- 1Endoscopic and Oncoplastic Breast Surgery Center, Department of Surgery, Changhua Christian Hospital, Changhua, Taiwan
- 2Division of General Surgery, Department of Surgery, Changhua Christian Hospital, Changhua, Taiwan
- 3Comprehensive Breast Cancer Center, Department of Surgery, Changhua Christian Hospital, Changhua, Taiwan
- 4Minimal Invasive Surgery Research Center, Department of Surgery, Changhua Christian Hospital, Changhua, Taiwan
- 5Department of Surgery, Kaohsiung Medical University, Kaohsiung, Taiwan
- 6Division of Breast Surgery, Department of Surgery, Yuanlin Christian Hospital, Yuanlin, Taiwan
- 7Department of Surgery, School of Medicine, National Yang Ming University, Taipei, Taiwan
- 8Department of Surgery, School of Medicine, Chung Shan Medical University, Taichung, Taiwan
- 9Department of Surgery, Chang Gung University College of Medicine, Taoyuan City, Taiwan
- 10Division of General Surgery, Department of Surgery, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, Taiwan
- 11Tumor Center, Changhua Christian Hospital, Changhua, Taiwan
- 12Division of Plastic and Reconstructive Surgery, Department of Surgery, Changhua Christian Hospital, Changhua, Taiwan
Objective: Endoscopic assisted breast surgery (EABS) or robotic assisted breast surgery (RABS) performed through minimal axillary and/or peri-areolar incisions has become the representative of minimal access breast surgery (MABS). We report the trend and clinical outcome of MABS for treatment of breast cancer.
Methods: Information on patients who underwent breast cancer operation by the principal investigator during the period of 2011 to 2020 was collected from a single institute for analysis. The clinical outcome, trend, and cost of MABS were analyzed and compared with conventional breast surgery (CBS).
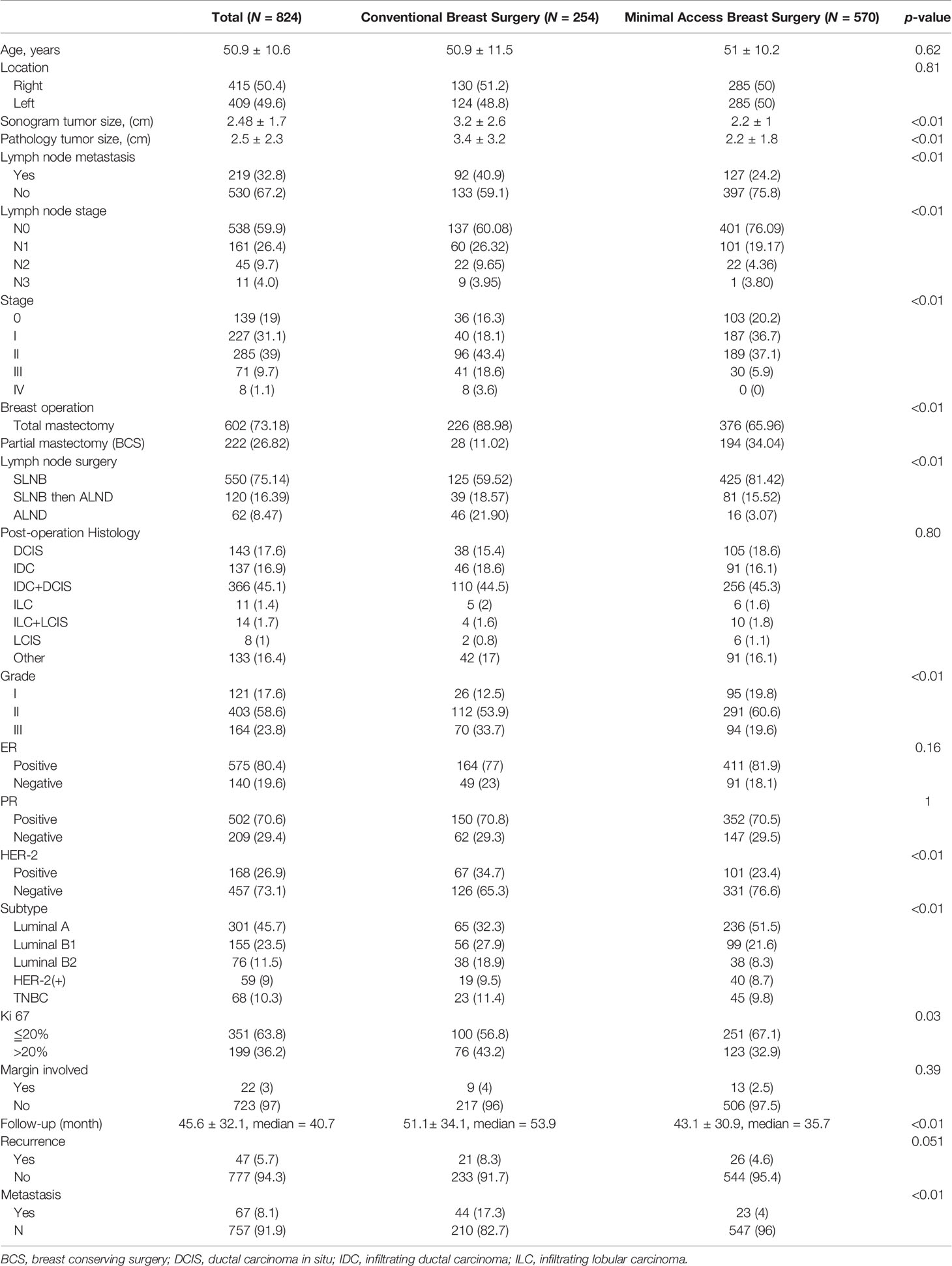
Results: A total of 824 breast cancer patients operated by a single surgeon were enrolled in this study: 254 received CBS and 570 received MABS, namely, 476 EABS and 94 RABS. From 2011 to 2020, the number of MABS performed annually has shown an increasing trend. Compared with CBS, MABS such as breast conserving surgery and nipple sparing mastectomy (NSM) have effectively reduced wound scar length. Since the sequential uprise from conventional NSM (C-NSM), dual-axillary-areolar-incision two dimensional (2D) endoscopic assisted NSM (E-NSM), single-axillary-incision E-NSM, robotic assisted NSM (R-NSM), and single-port 3D E-NSM, the development of minimal access mastectomies increasingly paralleled with NSM. The operation time of various MABS decreased significantly and showed no statistical difference compared with CBS. R-NSM was associated with highest cost, followed by 3D E-NSM, E-NSM, and C-NSM. The positive surgical margin rate and local recurrence rate of MABS and CBS were not statistically different.
Conclusion: MABS showed comparable clinical outcome and preliminary oncologic safety as CBS and has been increasingly performed as the surgical treatment of breast cancer, especially minimal access NSM.
Introduction
Minimal invasive/access surgery has become the mainstream of surgical practice in recent decades (1–3). Endoscopic assisted breast surgery (EABS) (4–6) or robotic assisted breast surgery (RABS) (7–9) performed through minimal axillary and/or peri-areolar incisions has become the representative of minimal access breast surgery (MABS) (10). MABS, either EABS or RABS, has been performed in breast conserving surgery (BCS) (4, 11, 12), mastectomy [mainly nipple sparing mastectomy (NSM) or skin sparing mastectomy (SSM) in some conditions] (7, 8, 13–17), and harvest of autologous flaps [latissimus dorsi flap (LDF) (18–20), omentum flap (21–23), or abdominal flap (14, 24, 25)]. Due to the wide spread of the breast screening program, the number of early breast cancers diagnosed increased dramatically and so is the reported use of MABS in literature around the world (15, 26, 27).
The advantages of MABS (EABS or RABS) include shortening of operation scar while hiding it in inconspicuous locations, which optimize aesthetic outcome and patients’ satisfaction (9, 11, 15, 17). However, the widespread use of MABS in the management of breast cancer is yet to be fully accepted; objections include limited working space, superficial nature of breast lesion, and relative low morbidity of breast surgery (28). Drawbacks include longer operation time, more instruments needed, and higher medical cost (9, 17, 29) Of utter importance, the long-term oncologic outcome is rarely reported (5, 26) and not yet confirmed by large randomized trials.
At our institution, we started performing EABS (4, 13–15, 26, 30, 31) for breast cancer treatment since 2010 and developed RABS (9, 17–19, 32–34) in 2017. After 10 years of work in the field of MABS, much experience and accumulated results of its applications in breast cancer were obtained. The development and trend of MABS (EABS or RABS) versus conventional breast surgery (CBS) in the past decade were evaluated from the principal investigator’s personal perspective. The clinical outcome, complications, and cost of MABS versus CBS were also analyzed and reported.
Materials and Methods
Patients
Patients who received CBS or MABS (EABS or RABS) for breast cancer by the principal investigator (H-WL) from January 2011 to December 2020 were retrieved from a prospectively collected endoscopic and robotic assisted breast surgery database at Changhua Christian Hospital (CCH) in Taiwan. Patient selection and enrollment process are shown in Figure 1.
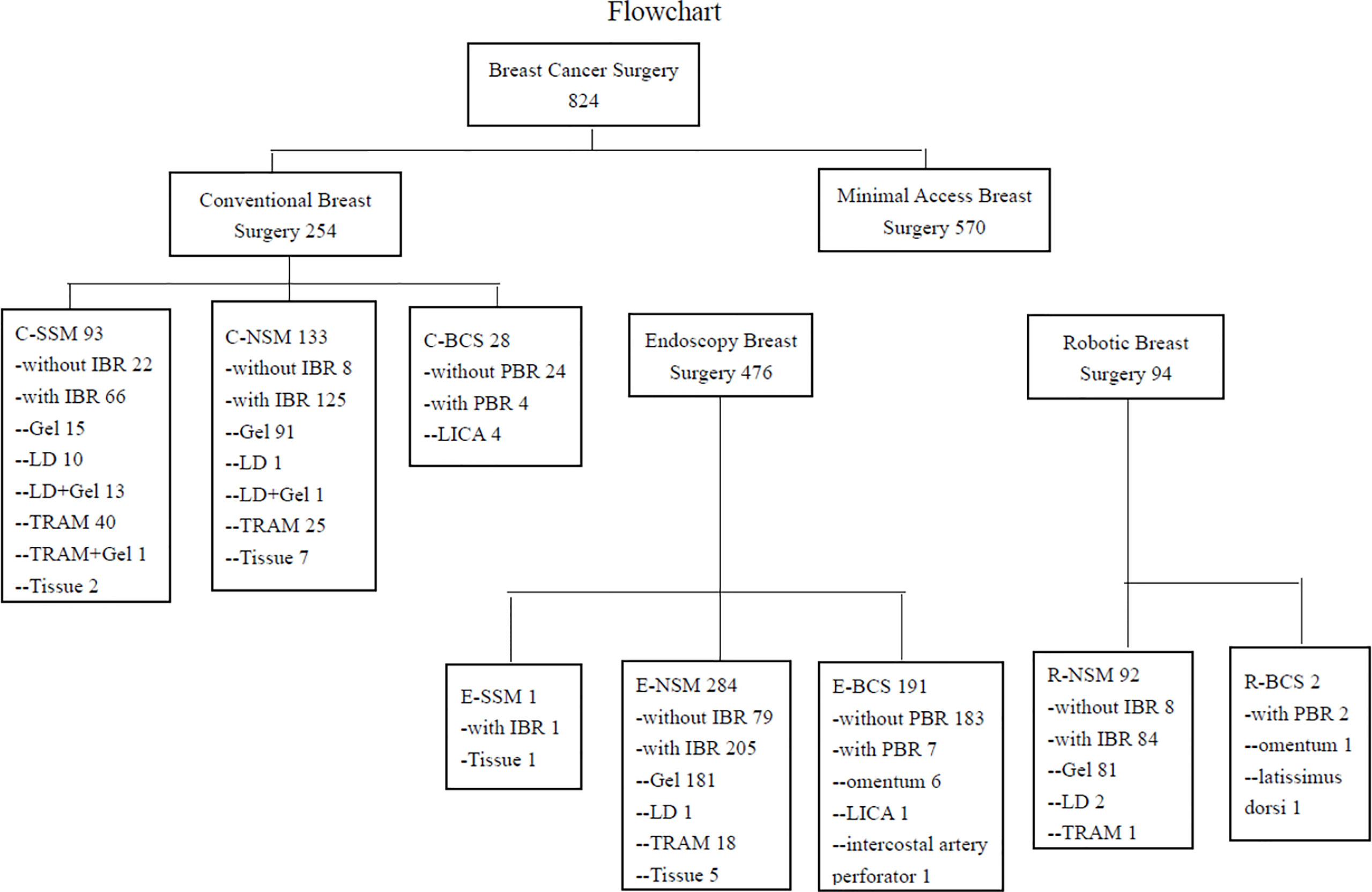
Figure 1 Flow chart of patients’ management in the current study. C-SSM, conventional skin sparing mastectomy; C-NSM, conventional nipple sparing mastectomy; C-BCS, conventional breast conserving surgery; PBR, partial breast reconstruction; LICA, lateral intercostal artery perforator flap; IBR, immediate breast reconstruction; LD, latissimus dorsi flap; TRAM, transverse abdominal rectus muscular flap; Tissue, tissue expander; E-SSM, endoscopic skin sparing mastectomy; E-NSM, endoscopic nipple sparing mastectomy; E-BCS, endoscopic breast conserving surgery; R-NSM, robotic nipple sparing mastectomy; R-BCS, robotic breast conserving surgery.
The data collection included clinicopathologic characteristics of patients, type of surgery, operative time, blood loss, length of hospital stays, recurrence, and survival status at last follow-up. All data were collected from chart review by specially trained nurses and subsequently confirmed by the principal investigator (H-WL). The study was approved by the Institutional Review Board of the CCH (CCH IRB No. 141224, 170806, and 190414). Written informed consent pertaining to the use of clinical records was obtained from each participant. This current report included photos of several patients who had agreed and signed the consent for publication of their pictures.
Indication for MABS (EABS or RABS)
Pre-operative sonography, mammography, and/or magnetic resonance imaging (MRI) were used to determine the eligibility of patients for MABS (EABS or RABS). Liver sonography, chest x-ray, and whole-body bone scan were used to exclude the possibility of distant metastasis. Indications for EABS or RABS include early-stage breast cancer (ductal carcinoma in situ, stage I, II or IIIA), a tumor size less than 3 cm (for BCS) or no larger than 5 cm (for mastectomy), absence of apparent multiple lymph nodes metastases, and absence of skin or chest wall invasion.
Patients for whom EABS or RABS was contraindicated include those with inflammatory breast cancer, breast cancer with chest wall or skin invasion, locally advanced breast cancer, breast cancer with extensive axillary lymph node metastasis (stage IIIB or later), and severe comorbidities, such as heart disease, renal failure, liver dysfunction, and poor performance status as assessed by their primary physicians. The inclusion and exclusion criteria were based on previous studies (11, 13–15, 27, 34) as well as current breast cancer treatment guidelines.
Minimal Access Endoscopic or Robotic Breast Surgery Technique
Details of the surgical technique used for EABS (11, 13–15, 31) or RABS (18, 19, 27, 32, 34) in the current study have been described previously. Endoscopic assisted or robotic assisted BCS and mastectomy (with or without breast reconstructions) were common MABS or aesthetic scar-less mastectomy (35) procedures for surgical treatments of breast cancers. Common incisions for MABS include axillary and/or areolar incisions via either dual incisions or single areolar, axillary, or lateral chest incision as per case indicated (Figure 2 and Supplementary Figure 1). Whether breast reconstructions were performed immediately or at a later time is decided with patients and physicians’ shared decision making. Breast reconstructions after minimal access mastectomy (or aesthetic scar-less mastectomy35) can be performed by using an implant (cohesive gel implants or tissue expander) (13, 15, 34) or autologous tissue with latissimus dorsi (LD) flap (18, 19) or abdominal flap (14, 24, 25) (Figure 2 and Supplementary Figure 1).
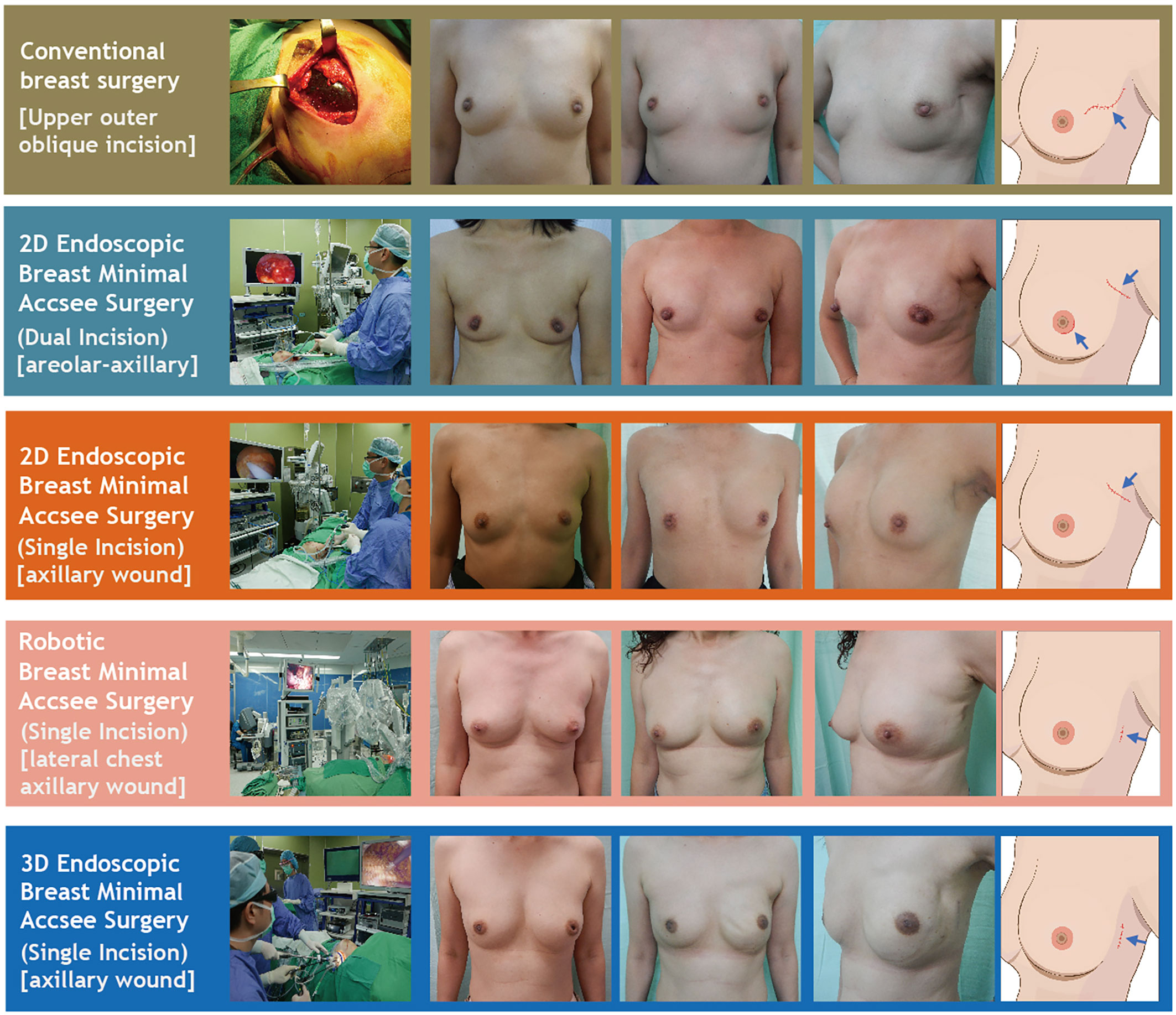
Figure 2 Various surgical technique of nipple sparing mastectomy (NSM). This figure showed various NSM techniques used in the current study, including conventional breast surgery, 2D endoscopic breast surgery (dual-areolar-axillary-incision), 2D endoscopic single-axillary-incision breast surgery, robotic assisted breast surgery, and single-port 3D assisted breast surgery. Photos are shown in each category including pre-operative frown view, post-operative front view, post-operative lateral view, and cartoon illustration of scar location.
Clinical Outcome of Minimal Access Breast Surgery
Peri-operative parameters, such as operation time, blood loss, complications, and hospital stay, of CBS, EABS, and RABS were analyzed and compared. Complications were also graded with the Clavien-Dindo classification (36) for severity evaluation. For oncological safety evaluation, we analyzed the rate of positive surgical margin involvement, local regional recurrence, distant metastasis, disease-free survival, and overall survival. Surgical margin involvement was defined as tumor on ink (37). Adjuvant chemotherapy and radiotherapy were given to patients based on recommendations from current breast cancer guidelines. Incidence of recurrence or death due to breast cancer was ascertained at the most recent follow-up, with a cutoff date in April 2021.
Statistical Analyses
Differences in continuous variables were tested by non-parametric Mann–Whitney U test and Kruskal–Wallis test, and are reported as means ± standard deviation (SD). The chi-square test was used for categorical comparisons of data when appropriate. A p-value of less than 0.05 was considered of statistical significance; all tests were two-tailed. Differences in cumulative survival were assessed using the log-rank test. All statistical analyses were performed with statistical package SPSS (Version 19.0, SPSS, Chicago) by a statistics personnel (Y-JL).
Results
During 2011 to 2020, a total of 824 breast cancer patients operated by a single surgeon (H-WL) were enrolled in the current study. Among them, 254 received conventional breast surgeries (CBS) and 570 received MABS. These included 476 EABS and 94 RABS. The characteristics and clinicopathologic parameters of enrolled patients are summarized in Table 1, and types of surgeries performed are shown in flow chart and photos (Figures 1, 2). The RABS mainly focusing on R-NSM (92/94) related, and most of the patients received immediate gel implant breast reconstruction (IGBR). The only two robotic assisted BCSs were related to partial breast reconstructions (robotic LD flap harvest or robotic omentum flap harvest). In our current MABS cohort, no case (either EABS nor RABS) required conversion to conventional operation method.
Trend of Minimal Access Breast Surgery
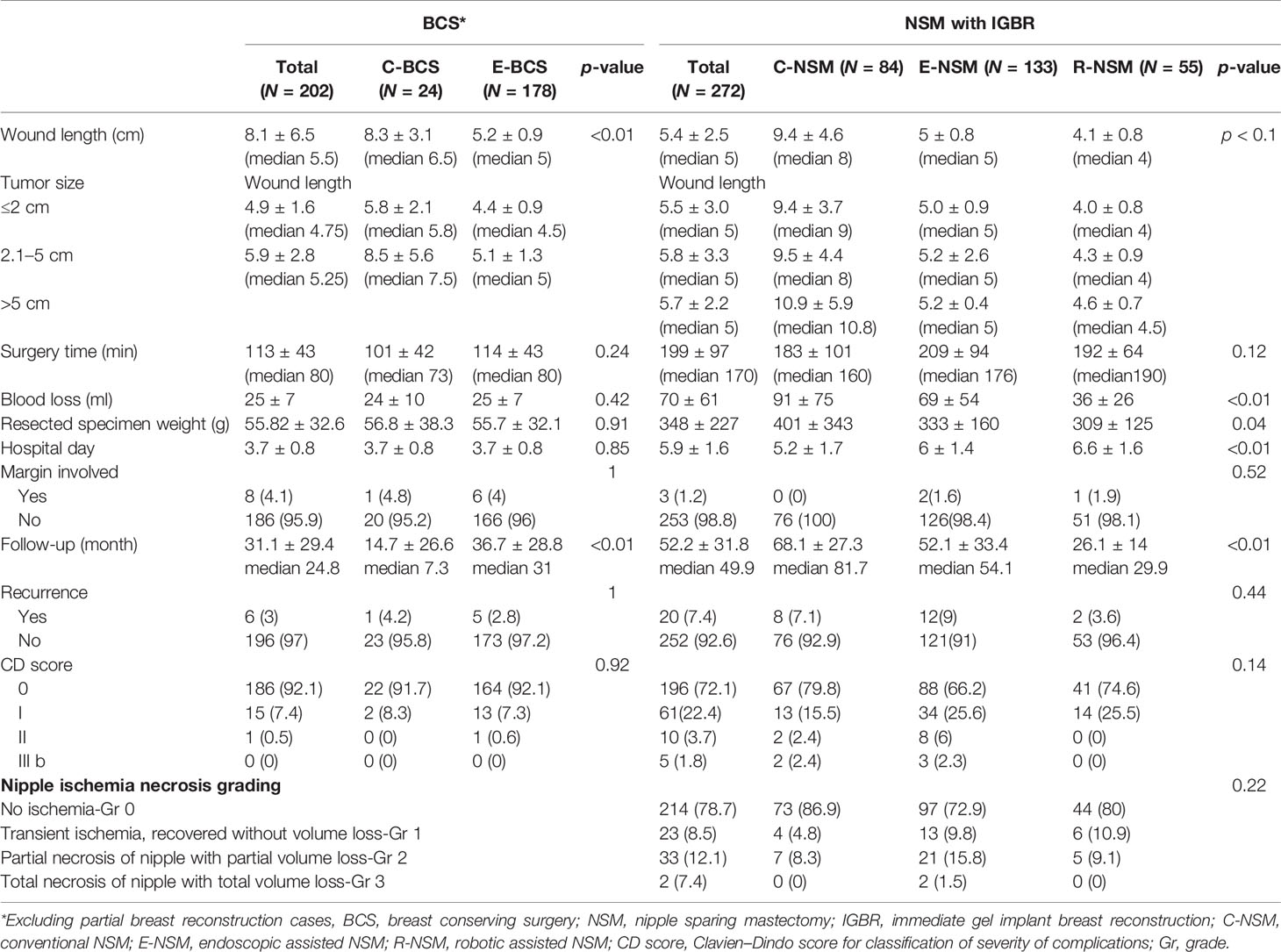
Breast cancer cases that received CBS or MABS were recorded and plotted from 2011 to 2020 (Figure 3A). Number of MABS performed increased annually and became the mainstream choice. Among this cohort of patients, 221 cases received BCS. Twenty-eight received conventional BCS (C-BCS), 191 received endoscopic assisted BCS (E-BCS), and 2 received robotic assisted BCS (R-BCS, Table 2). The mean incisional wound length of C-BCS was 8.3 ± 3.1 cm (median 6.5 cm) versus 5.2 ± 0.9 cm in E-BCS (median 5 cm, p < 0.01). The operation time of C-BCS versus E-BCS was 101 ± 42 versus 114 ± 43 min (p = 0.24), respectively. The median follow-up time of BCS patients was 24.8 months. Comparison between E-BCS and C-BCS showed no statistical difference between mean blood loss, resected specimen weight, hospital stay, positive margin rate, complication, and local recurrence (Table 2).
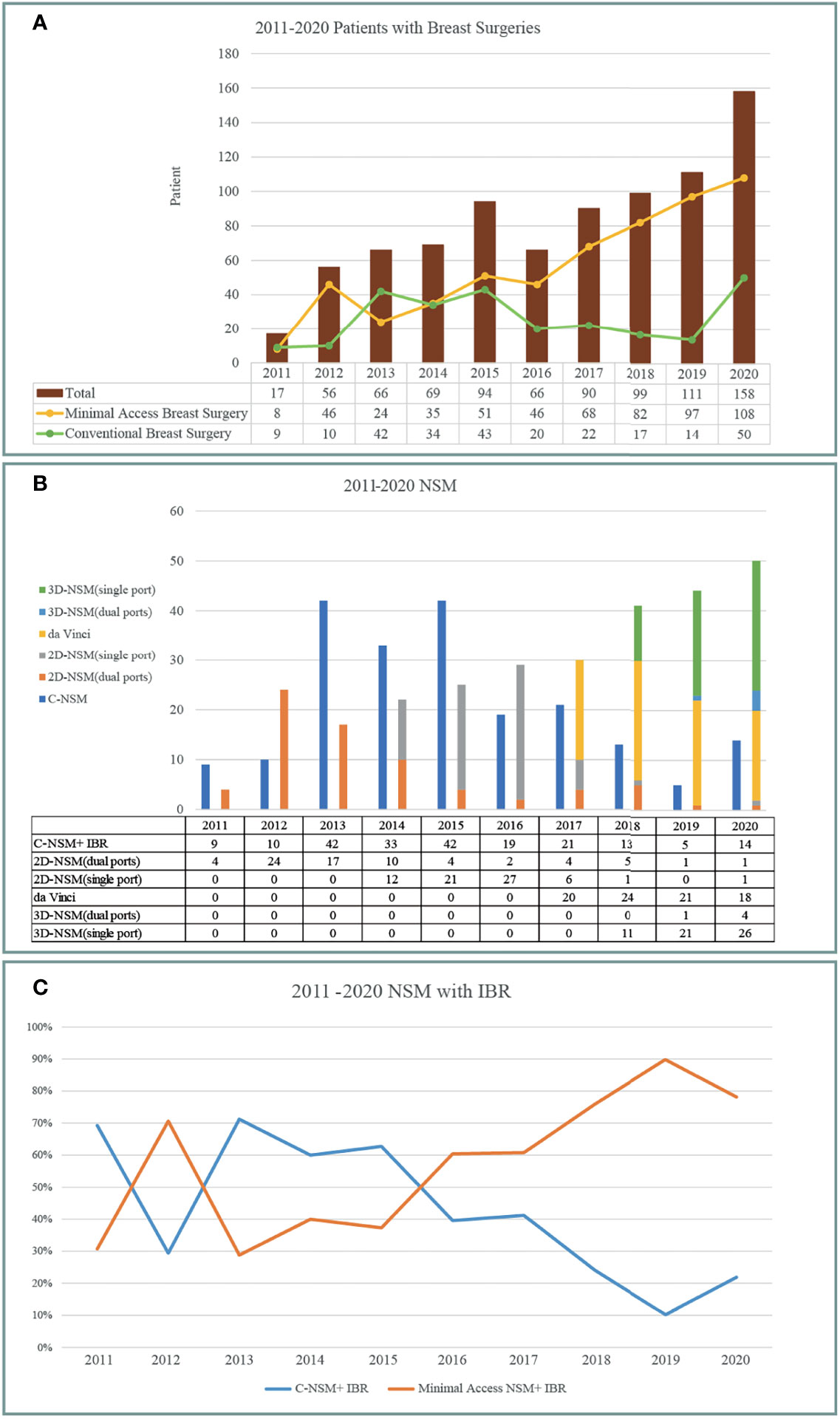
Figure 3 Development and trend of minimal access breast surgery over 2011–2020 from the principal investigator’s perspective. (A) Breast cancer operations performed per year according to conventional breast surgery (CBS) and minimal access (including endoscopic or robotic assisted) breast surgery (MABS). MABS increased gradually and became the mainstream of breast cancer operation. (B) Various nipple sparing mastectomy (NSM) operations performed per year. Following the sequences of conventional NSM, dual-areolar-axillary-incision 2D endoscopic assisted NSM (E-NSM), single-axillary-incision E-NSM, robotic assisted NSM (R-NSM), and single-port 3D videoscope-assisted E-NSM (3D E-NSM). (C) Trend of NSM with immediate breast reconstruction (IBR) during 2011–2020. Minimal access operation gradually became the dominant choices between patients receiving NSM+ IBR.
Development of Various Minimal Access Techniques of NSM
The amount and type of different NSMs performed during 2011–2020 are shown in Figure 3B. Initially, conventional NSM (C-NSM) was dominant, but dual-axillary-areolar-incision 2D endoscopic assisted NSM (E-NSM) became the predominant minimal access mastectomy during 2011–2014. In May 2014, we started using single-axillary-incision hybrid technique of E-NSM, and in March 2017, R-NSM operations were initiated (Figures 2, 3B). Single-port three-dimensional (3D) videoscope-assisted E-NSM was initiated in August 2018 and has become the main procedure of our NSM. As shown in Figure 3C, minimal access NSM gradually surpassed C-NSM and became the dominant procedures in the past decade.
Operation Time, Blood Loss, and Hospital Stay of Various NSMs
The wound length of C-NSM, E-NSM, and RNSM is 9.4 ± 4.6 (median 8) cm, 5 ± 0.8 (median 5) cm, and 4.1 ± 0.8 (median 4) cm (p < 0.01, Figure 2), respectively. The mean operation time for C-NSM and IGBR was 183 ± 101 (median 160) min, E-NSM and IGBR was 208 ± 94 (median 176) min, and R-NSM and IGBR was 191 ± 64 (median 190) min (p = 0.12, Table 2). Robotic NSM (36 ± 25 ml) and E-NSM (69 ± 54 ml) have less blood loss compared with C-NSM (91 ± 75 ml, p < 0.01). However, longer hospital stay was observed in the E-NSM (6 ± 1.4 days) and R-NSM (6.6 ± 1.6 days) group compared with C-NSM (5.2 ± 1.7 days, p < 0.01). Comparison between various groups of NSMs (Table 2) revealed no statistical difference in complications and nipple ischemia-related events, except that dual-areolar-axillary incision NSM had higher (22.8%) grade II+III nipple ischemia-necrosis compared with single axillary incision NSM (9.4%).
Operation Time Change and Overall Cost of Various NSMs
Figure 4A shows a decrease in operation time over the 10 years of experiences with BCS and NSM with and without breast reconstructions. The operation time of C-NSM, E-NSM, and R-NSM is plotted against cumulative patient number (Figure 4B), which reflects that as technique matures, the operation time for these three procedures all decreased and eventually merged without apparent difference (p = 0.12) The overall cost of C-NSM and IGBR (6,182 ± 453 USDs), 2D E-NSM and IGBR (6,664 ± 466 USDs), R-NSM and IGBR (10,672 ± 522 USDs), and single-port 3D E-NSM and IGBR (7,760 ± 740 USDs) is summarized and shown in Figure 5. R-NSM was significantly associated with higher cost than other NSMs (p < 0.01).
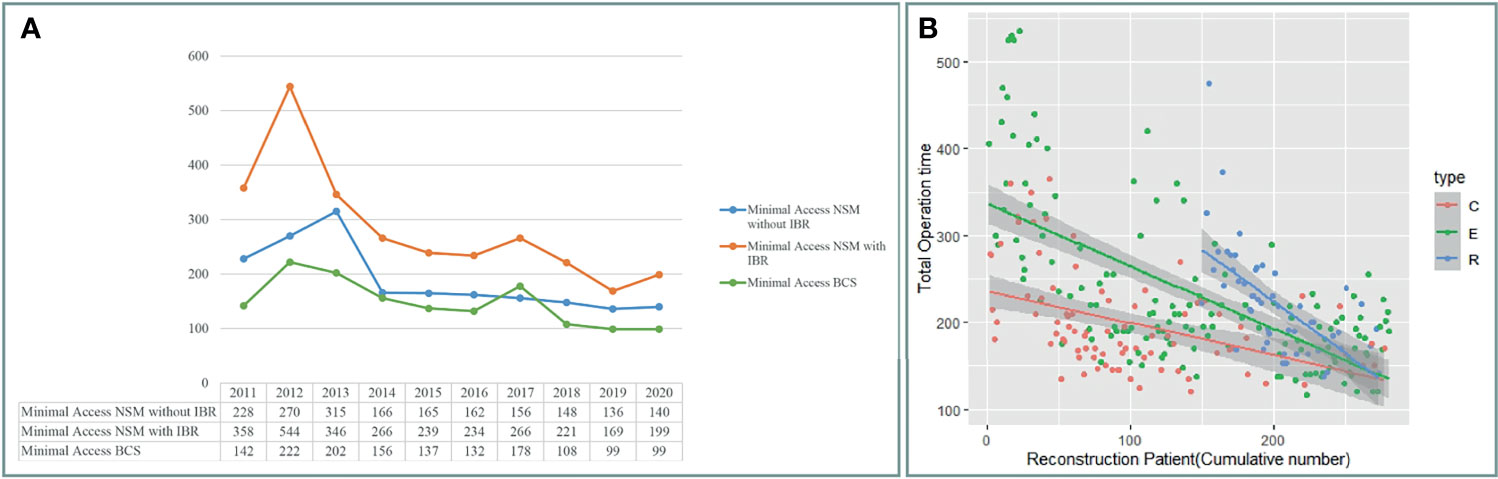
Figure 4 Operation time change after cases accumulation. (A) Following case accumulations over the past decade, various minimal access breast surgeries decreased operation time gradually. (B) The operation time of unilateral nipple sparing mastectomy with immediate gel implant breast reconstruction for conventional NSM (C-NSM), endoscopic assisted NSM (E-NSM), and robotic assisted NSM (R-NSM). The operation of each procedure performed was plotted in a timeline sequence, and all the operation time of three different types of NSM decreased and gradually merged together. C, C-NSM; E, E-NEM; R, R-NSM.

Figure 5 Cost analysis of various nipple sparing mastectomy (NSM) and immediate prothesis implant breast reconstruction (IPBR). With the introduction of two-dimensional endoscopic assisted NSM (2D E-NSM) retraction-type technique, the cost increased slightly from 6,182 [conventional NSM (C-NSM)] to 6,664 USD (about 500 USD more). Shift to robotic assisted NSM (R-NSM) dramatically increased medical cost to 10,672 USD (about 4,000 USD more). Inspired from R-NSM, we started the single-port 3D endoscopic assisted NSM (E-NSM) program in August 2018, and quickly this procedure became popular due to balanced clinical outcome and medical cost (7,760 USD), in which nearly 3,000 USD could be saved when compared with R-NSM. Following the advances of various minimal access breast surgeries, the overall cost also increased parallelly. R-NSM almost doubled the cost of C-NSM, and by adopting 3D E-NSM, the cost decreased near 3,000 USD without compromising the cosmetic result.
Oncologic Outcome of Conventional Versus Endoscopic or Robotic Assisted NSM
The positive margin rate was 0% in C-NSM, 1.6% in E-NSM, and 1.9% in R-NSM (p = 0.52). The median follow-up duration of NSM patients was 49.9 months. The local recurrence (Table 2), disease-free survival, and overall survival were not statistically different between C-NSM and minimal access (endoscopic or robotic assisted) NSMs (Supplementary Figure S2). In multivariate analysis for disease recurrence, minimal access breast surgery was not associated with increased disease recurrence risk (Supplementary Table S1).
Discussion
In the current study, we report the development and trend of MABS (EABS or RABS) for surgical treatment of breast cancer in the past 10 years from single surgeon at one institute. The proportion of breast cancer patients who received MABS (EABS or RABS) increased gradually (Figure 3A). The highlight of MABS is its small and inconspicuous operation wound (Figure 2). This value is more prominent when mastectomy is inevitable. When mastectomy is done via MABS, we can pursue an aesthetic scar-less mastectomy. With case accumulation, the overall operation time of various MABS decreased gradually (Figure 4).
BCS had become the mainstream for small early breast cancer, and our previous publication on the application of endoscopic (4, 38) or robotic (19) assisted techniques in BCS has shown promising results. Adopting E-BCS successfully decreased 23% of median wound length (Table 2) with incisional wound hidden in inconspicuous location (Supplementary Figure 1). We had applied robotic assisted technique in some breast cancer patients receiving BCS; these patients are mainly for partial breast reconstructions with either robotic assisted harvest of LDF (19) or omentum flap (23). From Figure 1, the major applications of robotic surgery in breast surgeries were total skin and NAC preserving mastectomy (R-NSM) (27) (Figure 2). The application of RABS in BCS alone, however, was not recommended due to high cost and long operation time.
NSM yields better cosmetic outcome and has proven oncologic safety; hence, it is increasingly used in breast cancer patients indicated for mastectomy (39, 40). Mastectomy usually requires larger incisional wound, by adopting E-NSM or R-NSM, about 46.8% to 57.5% of wound length was spared (Table 2), approaching the goal of aesthetic scar-less mastectomy (Figure 2) (35). Compared with about 23% reduction of BCS wound length, the value of MABS was more apparent in NSM, where scar size is nearly halved (Figures 1–3). This positive impact had been reflected in our previous patients-reported-outcome studies (9, 17): satisfaction rates of R-NSM (92% excellent, 8% good) and E-NSM (87.5% excellent, 12.5% good) were significantly higher than C-NSM (75.6% excellent, 24.4% good). Current optimal scar incision location had been shifted from inner breast to the anterior axillary line at the level of NAC. This location enables sentinel lymph node biopsy and NSM with IGBR. An asset of this location is that this scar could be well hidden along the bra-line (Figure 2).
Initially, the E-NSM was developed with non-gas inflation retraction type endoscope (5, 13, 41), and for vein harvest from posterior dissection (separation of pectoralis major and breast glandular tissue, Figure 2). Then, a semi-areolar incision was made for anterior skin flap dissection using optical port trocar with tunneling method. This dual-areolar-axillary-incision is well-adapted for small- to medium-sized breast, patients without breast reconstruction, and patients receiving tissue expander breast reconstruction. However, for direct gel implant breast reconstruction and large-sized breast patients, NAC ischemia/necrosis risk would be increased (4). The shift from initial dual-areolar-axillary-incision to single-axillary-incision in 2D E-NSM (13, 16) greatly decreased the rate of NAC ischemia/necrosis (Gr II+III) from 22.8% (1.4% total necrosis rate) to 9.4% (0.5% total necrosis rate, p < 0.01). However, this transition also increased operation difficulty and related operation time (Figure 4).
The development of R-NSM successfully solved the difficulties faced by single-axillary-incision 2D E-NSM (7, 8, 27, 34, 42) through better visual acuity with 3D videoscope and wristed articular robotic arms (Figures 2, 3B). The major drawback of R-NSM is the high cost, which is an extra 4,000 USD more (Figure 5) compared to C-NSM (17). Inspired from R-NSM, we started performing single-port 3D endoscopic assisted NSM (E-NSM) in August 2018 (Figure 3B) (31). This procedure became widely accepted due to balanced clinical outcome and medical cost (7,760 USD) (15), nearly 3,000 USD could be saved when compared with R-NSM (Figure 5). As medical cost remained an important consideration, we believe that R-NSM can work as a bridge and help surgeons be familiar with techniques of advanced endoscopic breast surgery such as single-port 3D E-NSM (15).
Extended operation time is one criticism of MABS (5, 6, 28, 29), especially towards R-NSM (29). Figure 4A showed that in the past 10 years, operation time had decreased dramatically. When we compare the first vs. last 10 cases of E-NSM and R-NSM, a reduction of 51.5% and 32% operation time was observed respectively (Figure 4B). Surgical length of MABS has now decreased to acceptable duration after much accumulated case (33), making surgeons more experienced and able to standardize the performed procedures (27) (Figure 4). From our perspective, air inflation system would be the predominant system in E-NSM (15, 16, 31) and R-NSM (8, 27, 34, 42); however, retraction system might be sufficient for E-BCS (11, 30).
The oncologic safety of E-BCS versus C-BCS was confirmed in our previous study (26) by adopting propensity score matching. Oncologic safety is not shown in the current cohort as patient-selection criteria and case number of C-BCS were quite different from E-BCS (Table 1). The preliminary oncologic safety of minimal access (endoscopic or robotic assisted) NSM versus C-NSM is shown in Table 2 and Supplementary Figure 2, which present comparable disease-free survival and overall survival for all three procedures. In multivariate analysis for disease recurrence, minimal access breast surgery was not associated with increased recurrence risk. However, difference in various groups and relative short follow-up time in R-NSM should be noted.
Our current study is limited in its retrospective nature, limited case numbers, and single-surgeon experience Nonetheless, data were collected prospectively over a 10-year-period, and the results derived from this study are solid and reliable. The observed trend of increasing MABS performed in early breast cancer and shortening of operation time in various MABS with case accumulated experience could be extrapolated to other centers in the world.
Conclusion
The era of minimal access (endoscopic and robotic assisted) breast surgery is approaching as an increasing number of hospitals around the world adopt these minimal access breast surgical techniques in the treatment of breast cancer. The highlight of MABS lies in its small inconspicuous operation scar and high patient satisfaction. The previously criticized extensive operation time had decreased steadily due to technique refinements and the surgical team’s procedural familiarity. The cost of minimal access mastectomy increased from conventional to E-NSM and nearly doubled in R-NSM. The newly developed single-port 3D E-NSM, which worked just as well as R-NSM while greatly decreased medical cost, could be an alternative choice.
Data Availability Statement
The data analyzed in this study are subject to the following licenses/restrictions: The dataset could be available only by asking the principal investigator, and will be released after permission. Requests to access these datasets should be directed to aHdsYWk2NTA0MjBAeWFob28uY29tLnR3.
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Review Board of the Changhua Christian Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
Study design: H-WL and D-RC. Data collection: S-TC and S-LL. Data analysis: Y-JL and S-JK. Manuscript: H-WL and D-RC. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the Ministry of Science and Technology of Taiwan, and the number of this funding was MOST 110-2314-B-371-009-. This study was also sponsored by research funding provided by the Changhua Christian Hospital 109-CCH-IRP-093 and 110-CCH-IRP-042. We also received research funding from Intuitive Surgery CRG09-08232019.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like thank Chin-Mei Tai, Yun-Ting Chang, Shu-Hsin Pai, Yi-Ru Ke, and Yi-Yuan Lee for the assistance in this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.739144/full#supplementary-material
Supplementary Figure 1 | Various operation photos. C: conventional breast surgery, E, endoscopic assisted breast surgery; PM, partial mastectomy; TM, total mastectomy.
Supplementary Figure 2 | Survival analysis of various nipple sparing mastectomy with immediate breast reconstruction.
References
1. Carbonell AM 2nd. Minimally Invasive Gastric Surgery. Surg Clin North Am (2011) 91(5):1089–103. doi: 10.1016/j.suc.2011.06.006
2. Lai HW, Tseng SH, Lee YT, Hsu CH, Chou DA, Wu HS, et al. Impact of AITS Laparoscopic Training Center on Surgeons’ Preference for Appendectomy. Surg Endosc (2010) 24(9):2210–5. doi: 10.1007/s00464-010-0930-4
3. Luketich JD, Pennathur A, Awais O, Levy RM, Keeley S, Shende M, et al. Outcomes After Minimally Invasive Esophagectomy: Review of Over 1000 Patients. Ann Surg (2012) 256(1):95–103. doi: 10.1097/SLA.0b013e3182590603
4. Lai HW, Chen ST, Chen DR, Chen SL, Chang TW, Kuo SJ, et al. Current Trends in and Indications for Endoscopy-Assisted Breast Surgery for Breast Cancer: Results From a Six-Year Study Conducted by the Taiwan Endoscopic Breast Surgery Cooperative Group. PLoS One (2016) 11(3):e0150310. doi: 10.1371/journal.pone.0150310
5. Mok CW, Lai HW. Endoscopic-Assisted Surgery in the Management of Breast Cancer: 20 Years Review of Trend, Techniques and Outcomes. Breast (2019) 46:144–56. doi: 10.1016/j.breast.2019.05.013
6. Leff DR, Vashisht R, Yongue G, Keshtgar M, Yang GZ, Darzi A. Endoscopic Breast Surgery: Where are We Now and What Might the Future Hold for Video-Assisted Breast Surgery? Breast Cancer Res Treat (2011) 125(3):607–25. doi: 10.1007/s10549-010-1258-4
7. Toesca A, Invento A, Massari G, Girardi A, Peradze N, Lissidini G, et al. Update on the Feasibility and Progress on Robotic Breast Surgery. Ann Surg Oncol (2019) 26(10):3046–51. doi: 10.1245/s10434-019-07590-7
8. Sarfati B, Struk S, Leymarie N, Honart JF, Alkhashnam H, Tran de Fremicourt K, et al. Robotic Prophylactic Nipple-Sparing Mastectomy With Immediate Prosthetic Breast Reconstruction: A Prospective Study. Ann Surg Oncol (2018) 25(9):2579–86. doi: 10.1245/s10434-018-6555-x
9. Lai HW, Chen ST, Tai CM, Lin SL, Lin YJ, Huang RH, et al. Robotic- Versus Endoscopic-Assisted Nipple-Sparing Mastectomy With Immediate Prosthesis Breast Reconstruction in the Management of Breast Cancer: A Case-Control Comparison Study With Analysis of Clinical Outcomes, Learning Curve, Patient-Reported Aesthetic Results, and Medical Cost. Ann Surg Oncol (2020) 27(7):2255–68. doi: 10.1245/s10434-020-08223-0
10. Mok CW, Lai HW. Evolution of Minimal Access Breast Surgery. Gland Surg (2019) 8(6):784–93. doi: 10.21037/gs.2019.11.16
11. Lai HW, Mok CW, Chang YT, Chen DR, Kuo SJ, Chen ST. Endoscopic Assisted Breast Conserving Surgery for Breast Cancer: Clinical Outcome, Learning Curve, and Patient Reported Aesthetic Results From Preliminary 100 Procedures. Eur J Surg Oncol (2020) 46(8):1446–55. doi: 10.1016/j.ejso.2020.02.020
12. Nakajima H, Fujiwara I, Mizuta N, Sakaguchi K, Hachimine Y. Video-Assisted Skin-Sparing Breast-Conserving Surgery for Breast Cancer and Immediate Reconstruction With Autologous Tissue. Ann Surg (2009) 249(1):91–6. doi: 10.1097/SLA.0b013e31818e3fa6
13. Lai HW, Lin SL, Chen ST, Kuok KM, Chen SL, Lin YL, et al. Single-Axillary-Incision Endoscopic-Assisted Hybrid Technique for Nipple-Sparing Mastectomy: Technique, Preliminary Results, and Patient-Reported Cosmetic Outcome From Preliminary 50 Procedures. Ann Surg Oncol (2018) 25(5):1340–9. doi: 10.1245/s10434-018-6383-z
14. Lai HW, Wu HS, Chuang KL, Chen DR, Chang TW, Kuo SJ, et al. Endoscopy-Assisted Total Mastectomy Followed by Immediate Pedicled Transverse Rectus Abdominis Musculocutaneous (TRAM) Flap Reconstruction: Preliminary Results of 48 Patients. Surg Innov (2015) 22(4):382–9. doi: 10.1177/1553350614546003
15. Lai HW, Chen ST, Mok CW, Chang YT, Lin SL, Lin YJ, et al. Single-Port Three-Dimensional (3D) Videoscope-Assisted Endoscopic Nipple-Sparing Mastectomy in the Management of Breast Cancer: Technique, Clinical Outcomes, Medical Cost, Learning Curve, and Patient-Reported Aesthetic Results From 80 Preliminary Procedures. Ann Surg Oncol (2021) 28(12):7331–44. doi: 10.1245/s10434-021-09964-2
16. Tukenmez M, Ozden BC, Agcaoglu O, Kecer M, Ozmen V, Muslumanoglu M, et al. Videoendoscopic Single-Port Nipple-Sparing Mastectomy and Immediate Reconstruction. J Laparoendosc Adv Surg Tech A (2014) 24(2):77–82. doi: 10.1089/lap.2013.0172
17. Lai HW, Chen ST, Mok CW, Lin YJ, Wu HK, Lin SL, et al. Robotic Versus Conventional Nipple Sparing Mastectomy and Immediate Gel Implant Breast Reconstruction in the Management of Breast Cancer- a Case Control Comparison Study With Analysis of Clinical Outcome, Medical Cost, and Patient-Reported Cosmetic Results. J Plast Reconstr Aesthet Surg (2020) 73(8):1514–25. doi: 10.1016/j.bjps.2020.02.021
18. Lai HW, Lin SL, Chen ST, Lin YL, Chen DR, Pai SS, et al. Robotic Nipple Sparing Mastectomy and Immediate Breast Reconstruction With Robotic Latissimus Dorsi Flap Harvest - Technique and Preliminary Results. J Plast Reconstr Aesthet Surg (2018) 71(10):e59–61. doi: 10.1016/j.bjps.2018.07.006
19. Lai HW, Chen ST, Lin SL, Lin YL, Wu HK, Pai SH, et al. Technique for Single Axillary Incision Robotic Assisted Quadrantectomy and Immediate Partial Breast Reconstruction With Robotic Latissimus Dorsi Flap Harvest for Breast Cancer: A Case Report. Med (Baltimore) (2018) 97(27):e11373. doi: 10.1097/MD.0000000000011373
20. Selber JC, Baumann DP, Holsinger FC. Robotic Latissimus Dorsi Muscle Harvest: A Case Series. Plast Reconstr Surg (2012) 129(6):1305–12. doi: 10.1097/PRS.0b013e31824ecc0b
21. Zaha H, Abe N, Sagawa N, Unesko M. Oncoplastic Surgery With Omentum Flap Reconstruction: A Study of 200 Cases. Breast Cancer Res Treat (2017) 162:267–74. doi: 10.1007/s10549-017-4124-9
22. Frey JD, Yu JW, Cohen SM, Zhao LC, Choi M, Levine JP. Robotically Assisted Omentum Flap Harvest: A Novel, Minimally Invasive Approach for Vascularized Lymph Node Transfer. Plast Reconstr Surg Glob Open (2020) 8:e2505. doi: 10.1097/GOX.0000000000002505
23. Lai HW, Mok CW. Minimal Invasive (Endoscopic & Robotic) Breast Surgery. Elsevier (2020) 209–18. doi: 10.1016/B978-0-323-73405-9.00012-1
24. Kuo WL, Huang JJ, Huang YT, Chueh LF, Lee JT, Tsai HP, et al. Robot-Assisted Mastectomy Followed by Immediate Autologous Microsurgical Free Flap Reconstruction: Techniques and Feasibility in Three Different Breast Cancer Surgical Scenarios. Clin Breast Cancer (2020) 20:e1–8. doi: 10.1016/j.clbc.2019.06.018
25. Selber JC. The Robotic DIEP Flap. Plast Reconstr Surg (2020) 145:340–3. doi: 10.1097/PRS.0000000000006529
26. Lai HW, Chen ST, Liao CY, Mok CW, Lin YJ, Chen DR, et al. Oncologic Outcome of Endoscopic Assisted Breast Surgery Compared With Conventional Approach in Breast Cancer: An Analysis of 3426 Primary Operable Breast Cancer Patients From Single Institute With and Without Propensity Score Matching. Ann Surg Oncol (2021) 28(12):7368–80. doi: 10.1245/s10434-021-09992-y
27. Lai HW, Toesca A, Sarfati B, Park HS, Houvenaeghel G, Selber JC, et al. Consensus Statement on Robotic Mastectomy-Expert Panel From International Endoscopic and Robotic Breast Surgery Symposium (IERBS) 2019. Ann Surg (2020) 271(6):1005–12. doi: 10.1097/SLA.0000000000003789
28. Ingram D. Is it Time for Breast Cancer Surgeons to Embrace Endoscopic-Assisted Mastectomy? ANZ J Surg (2008) 78(10):837–8. doi: 10.1111/j.1445-2197.2008.04676.x
29. Margenthaler JA. Robotic Mastectomy-Program Malfunction? JAMA Surg (2020) 155:461–2. doi: 10.1001/jamasurg.2019.6361
30. Lai HW, Lin HY, Chen SL, Chen ST, Chen DR, Kuo SJ. Endoscopy-Assisted Surgery for the Management of Benign Breast Tumors: Technique, Learning Curve, and Patient-Reported Outcome From Preliminary 323 Procedures. World J Surg Oncol (2017) 15(1):19. doi: 10.1186/s12957-016-1080-5
31. Lai HW, Chen ST, Mok CW, Lin SL, Tai CM, Chen DR, et al. Single-Port 3-Dimensional Videoscope-Assisted Endoscopic Nipple-Sparing Mastectomy in the Management of Breast Cancer. Plast Reconstr Surg Glob Open (2019) 7(8):e2367. doi: 10.1097/GOX.0000000000002367
32. Lai HW, Lin SL, Chen ST, Chen SL, Lin YL, Chen DR, et al. Robotic Nipple-Sparing Mastectomy and Immediate Breast Reconstruction With Gel Implant. Plast Reconstr Surg Glob Open (2018) 6:e1828. doi: 10.1097/GOX.0000000000001828
33. Lai HW, Wang CC, Lai YC, Chen CJ, Lin SL, Chen ST, et al. The Learning Curve of Robotic Nipple Sparing Mastectomy for Breast Cancer: An Analysis of Consecutive 39 Procedures With Cumulative Sum Plot. Eur J Surg Oncol (2019) 45(2):125–33. doi: 10.1016/j.ejso.2018.09.021
34. Lai HW, Chen ST, Lin SL, Chen CJ, Lin YL, Pai SH, et al. Robotic Nipple-Sparing Mastectomy and Immediate Breast Reconstruction With Gel Implant: Technique, Preliminary Results and Patient-Reported Cosmetic Outcome. Ann Surg Oncol (2019) 26(1):42–52. doi: 10.1245/s10434-018-6704-2
35. Yang JD, Lee JT, Lee JS, Kim EK, Park CS, Park HY. Aesthetic Scar-Less Mastectomy and Breast Reconstruction. J Breast Cancer (2021) 24:22–33. doi: 10.4048/jbc.2021.24.e11
36. Dindo D, Demartines N, Clavien P. Classification of Surgical Complications a New Proposal With Evaluation in a Cohort of 6336 Patients and Results of a Survey. Ann Surg (2004) 240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae
37. Moran MS, Schnitt SJ, Giuliano AE, Harris JR, Khan SA, Horton J, et al. Society of Surgical Oncology-American Society for Radiation Oncology Consensus Guideline on Margins for Breast-Conserving Surgery With Whole-Breast Irradiation in Stages I and II Invasive Breast Cancer. Ann Surg Oncol (2014) 21(3):704–16. doi: 10.1245/s10434-014-3481-4
38. Lee EK, Kook SH, Park YL, Bae WG. Endoscopy-Assisted Breast-Conserving Surgery for Early Breast Cancer. World J Surg (2006) 30(6):957–64. doi: 10.1007/s00268-005-0202-y
39. Petit JY, Veronesi U, Luini A, Orecchia R, Rey PC, Martella S, et al. When Mastectomy Becomes Inevitable: The Nipple-Sparing Approach. Breast (2005) 14(6):527–31. doi: 10.1016/j.breast.2005.08.028
40. Kim HJ, Park EH, Lim WS, Seo JY, Koh BS, Lee TJ, et al. Nipple Areola Skin-Sparing Mastectomy With Immediate Transverse Rectus Abdominis Musculocutaneous Flap Reconstruction is an Oncologically Safe Procedure: A Single Center Study. Ann Surg (2010) 251(3):493–8. doi: 10.1097/SLA.0b013e3181c5dc4e
41. Sakamoto N, Fukuma E, Higa K, Ozaki S, Sakamoto M, Abe S, et al. Early Results of an Endoscopic Nipple-Sparing Mastectomy for Breast Cancer. Ann Surg Oncol (2009) 16(12):3406–13. doi: 10.1245/s10434-009-0661-8
Keywords: endoscopy-assisted breast surgery (EABS), conventional breast surgery (CBS), endoscopy-assisted breast conserving surgery (E-BCS), endoscopic assisted nipple sparing mastectomy (E-NSM), robotic assisted nipple sparing mastectomy (R-NSM), single-port three-dimensional videoscope-assisted endoscopic nipple sparing mastectomy (3D E-NSM)
Citation: Lai H-W, Chen S-T, Lin Y-J, Lin S-L, Lin C-M, Chen D-R and Kuo S-J (2021) Minimal Access (Endoscopic and Robotic) Breast Surgery in the Surgical Treatment of Early Breast Cancer—Trend and Clinical Outcome From a Single-Surgeon Experience Over 10 Years. Front. Oncol. 11:739144. doi: 10.3389/fonc.2021.739144
Received: 10 July 2021; Accepted: 22 October 2021;
Published: 19 November 2021.
Edited by:
Masakazu Toi, Kyoto University, JapanReviewed by:
Masahiro Takada, Kyoto University, JapanHebatallah Gamal El Din Mohamed Mahmoud, Cairo University, Egypt
Copyright © 2021 Lai, Chen, Lin, Lin, Lin, Chen and Kuo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hung-Wen Lai, aHdsYWk2NTA0MjBAZ21haWwuY29t; MTQzODA5QGNjaC5vcmcudHc=
 Hung-Wen Lai
Hung-Wen Lai Shou-Tung Chen1,2,3
Shou-Tung Chen1,2,3