- Department of Gynecology, Jinan Maternal and Child Care Hospital, Jinan, China
Background: Many studies have investigated the prognostic role of the C-reactive protein/albumin ratio (CRP/Alb ratio) in patients with gynecological cancers; however, there is lack of consensus owing to conflicting results across studies. We performed a meta-analysis to determine the prognostic role of the CRP/Alb ratio in gynecological cancers.
Methods: We searched the PubMed, Embase, the Web of Science, Cochrane Library, China National Knowledge Infrastructure, and Wanfang electronic databases since inception to April 2021. Combined hazard ratios (HRs) and 95% confidence intervals (CIs) were used to estimate the prognostic effect of the CRP/Alb ratio in gynecological cancers. Pooled odds ratios (ORs) and 95% CIs were used to investigate the association between the CRP/Alb ratio and clinicopathological features.
Results: The meta-analysis included seven studies with 1,847 patients. The pooled results showed that a high pretreatment CRP/Alb ratio was associated with poor overall survival (HR, 1.84; 95% CI, 1.41–2.40; p < 0.001) and progression-/disease-free survival (HR, 2.58; 95% CI, 1.42–4.68; p = 0.002). Additionally, a high CRP/Alb ratio was significantly associated with stages III–IV disease (the International Federation of Gynecology and Obstetrics classification) (OR, 2.98; 95% CI, 1.45–6.14; p = 0.003). However, we observed a non-significant correlation between the CRP/Alb ratio and lymph node metastasis, tumor size, and histopathological grade.
Conclusions: The CRP/Alb ratio is a convenient and accurate predictor of survival outcomes in gynecological cancers. A high CRP/Alb ratio also predicts tumor progression.
Introduction
Gynecological cancers (GCs) represent the second most common cancer in women worldwide (1). Cervical cancer (CC), ovarian cancer (OC), and endometrial cancer (EC) are the predominant types of GC, which constitute a major public health concern globally (2). The 5-year survival rates in patients with GC are poor despite the availability of well-established surgical and chemoradiotherapeutic approaches (3). For example, the 5-year survival rate in patients with stages III–IV EC was only 17% (4). Therefore, it is important to identify effective novel biomarkers that predict survival outcomes and also establish individualized therapeutic regimens.
Accumulating evidence has shown an association between tumor-induced inflammatory responses and tumor development and progression (5). Reportedly, various inflammatory and immune response biomarkers serve as prognostic factors for solid tumors (6). For example, in a recent study, Marchetti et al. (7) observed that the neutrophil–lymphocyte ratio (NLR) was an independent prognostic marker for progression-free survival (PFS) in patients with high-grade advanced serous ovarian cancer. Another study (8), which recruited 1,266 patients with CC, showed that the platelet–lymphocyte ratio (PLR), NLR, derived NLR, and the PLR + NLR in combination showed equivalent efficacy as prognostic factors for overall survival (OS) in patients with locally advanced CC and are therefore considered promising prognostic biomarkers. The aforementioned studies (7, 8) highlight the role of systemic inflammatory biomarkers in the prognosis of GC. The C-reactive protein (CRP)/albumin ratio (CRP/Alb) is an index based on measurement of serum CRP and Alb levels (9) and is calculated as the serum CRP level divided by the serum Alb level. This ratio was initially proposed to predict outcomes in patients with acute medical admissions (9). In recent years, studies have investigated the utility of the CRP/Alb ratio as a prognostic factor in various cancers (10, 11). Previous studies have reported the independent prognostic value of the CRP/Alb ratio in colorectal cancer (CRC) (12), oral squamous cell carcinoma (13), gastric cancer (14), non-small-cell lung cancer (15), and gallbladder cancer (16). Many studies have also investigated the prognostic utility of the CRP/Alb ratio in patients with GC; however, the results remain inconclusive (17–23). We performed a meta-analysis to systemically investigate the prognostic and clinical significance of the CRP/Alb ratio in GC.
Materials and Methods
Study Guidelines and Ethics
This meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (24). All analyses were based on previously published studies; therefore, ethical approval and informed consent were waived for this study.
Literature Search
We performed a comprehensive search of the PubMed, Embase, the Web of Science, Cochrane Library, China National Knowledge Infrastructure, and Wanfang electronic databases from inception to April 2021. Based on relevant studies (25, 26), we used the following search terms: gynecological cancer OR gynecological carcinoma OR gynecological neoplasm OR endometrial neoplasm OR endometrial carcinoma OR endometrial cancer OR endometrium cancer OR endometrium carcinoma OR cervical cancer OR cervical carcinoma OR ovarian carcinoma OR ovarian cancer OR uterine neoplasm and C-reactive protein OR albumin OT CAR OR CRP/Alb OR C-reactive protein to albumin ratio, C-reactive protein/albumin ratio and prognosis OR prognostic OR survival OR outcome OR mortality. Both free text and medical subject heading terms were used for the literature search. We selected articles in English and Chinese. References from the identified publications were also retrieved for potential inclusion.
Inclusion and Exclusion Criteria
Following were the inclusion criteria: (1) histopathologically diagnosed GC; (2) studies that investigated the prognostic role of CRP/Alb ratio for survival outcomes including but not limited to OS, cancer-specific survival (CSS), disease-free survival (DFS), PFS, and recurrence-free survival; (3) availability of hazard ratios (HRs) and 95% confidence intervals (CIs) or sufficient data to calculate these values; and (4) availability of cutoff values of the CRP/Alb ratio. Following were the exclusion criteria: (1) reviews, meeting abstracts, case reports, and letters; (2) unavailability of data to calculate HRs and corresponding 95% CIs; (3) studies in languages other than English or Chinese; and (4) overlapping or duplication of studies.
Data Extraction and Quality Assessment
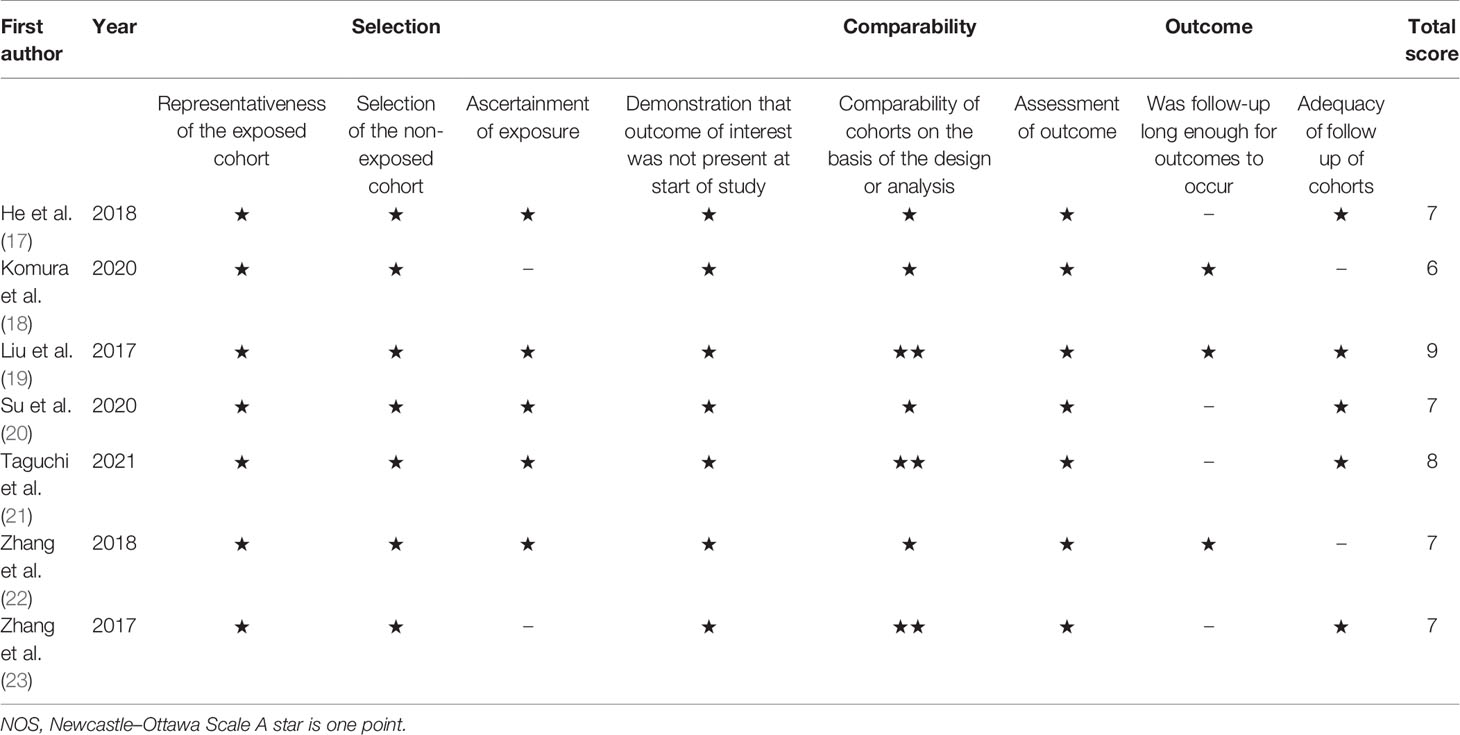
Two investigators (YF and TZ) independently screened all retrieved articles and extracted information using a predetermined form. All disagreements were resolved through discussion with a third investigator (CZ). The following data were recorded: first author’s name, year of publication, country, age, cancer type, the International Federation of Gynecology and Obstetrics (FIGO) stage, treatment, study period, cutoff value of the CRP/Alb ratio, method used to determine the cutoff value, duration of follow-up or the last date of follow-up, survival endpoints, and HRs and 95% CIs for OS, PFS, and DFS. HRs and 95% CIs were extracted from multivariable analyses depending on availability; HRs and 95% CIs were extracted from univariate analyses in the remaining cases. Two independent reviewers assessed the methodological quality of the included studies based on the Newcastle–Ottawa Quality Assessment Scale (NOS) (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp). The NOS consists of the following three dimensions: selection (four stars), comparability (two stars), and outcome assessment (three points). NOS scores range from 0 to 9, and scores ≥6 indicate high-quality studies.
Statistical Analysis
Combined HRs and 95% CIs were used to estimate the prognostic effect of the CRP/Alb ratio in GC. HR >1 without a 95% CI overlapping 1 indicates that a high CRP/Alb ratio predicts poor prognosis. Heterogeneity among studies was assessed using the Cochrane Q test and the I2 statistic. p < 0.10 and/or I2 > 50% was interpreted as indicative of significant heterogeneity, and a random-effects model was used in such cases; a fixed-effects model was used in the remaining cases. Subgroup analysis stratified by various factors was performed to determine the source of heterogeneity. Pooled odds ratios (ORs) and 95% CIs were used to determine the association between the CRP/Alb ratio and clinicopathological features. Publication bias was evaluated using Begg’s funnel plots. All statistical analyses were performed using the Stata software, version 12.0 (Stata Corporation, College Station, TX, USA). A p < 0.05 was considered statistically significant.
Results
Literature Search
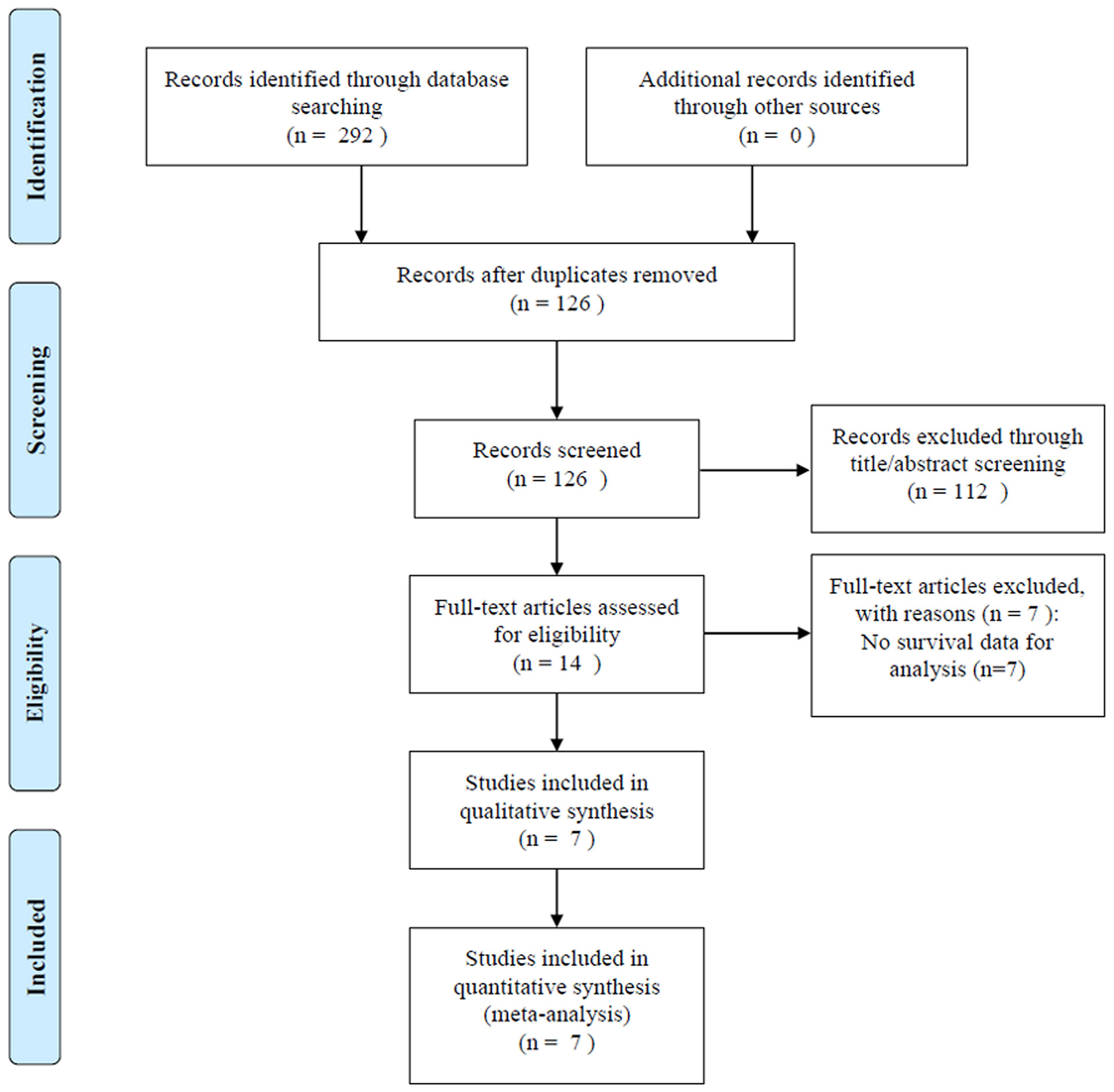
The initial literature search identified 292 studies from the aforementioned databases; 126 studies remained after exclusion of duplicate studies (Figure 1). After screening titles and abstracts, 112 studies were eliminated, and we reviewed the full text in 14 articles, of which 7 studies with insufficient data were excluded. Finally, seven studies with 1,847 patients (17–23) were included in this meta-analysis.
Characteristics of Included Studies
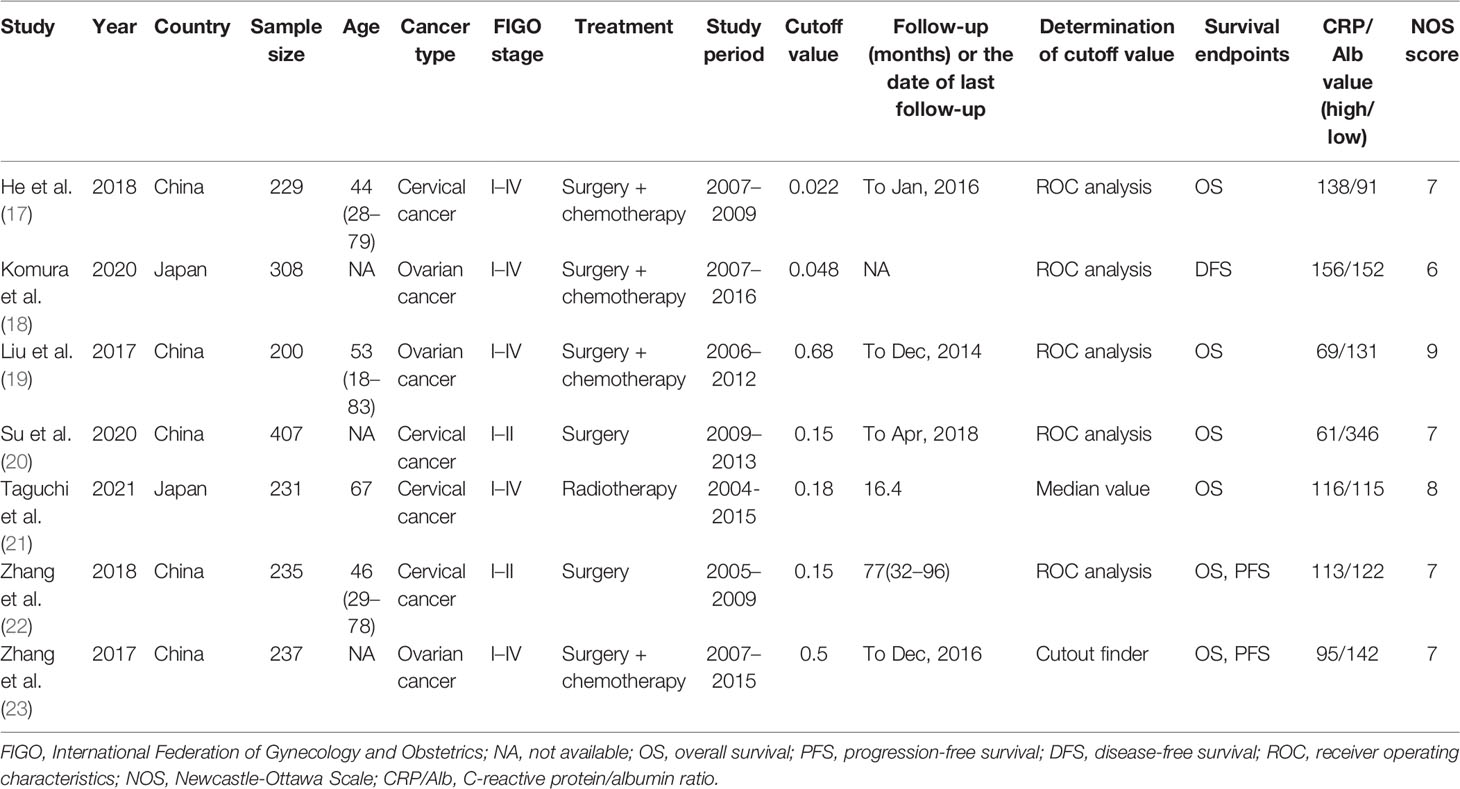
Table 1 summarizes the characteristics of studies included in this meta-analysis. The studies were published between 2017 and 2021. Five studies were performed in China (17, 19, 20, 22, 23) and two in Japan (18, 21). Six studies were published in English (17–19, 21–23) and one in Chinese (20). Four studies enrolled patients with CC (17, 20–22) and three recruited patients with OC (18, 19, 23). The sample size ranged from 200 to 407 (median, 235). The cutoff values of the CRP/Alb ratio ranged from 0.022 to 0.68 (median, 0.15). Therefore, we used 0.15 as the cutoff value of the CRP/Alb ratio for subgroup analysis. Five studies used receiver operating characteristic curve analysis to determine the cutoff value (17–20, 22), one study used the median value (21), and one study used cutoff finder software (23). Six studies (17, 19–23) reported the prognostic value of the CRP/Alb ratio for OS, two studies (22, 23) reported its prognostic value for PFS, and one study (18) for DFS. Five studies (17–19, 21, 23) enrolled patients with FIGO stages I–IV, and two studies (20, 22) included patients with FIGO stages I–II. NOS scores of the included studies ranged from 6 to 9 (median, 7), which indicates that all included studies were of high quality. Table 2 shows NOS score details.
Prognostic Role of the C-Reactive Protein/Albumin Ratio in Overall Survival and Progression- and Disease-Free Survival
A total of six studies that included 1,539 patients (17, 19–23) investigated the association between the CRP/Alb ratio and OS in GC. Pooled data showed HR of 1.84, 95% CI of 1.41–2.40, and p < 0.001 (Figure 2; Table 3). Owing to significant heterogeneity (I2 = 71.2%, p = 0.004), we used a random-effects model. Subgroup analyses based on various factors showed that a high CRP/Alb ratio remained a prognostic tool to determine poor survival in subgroups across different countries, cancer types, FIGO stage, cutoff values, cutoff value determination methods, and treatment modalities (Table 3). Three studies that included 780 patients (18, 22, 23) reported the role of the CRP/Alb ratio for prognosis of PFS/DFS (HR, 2.58; 95% CI, 1.42–4.68; p = 0.002) based on a random-effects model (Figure 3; Table 3). Similar to the findings associated with OS, subgroup analysis revealed that an elevated CRP/Alb ratio was an indicator of poor PFS/DFS in various subgroups of patients with GC.
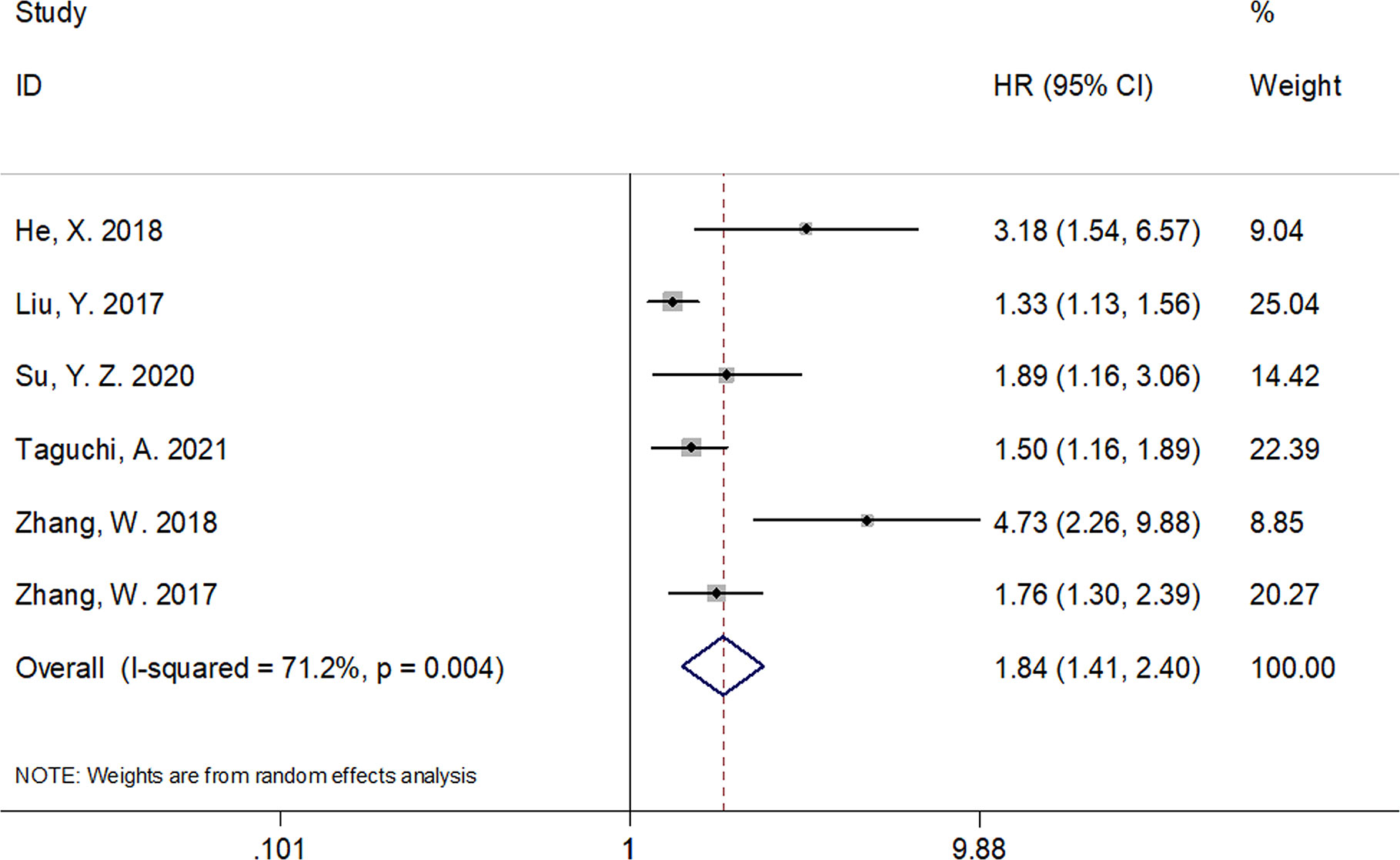
Figure 2 Forest plots of studies evaluating the hazard ratio for overall survival (OS) of patients with gynecological cancers of a high CRP/Alb ratio.
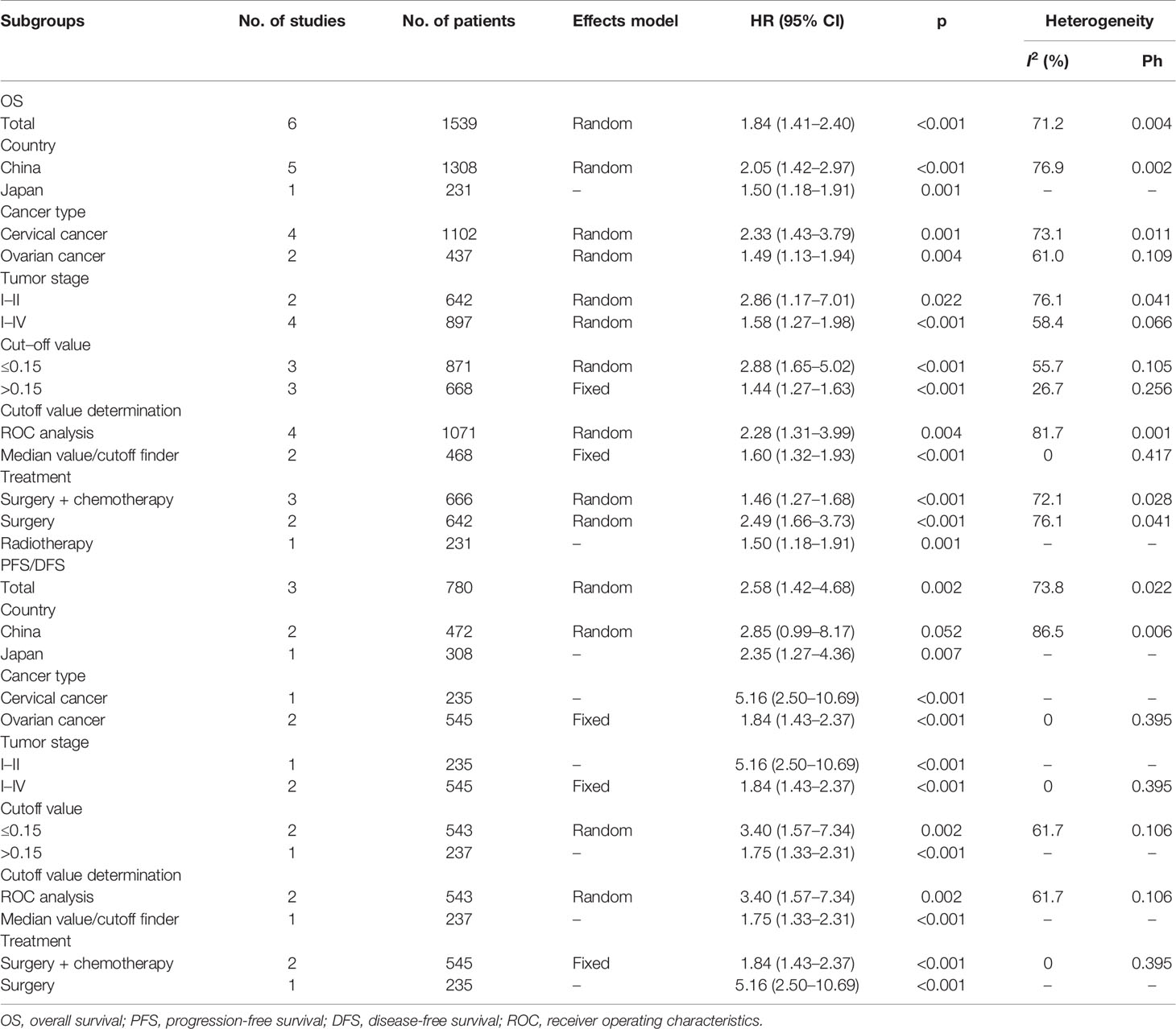
Table 3 Subgroup analysis of the prognostic value of CRP/Alb for OS and PFS/DFS in patients with gynecological cancers.
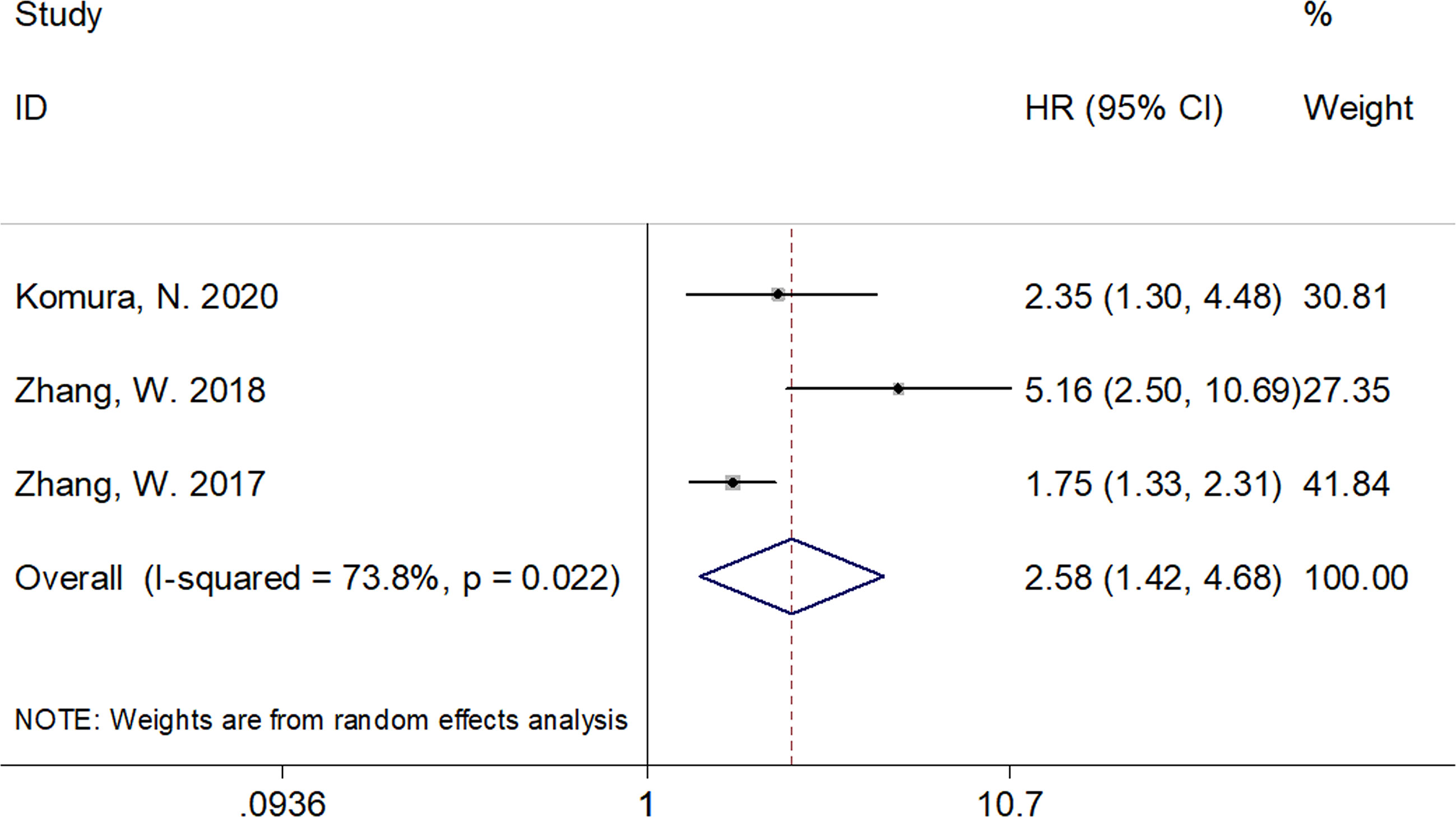
Figure 3 Forest plots of studies evaluating the hazard ratio for progression-free survival (PFS)/disease-free survival (DFS) of patients with gynecological cancers of a high CRP/Alb ratio.
Correlation Between the C-Reactive Protein/Albumin Ratio and Clinical Features
Using data from five studies, we investigated the association between the CRP/Alb ratio and clinicopathological characteristics (17–20, 22). A high CRP/Alb ratio was significantly associated with FIGO stages III–IV (OR, 2.98; 95% CI, 1.45–6.14; p = 0.003) (Figure 4; Table 4). However, we observed a non-significant correlation between the CRP/Alb ratio and lymph node metastasis (yes vs. no) (OR, 2.54; 95% CI, 0.59–10.90; p = 0.209), tumor size (≥4 vs. <4 cm) (OR, 2.54; 95% CI, 0.84–7.72; p = 0.100), and histopathological grade (G3 vs. G1/G2) (OR, 1.07; 95% CI, 0.75–1.53; p = 0.176) (Figure 4; Table 4).
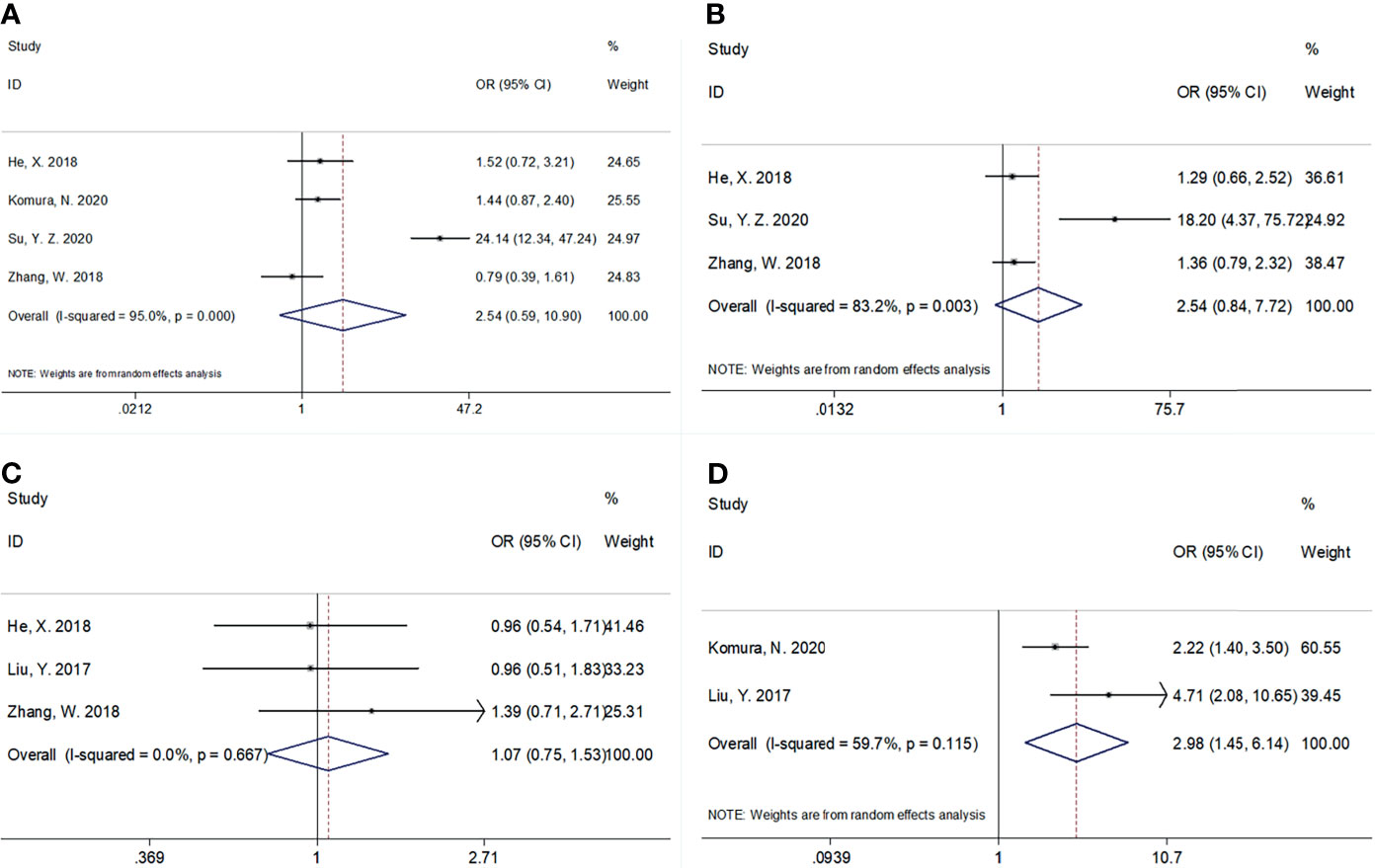
Figure 4 The association between CRP/Alb ratio and clinicopathological features in patients with gynecological cancers. (A) Lymph node metastasis (yes vs. no), (B) tumor size (≥4 vs. <4 cm), (C) histological grade (G3 vs. G1/G2), and (D) FIGO stage (III–IV vs. I–II).
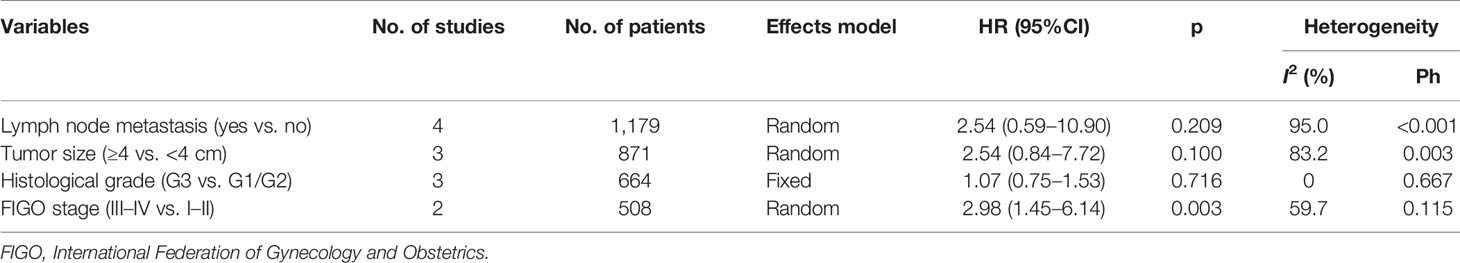
Table 4 The correlation between CRP/Alb ratio and clinicopathological features in patients with gynecological cancers.
Publication Bias
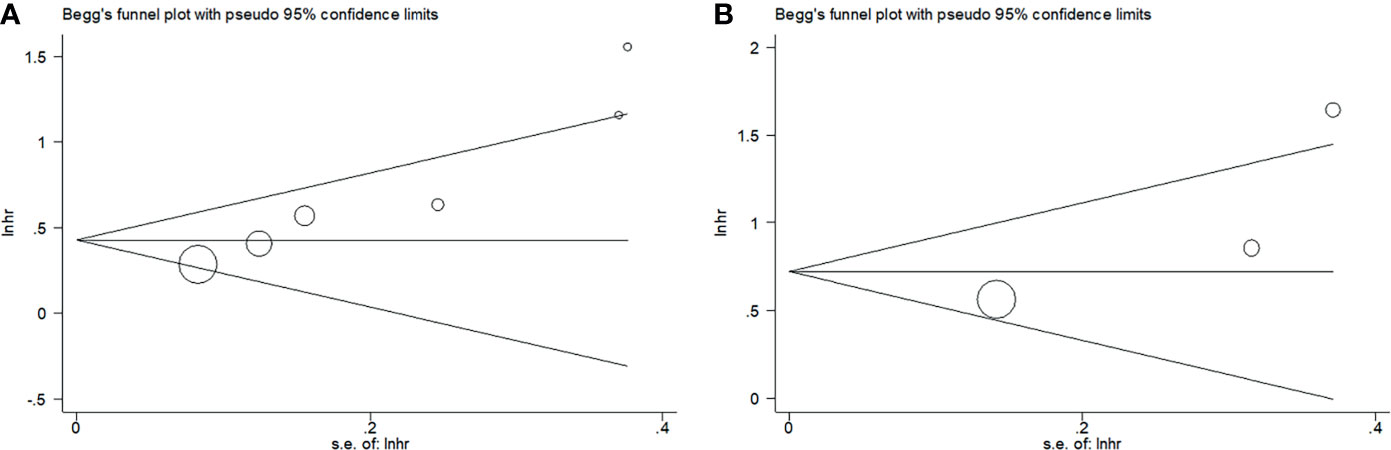
Begg’s funnel plots were used to assess the potential publication bias for OS and PFS/DFS. We observed no significant publication bias for OS (p = 0.135) or PFS/DFS (p = 0.296) in this meta-analysis (Figure 5). Therefore, the results of our meta-analysis are reliable.
Discussion
The CRP/Alb ratio has been investigated as a prognostic factor for patients with GC; however, there is lack of consensus owing to inconsistent results across studies. In the present meta-analysis, we used pooled data from seven studies that included 1,847 patients; our results showed that a high CRP/Alb ratio was an independent prognostic factor for poor OS, PFS, and DFS. Results of subgroup analyses performed after stratification based on country, cancer type, FIGO stage, cutoff values, cutoff value determination methods, and treatment modalities were in agreement with the results of the overall analysis. An elevated CRP/Alb ratio was also associated with advanced FIGO stages in GC. These results indicate that the CRP/Alb ratio may serve as an efficient and cost-effective prognostic biomarker in clinical settings for patients with GC. To our knowledge, this is the first meta-analysis that discusses the prognostic role of the CRP/Alb ratio in GC.
Inflammatory responses are known to promote tumorigenesis through their effects on the tumor microenvironment in GC (27). Tumor growth, invasion, necrosis, and hypoxia initiate immune responses in the tumor microenvironment, which consequently triggers the production of a variety of inflammatory cytokines (28). CRP is an acute-phase protein that is mediated by several proinflammatory cytokines, including interleukin-1 (IL-1), IL-6, and tumor necrosis factor-α (29). IL-6 can lead to inflammation and angiogenesis, which contribute to tumor progression (30). Serum Alb levels reflect patients’ nutritional status; a low serum Alb level indicates a state of malnutrition (31). Hypoalbuminemia is implicated in the nutritional decline observed in patients with cancer (17). Therefore, the CRP/Alb ratio (as a combination of CRP and Alb) provides a biological rationale to be considered a promising prognostic tool in GC.
Many studies have investigated the prognostic value of the CRP/Alb ratio in a variety of cancer types (26, 29, 32–35). Yu et al. showed that an elevated pretreatment CRP/Alb ratio was a prognostic marker of poor OS and CSS in patients with gastric cancer (26). A high CRP/Alb ratio was shown to be associated with clinicopathological features that reflect tumor progression in patients with gastric cancer (26). A meta-analysis of 15 studies that included 6,329 patients reported that a high CRP/Alb ratio was associated with various survival outcomes in patients with CRC (29). Another recent meta-analysis suggested that an elevated pretreatment CRP/Alb ratio could independently predict poor OS in patients with pancreatic cancer (33). Zhou et al. observed that a high pretreatment CRP/Alb ratio predicted poor survival in patients with renal cell carcinoma (36). In our meta-analysis, the CRP/Alb ratio was correlated with poor OS, PFS, and DFS and also predicted advanced FIGO stages. Therefore, the CRP/Alb ratio also serves as a promising tool for risk stratification of patients.
Following are the limitations of this meta-analysis: (a) we investigated studies that described only a few types of GC; only CC and OC were considered in this study. Other GCs, such as EC, were not included in our meta-analysis. Although the search items included all GCs; however, no eligible study on EC was identified. (b) All eligible studies were retrospectively designed, which may introduce selection bias in the meta-analysis. (c) All patients were from China and Japan; therefore, our findings may not be generalizable and are perhaps more applicable to Asian patients. The prognostic value of the CRP/Alb ratio in non-Asian patients warrants investigation.
Conclusions
Our meta-analysis showed that the CRP/Alb ratio is a convenient and accurate predictor of survival outcomes in GC. A high CRP/Alb ratio also predicts tumor progression. However, owing to several limitations of this study, large-scale trials that include patients of diverse ethnicities are warranted to validate our findings.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author Contributions
YF and TZ came up with the study. All authors collaborated on the design of the project. YF, TZ, and CZ collated, screened, and analyzed the data together and drafted the manuscript. YF and TZ reviewed the content, revised the manuscript, and approved the final manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Gultekin M, Kucukyildiz I, Karaca MZ, Dundar S, Boztas G, Turan SH, et al. Trends of Gynecological Cancers in Turkey: Toward Europe or Asia? Int J Gynecol Cancer (2017) 27(8S Suppl 1):S1–s9. doi: 10.1097/igc.0000000000001026
3. Lu JJ, Gu Y, Li Q, Zhong HX, Wang XX, Zheng ZX, et al. Wilms' Tumor 1 (WT1) as a Prognosis Factor in Gynecological Cancers A Meta-Analysis. Medicine (2018) 97(28):e11485. doi: 10.1097/md.0000000000011485
4. McDonald ME, Bender DP. Endometrial Cancer: Obesity, Genetics, and Targeted Agents. Obstet Gynecol Clinics North Am (2019) 46(1):89–105. doi: 10.1016/j.ogc.2018.09.006
5. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-Related Inflammation and Treatment Effectiveness. Lancet Oncol (2014) 15(11):e493–503. doi: 10.1016/s1470-2045(14)70263-3
6. Zou PJ, Yang E, Li ZY. Neutrophil-To-Lymphocyte Ratio Is an Independent Predictor for Survival Outcomes in Cervical Cancer: A Systematic Review and Meta-Analysis. Sci Rep (2020) 10(1):21917. doi: 10.1038/s41598-020-79071-x
7. Marchetti C, D'Indinosante M, Bottoni C, Di Ilio C, Di Berardino S, Costantini B, et al. NLR and BRCA Mutational Status in Patients With High Grade Serous Advanced Ovarian Cancer. Sci Rep (2021) 11(1):11125. doi: 10.1038/s41598-021-90361-w
8. Santos Thuler LC, Reis Wariss B, Nogueira-Rodrigues A, de Melo AC, Bergmann A. The Utility of Pretreatment Systemic Inflammatory Response Biomarkers on Overall Survival of Cervical Cancer Patients Stratified by Clinical Staging. Eur J Obstet Gynecol Reprod Biol (2021) 264:281–8. doi: 10.1016/j.ejogrb.2021.07.034
9. Fairclough E, Cairns E, Hamilton J, Kelly C. Evaluation of a Modified Early Warning System for Acute Medical Admissions and Comparison With C-Reactive Protein/Albumin Ratio as a Predictor of Patient Outcome. Clin Med (London England) (2009) 9(1):30–3. doi: 10.7861/clinmedicine.9-1-30
10. Li N, Tian GW, Wang Y, Zhang H, Wang ZH, Li G. Prognostic Role of the Pretreatment C-Reactive Protein/Albumin Ratio in Solid Cancers: A Meta-Analysis. Sci Rep (2017) 7:41298. doi: 10.1038/srep41298
11. Xu HJ, Ma Y, Deng F, Ju WB, Sun XY, Wang H. The Prognostic Value of C-Reactive Protein/Albumin Ratio in Human Malignancies: An Updated Meta-Analysis. Onco Targets Ther (2017) 10:3059–70. doi: 10.2147/ott.S137002
12. Tamai K, Okamura S, Makino S, Yamamura N, Fukuchi N, Ebisui C, et al. C-Reactive Protein/Albumin Ratio Predicts Survival After Curative Surgery in Elderly Patients With Colorectal Cancer. Updates Surg (2021). doi: 10.1007/s13304-021-01011-9
13. Keinänen A, Uittamo J, Marinescu-Gava M, Kainulainen S, Snäll J. Preoperative C-Reactive Protein to Albumin Ratio and Oral Health in Oral Squamous Cell Carcinoma Patients. BMC Oral Health (2021) 21(1):132. doi: 10.1186/s12903-021-01516-0
14. Yu Q, Li KZ, Fu YJ, Tang Y, Liang XQ, Liang ZQ, et al. Clinical Significance and Prognostic Value of C-Reactive Protein/Albumin Ratio in Gastric Cancer. Ann Surg Treat Res (2021) 100(6):338–46. doi: 10.4174/astr.2021.100.6.338
15. Araki T, Tateishi K, Sonehara K, Hirota S, Komatsu M, Yamamoto M, et al. Clinical Utility of the C-Reactive Protein:Albumin Ratio in Non-Small Cell Lung Cancer Patients Treated With Nivolumab. Thorac Cancer (2021) 12(5):603–12. doi: 10.1111/1759-7714.13788
16. Bao Y, Yang J, Duan Y, Chen Y, Chen W, Sun D. The C-Reactive Protein to Albumin Ratio Is an Excellent Prognostic Predictor for Gallbladder Cancer. Biosci Trends (2021) 14(6):428–35. doi: 10.5582/bst.2020.03326
17. He X, Li JP, Liu XH, Zhang JP, Zeng QY, Chen H, et al. Prognostic Value of C-Reactive Protein/Albumin Ratio in Predicting Overall Survival of Chinese Cervical Cancer Patients Overall Survival: Comparison Among Various Inflammation Based Factors. J Cancer (2018) 9(10):1877–84. doi: 10.7150/jca.23320
18. Komura N, Mabuchi S, Shimura K, Kawano M, Matsumoto Y, Kimura T. Significance of Pretreatment C-Reactive Protein, Albumin, and C-Reactive Protein to Albumin Ratio in Predicting Poor Prognosis in Epithelial Ovarian Cancer Patients. Nutr Cancer (2021) 73(8):1357–64. doi: 10.1080/01635581.2020.1798479
19. Liu Y, Chen S, Zheng C, Ding M, Zhang L, Wang L, et al. The Prognostic Value of the Preoperative C-Reactive Protein/Albumin Ratio in Ovarian Cancer. BMC Cancer (2017) 17(1):285. doi: 10.1186/s12885-017-3220-x
20. Su YZ, Xu ZY, Xiang HF, Wei ZL, Cao YX. Relationship Between CAR and Prognosis of Cervical Cancer Patients. Med Inf (2020) 33(08):80–3.
21. Taguchi A, Nakajima Y, Furusawa A, Yoshino Y, Takao M, Kashiyama T, et al. High Neutrophil-to-Lymphocyte Ratio Is a Predictor of Short-Term Survival for Patients With Recurrent Cervical Cancer After Radiation-Based Therapy. J Obstet Gynaecol Res (2021) 47(5):1862–70. doi: 10.1111/jog.14712
22. Zhang W, Liu K, Ye B, Liang W, Ren Y. Pretreatment C-Reactive Protein/Albumin Ratio Is Associated With Poor Survival in Patients With Stage IB-IIA Cervical Cancer. Cancer Med (2018) 7(1):105–13. doi: 10.1002/cam4.1270
23. Zhang W, Ye B, Liang W, Ren Y. Preoperative Prognostic Nutritional Index Is a Powerful Predictor of Prognosis in Patients With Stage III Ovarian Cancer. Sci Rep (2017) 7(1):9548. doi: 10.1038/s41598-017-10328-8
24. Moher D, Liberati A, Tetzlaff J, Altman DG, Grp P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med (2009) 6(7):e1000097. doi: 10.1371/journal.pmed.1000097
25. Hu JL, Wu XR, Huang PZ, Teng F, Wang YM, Xue FX. The Proportion and Prognostic Significance of T-Regulatory Cells in Patients With Gynecological Cancers: A Systematic Review and Meta-Analysis. J Cancer (2020) 11(11):3340–8. doi: 10.7150/jca.42472
26. Yu J, Liu H, Zeng X, Zhao Y, Jiang D, Lu H, et al. Prognostic and Clinicopathological Significance of C-Reactive Protein/Albumin Ratio (CAR) in Patients With Gastric Cancer: A Meta-Analysis. PLoS One (2021) 16(4):e0250295. doi: 10.1371/journal.pone.0250295
27. Polastro L, Closset C, Kerger J. Immunotherapy in Gynecological Cancers: Where Are We? Curr Opin Oncol (2020) 32(5):459–70. doi: 10.1097/cco.0000000000000661
28. Kinoshita A, Onoda H, Imai N, Iwaku A, Oishi M, Tanaka K, et al. The C-Reactive Protein/Albumin Ratio, a Novel Inflammation-Based Prognostic Score, Predicts Outcomes in Patients With Hepatocellular Carcinoma. Ann Surg Oncol (2015) 22(3):803–10. doi: 10.1245/s10434-014-4048-0
29. Liao CK, Yu YL, Lin YC, Hsu YJ, Chern YJ, Chiang JM, et al. Prognostic Value of the C-Reactive Protein to Albumin Ratio in Colorectal Cancer: An Updated Systematic Review and Meta-Analysis. World J Surg Oncol (2021) 19(1):139. doi: 10.1186/s12957-021-02253-y
30. Wakasaki T, Yasumatsu R, Masuda M, Takeuchi T, Manako T, Matsuo M, et al. Prognostic Biomarkers of Salvage Chemotherapy Following Nivolumab Treatment for Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma. Cancers (Basel) (2020) 12(8):2299. doi: 10.3390/cancers12082299
31. Saito H, Kono Y, Murakami Y, Shishido Y, Kuroda H, Matsunaga T, et al. Postoperative Serum Albumin Is a Potential Prognostic Factor for Older Patients With Gastric Cancer. Yonago Acta Med (2018) 61(1):72–8. doi: 10.33160/yam.2018.03.010
32. Luan CW, Yang HY, Tsai YT, Hsieh MC, Chou HH, Chen KS. Prognostic Value of C-Reactive Protein-To-Albumin Ratio in Head and Neck Cancer: A Meta-Analysis. Diagnostics (Basel Switzerland) (2021) 11(3):403. doi: 10.3390/diagnostics11030403
33. Zang Y, Fan Y, Gao Z. Pretreatment C-Reactive Protein/Albumin Ratio for Predicting Overall Survival in Pancreatic Cancer: A Meta-Analysis. Med (Baltimore) (2020) 99(23):e20595. doi: 10.1097/md.0000000000020595
34. Xie Q, Wang L, Zheng S. Prognostic and Clinicopathological Significance of C-Reactive Protein to Albumin Ratio in Patients With Pancreatic Cancer: A Meta-Analysis. Dose Response Publ Int Hormesis Soc (2020) 18(2):1559325820931290. doi: 10.1177/1559325820931290
35. Fang E, Wang X, Feng J, Zhao X. The Prognostic Role of Glasgow Prognostic Score and C-Reactive Protein to Albumin Ratio for Sarcoma: A System Review and Meta-Analysis. Dis Markers (2020) 2020:8736509. doi: 10.1155/2020/8736509
Keywords: gynecological cancers, meta-analysis, prognostic, CRP/Alb ratio, risk factors
Citation: Fang Y, Zheng T and Zhang C (2021) Prognostic Role of the C-Reactive Protein/Albumin Ratio in Patients With Gynecological Cancers: A Meta-Analysis. Front. Oncol. 11:737155. doi: 10.3389/fonc.2021.737155
Received: 13 July 2021; Accepted: 16 September 2021;
Published: 28 October 2021.
Edited by:
Dong Hoon SuhSeoul National University College of Medicine, South KoreaReviewed by:
Bala Audu, University of Maiduguri, NigeriaMarco D’Indinosante, Catholic University of the Sacred Heart, Italy
Copyright © 2021 Fang, Zheng and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yingji Fang, am5saDE5NzFAc2luYS5jb20=
 Yingji Fang
Yingji Fang Tingting Zheng
Tingting Zheng