
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 24 September 2021
Sec. Surgical Oncology
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.731336
This article is part of the Research Topic Virtual Surgical Planning and 3D Printing in Head and Neck Tumor Resection and Reconstruction View all 27 articles
 Lucas M. Ritschl1*
Lucas M. Ritschl1* Paul Kilbertus1
Paul Kilbertus1 Florian D. Grill1
Florian D. Grill1 Matthias Schwarz1
Matthias Schwarz1 Jochen Weitz1,2
Jochen Weitz1,2 Markus Nieberler1
Markus Nieberler1 Klaus-Dietrich Wolff1
Klaus-Dietrich Wolff1 Andreas M. Fichter1
Andreas M. Fichter1Background: Mandibular reconstruction is conventionally performed freehand, CAD/CAM-assisted, or by using partially adjustable resection aids. CAD/CAM-assisted reconstructions are usually done in cooperation with osteosynthesis manufacturers, which entails additional costs and longer lead time. The purpose of this study is to analyze an in-house, open-source software-based solution for virtual planning.
Methods and Materials: All consecutive cases between January 2019 and April 2021 that underwent in-house, software-based (Blender) mandibular reconstruction with a free fibula flap (FFF) were included in this cross-sectional study. The pre- and postoperative Digital Imaging and Com munications in Medicine (DICOM) data were converted to standard tessellation language (STL) files. In addition to documenting general information (sex, age, indication for surgery, extent of resection, number of segments, duration of surgery, and ischemia time), conventional measurements and three-dimensional analysis methods (root mean square error [RMSE], mean surface distance [MSD], and Hausdorff distance [HD]) were used.
Results: Twenty consecutive cases were enrolled. Three-dimensional analysis of preoperative and virtually planned neomandibula models was associated with a median RMSE of 1.4 (0.4–7.2), MSD of 0.3 (-0.1–2.9), and HD of 0.7 (0.1–3.1). Three-dimensional comparison of preoperative and postoperative models showed a median RMSE of 2.2 (1.5–11.1), MSD of 0.5 (-0.6–6.1), and HD of 1.5 (1.1–6.5) and the differences were significantly different for RMSE (p < 0.001) and HD (p < 0.001). The difference was not significantly different for MSD (p = 0.554). Three-dimensional analysis of virtual and postoperative models had a median RMSE of 2.3 (1.3–10.7), MSD of -0.1 (-1.0–5.6), and HD of 1.7 (0.1–5.9).
Conclusions: Open-source software-based in-house planning is a feasible, inexpensive, and fast method that enables accurate reconstructions. Additionally, it is excellent for teaching purposes.
The application of computer-aided design and computer-aided manufacturing (CAD/CAM) technology in primary and secondary mandibular reconstruction with the free fibula flap (FFF) following ablative surgery is considered to be state of the art nowadays. Several studies have proven its benefits and superiority in terms of operating time, ischemic time, symmetry, bony consolidation, and function (1–4), which may result in a positive cost–benefit balance sheet (5, 6) as well as better functional and aesthetic results (7–9).
After increasing standardization of the surgical processes and the integration of virtual planning processes in the last decade, the trend continues toward cost reduction, as patient-specific cutting guides and osteosynthesis plates are usually offered and produced by various osteosynthesis manufacturers and may even not have led to overall cost reduction (10). On the one hand, this necessitates a functioning infrastructure with nationwide coverage by these companies, and on the other hand, the effort expended entails additional costs for the surgical department and ultimately the healthcare system (10). In addition, the dependence on the industry reduces flexibility of planning timing and, depending on the complexity of the case, requires a lead time of at least seven to ten working days, during which one to three web meetings are held to discuss the planning and its implementation. In this context, two developments can be observed in the daily routine and more recent literature: first, the establishment of low-cost solutions for the in-house production of cutting guides using open-source software and in-house printers (11–14) and, second, the use of partially adjustable resection aids such as the ReconGuide (KLS Martin Group; Gebrüder Martin GmbH & Co. KG; Tuttlingen, Germany) and the MUC-Jig (15, 16).
The purpose of this study was to evaluate our workflow and results of in-house-planned mandibular reconstructions with the FFF and to describe potential pitfalls and solutions for a wider application of this versatile opportunity.
All clinical investigations were conducted according to the principles expressed in the Declaration of Helsinki. The exploratory cross-sectional study of a historical cohort was approved by the institutional ethics committee of the Technical University of Munich, Klinikum rechts der Isar (Approval number: 326/21 S-EB).
All patients who underwent mandibular resection and a reconstruction with an in-house-planned CAD/CAM FFF in our department for a benign or malignant disease between January 2019 and May 2021 were included. Patients without a postoperative computed tomography (CT) scan or with any otherwise planned and performed mandibular reconstruction (for example the use of partially adjustable resection aids or other microvascular bone flap) within this observation period were excluded (Figure 1). Data collection included: gender, age, indication for mandibular reconstruction, extent of resection according to Brown et al. (I–IV) (17), number of fibular segments, duration of surgery [min], ischemia time [min], and estimated resin volume and printing duration per case. Ischemia time was defined as the interval between ligation of the pedicle, mandibular reconstruction with completed osteosynthesis and opening of the vessel clamp following microvascular anastomoses.
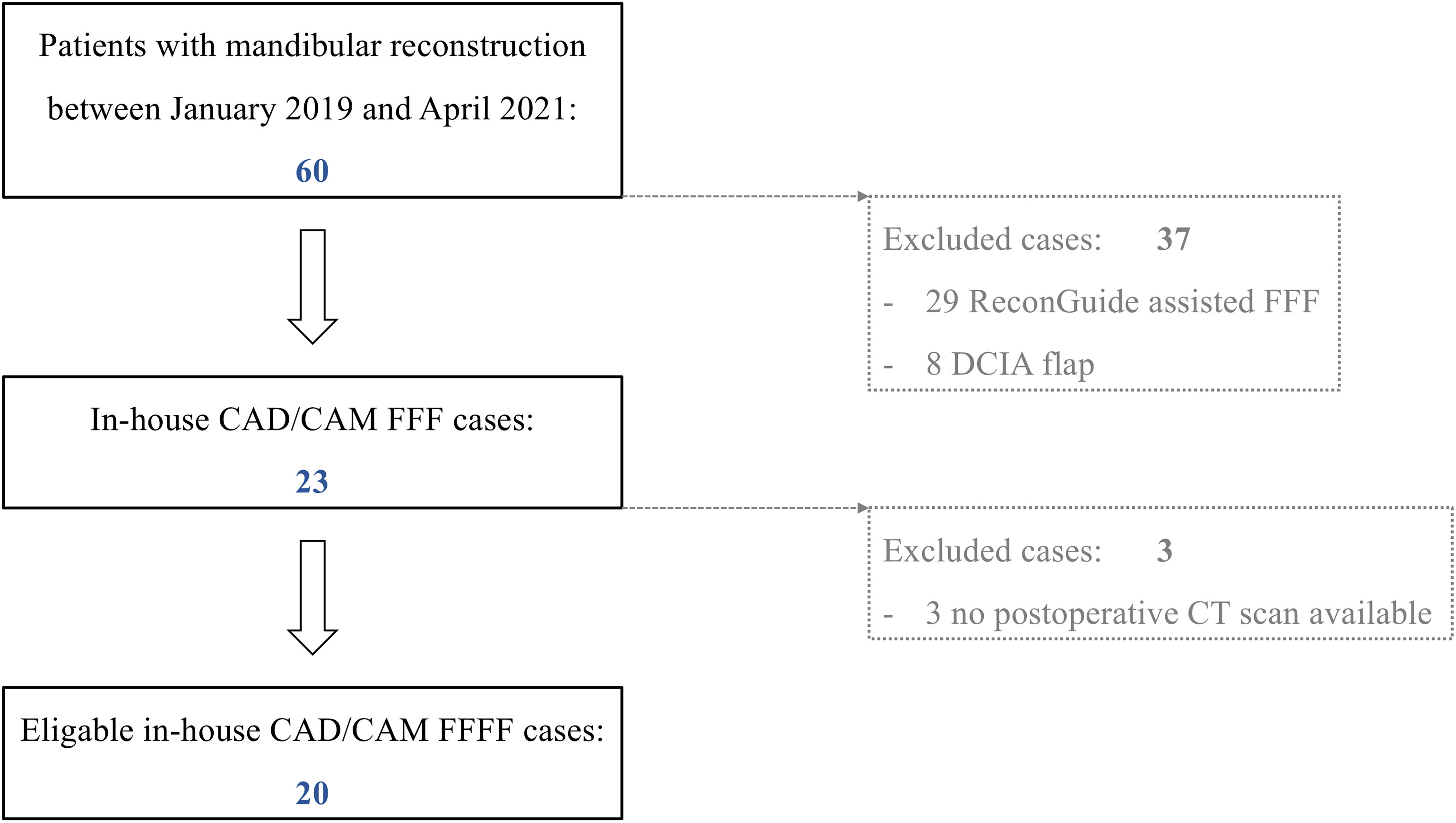
Figure 1 Patient enrollment protocol of this exploratory cross-sectional study of a historical cohort. (FFF, free fibula flap; DCIA, microvascular iliac crest flap, deep circumflex iliac artery).
All enrolled FFF cases were preoperatively planned and flaps were harvested by either the first- or last-named author (LMR, AMF), using the lateral approach (18) and using templates printed in-house (cutting guides and repositioning aids).
The digital workflow for the production of the in-house-planned and printed cutting and reconstruction guides complies with the general principles of CAD/CAM-assisted techniques as described elsewhere in detail (1, 19–21).
Available pre- and postoperative CT scans of enrolled patients were collected. Corresponding Digital Imaging and Communications in Medicine (DICOM) data sets of the CT scans were anonymized and converted to corresponding standard tessellation language (STL) files using Mimics® software (Mimics® 17.0, Materialise; Leuven, Belgium). The open-source software solution Blender (Blender® Version 2.79; Blender Foundation and Institute; Amsterdam, Netherlands) was used for the in-house computer planning and design of corresponding mandibular and fibular cutting guides and repositioning aids (Figure 2). No validation or comparison of the Blender-based planning with another software was performed.
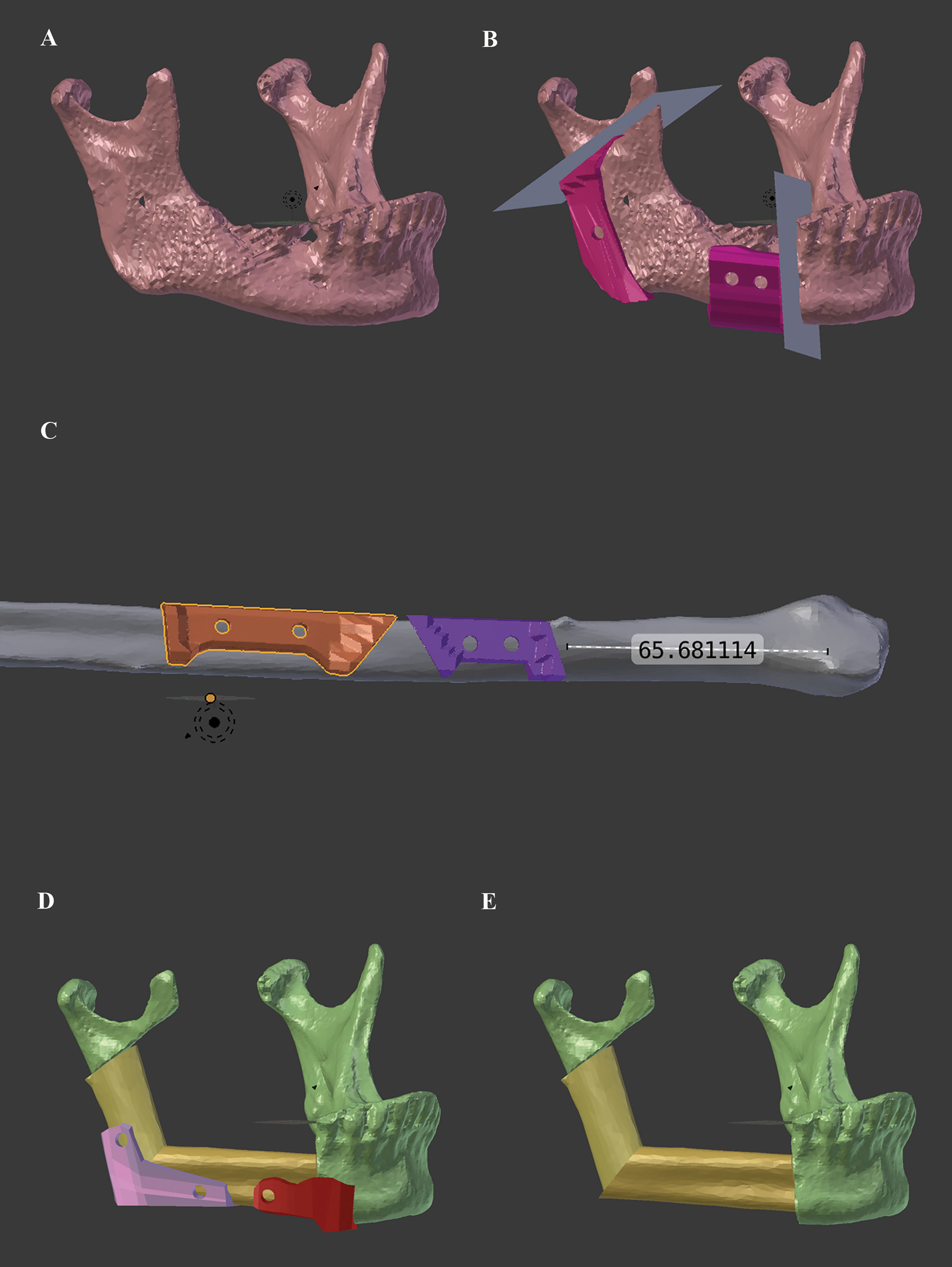
Figure 2 Workflow for in-house Blender-based (Blender® Version 2.79; Blender Foundation and Institute; Amsterdam, Netherlands) planning and design of corresponding mandibular and fibular cutting guides and repositioning aids for mandibular reconstruction with the free fibula flap. (A) preoperative mandibular situation, (B) simulated resection planes and designed mandibular cutting guides, (C) corresponding fibular cutting guides, (D) repositioning aids (at angle and neomandibular/mandibula body junction), and (E) final virtually reconstructed mandible.
The purely Blender-based planning procedure contained following key steps (Supplementary Data): First, the preoperative STL file of the mandible was imported to the Blender software and positioned in three-dimensional space (“Object_Transform_Geometry to Origin”). Then the necessary cutting planes were set and aligned as surgically required (paramedian, corpus, ramus, etc.) (“Create_Cube” → transform and manipulate in object and edit mode). Now, depending on the number of necessary fibula segments, the STL file of the fibula was imported and superimposed onto the expected mandibular defect according to the functional and reconstructive requirements. After simulation of the reconstructive result (= neomandibula) and final adjustment of the fibula segment positions, the corresponding cutting guides for the mandible and the fibula, as well as the repositioning aids for the final neomandibula, were designed (“Create_Cube” → transformation and manipulation in object and edit mode, and “Add Modifier_Boolean_Difference”). Repositioning aids were only created for the junction between neomandibula body and original mandibular as well as for the neomandibular angle. No repositioning aids were designed for the ramus/condylar junction due to lack of space. In this region, pre-bent miniplates were the only aid to transfer the virtual plan.
In addition, holes with a diameter of 4.2 mm were integrated to incorporate drill sleeves for safe intraoperative temporary fixation (KLS Martin Group; Gebrüder Martin GmbH & Co. KG; Tuttlingen, Germany).
The cutting guides and repositioning aids were printed in-house with a Form 2 stereolithographic printer (Form 2, Formlabs; USA) using a Class 1 autoclavable, biocompatible photopolymer resin (Dental SG, FLDGOR01; Formlabs; USA) with a layer thickness of 50 µm. The post-processing of the printed geometries was carried out according to the manufacturer’s instructions.
Postoperative analysis of the surgical results included conventional measurements and three-dimensional surface matching methods. The conventional measurements included the following distances: horizontal distance condylar head–condylar head (head–head) medial (1), lateral (2), and the condylar angles left (3) and right (4) according to Ueki et al. (22).
After the segmented pre- and postoperative mandibles and the virtual model were six-point-aligned, the following three three-dimensional parameters were determined for the comparison of three possible constellations [preoperative vs. virtual model (pre-virt); preoperative vs. postoperative model (pre-post); virtual model vs. postoperative (virt-post)]: root mean square error [RMSE, [mm)], mean surface distance [MSD, (mm)], and Hausdorff distance [HD, (voxel)] (23–25) (Figure 3). All conventional and three-dimensional analyses were performed using the open-source software MeshLab (MeshLab_64bit_fp v2020.12) and Blender.

Figure 3 Three-dimensional analyses were done with the open-source software MeshLab (MeshLab_64bit_fp v2020.12) showing root mean square error [RMSE, (mm)] in the left column and Hausdorff distance [HD, (voxel)] in the right column: (A, B) preoperative vs. virtual (pre-virt) model, (C, D) preoperative vs. postoperative (pre-post), and (E, F) virtual model vs. postoperative (virt-post).
All measurements were performed independently by two investigators (PK and FDG). All analyses were performed twice; the second round of analysis was performed at least seven to fourteen days later to minimize a habitual landmark setting and six-point alignment (26).
The intraclass correlation (ICC) coefficient (Cohen’s kappa = κ) was calculated to determine the intra- and interrater reliability and consistency of measurements performed by two raters applying a two-way mixed model. For the analysis of pre- and postoperative differences of the conventional parameters the Wilcoxon signed-rank test was used. For the differences of RMSE, MSD, and HD between pre-virt vs. pre-post models uni- and multivariate regression analyses were performed.
All statistical tests were performed on an exploratory two-sided 5% significance level. No adjustments were made for multiple testing. Analysis was done with IBM SPSS 24 for Mac software (IBM Corp, Armonk; New York, United States).
Twenty patients (9 female, 11 male) met the inclusion criteria (Figure 1). Age, indication for surgery, distribution of mandibular defect class according to Brown et al. (17), and the distribution of corresponding number of segments are shown in Table 1.
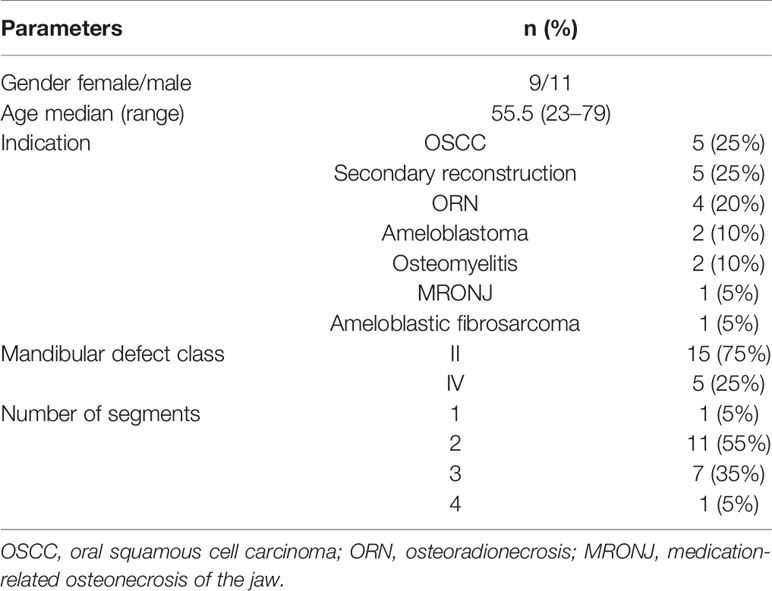
Table 1 Overview of enrolled patients with regard to registered parameters: gender, age, indication for surgery, mandibular defect class according to Brown et al. (17), number of segments.
The overall median estimated resin volume and printing duration per case were 43.6 ml (13.8–77.4) and 180 minutes (120–255). Median costs per case were EUR 14.30 (4.50–25.30). The median overall operation duration was 650 minutes (480–840) and ischemic time was 165 minutes (90–240). In the class II mandibular defect constellation the operation duration was 630 minutes (480–840) and ischemic time was 150 minutes (90–240). In the class IV mandibular defect constellation the operation duration was 660 minutes (600–750) and ischemic time was 180 minutes (120–185) (p = 0.180; p = 0.928, respectively).
The ICC coefficients (κ) for the horizontal distances head–head medial and lateral showed very good intra- and interrater reliabilities (κ>0.9). The condylar angle measurement showed only satisfactory intra- [between ICC κ 0.552, 95% CI -0.292–0.844 and ICC κ 0.866, 95% CI 0.655–0.948)] and good interrater reliability (between ICC κ 0.845, 95% CI 0.579–0.942), especially in the preoperative measurements. The postoperative condylar angle measurements again showed good to very good agreement (ICC κ>0.9).
ICC coefficients of all three-dimensional parameters (RMSE, MSD, and HD) consistently showed very good intra- and interrater reliability (ICC κ>0.9) (Supplementary Tables 1 and 2).
The detailed results of the conventional pre- and postoperative measurements (horizontal medial and lateral head–head distances and left and right condylar angles) are summarized in Table 2.
The three-dimensional alignment analyses of the preoperative and virtually planned neomandibula models (= pre-virt = expected deviation from “ground truth” model) were associated with a median RMSE of 1.4 (0.4–7.2), MSD of 0.3 (-0.1–2.9), and HD of 0.7 (0.1–3.1). The three-dimensional alignment analyses of preoperative and postoperative models (= pre-post = postoperative, real deviation from “ground truth” model) showed a median RMSE of 2.2 (1.5–11.1), MSD of 0.5 (-0.6–6.1), and HD of 1.5 (1.1–6.5) and the differences were significantly different for RMSE (p < 0.001) and HD (p < 0.001). The difference was not significantly different for MSD (p = 0.554).
Three-dimensional alignment analyses of the virtual and postoperative models (= virt-post = postoperative, real deviation from planned situation) had a median RMSE of 2.3 (1.3–10.7), MSD of -0.1 (-1.0–5.6), and HD of 1.7 (0.1–5.9) (Table 2 and Figure 3).
The results for the RMSE, MSD, and HD analyses as a function of number of bone segments or mandibular defect class (II vs. IV) are shown in Figure 4.
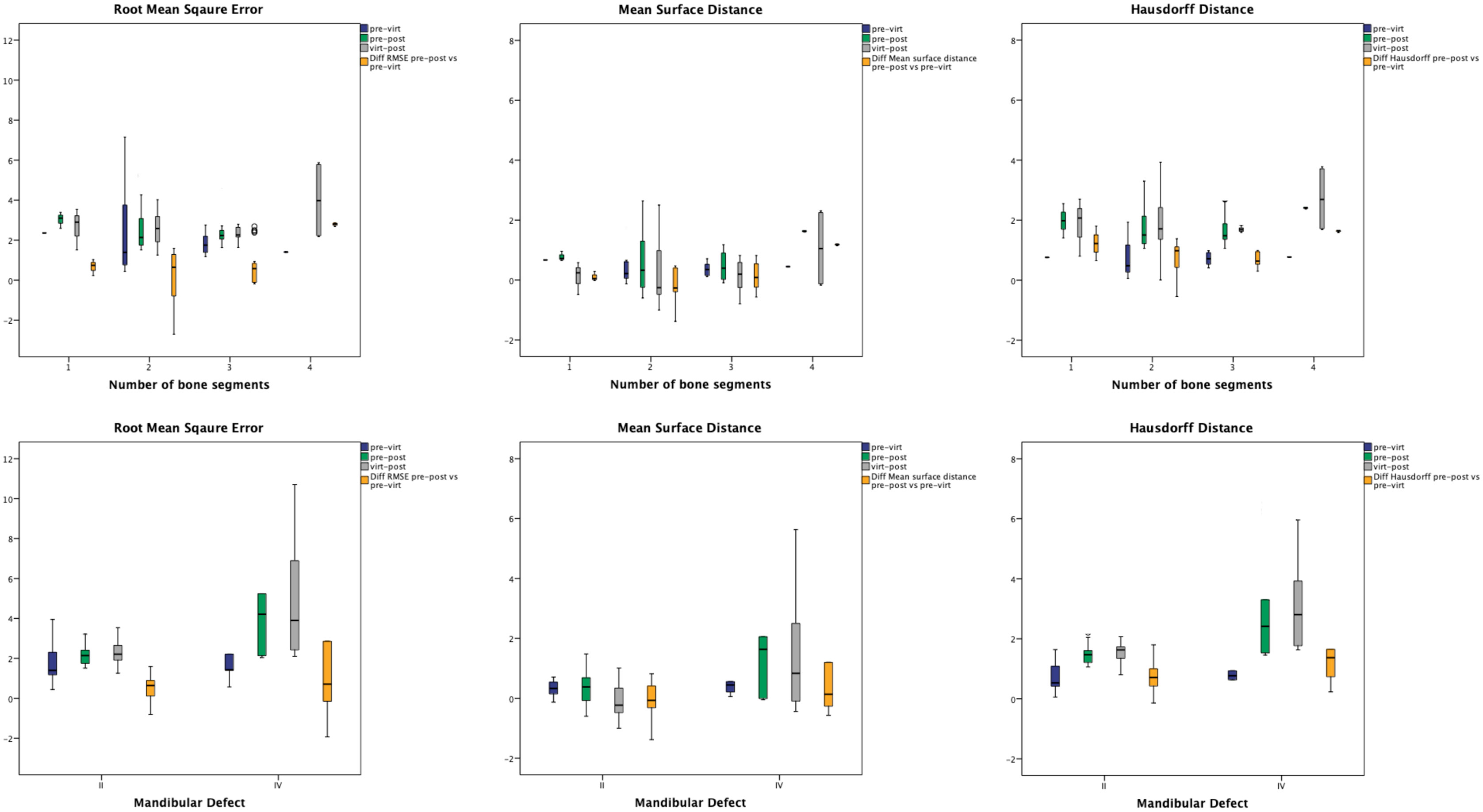
Figure 4 The results for the RMSE, MSD, and HD analyses as a function of number of bone segments (upper row) or mandibular defect class (lower row).
Uni- and multivariate regression analyses were performed to analyze possible confounding factors (gender, age, indication for surgery, dignity, mandibular defect class, and number of segments) on the RMSE, MSD, and HD of the virtual postoperative model alignment. Gender and number of segments did not have a significant influence on RMSE, MSD, or HD and were thus excluded from the multivariate linear regression analyses. Dignity (benign vs. malign) showed a significant influence on RMSE and MSD (p = 0.047 95% CI = 0.015–1.987 and p = 0.001 95% CI = 0.461–1.767), but not on HD (p = 0.223 95% CI = -0.210–0.886). The significant influence of the factors age, indication, dignity, and mandibular defect class remained in the multivariate linear regression analyses (Table 3).
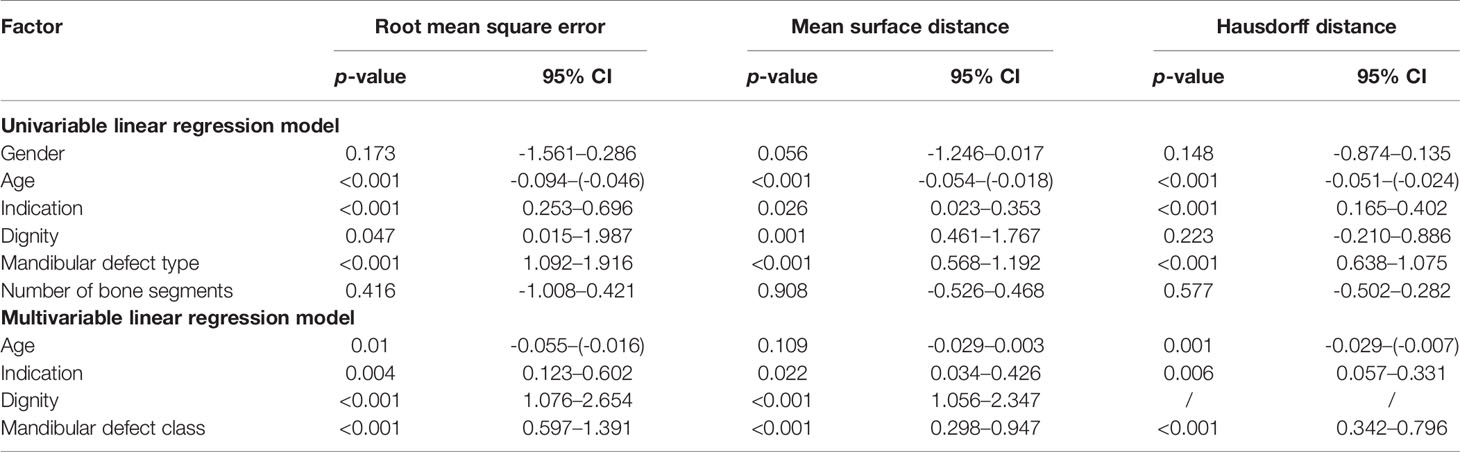
Table 3 Uni- and multivariable linear regression model of the virtual-postoperative RMSE, MSD, and HD results and possible confounding factors.
This study is one of the few studies on in-house CAD/CAM solutions that presents a critical contemporary three-dimensional evaluation of virtually planned mandibular reconstructions with FFF. Tarsitano et al. described that the use of CAD/CAM-assisted mandibular reconstruction is economically viable, as the money saved by the reduction of operation duration offsets the associated costs of approximately EUR 3,450 (5). Rommel et al. calculated the increased costs for planning and the use of patient-specific cutting guides offered and manufactured by osteosynthesis manufacturers to be EUR 2,250 per case (3). According to reviews and meta-analyses others reported the cost of virtual surgical planning to range from USD 3,000–8,200 (6, 10). These higher costs can be explained by the fact that the studies included in the reviews/meta-analyses were published between 2013 and 2016, at a time when 3D printing, for example, was even more expensive than it is today, and usually included the production of patient-specific reconstruction plates. Costs resulting from cost-effective in-house design and printing solutions are reported to be significantly lower. Bosc et al. calculated their cost per case for in-house design and printing at EUR 989 (13). In contrast, our reported median costs per case were EUR 14.30 (4.50–25.30). This represents an excellent cost–benefit ratio, We have deliberately omitted recent non-negligible cost items (acquisition and maintenance costs, sterilization time, and planning time of approximately five to six hours, salary, etc.) and did also not calculated overall potential cost savings for simplicity, as have many other authors, and focused exclusively on material costs. More recently, Moe et al. reported their cost per case to be USD 3.87 for in-house design and printing. Dell’Aversana Orabona et al. reported a cost per case of EUR 3 (27). At EUR 14.30, the calculated costs per case are comparable with data from the literature and are completely negligible in relation to the surgical costs. Our slightly higher costs can be explained by two factors: we created and printed repositioning aids for each osteotomy site, and the neomandibula segment was also printed in order to bend the miniplates preoperatively.
The total lead time (planning, designing, printing, post-processing, final preparation with drill sleeves, and sterilization) is two to three days, which agrees very well with the times reported in the literature (13, 14) – and can be expedited further with growing experience and optimization of the processes. Another essential aspect raised by Numajiri et al. is that this form of cost-effective planning is done in the surgeon’s time and is not outsourced to the osteosynthesis manufacturers and their clinical engineers as is usually the case (28). Geusens et al. reported that they have employed a full-time clinical engineer, which could be another solution to the altruistic behavior of interested and motivated surgeons (21). According to Tang et al., the economic benefits and limitations associated with the application of virtual surgical planning must be weighed against patient outcomes (4). The introduction of in-house solutions may represent the ideal approach in this respect, as costs can be saved and treatment optimized.
The study design and methodical analyses of the mandibular reconstruction is generally heterogenous among the available studies (4), which makes a direct comparison difficult. In a recent systematic review and meta-analysis on virtual surgical planning in mandibular reconstruction Barr et al. were not able to define a valuable parameter and result for “accuracy”, even though many authors highlight the introduction of CAD/CAM algorithms as beneficial (6). The reason is again the heterogeneity of parameters. The majority of studies compared the preoperative virtual plan to the postoperative situation by measuring the fibula segment lengths (21, 29–31), point-to-point distances and angles (3, 13, 14), intercondylar distance (32), or intercoronoid distance (21) or by comparing interfragmentary gap distances (33). Numajiri et al. analyzed an algorithm for the production of low-cost cutting guides designed and printed in-house (28). They reported an error deviation of 2.4 mm in 12 studied in-vitro model surgeries. For this laboratory setting under ideal conditions, an error deviation of 2.4 mm seems to be quite high. Four years later, Numajiri et al. published a clinical study comparing freehand and in-house-planned CAD/CAM FFF cases (14). Based on a point-to-point analysis, they describe their in-house Blender-based solution as accurate. Interestingly, the results of the more recent study are more precise than the results of their in vitro studies, which can probably be attributed to the research group’s growing experience with CAD/CAM-based surgical planning and the improvement of the processes. Yu et al. aligned the virtual plan and the postoperative result. But the evaluation was again limited only to distance measurements between corresponding points (8). Bosc et al. analyzed their postoperative results of in-house (Meshmixer or Blender) planned and printed cutting guides after surface alignment using CloudCompare, another potent open-source software solution for this purpose (13). However, the authors only used surface matching to get the maximum points of convergence for better correspondence of analyzed distances and angles. In all studies mentioned, the deviations between planning and operative results were 1–3 mm.
The disadvantage of all “conventional” measurement methods, which essentially involve only the analysis of distances (point-to-point or bone segment lengths) and angles, is that the effect of a small deviation on the entire three-dimensional geometry is neglected. An involuntary deviation from the planned osteotomy angle, fibula segment length, or fibula segment position will result in the derotation of the remaining mandible, and consequently in changes to the condyle angle.
For this reason and unlike most studies that have performed analyses of CAD/CAM-assisted mandibular reconstructions with the FFF, our focus has been on a three-dimensional analysis that encompasses the entire mandibular geometry. For example, Wallner et al. and van Eijnatten et al. applied three-dimensional parameters to compare the accuracy and comparability of open-source software solutions and influence of threshold setting for DICOM data set segmentation (23, 24). This approach seems to be a contemporary and objective way to compare two similar and rather complex objects but has not yet been used routinely in analyses of mandibular reconstructions (11, 12, 27). Each of our examined three-dimensional parameters – RMSE, MSD, and HD – had both excellent intra- and interobserver reliability, making the measured results valid for further analyses and discussion. In contrast, the conventional measurement of condylar angles in particular showed poorer intraobserver reliability for both examiners, which was confirmed by comparable interobserver reliability (Supplementary Tables 1, 2). Horizontal distances also revealed very good intra- and interobserver reliabilities. Dell’Aversana Orabona et al. used the open-source software InVesalius for in-house planning in four consecutive cases (27). They reported a distance between three-dimensional pre- and postoperative mesh points of 1.63 mm and a standard deviation of 5.45 mm in a volume overlay analysis. Recently, Moe et al. published results about their in-house workflow and described a mean surface overlay difference of 1.90 mm with an RMSE of 3.72 mm for 29 virtually planned cases, including 24 FFF (12). These results coincide with ours (virt-post MSD -0.1 (-1.0–5.6); virt-post RMSE 2.3 (1.3–10.7)). A novel aspect of our study is the comparison of preoperative situation and virtual model (pre-virt), which reflects the expected deviation from the “ground truth” model. Consequently, the difference between pre-post and pre-virt also reflects the accuracy when the value reaches 0. For this new parameter, we revealed excellent results for RMSE, MSD, and HD (Table 2).
In addition to potential cost savings and increased accuracy, we believe that this in-house planning has a great advantage especially in the training of junior surgeons. Dealing with virtual planning leads to the trainees intensively dealing with the clinical case, and potentially developing a higher demand for the final surgical result. Therefore, in our department, junior surgeons are introduced to virtual in-house fibula planning and learn to independently plan and create resection and repositioning guides. Unlike clinical engineers, they also perform flap elevation and mandibular reconstruction under the guidance of experienced specialists, so they will be involved in all steps of these complex reconstruction cases at an early stage in their careers. But this aspect (e.g. evaluation of junior surgeon´s satisfaction, confidence or virtual planning) was no not evaluated this study.
A major drawback of this study was the application of an license-based segmentation software that is associated with an additional acquisition cost (Mimics® 17.0, Materialise; Leuven, Belgium with an one-time acquisition cost in 2014 of EUR 11,700). But, we used Mimics in order to reduce potential software-based pitfalls in our in-house workflow. Nevertheless, there exist reliable and accurate open-source solutions for segmentation like Slicer, as described by Wallner et al. and Egger et al. (23, 34, 35).
Through precise planning the original position of the mandible should be imitated as closely as possible from an esthetic and functional point of view. With regard to occlusion and jaw movement, the correct position of the temporomandibular joint is important, and the course of the neo-alveolar ridge is decisive for subsequent dentoalveolar rehabilitation, while from an esthetic point of view the projection of the chin and jaw angle region is particularly decisive. After a learning period, the planning of complex and heterogenous reconstructive cases with the open-source software Blender was feasible and led to excellent results with regard to the realization of virtual plans (Figure 5). But incorrect virtual planning can lead to poor postoperative results even if all surgical steps are basically correct and performed as planned. In the experience of the authors, one key pitfall region is the mandibular ramus. When reconstructing it, the transition of the outer surfaces of the fibula and the remaining cranial ramus portion must blend perfectly. If this is not the case, the application of the osteosynthesis will derotate the ramus portion and consequently change the condyle angle and position. But condylar position changes may occur also later in a longer observational interval than it was in our study (36). But nevertheless, a derotation of the condylar head because of the cranial ramus rotation will be visible immediately. The functional sequalae is uncertain and needs further evaluation (37). A further prerequisite is the optimal design of ramus/collum resection guides. Aspects that should be taken into account here are that the mandibular angle is gripped caudally, so that the correct vertical positioning of the guide is ensured. In addition, the guide must not be too thick cranially to allow sawing; if necessary, an angled micro saw can be used, which is usually not a problem with regard to the general bone thickness in this area.
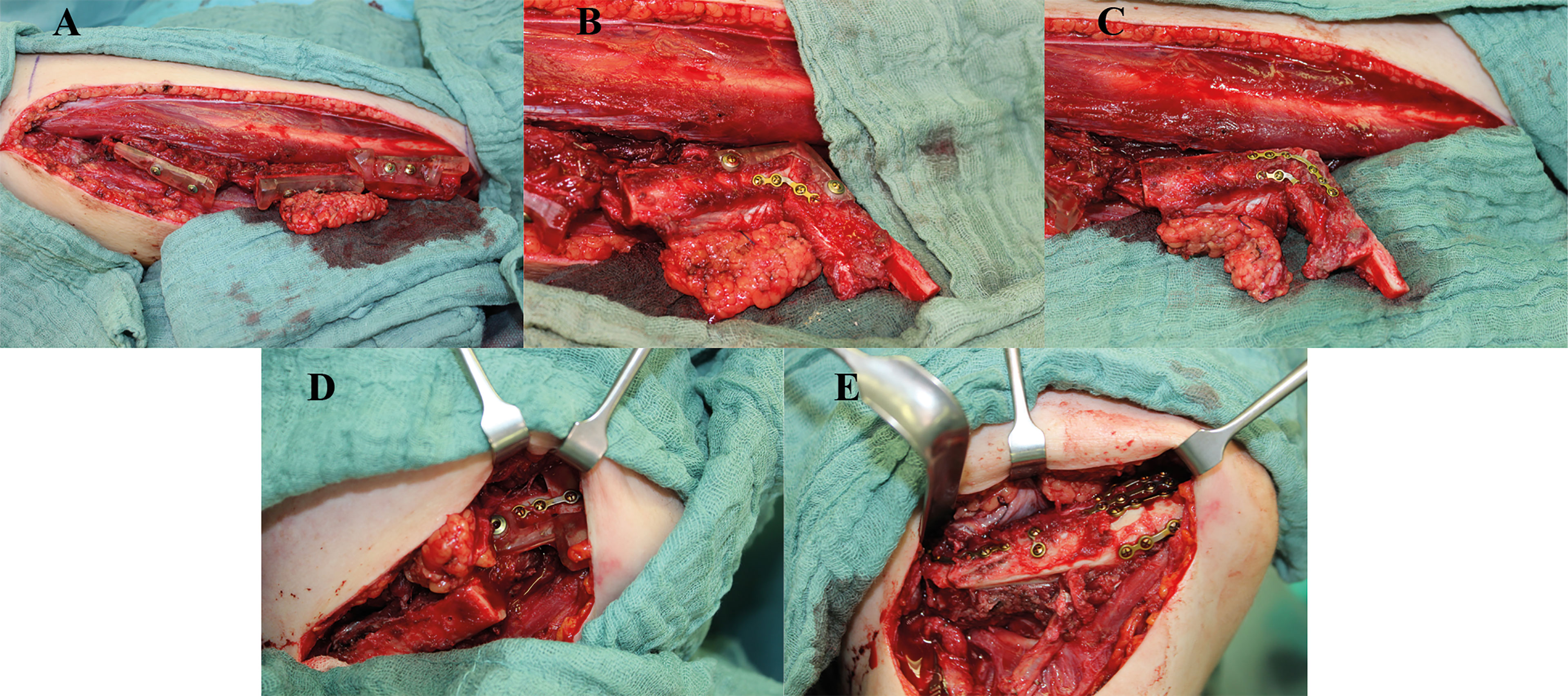
Figure 5 Clinical example with intraoperative images of a case with hemimandibulectomy with exarticulation (Brown IIc) due to chronic osteomyelitis. Reconstruction was performed with a three-segment free fibular flap (FFF), with the corpus section reconstructed with a double-barrel component for better neomandibula contouring and crestal bone position for secondary enossal implant insertion. (A) FFF following osteotomies with temporarily applied cutting guides, (B, C) reconstruction of the ramus including neo-condylar head and crestal corpus section applying a repositioning aid and pre-bent 2.0 miniplate osteosynthesis, (D) positioning of the neomandibula with another repositioning aid, and (E) final reconstructive result with the double-barreled caudal FFF section for optimized bony countering.
Lastly, we think that the usage of repositioning guides is an excellent alternative to intraoperative navigation as described by Yu et al. (8) to critically feedback the reconstructive result intraoperatively. Especially the combination of repositioning guides and pre-bent osteosynthesis plates enhance the overall accuracy. This is also reflected by the fact that the number of bone segments had no significant effect on RMSE, MSD, or HD (Table 3) and might explain our better results for MSD and RMSE compared to Dell’Aversana Orabona et al. and Moe et al. (12, 27). Regarding the design of the repositioning guides, we recognized that we achieved better fit of the guides when designing multiple, osteotomy-specific guides rather than one large, contiguous guide. The supposed inaccuracy of the large repositioning guides is most likely due to 3D printing itself.
After a certain learning period, open-source software facilitates cost-effective and precise in-house virtual planning of mandibular reconstructions with a short lead time and without the need for external companies. Even highly complex reconstructions are thus possible with favorable results. In addition, the open-source software offers an excellent possibility to illustrate the surgical procedure. This might enhance the understanding for younger colleagues and increase their likelihood and frequency of an ideal surgical result.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by The institutional ethics committee of the Technical University of Munich, Klinikum rechts der Isar (Approval number: 326/21 S-EB). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
LR: Study design/conduction, operations, data interpretation, and major contribution to manuscript writing and revision. PK: Surface matching and analysis, data acquisition and interpretation, contribution to revision. FG: Surface matching and analysis, data acquisition and interpretation, statistical analysis and contribution to revision. MS: Creation of figures, statistical analysis, contribution to revision. JW: Study design, data interpretation, contribution to revision. MN: Data interpretation, contribution to revision. K-DW: Data interpretation, contribution to revision. AF: Study design/conduction, operations, data interpretation, and major contribution to manuscript writing. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.731336/full#supplementary-material
1. Tarsitano A, Ciocca L, Scotti R, Marchetti C. Morphological Results of Customized Microvascular Mandibular Reconstruction: A Comparative Study. J Craniomaxillofac Surg (2016) 44:6. doi: 10.1016/j.jcms.2016.03.007
2. Haddock NT, Monaco C, Weimer KA, Hirsch DL, Levine JP, Saadeh PB. Increasing Bony Contact and Overlap With Computer-Designed Offset Cuts in Free Fibula Mandible Reconstruction. J Craniofac Surg (2012) 23:6. doi: 10.1097/SCS.0b013e318257576c
3. Rommel N, Kesting MR, Rohleder NH, Bauer FMJ, Wolff K-D, Weitz J. Mandible Reconstruction With Free Fibula Flaps: Outcome of a Cost-Effective Individual Planning Concept Compared With Virtual Surgical Planning. J Craniomaxillofac Surg (2017) 45:8. doi: 10.1016/j.jcms.2017.04.010
4. Tang NSJ, Ahmadi I, Ramakrishnan A. Virtual Surgical Planning in Fibula Free Flap Head and Neck Reconstruction: A Systematic Review and Meta-Analysis. J Plastic Reconstr Aesthet Surg JPRAS (2019) 9:1465–77. doi: 10.1016/j.bjps.2019.06.013
5. Tarsitano A, Battaglia S, Crimi S, Ciocca L, Scotti R, Marchetti C. Is a Computer-Assisted Design and Computer-Assisted Manufacturing Method for Mandibular Reconstruction Economically Viable? J Craniomaxillofac Surg (2016) 44:7. doi: 10.1016/j.jcms.2016.04.003
6. Barr ML, Haveles CS, Rezzadeh KS, Nolan IT, Castro R, Lee JC, et al. Virtual Surgical Planning for Mandibular Reconstruction With the Fibula Free Flap: A Systematic Review and Meta-Analysis. Ann Plast Surg (2020) 84:1. doi: 10.1097/SAP.0000000000002006
7. Ritschl LM, Mücke T, Fichter AM, Roth M, Kaltenhauser C, Pho Duc JM, et al. Axiographic Results of CAD/CAM-Assisted Microvascular, Fibular Free Flap Reconstruction of the Mandible: A Prospective Study of 21 Consecutive Cases. J Craniomaxillofac Surg (2017) 45:1. doi: 10.1016/j.jcms.2016.11.001
8. Yu Y, Zhang WB, Liu XJ, Guo CB, Yu GY, Peng X. Three-Dimensional Accuracy of Virtual Planning and Surgical Navigation for Mandibular Reconstruction With Free Fibula Flap. J Oral Maxillofac Surg (2016) 74:7. doi: 10.1016/j.joms.2016.02.020
9. Bouchet B, Raoul G, Julieron B, Wojcik T. Functional and Morphologic Outcomes of CAD/CAM-Assisted Versus Conventional Microvascular Fibular Free Flap Reconstruction of the Mandible: A Retrospective Study of 25 Cases. J Stomatol Oral Maxillofac Surg (2018) 119:6. doi: 10.1016/j.jormas.2018.07.003
10. Fatima A, Hackman TG, Wood JS. Cost-Effectiveness Analysis of Virtual Surgical Planning in Mandibular Reconstruction. Plast Reconstr Surg (2019) 143:4. doi: 10.1097/PRS.0000000000005418
11. Ganry L, Quilichini J, Bandini CM, Leyder P, Hersant B, Meningaud JP. Three-Dimensional Surgical Modelling With an Open-Source Software Protocol: Study of Precision and Reproducibility in Mandibular Reconstruction With the Fibula Free Flap. Int J Oral Maxillofac Surg (2017) 46:8. doi: 10.1016/j.ijom.2017.02.1276
12. Moe J, Foss J, Herster R, Powell C, Helman J, Ward BB, et al. An In-House Computer-Aided Design and Computer-Aided Manufacturing Workflow for Maxillofacial Free Flap Reconstruction is Associated With a Low Cost and High Accuracy. J Oral Maxillofac Surg (2021) 79:1. doi: 10.1016/j.joms.2020.07.216
13. Bosc R, Hersant B, Carloni R, Niddam J, Bouhassira J, De Kermadec H, et al. Mandibular Reconstruction After Cancer: An in-House Approach to Manufacturing Cutting Guides. Int J Oral Maxillofac Surg (2017) 46:1. doi: 10.1016/j.ijom.2016.10.004
14. Numajiri T, Morita D, Yamochi R, Nakamura H, Tsujiko S, Sowa Y, et al. Does an In-House Computer-Aided Design/Computer-Aided Manufacturing Approach Contribute to Accuracy and Time Shortening in Mandibular Reconstruction? J Craniofac Surg (2020) 7:317. doi: 10.1097/SCS.0000000000006699
15. Meyer S, Hirsch JM, Leiggener CS, Msallem B, Sigron GR, Kunz C, et al. Fibula Graft Cutting Devices: Are 3d-Printed Cutting Guides More Precise Than a Universal, Reusable Osteotomy Jig? J Clin Med (2020) 9:12. doi: 10.3390/jcm9124119
16. Weitz J, Wolff KD, Kesting MR, Nobis CP. Development of a Novel Resection and Cutting Guide for Mandibular Reconstruction Using Free Fibula Flap. J Craniomaxillofac Surg (2018) 46:11. doi: 10.1016/j.jcms.2018.09.007
17. Brown JS, Barry C, Ho M, Shaw R. A New Classification for Mandibular Defects After Oncological Resection. Lancet Oncol (2016) 17:1. doi: 10.1016/S1470-2045(15)00310-1
18. Wolff KD, Holzle F, Kolk A, Hohlweg-Majert B, Steiner T, Kesting MR. Raising the Osteocutaneous Fibular Flap for Oral Reconstruction With Reduced Tissue Alteration. J Oral Maxillofac Surg (2011) 69:6. doi: 10.1016/j.joms.2010.11.040
19. Ritschl LM, Mucke T, Fichter A, Gull FD, Schmid C, Duc JMP, et al. Functional Outcome of CAD/CAM-Assisted Versus Conventional Microvascular, Fibular Free Flap Reconstruction of the Mandible: A Retrospective Study of 30 Cases. J Reconstr Microsurg (2017) 33:4. doi: 10.1055/s-0036-1597823
20. Mottini M, Seyed Jafari SM, Shafighi M, Schaller B. New Approach for Virtual Surgical Planning and Mandibular Reconstruction Using a Fibula Free Flap. Oral Oncol (2016) 59:e6–9. doi: 10.1016/j.oraloncology.2016.06.001
21. Geusens J, Sun Y, Luebbers HT, Bila M, Darche V, Politis C. Accuracy of Computer-Aided Design/Computer-Aided Manufacturing-Assisted Mandibular Reconstruction With a Fibula Free Flap. J Craniofac Surg (2019) 30:8. doi: 10.1097/SCS.0000000000005704
22. Ueki K, Yoshizawa K, Moroi A, Iguchi R, Kosaka A, Ikawa H, et al. Changes in Computed Tomography Values of Mandibular Condyle and Temporomandibular Joint Disc Position After Sagittal Split Ramus Osteotomy. J Craniomaxillofac Surg (2015) 43:7. doi: 10.1016/j.jcms.2015.05.007
23. Wallner J, Hochegger K, Chen X, Mischak I, Reinbacher K, Pau M, et al. Clinical Evaluation of Semi-Automatic Open-Source Algorithmic Software Segmentation of the Mandibular Bone: Practical Feasibility and Assessment of a New Course of Action. PloS One (2018) 13:5. doi: 10.1371/journal.pone.0196378
24. van Eijnatten M, van Dijk R, Dobbe J, Streekstra G, Koivisto J, Wolff J. CT Image Segmentation Methods for Bone Used in Medical Additive Manufacturing. Med Eng Physics (2018) 51:6–16. doi: 10.1016/j.medengphy.2017.10.008
25. Ritschl LM, Wolff K-D, Erben P, Grill FD. Simultaneous, Radiation-Free Registration of the Dentoalveolar Position and the Face by Combining 3D Photography With a Portable Scanner and Impression-Taking. Head Face Med (2019) 15:1. doi: 10.1186/s13005-019-0212-x
26. Wolff K-D, Grill FD, Ritschl LM. Comparative Photographic, Retrospective Analysis of Nonsyndromic Cleft Noses Treated With or Without NAM. Plast Reconstr Surg Global Open (2020) 8:9. doi: 10.1097/GOX.0000000000003045
27. Dell’Aversana Orabona G, Abbate V, Maglitto F, Bonavolonta P, Salzano G, Romano A, et al. Low-Cost, Self-Made CAD/CAM-Guiding System for Mandibular Reconstruction. Surg Oncol (2018) 27:2. doi: 10.1016/j.suronc.2018.03.007
28. Numajiri T, Nakamura H, Sowa Y, Nishino K. Low-Cost Design and Manufacturing of Surgical Guides for Mandibular Reconstruction Using a Fibula. Plast Reconstr Surg Global Open (2016) 4:7. doi: 10.1097/GOX.0000000000000682
29. Hanasono MM, Skoracki RJ. Computer-Assisted Design and Rapid Prototype Modeling in Microvascular Mandible Reconstruction. Laryngoscope (2013) 123:3. doi: 10.1002/lary.23717
30. Succo G, Berrone M, Battiston B, Tos P, Goia F, Appendino P, et al. Step-By-Step Surgical Technique for Mandibular Reconstruction With Fibular Free Flap: Application of Digital Technology in Virtual Surgical Planning. Eur Arch Otorhinolaryngol (2015) 272:6. doi: 10.1007/s00405-014-3078-3
31. Schepers RH, Raghoebar GM, Vissink A, Stenekes MW, Kraeima J, Roodenburg JL, et al. Accuracy of Fibula Reconstruction Using Patient-Specific CAD/CAM Reconstruction Plates and Dental Implants: A New Modality for Functional Reconstruction of Mandibular Defects. J Craniomaxillofac Surg (2015) 43:5. doi: 10.1016/j.jcms.2015.03.015
32. Metzler P, Geiger EJ, Alcon A, Ma X, Steinbacher DM. Three-Dimensional Virtual Surgery Accuracy for Free Fibula Mandibular Reconstruction: Planned Versus Actual Results. J Oral Maxillofac Surg (2014) 72:12. doi: 10.1016/j.joms.2014.07.024
33. Stirling Craig E, Yuhasz M, Shah A, Blumberg J, Salomon J, Lowlicht R, et al. Simulated Surgery and Cutting Guides Enhance Spatial Positioning in Free Fibular Mandibular Reconstruction. Microsurgery (2015) 35:1. doi: 10.1002/micr.22229
34. Egger J, Pfarrkirchner B, Gsaxner C, Lindner L, Schmalstieg D, Wallner J. Fully Convolutional Mandible Segmentation on a Valid Ground- Truth Dataset. Conf Proc IEEE Eng Med Biol Soc (2018) 2018:29–33. doi: 10.1109/EMBC.2018.8512458
35. Wallner J, Schwaiger M, Hochegger K, Gsaxner C, Zemann W, Egger J. A Review on Multiplatform Evaluations of Semi-Automatic Open-Source Based Image Segmentation for Cranio-Maxillofacial Surgery. Comput Methods Programs Biomed (2019) 182:105102. doi: 10.1016/j.cmpb.2019.105102
36. Wang W, Shan XF, Liang J, Xie S, Zhang J, Cai ZG. Changes in Condylar Position After Mandibular Reconstruction With Condylar Head Preservation by Computed Tomography. J Oral Maxillofac Surg (2019) 77:6. doi: 10.1016/j.joms.2018.12.037
Keywords: in-house CAD/CAM planning, 3D printing, mandibular reconstruction, free fibula flap, open-source software
Citation: Ritschl LM, Kilbertus P, Grill FD, Schwarz M, Weitz J, Nieberler M, Wolff K-D and Fichter AM (2021) In-House, Open-Source 3D-Software-Based, CAD/CAM-Planned Mandibular Reconstructions in 20 Consecutive Free Fibula Flap Cases: An Explorative Cross-Sectional Study With Three-Dimensional Performance Analysis. Front. Oncol. 11:731336. doi: 10.3389/fonc.2021.731336
Received: 26 June 2021; Accepted: 31 August 2021;
Published: 24 September 2021.
Edited by:
Florian M. Thieringer, University Hospital Basel, SwitzerlandReviewed by:
Divya Mehrotra, King George’s Medical University, IndiaCopyright © 2021 Ritschl, Kilbertus, Grill, Schwarz, Weitz, Nieberler, Wolff and Fichter. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lucas M. Ritschl, bHVjYXMucml0c2NobEB0dW0uZGU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.