
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 01 October 2021
Sec. Breast Cancer
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.753209
Background: The purpose of this meta-analysis was to compare the safety and efficacy between hypofractionated and conventional fractionation radiotherapy in patients with early-stage breast cancer after breast-conserving surgery.
Methods: We conducted a comprehensive search of PubMed, Embase, Web of Science, and the Cochrane Library to identify relevant randomized controlled trials (RCTs) published before February 2021. At the same time, the hazard ratio (HR), risk ratio (RR), and 95% confidence interval (CI) were calculated to evaluate local recurrence (LR), relapse-free survival (RFS), overall survival (OS), adverse events, and cosmetic outcomes.
Results: A total of 14 articles were included in this meta-analysis. Four thousand eight hundred and sixty-nine patients were randomly assigned to the control group to receive conventional radiotherapy (CFRT); 6,072 patients were randomly assigned to the experimental group and treated with hypofractionated radiotherapy (HFRT). The results showed that there was no statistical difference between HFRT and CFRT in LR (HR = 0.99, 95%CI = 0.97–1.02, p = 0.476), RFS (HR = 0.99, 95%CI = 0.97–1.02, p = 0.485), OS (HR = 1.00, 95%CI = 0.97–1.03, p = 0.879), and cosmetic outcomes (RR = 1.03, 95%CI = 0.95–1.12, p = 0.53). In addition, HFRT showed fewer severe adverse reactions such as acute skin toxicity, induration, breast atrophy, and pain.
Conclusion: Our results suggest that there is no statistical difference between HFRT and CFRT in terms of LR, RFS, OS, and cosmetic outcomes. HFRT reduces the risk of developing toxicity reactions compared to CFRT. HFRT may be a better option for patients with early breast cancer after breast-conserving surgery.
According to the data released by the International Agency for Research on Cancer (IARC) of the World Health Organization in 2020, increased number of cases have made breast cancer become the world’s leading cancer by overtaking lung cancer (1). With the development of medical technology, the overall prognosis of breast cancer is good. In most countries and regions, the 5-year net survival rate is over 85%, and the trend is still rising (2). Patients in the early stage of breast cancer can choose between lumpectomy and mastectomy (3). Studies have shown that whole-breast radiotherapy following breast-conserving surgery is comparable to mastectomy in terms of overall survival (OS) in early breast cancer (4). Meanwhile, total mastectomy results in a larger wound area. Also, in addition to leading to a few unfavorable complications, total mastectomy also has negative impacts on patients’ social–emotional function and body image, which also affects patients’ self-esteem (5). Breast-conserving surgery, however, can improve the quality of life in a behavioral aspect compared to total mastectomy (6).
For patients in the early stage of breast cancer, radiotherapy after breast-conserving surgery is quite essential. The meta-analysis from the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) showed that the tumor recurrence and mortality rates were significantly reduced after breast-conserving surgery in patients who received whole-breast radiotherapy compared to those who did not (7). Based on this dominantly advantageous curative effect, it is time to consider the cosmetic outcomes, patients’ mental health, and the economic impact after surgery (8). The current conventional fractionated radiotherapy (CFRT) is 50 Gy fractionation divided into 25 fractions of 2 Gy over 5 weeks, once a day (9). However, this daily treatment takes five or more weeks, causing inconvenience to patients in terms of life and work, not to mention the negative impact on their social–emotional function (10). Hence, scholars have attempted to shorten the course of treatment. The aim of hypofractionated radiotherapy (HFRT) is to shorten the overall duration of treatment for patients by increasing the single dose of radiation, thereby providing greater convenience while bringing greater cost effectiveness and less resource waste to the entire healthcare system (11). The commonly used hypofractionated scheme is 43.5 Gy in 15 fractions over 15 days or 42.56 Gy in 16 fractions over 16 days (12). Due to the increase of the single dose and the decrease of the total dose, a great concern for HFRT has been whether to increase the toxicity and reduce the tumor control rate or not (13).
Four classical multicenter randomized controlled trials (RCTs) (14–17) have compared HFRT and CFRT and indicated equal local control, overall survival, and cosmetic outcomes, paving a promising future for the clinical application of HFRT (18). Although HFRT is theoretically promising in terms of shortening the course of treatment, it has no wide practical applications. This is probably due to the lingering concerns about the effectiveness and safety of HFRT (19). Thus, HFRT needs to be further evaluated in those aspects in order to improve our understanding and confidence in its clinical use. Herein, we conducted this meta-analysis to compare the treatment results of HFRT and CFRT in patients with early breast cancer after breast-conserving surgery in order to evaluate the safety and efficacy of HFRT.
This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (20). The objective was to directly compare the safety and effectiveness through RCTs between HFRT and CFRT among early breast cancer patients who had previous breast-conserving surgery.
Two investigators independently performed the systematic and comprehensive search of databases such as PubMed, Embase, Web of Science, and the Cochrane Library for articles published before February 2021 using the following keywords, individually or in combination: (“breast cancer” OR “breast neoplasms” OR “breast tumor”) AND (“radiotherapy” OR “conventional fractionation” OR “hypofractionated” OR “hypofractionation radiotherapy dosage” OR “dose fractionation”). Moreover, the bibliographies of relevant publications were manually searched for additional articles.
The included studies met the following criteria: 1) patients were histologically diagnosed with breast cancer; 2) patients have undergone breast-conserving surgery; 3) patients were treated with postoperative HFRT or CFRT; 4) HFRT should not be less than 2.3 Gy per fraction and the total dose should be more than 28 Gy; CFRT should be 1.8–2.0 Gy per fraction and the total dose should not be less than 45 Gy; and 5) relevant outcomes included but not limited to survival outcomes, tumor local control, toxicity, and cosmetic outcomes.
The exclusion criteria included the following: 1) the diagnosis was metastatic breast cancer; 2) data source was duplicated; 3) language of the article is non-English; 4) non-RCTs, including conference abstracts, observational research, review articles, and cases; 5) the efficacies of HFRT and CFRT were not directly compared; and 6) the data of the research are not feasible to be extracted.
Two investigators examined the data independently. The following pieces of information were extracted for each qualifying study: 1) study characteristics, including author, publication year, and country; 2) patient demographics, such as the clinical stage of breast cancer, sample size, radiation dose, median follow-up time, age range, systemic therapy, and time period of the clinical trials; 3) comparison of the clinical outcomes of HFRT and CFRT, including survival outcomes, tumor local control, toxicity, and cosmetic outcomes.
To evaluate the RCTs, we used the Cochrane Collaboration’s tool. Two investigators independently used “low risk,” “high risk,” or “unclear risk” to assess the risk of bias through six aspects: sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and free of other bias. Disagreements between the investigators were solved by consensus; if needed, a third investigator participated in the resolution process.
Meta-analysis was conducted by Review Manager, version 5.3, and Stata software, v.12.0. For categorical variables, such as adverse events and cosmetic outcomes, the risk ratio (RR) and 95% confidence interval (CI) were calculated for analysis. Meanwhile, survival data were analyzed by calculating the hazard ratio (HR) and 95% CI. I2 was used to evaluate heterogeneity among the studies. A random effects model or a fixed effects model was applied to the analysis when the I2 was greater than 50%. When more than 10 studies were included in the outcome indicators, the Begg’s and Egger’s tests are good tools to investigate publication bias. In addition, the stability and reliability of the results were evaluated using sensitivity analysis; thus, the sources of heterogeneity were further explored. P < 0.05 indicates statistical significance.
On the basis of our search strategy, a total of 6,746 records were identified from the four databases. After deleting duplicates, we screened the titles and abstracts of 1,794 records, then excluded 1,768 records and finally selected 26 to read through. We excluded 12 studies for the following reasons: four reported duplicate data sources; two were non-randomized controlled trials; two did not include relevant results; and the patients in four studies underwent mastectomy. Finally, we identified 14 studies that met the inclusion criteria. This meta-analysis is based on the quantitative and qualitative synthesis of the included 14 studies (14, 15, 21–32). A flowchart outlines the process in Figure 1.
More than 10,000 breast cancer cases were enrolled in the 14 included RCTs between 2004 and 2020. The characteristics of the studies are summarized in Table 1 and Supplementary Table S1. Among these studies, four were conducted in the UK, three were in China, and seven were from other countries. All cases involved adult patients with non-metastatic breast cancer, mostly at T1-2N0-1, and all of the patients had undergone breast-conserving surgery. Of the total patients, 4,869 were randomly assigned to the control group and received CFRT with a radiotherapy dose of 1.8–2.0 Gy per fraction and a total dose of not less than 45 Gy (with or without a boost); 6,072 were randomly assigned to the experimental group and received HFRT treatment. The HFRT dose was more than 2.3 Gy per fraction, with a total dose of more than 28 Gy (with or without a boost). The shortest median follow-up time was 6 weeks and the longest was 16.9 years. The primary endpoints of the studies generally included survival rate, local control, toxicity, and cosmetic outcome.
The quality of each study was appraised via the Cochrane Collaboration’s tool. Most studies have been evaluated as low risk in terms of sequence generation and allocation concealment. However, the blinding of these studies was evaluated as having high risk of bias because the blinding process cannot be implemented due to the nature of the intervention. One study had high risk resulting from incomplete outcome data, and one had unclear risk. When it comes to selective outcome reporting, one study had high risk and one study was unclear. Finally, all RCTs were unclear in terms of free of other bias. The assessment results are shown in detail in Supplementary Table S2.
Four trials, with 1,499 patients in the experimental group and 1,039 in the control group, were included to evaluate local recurrence (LR). The results showed that there was no statistically significant difference in LR between the two groups (HR = 0.99, 95%CI = 0.97–1.02, p = 0.476). No heterogeneity was found in the included studies (I2 = 0). A pooled analysis of 2,896 patients from five studies with low heterogeneity (I2 = 0) indicated that the difference between HFRT and CFRT in relapse-free survival (RFS) was not statistically significant (HR = 0.99, 95%CI = 0.97–1.02, p = 0.485). The results of LR and RFS are shown in Figures 2 and 3. As for the analysis of OS, seven trials involving 2,309 patients receiving HFRT and 2,317 patients receiving CFRT were included for evaluation. It was discovered that there was no significant relationship between OS and radiotherapy dose (HR = 1.00, 95%CI = 0.97–1.03, p = 0.879) (Figure 4).
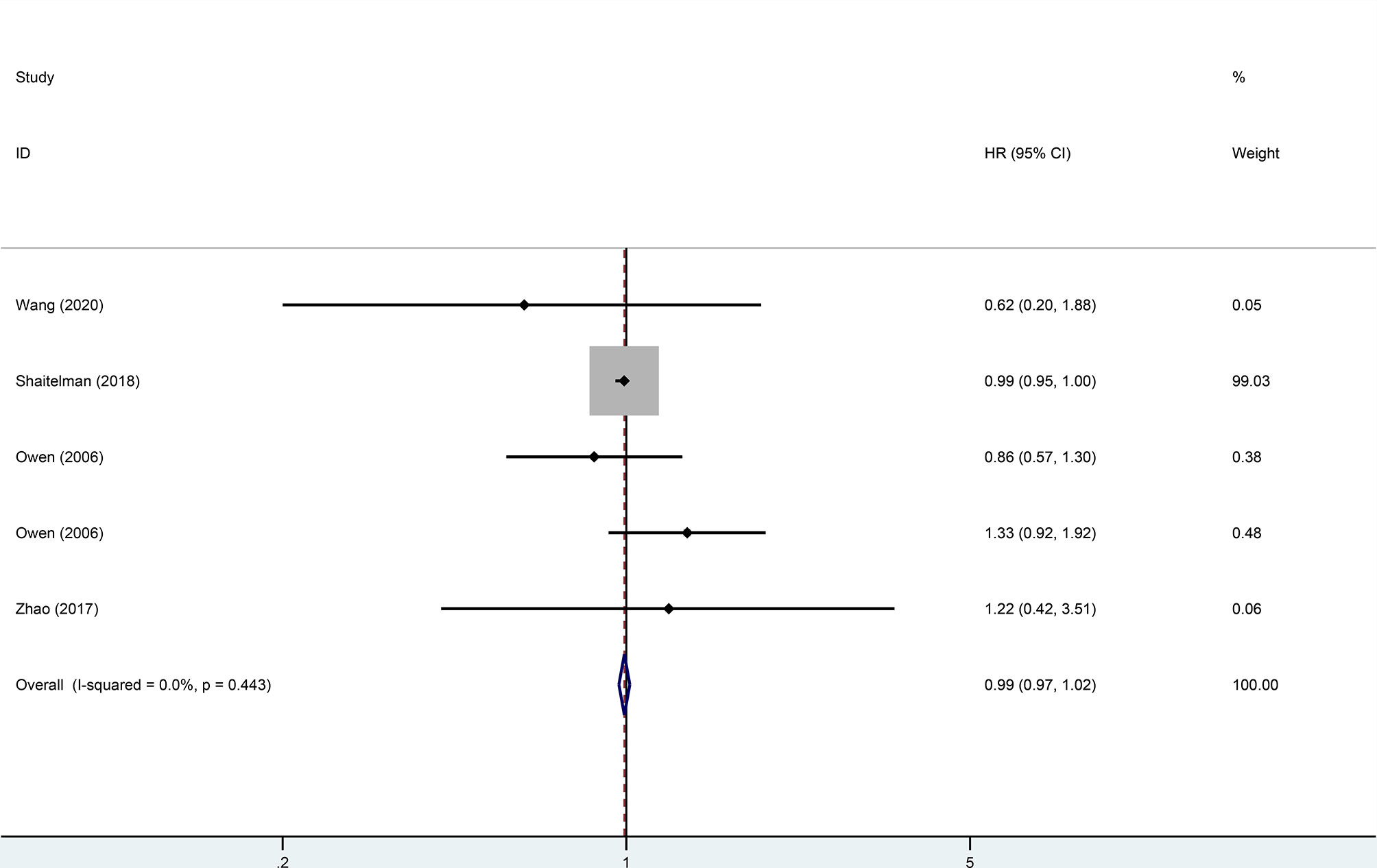
Figure 2 Forest plot of hypofractionated radiotherapy (HFRT) vs. conventional radiotherapy (CFRT) for local recurrence (p = 0.476).
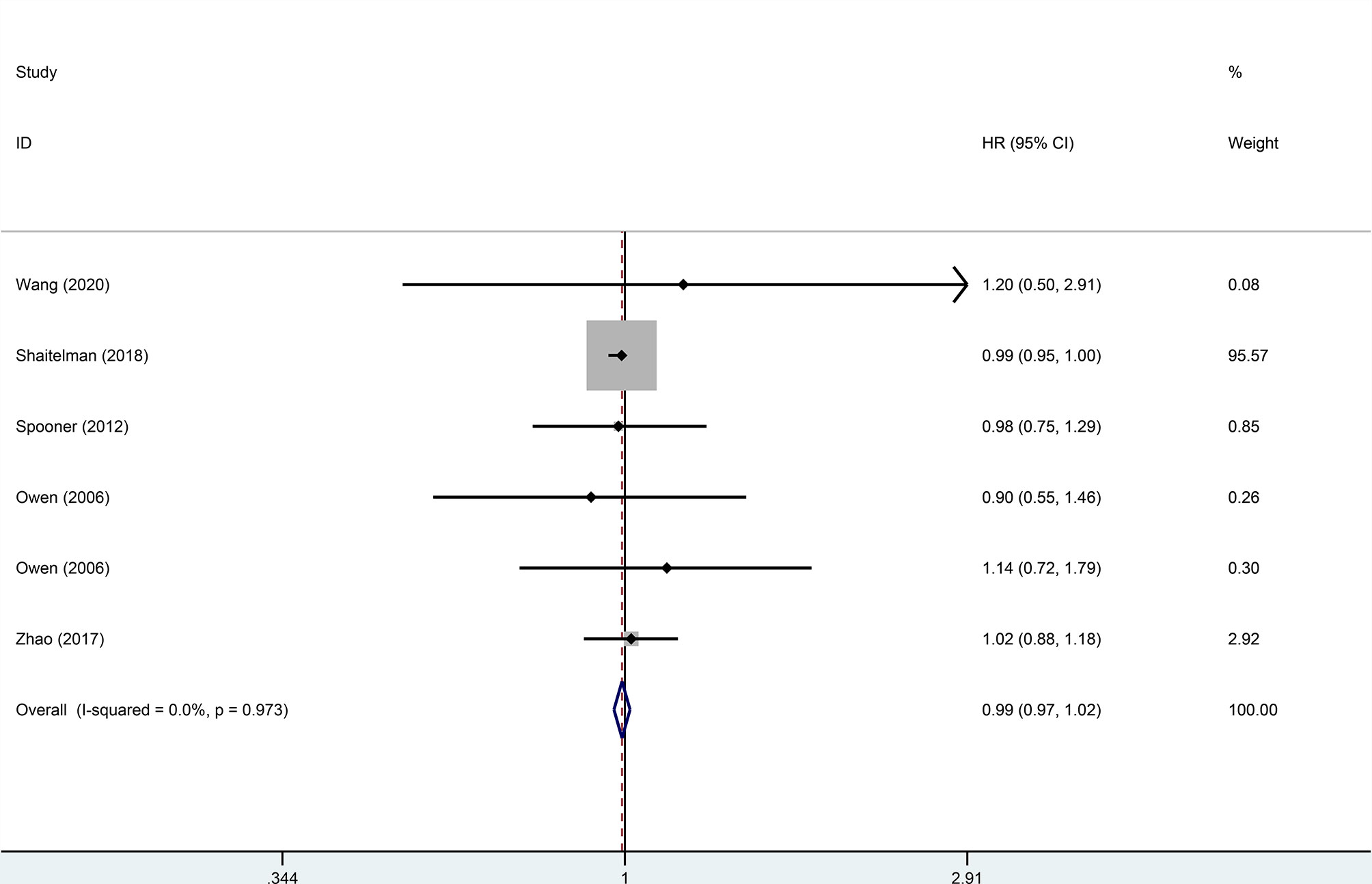
Figure 3 Forest plot of hypofractionated radiotherapy (HFRT) vs. conventional radiotherapy (CFRT) for relapse-free survival (p = 0.485).
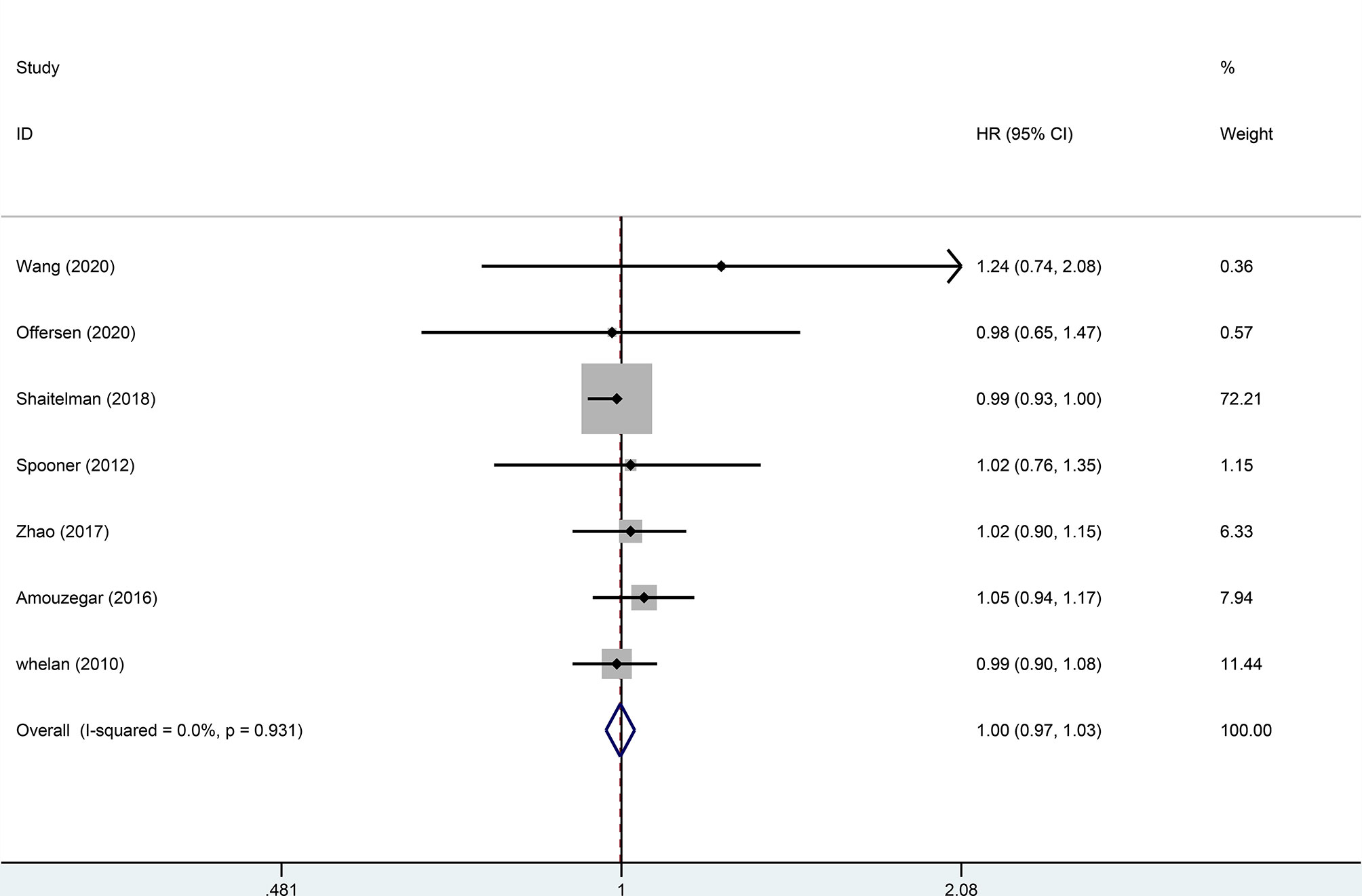
Figure 4 Forest plot of hypofractionated radiotherapy (HFRT) vs. conventional radiotherapy (CFRT) for overall survival (p = 0.879).
In addition to survival and recurrence, our primary concern was to assess the toxicity of HFRT. Compared with CFRT, less severe all grade acute skin toxicity (RR = 0.76, 95%CI = 0.61–0.94, p = 0.01) and breast atrophy (RR = 0.87, 95%CI = 0.80–0.95, p = 0.001), shorter induration (RR = 0.88, 95%CI = 0.79–0.97, p = 0.01), and fewer side effects in terms of pain (RR = 0.72, 95% CI = 0.51–1.01, p = 0.05) were observed for HFRT. The risk of pneumonitis (RR = 0.74, p = 0.08) was lower in patients receiving HFRT compared to those receiving CFRT, but the difference was not statistically significant. Additionally, the analysis results indicated that there was no statistical difference between HFRT and CFRT in many other adverse events, including all grade late skin toxicity (RR = 1.08, p = 0.55), dermatitis (RR = 0.79, p = 0.57), dyspigmentation (RR = 0.82, p = 0.15), edema (RR = 0.77, p = 0.10), telangiectasia (RR = 1.15, p = 0.35), and delayed toxic effects in subcutaneous tissues (RR = 0.95, p = 0.49). Detailed data are shown in Table 2.
With regard to moderate or marked adverse events, the results showed that HFRT can reduce the risk of acute skin toxicity (RR = 0.32, 95%CI = 0.15–0.69, p = 0.004) and breast atrophy (RR = 0.91, 95%CI = 0.84–0.98, p = 0.02) compared to CFRT. Also, there was no significant difference between modalities in moderate or marked late skin toxicity (RR = 0.95, p = 0.92), induration (RR = 0.93, p = 0.57), edema (RR = 0.81, p = 0.27), and delayed toxic effects in subcutaneous tissues (RR = 0.74, p = 0.54) after the two radiotherapies. Detailed data are shown in Table 3.
For early breast cancer patients who had undergone breast-conserving surgery, whether the cosmetic intervention after radiotherapy is excellent/good or not is also worth considering. Hence, the cosmetic outcomes of surgery were analyzed based on 3,841 patients from eight studies, with 2,064 patients in the HFRT group and 1,777 patients in the CFRT group. Patients’ cosmetic outcomes were scored on a scale of “excellent,” “good,” “fair,” and “poor”. Pooled RR revealed no significant difference in excellent/good cosmetic outcomes between HFRT and CFRT (RR = 1.03, 95%CI = 0.95–1.12, p = 0.53). More detailed information is shown in Figure 5.
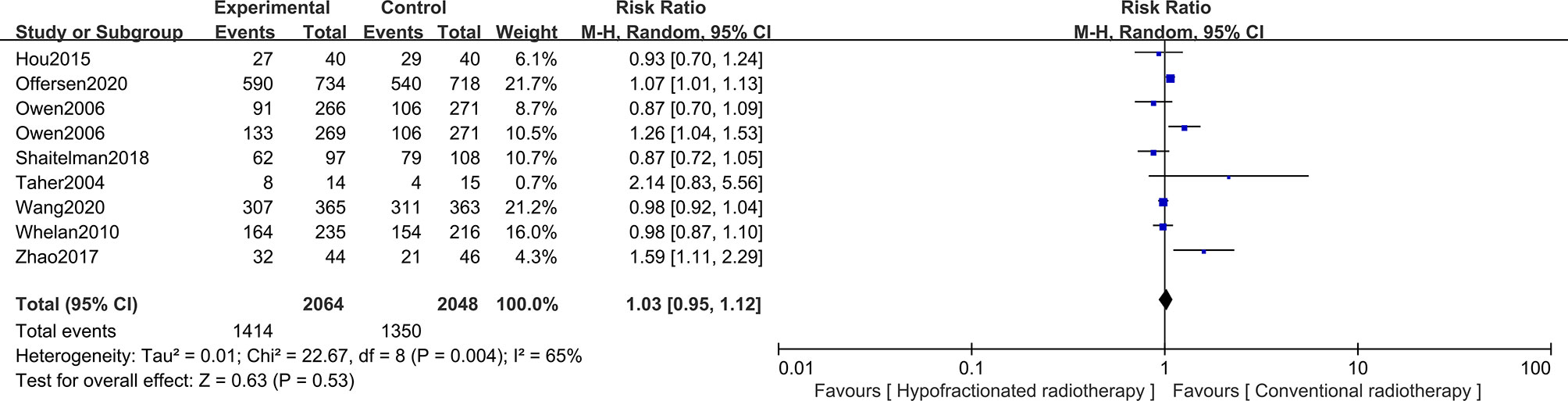
Figure 5 Forest plot of hypofractionated radiotherapy (HFRT) vs. conventional radiotherapy (CFRT) for cosmetic outcomes (p = 0.53).
In 2018, the evidence-based guidelines provided by the American Society of Radiation Oncology (ASTRO) strongly recommend that, for women undergoing whole-breast irradiation (WBI) with invasive breast cancer, the preferred protocol is hypofractionated whole-breast irradiation (HF-WBI) at 15 fractions of 40 Gy or 16 fractions of 42.5 Gy (33). The guidelines further expanded the range of eligibility of patients to treat breast cancer with HF-WBI and suggest that neither age, tumor grade, nor chemotherapy is a contraindication for HFRT application. Several large clinical trials have laid the foundation for the HFRT plan. The results have shown that the data on local control, survival rate, and recurrence for HFRT are as effective as those for CFRT. Compared with CFRT, HFRT is also advantageous in terms of being relevant to fewer adverse events. Although HFRT has shown superiority to a certain extent, its use is still controversial for patients who suffer from ductal carcinoma in situ (DCIS) and with tumor bed boost, and there are also a few problems in cosmetic outcomes and onset of symptoms of adverse events. For example, it has been suggested that HFRT may increase the development of fibrosis in breast cancer patients (34). There are also studies suggesting that HFRT will enhance the brachial plexopathy rate (35). Therefore, a large amount of evidence is still necessary to consolidate the decision on the proper use of HFRT.
Our meta-analysis evaluated 14 RCTs and showed no statistically significant differences in LR, RFS, OS, cosmetic outcomes, or other adverse events between HFRT and CFRT. In addition, HFRT, compared to CFRT, treats the disease with relatively lower acute skin toxicity and breast atrophy, shorter induration, and less pain.
In terms of effectiveness, two previously published meta-analyses in the same topic analyzed LR and OS (36, 37). The results of our meta-analysis were consistent with their results, showing that there was no statistically significant difference between HFRT and CFRT in LR and OS. Additionally, HFRT and CFRT also showed similar results in RFS in our study. Different fractionated dose schemes mainly depend on the proliferative state of tumor tissue and the tolerance of normal tissue (38). In radiobiology, tissues are divided into early responding and late responding tissues according to the linear quadratic (LQ) model (39). The sensitivity of tissues to the radiotherapy fraction dose was quantified by the α/β value. Tissues with high α/β (>10 Gy) values are the early responding tissues with fast cell proliferation, and vice versa (40). Withers proposed, through the LQ model in radiobiology, that late responding tissues were more sensitive to changes in the fraction dose, which laid the foundation for the treatment with HFRT (41). Then, Douglas applied radiobiology theory to clinical review and concluded that HFRT is potentially effective and safe for tumor treatment (42). It is widely believed that the α/β value of tumor tissue is generally 8–10 Gy. CFRT is based on the assumption that breast cancer is less sensitive to changes in the fraction dose than is normal tissue; therefore, 2 Gy per fraction with a total dose of 50 Gy is capable of protecting healthy tissue from being damaged (43). However, according to the study by Yarnold et al., the α/β value of breast cancer was calculated and was inferred to be low, approximately 4 Gy, which falls in the range 0.75–5.01 Gy. Also, the α/β value of normal breast tissue is about 3 Gy, suggesting that the sensitivity of breast cancer tissue to dose segmentation was similar to that of normal tissue (44). In other words, HFRT could theoretically be similarly effective without a significant increase in adverse effects, making it more beneficial to breast cancer patients (45). HFRT in breast cancer is proposed as an improved approach against traditional radiotherapy based on the dynamics of breast cancer proliferation. The main purpose of HFRT is to protect normal tissues and specifically kill tumor cells with maximum lethality. Therefore, in this study, we used data to verify the authenticity of the theory.
When analyzing adverse events, we divided them into all grades and moderate/marked adverse events. Ninety-five percent of cancer patients treated with radiotherapy would more or less develop radiation dermatitis, including erythema, dry desquamation, and wet desquamation (46). Our analysis revealed that, compared to CFRT, HFRT has significantly lower all grade and moderate/marked acute skin toxicity. Zhou et al. (37) and Andrade et al. (36) came to the same conclusion that HFRT was associated with less grade 2/3 acute skin toxicity. Moreover, we also observed a significant improvement in terms of less pain suffered by patients in the HFRT group. The improvement in acute skin toxicity and pain relief may be due to the fact that acute toxicity is more dependent on the total dose than the fraction size (47). Thus, HFRT may minify the acute toxicity by decreasing the total dose. Breast induration is the possible outcome of advanced fibrosis following radiotherapy for breast cancer (48), and the possibility of breast fibrosis increased for 2 years after radiotherapy (49). It has been suggested that HFRT increases the incidence of fibrosis (34), but our data showed that the incidence of breast induration was improved in the HFRT group. The mechanism of HFRT developing less induration is not yet clear. One conjecture is that the reduction in the total dose led to this beneficial characteristic, or, from a radiobiological point of view, the limit to avoid chronic toxicity increase is 3.2–3.3 Gy per segment (50).
Our analysis showed that, compared to CFRT, fewer side effects in terms of all grade breast atrophy and moderate/marked breast atrophy were observed for HFRT. Breast atrophy in patients with breast cancer may be linked to the reduced estrogen levels. Some patients have received anti-estrogen-based endocrine therapy, thus losing estrogen stimulation and producing atrophy (51). However, it is still difficult to explain why HFRT is superior to CFRT in terms of breast atrophy, and no relevant studies have been reported to date.
The lung can become a threatening organ. This is because pneumonia is a common and a serious complication after radiotherapy for breast cancer (52). Severe radiation pneumonia has been reported to have a negative impact on the survival of breast cancer patients who had undergone radiotherapy (53). In this analysis, there was no statistically significant difference in pneumonia between the HFRT and CFRT groups. In fact, the incidence of radiation-induced lung injury was much lower under the modern treatment method (54). Meanwhile, pneumonia caused by chemotherapy drugs and radiation damage may accumulate (55). Therefore, the radiation-pneumonia with HFRT still requires further investigation.
Radiotherapy for breast cancer may involve radioactive exposure of the heart, which can cause ischemic heart disease (56). After radiotherapy for cancer in the left breast, it is easy to develop ischemic heart disease. This has been recognized as a rare but associated sequelae and is generally considered disadvantageous in terms of survival benefits when looking much further afield (57). Studies have suggested that there is no safe threshold to the heart for breast cancer radiotherapy. The damage is potentially threatening as long as there is a dose of radiotherapy. However, in the studies we included, the incidence of ischemic heart disease was relatively rare, and it was only in the Canadian trial that a few deaths were observed (15). In the study of Zhou et al., there was no significant statistical difference between HFRT and CFRT in the incidence of developing ischemic heart disease (37). The α/β ratio of the heart is relatively low. Studies have found that when the α/β is equal to 3 Gy, the bioequivalent dose of HFRT to the heart is lower than that of CFRT, which may be the reason why there was no statistical difference between HFRT and CFRT regarding ischemic heart disease (58). Unfortunately, due to the lack of data, our study was unable to provide data in this regard.
Andrade et al. (36) reported that HFRT had a better outcome than CFRT in other adverse events such as telangiectasia and breast edema, but we failed to come up with a similar conclusion. The different results in the analysis of telangiectasia may be due to the small sample size. We included 1,709 patients, while the meta-analysis of Andrade et al. included 5,167 patients. The inconsistent results regarding edema may be due to the inclusion of patients with partial mastectomy in their studies, which is not comparable to patients with breast-conserving surgery.
Cosmetic outcomes were evaluated in several studies, primarily by clinicians or nurses, based on the standard European Organization for Research and Treatment of Cancer/Radiation Therapy Oncology Group (EORTC/RTOG) cosmetic scoring system. Radiotherapy may lead to breast volume reduction, breast invagination, pigmented skin telangiectasia, subcutaneous tissue fibrosis, and other adverse reactions. It is undeniable that radiotherapy can result in negative impacts on the appearance of the breast (59). Studies have shown that patients’ dissatisfaction with the cosmetic outcomes may be associated with higher rates of depression (60); thus, we also focused our attention to cosmetic outcomes. Cosmetic outcomes may be related to age, tumor size, cancer stage, and breast volume systemic therapy, among others. In our analysis, excellent/good results in cosmetic outcomes were not significantly different between the HFRT and CFRT groups, which may be due to the improvement of skin toxicity and induration in patients.
Due to lack of data, we did not analyze the cost effectiveness of HFRT versus CFRT, but it has been proven by other studies that HFRT is more cost-effective than CFRT (61). One study has built models to estimate cost effectiveness from the perspective of the social and health sectors; the results revealed that HFRT is more cost-effective than CFRT for women with early breast cancer who need adjuvant radiotherapy (62). A study from the USA reported that HFRT could save the US healthcare system millions of dollars in healthcare costs if it follows evidence-based practice guidelines and if expert advice is chosen appropriately (63). In addition, despite population differences, recent studies have demonstrated for the first time that post-mastectomy HFRT is often more cost-effective than CFRT for women at high risk of breast cancer in China, France, and the United States (64).
In previous studies, the Canadian trial did not use a boost, the START trial used a boost, and Yarnold’s (14) study indicated that a boost after HFRT may increase the risk of late toxicity. The optimal fraction dose scheme for a boost is not yet clear. The recent BIG 3-07/TROG 07.01 trial is exploring this issue (65).
Our search was comprehensive, and in order to improve the reliability of the results with a higher level of evidence, the studies we included were all RCTs. Compared with previously published meta-analysis on the same topic, the study by Andrade et al. (36) had a limited sample size and included only six studies, while that by Zhou et al. (37) included non-RCT studies; the quality of evidence needs to be further confirmed. Moreover, some patients with mastectomy were included in their analyses. However, there are limitations in our study as well. Our subgroup analysis was inadequate due to the lack of data. In terms of the tumor stage of patients, our meta-analysis was not very rigorous. Although Wang’s study included patients with N2-3, we still included this study in our analysis. Some of the hotspot issues were not further stratified for analysis, such as whether a boost was used, DCIS patients, systemic treatment, and a stratified follow-up time.
From what has been discussed above, in patients with early breast cancer after breast-conserving surgery, HFRT and CFRT showed consistent outcomes in LR, RFS, and OS. HFRT is generally safe and does not differ from CFRT in terms of adverse events such as pneumonia, telangiectasia, and breast edema. Also, HFRT has better outcomes in acute skin toxicity, induration, breast atrophy, and pain. In addition, HFRT and CFRT have shown similar results regarding cosmetic outcomes. Currently, the safety and efficacy of HFRT have been examined to some extent, but it has not been fully utilized in clinical practice and needs to be further improved.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
LG, FW, and WD contributed to research conception and design. LG, RF, and KJ collected the data. JS, YS, HL, and MZ interpreted the data. All authors contributed to the drafting of the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.753209/full#supplementary-material
RCT, randomized controlled trial; HR, hazard ratio; RR, risk ratio; CI, confidence interval; LR, local recurrence; RFS, relapse-free survival; OS, overall survival; CFRT, conventional radiotherapy; HFRT, hypofractionated radiotherapy; IARC, International Agency for Research on Cancer; EBCTCG, Early Breast Cancer Trialists’ Collaborative Group; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; ASTRO, American Society of Radiation Oncology; WBI, whole-breast irradiation; HF-WBI, hypofractionated whole-breast irradiation; DCIS, ductal carcinoma in situ; LQ, linear quadratic; EORTC/RTOG, European Organization for Research and Treatment of Cancer/Radiation Therapy Oncology Group.
1. Wild C, Weiderpass E, Stewart BW. World Cancer Report: Cancer Research for Cancer Prevention, IARC Press, 2020. World Cancer Report (2020).
2. Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, et al. Global Surveillance of Trends in Cancer Survival 2000-14 (CONCORD-3): Analysis of Individual Records for 37 513 025 Patients Diagnosed With One of 18 Cancers From 322 Population-Based Registries in 71 Countries. Lancet (London England) (2018) 391:1023–75. doi: 10.1016/S0140-6736(17)33326-3
3. Pusic A, Thompson TA, Kerrigan CL, Sargeant R, Slezak S, Chang BW, et al. Surgical Options for the Early-Stage Breast Cancer: Factors Associated With Patient Choice and Postoperative Quality of Life. Plast Reconstructive Surg (1999) 104:1325–33. doi: 10.1097/00006534-199910000-00013
4. van Maaren MC, de Munck L, de Bock GH, Jobsen JJ, van Dalen T, Linn SC, et al. 10 Year Survival After Breast-Conserving Surgery Plus Radiotherapy Compared With Mastectomy in Early Breast Cancer in the Netherlands: A Population-Based Study. Lancet Oncol (2016) 17:1158–70. doi: 10.1016/S1470-2045(16)30067-5
5. Sun Y, Kim SW, Heo CY, Kim D, Hwang Y, Yom CK, et al. Comparison of Quality of Life Based on Surgical Technique in Patients With Breast Cancer. Japanese J Clin Oncol (2014) 44:22–7. doi: 10.1093/jjco/hyt176
6. Kim MK, Kim T, Moon HG, Jin US, Kim K, Kim J, et al. Effect of Cosmetic Outcome on Quality of Life After Breast Cancer Surgery. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol (2015) 41:426–32. doi: 10.1016/j.ejso.2014.12.002
7. Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, et al. Effect of Radiotherapy After Breast-Conserving Surgery on 10-Year Recurrence and 15-Year Breast Cancer Death: Meta-Analysis of Individual Patient Data for 10,801 Women in 17 Randomised Trials. Lancet (London England) (2011) 378:1707–16. doi: 10.1016/S0140-6736(11)61629-2
8. Veronesi U, Stafyla V, Petit JY, Veronesi P. Conservative Mastectomy: Extending the Idea of Breast Conservation. Lancet Oncol (2012) 13:e311–7. doi: 10.1016/S1470-2045(12)70133-X
9. Whelan T, Levine M, Sussman J. Hypofractionated Breast Irradiation: What's Next? J Clin Oncol (2020) 38:3245–7. doi: 10.1200/JCO.20.01243
10. Royce TJ, Gupta GP, Marks LB. Breast Conservation Therapy Versus Mastectomy for Breast Cancer. Lancet Oncol (2020) 21:492–3. doi: 10.1016/S1470-2045(20)30172-8
11. Hunter D, Mauldon E, Anderson N. Cost-Containment in Hypofractionated Radiation Therapy: A Literature Review. J Med Radiat Sci (2018) 65:148–57. doi: 10.1002/jmrs.273
12. Gupta A, Ohri N, Haffty BG. Hypofractionated Radiation Treatment in the Management of Breast Cancer. Expert Rev Anticancer Ther (2018) 18:793–803. doi: 10.1080/14737140.2018.1489245
13. Jagsi R, Griffith KA, Boike TP, Walker E, Nurushev T, Grills IS, et al. Differences in the Acute Toxic Effects of Breast Radiotherapy by Fractionation Schedule: Comparative Analysis of Physician-Assessed and Patient-Reported Outcomes in a Large Multicenter Cohort. JAMA Oncol (2015) 1:918–30. doi: 10.1001/jamaoncol.2015.2590
14. Owen JR, Ashton A, Bliss JM, Homewood J, Harper C, Hanson J, et al. Effect of Radiotherapy Fraction Size on Tumour Control in Patients With Early-Stage Breast Cancer After Local Tumour Excision: Long-Term Results of a Randomised Trial. Lancet Oncol (2006) 7:467–71. doi: 10.1016/S1470-2045(06)70699-4
15. Whelan TJ, Pignol JP, Levine MN, Julian JA, MacKenzie R, Parpia S, et al. Long-Term Results of Hypofractionated Radiation Therapy for Breast Cancer. New Engl J Med (2010) 362:513–20. doi: 10.1056/NEJMoa0906260
16. Bentzen SM, Agrawal RK, Aird EG, Barrett JM, Barrett-Lee PJ, Bentzen SM, et al. The UK Standardisation of Breast Radiotherapy (START) Trial B of Radiotherapy Hypofractionation for Treatment of Early Breast Cancer: A Randomised Trial. Lancet (London England) (2008) 371:1098–107. doi: 10.1016/S0140-6736(08)60348-7
17. Bentzen SM, Agrawal RK, Aird EG, Barrett JM, Barrett-Lee PJ, Bliss JM, et al. The UK Standardisation of Breast Radiotherapy (START) Trial A of Radiotherapy Hypofractionation for Treatment of Early Breast Cancer: A Randomised Trial. Lancet Oncol (2008) 9:331–41. doi: 10.1016/S1470-2045(08)70077-9
18. Mitin T, Kubicky CD. The Use of Hypofractionated Whole Breast Irradiation in Treatment of Patients With Early-Stage Breast Cancer in the United States. JAMA Oncol (2015) 1:245–6. doi: 10.1001/jamaoncol.2014.321
19. Bekelman JE, Sylwestrzak G, Barron J, Liu J, Epstein AJ, Freedman G, et al. Uptake and Costs of Hypofractionated vs Conventional Whole Breast Irradiation After Breast Conserving Surgery in the United States, 2008-2013. Jama (2014) 312:2542–50. doi: 10.1001/jama.2014.16616
20. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PloS Med (2009) 6:e1000100. doi: 10.1371/journal.pmed.1000100
21. Spooner D, Stocken DD, Jordan S, Bathers S, Dunn JA, Jevons C, et al. A Randomised Controlled Trial to Evaluate Both the Role and the Optimal Fractionation of Radiotherapy in the Conservative Management of Early Breast Cancer. Clin Oncol (R Coll Radiol) (2012) 24:697–706. doi: 10.1016/j.clon.2012.08.003
22. Hou HL, Song YC, Li RY, Zhu L, Zhao LJ, Yuan ZY, et al. Similar Outcomes of Standard Radiotherapy and Hypofractionated Radiotherapy Following Breast-Conserving Surgery. Med Sci Monit (2015) 21:2251–6. doi: 10.12659/MSM.893585
23. Amouzegar Hashemi F, Barzegartahamtan M, Mohammadpour RA, Sebzari A, Kalaghchi B, Haddad P. Comparison of Conventional and Hypofractionated Radiotherapy in Breast Cancer Patients in Terms of 5-Year Survival, Locoregional Recurrence, Late Skin Complications and Cosmetic Results. Asian Pac J Cancer Prev (2016) 17:4819–23. doi: 10.22034/APJCP.2016.17.11.4819
24. De Felice F, Ranalli T, Musio D, Lisi R, Rea F, Caiazzo R, et al. Relation Between Hypofractionated Radiotherapy, Toxicity and Outcome in Early Breast Cancer. Breast J (2017) 23:563–8. doi: 10.1111/tbj.12792
25. Zhao S, Liu Y, Huang F, Chen X, Cao X, Yu J. The Long-Term Outcome of Adjuvant Hypofractionated Radiotherapy and Conventional Fractionated Radiotherapy After Breast-Conserving Surgery for Early Breast Cancer: A Prospective Analysis of 107 Cases. J Thorac Dis (2017) 9:3840–50. doi: 10.21037/jtd.2017.09.125
26. Shaitelman SF, Lei X, Thompson A, Schlembach P, Bloom ES, Arzu IY, et al. Three-Year Outcomes With Hypofractionated Versus Conventionally Fractionated Whole-Breast Irradiation: Results of a Randomized, Noninferiority Clinical Trial. J Clin Oncol (2018) 36:JCO1800317. doi: 10.1200/JCO.18.00317
27. Schmeel LC, Koch D, Schmeel FC, Rohner F, Schoroth F, Bucheler BM, et al. Acute Radiation-Induced Skin Toxicity in Hypofractionated vs. Conventional Whole-Breast Irradiation: An Objective, Randomized Multicenter Assessment Using Spectrophotometry. Radiother Oncol (2020) 146:172–9. doi: 10.1016/j.radonc.2020.02.018
28. Haviland JS, Owen JR, Dewar JA, Agrawal RK, Barrett J, Barrett-Lee PJ, et al. The UK Standardisation of Breast Radiotherapy (START) Trials of Radiotherapy Hypofractionation for Treatment of Early Breast Cancer: 10-Year Follow-Up Results of Two Randomised Controlled Trials. Lancet Oncol (2013) 14:1086–94. doi: 10.1016/S1470-2045(13)70386-3
29. Taher AN, El-Baradie MM, Essa H, Zaki O, Ezzat S. Hypofractionation Versus Conventional Fractionation Radiotherapy After Conservative Treatment of Breast Cancer: Early Skin Reactions and Cosmetic Results. J Egyptian Natl Cancer Institute (2004) 16:178–87.
30. Fragkandrea I, Kouloulias V, Mavridis P, Zettos A, Betsou S, Georgolopoulou P, et al. Radiation Induced Pneumonitis Following Whole Breast Radiotherapy Treatment in Early Breast Cancer Patients Treated With Breast Conserving Surgery: A Single Institution Study. Hippokratia (2013) 17:233–8. doi: 10.6061/clinics/2013(11)15
31. Wang SL, Fang H, Hu C, Song YW, Wang WH, Jin J, et al. Hypofractionated Versus Conventional Fractionated Radiotherapy After Breast-Conserving Surgery in the Modern Treatment Era: A Multicenter, Randomized Controlled Trial From China. J Clin Oncol (2020) 38:3604–14. doi: 10.1200/JCO.20.01024
32. Offersen BV, Alsner J, Nielsen HM, Jakobsen EH, Nielsen MH, Krause M, et al. Hypofractionated Versus Standard Fractionated Radiotherapy in Patients With Early Breast Cancer or Ductal Carcinoma In Situ in a Randomized Phase III Trial: The DBCG HYPO Trial. J Clin Oncol (2020) 38:3615–25. doi: 10.1200/JCO.20.01363
33. Smith BD, Bellon JR, Blitzblau R, Freedman G, Haffty B, Hahn C, et al. Radiation Therapy for the Whole Breast: Executive Summary of an American Society for Radiation Oncology (ASTRO) Evidence-Based Guideline. Pract Radiat Oncol (2018) 8:145–52. doi: 10.1016/j.prro.2018.01.012
34. Digesù C, Deodato F, Macchia G, Cilla S, Pieri M, Zamagni A, et al. Hypofractionated Radiotherapy After Conservative Surgery May Increase Low-Intermediate Grade Late Fibrosis in Breast Cancer Patients. Breast Cancer (Dove Med Press) (2018) 10:143–51. doi: 10.2147/BCTT.S167914
35. Koulis TA, Phan T, Olivotto IA. Hypofractionated Whole Breast Radiotherapy: Current Perspectives. Breast Cancer (Dove Med Press) (2015) 7:363–70. doi: 10.2147/BCTT.S81710
36. Andrade TRM, Fonseca MCM, Segreto HRC, Segreto RA, Martella E, Nazário ACP. Meta-Analysis of Long-Term Efficacy and Safety of Hypofractionated Radiotherapy in the Treatment of Early Breast Cancer. Breast (Edinburgh Scotland) (2019) 48:24–31. doi: 10.1016/j.breast.2019.08.001
37. Zhou ZR, Mei X, Chen XX, Yang ZZ, Hou J, Zhang L, et al. Systematic Review and Meta-Analysis Comparing Hypofractionated With Conventional Fraction Radiotherapy in Treatment of Early Breast Cancer. Surg Oncol (2015) 24:200–11. doi: 10.1016/j.suronc.2015.06.005
38. Schoenfeld JD, Harris JR. Abbreviated Course of Radiotherapy (RT) for Breast Cancer. Breast (Edinburgh Scotland) (2011) 20 Suppl 3:S116–27. doi: 10.1016/S0960-9776(11)70308-3
39. Bruni C, Conte F, Papa F, Sinisgalli C. Optimal Number and Sizes of the Doses in Fractionated Radiotherapy According to the LQ Model. Math Med Biol J IMA (2019) 36:1–53. doi: 10.1093/imammb/dqx020
40. van Leeuwen CM, Oei AL, Crezee J, Bel A, Franken NAP, Stalpers LJA, et al. The Alfa and Beta of Tumours: A Review of Parameters of the Linear-Quadratic Model, Derived From Clinical Radiotherapy Studies. Radiat Oncol (London England) (2018) 13:96. doi: 10.1186/s13014-018-1040-z
41. Fowler JF. The Linear-Quadratic Formula and Progress in Fractionated Radiotherapy. Br J Radiol (1989) 62:679–94. doi: 10.1259/0007-1285-62-740-679
42. Douglas BG. Superfractionation: Its Rationale and Anticipated Benefits. Int J Radiat Oncol Biol Phys (1982) 8:1143–53. doi: 10.1016/0360-3016(82)90062-1
43. Nahum AE. The Radiobiology of Hypofractionation. Clin Oncol (R Coll Radiol) (2015) 27:260–9. doi: 10.1016/j.clon.2015.02.001
44. Wei NN, Li F, Cai P, Yin HM, Zhu CM, Zhang Q, et al. Progress of Clinical Study on Hypofractionated Radiotherapy After Breast-Conserving Surgery. Ann Palliative Med (2020) 9:463–71. doi: 10.21037/apm.2020.02.18
45. Qi XS, White J, Li XA. Is α/β for Breast Cancer Really Low? Radiother Oncol (2011) 100:282–8. doi: 10.1016/j.radonc.2011.01.010
46. Singh M, Alavi A, Wong R, Akita S. Radiodermatitis: A Review of Our Current Understanding. Am J Clin Dermatol (2016) 17:277–92. doi: 10.1007/s40257-016-0186-4
47. Arsenault J, Parpia S, Goldberg M, Rakovitch E, Reiter H, Doherty M, et al. Acute Toxicity and Quality of Life of Hypofractionated Radiation Therapy for Breast Cancer. Int J Radiat Oncol Biol Phys (2020) 107:943–8. doi: 10.1016/j.ijrobp.2020.03.049
48. Padhani AR, Yarnold JR, Regan J, Husband JE. Magnetic Resonance Imaging of Induration in the Irradiated Breast. Radiother Oncol (2002) 64:157–62. doi: 10.1016/S0167-8140(02)00137-8
49. Lam E, Yee C, Wong G, Popovic M, Drost L, Pon K, et al. A Systematic Review and Meta-Analysis of Clinician-Reported Versus Patient-Reported Outcomes of Radiation Dermatitis. Breast (Edinburgh Scotland) (2020) 50:125–34. doi: 10.1016/j.breast.2019.09.009
50. Sanz J, Zhao M, Rodríguez N, Granado R, Foro P, Reig A, et al. Once-Weekly Hypofractionated Radiotherapy for Breast Cancer in Elderly Patients: Efficacy and Tolerance in 486 Patients. BioMed Res Int (2018) 2018:8321871. doi: 10.1155/2018/8321871
51. Falk SJ, Bober S. Vaginal Health During Breast Cancer Treatment. Curr Oncol Rep (2016) 18:32. doi: 10.1007/s11912-016-0517-x
52. Omarini C, Thanopoulou E, Johnston SR. Pneumonitis and Pulmonary Fibrosis Associated With Breast Cancer Treatments. Breast Cancer Res Treat (2014) 146:245–58. doi: 10.1007/s10549-014-3016-5
53. Werner EM, Eggert MC, Bohnet S, Rades D. Prevalence and Characteristics of Pneumonitis Following Irradiation of Breast Cancer. Anticancer Res (2019) 39:6355–8. doi: 10.21873/anticanres.13847
54. Meattini I, Lambertini M, Desideri I, De Caluwé A, Kaidar-Person O, Livi L. Radiation Therapy for Young Women With Early Breast Cancer: Current State of the Art. Crit Rev Oncol/Hematol (2019) 137:143–53. doi: 10.1016/j.critrevonc.2019.02.014
55. Robnett TJ, Machtay M, Vines EF, McKenna MG, Algazy KM, McKenna WG. Factors Predicting Severe Radiation Pneumonitis in Patients Receiving Definitive Chemoradiation for Lung Cancer. Int J Radiat Oncol Biol Phys (2000) 48:89–94. doi: 10.1016/S0360-3016(00)00648-9
56. Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Brønnum D, et al. Risk of Ischemic Heart Disease in Women After Radiotherapy for Breast Cancer. New Engl J Med (2013) 368:987–98. doi: 10.1056/NEJMoa1209825
57. Piroth MD, Baumann R, Budach W, Dunst J, Feyer P, Fietkau R, et al. Heart Toxicity From Breast Cancer Radiotherapy : Current Findings, Assessment, and Prevention. Strahlentherapie Und Onkol Organ Der Deutschen Rontgengesellschaft (2019) 195:1–12. doi: 10.1007/s00066-018-1378-z
58. James M, Swadi S, Yi M, Johansson L, Robinson B, Dixit A. Ischaemic Heart Disease Following Conventional and Hypofractionated Radiation Treatment in a Contemporary Breast Cancer Series. J Med Imaging Radiat Oncol (2018) 62:425–31. doi: 10.1111/1754-9485.12712
59. Ciammella P, Podgornii A, Galeandro M, Micera R, Ramundo D, Palmieri T, et al. Toxicity and Cosmetic Outcome of Hypofractionated Whole-Breast Radiotherapy: Predictive Clinical and Dosimetric Factors. Radiat Oncol (London England) (2014) 9:97. doi: 10.1186/1748-717X-9-97
60. Ho PJ, Hartman M, Young-Afat DA, Gernaat SAM, Lee SC, Verkooijen HM. Determinants of Satisfaction With Cosmetic Outcome in Breast Cancer Survivors: A Cross-Sectional Study. PloS One (2018) 13:e0193099. doi: 10.1371/journal.pone.0193099
61. Monten C, Lievens Y. Adjuvant Breast Radiotherapy: How to Trade-Off Cost and Effectiveness? Radiother Oncol (2018) 126:132–8. doi: 10.1016/j.radonc.2017.11.005
62. Deshmukh AA, Shirvani SM, Lal L, Swint JM, Cantor SB, Smith BD, et al. Cost-Effectiveness Analysis Comparing Conventional, Hypofractionated, and Intraoperative Radiotherapy for Early-Stage Breast Cancer. J Natl Cancer Institute (2017) 109:1–9. doi: 10.1093/jnci/djx068
63. Niska JR, Keole SR, Pockaj BA, Halyard MY, Patel SH, Northfelt DW, et al. Choosing Wisely After Publication of Level I Evidence in Breast Cancer Radiotherapy. Breast Cancer (Dove Med Press) (2018) 10:31–7. doi: 10.2147/BCTT.S153117
64. Yang J, Qi SN, Fang H, Song YW, Jin J, Liu YP, et al. Cost-Effectiveness of Postmastectomy Hypofractionated Radiation Therapy vs Conventional Fractionated Radiation Therapy for High-Risk Breast Cancer. Breast (Edinburgh Scotland) (2021) 58:72–9. doi: 10.1016/j.breast.2021.04.002
65. King MT, Link EK, Whelan TJ, Olivotto IA, Kunkler I, Westenberg AH, et al. Quality of Life After Breast-Conserving Therapy and Adjuvant Radiotherapy for non-Low-Risk Ductal Carcinoma In Situ (BIG 3-07/TROG 07.01): 2-Year Results of a Randomised, Controlled, Phase 3 Trial. Lancet Oncol (2020) 21:685–98. doi: 10.1016/S1470-2045(20)30085-1
Keywords: breast cancer, hypofractionated radiotherapy, conventional fractionated radiotherapy, breast-conserving surgery, meta-analysis
Citation: Gu L, Dai W, Fu R, Lu H, Shen J, Shi Y, Zhang M, Jiang K and Wu F (2021) Comparing Hypofractionated With Conventional Fractionated Radiotherapy After Breast-Conserving Surgery for Early Breast Cancer: A Meta-Analysis of Randomized Controlled Trials. Front. Oncol. 11:753209. doi: 10.3389/fonc.2021.753209
Received: 05 August 2021; Accepted: 09 September 2021;
Published: 01 October 2021.
Edited by:
Tadahiko Shien, Okayama University, JapanReviewed by:
Ikuno Nishibuchi, Hiroshima University, JapanCopyright © 2021 Gu, Dai, Fu, Lu, Shen, Shi, Zhang, Jiang and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng Wu, SGZydHNsanM4OEAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.