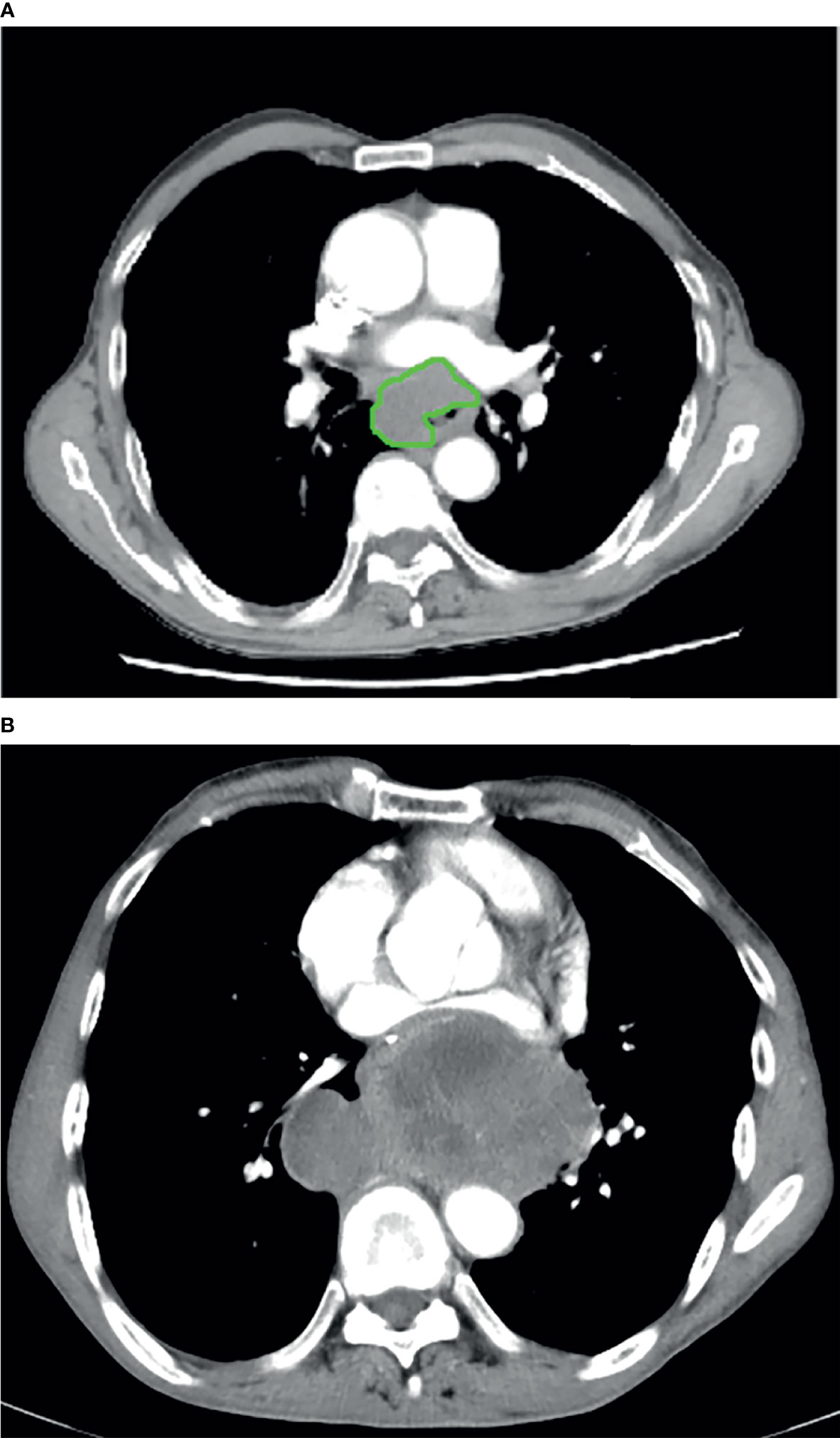
Figure 1 In a 61-year-old male with esophageal squamous cell carcinoma at cT2N0M0, the preoperative thoracic contrast-enhanced CT scans depict the gross tumor volume obtained by manual delineation along the margin of the abnormal esophageal wall (A) slice-by-slice, and the gross tumor volume is 58.66 cm3. Regional and local recurrence has been found 12 months after radical esophagectomy during the follow-up period (B).
Figure 2 In a 66-year-old male with esophageal squamous cell carcinoma at cT3N0M0, the preoperative thoracic contrast-enhanced CT scans depict the gross tumor volume obtained by manual delineation along the margin of the abnormal esophageal wall slice-by-slice, and the gross tumor volume is 12.83 cm3. During the follow-up period, there was no recurrence as shown on follow-up CT after radical esophagectomy.
To ensure the accuracy of the tumor volume measurement, two experienced radiologists (Observer 1 with 3 years of radiology expertise, and Observer 2 with 5 years of experience in radiology) measured the GTV of all patients in the TC independently to verify the interobserver reproducibility. To verify the intraobserver reproducibility, the first radiologist remeasured the GTV of all patients 1 month later. Before the radiologists outlined the tumor to obtain the GTV, a professor of radiology with 23 years of experience in body radiology trained them how to draw the outlines of the tumor in 10 patients at random. All measurements were carried out without knowing the histologic results.
Statistical Analysis
The IBM SPSS statistics software (version 25.0 for Windows; SPSS, Chicago, IL, USA) was used for the statistical analysis of data. A P value less than 0.05 was defined as a significant difference for all data. The reliability of repeated measurements of GTV was assessed via the intraclass correlation coefficient (ICC). ICCs less than 0.5, between 0.5 and 0.75, between 0.75 and 0.9, and greater than 0.90 are indicative of poor, moderate, good, and excellent reliability, respectively (24).
The continuous variables were expressed as mean ± standard deviation (SD). Categorical variables were shown as numbers and percentages. For the TC, univariate and multivariate analyses were performed to determine factors associated with ER. The Chi-square test or Fisher test was used to assess the univariate associations of possible factors with recurrence of resectable ESCC 1 year after the surgery. If the variables were statistically different at a P value less than 0.05 in the univariate analysis, they were enrolled in the multivariate analysis, which was performed by the binary logistic regression analysis to clarify the independent risk factors of the postoperative ER of resectable ESCC. The Mann-Whitney U test was used to compare GTV between patients with and without ER on the basis of different T stages. When a significant difference was proved by the previous Mann-Whitney U test, receiver operating characteristic (ROC) analyses were then carried out to determine if the cutoff values of GTV could be helpful to predict ER. Unweighted Cohen’s Kappa tests were conducted to evaluate the performances of the previous ROC predictive models to predict ER in the VC dataset independently. We used unweighted Cohen’s Kappa test according to the following rating scheme: less than 0.20, poor agreement; 0.21 to 0.40, fair agreement; 0.41 to 0.60, moderate agreement; 0.61 to 0.80, good agreement; and greater than 0.81, very good agreement (25).
Results
Patterns of ER of ESCC
In TC, ER occurred in 63 cases, and the rate of ER was 39.62% (63/159) while the remaining 96 patients did not have the recurrence. In detail, there were 4 (6.3%), 30 (47.6%), 6 (9.5%), and 23 (36.5%) patients who had local recurrence, regional recurrence, distant recurrence, and recurrence of two or more patterns 1 year after surgery, respectively. Of the 23 patients with recurrences of two or more patterns, there were 3 (13.0%) patients with local recurrence and regional recurrence; 8 (34.8%) patients had local and regional recurrence and distant recurrence in lung, liver, or bone; and 12 (52.2%) patients had regional and distant recurrence in lung, liver, or bone. In VC, ER occurred in 18 cases, and the rate of ER was 40.0% (18/45), among which regional recurrence was the most common recurrence pattern (11/18).
Intra- and Interobserver Variability of GTV Measurements in the Training Cohort
For the initial measurement of the first observer, the mean GTV was 16.90 ± 10.8 cm3. To ensure the accuracy of GTV measurement, the intra- and interobserver ICC values of the GTV repeated measurements were 0.984 [95% confidence interval (95%CI), 0.968–0.992] and 0.978 (95%CI, 0.913–0.978), respectively, each with a P-value less than 0.001, indicating excellent repeatability of the GTV measurements in the TC. Thus, the first measurement of the first observer was reproducible and could be subsequently used for the further statistical analyses.
Univariate Analysis of Correlation of Both Clinicopathological Factors and Tumor Stage-Based GTV With ER of ESCC in the Training Cohort
The correlation of both the clinicopathological characteristics and GTV with ER is summarized in Table 2. The histologic differentiation, cT stage, cN stage, and the GTV were associated with ER. In detail, ER was more common in poorly differentiated patients than in well-differentiated patients, patient with a higher cT stage was associated with a higher likelihood of ER, patient with a higher cN stage was more likely to be with ER, and the larger the tumor, the higher the incidence of ER (Figure 1B) (all P-values < 0.05). However, statistical analysis showed no significant association between ER and postoperative therapy with the P-value of 0.588, suggesting that not postoperative therapy but radical surgical resection could be a very effective treatment strategy for resectable ESCC.
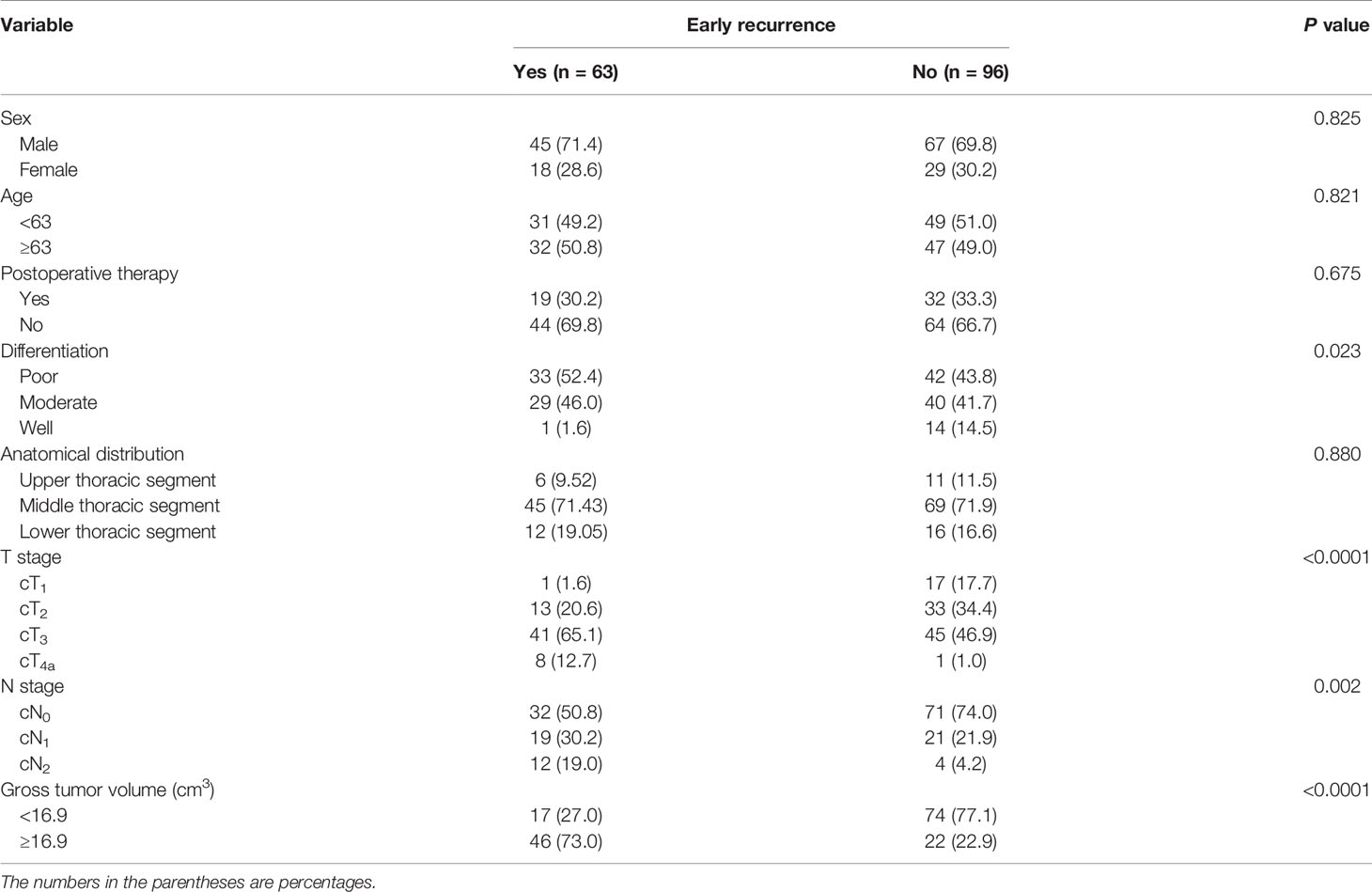
Table 2 Univariate analysis of clinicopathological factors and gross tumor volume correlated with early recurrence in the training cohort.
Multivariate Analysis of ER in Resectable ESCC in the Training Cohort
Based on the above significant factors obtained by the univariate analysis, histologic differentiation, cT stage, cN stage, and GTV were selected as potential independent risk factors, and the binary logistic regression analysis was performed to determine the independent risk factors of ER. The logistic regression analysis showed that cT stage and GTV were independent risk factors for ER after radical resection of resectable ESCC (P = 0.003 and <0.0001, hazard ratio = 3.382 and 1.222, 95% CI of 1.533–7.459 and 1.125–1.327, respectively).
Association of Tumor Stage-Based GTV With ER of Resectable ESCC in the Training Cohort
Associations of tumor stage-based GTV with ER of resectable ESCC after surgery were analyzed by the Mann-Whitney U test. Because most of the patients in our study were in the cT2 and cT3 category whereas numbers of patients in stages cT1 and cT4a were small, we did not perform the Mann-Whitney U tests separately focusing on stages cT1 and cT4a but on the stages cT2 and cT3 (Table 3). As depicted by the Mann-Whitney U tests, the GTV could help identify patients with ER in stages cT1-4a, cT2, cT3 (all P-values < 0.0001).
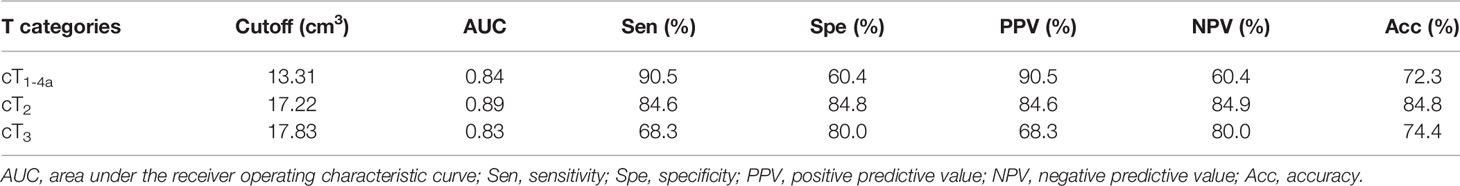
Table 3 Receiver operating characteristic analysis of tumor stage-based gross tumor volume for predicting early recurrence of esophageal squamous cell carcinoma in the training cohort.
ROC Analysis of Tumor Stage-Based GTV to Discriminate ESCC Between Patients With and Without ER in the Training Cohort
In order to demonstrate the accuracy of tumor stage-based GTV in predicting postoperative ER of resectable ESCC after esophagectomy, the ROC analysis was performed. According to the ROC analysis (Figures 3A–C), the GTV values were helpful for predicting ER of resectable ESCC after the radical surgery in patients in stages cT1-4a, cT2, and cT3 with the cutoff values of 13.31, 17.22, and 17.83 cm3, respectively. The area under the ROC curve (AUC), sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of GTV for predicting ER of resectable ESCC are shown in Table 3.
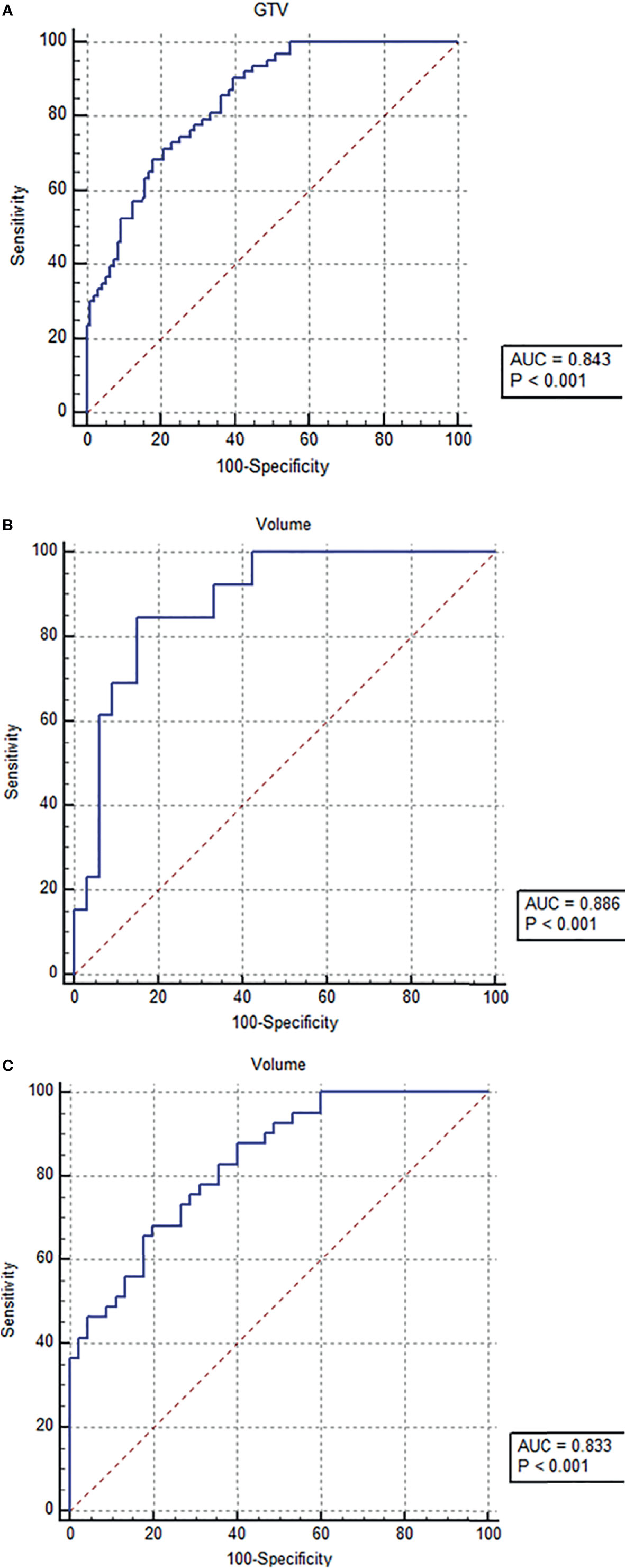
Figure 3 Receiver operating characteristic (ROC) analysis of tumor stage-based gross tumor volume (GTV) has been performed for predicting early recurrence of esophageal squamous cell carcinoma after esophagectomy, and the ROC curves show that the GTV can help predict early recurrence in tumor stages cT1-4a (A), cT2 (B), and cT3 (C) with the cutoff values of 13.31, 17.22, and 17.83 cm3, respectively.
Unweighted Cohen’s Kappa Tests in the Validation Cohort for Validating the Performance of the ROC Predictive Models
In order to validate the extent of agreement in the diagnostic efficiency of the ROC models of tumor stage-based GTV for differentiating between ESCC with and without ER in stages cT1-4a, cT2, and cT3 with clinicopathological and follow-up results, unweighted Cohen’s Kappa tests were conducted in the VC according to the cutoff values obtained by ROC analyses of the TC. The tests revealed that the models obtained good agreements with clinicopathological and follow-up results in VC shown in Table 4.

Table 4 Unweighted Cohen’s kappa tests in the validation cohort for validating the performance of the predictive models.
Discussion
The current study shows that the incidence of ER of resectable ESCC after radical esophagectomy could be associated with the cN stage, cT stage, degree of differentiation of the primary tumor, and GTV according to the univariate analysis. The GTV and cT stage are independent risk factors of ER in resectable ESCC. Except the GTV, the associations of the clinicopathological factors with ER of ESCC after radical esophagectomy were in accordance with published studies (19, 20, 26). In this study, for the first time, we found the association of the GTV with ER of ESCC after radical esophagectomy. In consideration for both independent risk factors (the cT stage and GTV), we further illustrated the feasibility of tumor stage-based GTV to predict ER of resectable ESCC after radical esophagectomy in TC.
As shown in our study, the GTV measured on CT could be an independent risk factor of ER in resectable ESCC. Tumor volume might be a comprehensive indicator reflecting the invasion length, tumor diameter, and the tumor depth of invasion. Hsu PK et al. found that the length of primary tumor is an important factor affecting recurrence of ESCC (27). And a previously published study showed that tumor length >6 cm was associated with distant recurrence (28). Both published papers suggest that the longer the tumor, the more likely the recurrence after esophagectomy. Gotohda et al. reported that the invasion of ESCC exceeded the submucosa, the lymph node metastasis rate increased significantly (29), and other published literatures showed the presence of metastatic lymph nodes was probably the main risk factor for the high incidence of recurrence in esophageal cancer (30, 31). We can presume that the invasion of ESCC exceeding the submucosa might be associated with ER of ESCC after esophagectomy. In addition, a study demonstrated that angiolymphatic invasion, submucosal invasion depth, and the largest diameter of invasion were independent predictors of early recurrence of ESCC (32). Based on the published studies on the relationship of early recurrence of ESCC with the invasion length, tumor diameter, and depth of invasion, we took all the possible risk factors of tumor size into consideration and chose the GTV as alternative parameter to explore the association of tumor size with ER after esophagectomy for the first time. Our study depicted that the GTV of ESCC was associated with ER after esophagectomy.
Our study showed that the GTV and tumor stage could be independent risk factors for ER of ESCC after esophagectomy. In consideration for both the cT stage and GTV, for the first time, we performed the stratification analysis of the GTV according to the cT stage to provide a new quantitative method for predicting ER of resectable ESCC after radical esophagectomy. Our study demonstrated that the stratification of GTV according to the tumor stage could obtain a good predictive performance on ER of ESCC. The ROC analyses showed that tumor-stage-based GTV measured on CT could predict ER of ESCC after radical esophagectomy with the AUC of more than 0.8.
Our study had several limitations. Firstly, this study enrolled a total of consecutive 204 patients in more than 2 years; the sample size was a little small. Especially, the numbers of patients in stage cT1 in the group of ER and patients in stage cT4a in group of non-ER in both TC and VC are small, so we did not perform the ROC analyses separately. We will collect more patients in different cT stages in the future to confirm our findings. Secondly, GTV was obtained by manual delineation along the margin of the abnormal esophageal wall in our study. GTV measured by machine learning algorithm might be more reproducible, and the measurement time can be greatly reduced compared with the measurement in our study, but the repeatability of the GTV measurements in this cohort was still excellent, suggesting that our results were reproducible. Thirdly, because the routine CT scan thickness of thorax in our hospital was 5 mm rather than 1 mm, we performed this retrospective study by using 5 mm thick slices rather than 1 mm thick slices for tumor delineation. We will perform a prospective study by using 1 mm thick slices in the future to confirm our findings.
In conclusion, we found that GTV and cT stage can be independent risk factors of ER in resectable ESCC after esophagectomy for the first time. GTV measured on CT could predict ER of ESCC after radical esophagectomy, and the cT stage-based GTV has a better predictive performance on ER of resectable ESCC. We hope that our findings could help clinicians to generate new thoughts on the recurrence of ESCC after radical esophagectomy based on tumor stage-based GTV for timely appropriate intervention decision-making to improve the prognosis of ESCC.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Affiliated Hospital of North Sichuan Medical College. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
T-WC, Y-PW, and ST conceived and designed the study. B-GT, L-QY, and F-LL collected the data. JO and DG analyzed the data and drafted the manuscript. All authors reviewed the manuscript, and T-WC revised the final manuscript. T-WC provided funding for the study. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (grant no. 81571645), the Sichuan Province Special Project for Youth Team of Science and Technology Innovation (grant no. 2015TD0029), and the Nanchong-University Cooperative Research Project (grant no: 20SXQT0329).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Corvera C, Das P, et al. Esophageal and Esophagogastric Junction Cancers, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw (2019) 17(7):855–83. doi: 10.6004/jnccn.2019.0033
2. Chen W, Zheng R, Zeng H, Zhang S. The Updated Incidences and Mortalities of Major Cancers in China, 2011. Chin J Cancer (2015) 34(11):502–7. doi: 10.1186/s40880-015-0042-6
3. Zeng H, Zheng R, Zhang S, Zuo T, Xia C, Zou X, et al. Esophageal Cancer Statistics in China, 2011: Estimates Based on 177 Cancer Registries. Thorac Cancer (2016) 7(2):232–7. doi: 10.1111/1759-7714.12322
4. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Globalcancer Statistics, 2012. CA Cancer J Clin (2015) 65(2):87–108. doi: 10.3322/caac.21262
5. Borggreve AS, Kingma BF, Domrachev SA, Koshkin MA, Ruurda JP, van Hillegersberg R, et al. Surgical Treatment of Esophageal Cancer in the Era of Multimodality Management. Ann N Y Acad Sci (2018) 1434(1):192–209. doi: 10.1111/nyas.13677
6. Nakagawa S, Kanda T, Kosugi S, Ohashi M, Suzuki T, Hatakeyama K. Recurrence Pattern of Squamous Cell Carcinoma of the Thoracic Esophagus After Extended Radical Esophagectomy With Three-Field Lymphadenectomy. J Am Coll Surg (2004) 198(2):205–11. doi: 10.1016/j.jamcollsurg.2003.10.005
7. Oezcelik A, Kaiser GM, Niebel W, Sleyman C, Treckmann JW, Sotiropoulos GC, et al. Ten-Year Survival of Esophageal Cancer After an En-Bloc Esophagectomy. J Surg Oncol (2012) 105(3):284–7. doi: 10.1002/jso.22096
8. Lou F, Sima CS, Adusumilli PS, Bains MS, Sarkaria IS, Rusch VW, et al. Esophageal Cancer Recurrence Patterns and Implications for Surveillance. J Thorac Oncol (2013) 8(12):1558–62. doi: 10.1097/01.JTO.0000437420.38972.fb
9. Mariette C, Balon JM, Piessen G, Fabre S, Van Seuningen I, Triboulet JP. Pattern of Recurrence Following Complete Resection of Esophageal Carcinoma and Factors Predictive of Recurrent Disease. Cancer (2003) 97(7):1616–23. doi: 10.1002/cncr.11228
10. Abate E, DeMeester SR, Zehetner J, Oezcelik A, Ayazi S, Costales J, et al. Recurrence After Esophagectomy for Adenocarcinoma: Defining Optimal Follow-Up Intervals and Testing. J Am Coll Surg (2010) 210(4):428–35. doi: 10.1016/j.jamcollsurg.2010.01.006
11. Ou J, Li R, Zeng R, Wu CQ, Chen Y, Chen TW, et al. CT Radiomic Features for Predicting Resectability of Esophageal Squamous Cell Carcinoma as Given by Feature Analysis: A Case Control Study. Cancer Imaging (2019) 19(1):66. doi: 10.1186/s40644-019-0254-0
12. Umeoka S, Koyama T, Togashi K, Saga T, Watanabe G, Shimada Y, et al. Esophageal Cancer: Evaluation With Triple-Phase Dynamic CT–initial Experience. Radiology (2006) 239(3):777–83. doi: 10.1148/radiol.2393050222
13. Tullie LG, Sohn HM, Zylstra J, Mattsson F, Griffin N, Sharma N, et al. A Role for Tumor Volume Assessment in Resectable Esophageal Cancer. Ann Surg Oncol (2016) 23(9):3063–70. doi: 10.1245/s10434-016-5228-x
14. Lagarde SM, ten Kate FJ, Reitsma JB, Busch OR, van Lanschot JJ. Prognostic Factors in Adenocarcinoma of the Esophagus or Gastroesophageal Junction. J Clin Oncol (2006) 24(26):4347–55. doi: 10.1200/JCO.2005.04.9445
15. Blom RL, Steenbakkers IR, Lammering G, Vliegen RF, Belgers EJ, de Jonge C, et al. PET/CT-Based Metabolic Tumor Volume for Response Prediction of Neoadjuvant Chemoradiotherapy in Esophageal Carcinoma. Eur J Nucl Med Mol Imaging (2013) 40(10):1500–6. doi: 10.1007/s00259-013-2468-x
16. Wu L, Ou J, Chen TW, Li R, Zhang XM, Chen YL, et al. Tumor Volume of Resectable Esophageal Squamous Cell Carcinoma Measured With MRI Correlates Well With T Category and Lymphatic Metastasis. Eur Radiol (2018) 28(11):4757–65. doi: 10.1007/s00330-018-5477-0
17. Chen TW, Yang ZG, Dong ZH, Li Y, Yao J, Wang QL, et al. Whole Tumour First-Pass Perfusion Using a Low-Dose Method With 64-Section Multidetector Row Computed Tomography in Oesophageal Squamous Cell Carcinoma. Eur J Radiol (2011) 80(2):284–91. doi: 10.1016/j.ejrad.2010.07.006
18. Rice TW, Ishwaran H, Ferguson MK, Blackstone EH, Goldstraw P. Cancer of the Esophagus and Esophagogastric Junction: An Eighth Edition Staging Primer. J Thorac Oncol (2017) 12(1):36–42. doi: 10.1016/j.jtho.2016.10.016
19. Hamai Y, Emi M, Ibuki Y, Murakami Y, Nishibuchi I, Nagata Y, et al. Early Recurrence and Cancer Death After Trimodal Therapy for Esophageal Squamous Cell Carcinoma. Anticancer Res (2019) 39(3):1433–40. doi: 10.21873/anticanres.13259
20. Yoshida N, Baba Y, Shigaki H, Harada K, Iwatsuki M, Sakamoto Y, et al. Risk Factors of Early Recurrence Within 6 Months After Esophagectomy Following Neoadjuvant Chemotherapy for Resectable Advanced Esophageal Squamous Cell Carcinoma. Int J Clin Oncol (2016) 21(6):1071–8. doi: 10.1007/s10147-016-0994-9
21. Jiang Y, Chen YL, Chen TW, Wu L, Ou J, Li R, et al. Is There Association of Gross Tumor Volume of Adenocarcinoma of Oesophagogastric Junction Measured on Magnetic Resonance Imaging With N Stage? Eur J Radiol (2019) 110:181–6. doi: 10.1016/j.ejrad.2018.05.023
22. Li R, Chen TW, Hu J, Guo DD, Zhang XM, Deng D, et al. Tumor Volume of Resectable Adenocarcinoma of the Esophagogastric Junction at Multidetector CT: Association With Regional Lymph Node Metastasis and N Stage. Radiology (2013) 269(1):130–8. doi: 10.1148/radiol.13122269
23. Moss AA, Schnyder P, Thoeni RF, Margulis AR. Esophageal Carcinoma: Pretherapy Staging by Computed Tomography. AJR Am J Roentgenol (1981) 136(6):1051–6. doi: 10.2214/ajr.136.6.1051
24. Koo TK, Li MY. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med (2016) 15(2):155–63. doi: 10.1016/j.jcm.2016.02.012
25. Qiao J, Qin J, Xing D, Li S, Wu D. Diagnosis of Retrolingual Obstruction During Drug-Induced Sleep Endoscopy Versus Polysomnography With Nasopharyngeal Tube in Patients With Obstructive Sleep Apnea. Ann Otol Rhinol Laryngol (2021) 130(11):1285–91. doi: 10.1177/00034894211005944
26. Liu Q, Cai XW, Wu B, Zhu ZF, Chen HQ, Fu XL. Patterns of Failure After Radical Surgery Among Patients With Thoracic Esophageal Squamous Cell Carcinoma: Implications for the Clinical Target Volume Design of Postoperative Radiotherapy. PloS One (2014) 9(5):e97225. doi: 10.1371/journal.pone.0097225
27. Hsu PK, Wang BY, Huang CS, Wu YC, Hsu WH. Prognostic Factors for Post-Recurrence Survival in Esophageal Squamous Cell Carcinoma Patients With Recurrence After Resection. J Gastrointest Surg (2014) 9(5):e97225. doi: 10.1371/journal.pone.0097225
28. Thomson IG, Smithers BM, Gotley DC, Martin I, Thomas JM, O'Rourke P, et al. Thoracoscopic-Assisted Esophagectomy for Esophageal Cancer: Analysis of Patterns and Prognostic Factors for Recurrence. Ann Surg (2010) 252(2):281–91. doi: 10.1097/SLA.0b013e3181e909a2
29. Gotohda N, Nishimura M, Yoshida J, Nagai K, Tanaka N. The Pattern of Lymphatic Metastases in Superficial Squamous Cell Carcinoma of the Esophagus. Hepatogastroenterology (2005) 52(61):105–7.
30. López-Sebastián J, Martí-Obiol R, López-Mozos F, Ortega-Serrano J. Recurrence of Esophageal Cancer After R0 Surgery. Risk Factors and Evolution. Rev Esp Enferm Dig (2013) 105(6):318–25. doi: 10.4321/s1130-01082013000600002
31. Hiyoshi Y, Yoshida N, Watanabe M, Kurashige J, Karashima R, Iwagami S, et al. Late Recurrence After Radical Resection of Esophageal Cancer. World J Surg (2016) 40(4):913–20. doi: 10.1007/s00268-015-3334-8
Keywords: esophagus, squamous cell carcinoma, recurrence, esophagectomy, tomography, X-ray computed
Citation: Wu Y-p, Tang S, Tan B-g, Yang L-q, Lu F-l, Chen T-w, Ou J, Zhang X-m, Gao D, Li K-y, Yu Z-y and Tang Z (2021) Tumor Stage-Based Gross Tumor Volume of Resectable Esophageal Squamous Cell Carcinoma Measured on CT: Association With Early Recurrence After Esophagectomy. Front. Oncol. 11:753797. doi: 10.3389/fonc.2021.753797
Received: 05 August 2021; Accepted: 04 October 2021;
Published: 22 October 2021.
Edited by:
Chuanming Li, Chongqing Medical University, ChinaReviewed by:
Lian-Ming Wu, Shanghai JiaoTong University, ChinaQing Lu, Shanghai Jiao Tong University, China
Copyright © 2021 Wu, Tang, Tan, Yang, Lu, Chen, Ou, Zhang, Gao, Li, Yu and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tian-wu Chen, dGlhbnd1Y2hlbl9uc21jQDE2My5jb20=
 Yu-ping Wu
Yu-ping Wu Tian-wu Chen
Tian-wu Chen Xiao-ming Zhang
Xiao-ming Zhang