
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 15 September 2021
Sec. Surgical Oncology
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.737885
This article is part of the Research Topic Improving Post-Surgical Outcomes for Patients with Primary Gastrointestinal Stromal Tumors View all 5 articles
 Hao Wu1†‡
Hao Wu1†‡ Han Li2‡
Han Li2‡ Qinfeng Xu3
Qinfeng Xu3 Liang Shang1,4,5,6
Liang Shang1,4,5,6 Ronghua Zhang1,5
Ronghua Zhang1,5 Chen Li7
Chen Li7 Mengdi Fu3
Mengdi Fu3 Wandi Xu3
Wandi Xu3 Jianfeng Chen3
Jianfeng Chen3 Jin Liu8,9*
Jin Liu8,9* Leping Li1,4,5,6*†
Leping Li1,4,5,6*†Background: The management of 2-5 cm gastric gastrointestinal stromal tumours (GISTs) is still debated between surgeons and endoscopists. We aimed to investigate short-term and long-term outcomes between surgical resection (SR) and endoscopic resection (ER).
Methods: This study included 67 and 215 patients between 2010 and 2020 who underwent ER and SR, respectively. After propensity score matching, the clinical outcomes were compared. Individual patient information that requires special instructions is also summarized.
Results: After matching, the operation time (P=0.005) and postoperative hospital stay (P=0.005) were significantly longer in the SR group than in the ER group. However, there were no significant differences in blood loss (P=0.741), resection margin (P=1.000) or time to liquid diet (P=0.055). Statistical differences were also seen in en bloc resection (P<0.001) and adverse events (P=0.027). The recurrence rate did not differ significantly between the two techniques, and the mitotic index and ulceration were identified as independent prognostic factors of progression-free survival.
Conclusions: ER might be comparable to SR for the treatment of 2-3 cm gastric GISTs. SR is still considered the standard treatment for 3-5 cm gastric GISTs, while the intraoperative and postoperative information of ER should be recorded in detail and closely evaluated. Surgical resection is recommended if the tumour has a high mitotic index or mucosal ulceration.
As one of the most common mesenchymal neoplasms, gastrointestinal stromal tumours (GISTs) have attracted increasing attention in recent years (1–4). GISTs can be found anywhere of the gastrointestinal (GI) tract, but most appear in the stomach (5). Although the majority of gastric GISTs are indolent, all GISTs are believed to have malignant potential (6).
According to the latest guidelines of the National Comprehensive Cancer Network (NCCN) (7) and European Society for Medical Oncology (ESMO) (8), gastric GISTs smaller than 2 cm without high-risk features (irregular borders, cystic spaces, ulceration, echogenic foci, heterogeneity) should receive periodic surveillance, while surgical resection (SR) is the recommended treatment for primary, localized gastric GISTs larger than 2 cm.
In recent years, endoscopic resection (ER) has gradually been used to remove small GISTSs (9). However, there is no guarantee of en bloc resection, and the potential risk of recurrence is an issue that doctors continue to pay attention to (10, 11). Moreover, many studies have compared the clinical outcomes of ER with those of SR in the treatment of gastric GISTs smaller than 2 cm, and there are currently no high-level studies supporting a clear best choice for 2-5 cm GISTs.
We aimed to compare short-term and long-term outcomes between SR and ER for 2-5 cm gastric GISTs and provide a potential reference for the standardization of the treatment.
To ensure suitable randomization in the evaluation of short- and long-term outcomes in GIST patients who underwent SR or ER, we applied propensity score matching to equalize the baseline clinicopathological characteristics.
This retrospective cohort study was performed based on a prospectively collected database of GISTs at Shandong Provincial Hospital (SPH). All relevant procedures were approved by the Institutional Review Board (IRB). This study was designed in compliance with the Helsinki Declaration and approved by the Ethics Committee of Shandong Provincial Hospital, China (SWYX: No. 2021-035). The Reporting and Guidelines in Propensity Score Analysis were also followed (12).
A total of 1163 consecutive patients diagnosed with GISTs and undergoing resection at Shandong Provincial Hospital between March 2010 and January 2020 were initially pooled. Among them, 302 were classified as having 2-5 cm primary GISTs in our institution (Figure 1). All oncologic resections with curative intent were performed by senior experts to reach the rigorous standard at our institution.
Computed tomography (CT) examination was used to exclude adjacent organ infiltration and metastases before surgery. In addition, gastroscopy or ultrasound gastroscopy was performed to determine the location and size of the gastric GISTs. Cardiopulmonary examinations were routinely performed to assess tolerance to surgery.
Patients with the following criteria were included: (1) age over eighteen years; (2) pathological diagnosis of gastric GISTs; (3) tumour size 2–5 cm in diameter; (4) no evidence of recurrent GISTs or distant metastasis before treatment; and (5) American Society of Anesthesiology (ASA) score ≤ 3.
Patients having the following criteria were excluded: (1) any previous or concurrent malignancies; (2) underwent the first operation at another institution; (3) endoscopic resection conversion to surgical resection; (4) combined resection with other organs; (5) received neoadjuvant therapy; (6) severely missing or illegible medical data; and (7) missing follow-up data.
Finally, 282 patients with regular follow-up were included and analysed in the entire cohort. The follow-up was performed every 3 months for the first 3 years, then every 6 months up to 5 years, and then every year or until death. in the following years. The latest follow-up date was updated to December 2020.
Following clinicopathological characteristics of patients were routinely collected from database of GISTs, including age, sex, body mass index (BMI), comorbidities, chief complaint, tumour location, tumour size, growth type, tumour shape, tumour origin, ulceration or high-risk imageology features, mitotic index (per 50 high power field), modified NIH (National Institutes of Health) risk category, surgical and endoscopic methods, estimated blood loss, operation time, resection margin, adverse events, time to liquid diet, hospitalization time, postoperative imatinib, immunohistochemistry (IHC) result, haematological indices and so on. The patients’ BMIs were classified into the following categories: < 18.5, 18.5–24.9 and ≥ 25 kg/m2, based on the World Health Organization (WHO) classification standards. The comorbidities analysed in this study comprised hypertension, diabetic mellitus, anaemia, pulmonary disease (asthma, pneumonia, chronic obstructive pulmonary disease, etc.), heart disease (arrhythmia, coronary atherosclerotic heart disease, etc.), liver disease (hepatitis, cirrhosis, etc.), renal disease (nephritis, chronic kidney disease, etc.) and central nervous system disease (cerebrovascular disease, neurodegenerative disease, etc.).
The primary outcome was progression-free survival (PFS), which was defined as the interval between the date of resection and confirmed disease progression or death. Patients were censored at the date of the last follow-up without the above event.
The surgical resection method is determined mainly based on the evaluation of tumour location and patient’s condition. In recent years, laparoscopic wedge resection has been the main surgical method. When the tumour was located adjacent to the pylorus or oesophagogastric junction, distal, proximal or gastrectomy was performed. Open surgery was usually performed when the adhesion was severe or the tumour location was inappropriate, and it was also performed in the early years.
For the endoscopic resection group, 5 types of endoscopic procedures were performed, including endoscopic submucosal dissection (ESD), endoscopic submucosal excavation (ESE), endoscopic full-thickness resection (EFR) and submucosal tunnelling endoscopic resection (STER). The method of endoscopic resection was also selected according to the characteristics of the tumour.
All resection procedures were performed by experts with similarly high levels of experience.
According to the expected values, categorical variables were analysed by Pearson’s chi-squared test or Fisher’s exact test. Continuous variables were compared by utilizing the Mann–Whitney U-test, which are presented as medians and interquartile ranges (IQRs) and as the mean ± standard deviation (SD). The Kaplan–Meier method and log-rank test were performed to conduct survival analyses and evaluate differences in survival time, respectively. A Cox proportional hazards model was used to perform univariate and multivariate analyses. Univariate analysis was primarily performed, and variables with P < 0.1 were subsequently computed into multivariate analysis to determine the independent prognostic factors. Hazard ratios (HRs) with their 95% confidence intervals (CIs) were also derived. Statistical significance was considered when P < 0.05.
R software version 3.5.3 (The R Foundation for Statistical Computing, Vienna, Austria) and SPSS 26.0 (IBM SPSS Statistics, Armonk, NY: IBM Corp) were used in this study.
Propensity score matching was performed due to the inhomogeneous distribution of several baseline characteristics and uneven group sizes between SR and ER. First, the endoscopic resection group was regressed as a dependent variable on relevant baseline parameters, and a logistic regression model was used to calculate a propensity score. The propensity score matching ratio was set to a 1:1 ratio to minimize the differences due to age, sex, comorbidities, BMI, tumour location, tumour size, mitotic index and growth type with the nearest neighbour method. The assessment of propensity score matching is also shown (Supplemental Figure 1).
As shown in the flow chart (Figure 1), of the 282 consecutive patients who pooled into the entire cohort at our institution between March 2010 and January 2020, 67 patients in the ER group and 215 patients in the SR group were included.
The clinical characteristics are described in detail (Table 1). Before matching, the baseline characteristics of the patients, such as age, sex, BMI and comorbidities, were similar between the two groups. Regarding tumour characteristics, there were statistically significant differences in tumour size (P<0.001), tumour location (P=0.024), mitotic index (P=0.048), modified NIH risk category (P<0.001) and growth type (P=0.038). Regarding the clinicopathological characteristics of the tumour (Supplemental Table 1), significant differences were observed in ulceration (P<0.001) and high-risk imaging features (P<0.001), but there was no significant difference in the immunohistochemical results. Among the blood indicators, some were also found to be significantly different (Supplemental Table 2).
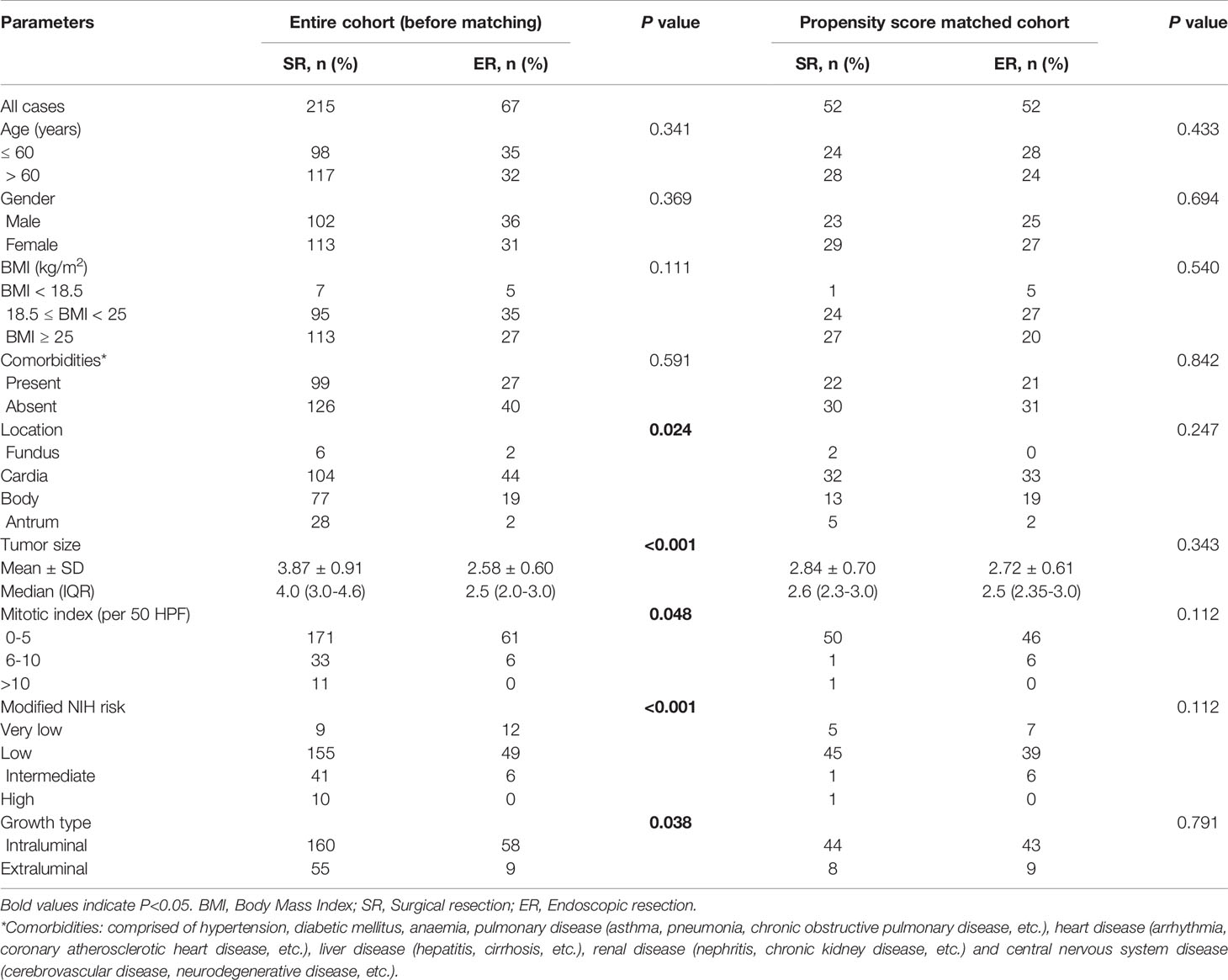
Table 1 Baseline clinicopathologic characteristics of SR and ER group in the entire cohort and after propensity score matching.
After propensity score matching, there were no significant differences among all variables, and all baseline and tumour characteristics of 52 patients in the ER group were compared with 52 patients in the SR group. Supplemental clinicopathological characteristics and blood indicators were also compared, and there was a difference only in the high-risk imaging features (P=0.016).
The resection methods of the SR and ER groups in the entire cohort and after propensity score matching are also summarized (Supplemental Table 3). The GISTs of 5 patients were unsuccessfully resected in the first attempt at endoscopic resection and switched to surgical resection. We did not include these patients in entire cohort, but they are very important for evaluating resection methods, which we also summarized (Table 2). In addition, tumours with transverse diameters >3.5 cm were very difficult to remove completely through the cardia. Patients with tumours larger than 3.5 cm were also included in the analysis presented in Table 3.
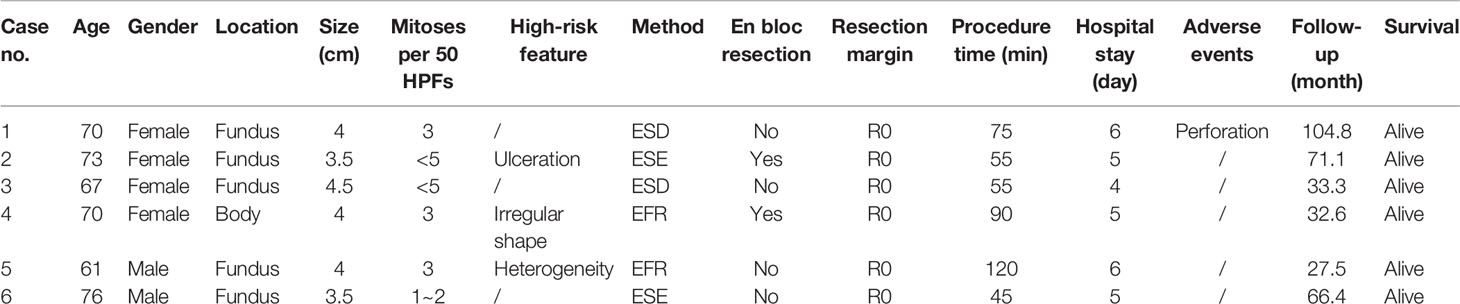
Table 3 Clinical characteristics of patients with tumours larger than 3.5 cm and undergoing endoscopic resection.
The comparison of short-term outcomes between the ER and SR groups is summarized in Table 4. Before PSM, the operation time (P=0.001), time to liquid diet (P=0.001) and postoperative hospital stays (P=0.001) were significantly shorter in the ER group than in the SR group. No significant differences were observed in the achievement of a negative resection margin. Furthermore, the ER group also showed significantly less bleeding (P=0.037), although there were deficiencies in en bloc resection (P<0.001) and an increased number of adverse events (P=0.001) compared with the SR group. The clinical characteristics and treatments of patients with adverse events are also summarized (Table 5).
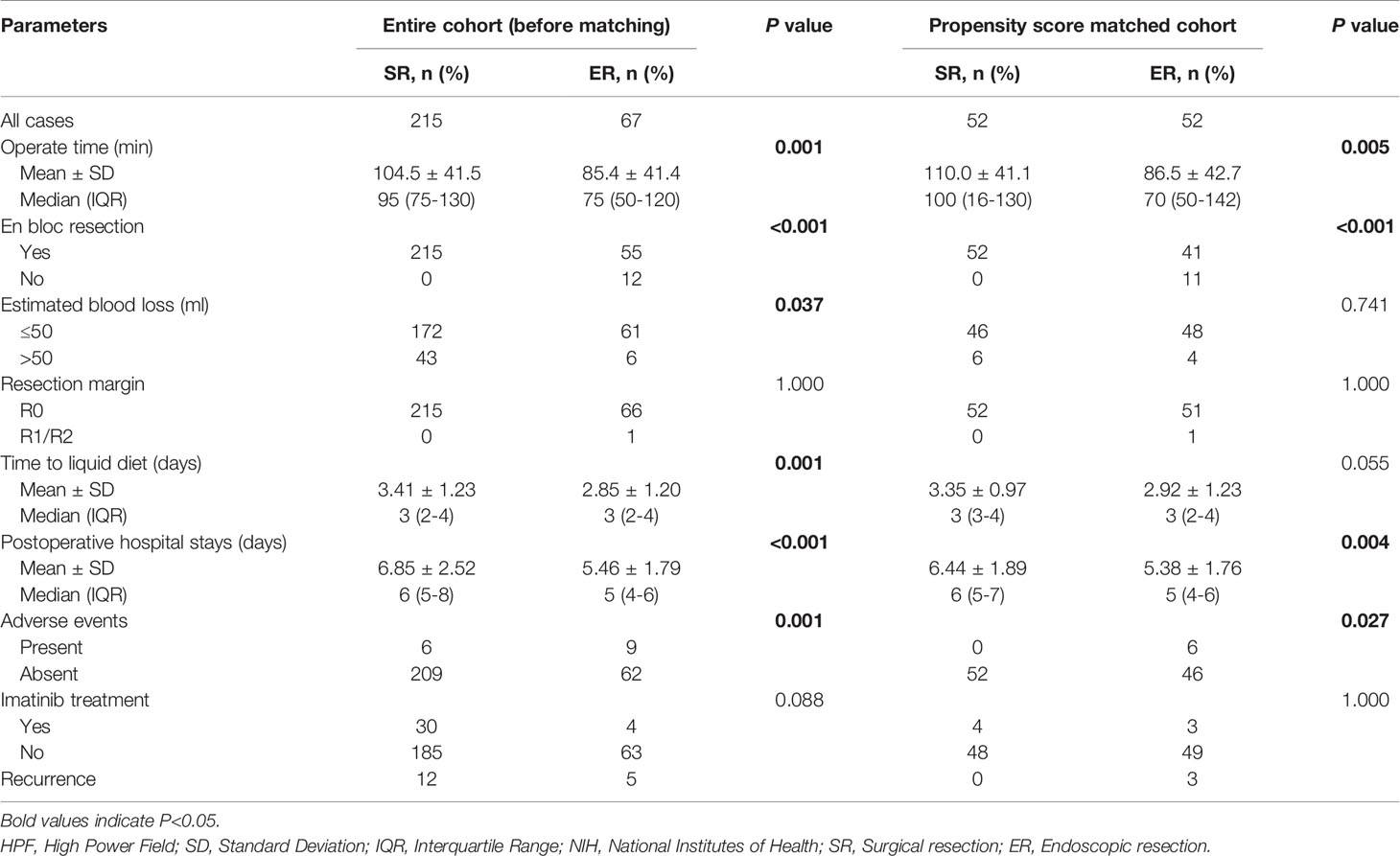
Table 4 Perioperative characteristics and long-term outcomes of SR and ER group in the entire cohort and after propensity score matching.
After matching, the operation time (P=0.005) and postoperative hospital stay (P=0.005) were also significantly longer in the SR group than in the ER group. However, there were no significant differences in blood loss (P=0.741), resection margin (P=1.000) or time to liquid diet (P=0.055). Statistical differences were also seen in en bloc resection (P<0.001) and adverse events (P=0.027).
The median follow-up time of the entire matched cohort was 1660 days (IQR, 950-2399), and the 1-, 3- and 5-year PFS rates were 98.94%, 95.39% and 94.33%, respectively. Prior to the last follow-up, a recurrence of GISTs occurred in 12 patients in the SR group (5.58%, 12/215) and 5 patients in the ER group (7.46%, 5/67), but the difference was not significant (P=0.660) (Figure 2). For the propensity score matched cohort, significant differences could also not be observed (P=0.077).
We continued to utilize univariate analysis to identify the mitotic index (P=0.004) and ulceration (P=0.001) as prognostic factors for PFS (Table 6). On multivariate analysis, mitotic index (HR=2.823, 95% CI=0.083-9.122, P=0.083; HR=6.385, 95% CI=1.733-23.523, P=0.005, respectively) and ulceration (HR=4.909, 95% CI=1.873-12.867, P=0.001) were eventually identified as independent prognostic factors.
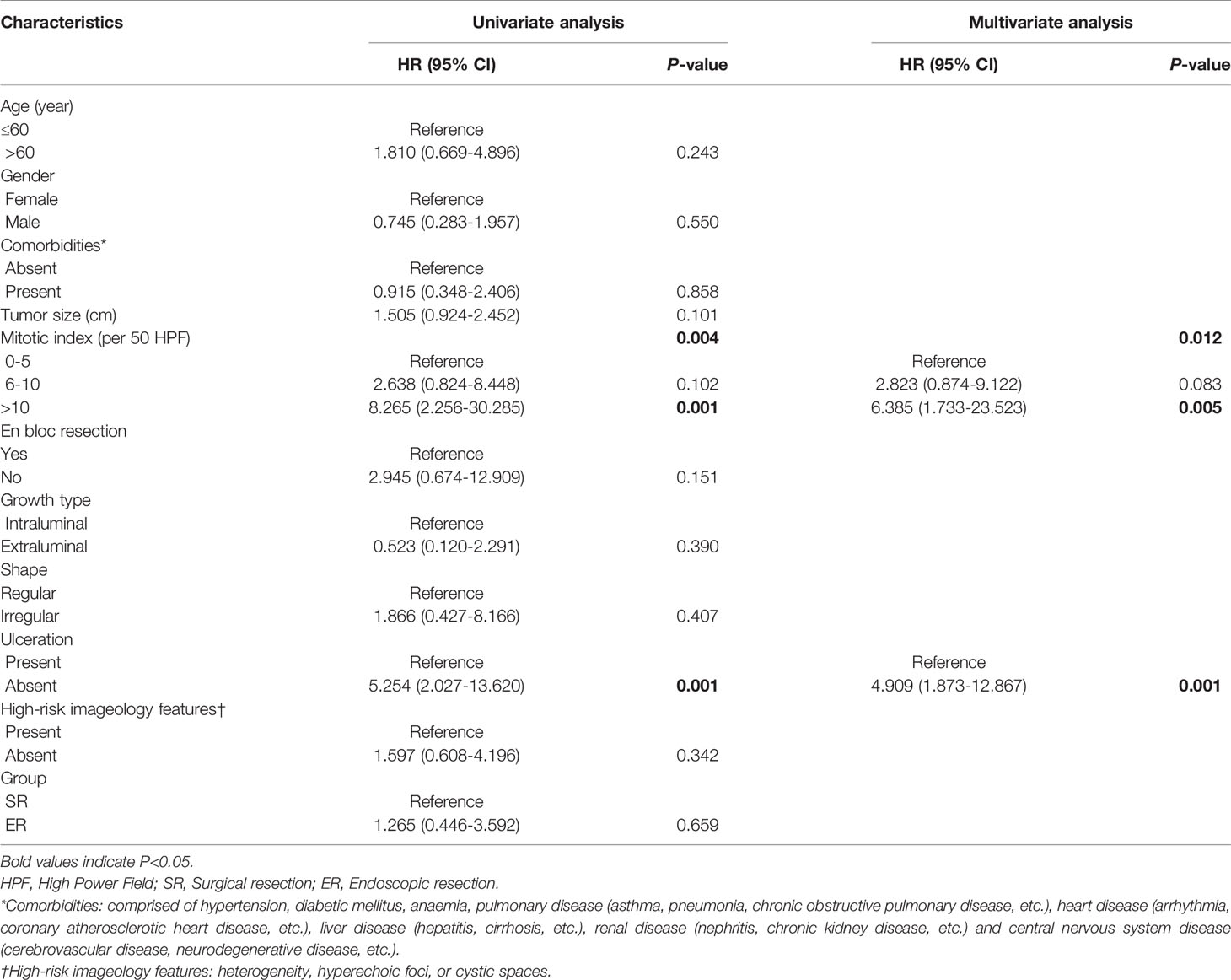
Table 6 Univariate and multivariate of the clinicopathological factors for progression-free survival.
We found that in the cohort after propensity score, most of the tumour diameters were between 2 and 3 cm. Then, we continued to compare surgical and endoscopic resection for patients with 2-3 cm gastric gastrointestinal stromal tumours (Supplemental Table 4).
After propensity score matching, there were no significant differences among all characteristic variables. For short−term outcomes (Supplemental Table 5), operation time (P=0.062), estimated blood loss (P=0.485) and adverse events (P=0.056) were comparable. Although the ER group still had significant advantages in time to liquid diet (P=0.043) and postoperative hospital stays (P=0.031), the achievement of en bloc resection (P=0.026) still needs to be improved.
In recent years, with the development of medical devices and the advancement of surgical techniques, laparoscopic resection and advanced endoscopic resection, including ESE, EFR and STER, have gradually become the primary choices for the treatment of muscularis propria tumours. Therefore, we compared laparoscopic and advanced endoscopic resection for gastrointestinal stromal tumours originating from the muscularis propria (Supplemental Table 6).
There were no significant differences among all characteristic variables after propensity score matching. The ER group still had a significantly shorter operation time (P=0.002) and postoperative hospital stay (P=0.009). The two groups were equal in blood loss (P=1.000), resection margin (P=1.000), time to liquid diet (P=0.072) and adverse events (P=0.237). However, in the ER group, there was a risk of difficult resection and recurrence (Supplemental Table 7).
For gastric GISTs larger than 3 cm, we also tried to make some reports descriptively (Supplemental Table 8). Although the patients in the SR group were older and had a higher rate of malignancy, surprisingly, there were no statistical differences in the operation time, blood loss, liquid diet time, and postoperative hospital stay. Of course, this has not yet taken into account that patients in the ER group have failed resections and converted to surgical resections (Supplemental Table 9). Although we also tried propensity score matching, there were too few patients in the ER group to obtain convincing results.
The management of small GISTs is still debated between surgeons and endoscopists (13, 14). In recent years, many studies have demonstrated that ER could be an effective, safe, and feasible therapeutic method for small gastric GISTs (15–17). Currently, there are also some studies aimed at comparing the clinical outcomes of ER and SR (18, 19). However, we found that the mean size of tumours was far below 5 cm, and the majority of them were even below 2 cm, which may have biased the results in favour of ER. At the same time, there are few descriptions of tumour origin and high-risk characteristics in existing studies. Therefore, the comparison of outcomes is often affected by clinical judgement; for example, patients with extraluminal tumours or imaging findings indicating highly malignant potential often undergo surgical resection.
In the present study, we performed propensity score matching using seven covariates, including age, sex, comorbidities, BMI, tumour location, tumour size, mitotic index and growth type, to compare the safety and efficacy of ER and SR for 2-5 cm gastric GISTs. Individual patient information that requires special instructions is also summarized.
Regarding short-term outcomes, ER had certain advantages over SR, such as shorter operation time (86.5 ± 42.7 vs 110.0 ± 41.1 minutes, P=0.005) and shorter postoperative hospital stays (5.38 ± 1.76 vs 6.44 ± 1.89 days, P=0.027). In our hospital, patients were routinely kept several days to ensure the recovery of gastrointestinal function and no postoperative complications. However, in recent years, laparoscopic resection has gradually become the recommended surgical method for GISTs in favourable anatomic locations, which is associated with shorter operation times and hospital stays. In addition, with the development and promotion of enhanced recovery after surgery (ERAS), the postoperative hospital stays after surgeries have been greatly reduced (20). In the subgroup analysis of LR and ER, we found that for 2-3 cm GISTs, the difference in operation time between the two groups was not statistically significant. For similar reasons, we found that the estimated blood loss in the ER group and the SR group were similar. In terms of time to liquid diet, there was a significant difference only in 2-3 cm GISTs.
Moreover, our data show that the incidence rate of adverse events in the ER group was relatively higher than that in the SR group. For the ER group, perforation was common and increasingly considered a minor adverse event due to endoscopic clipping and suturing techniques. In this study, most endoscopic perforation patients were successfully repaired endoscopically without conversions to emergency surgical operation, and we recorded only perforations that required surgical intervention. A total of four patients had macroperforation during endoscopic resection and underwent emergency surgery immediately.
Bleeding is also a common adverse event. One patient had bleeding after endoscopy resection and underwent a second endoscopic haemostasis procedure. In addition, there were 2 cases of delayed bleeding after surgery: one patient underwent exploratory surgery, and the other was treated conservatively after blood transfusion. All patients were fully discharged from the hospital. In our study, anastomotic stricture occurred in a patient one month after laparoscopic resection, and conservative treatment was performed.
Moreover, two patients suffered from peritonitis and recovered after fasting, nutritional support, antibiotic treatment and so on. In addition, some uncommon adverse events were recorded, such as jaundice and subcutaneous emphysema. Ultimately, all patients recovered and were discharged from the hospital after proper treatment.
In addition, due to massive intraoperative bleeding in 4 patients and resection difficulty in 1 patient, five GISTs were unsuccessfully resected in the first attempt at endoscopic resection. Therefore, surgeons had to perform surgical operations immediately to remove the tumour completely to achieve R0 resection.
The risk of tumour rupture and remnants is a major concern for ER. The R0 resection rates of ER are slightly lower than those of SR. However, a meta-analysis performed by our team showed that R1 resection did not influence the recurrence of GISTs.Tumour rupture is significantly associated with the occurrence of R1 resection and may be a confounder of R1 resection in GISTs (21). Tumour rupture could be caused by the removal of GISTs larger than 3 cm under peroral endoscopy. Due to the limited operating space and visual field, it might be difficult for endoscopists to maintain the integrity of a 2-5 cm gastric GIST. Although some large GISTs were resected in one piece (en bloc resection) successfully, they had to be divided into small pieces for removal. The en bloc resection rate was 82.09% (55/67) in our study. Considering that the tumours we included were all larger than 2 cm, this rate is acceptable. Of the 12 patients, two recurrences were noted during follow-up, and both were diagnosed with moderate-risk GISTs. Du et al. evaluated risk factors associated with piecemeal resection and demonstrated that large size and irregular shape may play a role (22).
Considering the risk of tumour rupture, tumour remnants, perforation, and bleeding, some experts consider the indications for endoscopic treatment for GISTs to be as follows: tumour size less than 3 cm, mainly an intraluminal growth pattern, a clear tumour boundary and uniform texture, and no symptoms of invasion and metastasis to other locations (23). Due to the above restrictions, ER is not currently recommended as a routine treatment for GISTs, and SR is still considered the standard treatment for 2-5 cm GISTs by most guidelines.
Regarding long-term outcomes, there are currently still insufficient studies. After following up with the patients in our hospital for the last decade, we found that the recurrence rate did not differ significantly between the those undergoing the two techniques. Our findings are consistent with the published articles thus far (23, 24).
We further performed univariate and multivariate analyses, and mitotic index (HR=2.823, 95% CI=0.083-9.122, P=0.083; HR=6.385, 95% CI=1.733-23.523, P=0.005, respectively) and ulceration (HR=4.909, 95% CI=1.873-12.867, P=0.001) were eventually identified as independent prognostic factors of PFS, which was partly similar to the prediction model for high malignancy potential of Yang et al (25).
In the subgroup analysis, we also obtained some inspiring findings. For 2-3 cm gastric GISTs, although the advantage of the ER in operation time is no longer significant, the number of adverse events do not seem to be significantly different. ER might be an effective strategy for improving postoperative recovery without increasing the risk of surgery and recurrence for these selected GISTs.
SR and ER comprise two alternative options for the treatment of GISTs originating from the muscularis propria (MP) layer, and there is currently no study comparing the two methods. Currently, ESE, EFR and STER are the three main endoscopic methods to resect these GISTs (26). Based on the limited information available, we found that ER still has advantages in terms of operating time and hospital stay, but there is no significant advantage in liquid diet. Of course, the most surprising thing is that these advanced endoscopic techniques are excellent in reducing the occurrence of adverse events.
Recently, the nonexposure simple suturing endoscopic full-thickness resection (NESS-EFTR) method was developed to avoid tumour exposure to the peritoneal cavity (27). In the future, it will be more exciting to look forward to the emergence of technologies that can avoid tumour exposure under complete endoscopic full-thickness resection. There are an increasing number of options available for doctors to choose, but these multiple options also bring difficulties to the evaluation of the most suitable method (28–30).
Our results indicate that SR is safer and results in fewer intraoperative adverse events than ER. It might be appropriate to determine the method of resection according to tumour location and mucosal ulceration. Regardless of the technique chosen, detailed routine physical examination should be carried out, such as endoscopic ultrasonography (EUS) and abdominal CT, to assess the location, border, size, origin and even blood supply of the lesions (6).
There are many highlights of our research. The general distribution of tumour size was usually not clear and precise, as stated in previous studies. In addition, previous studies did not pay attention to the growth type of tumours, and the malignant potential was not well stated, which may lead to some biases. We provided a detailed and comprehensive description of the tumours included in the study and applied PSM to ensure suitable randomization in the evaluation of short- and long-term outcomes.
Our study also has several limitations. First, it is difficult for a single centre to enrol a very large cohort for GISTs, a relatively rare neoplasm. To the best of our knowledge, our cohort is one of the largest samples in the relevant field. Second, we excluded five patients who had additional surgery because of incomplete resection of the GISTs after ER, which may have biased the results in favour of ER. Third, with a small number of events, it was difficult to have good reliability and validity. Propensity match score could also not eliminate all biases of the study. Last, as a retrospective study, some intraoperative and postoperative information may not be assiduously observed and recorded in detail. We will carry out multi-centre trials for comparison in the future.
In conclusion, ER might be a safe and feasible method that is comparable to SR for the treatment of 2-3 cm gastric GISTs. SR is still considered the standard treatment for 3-5 cm gastric GISTs, while the intraoperative and postoperative information of ER should be recorded in detail and closely evaluated. Surgical resection is recommended if the tumour has a high mitotic index or mucosal ulceration. All patients should be carefully evaluated preoperatively to select the most appropriate resection procedure, and more prospective multi-centre random control trials with long-term follow-up are warranted to determine the best management of GISTs in the future.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
This study was approved by the Ethics Committee of Shandong Provincial Hospital (SWYX: No. 2021-035). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
All authors helped to perform the research; HW and HL were involved in the conception and design; MF and WX were involved in the follow-up of patients; QX and RZ were involved in preliminary medical record screening and entry; LS was involved in the verification of the included data; CL and JC were involved in the drafting of the paper or revising it critically for intellectual content; JL and LL were involved in the final approval of the version to be published. All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Supported by Key Research and Development Program of Shandong Province (No.2019JZZY010104; No.2019GSF108146); Special Foundation for Taishan Scholars Program of Shandong Province (No.ts20190978); Academic promotion programme of Shandong First Medical University (No. 2019QL021): Science and Technology Development Plan of Jinan 202019082.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We give our respect to all patients involved in the study. We would also like to thank the Medical Records Department of Shandong Provincial Hospital for providing data support and the efforts of medical workers over the past decades.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.737885/full#supplementary-material
Supplementary Figure 1 | Evaluation of propensity score matching. (A) Jitter plot of individual cases; (B) Line plot of individual differences; (C) Histogram of propensity score; (D) Dot plot of standardized mean differences.
GISTs, Gastrointestinal Stromal Tumors; GI, gastrointestinal; NCCN, National Comprehensive Cancer Network; ESMO, European Society for Medical Oncology; SR, Surgical Resection; ER, Endoscopic Resection; LR, Laparoscopic Resection; CT, Computed Tomography; BMI, Body Mass Index; ASA, American Society of Anesthesiology; ESD, Endoscopic Submucosal Dissection; ESE, Endoscopic Submucosal Excavation; EFR, Endoscopic Full-Thickness Resection; STER, Submucosal Tunneling Endoscopic Resection; HPF, High Power Field; SD, Standard Deviation; IQR, Interquartile Range; CI, Confidence Interval; HR, Hazard ratios; NIH, National Institutes of Health; PFS, Progression-free Survival; IHC, Immunohistochemistry; ERAS, Enhanced Recovery After Surgery; MP, muscularis propria; NESS-EFTR, Non-Exposure Simple Suturing Endoscopic Full-Thickness Resection; EUS, Endoscopic Ultrasonography; NA, Not Available.
1. Joensuu H, Hohenberger P, Corless CL. Gastrointestinal Stromal Tumour. Lancet (2013) 382:973–83. doi: 10.1016/S0140-6736(13)60106-3
2. Ma GL, Murphy JD, Martinez ME, Sicklick JK. Epidemiology of Gastrointestinal Stromal Tumors in the Era of Histology Codes: Results of a Population-Based Study. Cancer Epidemiol Biomarkers Prev (2015) 24:298–302. doi: 10.1158/1055-9965.EPI-14-1002
3. Serrano C, George S. Gastrointestinal Stromal Tumor: Challenges and Opportunities for a New Decade. Clin Cancer Res (2020) 26:5078–85. doi: 10.1158/1078-0432.CCR-20-1706
4. Scarpa M, Bertin M, Ruffolo C, Polese L, D’Amico DF, Angriman I. A Systematic Review on the Clinical Diagnosis of Gastrointestinal Stromal Tumors. J Surg Oncol (2008) 98:384–92. doi: 10.1002/jso.21120
5. Soreide K, Sandvik OM, Soreide JA, Giljaca V, Jureckova A, Bulusu VR. Global Epidemiology of Gastrointestinal Stromal Tumours (GIST): A Systematic Review of Population-Based Cohort Studies. Cancer Epidemiol (2016) 40:39–46. doi: 10.1016/j.canep.2015.10.031
6. Rajravelu RK, Ginsberg GG. Management of Gastric GI Stromal Tumors: Getting the GIST of it. Gastrointest Endosc (2020) 91:823–5. doi: 10.1016/j.gie.2019.11.036
7. von Mehren M, Kane JM, Bui MM, Choy E, Connelly M, Dry S, et al. NCCN Guidelines Insights: Soft Tissue Sarcoma, Version 1.2021. J Natl Compr Canc Netw (2020) 18:1604–12. doi: 10.6004/jnccn.2020.0058
8. Casali PG, Abecassis N, Aro HT, Bauer S, Biagini R, Bielack S, et al. Gastrointestinal Stromal Tumours: ESMO-EURACAN Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann Oncol (2018) 29:iv68–78. doi: 10.1093/annonc/mdy095
9. Joo MK, Park JJ, Kim H, Koh JS, Lee BJ, Chun HJ, et al. Endoscopic Versus Surgical Resection of GI Stromal Tumors in the Upper GI Tract. Gastrointest Endosc (2016) 83:318–26. doi: 10.1016/j.gie.2015.07.034
10. Bialek A, Wiechowska-Kozlowska A, Pertkiewicz J, Polkowski M, Milkiewicz P, Karpinska K, et al. Endoscopic Submucosal Dissection for Treatment of Gastric Subepithelial Tumors (With Video). Gastrointest Endosc (2012) 75:276–86. doi: 10.1016/j.gie.2011.08.029
11. An W, Sun PB, Gao J, Jiang F, Liu F, Chen J, et al. Endoscopic Submucosal Dissection for Gastric Gastrointestinal Stromal Tumors: A Retrospective Cohort Study. Surg Endosc (2017) 31:4522–31. doi: 10.1007/s00464-017-5511-3
12. Yao XI, Wang X, Speicher PJ, Hwang ES, Cheng P, Harpole DH, et al. Reporting and Guidelines in Propensity Score Analysis: A Systematic Review of Cancer and Cancer Surgical Studies. J Natl Cancer Inst (2017) 109:1–9. doi: 10.1093/jnci/djw323
13. Zhu H, Zhao S, Jiao R, Zhou J, Zhang C, Miao L. Comparison of Endoscopic Versus Laparoscopic Resection for Gastric Gastrointestinal Stromal Tumors: A Preliminary Meta-Analysis. J Gastroenterol Hepatol (2020) 35:1858–68. doi: 10.1111/jgh.15106
14. Wang C, Gao Z, Shen K, Cao J, Shen Z, Jiang K, et al. Safety and Efficiency of Endoscopic Resection Versus Laparoscopic Resection in Gastric Gastrointestinal Stromal Tumours: A Systematic Review and Meta-Analysis. Eur J Surg Oncol (2020) 46:667–74. doi: 10.1016/j.ejso.2019.10.030
15. He Z, Sun C, Zheng Z, Yu Q, Wang T, Chen X, et al. Endoscopic Submucosal Dissection of Large Gastrointestinal Stromal Tumors in the Esophagus and Stomach. J Gastroenterol Hepatol (2013) 28:262–7. doi: 10.1111/jgh.12056
16. Yu C, Liao G, Fan C, Yu J, Nie X, Yang S, et al. Long-Term Outcomes of Endoscopic Resection of Gastric GISTs. Surg Endosc (2017) 31:4799–804. doi: 10.1007/s00464-017-5557-2
17. Abe N, Takeuchi H, Ohki A, Hashimoto Y, Mori T, Sugiyama M. Comparison Between Endoscopic and Laparoscopic Removal of Gastric Submucosal Tumor. Dig Endosc (2018) 30 Suppl 1:7–16. doi: 10.1111/den.13010
18. Kim GH, Choi KD, Gong CS, Lee IS, Park YS, Han M, et al. Comparison of the Treatment Outcomes of Endoscopic and Surgical Resection of GI Stromal Tumors in the Stomach: A Propensity Score-Matched Case-Control Study. Gastrointest Endosc (2020) 91:527–36. doi: 10.1016/j.gie.2019.10.020
19. Zhao Y, Pang T, Zhang B, Wang L, Lv Y, Ling T, et al. Retrospective Comparison of Endoscopic Full-Thickness Versus Laparoscopic or Surgical Resection of Small (</= 5 Cm) Gastric Gastrointestinal Stromal Tumors. J Gastrointest Surg (2020) 24:2714–21. doi: 10.1007/s11605-019-04493-6
20. Triantafyllou T, Olson MT, Theodorou D, Schizas D, Singhal S. Enhanced Recovery Pathways vs Standard Care Pathways in Esophageal Cancer Surgery: Systematic Review and Meta-Analysis. Esophagus (2020) 17:100–12. doi: 10.1007/s10388-020-00718-9
21. Kong M, Liu G, Zhuo H, Xin Y, Chen H, Sheng H, et al. Association Between R1 Resection and Oncological Outcome in Resectable Gastrointestinal Stromal Tumors Without Tumor Rupture: A Systematic Review and Meta-Analysis. Eur J Surg Oncol (2021). doi: 10.1016/j.ejso.2021.01.032
22. Du C, Chai N, Linghu E, Li H, Zhai Y, Li L, et al. Clinical Outcomes of Endoscopic Resection for the Treatment of Gastric Gastrointestinal Stromal Tumors Originating From the Muscularis Propria: A 7-Year Experience From a Large Tertiary Center in China. Surg Endosc (2021). doi: 10.1007/s00464-021-08443-9
23. Dong X, Chen W, Cui Z, Chen T, Liu X, Chen D, et al. Laparoscopic Resection is Better Than Endoscopic Dissection for Gastric Gastrointestinal Stromal Tumor Between 2 and 5 Cm in Size: A Case-Matched Study in a Gastrointestinal Center. Surg Endosc (2020) 34:5098–106. doi: 10.1007/s00464-019-07251-6
24. Lei T, Tan F, Liu H, Ouyang M, Zhou H, Liu P, et al. Endoscopic or Surgical Resection for Patients With 2-5cm Gastric Gastrointestinal Stromal Tumors: A Single-Center 12-Year Experience From China. Cancer Manag Res (2020) 12:7659–70. doi: 10.2147/CMAR.S266898
25. Yang Z, Gao Y, Fan X, Zhao X, Zhu S, Guo M, et al. A Multivariate Prediction Model for High Malignancy Potential Gastric GI Stromal Tumors Before Endoscopic Resection. Gastrointest Endosc (2020) 91:813–22. doi: 10.1016/j.gie.2019.09.032
26. Du C, Linghu E. Submucosal Tunneling Endoscopic Resection for the Treatment of Gastrointestinal Submucosal Tumors Originating From the Muscularis Propria Layer. J Gastrointest Surg (2017) 21:2100–9. doi: 10.1007/s11605-017-3579-7
27. Eom BW, Kim CG, Kook MC, Yoon HM, Ryu KW, Kim YW, et al. Feasibility of Non-Exposure Simple Suturing Endoscopic Full-Thickness Resection in Comparison With Laparoscopic Endoscopic Cooperative Surgery for Gastric Subepithelial Tumors: Results of Two Independent Prospective Trials. Cancers (Basel) (2021) 13:1–10. doi: 10.3390/cancers13081858.
28. Xiong W, Xu Y, Chen T, Feng X, Zhou R, Wan J, et al. Laparoscopic vs. Open Surgery for Gastrointestinal Stromal Tumors of Esophagogastric Junction: A Multicenter, Retrospective Cohort Analysis With Propensity Score Weighting. Chin J Cancer Res (2021) 33:42–52. doi: 10.21147/j.issn.1000-9604.2021.01.05
29. Ojima T, Nakamura M, Nakamori M, Takifuji K, Hayata K, Katsuda M, et al. Laparoscopic and Endoscopic Cooperative Surgery is a Feasible Treatment Procedure for Intraluminal Gastric Gastrointestinal Stromal Tumors Compared to Endoscopic Intragastric Surgery. Surg Endosc (2018) 32:351–7. doi: 10.1007/s00464-017-5683-x
30. Balde AI, Chen T, Hu Y, Redondo NJ, Liu H, Gong W, et al. Safety Analysis of Laparoscopic Endoscopic Cooperative Surgery Versus Endoscopic Submucosal Dissection for Selected Gastric Gastrointestinal Stromal Tumors: A Propensity Score-Matched Study. Surg Endosc (2017) 31:843–51. doi: 10.1007/s00464-016-5042-3
Keywords: gastrointestinal stromal tumours, surgical, endoscopic, propensity score matching, gastric tumours
Citation: Wu H, Li H, Xu Q, Shang L, Zhang R, Li C, Fu M, Xu W, Chen J, Liu J and Li L (2021) Surgical Resection Is Still Better Than Endoscopic Resection for Patients With 2-5 cm Gastric Gastrointestinal Stromal Tumours: A Propensity Score Matching Analysis. Front. Oncol. 11:737885. doi: 10.3389/fonc.2021.737885
Received: 07 July 2021; Accepted: 30 August 2021;
Published: 15 September 2021.
Edited by:
Cesare Ruffolo, University Hospital of Padua, ItalyReviewed by:
Giovanni Capovilla, Johannes Gutenberg University Mainz, GermanyCopyright © 2021 Wu, Li, Xu, Shang, Zhang, Li, Fu, Xu, Chen, Liu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jin Liu, bWlyYWNsZTcxM0AxNjMuY29t; Leping Li, bGlsZXBpbmdAc2R1LmVkdS5jbg==
†ORCID: Hao Wu, orcid.org/0000-0001-9222-2521
Leping Li, orcid.org/0000-0003-2329-6791
‡These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.