
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
HYPOTHESIS AND THEORY article
Front. Oncol. , 18 November 2021
Sec. Neuro-Oncology and Neurosurgical Oncology
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.746416
This article is part of the Research Topic Insights in Neuro-Oncology and Neurosurgical Oncology: 2021 View all 19 articles
Purpose: The safety and effectiveness of laser interstitial thermal therapy (LITT) relies critically on the ability to continuously monitor the ablation based on real-time temperature mapping using magnetic resonance thermometry (MRT). This technique uses gradient recalled echo (GRE) sequences that are especially sensitive to susceptibility effects from air and blood. LITT for brain tumors is often preceded by a biopsy and is anecdotally associated with artifact during ablation. Thus, we reviewed our experience and describe the qualitative signal dropout that can interfere with ablation.
Methods: We retrospectively reviewed all LITT cases performed in our intraoperative MRI suite for tumors between 2017 and 2020. We identified a total of 17 LITT cases. Cases were reviewed for age, sex, pathology, presence of artifact, operative technique, and presence of blood/air on post-operative scans.
Results: We identified six cases that were preceded by biopsy, all six had artifact present during ablation, and all six were noted to have air/blood on their post-operative MRI or CT scans. In two of those cases, the artifactual signal dropout qualitatively interfered with thermal damage thresholds at the borders of the tumor. There was no artifact in the 11 non-biopsy cases and no obvious blood or air was noted on the post-ablation scans.
Conclusion: Additional consideration should be given to pre-LITT biopsies. The presence of air/blood caused an artifactual signal dropout effect in cases with biopsy that was severe enough to interfere with ablation in a significant number of those cases. Additional studies are needed to identify modifying strategies.
Laser interstitial thermal therapy (LITT) is a minimally invasive therapeutic option for treatment of brain tumors. The safety and effectiveness of LITT rely critically on the ability to continuously monitor the ablation in real time based on accurate temperature mapping of the region of interest. Currently, this is achieved by measuring phase change using a magnetic resonance imaging technique called MR thermometry (MRT) (1, 2), which uses a gradient recalled echo (GRE) sequence to leverage six temperature-sensitive MR parameters, namely, the proton resonance frequency (PRF), the diffusion coefficient (D), T1 and T2 relaxation times, magnetization transfer, and proton density. The temperature measurement is then used to estimate tissue damage using a thermal damage threshold (TDT) model that utilizes temperature and time in a non-linear manner to quantify the damage by relating it to an equivalent heating time at 43°C (Figure 1A) (3). This allows the operator to determine when the tumor has been sufficiently ablated without injuring the surrounding normal brain.
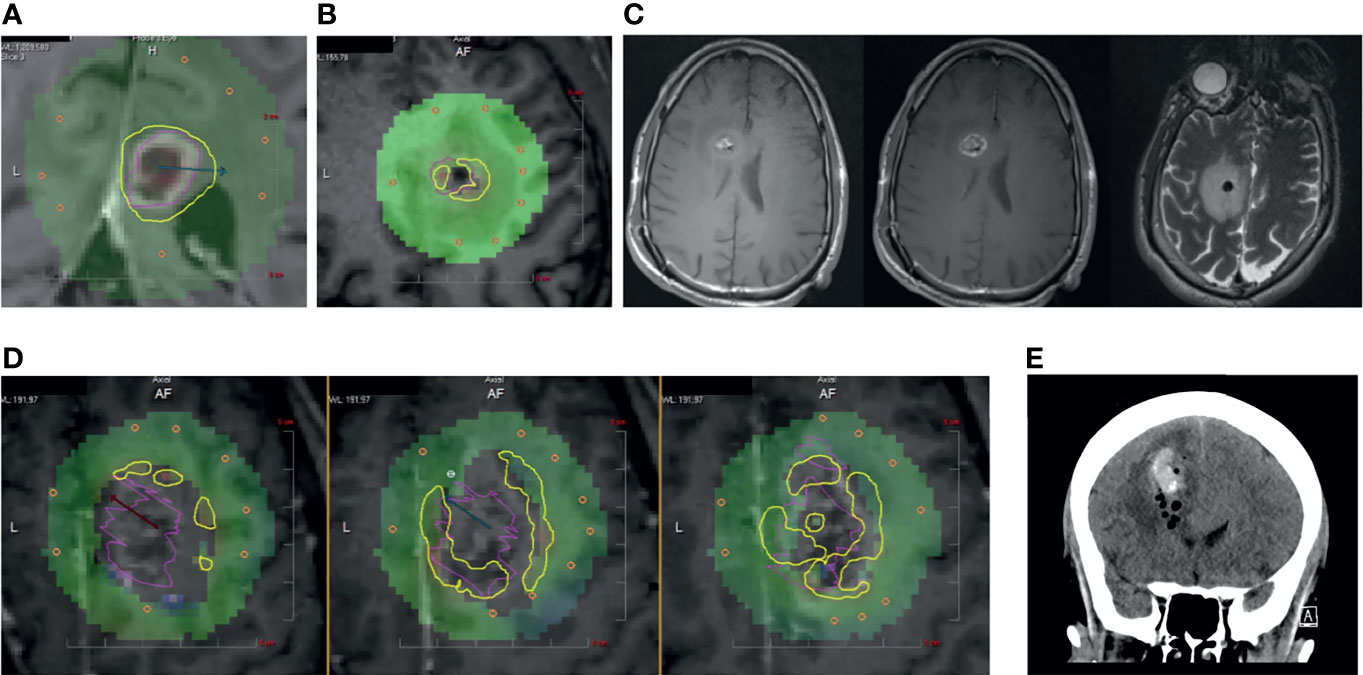
Figure 1 (A) Panel displaying a typical inline view on a contrast-enhanced T1-weighted MRI that is perpendicular to the laser catheter. Yellow TDT lines indicate the areas where tumor (pink) has reached 43°C as measured by MRT. (B) Patient 1. Intraoperative ablation showing central zones of signal “dropout” (gray voxels) on MRT and interference with TDT lines at the tumor (pink) borders. (C) (left) Unenhanced T1-weighted MRI, (middle) Contrast-enhanced T1-weighted MRI, and (right) T2-weighted MR image showing mixed hyper- and hypo-intensities in biopsy cavity. (D) Patient 2. Intraoperative panels showing three sequential inline cuts along the laser catheter with zones of signal dropout and interference with MRT at the tumor (pink) borders. (E) Post-operative coronal CT showing air and blood within the ablated tumor.
LITT for brain tumors is often preceded by a biopsy for histologic and molecular characterization. We have anecdotally found biopsies to be associated with artifact during ablation and thus sought to review the incidence in our series and describe the qualitative signal dropout that can interfere with TDT assessment during LITT ablation.
We retrospectively reviewed all LITT cases performed in our intraoperative MRI suite between 2017 and 2020 (IRB number: 2002P001238) using the NeuroBlate system (Plymouth, MN, USA). We identified a total of 17 LITT cases. We identified six patients in which LITT was preceded by biopsy. Two out of six of those cases were noted to have clinically significant ablation artifact as determined by the neurosurgeon performing the procedure. Clinically significant artifact was defined as ablations where grayed-out voxels were present from the onset of ablation on MRT extended outside of the volumetric limits of the contrast-enhancing portion of the tumor. Post-ablation scans were read by a neuroradiologist. None of the 11 cases without biopsy had ablation artifact and all but one (complicated by IVH) had no obvious blood or air noted on the post-ablation scans (Table 1). All ablations were performed in an IMRIS 3T Siemen’s Verio scanner (Erlangen, Germany).
Patient 1: A right-handed male in his 30s had a 3-year history of a recurrent glioblastoma multiforme (GBM) initially treated with standard radiation and temozolomide. He had several recurrences including one at 36 months after his initial diagnosis in the right frontal region and elected to undergo biopsy for tumor restaging and LITT. He underwent three biopsies approximately 5 mm into the lesion for frozen section, followed by three more biopsies for permanent pathology using a standard suction aspiration technique; there was no sense that bleeding had occurred. Immediately following biopsy, he underwent laser ablation during which there appeared grayed-out voxels within the resection cavity and difficulty measuring TDT around the margins of the tumor (Figure 1B). Post-ablation T1w and T2w MRI scans were noted to have blood and air within the ablated tumor (Figure 1C).
Patient 2: A right-handed female in her 60s presented with a large T1w contrast-enhancing bifrontal lesion with extension through the corpus callosum. She initially underwent four biopsies using a standard suction aspiration. To perform molecular analysis, the biopsy catheter was withdrawn 8 mm and 2 more cores were taken. There was no bleeding noted. A laser catheter was carefully exchanged into the deepest part of the tumor. After initial ablation, the catheter was withdrawn 5 mm for additional ablation to cover more of the tumor volume. This occurred two more times and, in each instance, there were significant signal dropouts that interfered with MRT and TDT (Figure 1D). A post-ablation MRI showed blood and air in the cavity, which was demonstrated as stable on a CT on post-operative day 1 (Figure 1E). Histopathology resulted as GBM.
All patients who underwent biopsy prior to LITT (Patients 1–6) were found to have some degree of artifact during ablation (Table 1). Patients 1 and 2 were specifically described as having clinically significant artifact, as defined in the Methods section, and thus were discussed as specific case examples. Interestingly, ablation artifacts were observed regardless of the number of biopsies drawn, including for Patient 6, who underwent one single biopsy. For 50% of the cohort (Patients 3–5), four biopsies were obtained. The remaining subjects, Patients 1 and 2, underwent three and six biopsies, respectively. There also does not appear to be correlation with presence of artifact and pathology of lesion. Of the patients in the biopsy cohort, 66% were treated for GBM. The remaining patients were found to have metastatic lesions. However, since this is a low-powered study with a small cohort of patients, trends correlating ablation artifacts with number of biopsies obtained and tumor pathology likely cannot be inferred from this dataset alone.
Importantly, all biopsy patients were found to have either blood or air on their post-ablation scan, whether by MRI or CT. Of the remaining patients who did not undergo biopsy, all but one had no evidence of blood or air on post-ablation imaging. The singular case of LITT without preceding biopsy that did show post-ablative blood on follow up imaging had intraventricular hemorrhage as a confounding factor.
Munier et al. found that when there was artifact present on MRT, the TDT overestimated the cross-sectional ablation area of the tumor (4). They postulated that the aberrations stemmed from local tissue trauma. Indeed, in our series, we observed this artifact to occur during all LITT cases that were preceded by a biopsy and associated with the presence of blood and air on post-ablation scans in all six cases. Similar to iron within heme molecules, the oxygen content of air is paramagnetic and can cause dephasing in T2*-sensitive MR sequences, resulting in magnitude loss, phase shifts, as well as geometric distortion during MRT (5). Not only does this artifact cause a quantitative overestimation, but we demonstrate two examples of how it can cause qualitative interference particularly at the tumor margins during ablation (Figures 1B, D). The signal dropout artifact that occurs during ablation could be detrimental to the patient when artifact extends outside of the tumor (Figure 1D). The operator is faced with a dilemma of waiting until a damage estimate line suddenly appears outside the grayed-out voxels or cut the ablation short not knowing where within the voxels the damage has occurred up to.
There is increasing evidence suggesting the clinical benefit of the addition of LITT to biopsy in patients with primary brain tumors (6, 7) which makes finding strategies to avoid this artifact increasingly important. In our anecdotal experience, we find that a slow speed of catheter exchange helps to prevent significant air leaks, and that repositioning the catheter by a few millimeters between ablations may also provide enough of a readjustment of the local environment to restore MRT. Another potential mitigating strategy, particularly for a small lesion in a functional area, may be to add a few milliliters of saline or thrombin (8, 9) to tamponade and restore the local architecture. There are currently no studies to the authors’ knowledge that have described using either during LITT.
Although there is currently no understanding of how the artifact affected the software’s ability to calculate the ablation zone, there are studies in the literature that have quantified discrepancies between TDT and postoperative MRI contrast-enhancing area.
There are also several newer MRT techniques that may provide a potential solution including spectroscopic imaging and measuring water–fat proton chemical shifts since they do not depend on relative phase shifts (10).
The location of the planned ablation will influence the challenge introduced by the signal dropout. Both patients in whom there was significant loss of MRT data were in non-eloquent brain areas. In cases where lesions may be close to eloquent structures like the corticospinal tracts, the clinician may consider surgical planning with tractography. Future studies should address functional outcomes and factors that influence the degree of artifact such as the size of the lesion, number of biopsies, and novel MRT strategies.
Pre-LITT biopsies should be limited to patients in whom the tissue diagnosis will impact treatment decisions. Although the presence of air/blood in the cavity does not preclude LITT, it caused a qualitatively significant signal dropout effect that interfered with MRT at the tumor’s margins.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
IRB was obtained before the procedure (2002P001238). The data have been anonymized so that no individual or anyone who knows them could identify them.
TN: first author. PJ: data gathering and figures. RH: interpretation, writing, and figures. GL: equal contributions. CO: equal contributions. AG: last authorship. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. de Senneville BD, Mougenot C, Quesson B, Dragonu I, Grenier N, Moonen CTW. MR Thermometry for Monitoring Tumor Ablation. Eur Radiol (2007) 17:2401–10. doi: 10.1007/s00330-007-0646-6
2. Patel NV, Mian M, Stafford RJ, Nahed BV, Willie JT, Gross RE, et al. Laser Interstitial Thermal Therapy Technology, Physics of Magnetic Resonance Imaging Thermometry, and Technical Considerations for Proper Catheter Placement During Magnetic Resonance Imaging-Guided Laser Interstitial Thermal Therapy. Neurosurgery (2016) 79(Suppl 1):S8–S16. doi: 10.1227/NEU.0000000000001440
3. Peters RD, Chan E, Trachtenberg J, Jothy S, Kapusta L, Kucharczyk W, et al. Magnetic Resonance Thermometry for Predicting Thermal Damage: An Application of Interstitial Laser Coagulation in an In Vivo Canine Prostate Model. Magn Reson Med (2000) 44:873–83. doi: 10.1002/1522-2594(200012)44:6<873::aid-mrm8>3.0.co;2-x
4. Munier SM, Desai AN, Patel NV, Danish SF. Effects of Intraoperative Magnetic Resonance Thermal Imaging Signal Artifact During Laser Interstitial Thermal Therapy on Thermal Damage Estimate and Postoperative Magnetic Resonance Imaging Ablative Area Concordance. Oper Neurosurg (Hagerstown) (2019) 18:524–30. doi: 10.1093/ons/opz182
5. Czervionke LF, Daniels DL, Wehrli FW, Mark LP, Hendrix LE, Strandt JA, et al. Magnetic Susceptibility Artifacts in Gradient-Recalled Echo MR Imaging. Am J Neuroradiol (1988) 9:1149–55.
6. Ahluwalia M, Barnett GH, Deng D, Tatter SB, Laxton AW, Mohammadi AM, et al. Laser Ablation After Stereotactic Radiosurgery: A Multicenter Prospective Study in Patients With Metastatic Brain Tumors and Radiation Necrosis. J Neurosurg (2018) 130:804–11. doi: 10.3171/2017.11.JNS171273
7. Shah AH, Burks JD, Buttrick SS, Debs L, Ivan ME, Komotar RJ. Laser Interstitial Thermal Therapy as a Primary Treatment for Deep Inaccessible Gliomas. Neurosurgery (2019) 84:768–77. doi: 10.1093/neuros/nyy238
8. Chimowitz MI, Barnett GH, Palmer J. Treatment of Intractable Arterial Hemorrhage During Stereotactic Brain Biopsy With Thrombin: Report of Three Patients. J Neurosurg (1991) 74:301–3. doi: 10.3171/jns.1991.74.2.0301
9. de Quintana-Schmidt C, Leidinger A, Teixidó JM, Bertrán GC. Application of a Thrombin-Gelatin Matrix in the Management of Intractable Hemorrhage During Stereotactic Biopsy. World Neurosurg (2019) 121:180–5. doi: 10.1016/j.wneu.2018.10.053
Keywords: laser interstitial thermal therapy, LITT, magnetic resonance thermometry, MRT, brain tumors, biopsy, artifact, signal dropout
Citation: Noh T, Juvekar P, Huang R, Lee G, Ogasawara CT and Golby AJ (2021) Biopsy Artifact in Laser Interstitial Thermal Therapy: A Technical Note. Front. Oncol. 11:746416. doi: 10.3389/fonc.2021.746416
Received: 23 July 2021; Accepted: 28 October 2021;
Published: 18 November 2021.
Edited by:
Alireza Mansouri, The Pennsylvania State University (PSU), United StatesReviewed by:
Emanuele La Corte, University of Bologna, ItalyCopyright © 2021 Noh, Juvekar, Huang, Lee, Ogasawara and Golby. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thomas Noh, bm9odEBoYXdhaWkuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.