
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 21 October 2021
Sec. Radiation Oncology
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.742971
 Guixiang Liao1
Guixiang Liao1 Yuting Qian2
Yuting Qian2 Sumbal Arooj1,3
Sumbal Arooj1,3 Zhihong Zhao4
Zhihong Zhao4 Maosheng Yan1
Maosheng Yan1 Zihuang Li1
Zihuang Li1 Hongli Yang1
Hongli Yang1 Tao Zheng1
Tao Zheng1 Gang Li5
Gang Li5 Xianming Li1*
Xianming Li1* Muhammad Khan1,6*
Muhammad Khan1,6*Background: Radiation therapy (RT) is the mainstay of brain metastases (BMs), and anti-PD-1 blockade has led to intracranial responses in non-small cell lung carcinoma (NSCLC) patients with BMs.
Objective: This study aimed to evaluate the efficacy and safety of adding anti-PD-1 blockade to RT in the management of NSCLC patients with BM in terms of survival outcome.
Materials and Methods: We retrospectively reviewed 70 NSCLC patients with BMs who were treated with whole brain radiation therapy (WBRT) between January 2016 and January 2021. Of the 70 patients, 29 additionally received anti-PD-1 therapy within 30 days of WBRT initiation. Baseline characteristics of the patients and efficacy outcomes such as progression-free survival (PFS) and overall survival (OS) were statistically compared using SPSS v26. Results were obtained using the Chi-square test/Fisher exact test, t-test, Kaplan-Meier, and Cox regression survival analyses.
Results: The median survival for the entire cohort was 24 months (95% CI, 19.5–28.5). The median survival times for WBRT alone and WBRT plus anti-PD-1 therapy cohorts were 20 months (95% CI, 11.6–28.3) and 27 months (95% CI, 19.5–28.5), respectively (p=0.035). There was no statistical difference in PFS for the treatment cohorts (median PFS for WBRT alone: 7 months vs. 12 months for WBRT plus anti-PD-1, p=0.247). In EGFR wild-type subgroup (n=31), both PFS (p=0.037) and OS (p=0.012) were significantly improved. Only the treatment group (WBRT plus anti-PD-1) was a significant predictor of OS on univariate and multivariate analyses (p=0.040). There were no significant differences in adverse events among the treatment groups.
Conclusions: NSCLC patients with BM receiving additional anti-PD-1 therapy may derive better OS than WBRT alone without any increase in adverse events. Prospective well-designed studies are warranted to validate and elucidate the additive effects of the two modalities in this group of patients.
Lung cancer is the second most common cancer type in terms of incidence rate (T: 228820, 12.7%; M: 116, 300 13%; F: 112, 520 12%), and is the leading cause of death (T: 135720, 22%; M: 72,500 23%; F: % 63220 22%) in both sexes according to newly estimated new cancer cases and deaths by sex in the United States in 2020 (1). Non-small cell lung carcinoma (NSCLC) accounts for 85% of lung cancer cases and is the most frequent primary site for brain metastasis (BM) (1–3). The relative incidence of BMs accounts for 40% of all NSCLC patients and is increasing with the development of advanced imaging technology, targeted agents, and immunotherapy (IT) (3–5). Radiation therapy (RT) has been predominantly used for the management of BMs (5–13).
BM patients with a high intracranial burden are primarily offered whole brain radiation therapy (WBRT). Generally, stereotactic radiosurgery (SRS) has been restricted to patients with up to 3 BMs. However, recent trends indicate that SRS/stereotactic radiotherapy (SRT) has been increasingly offered to patients with >3 BMs with WBRT used as salvage therapy (5, 9, 10, 12). In BM patients with NSCLC, a combination of the two may result in improved outcomes compared to being treated with either of the two (5, 7–12). Next-generation tyrosine kinase inhibitors (TKIs) and immune checkpoint blockades (ICBs) have shown efficacy in treating BMs (14, 15). The addition of up-front radiotherapy to TKIs may improve outcomes compared with TKI alone in EGFR-mutated NSCLC (14). Despite increased usage of SRS in recent times, BM patients are still managed with WBRT alone because of multiple brain lesions at presentation and the feasibility of SRS treatment (13).
ICB targeting the CTLA-4 and PD-1 checkpoint pathways has shown significant improvement in the survival of NSCLC patients (16). As a result, anti-PD-1/PD-L1 therapy has been approved as a first-line or second-line monotherapy treatment or given in combination with chemotherapy (16). Anti-PD-1 monoclonal antibodies (nivolumab/pembrolizumab) as monotherapy have also displayed an intracranial response with an objective response rate (ORR) of 9–30% in patients with NSCLC BMs (15, 17, 18). A rationale has been developed for the combination of immune checkpoint inhibitors (ICIs) and RT to seek synergistic anti-cancer responses (19). So far, there are limited reports of improvement in outcome with a combination of the two treatments (20–25). Hence, we are attempting to conduct a retrospective review involving NSCLC patients with BM to analyze the addition of anti-PD-1 therapy to WBRT compared to RT alone.
A total of 70 stage IV non-small cell lung cancer patients with newly diagnosed brain metastases (≥ three BMs), and who had received WBRT alone (n=41) or in combination with anti-PD-1 therapy (n=29) for BMs during the time period between January 2016 and January 2021 at the “Shenzhen People’s Hospital, The First Affiliated Hospital Of Southern University Of Science And Technology, Shenzhen, China”, and “The First Affiliated Hospital Of Wenzhou Medical University, Wenzhou, China”, were identified by conducting a retrospective review of the database following ethics approval from the two hospitals. All the included patients had developed brain metastases after being previously treated with first line platinum-based chemotherapy at initial lung cancer diagnosis. Only six patients in control group and none in the anti-PD-1 group were diagnosed with synchronous brain metastases who were offered first line platinum-based chemotherapy or anti-EGFR therapy along with WBRT to the brain. The remaining patients in control group were offered second line docetaxel chemotherapy and/or anti-EGFR treatment for systemic disease, and WBRT for brain metastases. Patients in the anti-PD-1 group only received anti-PD-1 antibody treatment (nivolumab) that was started within 30 days of WBRT induction. At subsequent disease progression, all patients were offered best supportive care. WBRT was delivered with a median dose of 30 Gy/10 F. Clinicopathological information and follow-up time for all patients were recorded and are presented in Table 1. Written informed consent for participation was obtained from the patients or their guardians according to the Declaration of Helsinki (26). The STROBE guidelines for cohort studies were followed for reporting (27).
Follow-up included clinical evaluation and radiological imaging tests (CT and MRI) obtained at 3-, 6-month-, and 1-year intervals. Overall survival (OS) was termed as the primary endpoint and defined as the time from BM diagnosis to death. Progression-free survival (PFS) was termed as the secondary endpoint and defined as the time from BM diagnosis to disease progression on clinical and radiological evaluation during follow-up or death following treatment induction. Progressive disease was defined according to the RECIST 1.1, in which new BM occurrence was also characterized as disease progression (28). Adverse events experienced by patients after receiving treatment were also assessed and graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 criteria (29).
Median survival time and confidence intervals were obtained for OS and PFS using Kaplan-Meier analysis available in SPSS software version 26. The log-rank test was used to determine statistical differences between the groups’ OS and PFS outcomes. To determine the differences between the cohorts, Chi-square test/Fisher exact test for categorical covariates, and t-tests for numerical covariates were applied if tests of normality and homogeneity of variance were satisfied. Otherwise, the Mann-Whitney U test or Kruskal-Wallis H test was used. The Cox proportional hazard model was used for univariate and multivariate factor analyses. Factors with p-values less than 030 (p<0.30) were selected for multivariate analysis.
Our retrospective review included 70 patients with stage IV NSCLC and ≥ three BMs. All patients were treated with WBRT between 2016 and 2021. Of the 70 NSCLC BM patients, 29 (41%) received a median number of 6 cycles of anti-PD-1 monoclonal antibody treatment (nivolumab) within 30 days of WBRT initiation. The entire cohort was followed up for an average of 17 months (standard deviation, ± 8.0 m). The mean age of the entire cohort was 58.4 years (standard deviation, ± 11.3 y). The majority of the patients in the WBRT alone group were EGFR+ (25 vs. 5), and the difference was significant between the treatment groups according to EGFR status (p=0.004). No significant association was found between the treatment groups for other baseline characteristics such as age, sex, smoking status, histopathology, tumor differentiation, Karnofsky performance status (KPS), number of metastatic organs, and follow-up duration (Table 1) BMI.
The median survival for the entire cohort was 24 months (95% CI, 19.5–28.5) (Figure 1). The median survival time for the WBRT alone cohort was 20 months (95% CI, 11.6–28.3) and 27 months (95% CI, 19.5–28.5) for the WBRT plus anti-PD-1 cohort. OS was significant for the treatment difference (p=0.035). In the univariate analysis, age, sex, pathohistological type, pathological differentiation, KPS performance status, body mass index (BMI), and presence of extracranial metastatic sites had no impact on survival (Table 2). Additional anti-PD-1 antibody administration was the only predictor of OS identified on univariate analysis. Other factors that showed a close relationship with OS included sex (p=0.095) and histopathologic differentiation (p=0.075). These three factors were included in the multivariate analysis. Only the combined treatment remained significant for predicting OS on multivariate analysis.
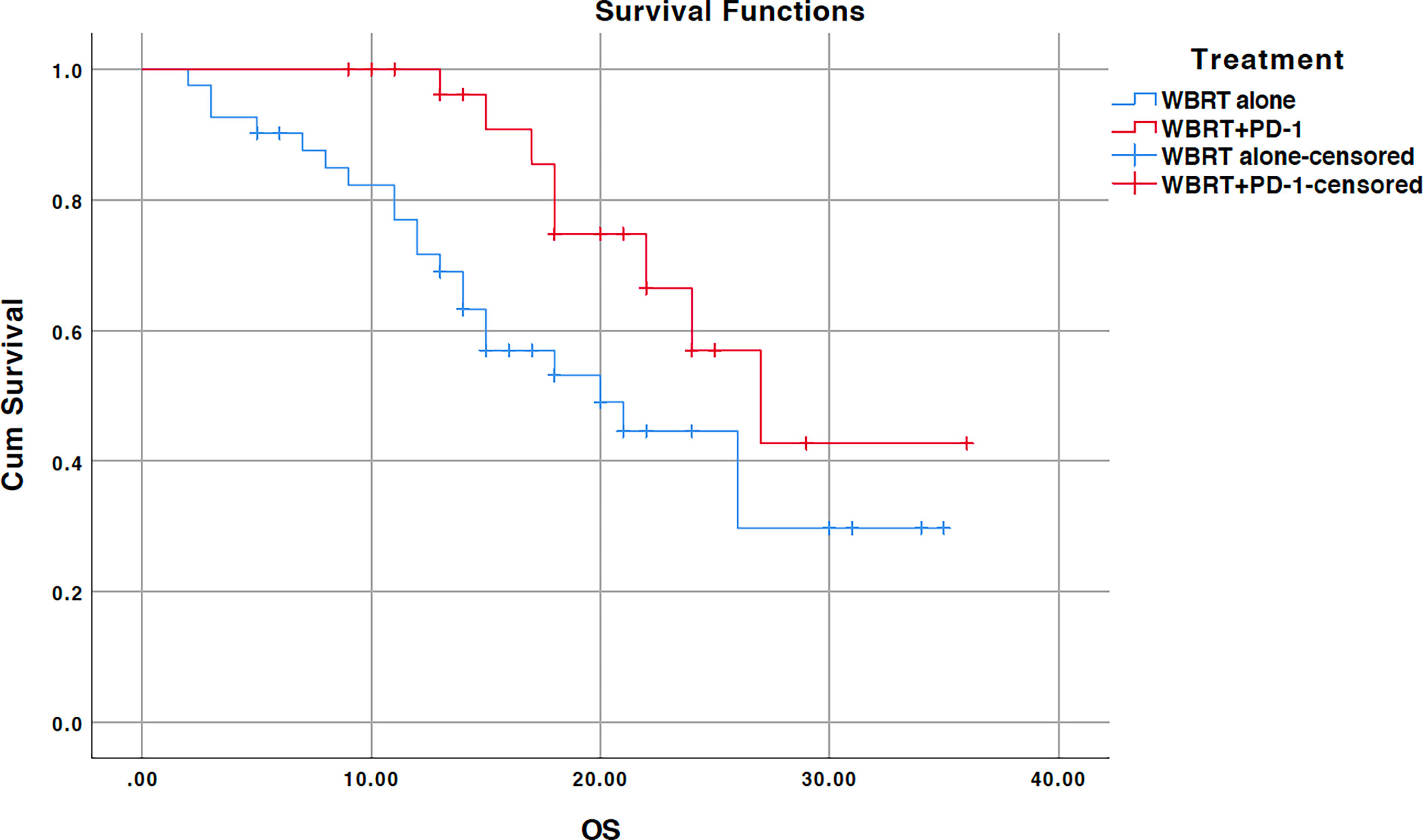
Figure 1 Kaplan-Meier overall survival (OS) curve for treatments; WBRT alone (No PD-1), and WBRT plus PD-1 inhibition therapy (WBRT+PD-1). Cum, cumulative.
We further performed subgroup analysis as there was a significant difference between two cohorts according to the EGFR mutation status (p=0.004). In subgroup analysis, significant improvement in OS for the treatment difference was unraveled for NSCLC patients negative for EGFR mutation (n=31, p=0.012). WBRT alone cohort (n=15) demonstrated a median OS of 15 months (95% CI, 11.5–18.4) while median OS for WBRT plus anti-PD-1 cohort (n=16) was not reached as shown in Figure 2. There was no difference when analysis was restricted to EGFR positive NSCLC patients (p=0.096). Median OS couldn’t be calculated as no events were reported in WBRT plus anti-PD-1 cohort (n=5) as illustrated in Supplementary Figure 1.
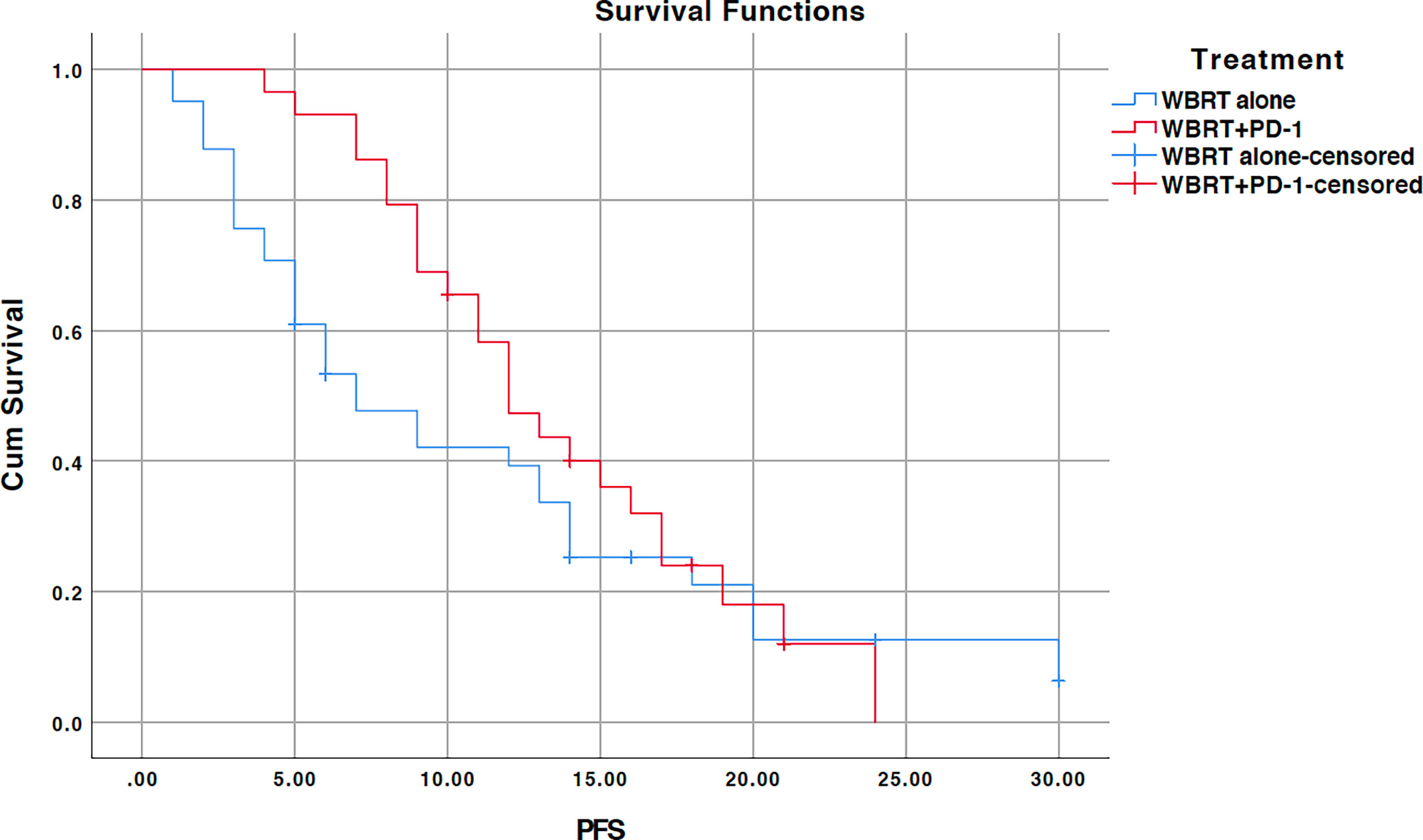
Figure 2 Kaplan-Meier overall survival (OS) curve for treatments in EGFR wildtype NSCLC subgroup; WBRT alone (No PD-1), and WBRT plus PD-1 inhibition therapy (WBRT+PD-1). Cum, cumulative.
The median PFS for the entire cohort was 11 months (95% CI, 8.4–13.6) (Figure 3). The median PFS for the WBRT alone cohort was 7 months (95% CI, 3.7–10.3) and 12 months (95% CI, 9.4–14.5) for the WBRT plus anti-PD-1 cohort. The difference between the median PFS of the treatments was not significant (p=0.247). On univariate analysis, pathological differentiation (p=0.021) and KPS score (p=0.047) appeared to be predictive of better PFS. Both predictors lost statistical significance in the multivariate analysis (Table 3). Treatment (p=0.061), smoking (p=0.066), and pathological differentiation (p=0.088) showed close association with PFS on multivariate analysis.
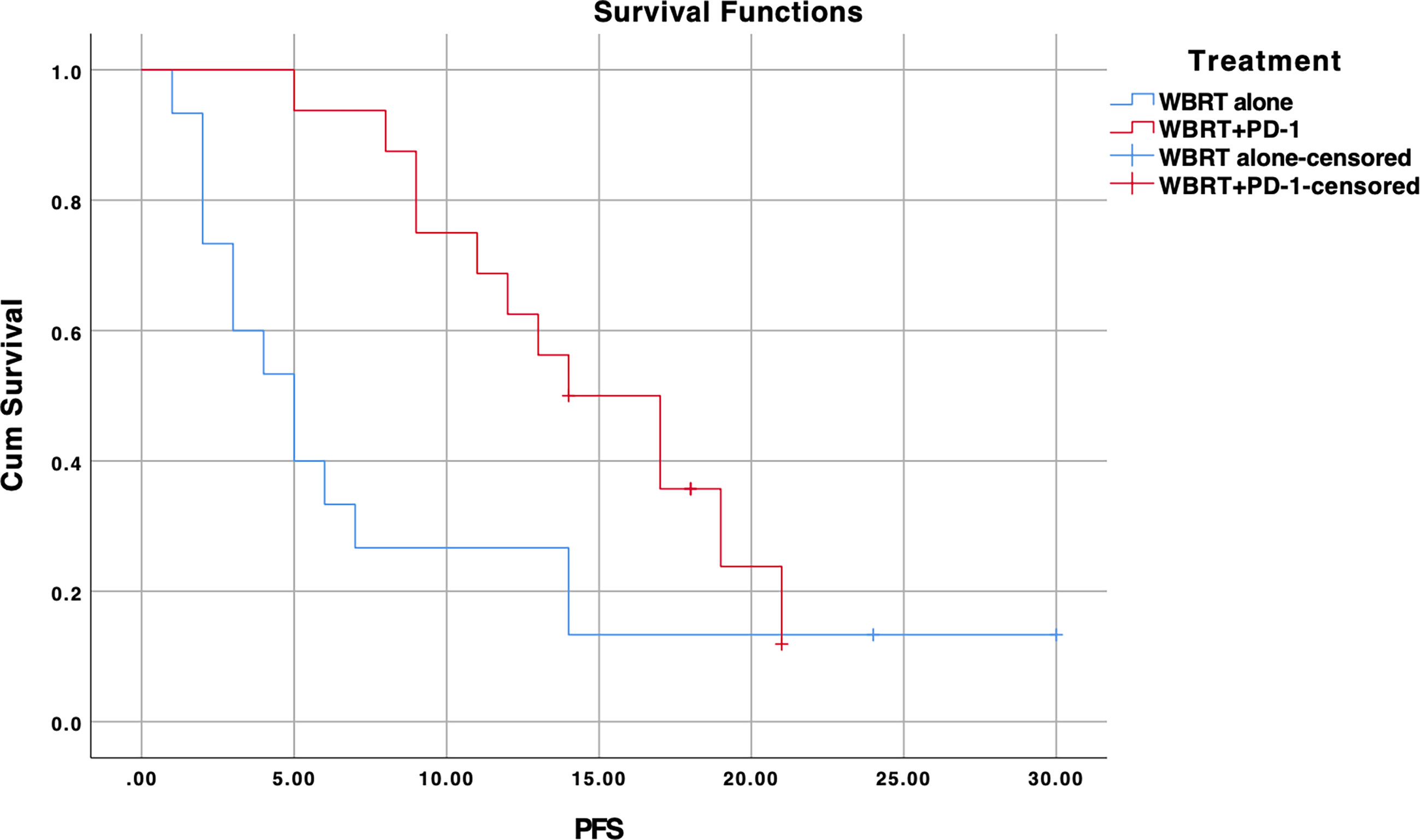
Figure 3 Kaplan-Meier progression-free survival (PFS) curve for treatments; WBRT alone (No PD-1), and WBRT plus PD-1 inhibition therapy (WBRT+PD-1). Cum, cumulative.
In subgroup analysis, NSCLC patients lacking EGFR mutation (n=31) showed significant improvement in PFS for the treatment difference (p=0.037) (Figure 4). WBRT plus anti-PD-1 cohort demonstrated a median PFS of 14 months (95% CI, 9.2–18.7) as compared to 5 months in WBRT alone (95% CI, 2.5–7.5). There was no difference when analysis was restricted to EGFR positive NSCLC patients (median PFS for WBRT alone (n=25): 9 months (95% CI, 3.6–14.4) vs. 12 months (95% CI, 8.0–15.9) for WBRT plus anti-PD-1 (n=5), p=0.510) (Supplementary Figure 2).
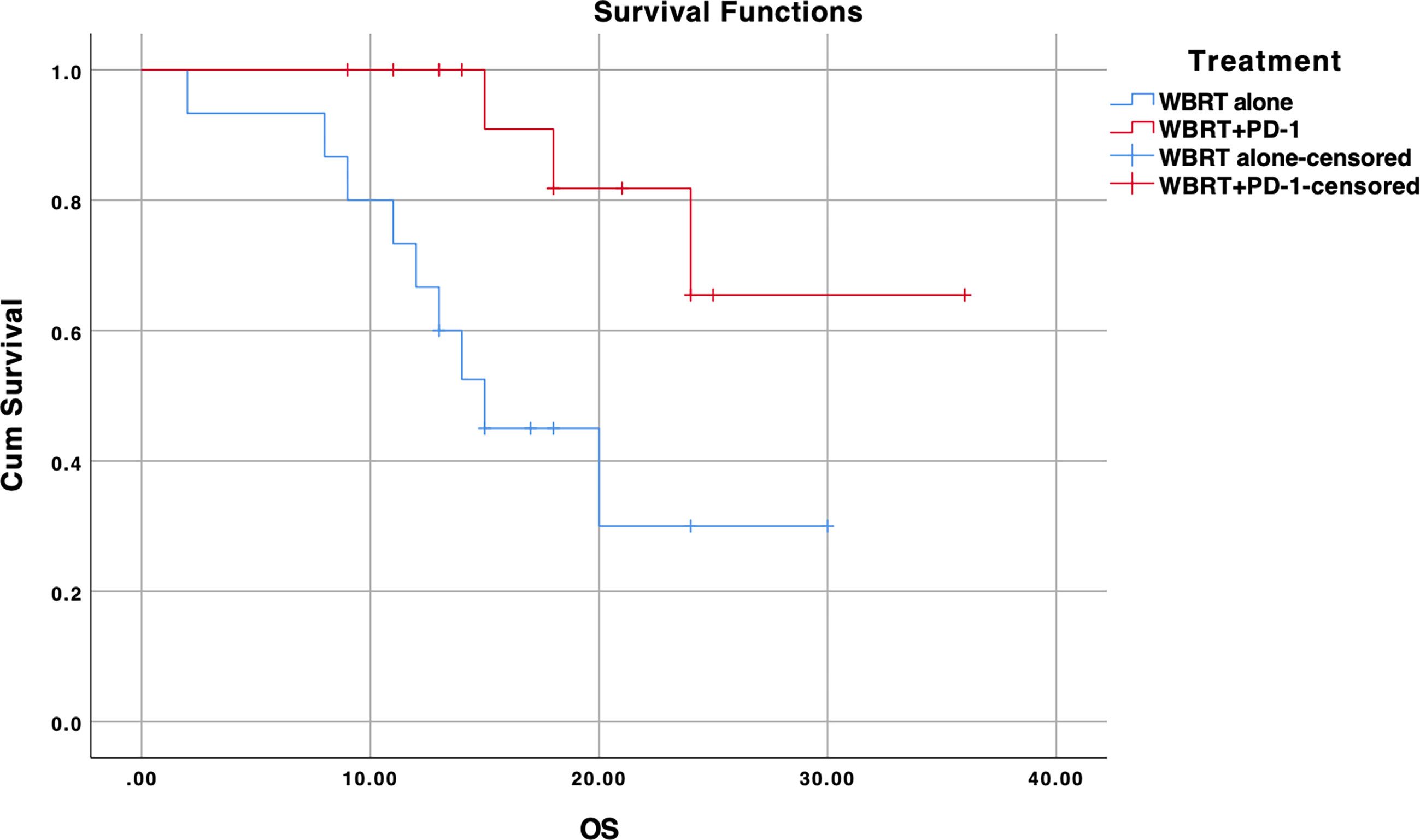
Figure 4 Kaplan-Meier progression-free survival (PFS) curve for treatments in EGFR wildtype NSCLC subgroup; WBRT alone (No PD-1), and WBRT plus PD-1 inhibition therapy (WBRT+PD-1). Cum, cumulative.
Overall, 47 patients (67%) experienced at least one adverse event. In the WBRT plus anti-PD-1 group, 18 (62%) experienced 34 (47.8%) adverse events, while 27 (65.8%) patients in the WBRT alone cohort suffered from 37 (52%) adverse events. There was no significant difference between the treatment cohorts in terms of adverse events (ORR, 0.94 [95% CI, 0.44–2.02), p=0.879). Most adverse events were of grade 1 or 2 severity. Only two grade 3 events were reported, and both were in patients receiving additional anti-PD-1 therapy. Rash, pruritis, hyperthyroidism, and hypothyroidism were mainly observed in the combined treatment group. The reported adverse events are listed in Table 4.
Lung cancer is often diagnosed at an advanced stage, with a 5-year survival rate of 5% (1). Advanced stage NSCLC patients lacking molecular markers are offered chemotherapy alone or chemotherapy in combination with immune checkpoint inhibitors (ICIs) as first-line treatment (16). Moreover, the addition of stereotactic ablative radiotherapy (SABR) to IT has also been tested in metastatic NSCLC (30, 31). This combination has demonstrated abscopal responses in metastatic sites and delayed disease progression (30, 31). In fact, multiple-lesion radiotherapy has shown to enhance the efficacy of PD-1 checkpoint inhibitors as compared to single-lesion receiving radiotherapy indicating synergism (32). However, the efficacy of this combination of RT and ICI in the brain remains to be elucidated. Our retrospective analysis of 70 NSCLC patients with multiple BMs (>3) revealed a better PFS (though not significant) and significantly improved OS in patients receiving concurrent treatment without any increase in toxicity.
The benefit in response can be explained based on preclinical and clinical evidence. Clinical response to ICIs is predicated on PD-L1 expression and the density of tumor-infiltrating lymphocytes (TILs), with a correlation between the primary tumor and BMs in lung adenocarcinoma (33–37). Moreover, RT promotes PD-L1 expression in metastatic sites by improving antigen presentation and tumor-specific immunity, which could be further augmented by ICIs to overcome the acquired resistance to RT (38–40). Third, local damage caused to the blood-brain-barrier during RT could also provide a window for IT drugs to be effective in the brain (41). For example, secondary analysis of the KEYNOTE-001 phase I trial demonstrated a comparatively superior PFS (median PFS: 4.4 vs. 2.1 months) and OS (median OS: 10.7 vs. 5.3 month) in the cohort that had previously received RT to the brain or extracerebral in addition to pembrolizumab, as compared to pembrolizumab alone (21). Moreover, the two concurrently administered treatments showed superior efficacy compared to use of only one of either treatment. In a retrospective study of BM patients (n=260) with primary tumor sites such as NSCLC, melanoma, and renal cell carcinoma (RCC), the use of ICIs with SRS/SRT (n=79) improved the median OS compared to SRS/SRT alone (14.5 vs. 12 months) (22). The improvement in OS (24.7 months) was significantly higher for the concurrent (within 2 weeks) IT (CI) cohort (n=28) than in the non-concurrent IT (nCI) cohort (HR 2.40, p=0.006) and SRS/SRT alone group (HR 2.69, p=0.002) (22). The median survival for concurrent group (n=28, median OS= 24.7 months) reported in the study by Chen et al. was similar to our study (n=29, median OS=27 months) (22). Likewise, no statistical difference in PFS (PFS: CI=2.3 vs. nCI=2.3 vs. SRS alone=3.7 months) was revealed between the cohorts (7 vs. 12 months, p=0.264) (22). However, the melanoma patients were prevalent in concurrent group (83%) in their study, which was also significant predictor of survival on multivariate analysis (HR 2.7, 95% CI: 1.6–4.7 for NSCLC; 3.6, 95% CI: 1.4–8.3 for RCC) (22). Melanoma BM patients (n=48, median OS=394 days) were also a significant driver of OS from the first anti-PD-1 therapy in comparison to NSCLC (n=79, median OS=192 days) and RCC (n=10, median OS=121 days) in a retrospective study by Pike et al. (23). In their study, among 59 BM patients who received RT following PD-1 inhibition, 25 continued to receive anti-PD-1 therapy for a median of 179 days and showed an improved median survival (additional 238 days) (23). In another retrospective matched cohort study of NSCLC-derived BM, the concurrent use of ICI (n=17, BMs=45) within 3-months of SRS provided a significantly rapid regression of BM (2.5 vs. 3.1 months, p<0.0001) and improved CNS complete response (CR) [8/16 (50%) vs. 5/32 (15.6%), p=0.012] compared to SRS alone (n=34, BM =92) (24). However, this benefit was not translated clinically in terms of PFS (HR 2.18; 95% CI, 0.72–6.62; p=0.11) and OS (HR 0.99, 95% CI: 0.39–2.52, p=0.99). Similarly, no statistical difference in median survival was found between the IT group (n=39) and CT group (n=46) in a retrospective study of 85 NSCLC patients with BMs (median OS: 10 vs. 11.6 months, p=0.23) (25). However, lesion shrinkage was significantly higher in the IT group than in the CT group in a subset of patients with lesion volume > 500 mm3 (90% vs. 47.8%, p=0.001). In both of these studies, even though no survival advantage was achieved, intracranial responses were observed with the combined approach. Similarly, the 6-month distant brain control rate for the before/concurrent cohort was significant compared to the post-RT cohort (57% vs. 0%, p=0.05) in a small cohort of NSCLC BM patients (n=17) receiving anti-PD-1 (nivolumab/durvalumab) and/or SRS/fractionated stereotactic radiation therapy (42). The timing (before/concurrent vs. after) was also significant for OS on univariate analysis (HR 9.2, 95% CI: 1.9–65.3, p=0.006) but not on multivariate analysis (HR 3.6; 95% CI, 0.74–26.9; p=0.11) (42). Another retrospective study on metastatic NSCLC patients revealed that delivering radiation before (6 months) or during/after (3 months) nivolumab administration was not associated with better OS or PFS (43). These outcomes endorse the observation that a window of at least 14 days was essential for palliative RT prior to the administration of nivolumab to take advantage of the RT-induced tumor antigenic stimulation effect (44, 45). Preclinical evidence also suggests that concurrent RT/anti-PD-1 inhibition may induce better anti-cancer effects compared to RT undertaken prior to PD-1 inhibition (30). Therefore, the literature implies that these patients may benefit from a combination of both treatments.
Investigation of factors affecting OS or PFS revealed no impact for several factors including age, gender, smoking, pathology, KPS, and the presence of EGFR mutation and extracranial metastases. Nonetheless, histopathologic differentiation showed slight association with worst PFS and OS. Likewise, smoking and KPS have also shown to negatively impact PFS on univariate and/or multivariate analysis. Importantly, WBRT alone group had more EGFR-positive participants, which may have confounded survival advantage as EGFR inhibitor plus WBRT treatment yields better survival compared to WBRT alone in NSCLC BM patients (14). For this reason, subgroup analysis was performed which revealed a significant improvement in PFS and OS for EGFR negative NSCLC patients. This result in concordance to a previously published meta-analysis comprising seven randomized controlled trials, in which immune checkpoint blockade had resulted in significantly better PFS and OS as compared to chemotherapy in EGFR wild-type stage IV NSCLC patients (PFS: HR 0.83, 95% CI 0.73-0.95); OS: HR 0.67, 95% CI 0.60-0.76; p<00001) (46). In this meta-analysis, EGFR mutant responded better to chemotherapy as opposed to immunotherapy in terms of PFS and OS. Likewise, no significant improvement in PFS and OS was found in EGFR positive patients in our study. Nonetheless, the cohort for combined treatment (n=5) was very small and further investigation would be required to establish presence or lack of benefit for the combined treatment in EGFR positive NSCLC patients with brain metastases.
Our study showed the safety of the combined approach with no increase in toxicity. Other studies have also revealed that the combination of ICIs and RT does not lead to an increase in toxicity compared to RT alone or ICI alone (47). In a single-center secondary analysis of a phase 1/2 trial (n=10), a combination of palliative RT (3DCRT, 79% and SRS–SRT, 21%; 28 Gy/5 fraction) plus durvalumab (10 mg/kg every 2 weeks via intravenous infusion) led to no grade 3 RT-related adverse events (AEs) (NCT01693562) (47). All AEs were transient and manageable according to the standard guidelines (47). Concurrent ICI (nivolumab within 3-month of RT) was also not associated with any increase in the rate of radiation necrosis or intratumoral hemorrhage in NSCLC-derived BM patients (5.9% vs. 2.9% in ICI-naive cohort, p=0.99) (24). There were no significant differences in the rates of all-grade AEs and grade ≥3 AEs between the ICI-naive (n=113) and ICI-treated NSCLC BM patients (n=50) across different cranial RT types (grade ≥3 AEs in 8% vs. 9% for SRS, p=1.00; 8% vs. 10% for WBRT, p=0.71) (48). Additionally, there was no difference in AE rates based on the timing of ICI administration with respect to RT.
Our study is limited by inherent biases of retrospective research design, which include selection, information, recall, and/or observation biases (49). The small sample size also limits our study. There is also chronological bias since WBRT alone participants were diagnosed earlier, whereas the PD-1 inhibition therapy group participants were diagnosed later. Moreover, certain outcomes were not reported such as intracranial response rate and subsequent therapies undertaken after completion or withdrawal from either treatment.
Our results indicate that concurrent WBRT and anti-PD-1 therapy may enhance OS in NSCLC patients with BM particularly in EGFR negative patients. The addition of anti-PD-1 therapy to RT may not increase the toxicity. Further studies are warranted to validate and elucidate the effect of using the treatments in combination.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics approval was obtained from the Ethical review board of “Shenzhen People’s Hospital, The First Affiliated Hospital Of Southern University Of Science And Technology, Shenzhen, China”, and “The First Affiliated Hospital Of Wenzhou Medical University, Wenzhou, China. The patients/participants provided their written informed consent to participate in this study.
GXL and GL provided the data. GXL and MK wrote the manuscript. All authors approved the design, data collection, data analysis, and final manuscript for publication.
The Natural Science Foundation of Shenzhen (No.JCYJ2017 0307095828424); Shenzhen Health and Family Planning System Research Project (No.SZBC2017024) were providing support for this work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.742971/full#supplementary-material
Supplementary Figure 1 | Kaplan-Meier overall survival (OS) curve for treatments in EGFR mutant NSCLC subgroup; WBRT alone (No PD-1), and WBRT plus PD-1 inhibition therapy (WBRT+PD-1). Cum, cumulative.
Supplementary Figure 2 | Kaplan-Meier progression-free survival (PFS) curve for treatments in EGFR mutant NSCLC subgroup; WBRT alone (No PD-1), and WBRT plus PD-1 inhibition therapy (WBRT+PD-1). Cum, cumulative.
1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2020. CA Cancer J Clin (2020) 70(1):7–30. doi: 10.3322/caac.21590
2. Petersen I. The Morphological and Molecular Diagnosis of Lung Cancer. Dtsch Arztebl Int (2011) 108(31-32):525–31. doi: 10.3238/arztebl.2011.0525
3. Eichler AF, Chung E, Kodack DP, Loeffler JS, Fukumura D, Jain RK. The Biology of Brain Metastases-Translation to New Therapies. Nat Rev Clin Oncol (2011) 8(6):344–56. doi: 10.1038/nrclinonc.2011.58
4. Liao BC, Lin CC, Yang JC. Treating Brain Metastases in Non-Small Cell Lung Cancer Patients: What Have We Learnt From Pharmaceutical Recent Clinical Trials? Expert Opin Pharmacother (2018) 19(8):851–64. doi: 10.1080/14656566.2018.1472765
5. Khan M, Arooj S, Li R, Tian Y, Zhang J, Lin J, et al. Tumor Primary Site and Histology Subtypes Role in Radiotherapeutic Management of Brain Metastases. Front Oncol (2020) 10:781(781). doi: 10.3389/fonc.2020.00781
6. Khan M, Lin J, Liao G, Li R, Wang B, Xie G, et al. Comparison of WBRT Alone, SRS Alone, and Their Combination in the Treatment of One or More Brain Metastases: Review and Meta-Analysis. Tumour Biol J Int Soc Oncodevelopmental Biol Med (2017) 39(7):1010428317702903. doi: 10.1177/1010428317702903
7. Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, et al. Whole Brain Radiation Therapy With or Without Stereotactic Radiosurgery Boost for Patients With One to Three Brain Metastases: Phase III Results of the RTOG 9508 Randomised Trial. Lancet (London England) (2004) 363(9422):1665–72. doi: 10.1016/s0140-6736(04)16250-8
8. Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, et al. Stereotactic Radiosurgery Plus Whole-Brain Radiation Therapy vs Stereotactic Radiosurgery Alone for Treatment of Brain Metastases: A Randomized Controlled Trial. Jama (2006) 295(21):2483–91. doi: 10.1001/jama.295.21.2483
9. Linskey ME, Andrews DW, Asher AL, Burri SH, Kondziolka D, Robinson PD, et al. The Role of Stereotactic Radiosurgery in the Management of Patients With Newly Diagnosed Brain Metastases: A Systematic Review and Evidence-Based Clinical Practice Guideline. J Neurooncol (2010) 96(1):45–68. doi: 10.1007/s11060-009-0073-4
10. Lippitz B, Lindquist C, Paddick I, Peterson D, O’Neill K, Beaney R. Stereotactic Radiosurgery in the Treatment of Brain Metastases: The Current Evidence. Cancer Treat Rev (2014) 40(1):48–59. doi: 10.1016/j.ctrv.2013.05.002
11. Khan M, Lin J, Liao G, Tian Y, Liang Y, Li R, et al. Whole Brain Radiation Therapy Plus Stereotactic Radiosurgery in the Treatment of Brain Metastases Leading to Improved Survival in Patients With Favorable Prognostic Factors. Front Oncol (2019) 9:205(205). doi: 10.3389/fonc.2019.00205
12. Ni M, Liu W, Jiang A, Wang Y, Sheng Y, Zeng H, et al. Whole Brain Radiation Therapy Plus Focal Radiation Boost May Generate Better Survival Benefit for Brain Metastases From Non-Small Cell Lung Cancer. Front Oncol (2020) 10:576700. doi: 10.3389/fonc.2020.576700
13. Barbour AB, Jacobs CD, Williamson H, Floyd SR, Suneja G, Torok JA, et al. Radiation Therapy Practice Patterns for Brain Metastases in the United States in the Stereotactic Radiosurgery Era. Adv Radiat Oncol (2020) 5(1):43–52. doi: 10.1016/j.adro.2019.07.012
14. Wang C, Lu X, Lyu Z, Bi N, Wang L. Comparison of Up-Front Radiotherapy and TKI With TKI Alone for NSCLC With Brain Metastases and EGFR Mutation: A Meta-Analysis. Lung Cancer (Amsterdam Netherlands) (2018) 122:94–9. doi: 10.1016/j.lungcan.2018.05.014
15. Goldberg SB, Gettinger SN, Mahajan A, Chiang AC, Herbst RS, Sznol M, et al. Pembrolizumab for Patients With Melanoma or Non-Small-Cell Lung Cancer and Untreated Brain Metastases: Early Analysis of a Non-Randomised, Open-Label, Phase 2 Trial. Lancet Oncol (2016) 17(7):976–83. doi: 10.1016/s1470-2045(16)30053-5
16. Huang Z, Su W, Lu T, Wang Y, Dong Y, Qin Y, et al. First-Line Immune-Checkpoint Inhibitors in Non-Small Cell Lung Cancer: Current Landscape and Future Progress. Front Pharmacol (2020) 11:578091(1591). doi: 10.3389/fphar.2020.578091
17. Dudnik E, Yust-Katz S, Nechushtan H, Goldstein DA, Zer A, Flex D, et al. Intracranial Response to Nivolumab in NSCLC Patients With Untreated or Progressing CNS Metastases. Lung Cancer (2016) 98:114–7. doi: 10.1016/j.lungcan.2016.05.031
18. Gauvain C, Vauléon E, Chouaid C, Le Rhun E, Jabot L, Scherpereel A, et al. Intracerebral Efficacy and Tolerance of Nivolumab in Non-Small-Cell Lung Cancer Patients With Brain Metastases. Lung Cancer (2018) 116:62–6. doi: 10.1016/j.lungcan.2017.12.008
19. Li W, Yu H. Separating or Combining Immune Checkpoint Inhibitors (ICIs) and Radiotherapy in the Treatment of NSCLC Brain Metastases. J Cancer Res Clin Oncol (2020) 146(1):137–52. doi: 10.1007/s00432-019-03094-9
20. Khan M, Lin J, Liao G, Tian Y, Liang Y, Li R, et al. SRS in Combination With Ipilimumab: A Promising New Dimension for Treating Melanoma Brain Metastases. Technol Cancer Res Treat (2018) 17:1533033818798792. doi: 10.1177/1533033818798792
21. Shaverdian N, Lisberg AE, Bornazyan K, Veruttipong D, Goldman JW, Formenti SC, et al. Previous Radiotherapy and the Clinical Activity and Toxicity of Pembrolizumab in the Treatment of Non-Small-Cell Lung Cancer: A Secondary Analysis of the KEYNOTE-001 Phase 1 Trial. Lancet Oncol (2017) 18(7):895–903. doi: 10.1016/s1470-2045(17)30380-7
22. Chen L, Douglass J, Kleinberg L, Ye X, Marciscano AE, Forde PM, et al. Concurrent Immune Checkpoint Inhibitors and Stereotactic Radiosurgery for Brain Metastases in Non-Small Cell Lung Cancer, Melanoma, and Renal Cell Carcinoma. Int J Radiat Oncol Biol Phys (2018) 100(4):916–25. doi: 10.1016/j.ijrobp.2017.11.041
23. Pike LRG, Bang A, Ott P, Balboni T, Taylor A, Catalano P, et al. Radiation and PD-1 Inhibition: Favorable Outcomes After Brain-Directed Radiation. Radiother Oncol J Eur Soc Ther Radiol Oncol (2017) 124(1):98–103. doi: 10.1016/j.radonc.2017.06.006
24. Shepard MJ, Xu Z, Donahue J, Eluvathingal Muttikkal TJ, Cordeiro D, Hansen L, et al. Stereotactic Radiosurgery With and Without Checkpoint Inhibition for Patients With Metastatic Non-Small Cell Lung Cancer to the Brain: A Matched Cohort Study. J Neurosurg (2019) 26:1–8. doi: 10.3171/2019.4.Jns19822
25. Singh C, Qian JM, Yu JB, Chiang VL. Local Tumor Response and Survival Outcomes After Combined Stereotactic Radiosurgery and Immunotherapy in Non-Small Cell Lung Cancer With Brain Metastases. J Neurosurg (2019) 132(2):512–7. doi: 10.3171/2018.10.Jns181371
26. World Medical Association Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects. Jama (2013) 310(20):2191–4. doi: 10.1001/jama.2013.281053
27. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. J Clin Epidemiol (2008) 61(4):344–9. doi: 10.1016/j.jclinepi.2007.11.008
28. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur J Cancer (2009) 45(2):228–47. doi: 10.1016/j.ejca.2008.10.026
29. Yu Y, Ruddy KJ, Tsuji S, Hong N, Liu H, Shah N, et al. Coverage Evaluation of CTCAE for Capturing the Immune-Related Adverse Events Leveraging Text Mining Technologies. AMIA Jt Summits Transl Sci Proc (2019) 2019:771–8.
30. Chicas-Sett R, Morales-Orue I, Castilla-Martinez J, Zafra-Martin J, Kannemann A, Blanco J, et al. Stereotactic Ablative Radiotherapy Combined With Immune Checkpoint Inhibitors Reboots the Immune Response Assisted by Immunotherapy in Metastatic Lung Cancer: A Systematic Review. Int J Mol Sci (2019) 20(9):2173. doi: 10.3390/ijms20092173
31. Luke JJ, Lemons JM, Karrison TG, Pitroda SP, Melotek JM, Zha Y, et al. Safety and Clinical Activity of Pembrolizumab and Multisite Stereotactic Body Radiotherapy in Patients With Advanced Solid Tumors. J Clin Oncol Off J Am Soc Clin Oncol (2018) 36(16):1611–8. doi: 10.1200/jco.2017.76.2229
32. Schubert P, Rutzner S, Eckstein M, Frey B, Schweizer C, Haderlein M, et al. Prospective Evaluation of All-Lesion Versus Single-Lesion Radiotherapy in Combination With PD-1/PD-L1 Immune Checkpoint Inhibitors. Front Oncol (2020) 10:576643. doi: 10.3389/fonc.2020.576643
33. Téglási V, Reiniger L, Fábián K, Pipek O, Csala I, Bagó AG, et al. Evaluating the Significance of Density, Localization, and PD-1/PD-L1 Immunopositivity of Mononuclear Cells in the Clinical Course of Lung Adenocarcinoma Patients With Brain Metastasis. Neuro Oncol (2017) 19(8):1058–67. doi: 10.1093/neuonc/now309
34. Yi M, Jiao D, Xu H, Liu Q, Zhao W, Han X, et al. Biomarkers for Predicting Efficacy of PD-1/PD-L1 Inhibitors. Mol Cancer (2018) 17(1):129. doi: 10.1186/s12943-018-0864-3
35. Batur S, Dulger O, Durak S, Yumuk PF, Caglar HB, Bozkurtlar E, et al. Concordance of PD-L1 Expression and CD8+ TIL Intensity Between NSCLC and Synchronous Brain Metastases. Bosn J Basic Med Sci (2020) 20(3):329–35. doi: 10.17305/bjbms.2019.4474
36. Berghoff AS, Fuchs E, Ricken G, Mlecnik B, Bindea G, Spanberger T, et al. Density of Tumor-Infiltrating Lymphocytes Correlates With Extent of Brain Edema and Overall Survival Time in Patients With Brain Metastases. Oncoimmunology (2016) 5(1):e1057388. doi: 10.1080/2162402x.2015.1057388
37. Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The Immune Contexture in Human Tumours: Impact on Clinical Outcome. Nat Rev Cancer (2012) 12(4):298–306. doi: 10.1038/nrc3245
38. Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ, et al. Acquired Resistance to Fractionated Radiotherapy can be Overcome by Concurrent PD-L1 Blockade. Cancer Res (2014) 74(19):5458–68. doi: 10.1158/0008-5472.Can-14-1258
39. Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, et al. Irradiation and Anti-PD-L1 Treatment Synergistically Promote Antitumor Immunity in Mice. J Clin Invest (2014) 124(2):687–95. doi: 10.1172/jci67313
40. Takamori S, Toyokawa G, Okamoto I, Takada K, Kozuma Y, Matsubara T, et al. Discrepancy in Programmed Cell Death-Ligand 1 Between Primary and Metastatic Non-Small Cell Lung Cancer. Anticancer Res (2017) 37(8):4223–8. doi: 10.21873/anticanres.11813
41. Sprowls SA, Arsiwala TA, Bumgarner JR, Shah N, Lateef SS, Kielkowski BN, et al. Improving CNS Delivery to Brain Metastases by Blood–Tumor Barrier Disruption. Trends Cancer (2019) 5(8):495–505. doi: 10.1016/j.trecan.2019.06.003
42. Ahmed KA, Kim S, Arrington J, Naghavi AO, Dilling TJ, Creelan BC, et al. Outcomes Targeting the PD-1/PD-L1 Axis in Conjunction With Stereotactic Radiation for Patients With Non-Small Cell Lung Cancer Brain Metastases. J Neurooncol (2017) 133(2):331–8. doi: 10.1007/s11060-017-2437-5
43. Lesueur P, Escande A, Thariat J, Vauléon E, Monnet I, Cortot A, et al. Safety of Combined PD-1 Pathway Inhibition and Radiation Therapy for Non-Small-Cell Lung Cancer: A Multicentric Retrospective Study From the GFPC. Cancer Med (2018) 7(11):5505–13. doi: 10.1002/cam4.1825
44. Gerber DE, Urbanic JJ, Langer C, Hu C, Chang IF, Lu B, et al. Treatment Design and Rationale for a Randomized Trial of Cisplatin and Etoposide Plus Thoracic Radiotherapy Followed by Nivolumab or Placebo for Locally Advanced Non-Small-Cell Lung Cancer (RTOG 3505). Clin Lung Cancer (2017) 18(3):333–9. doi: 10.1016/j.cllc.2016.10.009
45. Iyengar P, Gerber DE. Locally Advanced Lung Cancer: An Optimal Setting for Vaccines and Other Immunotherapies. Cancer J (Sudbury Mass) (2013) 19(3):247–62. doi: 10.1097/PPO.0b013e318292e51a
46. Khan M, Lin J, Liao G, Tian Y, Liang Y, Li R, et al. Comparative Analysis of Immune Checkpoint Inhibitors and Chemotherapy in the Treatment of Advanced Non-Small Cell Lung Cancer: A Meta-Analysis of Randomized Controlled Trials. Medicine (2018) 97(33):e11936. doi: 10.1097/md.0000000000011936
47. Levy A, Massard C, Soria JC, Deutsch E. Concurrent Irradiation With the Anti-Programmed Cell Death Ligand-1 Immune Checkpoint Blocker Durvalumab: Single Centre Subset Analysis From a Phase 1/2 Trial. Eur J Cancer (2016) 68:156–62. doi: 10.1016/j.ejca.2016.09.013
48. Hubbeling HG, Schapira EF, Horick NK, Goodwin KEH, Lin JJ, Oh KS, et al. Safety of Combined PD-1 Pathway Inhibition and Intracranial Radiation Therapy in Non-Small Cell Lung Cancer. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer (2018) 13(4):550–8. doi: 10.1016/j.jtho.2018.01.012
Keywords: non-small cell lung cancer (NSCLC), immunotherapy (IT), anti-PD-1 immunotherapy, whole brain radiation therapy (WBRT), immune checkpoint blockade (ICB), brain metastasis (BM)
Citation: Liao G, Qian Y, Arooj S, Zhao Z, Yan M, Li Z, Yang H, Zheng T, Li G, Li X and Khan M (2021) Radiation Plus Anti-PD-1 Therapy for NSCLC Brain Metastases: A Retrospective Study. Front. Oncol. 11:742971. doi: 10.3389/fonc.2021.742971
Received: 17 July 2021; Accepted: 28 September 2021;
Published: 21 October 2021.
Edited by:
Minesh P. Mehta, Baptist Health South Florida, United StatesReviewed by:
Aidan D. Meade, Technological University Dublin, IrelandCopyright © 2021 Liao, Qian, Arooj, Zhao, Yan, Li, Yang, Zheng, Li, Li and Khan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Muhammad Khan, ZHJraGFuX29uY29Ab3V0bG9vay5jb20=; Xianming Li, Y2hlbmxobmZ5QDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.