
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 03 January 2022
Sec. Head and Neck Cancer
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.746776
Background: Ultrasound, cytology, and BRAFV600E mutation analysis were applied as valuable tools in the differential diagnosis of thyroid nodules. The aim of the present study was to evaluate the diagnostic efficiency of the three methods and their combined use in screening for papillary thyroid microcarcinoma (PTMC).
Methods: A total of 1,081 patients with 1,157 thyroid nodules (0.5–1 cm in maximum diameter) classified as thyroid imaging reporting and data system (TIRADS) 4–5 were recruited. All patients underwent ultrasound, fine-needle aspiration (FNA) examination, and an additional BRAFV600E mutation test. TIRADS and Bethesda System for Reporting Thyroid Cytopathology (BSRTC) were adopted to judge the ultrasound and cytological results. The receiver operating characteristic (ROC) curve was established to assess the diagnostic values of different methods.
Results: Of the 1,157 nodules, 587 were benign and 570 were PTMCs. BRAFV600E mutation test had highest sensitivity (85.4%), specificity (97.1%), accuracy (91.4%), and area under the ROC curve (Az) value (0.913) among the three methods. The combination of BSRTC and BRAFV600E mutation analysis yielded a considerably high sensitivity (96.0%), accuracy (94.3%), and negative predictive value (95.9%) than either BSRTC or BRAFV600E mutation alone (P < 0.0001 for all comparisons). Of all the methods, the combined use of the three methods produced the best diagnostic performance (Az = 0.967), which was significantly higher than that (Az = 0.943) for the combination of BSRTC and BRAFV600E mutation (P < 0.0001). The diagnostic accuracy of the molecular method in the 121 nodules with indeterminate cytology was 90.1% (109/121), which was significantly higher than that of TIRADS classification, 74.4% (90/121) (P = 0.002).
Conclusion: The combined use of ultrasound, cytology, and BRAFV600E mutation analysis is the most efficient and objective method for diagnosing PTMC. Both BRAFV600E mutation and TIRADS classification are potentially useful adjuncts to differentiate thyroid nodules, especially indeterminate samples classified as BSRTC III.
Papillary thyroid microcarcinoma (PTMC) is defined as papillary thyroid carcinoma (PTC) that is less than 1 cm in its greatest diameter (1). It is the most common form of thyroid cancer, accounting for approximately half of the increased incidence in PTC (2). The early clinical recognition of PTMC is important due to high risk of lymph node metastasis and multicentricity. According to the previous reports, lymph node metastasis is highly prevalent in PTMC, resulting in a poor prognosis (3–5).
Ultrasound is the most sensitive modality available to detect thyroid nodules and useful in selecting the high-risk lesions for fine-needle aspiration (FNA) by detecting ultrasonographic features suggestive of malignancy. Thyroid imaging reporting and data system (TIRADS) was carried out and utilized by radiologists worldwide on the basis of ultrasonographic imaging features to stratify malignant risks of thyroid nodules (6). The diagnosis of PTMC relies on ultrasound examinations, whereas the lesions are difficult to assess and easily misdiagnosed due to their small size and atypical ultrasonographic features (7, 8).
Ultrasound-guided FNA is a routine and reliable approach for preoperative evaluation of thyroid nodules (9). Bethesda System for Reporting Thyroid Cytopathology (BSRTC) is developed to provide uniform terminology and diagnostic criteria, which has proven to be an effective and robust thyroid FNA classification scheme to guide the clinical management of patients with thyroid nodules (10). Despite the high sensitivity and specificity of thyroid cytology, the highlighted limitation is that about 30% of FNA cytologic findings remain non-diagnostic or indeterminate and may complicate the management of thyroid nodules (11). The prevalence of malignant tumors among these lesions varies from 10% to 52%, being mostly represented by PTCs that are diagnosed postoperatively (12).
A number of genetic analyses have been found to enhance the diagnostic accuracy of cytology and as a predictor of poor prognosis in patients with thyroid carcinomas. The most promising of these is the application of BRAFV600E mutation analysis as a molecular marker for the detection of PTC. Numerous researchers have proven that the detection of BRAFV600E mutation in FNA specimens refines the FNA-cytology diagnosis, especially in a BRAFV600E mutation-prevalent area (13, 14), while others believe that its utility is limited by low prevalence of BRAFV600E mutation in indeterminate nodules (15–17). Further studies are needed to identify which thyroid nodules should be rescreened using molecular tests.
The positive detection rate of BRAFV600E mutation is associated with tumor size (18, 19). Moreover, the size of thyroid lesions might also have an impact on the diagnostic efficiency of ultrasound and FNA. To overcome and supplement the individual shortcomings of conventional ultrasound, ultrasound-guided FNA, and BRAF gene mutation analysis, several previous studies have tried to predict the probability of PTC by combining them (15, 20, 21), but none has focused on PTMC.
The purpose of this study, therefore, was to compare the diagnostic efficacy of ultrasound, cytology, and BRAFV600E mutation analysis in the detection of PTMC and to explore the combined use of these methods for better diagnostic performance, especially focusing our attention on indeterminate thyroid nodules classified as BSRTC category III.
This prospective study was approved by the Ethics Committee of Renji Hospital, and written informed consent was obtained from each patient for ultrasound-guided FNA and BRAFV600E mutation analysis prior to each procedure. This investigation was carried out following the rules of the Declaration of Helsinki. Between April 2019 and September 2020, 1,251 consecutive patients with 1,335 nodules (TIRADS categories 4 and 5, measured 5–10 mm in maximum diameter) underwent ultrasound and ultrasound-guided FNA with an additional BRAFV600E mutation test. Of the 1,251 patients, only patients who met the following criteria were included: those who underwent surgery after thyroid ultrasound and FNA, those who underwent FNA at least twice with a 1-year interval for a benign thyroid lesion, and those who underwent FNA and follow-up (F/U) ultrasound (>12 months after FNA cytology diagnosis) for a benign thyroid lesion. All the patients with malignant lesions underwent thyroid lobectomy or total thyroidectomy with unilateral or bilateral prophylactic central neck dissection. Among the 1,251 patients with 1,335 nodules, 80 nodules in 79 patients were excluded because they showed cytologic results of non-diagnostic (n = 4), atypia undetermined significance/follicular lesion of undetermined significance (AUS/FLUS) (n = 18), follicular neoplasm or suspicious for a follicular neoplasm (FN/SFN) (n = 12), suspicious for malignancy (n = 27) and malignancy (n = 19) without further histopathological diagnosis or FNA and ultrasound F/U. Among the 478 nodules with benign cytologic results and negative BRAF gene mutation, 76 nodules in 69 patients were excluded because they did not undergo F/U FNA or at least 1-year F/U ultrasound, and another 17 nodules in 17 patients were excluded because they showed an increase in size in F/U ultrasound without further cytologic or hispathologic evaluation. Other types of thyroid malignancies were also excluded including two medullary thyroid carcinomas and three follicular adenocarcinomas. Finally, 1,081 patients with 1,157 thyroid nodules were included in this study. The mean maximum diameter of the nodules was 7.6 ± 2.3 mm (range, 5.2–9.9 mm). Among the 1,157 nodules, 639 nodules were confirmed by operation (Surgery group) and the other 518 nodules were observed by F/U FNA with an additional BRAFV600E mutation test or F/U ultrasound after a year (Observation group) (Figure 1). Patient characteristics including age, sex, multifocality, lymph node metastasis, association with Hashimoto’s thyroiditis, and family history of thyroid cancer were also recorded.
Conventional ultrasound examination and measurement were performed by an ultrasound radiologist (JD) with 14 years of experience in thyroid ultrasound. A Philips EPIQ 7 scanner (Philips Medical Systems, Bothell, WA, USA) equipped with a 3- to 12-MHz linear-array transducer was used for careful ultrasound examination of the thyroid gland and neck region. The ultrasound criteria that we used in this study for the evaluation of solid and partially cystic thyroid nodules were based on a number of previous studies (22–24). Suspicious ultrasound features of thyroid malignancy were as follows: (a) solid component, hypoechogenicity or marked hypoechogenicity; (b) irregular margins or perinodular thyroid parenchyma invasion; (c) calcifications; (d) taller-than-wide shape; (e) partially cystic nodule with eccentric location of the fluid portion and lobulation of the solid component; (f) intranodular hypervascularization and peripherally penetrating vessels; (g) irregular thick halo; and (h) cervical lymphadenopathy with intranodal cystic components or microcalcifications.
According to TIRADS proposed by Kwak et al. (23) and Sánchez (24), all nodules were classified into four categories: 4a (one suspicious ultrasound feature), 4b (two suspicious ultrasound features), 4c (three or four suspicious ultrasound features), and 5 (five suspicious ultrasound features). Two radiologists (RH and FL, with 11 and 31 years of experience in thyroid ultrasound, respectively) did all the classifications. Every investigator clarified the reasons why they made the classifications, and a consensus was reached in cases of discrepancy. The nodules with TIRADS 4c and 5 were classified as malignant lesions, while those with TIRADS 4a and 4b were considered benign lesions.
Ultrasound-guided FNA with an additional BRAFV600E mutation analysis was performed by an experienced radiologist (JD) using a standardized protocol (20). At our institution, ultrasound-guided FNA was performed in the thyroid nodule with most suspicious ultrasound features. If a patient had multiple suspicious nodules, the one in the same lobe showing the highest risk of malignancy or two nodules in the bilateral thyroid lobes were selected for ultrasound-guided FNA.
Based on BSRTC published by Cibas and Ali (25), FNA cytology results were categorized by two experienced pathologists as I–VI: non-diagnostic, benign, AUS/FLUS, FN/SFN, suspicious for malignancy, and malignancy. BSRTC I–IV were considered benign, while BSRTC V–VI were considered malignant.
BRAFV600E mutation detection was performed using a China Food and Drug Administration (CFDA)-approved human BRAF gene V600E mutation fluorescence polymerase chain reaction (PCR) diagnostic kit (AmoyDX, Xiamen, China). The quantity of isolated DNA was assessed by using a NanoDrop2000 spectrophotometer (Thermo, Los Angeles, CA, USA). The samples were analyzed by applying the amplification refractory mutation system (ARMS) technique (20).
When two diagnostic methods combined, a nodule was considered positive as long as either method was positive. In our study, BRAFV600E mutation showed the highest diagnostic performance for discrimination between benign and malignant thyroid lesions. Cytology or ultrasound alone was not enough to make a definite diagnosis when BRAFV600E mutation was negative. Therefore, the following criteria were included in the combined use of the three methods: (a) when BRAFV600E mutation was positive, thyroid nodules were considered malignant tumors regardless of cytology and ultrasound; (b) when BRAFV600E mutation was negative, thyroid nodules were considered malignant tumors if the results of both cytology and ultrasound were highly suggestive of malignancy; and (c) when BRAFV600E mutation was negative, thyroid nodules were considered an uncertain diagnosis and suggestive of benign possibility if cytology or ultrasound alone was suspicious for malignancy.
The Student’s t-test and Mann–Whitney test were used to analyze numeric variables. Differences in the distribution of categorical variables between groups were evaluated by using a chi-square test. The McNemar chi-square test was used to compare the diagnostic sensitivity, specificity, and accuracy. The receiver operating characteristic (ROC) curve analysis was performed by using MedCalc V.16.4 statistical software (MedCalc Software, Ostend, Belgium). SPSS software (version 13.0, SPSS) was used for the statistical analysis, and a P value of < 0.05 was defined as statistical significance.
The 1,081 patients (815 women, 266 men; mean age, 48.8 years; range, 16–79 years) with 1,157 thyroid nodules were included in this study. There were 554 (51.2%) cases of benign nodules and 527 (48.8%) cases of malignancy. Of the 1,157 nodules, 587 were benign and 570 were malignant. All the diagnoses of malignant lesions were PTMCs. Diagnoses of benign lesions included nodular goiter (n = 376), Hashimoto’s thyroiditis (n = 28), adenoma (n = 102), subacute thyroiditis (n = 49), absorption phase cyst (n = 31), and follicular epithelial dysplasia (n = 1).
Detailed information on demographic and clinicopathologic characteristics of all the subjects enrolled in this study was summarized in Table 1. Patients with benign nodules were older than those with malignant nodules (mean age, 51.4 years ± 13.1 vs. 46.1 years ± 12.6, respectively; P < 0.0001). There was no statistically significant relationship between the risk of malignancy and sex or size (P = 0.452 and P = 0.741, respectively). Tumor multifocality was observed in 43 (8.2%) cases, with PTMCs and lymph node metastases in 150 (28.5%) patients. A total of 112 (21.3%) cases with PTMCs were combined with Hashimoto’s thyroiditis, and 21 (4.0%) had familial genetic history.
A total of 1,157 thyroid nodules were prospectively classified as 4a (n = 322), 4b (n = 354), 4c (n = 439), and 5 (n = 42). The malignancy risk increased as the level of TIRADS classification increased. The malignancy risks of categories 4a, 4b, 4c, and 5 were 14.6%, 36.4%, 80.2%, and 100%, respectively. The ROC curve demonstrated that the optimal cutoff point of TIRADS was 4c. The Az value, sensitivity, specificity, accuracy, positive predictive value (PPV), and negative predictive value (NPV) were 0.814, 69.1%, 85.2%, 77.3%, 81.9%, and 74.0%, respectively.
As shown in Table 2, the detection rates of BSRTC and BRAFV600E mutation for PTMCs increased as the level of TIRADS classification increased. In nodules classified as TIRADS 4a, 4b, 4c, and 5, the detection rates of BRAFV600E mutation were higher than those of BSRTC, whereas a statistical difference was found in only nodules classified as TIRADS 4c (P = 0.016), and there was no statistical difference in nodules classified as TIRADS 4a, 4b, and 5 (72.3% vs. 78.7%, P = 0.629; 74.4% vs. 83.7%, P = 0.088; 83.3% vs. 85.7%, P = 1, respectively). The combination of BSRTC and BRAFV600E mutation significantly increased the detection rates for PTMCs with various levels of TIRADS classification compared with either BSRTC or BRAFV600E mutation alone, and all the detection rates for PTMCs were above 90%. However, in nodules classified as TIRADS 5, no statistical difference was found between the combination of two methods and BSRTC or BRAFV600E mutation alone (P = 0.063 and P = 0.125, respectively). This result indicated that when the nodule was classified as TIRADS category 5, a single detection method could be used to make a definite diagnosis without the need for a combination test.
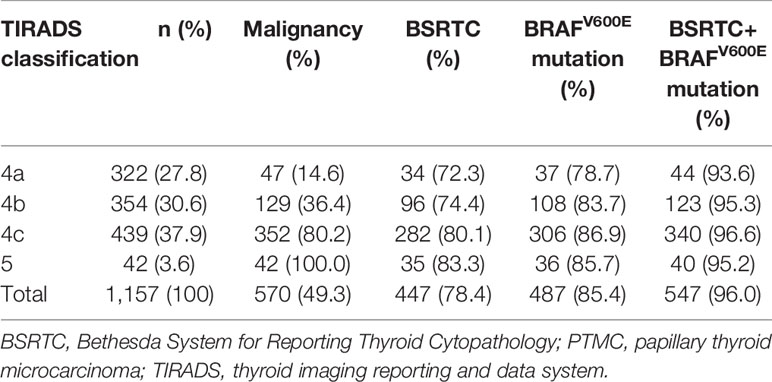
Table 2 Correlations of TIRADS classification with detection rates of BSRTC and BRAFV600E mutation for PTMCs.
Correlations of BSRTC categories with BRAFV600E mutation and histopathological results were shown in Table 3. Only 33 (2.9%) of the thyroid nodules were diagnosed as non-diagnostic based on the FNA cytology; 12 (36.4%) of them were identified as PTMCs by postoperative histopathology. A total of 51 (42.1%) of the 121 nodules with indeterminate cytology were confirmed after surgery as PTMCs. Non-diagnostic and indeterminate cytology were found for 13.3% (154/1,157) of thyroid nodules, and the malignancy rates among these lesions were 40.9% (63/154). The malignancy rates in BSRTC I–VI lesions were 36.4%, 5.8%, 42.1%, 33.6%, 89.7%, and 98.1%, respectively. The rates of BRAFV600E mutation in BSRTC I–VI lesions were 27.3%, 6.5%, 35.5%, 29.9%, 78.2%, and 87.0%, respectively. In BSRTC V–VI thyroid lesions, both the malignancy rates and the BRAFV600E mutation rates increased obviously. The 28 nodules categorized as BSRTC V and four nodules categorized as BSRTC VI proved to be benign lesions postoperatively, including nodular goiter (n = 6), Hashimoto’s thyroiditis with fibrosis and calcification (n = 8), atypical adenoma with fibrosis, calcification, cystic degeneration or papillary hyperplasia (n = 14), subacute thyroiditis (n = 3), and atypical follicular epithelial hyperplasia (n = 1). The ROC curve demonstrated that the optimal cutoff point of cytologic results was BSRTC category V. The Az value, sensitivity, specificity, accuracy, PPV, and NPV were 0.910, 78.4%, 94.5%, 86.6%, 93.3%, and 81.9%, respectively.
Of the 1,157 thyroid nodules, 1,153 (99.7%) specimens had definite genetic results and the remaining four (0.3%) nodules showed suspicious positivity for BRAFV600E mutation. BRAFV600E mutation was detected in 487 (85.4%) of 570 PTMCs. Seventeen (2.9%) of the 587 benign thyroid nodules harbored the BRAFV600E mutation. Of the 678 nodules with negative cytologic results, 111 (16.4%) nodules harbored the BRAFV600E mutation, 100 (90.1%) of them were validated as PTMCs after surgery, while the remaining 11 (9.9%) nodules were diagnosed as benign lesions by surgery (n = 7) or F/U FNA and ultrasound (n = 4). The BRAFV600E mutation rates were more frequent in the malignant category compared with the indeterminate category (BSRTC V vs. BSRTC III, P < 0.0001; BSRTC VI vs. BSRTC III, P < 0.0001) (Table 3). The Az value, sensitivity, specificity, accuracy, PPV, and NPV for BRAFV600E mutation were 0.913, 85.4%, 97.1%, 91.4%, 96.6%, and 87.3%, respectively.
As shown in Table 4, sensitivity, specificity, accuracy, PPV, and NPV for BSRTC categories were significantly higher than those for TIRADS classification (P < 0.0001 for all comparisons). Moreover, BRAFV600E mutation showed higher sensitivity, specificity, accuracy, PPV, and NPV than BSRTC categories (P = 0.002, P = 0.02, P < 0.0001, P = 0.017, and P = 0.006, respectively).
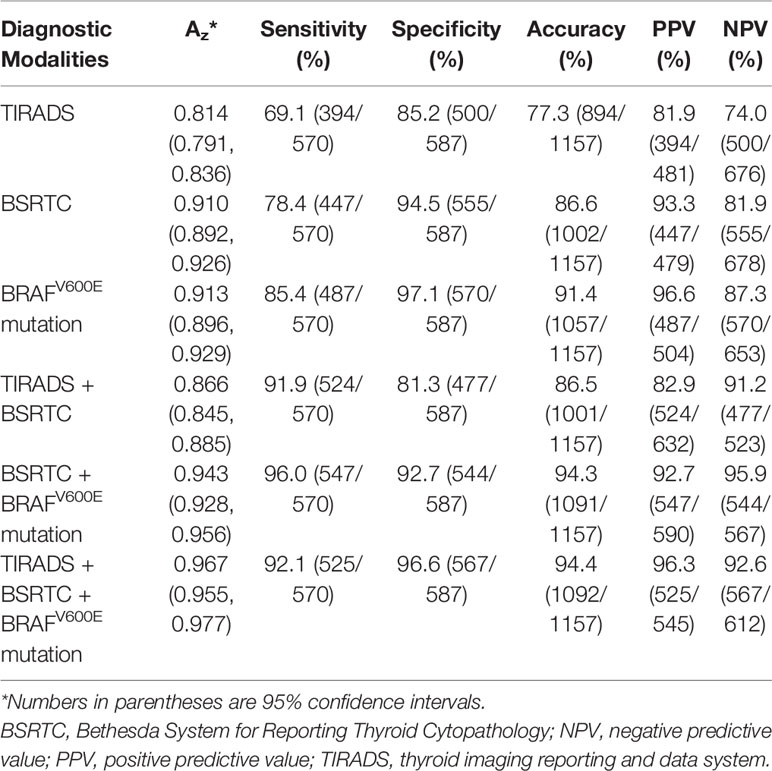
Table 4 Comparison of the diagnostic performance of different modalities for discrimination between benign and malignant thyroid nodules.
Using TIRADS classification, BSRTC categories, and BRAFV600E mutation as the criteria to distinguish malignant from benign thyroid nodules, the Az value was 0.814 for TIRADS classification and 0.910 for BSRTC categories, and the Az value for BRAFV600E mutation was 0.913. The Az values of both BSRTC and BRAFV600E mutation analysis were significantly higher as compared with TIRADS classification (P < 0.0001 and P < 0.0001, respectively), whereas no statistically significant difference was found between them (P = 0.788).
The combined use of TIRADS and BSRTC significantly increased the sensitivity (91.9%) compared to cytology alone, while specificity (81.3%) and accuracy (86.5%) were not improved obviously. The combination of BSRTC and BRAFV600E mutation analysis yielded a considerably high sensitivity, accuracy, and NPV than either BSRTC or BRAFV600E mutation alone (P < 0.0001 for all comparisons). When the three methods were combined, accuracy increased to 94.4%, with an increased specificity and PPV to 96.6% and 96.3% (Figure 2). The combination of the three methods yielded a higher specificity and PPV (P < 0.0001 and P = 0.008, respectively), whereas sensitivity and NPV were lower than those of the combination of BSRTC and BRAFV600E mutation (P < 0.0001 and P = 0.015, respectively). The combination of the three methods could not improve the diagnostic accuracy compared with the combination of BSRTC and BRAFV600E mutation (P = 1) (Table 4).
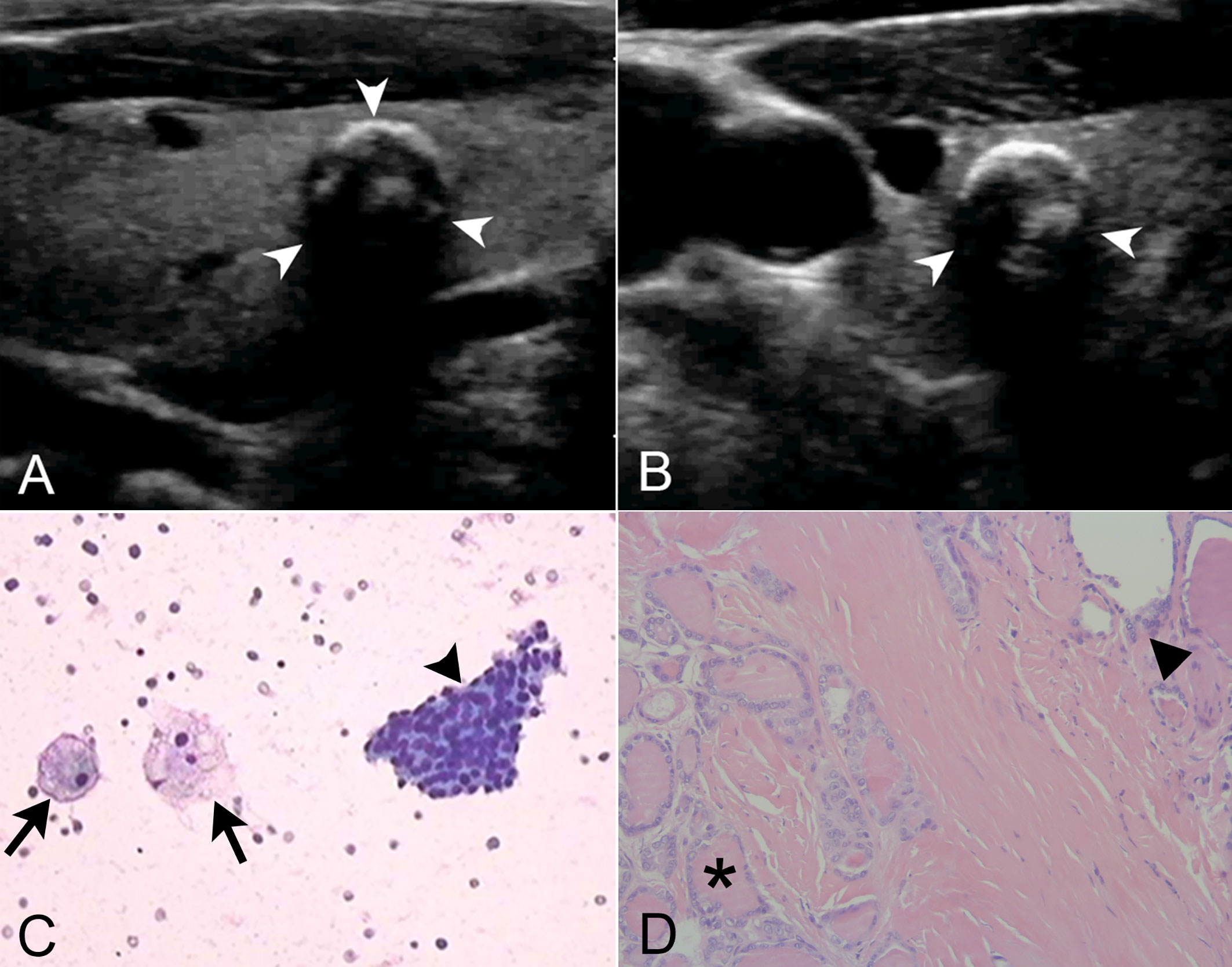
Figure 2 The combined use of thyroid imaging reporting and data system (TIRADS), Bethesda System for Reporting Thyroid Cytopathology (BSRTC), and BRAFV600E mutation analysis in a 59-year-old man with 7.2 mm × 6.1 mm papillary thyroid microcarcinoma (PTMC). (A) Longitudinal ultrasound image showed a regularly shaped, indistinctly marginated, hypoechoic nodule with multiple macrocalcifications in the right lobe of the thyroid gland (arrow heads). (B) Transrectal ultrasound image more clearly showed the peripheral half ring-like macrocalcifications (arrow heads). (C) Cytological pathology showed nodular goiter with cystic change. Lamellar follicular epithelial cells (arrow head) and phagocytes (arrows) were seen, which was consistent with benign lesions (original magnification, ×20). (D) Tumor cells were seen at the lower left (asterisk), and normal thyroid follicles were at the upper right (triangle). Tumor cells were ground glass-like with large and crowded nuclei (original magnification, ×200). This nodule was classified as TIRADS 4a. The fine-needle aspiration (FNA) cytology result was categorized as BSRTC II, suggestive of a benign lesion. However, this nodule with negative cytologic result harbored the BRAFV600E mutation and proven to be PTMC postoperatively. By the combined use of BRAFV600E mutation analysis, this malignant thyroid nodule that was misdiagnosed as benign by ultrasound and cytology got a correct diagnosis.
The Az value for the combined use of the three methods was maximal and that for the combination of BSRTC and BRAFV600E mutation analysis was slightly lower; a statistical difference was found between them (P < 0.0001). The Az value of the combination of BSRTC and BRAFV600E mutation analysis was significantly higher as compared with each method alone or the combination of TIRADS and BSRTC (TIRADS, P < 0.0001; BSRTC, P = 0.0001; BRAFV600E mutation, P = 0.0001; TIRADS and BSRTC, P < 0.0001) (Figure 3).
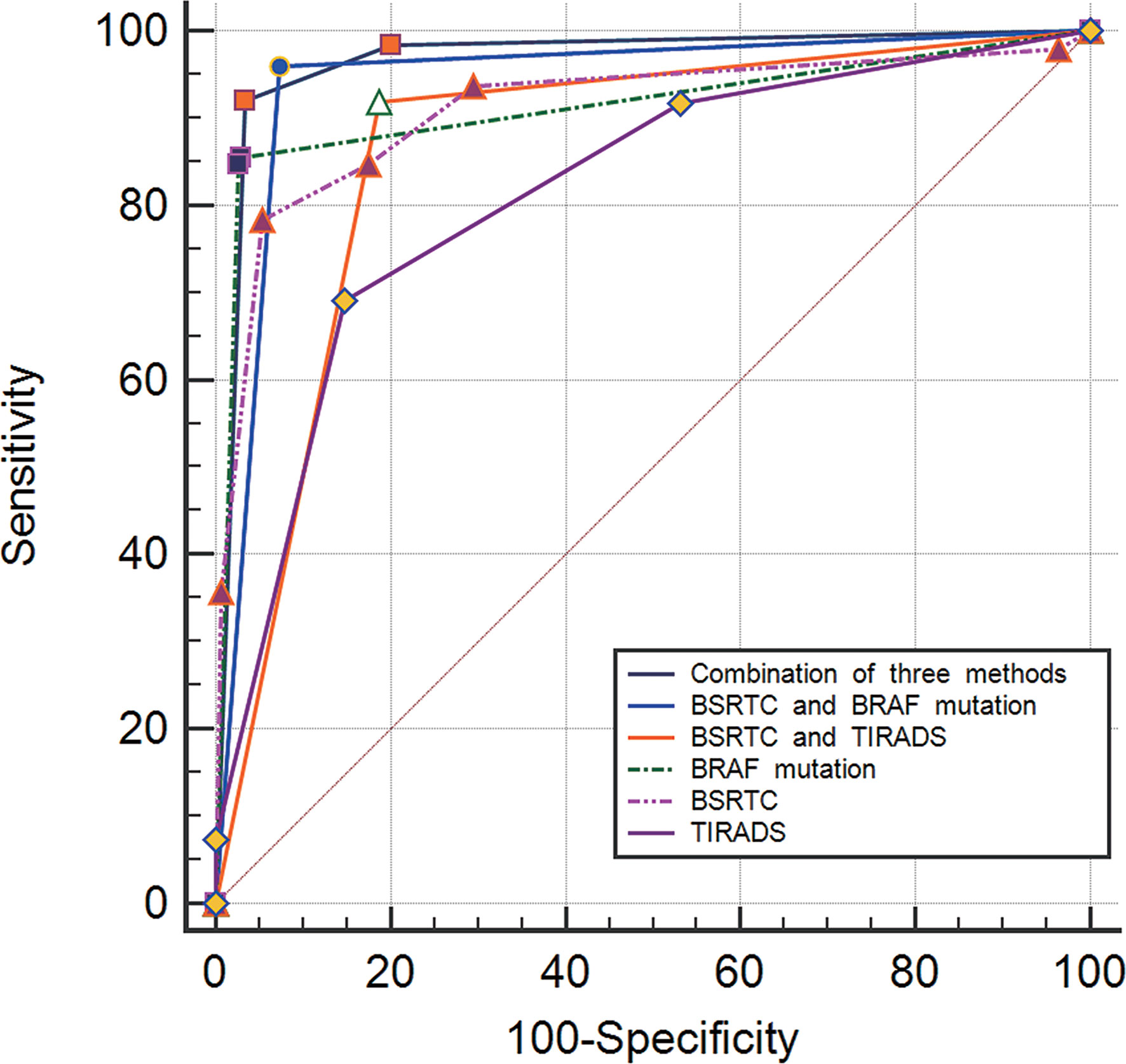
Figure 3 Graph of receiver operating characteristic (ROC) analyses of different diagnostic methods for distinguishing between benign and malignant thyroid nodules.
Of 1,157 thyroid lesions, 121 (10.5%) nodules were classified as BSRTC III, 51 (42.1%) of them were malignant and 70 (57.9%) were benign. TIRADS classification and BRAFV600E mutation status in 121 thyroid nodules with indeterminate cytology were shown in Table 5. The malignancy rates of nodules categorized as TIRADS 4a, 4b, 4c, and 5 were 11.6%, 42.9%, 76.5%, and 100%, respectively. BRAFV600E mutation status was significantly associated with TIRADS classification. Except for only two nodules classified as TIRADS 5, BRAFV600E mutation rate increased as the level of TIRADS classification increased. In nodules classified as TIRADS 4a, 4b, and 4c, the BRAFV600E mutation rates were 11.6%, 38.1%, and 61.8%, respectively. Thyroid nodules with different TIRADS categories showed significant differences in the BRAFV600E mutation rate (4a vs. 4b, P = 0.005; 4b vs. 4c, P = 0.04, respectively).
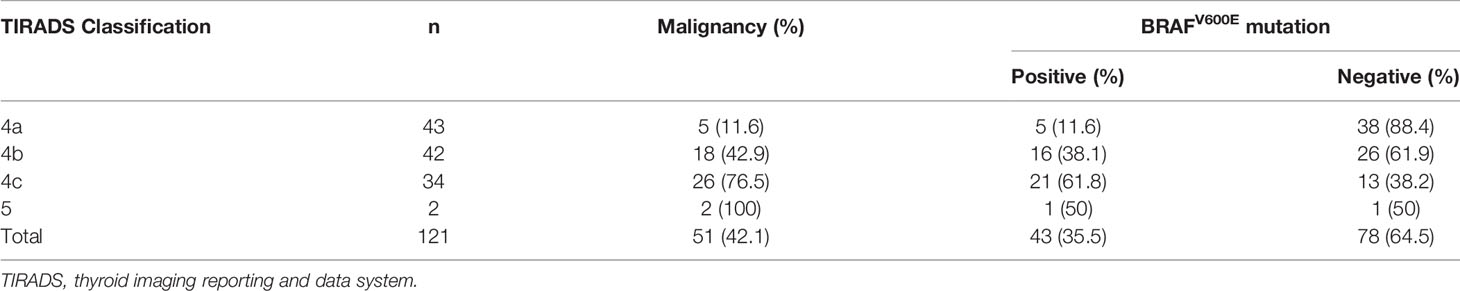
Table 5 TIRADS classification and BRAF mutation status in 121 thyroid nodules with indeterminate cytology.
Of the 51 nodules that were classified as BSRTC III but proven to be PTMCs by histopathology, 10 (19.6%) and 32 (62.7%) were found to associated with Hashimoto’s thyroiditis and calcifications. No significant difference was found between malignant and benign nodules with respect to Hashimoto’s thyroiditis and calcifications (P = 0.133 and P = 0.278, respectively). The malignancy rates of thyroid nodules with BSRTC III increased as the level of TIRADS classification increased (P < 0.0001). Forty-one (80.4%) of 51 PTMCs were positive for BRAFV600E mutation, indicating that BRAFV600E mutation was very helpful for the detection of PTMCs with BSRTC III. Of the 51 PTMCs with indeterminate cytology, TIRADS categories were suggestive of malignancy in 28 (54.9%) and benign lesion in 23 (45.1%). The diagnostic accuracy of the molecular method in the 121 indeterminate nodules was 90.1% (109/121), which was significantly higher than that of TIRADS classification, 74.4% (90/121) (P = 0.002) (Table 6).
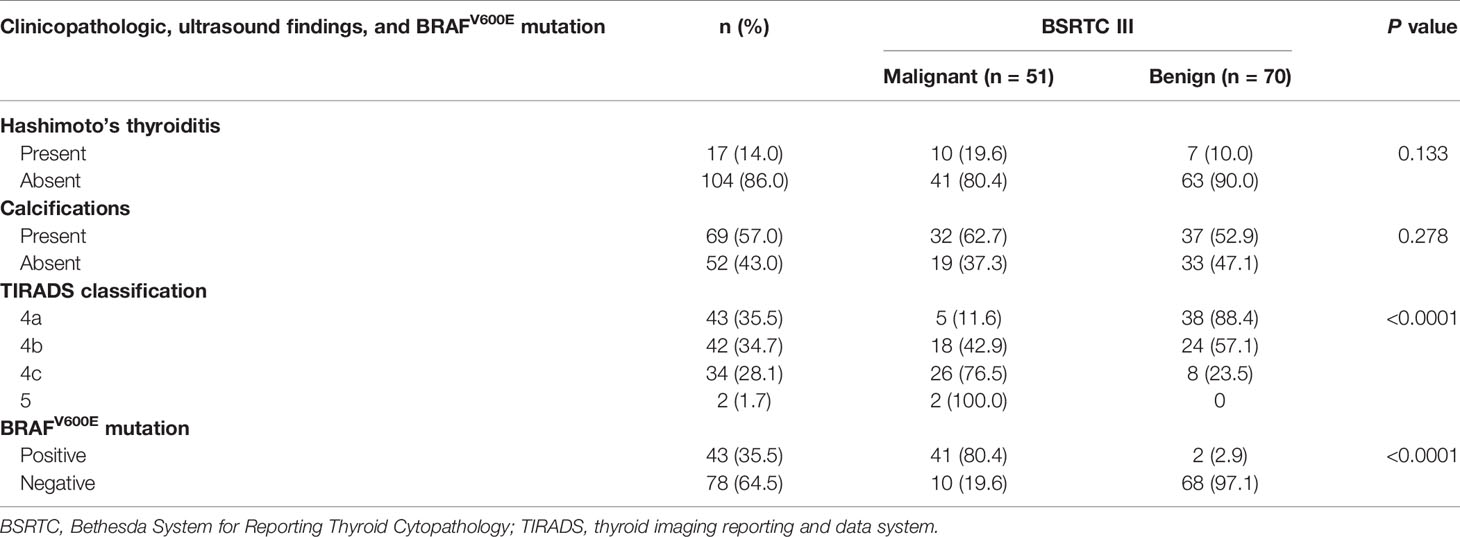
Table 6 Correlations of clinicopathologic and ultrasound findings and BRAFV600E mutation with final diagnosis in BSRTC III category.
It has been reported that 30%–65% of patients with PTMC had central lymph node metastasis (detected only on pathology) (4). Our results suggested that lymph node metastases were found in 150 (28.5%) patients with PTMC, which was slightly lower than those in literature reports. However, in our study, only four (2.7%) patients with cervical lymph node metastasis were observed by preoperative ultrasound because they were usually small and were obscured by the overlying thyroid gland. Hashimoto’s thyroiditis was an independent risk factor for PTC in children and adolescents (26). Our results had also shown that the proportion of patients combined with Hashimoto’s thyroiditis was significantly higher in the PTMC group than in the benign thyroid disease group. Multifocality is another independent risk factor that could predict lymph node metastasis and recurrence (27). In our study, tumor multifocality was observed in 43 (8.2%) cases with PTMC.
Surgical treatment strategies, such as hemithyroidectomy or total thyroidectomy and whether to perform lymph node dissection, depend on the qualitative diagnosis of thyroid nodules, the degree of risk of nodules, lymph nodal involvement, or the presence of multiglandular disease or thyroiditis (28). In the treatment of differentiated thyroid cancer, in the absence of enlarged lymph nodes, the role of routine central lymph node dissection (RCLD) remains controversial. In more demolitive surgery, interventions are exposed to more severe complications, such as permanent hypoparathyroidism and unilateral temporary laryngeal nerve paralysis. RCLD for differentiated thyroid cancer is not recommended because a simple thyroidectomy not combined with RCLD is associated with a low locoregional recurrence rate (29). In all patients with incidental PTMCs, the surgical indication is given for symptomatic disease, for impairment of thyroid function, or for failure to respond to medical therapy or unable to continue, since incidental PTMCs have little biological aggressiveness and are susceptible to metabolic radioiodine therapy (30).
Risk stratification of thyroid malignancy by using the number of suspicious ultrasound features allows for a practical and convenient TIRADS. The observation indices of malignant thyroid nodules used in this study were more comprehensive and detailed, including more meaningful ultrasonographic features such as irregular thick halo, suspicious cervical lymph nodes, and findings for predicting malignant partially cystic thyroid nodules. In the present study, the malignancy risk increased as the level of TIRADS classification increased. The malignancy risks of categories 4a, 4b, 4c, and 5 were 14.6%, 36.4%, 80.2%, and 100%, respectively, which were almost exactly in agreement with the ideal range (4a: 2–10, 4b: 10–50, 4c: 50–95, 5: >95) of TIRADS classification proposed by Kwak et al. (23). This result demonstrated that the classification method used in this study and the interpretation of ultrasound images were accurate. The present study indicated that TIRADS classification had a better diagnostic performance for the differential diagnosis of thyroid nodules, and the Az value, sensitivity, specificity, and accuracy were 0.815, 69.1%, 85.2%, and 77.3%, respectively. A recent study reveals that TIRADS classification can be used as a clinical parameter for deciding the BRAF mutation test in thyroid nodules with AUS/FLUS cytology (31). A similar conclusion was also drawn from our present study because the BRAFV600E mutation rate increased as the level of TIRADS classification increased in 121 thyroid nodules with indeterminate cytology and different TIRADS categories showed significant differences in the BRAFV600E mutation rate.
Thyroid FNA cytology is a cost-effective procedure that provides specific diagnosis rapidly with minimal complications. It plays an important role in the determination of clinical treatment and management plan. A reliable definitive diagnosis of PTC is usually straightforward in fine-needle aspirates of conventional PTC whenever the characteristic papillary and/or flat honeycomb sheet-like architecture and the typical nuclear features of chromatin pallor, nuclear enlargement, crowding, grooves, and pseudoinclusions are encountered. Cytology could help distinguish conventional PTC and the different PTC subtypes, such as “noninvasive follicular thyroid neoplasm with papillary-like nuclear features” (NIFTP) and follicular variant of PTC (FVPTC) by observing cytomorphologic features (32). Different subtypes of PTC have different molecular features. Conventional PTC with BRAFV600E mutations readily demonstrates nuclear features of PTC in cytology specimens as compared to RAS-driven tumors (NIFTP and FVPTC). At the molecular level, NIFTP shows a very high association with other follicular-pattern neoplasms, with RAS mutations being the most common, followed by PAX8/PPARγ translocations, THADA fusions, and BRAFK601E mutations (33–36). In contrast, BRAFV600E mutations and RET fusions (common in conventional PTC) are absent in NIFTP. Different genetic characteristics of PTC subtypes also explain why some PTMCs in our study fail to detect BRAF mutations.
The BSRTC established a standardized category-based reporting system for thyroid FNA specimens and has proven to be an effective and robust thyroid FNA classification scheme to guide the clinical treatment. Lin et al. (11) has shown that the proportion of non-diagnostic and indeterminate samples accounted for 30%. However, the results of our study indicated that non-diagnostic and indeterminate cytology was found for 13.3% (154/1157) of thyroid nodules, which was much lower than that of the previous report. This finding may be due to years of experience of pathologists and their training in cytopathology or the proficiency of the FNA operators. A recent study of Cibas and Ali (25) has shown that the rates of malignancy in BSRTC I–VI were 5%–10%, 0%–3%, 10%–30%, 25%–40%, 50%–75%, and 97%–99%, respectively. The rates of malignancy in nodules that were classified as BSRTC I, III, and V in this present study were significantly higher than the recommended range, whereas the rates of malignancy in BSRTC II, IV, and VI thyroid nodules were close to the ideal range. The malignancy rates among BSRTC I and III lesions were as high as 40.9%, indicating a relatively conservative attitude of the pathologist for interpreting the cytology. In our study, the high risk of malignancy associated with AUS/FLUS cases was even higher than cases classified as Bethesda IV (42.1% vs. 33.6%). Therefore, it was more important to further combine genetic testing methods to improve the detection rates for PTMCs with BSRTC III.
The mutation rate of BRAFV600E in PTMCs in this study population was 85.4%, which is close to the Korea population with 52%–83% prevalence of BRAF mutation (37). The rates of BRAF gene mutation in Chinese and Korea patients are significantly higher than those in western populations (USA and Europe) with the consistent prevalence of 40%–45% (38). Furthermore, this difference in prevalence might also be due to the different methods and techniques used for gene mutation testing. In this study, polymerase chain reaction (PCR)-based method amplification refractory mutation system (ARMS) was used for BRAFV600E mutation test but not traditional DNA sequencing. The ARMS assays could maximize the number of samples and improve both the sensitivity and speed of analysis compared with DNA sequencing (39). In our study, the specificity (97.1%) of BRAFV600E mutation was slightly lower than the result of the meta-analysis (pooled specificity 99.0%) by Jia et al. (37). This finding may be related to the oversensitivity of ARMS method so that the BRAFV600E mutation has been detected in a small number of benign nodules. BRAFV600E mutation analysis could also significantly increase the detection rates of FNA for PTMCs with various levels of TIRADS classification, especially those lesions classified as TIRADS 4.
The prevalence of BRAFV600E mutation (80.4%) among cytologically indeterminate samples in our study was significantly higher compared with that recently reported by Su et al. (9), who found a 40.1% incidence in BSRTC III nodules. The result of our study indicated that BRAFV600E status would be helpful for diagnosing the status of nodules with indeterminate cytology, which was consistent with those of some previous studies (13, 40) but conflicted with the findings of other researchers (15–17), who had reported that BRAF mutation analysis on an FNA specimen with indeterminate cytology might not be as helpful as indicated by previous studies (13, 40). Our data suggested that both BRAFV600E mutation analysis and TIRADS classification were potentially useful adjuncts to evaluate thyroid nodules that were difficult to diagnose. Moreover, the diagnostic accuracy of the molecular method for the indeterminate nodules was significantly higher than that of TIRADS classification. The application of BRAFV600E mutation analysis in FNA specimens classified as BSRTC III was more effective for thyroid nodules with higher TIRADS classification as compared with those nodules with lower TIRADS classification, demonstrating that TIRADS classification could be used to select patients for molecular analysis. Hashimoto’s thyroiditis and calcifications might be some factors that influence the differential diagnosis of benign and malignant thyroid nodules classified as BSRTC III. However, in our study, no significant relationship was observed between malignant and benign nodules with respect to Hashimoto’s thyroiditis and calcifications.
We found that BRAFV600E mutation detection could significantly increase the sensitivity, accuracy, and NPV when combined with BSRTC. Of all the methods, the combined use of the three methods produced the best diagnostic performance, which was significantly higher than that for the combination of BSRTC and BRAFV600E mutation analysis. However, Zhang et al. (20) has reported that the diagnostic performance of BSRTC combined with BRAFV600E mutation analysis for differentiating high-risk thyroid nodules is significantly higher as compared with the combination of the three methods, which is not consistent with the result of our study. This finding, in part, might be due to the fact that investigators at different institutions use different criteria for the combined use of the three methods. In our study, the criteria for the combined use of the three methods were developed when considering the diagnostic performances of different methods and different combined situations that BRAFV600E mutation, BSRTC, or TIARDS classification was suggestive of malignancy or benign possibility. This combined criteria allowed for a high sensitivity while improving specificity and accuracy for the differentiation of thyroid lesions. However, in the previous study of Zhang et al. (20), a nodule was considered positive as long as either method was positive when three diagnostic methods were used in combination. Such a combined criteria would inevitably lead to a certain degree increase in sensitivity, whereas the specificity was significantly reduced and the accuracy was not improved obviously.
This study had several limitations. Firstly, there was a selection bias because we excluded thyroid nodules without further histopathological diagnosis or F/U FNA and ultrasound to evaluate diagnostic performance. Secondly, not all thyroid nodules obtained histopathological results. Some nodules were classified as benign based on cytological diagnosis, F/U ultrasound, and BRAFV600E gene mutation test, which might cause some false negative results and overestimate the sensitivity of BRAFV600E mutation analysis. Thirdly, only high-risk thyroid nodules classified as TIRADS categories 4 and 5 were included in our study, so that the rate of malignant lesions was higher as compared to the general population with thyroid nodules.
In summary, BRAFV600E mutation analysis has the best diagnostic performance among the three methods. The combined use of ultrasound, cytology, and BRAFV600E mutation analysis is the most efficient and objective method for diagnosing PTMC. Both BRAFV600E mutation analysis and TIRADS classification are potentially useful adjuncts to differentiate thyroid nodules, especially indeterminate samples classified as BSRTC III, and select malignancy to guide the surgery.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Ethics Committee of Renji Hospital. The patients/participants provided their written informed consent to participate in this study.
Study concept and study design, JD, RH, and FL. Data acquisition, analysis, and interpretation, JD and RH. Article drafting or article revision for important intellectual content, all authors. Literature research, JD, RH, CC, and XM. Clinical studies, YS and JC. Statistical analysis, JD and RH. All authors contributed to the article and approved the submitted version.
This study was supported by the Health and Family Planning Joint Research Project of Shanghai Pudong New Area Health Commission (grant number PW2019D-6) and the National Natural Science Foundation of China (grant numbers 82071954 and 81102014).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.746776/full#supplementary-material
1. Brito JP, Hay ID. Management of Papillary Thyroid Microcarcinoma. Endocrinol Metab Clin North Am (2019) 48(1):199–213. doi: 10.1016/j.ecl.2018.10.006
2. Li F, Chen G, Sheng C, Gusdon AM, Huang Y, Lv Z, et al. BRAFV600E Mutation in Papillary Thyroid Microcarcinoma: A Meta-Analysis. Endocr Relat Cancer (2015) 22:159–68. doi: 10.1530/ERC-14-0531
3. Wang WH, Xu SY, Zhan WW. Clinicopathologic Factors and Thyroid Nodule Sonographic Features for Predicting Central Lymph Node Metastasis in Papillary Thyroid Microcarcinoma: A Retrospective Study of 1204 Patients. J Ultrasound Med (2016) 35:2475–81. doi: 10.7863/ultra.15.10012
4. Zhang Q, Wang Z, Meng X, Duh QY, Chen G. Predictors for Central Lymph Node Metastases in CN0 Papillary Thyroid Microcarcinoma (mPTC): A Retrospect Analysis of 1304 Cases. Asian J Surg (2019) 42(4):571–6. doi: 10.1016/j.asjsur.2018.08.013
5. Pelizzo MR, Boschin IM, Toniato A, Pagetta C, Piotto A, Bernante P, et al. Natural History, Diagnosis, Treatment and Outcome of Papillary Thyroid Microcarcinoma (PTMC): A Mono-Institutional 12-Year Experience. Nucl Med Commun (2004) 25:547–52. doi: 10.1097/01.mnm.0000126625.17166.36
6. Wei X, Li Y, Zhang S, Gao M. Meta-Analysis of Thyroid Imaging Reporting and Data System in the Ultrasonographic Diagnosis of 10,437 Thyroid Nodules. Head Neck (2016) 38:309–15. doi: 10.1002/hed.23878
7. Chen HY, Liu WY, Zhu H, Jiang DW, Wang DH, Chen Y, et al. Diagnostic Value of Contrast-Enhanced Ultrasound in Papillary Thyroid Microcarcinoma. Exp Ther Med (2016) 11:1555–62. doi: 10.3892/etm.2016.3094
8. Ha SM, Kim JK, Baek JH. Detection of Malignancy Among Suspicious Thyroid Nodules <1 Cm on Ultrasound With Various Thyroid Image Reporting and Data Systems. Thyroid (2017) 27:1307–15. doi: 10.1089/thy.2017.0034
9. Su X, Jiang X, Xu X, Wang W, Teng X, Shao A, et al. Diagnostic Value of BRAF (V600E)-Mutation Analysis in Fine-Needle Aspiration of Thyroid Nodules: A Meta-Analysis. Onco Targets Ther (2016) 9:2495–509. doi: 10.2147/OTT.S101800
10. Bongiovanni M, Spitale A, Faquin WC, Mazzucchelli L, Baloch ZW. The Bethesda System for Reporting Thyroid Cytopathology: A Meta-Analysis. Acta Cytol (2012) 56:333–9. doi: 10.1159/000339959
11. Lin K, Xiang Y, Qiao L, Liu R, Dong S, Zhang X. A Predictive Model for Selecting Malignant Thyroid Nodules in Patients With Nondiagnostic or Indeterminate Fine-Needle Aspiration Cytologic Findings. J Ultrasound Med (2015) 34:1245–51. doi: 10.7863/ultra.34.7.1245
12. Rossi M, Buratto M, Bruni S, Filieri C, Tagliati F, Trasforini G, et al. Role of Ultrasonographic/Clinical Profile, Cytology, and BRAF V600E Mutation Evaluation in Thyroid Nodule Screening for Malignancy: A Prospective Study. J Clin Endocrinol Metab (2012) 97:2354–61. doi: 10.1210/jc.2011-3494
13. Chung KW, Yang SK, Lee GK, Kim EY, Kwon S, Lee SH, et al. Pathology Diagnosis, Especially in BRAFV600E Mutation-Prevalent Area. Clin Endocrinol (Oxf) (2006) 65:660–6. doi: 10.1111/j.1365-2265.2006.02646.x
14. Adeniran AJ, Hui P, Chhieng DC, Prasad ML, Schofield K, Theoharis C. BRAF Mutation Testing of Thyroid Fine-Needle Aspiration Specimens Enhances the Predictability of Malignancy in Thyroid Follicular Lesions of Undetermined Significance. Acta Cytol (2011) 55:570–5. doi: 10.1159/000333274
15. Moon WJ, Choi N, Choi JW, Kim SK, Hwang TS. BRAF Mutation Analysis and Sonography as Adjuncts to Fine-Needle Aspiration Cytology of Papillary Thyroid Carcinoma: Their Relationships and Roles. AJR Am J Roentgenol (2012) 198:668–74. doi: 10.2214/AJR.11.7185
16. Trimboli P, Treglia G, Condorelli E, Romanelli F, Crescenzi A, Bongiovanni M, et al. BRAF-Mutated Carcinomas Among Thyroid Nodules With Prior Indeterminate FNA Report: A Systematic Review and Meta-Analysis. Clin Endocrinol (Oxf) (2016) 84:315–20. doi: 10.1111/cen.12806
17. Jinih M, Foley N, Osho O, Houlihan L, Toor AA, Khan JZ, et al. BRAFV600E Mutation as a Predictor of Thyroid Malignancy in Indeterminate Nodules: A Systematic Review and Meta-Analysis. Eur J Surg Oncol (2017) 43:1219–27. doi: 10.1016/j.ejso.2016.11.003
18. Kwak JY, Kim EK, Chung WY, Moon HJ, Kim MJ, Choi JR. Patients With Papillary Thyroid Microcarcinoma. Radiology (2009) 253:854–60. doi: 10.1148/radiol.2533090471
19. Koh J, Choi JR, Han KH, Kim EK, Yoon JH, Moon HJ, et al. Proper Indication of BRAF(V600E) Mutation Testing in Fine-Needle Aspirates of Thyroid Nodules. PLoS One (2013) 8:e64505. doi: 10.1371/journal.pone.0064505
20. Zhang YZ, Xu T, Cui D, Li X, Yao Q, Gong HY, et al. Value of TIRADS, BSRTC and FNA-BRAFV600E Mutation Analysis in Differentiating High-Risk Thyroid Nodules. Sci Rep (2015) 5:16927. doi: 10.1038/srep16927
21. Seo JY, Kim EK, Baek JH, Shin JH, Han KH, Kwak JY. Can Ultrasound be as a Surrogate Marker for Diagnosing a Papillary Thyroid Cancer? Comparison With BRAF Mutation Analysis. Yonsei Med J (2014) 55:871–8. doi: 10.3349/ymj.2014.55.4.871
22. Kim DW, Park JS, In HS, Choo HJ, Ryu JH, Jung SJ. Ultrasound-Based Diagnostic Classification for Solid and Partially Cystic Thyroid Nodules. AJNR Am J Neuroradiol (2012) 33:1144–9. doi: 10.3174/ajnr.A2923
23. Kwak JY, Han KH, Yoon JH, Moon HJ, Son EJ, Park SH, et al. Thyroid Imaging Reporting and Data System for US Features of Nodules: A Step in Establishing Better Stratification of Cancer Risk. Radiology (2011) 260:892–9. doi: 10.1148/radiol.11110206
24. Sánchez JF. TI-RADS Classification of Thyroid Nodules Based on a Score Modified According to Ultrasound Criteria for Malignancy. Rev Argent Radiol (2014) 78:138–48.
25. Cibas ES, Ali SZ. The 2017 Bethesda System for Reporting Thyroid Cytopathology. Thyroid (2017) 27:1341–6. doi: 10.1089/thy.2017.0500
26. Zeng R, Zhao M, Niu H, Yang KX, Shou T, Zhang GQ, et al. Relationship Between Hashimoto’s Thyroiditis and Papillary Thyroid Carcinoma in Children and Adolescents. Eur Rev Med Pharmacol Sci (2018) 22:778–87. doi: 10.26355/eurrev_201811_16401
27. Mantinan B, Rego-Iraeta A, Larrañaga A, Fluiters E, Sánchez-Sobrino P, Garcia-Mayor RV. Factors Influencing the Outcome of Patients With Incidental Papillary Thyroid Microcarcinoma. J Thyroid Res (2012) 2012:469397. doi: 10.1155/2012/469397
28. Giovanni C, Giorgio Calò P, Gambardella C, Tartaglia E, Mauriello C, Della Pietra C, et al. Controversies in the Surgical Management of Thyroid Follicular Neoplasms. Retrospective Analysis of 721 Patients. Int J Surg (2014) 12:S29–34. doi: 10.1016/j.ijsu.2014.05.013
29. Conzo G, Docimo G, Ruggiero R, Napolitano S, Palazzo A, Gambardella C, et al. Surgical Treatment of Papillary Thyroid Carcinoma Without Lymph Nodal Involvement. G Chir (2012) 33:339–42.
30. Pezzolla A, Docimo G, Ruggiero R, Monacelli M, Cirocchi R, Parmeggiani D, et al. Incidental Thyroid Carcinoma: A Multicentric Experience. Recenti Prog Med (2010) 101:194–8.
31. Rho M, Kim EK, Moon HJ, Yoon JH, Park VY, Han K, et al. Clinical Parameter for Deciding the BRAFV600E Mutation Test in Atypia of Undetermined Significance/Follicular Lesion of Undetermined Significance Thyroid Nodules: US Features According to TIRADS. Ultrasound Q (2017) 33:284–8. doi: 10.1097/RUQ.0000000000000313
32. Pusztaszeri M, Auger M. Update on the Cytologic Features of Papillary Thyroid Carcinoma Variants. Diagn Cytopathol (2017) 45:714–30. doi: 10.1002/dc.23703
33. Nikiforov YE, Seethala RR, Tallini G, Baloch ZW, Basolo F, Thompson LD, et al. Nomenclature Revision for Encapsulated Follicular Variant of Papillary Thyroid Carcinoma. A Paradigm Shift to Reduce Overtreatment of Indolent Tumors. JAMA Oncol (2016) 2:1023–9. doi: 10.1001/jamaoncol
34. Baloch ZW, Seethala RR, Faquin WC, Papotti MG, Basolo F, Fadda G, et al. Noninvasive Follicular Thyroid Neoplasm With Papillary-Like Nuclear Features (NIFTP): A Changing Paradigm in Thyroid Surgical Pathology and Implications for Thyroid Cytopathology. Cancer Cytopathol (2016) 124:616–20. doi: 10.1002/cncy
35. Afkhami M, Karunamurthy A, Chiosea S, Nikiforova MN, Seethala R, Nikiforov TE, et al. Histopathologic and Clinical Characterization of Thyroid Tumors Carrying the BRAFK601E Mutation. Thyroid (2016) 26:242–7. doi: 10.1089/thy
36. Jiang XS, Harrison GP, Datto MB. Young Investigator Challenge: Molecular Testing in Noninvasive Follicular Thyroid Neoplasm With Papillary-Like Nuclear Features. Cancer (2016) 124:893–900. doi: 10.1002/cncy.21802
37. Jia Y, Yu Y, Li X, Wei S, Zheng X, Yang X, et al. Diagnostic Value of B-RAF(V600E) in Difficult-to-Diagnose Thyroid Nodules Using Fine-Needle Aspiration: Systematic Review and Meta-Analysis. Diagn Cytopathol (2014) 42:94–101. doi: 10.1002/dc.23044
38. Bychkov A. Prevalence of BRAFV600E Mutation in Asian Patients With Thyroid Cancer. Malays J Pathol (2017) 39:95–6.
39. Ellison G, Donald E, McWalter G, Knight L, Fletcher L, Sherwood J, et al. A Comparison of ARMS and DNA Sequencing for Mutation Analysis in Clinical Biopsy Samples. J Exp Clin Cancer Res (2010) 29:132. doi: 10.1186/1756-9966-29-132
Keywords: thyroid nodule, papillary thyroid microcarcinoma, ultrasound, fine-needle aspiration, BRAFV600E mutation
Citation: Du J, Han R, Chen C, Ma X, Shen Y, Chen J and Li F (2022) Diagnostic Efficacy of Ultrasound, Cytology, and BRAFV600E Mutation Analysis and Their Combined Use in Thyroid Nodule Screening for Papillary Thyroid Microcarcinoma. Front. Oncol. 11:746776. doi: 10.3389/fonc.2021.746776
Received: 24 July 2021; Accepted: 22 October 2021;
Published: 03 January 2022.
Edited by:
Avraham Eisbruch, University of Michigan, United StatesReviewed by:
Ludovico Docimo, University of Campania Luigi Vanvitelli, ItalyCopyright © 2022 Du, Han, Chen, Ma, Shen, Chen and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fenghua Li, ZmVuZ2h1YS1saUAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.