
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 02 December 2021
Sec. Cancer Molecular Targets and Therapeutics
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.732782
This article is part of the Research Topic Molecular Targets and Therapeutic Strategies in Gynecological Cancers View all 7 articles
Objective: Clear cell carcinoma (CCC) of the endometrium is an uncommon yet aggressive tumor. Few cohort studies are reporting the overall survival time of CCC patients. This study aimed to retrospectively analyze the clinicopathologic features, molecular characteristics and survival data of 27 endometrial CCC patients to improve the understanding of CCC.
Methods: The clinicopathologic features, molecular characteristics and survival data total of 27 CCC patients admitted to the BBMU affiliated hospital (Anhui, China) between January 2005 and December 2018 were retrospectively analyzed. Kaplan-Meier method was used to analyze the prognosis-related factors.
Results: The median age of the patients was 60 years (range; 39 to 81 years). The average tumor size was 3.8 cm (range; 0.8 to 13.0cm). Myometrial infiltration greater than 50% was reported in 55.6% of the patients, while the Ki-67 index greater than 50% was reported in 70.4% of the patients. The patients’ FIGO (2009) surgical stages were as follows: 18 I, 3 II, 4 III, and 2 IV. Besides, 7 (25.6%) patients had lymphovascular invasion, 3 (11.1%) patients with distant metastasis, including 1 patient with bone metastasis, and 2 with liver metastasis. Adjuvant treatment included 7 with chemotherapy alone, 9 with radiotherapy alone, and 9 with both radiotherapy and chemotherapy. The median overall survival time from the time of CCC diagnosis was 56 months. ER and PR showed negative expression and P16 showed patchy immunostaining. 18 (63%) cases showed Napsin A positive expression. Loss of MSH2, MSH6 and PTEN were seen in 5, 4 and 7 cases respectively. All cases showed HER-2/nue negative expression.
Conclusion: CCC is a rare and invasive tumor. Age of diagnosis, FIGO stage, tumor size, myometrial infiltration, lymphovascular invasion, distant metastasis, Ki-67 index and P53 expression are important indicators to evaluate patient’s prognosis (P = 0.048, P < 0.001, P = 0.016, P = 0.043, P = 0.001, P < 0.001, P = 0.026, and P = 0.007, respectively). CCC has a worse prognosis than endometrioid carcinoma (P = 0.002), and there is no significant difference when compared with uterine papillary serous carcinoma (P = 0.155).
Clear cell carcinoma (CCC) of the endometrium is accounting for 1%–5.5% of all endometrial carcinomas (1–3). Due to the lack of exploratory research, the incidence of CCC may be underestimated. Most CCC are a mixture of at least two architectural forms, the most common form is papillary and solid mixed growth (4). Compared with endometrioid carcinoma (EC), patients with CCC have a worse prognosis (5). The 5-year overall survival rate of patients with higher International Federation of Gynecology and Obstetrics (FIGO) stage is below 50% (6).
CCC can occur in different tissues, such as the vagina, uterus, cervix, and ovary (7). Due to the low incidence rate of CCC of the endometrium, data on prognosis-related factors of CCC is still controversial. Therefore, in this study, we retrospectively analyzed the clinicopathological parameters, immunohistochemical analysis results and survival data of 27 CCC patients diagnosed by immunohistochemistry to identify significant prognostic parameters.
Tissue sample collection was approved by the Ethics Committee of the First Affiliated Hospital of Bengbu Medical College and informed consent was obtained from all the patients. A retrospective review of endometrial cancer cases diagnosed from January 2005 to December 2018 was conducted. A total of 222 cases with a diagnosis of endometrial cancer were included, 27 cases with CCC, 45 cases with uterine papillary serous carcinoma (UPSC) and 150 cases with EC, and those cases without histological and cytological confirmation were excluded. Prognostic parameters including, age, tumor size, FIGO stage, myometrial invasion (MI), lymphovascular invasion (LVI), distant metastasis, adjuvant therapy was reviewed to determine vital status and overall survival (OS). Depth of MI was measured from the deepest part of the lesion to the serosa with the naked eye. An expert group comprising of three experienced pathologists who were blinded to the patients’ clinical results, re-evaluated the pathological sections according to the World Health Organization (WHO) criteria of CCC.
A total of 222 specimens were processed using classical histological methods, including 10% buffered formalin fixation, paraffin embedding, HE staining, and immunohistochemical staining. Immunohistochemical analysis was performed using the ElivisionTM Plus detection kit (Lab Vision, USA) according to the manufacturer’s instructions to determine the expression of estrogen receptors (ER), progesterone receptors (PR), P53 protein, Napsin A, and Ki-67. In addition, we performed the immunohistochemical analysis of mismatch repair (MMR) proteins (MSH2, MSH6), p16 protein, PTEN, and HER-2/neu in 27 CCC cases. The antibodies were purchased from Maixin Biotechnology Co., Ltd. (Fuzhou, China). Immunohistochemical staining can clearly distinguish CCC, UPSC and EC.
Upon immunohistochemical staining, the positive expression results showed brownish-yellow granule deposition, while cases without staining were considered negative. The Allred scoring system was used to evaluate ER and PR. Score according to the intensity and degree of staining. The intensity of staining score: 0 – no-staining, 1 – weak, 2 – moderate, 3 – strong. The extent of positive staining was graded as follows: 1, ≤ 1%; 2, 1% - 10%; 3, 10% - 33%; 4, 33% - 66% and 5, 66% - 100%. The score was interpreted as > 2 is positive expression (8). HER2/neu, P16, P53 and Napsin A expression was evaluated as described previously. If <10% of the tumor cells was stained considered as negative expression (9–11). ER, PR and P53 were nuclear expression. HER2/neu was located in membrane. Napsin A was expressed in the cytoplasm. P16 was presented as nucleus and cytoplasm. MMR proteins (MSH2 and MSH6) and PTEN were considered abnormal if loss of nuclear expression. Peripheral lymphocytes and normal endometrium were regarded as positive internal control. Ki-67 was presented as nuclear expression. Immunostaining for Ki-67 was defined as a high expression if > 50% of the tumor cells were stained, but if < 50% stained, this was considered as low expression of Ki-67.
All patients were followed up by telephone calls. OS was defined as the date of surgery to the date of death or last follow-up. The follow-up was completed in December 2020.
SPSS 25.0 (IBM Corp., NY) statistical software was used to perform statistical analysis. Kaplan-Meier method was used to conduct univariate analysis to evaluate the relationship between clinicopathological data and survival rate. The Chi-square test was used for classified variables, while the independent sample t-test was used for continuous variables. P < 0.05 was considered to be statistically significant.
A total of 27 patients diagnosed with CCC, and confirmed by immunohistochemical analysis of ER, PR, P16, Napsin A, P53 and Ki-67. The clinicopathological parameters of the included patients are summarized in Table 1. Typical clinical presentations included abnormal bleeding, pelvic pain, abdominal distension and pain. The most common clinical manifestation was postmenopausal bleeding or dysfunctional uterine bleeding. All the included patients were post-menopausal women except one. According to the FIGO staging system, the proportion of stage I was 18 cases (66.7%), there were 3 cases (11.1%) in stage II, and 6 (22.2%) patients in stage III and IV. Until December 2020, 9 (33.3%) patients died, with an average survival time of 18.4 months. The OS of patients with stage III and IV was significantly shorter than patients with stage I and II (median OS, 26 months compared with 67 months, P < 0.001).
The average age at diagnosis was 60 years (range: 39 - to 81 years), 14 (52%) of the patients were older than 60 years, and the median age was 66 years. There were 14 cases with tumor diameter < 3.5cm and 13 cases with tumor diameter ≥ 3.5cm. The average tumor size was 3.5cm (range: 0.8cm to 13.0cm), and 14 (52%) of the tumors were more than 3.5cm. Myometrial invasion (MI) > 1/2 (extending to the outer half) was reported in 15 (55%) patients, while 9 (33%) patients had MI < 1/2 (inner half involvement), and 3 (11%) patients reported no invasion. Lymphovascular invasion was observed in 26% (7/27) of the cases, and distant metastases were reported in 3 (11.1%) patients.
The CCC of the endometrium can be observed in four morphological structures under a microscope, with the most common being papillary, followed by tubular cystic and solid structures. Cytoplasm clarity intermixed with eosinophilic cells and hobnail cells are the most prominent diagnostic feature of CCC. In this study, the majority of cases were mainly composed of hobnail cells (Figure 1A); a few cases intermixed with other cell types such as cubic cell and flat cell.
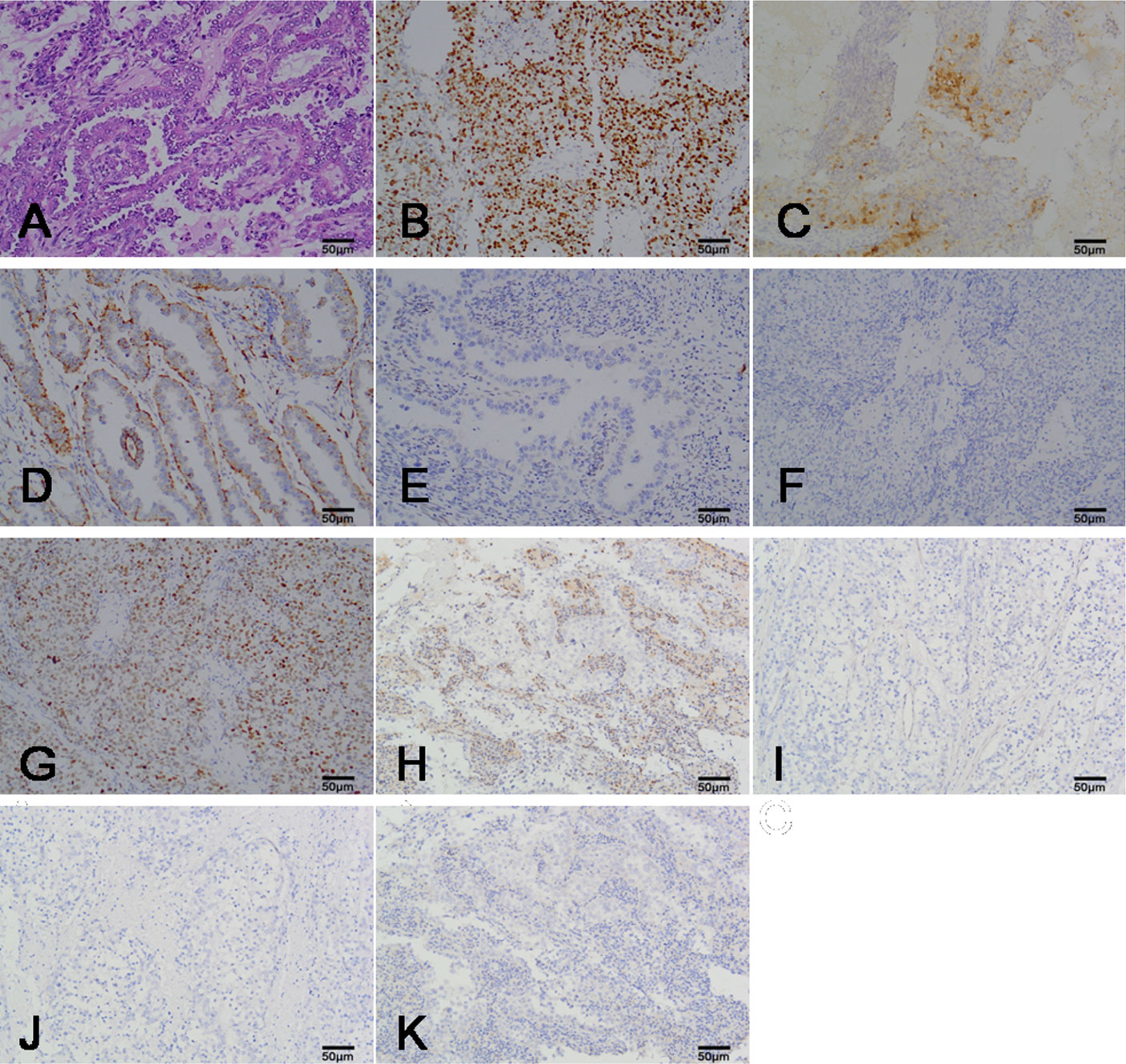
Figure 1 The representation micrographs showing of clear cell carcinoma of the endometrium (hematoxylin eosin stain for A, ×200; immumohistochemical stain, ×200 for B-K). (A) Papillary architecture and hobnail cells. (B) P16 staining. Nuclear and cytoplasm is patchy positive. (C) Ki-67 staining. Nuclear expression is positive. (D) Napsin A staining. Cytoplasm expression is positive. (E) ER staining. Nuclear expression is negative. (F) PR staining. Nuclear expression is negative. (G) P53 staining. Nuclear expression is positive. (H) PTEN staining. Nuclear expression is negative. Peripheral lymphocyte is positive. (I) MSH2 staining. Nuclear expression is negative. (J) MSH6 staining. Nuclear expression is negative. (K) HER-2/nue staining. Membrane expression is negative. Scar bar = 50μm. ER, estrogen receptors; PR, progesterone receptors.
A total of 19 cases had a Ki-67 proliferation index higher than 50% (high expression), while 8 cases had a Ki-67 proliferation index lower than 50% (low expression). Patients with Ki-67 high expression had a shorter survival time compared with those with low Ki-67 proliferation indices (P = 0.026). All cases showed patchy immunostaining for P16. Positive expression of Napsin A was observed in 18 (63.0%) cases and Napsin A positive expression was unrelated to prognosis (P = 0.119). ER, PR is negative expression or focal expression in CCC. The immunohistochemical stain of P16, Ki-67, Napsin A, ER and PR in CCC was showed in Figures 1B–F.
In addition, we performed the immunohistochemical analysis of mismatch repair (MMR) proteins (MSH2, MSH6), P53, PTEN, and HER-2/neu to understand CCC from the perspective of molecular genetics. The immunohistochemistry of the 27 CCC patients are summarized in Table 2. Positive expression of P53 was observed in 17 (63.0%) cases and most of the cases were weakly or moderately positively expressed. Patients with positive expression of P53 had worse outcomes than those with negative expression of P53 (P = 0.007). Losses of MSH2 and MSH6 were seen in 5 and 4 cases, respectively. Loss of both MSH2 and MSH6 was observed in 7 cases (25.9%). PTEN loss was observed in 12 (44.4%) cases. Overexpression of HER2/neu was not found in all 27 cases. The immunohistochemical stain of P53, PTEN, MSH2, MSH2 and HER-2/neu in CCC was showed in Figures 1G–K.
Preoperative pelvic magnetic resonance imaging of the uterus is shown in Figures 2A–C. Magnetic resonance imaging (MRI) showed hypointense or isointense on T1-weighted images, hyperintense on T2-weighted and T1 enhancement images and high signal intensity on diffusion weighted images. The normal postmenopausal endometrium is thin and homogeneous, the uterus with a thicken endometrium, irregular margins and irregular endometrial-myometrial border suggest endometrial carcinoma.
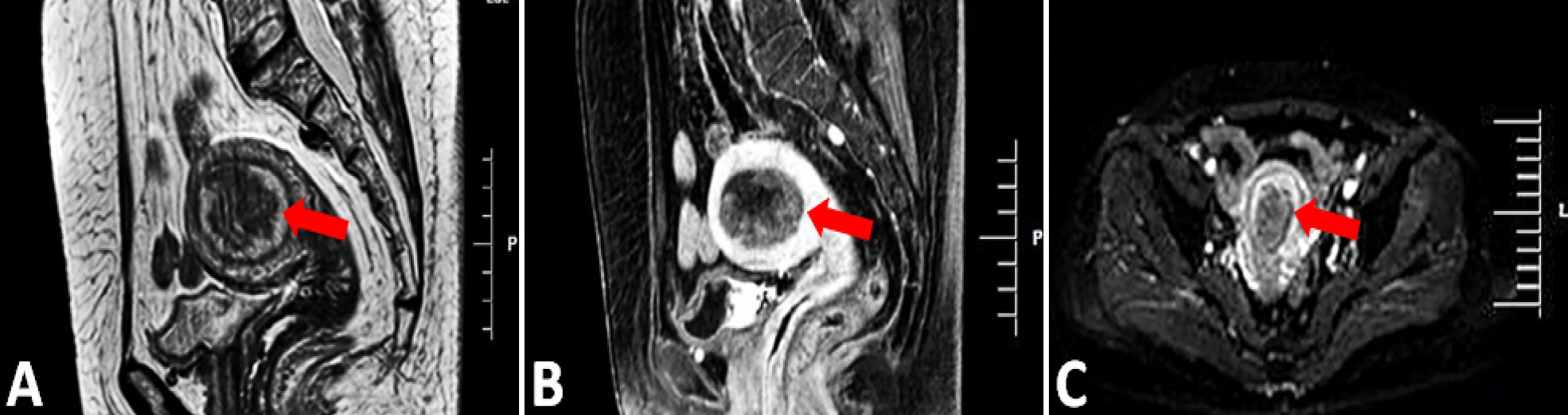
Figure 2 MRI, Magnetic resonance imaging. (A, B) Sagittal T2-weighted and T1 enhancement image showing the 5.2CM*3.5CM mass in the uterine cavity, the abnormal signal affected more than 1/2 of the myometrium (arrow). (C) Axial diffusion weighted image showing hyperintense endometrium (red arrow).
The cohort consisted of 27 patients, all patients were treated with abdominal hysterectomy (TAH) and bilateral salpingo-oophorectomy (BSO), and 22 (82%) patients underwent lymph node dissection. A total of 21 (78%) patients were stage I – II, 9 (42%) patients received radiation theory along, 5 (24%) patients were treated with chemotherapy along [cisplatin + cyclophosphamide + epirubicin (CAP), cisplatin + epirubicin (AP) and carboplatin + paclitaxel (TC). 5 (24%) patients received both radiation and chemotherapy. Besides, 2 (10%) patients received no adjuvant therapy. 6 (22%) patients were stage III - IV, 2 (33.3%) patients were treated with chemotherapy alone, and 4 (67%) patients received both radiation and chemotherapy. Chemotherapy was applied for at least six cycles. The sample size was small, therefore, it was impossible to determine if there was a significant difference between management and prognosis through subgroup analysis (P = 0.180).
In this study, three patients developed tumor recurrence: one developed bone metastasis following systemic treatment and died 3 months following recurrence; two patients developed liver metastasis after hysterectomy and died 5 months and 8 months after recurrence, respectively. No patient developed lung or brain metastasis. Patients with recurrent CCC were treated with systemic chemotherapy supplemented with external beam radiotherapy, and a chemotherapy regimen dominated by carboplatin and paclitaxel.
A total of 27 patients were followed up until December 2020, and none of the patients was lost to follow-up. The patients had a median follow-up of 56 months (range: 5 - 128 months). During the follow-up interval, tumor-related deaths were observed in 9 (33.3%) of the patients, and the survival time was 5 – 32 months. The average survival time was 18.4 months, the median survival time was 24 months, and the 2-year OS rate was 81.5%.
The Kaplan-Meier method was used for univariate analysis of the prognosis-related factors and OS of the patients. Older age, advanced FIGO stage (III - IV), big tumor size, deep MI, high Ki-67 index, positive expression of P53, lymphovascular invasion, and distant metastasis of the patients were significantly associated with shorter OS ((P = 0.048, P < 0.001, P = 0.016, P = 0.043, P = 0.026, P = 0.007, P = 0.001, and P < 0.001 respectively). The positive expression of Napsin A (P = 0.119), adjuvant treatment (P = 0.180), loss of MSH2 (P = 0.472), MSH6 (P = 0.524) and PTEN (P = 0.472) were not statistically significant in this study. Figure 3 and Table 3 shows the clinicopathologic features and univariate analysis of prognostic parameters in CCC, and the survival curves are shown in Figures 4A–M.
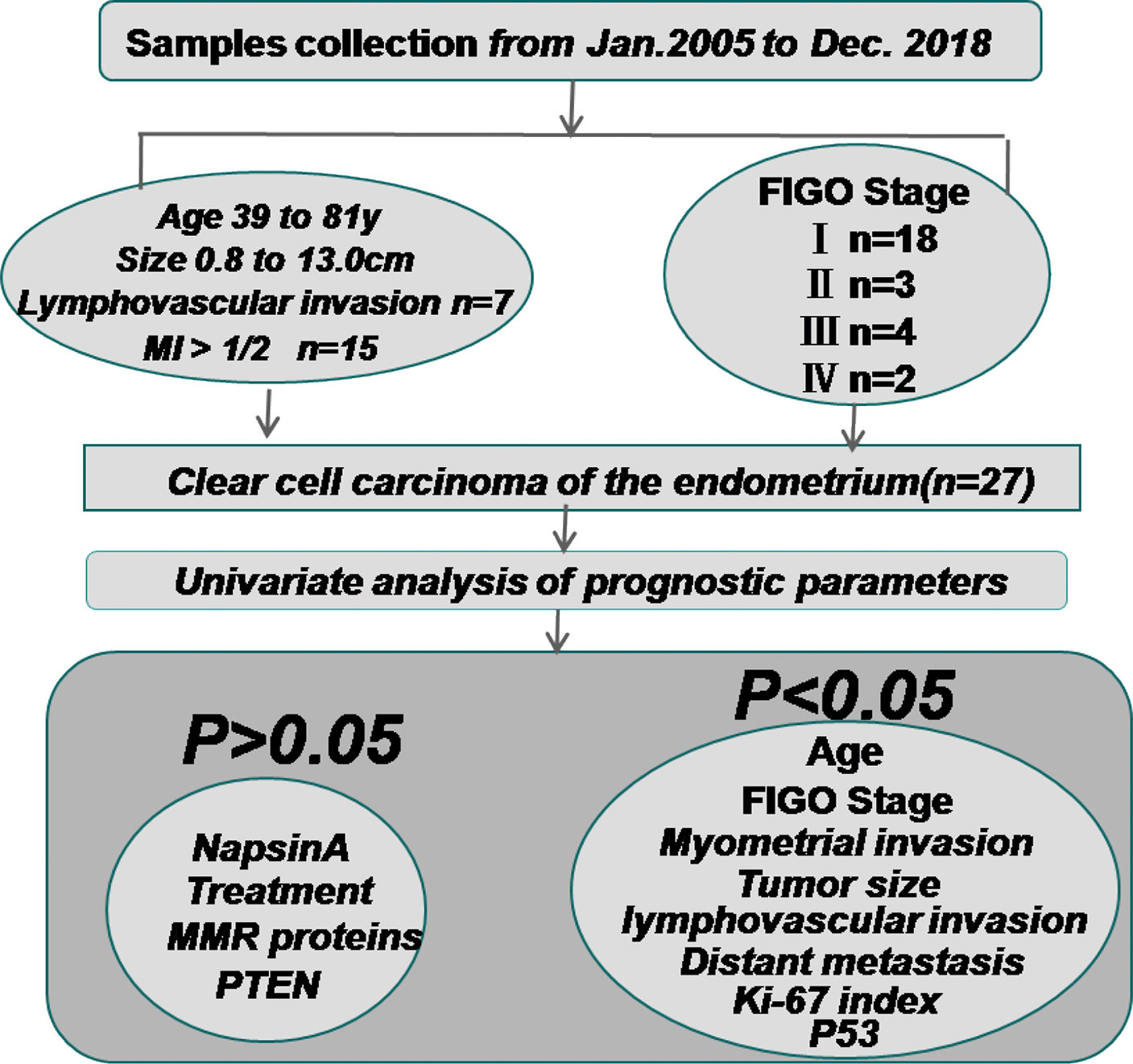
Figure 3 The clinicopathologic features and univariate analysis of prognostic parameters in clear cell carcinoma of the endometrium. Sample collection was approved by the Ethics Committee of the First Affiliated Hospital of Bengbu Medical College from January 2005 to December 2018. The patients’ FIGO (2009) surgical stages were as follows: 18 I, 3 II, 4 III, and 2 IV. The average age at diagnosis was 60 years (range: 39 - to 81 years). 7 patients had lymphovascular invasion and 15 patients had MI > 1/2. The Kaplan-Meier method was used for univariate analysis of the prognosis-related factors and OS. Age, FIGO stage, tumor size, MI, Ki-67 index, positive expression of P53, lymphovascular invasion, and distant metastasis of the patients were significantly associated with shorter OS ((P = 0.048, P < 0.001, P = 0.016, P = 0.043, P = 0.026, P = 0.007, P = 0.001, and P < 0.001 respectively). The positive expression of Napsin A (P = 0.119), adjuvant treatment (P = 0.180), loss of MSH2 (P = 0.472), MSH6 (P = 0.524) and PTEN (P = 0.472) were not statistically associated with OS. MI, myometrial invasion; MMR proteins, mismatch repair proteins.
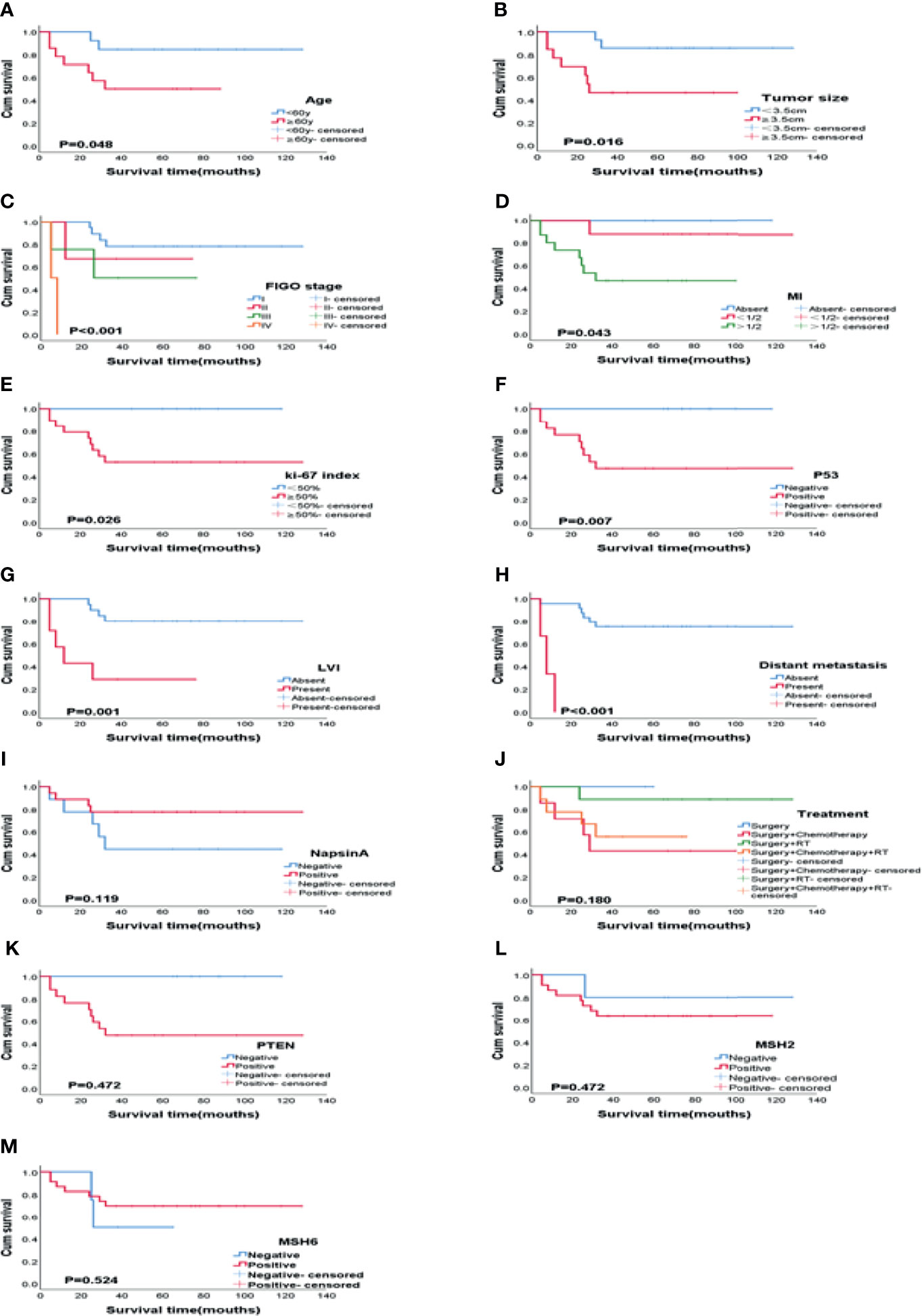
Figure 4 Kaplan-Meier plots of OS according to variables on univariate analysis. (A) Age (P = 0.048), (B) Tumor size (P = 0.016), (C) FIGO stage (P < 0.001), (D) MI (P = 0.043), (E) Ki-67 index (P = 0.026), (F) P53 (P = 0.007), (G) LVI (P = 0.001), (H) Distant metastasis (P < 0.001), (I) Napsin A (P = 0.119), (J) Treatment (P = 0.180), (K) PTEN (P = 0.472), (L) MSH2 (P = 0.472), (M) MSH6 (P = 0.524). MI, myometrial invasion; LVI, Lymphovascular invasion.
A total of 45 patients were diagnosed with uterine papillary serous carcinoma (UPSC), and the average age at diagnosis was 60 years (range: 44 - 76 years). A negative expression of ER was reported in 3 UPSC patients, while 38 patients showed P53 positive. Lymphovascular invasion was observed in 20 patients. There were 6 patients with distant metastasis, the common sites of metastasis were abdominal para-aortic lymph nodes, lung, liver and bone. Patients had an average survival time of 60 months. There was no difference in age, MI, treatment, LVI, distant metastasis, and OS between CCC and UPSC (P > 0.05). There was a significant difference in the expression of ER and P53 between CCC and UPSC (P < 0.001 and P = 0.038; Table 4). To detect ER/PR, P53 and Napsin A expression is important for the diagnosis and differentiation of UPSC and CCC. In USPC, P53 has a diffuse immunoreactivity.

Table 4 Comparative of clear cell carcinoma (CCC) of the endometrium, uterine papillary serous carcinoma (UPSC) and endometrioid carcinoma (EC).
In our cohort, we eliminated lost follow-up patients and selected 150 patients diagnosed with endometrioid carcinoma (EC) with an average age of 56 years (31 - 79 years). 65% (98/150) cases have myometrial invasion inner half involvement. The proportion of ER positive expression was higher 92% (138/150), P53 positive expression was lower 31% (47/150). There were 11 of cases with LVI and 9 of cases with distant metastasis. Patients with an average survival time of 63 months. Compared with CCC, age, treatment, distant metastasis between the two groups had no significance (P > 0.05). There was significance in myometrial invasion, the expression of ER and P53, LVI, and survival time between CCC and EC (P = 0.039, P < 0.001, P = 0.002, P = 0.003, P = 0.002 respectively; Table 4). The diagnosis of CCC and its distinction from UPSC and EC is outlined in Figure 5
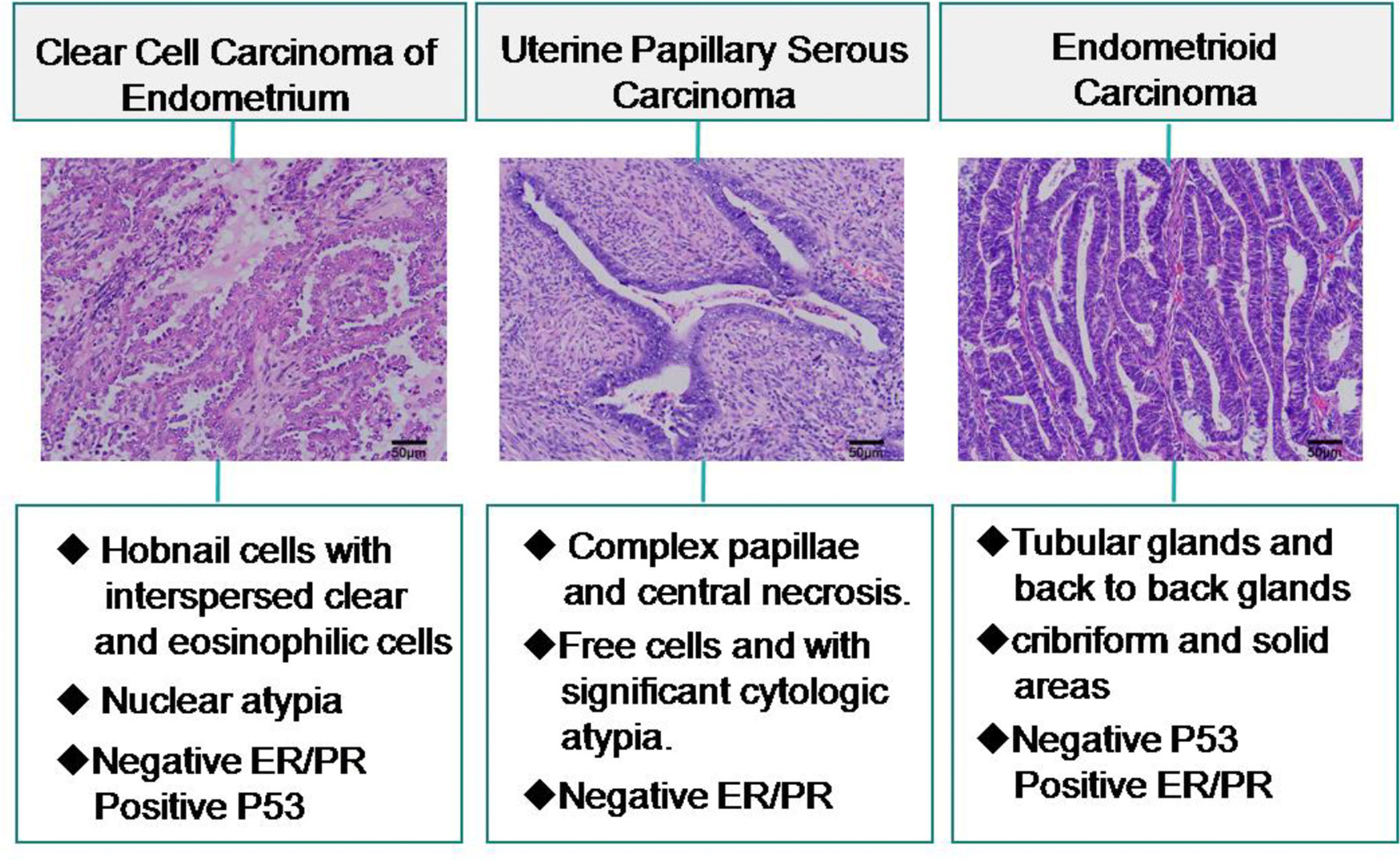
Figure 5 Differential diagnosis of clear cell carcinoma of the endometrium and relevant histopathologic features.
Staging of endometrial cancer follows the surgical and pathological staging adopted by the FIGO, even though it is only based on the depth of myometrial invasion and does not take into account the histological type, tumor grade, tumor size, and other relevant factors affecting prognosis. In recent years, new perspectives based on histology, immunohistochemistry, molecular genetics, prognosis-related factors, and the effect of different management practices on prognosis have emerged. In 2018, Maheshwari performed surgical/pathological staging of endometrial cancer based on important prognosis-related factors (12). He proposed that type II non-endometrioid carcinomas, including clear cell endometrial carcinoma, should be classified as “high level” stage (12). However, to date, there is no consensus on this view.
WHO classifies endometrial cancer into two categories: Type I also known as a classical pathway, occurs under the stimulation of estrogen, with atypical hyperplasia of the endometrium as a precursor, and endometrioid carcinoma is the most common histological type. Type II account for 10%-21% of all endometrial cancers, it is independent of estrogen stimulation, and usually present in older people with endometrial atrophy (13–15). Type II tumors include UPSC, CCC, and undifferentiated carcinoma. CCC is more aggressive and has a worse prognosis compared to other types of endometrial cancer (5, 16, 17). Therefore, our control group comprised UPSC and EC patients. We found that there was no significance in survival time between CCC and UPSC (P > 0.05). Compared to EC, the survival time in CCC was significantly shorter (P = 0.002).
In our cohort, a total of 27 CCC patients performed survival analysis. 14 (52%) of the patients were older than average age at diagnosis (60y), 14 (52%) of the patients were more than average tumor size (3.5cm). 15 (55%) of the patients were MI > 1/2. Older age, big tumor size, deep MI significantly associated with poor prognosis (P = 0.048, P = 0.016, and P = 0.043 respectively). The OS was significantly shorter for CCC patients with stage III and IV than for those with stage I and II (P < 0.001). 7 (26%) of the patients had LVI, and 3 (11.1%) of the patients had distant metastasis. LVI (P = 0.001) and distant metastasis (P < 0.001) were prognostic factors for the OS of CCC patients. 3 (11.1%) patients developed tumor recurrence and the most common metastatic site was liver. These results suggest that CCC have invasive potential.
In this study, the typical clinical presentation of patients with CCC was postmenopausal bleeding before diagnosis. The diagnosis of CCC can be achieved through clinical manifestation, preoperative diagnostic curettage, and endometrial biopsy, as well as in other types of endometrial carcinoma. Endometrial biopsy is a sensitive and accurate method used to evaluate abnormal bleeding (18). Pap smear is not a reliable diagnostic method for endometrioid carcinoma, but it appears that diagnosis can be made following an abnormal smear in CCC patients (19). However, the final diagnosis should rely on examination of histopathological sections. Delaying the diagnosis can have serious consequences, and the 5-year survival rate significantly decreases as the disease progresses (20).
Transvaginal ultrasonography is the first choice for patients with abnormal postmenopausal bleeding (21). Preoperative imaging examination is crucial for evaluating the depth of myometrial invasion and the presence of adnexal and distant metastasis. Magnetic resonance imaging (MRI) and positron emission tomography (PET)/computer tomography (CT) are used in advanced-stage patients with lymphovascular invasion and distant metastasis.
Taking into consideration the rarity of CCC, diagnosis remains a challenge. In this study, the immunohistochemical performance for Napsin A was high, low for p53, a high Ki-67 index, and absence or focal nuclear expression of ER and PR. This is one of the reasons why hormone therapy is not routinely used for CCC treatment (22). Napsin A was higher frequent expression in CCC (67% – 93%) and lower frequent expression in UPSC (8% – 22%) and EC (0 –10%) (23, 24). In EC, ER/PR is positive, while in CCC ER and PR showed negative expression or focal nuclear expression. In UPSC, P53 has a diffuse immunoreactivity, while in CCC P53 showed weakly or moderately positive expression (22, 25). The immunohistochemical features is consistent with our research. These studies contribute to a deeper understanding to distinguish CCC from other subtypes of endometrial cancer.
A previous study suggested that high Ki-67 indices were related to increased tumor proliferation, poor prognosis (26), and decreased survival time. Positive expression of P53 is associated with unfavorable outcomes in CCC (22), and this was confirmed in the present research. The expression of E-cadherin in CCC is significantly lower than that in endometrioid carcinoma, which demonstrates that reduced cohesion of tumor cells is responsible for the more aggressive behavior of CCC (27). Napsin A is located in the cytoplasm and unrelated to patient age, pathological subtype, FIGO stage, degree of infiltration, lymphovascular invasion and OS (28). In our research, the OS was shorter for CCC patients with high Ki-67 index than for those with low Ki-67 index (P = 0.026), positive expression of P53 significantly associated with poor prognosis (P = 0.007), and Napsin A positive expression was unrelated to prognosis (P = 0.119), which is consistent with previous studies.
In the 2013, the Global Cancer Genome (TCGA) study, divided endometrial cancer into four types based on histomorphology and molecular genetics, including polar hyper-mutation, microsatellite instability, low copy number, and high copy number types (29). TCGA emphasizes the importance of classification in prognosis to provide better-individualized treatment. To develop more efficacious molecular targeted therapies, there is an urgent need to determine the molecular characteristics of endometrial cancer.
The molecular features of CCC were not been analyzed by TCGA. Therefore, the molecular characteristics of CCC remain not clearly explored compared to EC and UPSC. Therefore, we performed the immunohistochemical analysis of mismatch repair (MMR) proteins (MSH2, MSH6), P53, PTEN and HER-2 to understand CCC from the perspective of molecular genetics.
Based on molecular profile analysis, the most frequently mutated gene is TP53, followed by KRAS and PIk3CA (30–32). However, in the majority of uterine clear cell carcinoma, there is an absence of mutations in the P53 (13). Positive P53 immunohistochemical staining can indicate P53 gene mutation. Soyoun et al. have reported that P53-mutated was observed in to 18 cases (35%) and P53 wild-type was observed in to 28 cases (54%) (33). Deborah et al. have reported that 11 (34%) cases displayed P53-mutated (31). In this study, approximately 2/3 of CCC patients showed a mutated P53 immunostaining pattern and P53-mutated cases had a shorter survival time compared to P53 wild-type cases.
HER2/neu, also named asc-erbB2. HyoSookBae et al. have reported that overexpression of HER2/neu was observed in only to 2 cases (12.5%), but the amplification of HER2/neu gene was not detected by situ hybridization (FISH) in all 16 CCC cases (10). They also discovered that PTEN loss was seen in 81.3% of 16 CCC cases but the LOH of PTEN was only 6.3% (10). Lien et al. reported that PTEN mutations were not detected in 14 CCC cases (34). 6 (19%) cases showed abnormal expression of MMR proteins and 11% ERBB2 amplifications was detected in 32 CCC cases (31). Also have immunohistochemistry analysis revealed 15 (33.3%) cases loss of MMR proteins in 45 CCC cases (4). In immunohistochemical staining, overexpression of HER2/neu was not found in of our cases, negative expression was found in all 27 cases in our study. Loss of MMR proteins (MSH2 and MSH6) were observed in 7 cases (25.9%). 12 (44.4%) cases showed PTEN loss. MSH2, MSH6 and PTEN have no statistical significance with OS (P = 0.472, P = 0.524 and P = 0.472, respectively) in our study.
Currently, total abdominal hysterectomy and bilateral salpingo-oophorectomy have been established as first-line treatment. Systematic pelvic and para-aortic lymphadenectomy are reported to improve patients prognoses (35). Pre-and/or postoperative chemotherapy and/or radiation have been widely employed. Patients diagnosed with CCC are administered with adriamycin, cisplatinum, and paclitaxel either in a double or triple combination. The triple combination, however, is demonstrated to cause neurologic and hematologic toxicities (1, 36). Radiation therapy is primarily administered in the postoperative adjuvant setting (37), depending on the risk of recurrence and patient-related factors. To explore the relationship between adjuvant therapy and prognosis, a multi-institutional retrospective study reported that brachytherapy was beneficial for survival in stages I – II patients, while chemotherapy was significant for stage III patients (38). On the contrary, Abdulfatah reported that adjuvant radiotherapy alone had a significant impact on a patient’s prognosis (P = 0.012), while the prognosis of patients receiving chemotherapy alone or combined with radiotherapy showed no significant improvement on OS (P=0.202, P=0.229, respectively) (39). External beam radiation (EBRT) or vaginal brachytherapy (VBT) can reduce vaginal stump recurrence, and EBRT is recommended for patients who are not eligible for chemotherapy (40). Some institutional studies have shown that both adjuvant chemotherapy and radiotherapy are found to be beneficial in CCC and UPSC patients (41, 42), while other studies found that the patients treated with chemotherapy had no added benefit (43, 44). There are no universally accepted guidelines for patient’s management (39).
There remain limitations in this article. Firstly, our results show that any adjuvant therapy cannot effectively improve the prognosis of CCC. It is great possibility that this is the single agency study with small sample size, and has the limitation of data accessibility. Secondly, the radiotherapy or chemotherapy regimens for patients of CCC were different. We did not collect the specific radiotherapy regimens and not clear about whether pelvic radiotherapy or vaginal brachytherapy is related to the prognosis of patients. Similarly, patients of CCC receive different chemotherapy cycles. We have made an immunohistochemical analysis of CCC instead of whole exome sequencing, which cannot identify the degree of genes mutation. In our cohort, there was no significant difference in survival outcomes between UPSC and CCC. Therefore, further stratified analyses with larger populations by combination with other medical units are required.
Considering the rarity of CCC, prospective retrospective studies are difficult to perform. Therefore, this retrospective study is valuable, as a representative sample of Eastern China, to make up for deficiencies in clinical parameters, pathological variables, immunohistochemical characteristics and survival data. We discussed 10 markers and evaluated molecular characteristics of CCC. PTEN, MSH2, MSH6 and HER-2 are not specific and sensitive antibodies for detecting CCC, and have no statistical significance with prognosis. This study demonstrates that age at diagnosis, FIGO stage, MI, tumor size, high Ki-67 index, positive expression of P53, lymphovascular invasion and distant metastasis are significantly associated with OS.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by First Affiliated Hospital of Bengbu Medical University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
DC studied concept and design; ZZ, ZB, PG, LZ acquired data. ZZ, ZB and JY analyzed data. ZZ drafted the manuscript. TL revises the manuscript. ZZ, PG provided acquisition, analysis and interpretation of data, and statistical analysis. All authors read and approved the final manuscript.
This work was supported in part by the Nature Science Major and Key Program of College and University of Anhui Province (No.KJ2020A0559), the Natural Science Research Program of Education Bureau of Anhui Province (NO. gxyq2017032), the Nature Science Key Program of College and University of Anhui Province (NO.KJ2016A460).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank Home for Researchers editorial team (www.home-for-researchers.com) for English Language Editing Service.
1. Gadducci A, Cosio S, Spirito N, Cionini L. Clear Cell Carcinoma of the Endometrium: A Biological and Clinical Enigma. Anticancer Res (2010) 30(4):1327–34.
2. Khalique S, Lord CJ, Banerjee S, Natrajan R. Translational Genomics of Ovarian Clear Cell Carcinoma. Semin Cancer Biol (2020) 61:121–31. doi: 10.1016/j.semcancer.2019.10.025
3. Le Saux O, Ray-Coquard I, Labidi-Galy SI. Challenges for Immunotherapy for the Treatment of Platinum Resistant Ovarian Cancer. Semin Cancer Biol (2020). doi: 10.1016/j.semcancer.2020.08.017
4. Zannoni GF, Santoro A, Angelico G, Spadola S, Arciuolo D, Valente M, et al. Clear Cell Carcinoma of the Endometrium: An Immunohistochemical and Molecular Analysis of 45 Cases. Hum Pathol (2019) 92:10–7. doi: 10.1016/j.humpath.2019.06.005
5. Cirisano FD, Robboy SJ, Dodge RK, Bentley RC, Krigman HR, Synan IS, et al. The Outcome of Stage I–II Clinically and Surgically Staged Papillary Serous and Clear Cell Endometrial Cancers When Compared With Endometrioid Carcinoma. Gynecol Oncol (2000) 77(1):55–65. doi: 10.1006/gyno.2000.5737
6. Xu Y, Hanna RK, Elshaikh MA. Adjuvant Therapy of Uterine Clear Cell Carcinoma: A Review. Arch Gynecol Obstet (2016) 293(3):485–92. doi: 10.1007/s00404-015-3973-x
7. Ju B, Wang J, Yang B, Sun L, Guo Y, Hao Q, et al. Morphologic and Immunohistochemical Study of Clear Cell Carcinoma of the Uterine Endometrium and Cervix in Comparison to Ovarian Clear Cell Carcinoma. Int J Gynecol Pathol (2018) 37(4):388–96. doi: 10.1097/PGP.0000000000000430
8. Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen Receptor Status by Immunohistochemistry is Superior to the Ligand-Binding Assay for Predicting Response to Adjuvant Endocrine Therapy in Breast Cancer. J Clin Oncol (1999) 17(5):1474–81. doi: 10.1200/JCO.1999.17.5.1474
9. Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer. J Clin Oncol (2007) 25(1):118–45. doi: 10.1200/JCO.2006.09.2775
10. Bae HS, Kim H, Young Kwon S, Kim KR, Song JY, Kim I. Should Endometrial Clear Cell Carcinoma be Classified as Type II Endometrial Carcinoma? Int J Gynecol Pathol (2015) 34(1):74–84. doi: 10.1097/PGP.0000000000000111
11. Murali R, Davidson B, Fadare O, Carlson JA, Crum CP, Gilks CB, et al. High-Grade Endometrial Carcinomas: Morphologic and Immunohistochemical Features, Diagnostic Challenges and Recommendations. Int J Gynecol Pathol (2019) 38 Suppl 1:S40–63. doi: 10.1097/PGP.0000000000000491
12. Maheshwari A, Gupta S, Prat J. A Proposal for Updating the Staging of Endometrial Cancer. Int J Gynaecol Obstet (2019) 145(2):245–52. doi: 10.1002/ijgo.12789
13. An HJ, Logani S, Isacson C, Ellenson LH. Molecular Characterization of Uterine Clear Cell Carcinoma. Mod Pathol (2004) 17(5):530–7. doi: 10.1038/modpathol.3800057
14. Bokhman JV. Two Pathogenetic Types of Endometrial Carcinoma. Gynecol Oncol (1983) 15(1):10–7. doi: 10.1016/0090-8258(83)90111-7
15. Colomer R, Mondejar R, Romero-Laorden N, Alfranca A, Sanchez-Madrid F, Quintela-Fandino M. When Should We Order a Next Generation Sequencing Test in a Patient With Cancer? EClinicalMedicine (2020) 25:100487. doi: 10.1016/j.eclinm.2020.100487
16. Wahid M, Dar SA, Jawed A, Mandal RK, Akhter N, Khan S, et al. Microbes in Gynecologic Cancers: Causes or Consequences and Therapeutic Potential. Semin Cancer Biol (2021). doi: 10.1016/j.semcancer.2021.07.013
17. Oien DB, Pathoulas CL, Ray U, Thirusangu P, Kalogera E, Shridhar V. Repurposing Quinacrine for Treatment-Refractory Cancer. Semin Cancer Biol (2021) 68:21–30. doi: 10.1016/j.semcancer.2019.09.021
18. Huang GS, Gebb JS, Einstein MH, Shahabi S, Novetsky AP, Goldberg GL. Accuracy of Preoperative Endometrial Sampling for the Detection of High-Grade Endometrial Tumors. Am J Obstet Gynecol (2007) 196(3):243.e1–5. doi: 10.1016/j.ajog.2006.09.035
19. Gu M, Shi W, Barakat RR, Thaler HT, Saigo PE. Pap Smears in Women With Endometrial Carcinoma. Acta Cytol (2001) 45(4):555–60. doi: 10.1159/000327864
20. Wilczynski M, Danielska J, Wilczynski J. An Update of the Classical Bokhman’s Dualistic Model of Endometrial Cancer. Prz Menopauzalny (2016) 15(2):63–8. doi: 10.5114/pm.2016.61186
21. Bartosch C, Pires M, Jeronimo C, Lopes JM. The Role of Pathology in the Management of Patients With Endometrial Carcinoma. Future Oncol (2017) 13(11):1003–20. doi: 10.2217/fon-2016-0570
22. Arai T, Watanabe J, Kawaguchi M, Kamata Y, Nishimura Y, Jobo T, et al. Clear Cell Adenocarcinoma of the Endometrium Is a Biologically Distinct Entity From Endometrioid Adenocarcinoma. Int J Gynecol Cancer (2006) 16(1):391–5. doi: 10.1111/j.1525-1438.2006.00494.x
23. Hariri N, Qarmali M, Fadare O. Endometrial Serous Carcinoma With Clear-Cell Change: Frequency and Immunohistochemical Analysis. Int J Surg Pathol (2018) 26(2):126–34. doi: 10.1177/1066896917731862
24. Al-Maghrabi JA, Butt NS, Anfinan N, Sait K, Sait H, Marzouki A, et al. Infrequent Immunohistochemical Expression of Napsin A in Endometrial Carcinomas. Appl Immunohistochem Mol Morphol (2017) 25(9):632–8. doi: 10.1097/PAI.0000000000000350
25. Lax SF, Pizer ES, Ronnett BM, Kurman RJ. Clear Cell Carcinoma of the Endometrium is Characterized by a Distinctive Profile of P53, Ki-67, Estrogen, and Progesterone Receptor Expression. Hum Pathol (1998) 29(6):551–8. doi: 10.1016/s0046-8177(98)80002-6
26. Visser NC, Bulten J, van der Wurff AA, Boss EA, Bronkhorst CM, Feijen HW, et al. PIpelle Prospective ENDOmetrial Carcinoma (PIPENDO) Study, Pre-Operative Recognition of High Risk Endometrial Carcinoma: A Multicentre Prospective Cohort Study. BMC Cancer (2015) 15:487. doi: 10.1186/s12885-015-1487-3
27. Holcomb K, Delatorre R, Pedemonte B, McLeod C, Anderson L. Chambers J. E-Cadherin Expression in Endometrioid, Papillary Serous, and Clear Cell Carcinoma of the Endometrium. Obstet Gynecol (2002) 100(6):1290–5. doi: 10.1016/s0029-7844(02)02391-8
28. Fadare O, Desouki MM, Gwin K, Hanley KZ, Jarboe EA, Liang SX, et al. Frequent Expression of Napsin A in Clear Cell Carcinoma of the Endometrium: Potential Diagnostic Utility. Am J Surg Pathol (2014) 38(2):189–96. doi: 10.1097/PAS.0000000000000085
29. Cancer Genome Atlas Research N, Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, et al. Integrated Genomic Characterization of Endometrial Carcinoma. Nature (2013) 497(7447):67–73. doi: 10.1038/nature12113
30. Le Gallo M, Rudd ML, Urick ME, Hansen NF, Zhang S, Program NCS, et al. Somatic Mutation Profiles of Clear Cell Endometrial Tumors Revealed by Whole Exome and Targeted Gene Sequencing. Cancer (2017) 123(17):3261–8. doi: 10.1002/cncr.30745
31. DeLair DF, Burke KA, Selenica P, Lim RS, Scott SN, Middha S, et al. The Genetic Landscape of Endometrial Clear Cell Carcinomas. J Pathol (2017) 243(2):230–41. doi: 10.1002/path.4947
32. Baniak N, Fadare O, Kobel M, DeCoteau J, Parkash V, Hecht JL, et al. Targeted Molecular and Immunohistochemical Analyses of Endometrial Clear Cell Carcinoma Show That POLE Mutations and DNA Mismatch Repair Protein Deficiencies Are Uncommon. Am J Surg Pathol (2019) 43(4):531–7. doi: 10.1097/PAS.0000000000001209
33. Kim SR, Cloutier BT, Leung S, Cochrane D, Britton H, Pina A, et al. Molecular Subtypes of Clear Cell Carcinoma of the Endometrium: Opportunities for Prognostic and Predictive Stratification. Gynecol Oncol (2020) 158(1):3–11. doi: 10.1016/j.ygyno.2020.04.043
34. Hoang LN, McConechy MK, Meng B, McIntyre JB, Ewanowich C, Gilks CB, et al. Targeted Mutation Analysis of Endometrial Clear Cell Carcinoma. Histopathology (2015) 66(5):664–74. doi: 10.1111/his.12581
35. Todo Y, Kato H, Kaneuchi M, Watari H, Takeda M, Sakuragi N. Survival Effect of Para-Aortic Lymphadenectomy in Endometrial Cancer (SEPAL Study): A Retrospective Cohort Analysis. Lancet (2010) 375(9721):1165–72. doi: 10.1016/s0140-6736(09)62002-x
36. Fleming GF, Filiaci VL, Bentley RC, Herzog T, Sorosky J, Vaccarello L, et al. Phase III Randomized Trial of Doxorubicin + Cisplatin Versus Doxorubicin + 24-H Paclitaxel + Filgrastim in Endometrial Carcinoma: A Gynecologic Oncology Group Study. Ann Oncol (2004) 15(8):1173–8. doi: 10.1093/annonc/mdh316
37. Fleming GF, Brunetto VL, Cella D, Look KY, Reid GC, Munkarah AR, et al. Phase III Trial of Doxorubicin Plus Cisplatin With or Without Paclitaxel Plus Filgrastim in Advanced Endometrial Carcinoma: A Gynecologic Oncology Group Study. J Clin Oncol (2004) 22(11):2159–66. doi: 10.1200/jco.2004.07.184
38. Olawaiye AB, Boruta DM. 2nd. Management of Women With Clear Cell Endometrial Cancer: A Society of Gynecologic Oncology (SGO) Review. Gynecol Oncol (2009) 113(2):277–83. doi: 10.1016/j.ygyno.2009.02.003
39. Abdulfatah E, Sakr S, Thomas S, Al-Wahab Z, Mutch DG, Dowdy S, et al. Clear Cell Carcinoma of the Endometrium: Evaluation of Prognostic Parameters in a Multi-Institutional Cohort of 165 Cases. Int J Gynecol Cancer (2017) 27(8):1714–21. doi: 10.1097/IGC.0000000000001050
40. Xiang M, English DP, Kidd EA. National Patterns of Care and Cancer-Specific Outcomes of Adjuvant Treatment in Patients With Serous and Clear Cell Endometrial Carcinoma. Gynecol Oncol (2019) 152(3):599–604. doi: 10.1016/j.ygyno.2018.12.007
41. Velker V, D’Souza D, Prefontaine M, McGee J, Leung E. Role of Adjuvant Therapy for Stage IA Serous and Clear Cell Uterine Cancer: Is Observation a Valid Strategy? Int J Gynecol Cancer (2016) 26(3):491–6. doi: 10.1097/IGC.0000000000000643
42. Rodrigues da Cunha Colombo Bonadio R, Gondim Meira Velame Azevedo R, Harada G, Cabral Severino da Costa S, Costa Miranda V, de Freitas D, et al. Adjuvant Carboplatin and Paclitaxel Chemotherapy Followed by Radiotherapy in High-Risk Endometrial Cancer: A Retrospective Analysis. J Glob Oncol (2018) 4:1–8. doi: 10.1200/JGO.17.00146
43. Randall ME, Filiaci V, McMeekin DS, von Gruenigen V, Huang H, Yashar CM, et al. Phase III Trial: Adjuvant Pelvic Radiation Therapy Versus Vaginal Brachytherapy Plus Paclitaxel/Carboplatin in High-Intermediate and High-Risk Early Stage Endometrial Cancer. J Clin Oncol (2019) 37(21):1810–8. doi: 10.1200/JCO.18.01575
44. de Boer SM, Powell ME, Mileshkin L, Katsaros D, Bessette P, Haie-Meder C, et al. Adjuvant Chemoradiotherapy Versus Radiotherapy Alone in Women With High-Risk Endometrial Cancer (PORTEC-3): Patterns of Recurrence and Post-hoc Survival Anal Randomised Phase 3 Trial. Lancet Oncol (2019) 20(9):1273–85. doi: 10.1016/s1470-2045(19)30395-x
Keywords: clear cell endometrial carcinoma, clinicopathology, prognosis, overall survival, clinical study
Citation: Zhang Z, Gao P, Bao Z, Zeng L, Yao J, Chai D and Li T (2021) Clear Cell Carcinoma of the Endometrium: Evaluation of Prognostic Parameters in 27 Cases. Front. Oncol. 11:732782. doi: 10.3389/fonc.2021.732782
Received: 29 June 2021; Accepted: 12 November 2021;
Published: 02 December 2021.
Edited by:
Hanqing Liu, Jiangsu University, ChinaReviewed by:
Sarah Adel Hakim, Ain Shams University, EgyptCopyright © 2021 Zhang, Gao, Bao, Zeng, Yao, Chai and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Damin Chai, Y2hhaWRtMjAyMEAxNjMuY29t; Tian Li, Zm1tdWx0QGZveG1haWwuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.