
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol., 23 September 2021
Sec. Genitourinary Oncology
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.731002
This article is part of the Research TopicCase Reports in Prostate CancerView all 12 articles
The treatment landscape of metastatic castration-resistant prostate cancer (mCRPC) has dramatically improved over the last decade; however, patients with visceral metastases are still faced with poor outcomes. Phosphatase and tensin homolog (PTEN) loss is observed in 40%–60% of mCRPC patients and is also associated with a poor prognosis. Several PI3K/AKT/mTOR pathway inhibitors have been studied, with disappointing anti-tumor activity. Here, we present a case of a patient with heavily treated mCRPC who had a modest tumor response to concurrent carboplatin, abiraterone acetate/prednisone, and liver-directed radiation therapy. We discuss the potential rationale supporting the use of this combination therapy and its safety in mCRPC. While the underlying basic mechanism of our patient’s anti-tumor response remains uncertain, we suggest that further prospective studies are warranted to evaluate whether this combination therapy is effective in this population of patients with pre-treated mCRPC and PTEN loss.
Visceral metastases in men with metastatic castration-resistant prostate cancer (mCRPC) occur at a very late stage of disease. One retrospective study showed that the rate of radiologically detected visceral metastases before death from prostate cancer was 32% (1). In the vast majority of patients with visceral metastases, there are also detectable metastases at other sites such as bone and regional lymph nodes (1). The site of metastases impacts the expected survival of a patient. A prior meta-analysis showed that the poorest overall survival is seen in men with liver metastases (13.5 months) followed by lung metastases (19.4 months), non-visceral bone metastases (21.3 months), and lymph node-only metastases (31.6 months) (2).
The treatment landscape of mCRPC has dramatically improved over the last decade. The development of potent androgen synthesis and receptor inhibitors (3, 4); chemotherapy with taxanes alone or in combination with a platinum (5–8); poly (ADP-ribose) polymerase (PARP) inhibitors in patients who carry DNA homologous recombination repair gene-mutations (9); immunotherapy in men with high microsatellite instability (10); and prostate-specific membrane antigen (PSMA) targeted radiopharmaceutical agents (11) have significantly prolonged overall survival and progression-free survival in mCRPC. Still, the disease is incurable and patients with visceral metastases have a limited expected survival.
Loss of the tumor suppressor gene phosphatase and tensin homolog (PTEN) is identified in 15%–20% of primary prostate tumor samples. Upon progression to castrate-resistant disease, the incidence increases to 40%–60% (12). Loss of function of PTEN leads to an activation of the PI3K/AKT/mTOR pathway precipitating cell proliferation, growth, and survival. PTEN loss is associated with a poor prognosis and is an independent prognostic indicator of prostate-cancer-specific death (12). While several PI3K/AKT/mTOR pathway inhibitors have been studied in mCRPC, the majority of outcomes have been disappointing, with no significant anti-neoplastic activity (13–19). However, early-phase studies show that ipatasertib, a new oral small-molecule inhibitor of AKT (protein kinase B), has promising anti-tumor activity when combined with novel hormonal agents; the activity appears to be significantly increased in mCRPC patients with PTEN loss (20). A Phase III randomized study of ipatasertib plus abiraterone is currently ongoing (21). Currently, treatment of mCRPC patients with PTEN loss is challenging.
Here, we present the case of a patient with PTEN loss castration-resistant prostate cancer with liver-only metastases who failed multiple lines of treatment but demonstrated some modest response to the combination of abiraterone acetate/prednisone plus carboplatin and liver-directed radiation therapy.
A 62-year-old gentleman with a family history of prostate cancer and a personal history of stage II chronic lymphocytic leukemia (CLL) under observation (not required any treatment) and a right nephrectomy for stage I clear cell renal cell carcinoma (not required any systemic treatment) was diagnosed with a localized, high-risk prostate adenocarcinoma. His pre-surgery prostatic specific antigen (PSA) was 19.7 ng/ml, and he had a Gleason score of 4 + 5 = 9 in all prostate biopsy cores. He underwent a radical prostatectomy with bilateral pelvic lymphadenectomy in September 2016. Final pathology confirmed prostate adenocarcinoma with a Gleason score of 4 + 5 = 9 with invasion of periprostatic fat and the seminal vesicle as well as perineural invasion: pT3bN0. The pathology also revealed positive surgical margins. Post-surgery PSA was 2.39 ng/ml. He received bicalutamide and androgen deprivation therapy (ADT) with leuprolide for 6 months from his local urologist but did not receive salvage radiation. His PSA became undetectable with ADT. Germline genetic testing was performed using the Ambry CancerNext® test and was negative.
The patient presented to our medical oncology clinic in January 2020 with a 2-month history of lower abdominal pain, anal spasms, constipation, and significant lower urinary tract symptoms with a severe International Prostate Symptom Score (IPSS) of 33. He denied loss of appetite and his weight had been maintained. His PSA had risen to 2.3 in December 2019 in a previous record, but at our initial visit, it was elevated to 11.70 ng/ml and further elevated to 41.78 ng/ml within 4 weeks. A computerized tomography (CT) scan of his chest and abdomen and pelvis demonstrated numerous liver lesions and extensive sub-centimeter supraclavicular, mediastinal, and bilateral axillary lymphadenopathy. A nuclear bone scan was negative for bony metastases. Subsequently, a whole-body positron emission tomography/CT scan was performed to evaluate for a Richter’s transformation given his CLL history. The scan showed hypermetabolic changes in a left hepatic lesion with an standardized uptake value (SUV) of 10.2; a caudate lobe lesion with an SUV of 11; a right dome of the liver lesion with an SUV of 8.0; hypermetabolic bilateral iliac nodes with SUVs of 3.8 and 2.7; and no fluorodeoxyglucose avidity above blood pool in his supraclavicular, bilateral axillary, mediastinal, and bilateral perihilar nodes. There was no evidence of bone marrow infiltration. A liver biopsy was obtained given it demonstrated the highest uptake value and showed metastatic carcinoma that was strongly positive for the prostate-specific immunohistochemical (IHC) markers NKX3.1 and PSA and negative for the neuroendocrine markers chromogranin and synaptophysin (Figure 1).
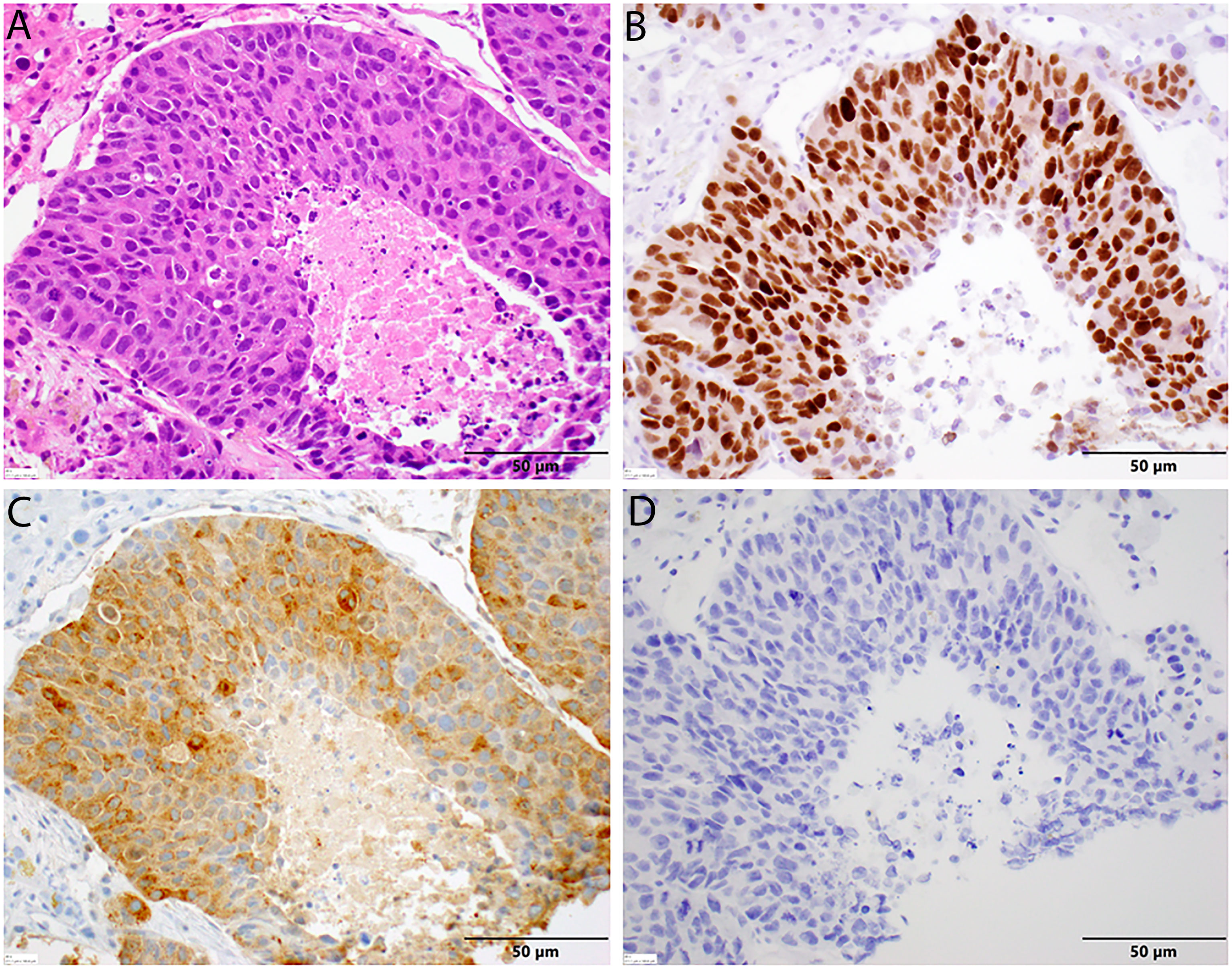
Figure 1 Histopathology of liver biopsy. (A) Metastatic prostate adenocarcinoma displaying significant nuclear enlargement and pleomorphism, prominent nucleoli, mitotic figures, and comedo-type central necrosis in this representative field. Note the absence in neuroendocrine features and the surrounding benign hepatocytes [H&E stain, 40× magnification]. (B) Diffuse nuclear positivity with NKX3.1 in tumor cells [NKX3.1 stain, 40× magnification]. (C) Diffuse cytoplasmic positivity with PSA in tumor cells. (D) No cytoplasmic staining with chromogranin in tumor cells [chromogranin, 40× magnification].
In light of these findings, the patient was started on leuprolide 22.5 mg plus docetaxel 75 mg/m2. He received six cycles of docetaxel from March 2020 to June 2020 and tolerated the treatment well with no major treatment-related side effects except some mild fatigue. PSA trends shown in Figure 2. Repeat CT scans after three cycles of docetaxel + ADT showed stable disease; however, the scans repeated after six cycles showed disease progression in the liver (Figures 3A, B).
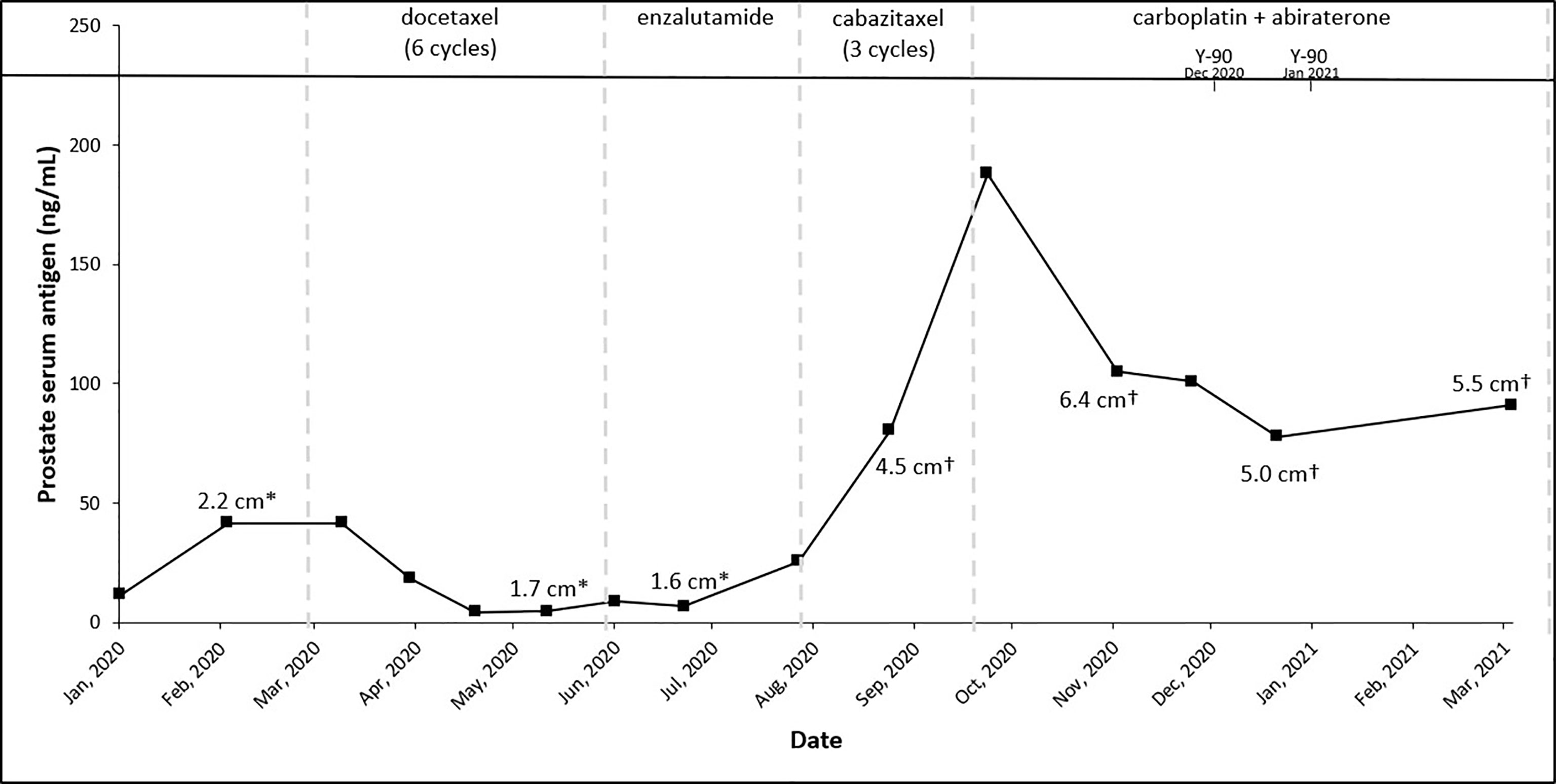
Figure 2 Trends of treatment, prostate serum antigen, and tumor size across patient’s treatment course. *Right inferior lobe lesion and †segment 7 lesion.
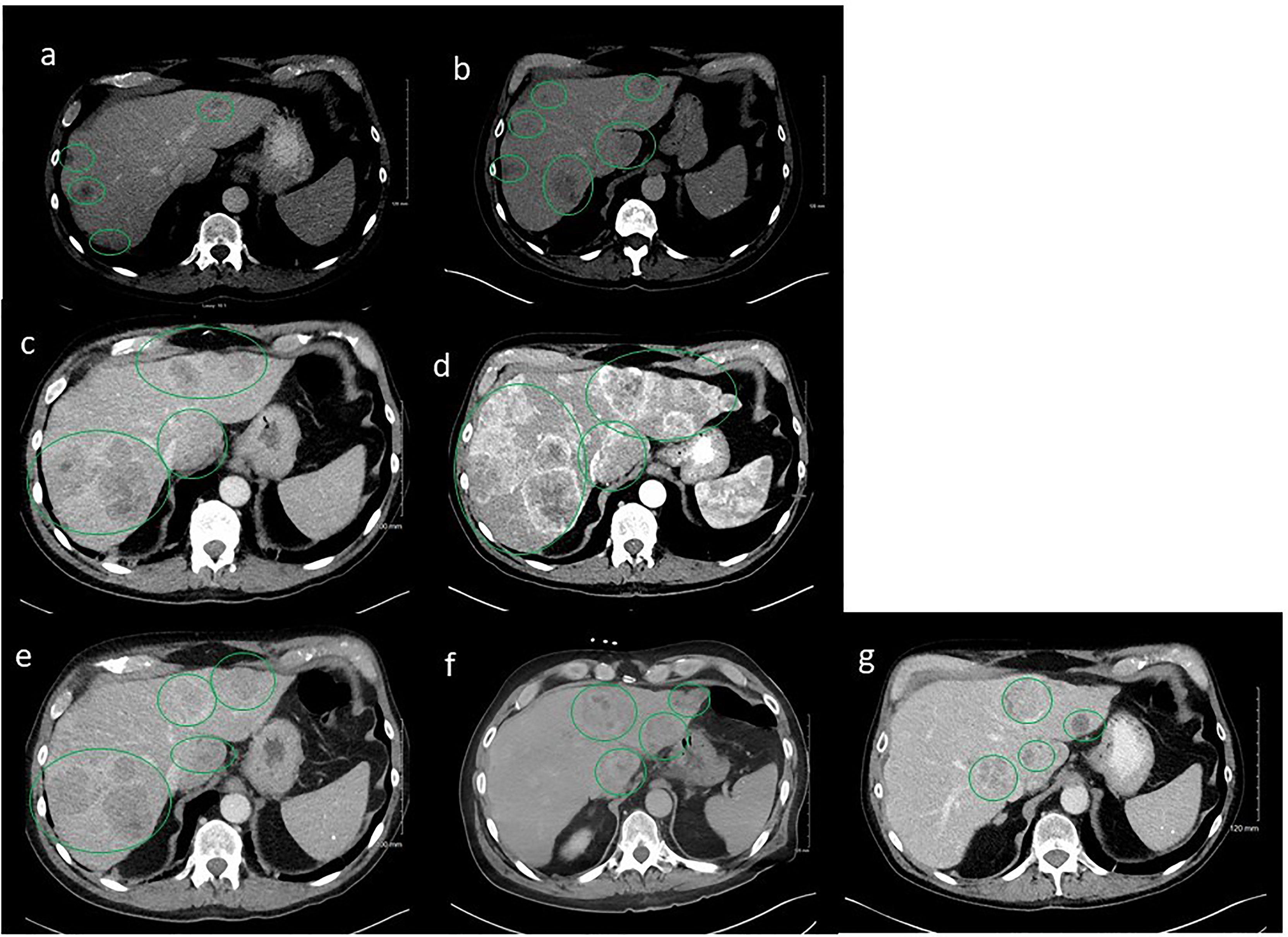
Figure 3 Serial computerized tomography (CT) images of abdomen and pelvis with contrast of the patient’s tumor after sequencing treatments. Green oval cycles represent tumors. (A) Prior to docetaxel, March 2020. (B) Six cycles after docetaxel, June 2020. (C) Two months after enzalutamide, August 2020. (D) After three cycles of cabazitaxel, October 2020. (E) After Y-90 embolization to the right lobe of liver concurrent with carboplatin/abiraterone acetate, December 2020. (F) After Y-90 embolization to the left lobe of liver concurrent with carboplatin/abiraterone acetate, January 2021. (G) On carboplatin/abiraterone acetate, May 2021.
A repeat liver biopsy was again consistent with metastatic prostatic adenocarcinoma with similar morphology and IHC profile to the previous biopsy (Figure 4). Molecular testing through Caris Life Sciences was performed, and it showed positive for androgen receptor, PTEN loss in exon 2c.164, CDKN1B exon 1p.p92fs, tumor mutation burden (5 mutations/Mb), and stable microsatellite instability, and negative for NTRK1/2/3, ATM, BRCA1, BRCA2, FANCA, PALB2, RAD51C, and RAD51D. Second-line therapy with enzalutamide 160 mg was started in July 2020, but in August 2020, he presented to the emergency department with intractable right upper quadrant pain and CT scans showed progression of the hepatic masses with small, new infiltrative lesions (Figure 3C). PSA trends are shown in Figure 2. Due to the rapid progression, we switched to cabazitaxel 20 mg/m2 with G-CSF as third-line therapy; the patient received three cycles from September to October 2020. While on cabazitaxel, his PSA dramatically increased to 188 ng/ml and repeat scans again demonstrated worsening of his extensive hepatic metastases (Figure 3D). AR-V7 testing was sent and was negative. We initiated abiraterone acetate 1000 mg daily/prednisone 5 mg twice daily combined with carboplatin AUC 5 every 3 weeks in November 2020 given PTEN loss. He also underwent Y-90 embolization of the right lobe of his liver in December 2020 (Figure 3E) and a second Y-90 embolization of the left lobe of his liver in January 2021 (Figure 3F). He was instructed to continue his abiraterone acetate/prednisone regimen throughout his Yttrium-90 (Y-90) embolization. Y-90 embolization is a type of radiation using resin or glass microspheres containing 90Y administered directly into the hepatic arteries. However, the carboplatin was held 2 weeks prior and 2 weeks after Y-90 embolization. He received continuous ADT as backbone. He tolerated the treatment well without having any major side effects except grade 2 fatigue and grade 2 nausea/vomiting. His PSA slowly trended down and became stable, as shown in Figure 2. Repeat CT scans showed a partial response in the liver. He remains on the same chemotherapy/hormonal therapy combination at the time of writing with stable response (Figure 3G).
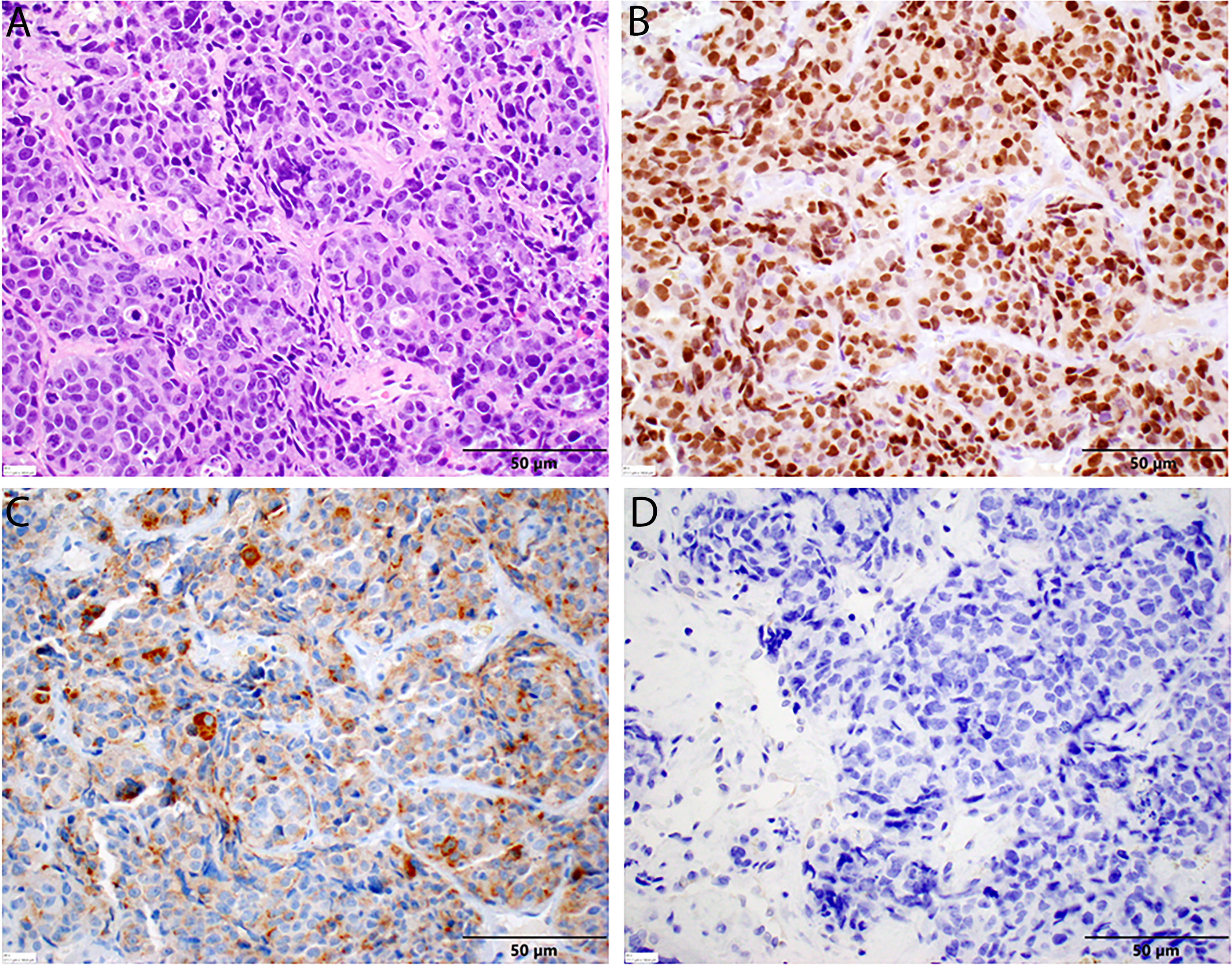
Figure 4 Histopathology of second liver biopsy. (A) Metastatic prostate adenocarcinoma displaying similar features to the previous sample, including significant nuclear enlargement and pleomorphism, prominent nucleoli, mitotic figures, and single-cell necrosis in this representative field. Again, note the absence of neuroendocrine features [H&E stain, 40× magnification]. (B) Diffuse nuclear positivity with NKX3.1 in tumor cells [NKX3.1 stain, 40× magnification]. (C) Diffuse cytoplasmic positivity with PSA in tumor cells. (D) No cytoplasmic staining with chromogranin in tumor cells [chromogranin, 40× magnification].
To our knowledge, abiraterone acetate/carboplatin/radiation combination therapy has never been studied in mCRPC patients; we report the first case on this chemotherapy/hormonal therapy/radiation therapy combination. This case demonstrates the clinical utility of the above combination therapy in patients with metastatic CRPC with PTEN loss.
Prostate cancer is a heterogenous disease and a small subset of the prostate adenocarcinoma population can present with aggressive clinical features like neuroendocrine origin. This subpopulation frequently carries some driver molecular alterations in retinoblastoma-associated protein 1 (RB1), tumor protein 53 (TP53), and/or PTEN (22). These alterations have been associated with abnormal cell proliferation and increased DNA damage response defects through activation of Akt signaling (23).
Loss of PTEN by mono- and biallelic deletions or mutations is among the most frequently observed molecular aberrations in localized and metastatic prostate cancer. PTEN loss is identified in 15%–20% of primary prostate tumor samples (12). Upon progression to castrate-resistant disease, the incidence increases to 40%–60% (12). PTEN loss is known to be associated with a poor prognosis (12). PTEN plays a crucial role as a tumor suppressor in cell cycle by controlling both G1/S and G2/M transitions (24). Loss of PTEN promotes activation of the PI3K/AKT/mTOR signaling pathway, which modulates several downstream pathways. This signaling pathway also causes abnormal cell proliferation and survival (25–27). PTEN regulates p53 by modulating its DNA binding activity. PTEN and p53 both regulate the DNA damage response pathway by promoting nucleotide excision repair (NER) following ionizing radiation damage (28). When PTEN function is lost, Akt signaling pathways are activated through inappropriate activation of Chk1 (29) and, thus, impairs DNA damage repair and the DNA damage response pathway (28).
Platinum compounds as monotherapy or combination therapy have shown promising activity in mCRPC (7, 8). Platinum-based agents cause mono-, inter-, or intra-strand crosslinking of DNA triggering DNA damage, which activates ATM/Chk2/p53 signaling, inducing apoptosis and cell cycle arrest (30). Interestingly, androgen receptor signaling also regulates DNA repair genes of both non-homologous end joining (NHEJ) and homologous recombination (HR) repair pathways (31). Preclinical studies (both in vitro and in vivo models) have demonstrated the synergistic combinations of radiation and novel androgen synthesis inhibitors (abiraterone acetate or enzalutamide) in both androgen-dependent and androgen-independent prostate cancer (31, 32). The rationale is that the ionizing radiation enhances DNA damage, which then activates the ATM/Chk2/p53 signaling pathway promoting cell cycle arrest (33, 34). Anti-androgen therapy further augments by decreasing DNA repair genes and, thus, inducing synthetic lethality and causing apoptosis of prostate cancer cells (31, 35). Recent early-phase studies further confirmed the clinical efficacy data of these synergistic combinations (36–38). Phase III studies are ongoing.
We hypothesize that a response was seen in this case because carboplatin and radiation both induce DNA damage through the ATM/Chk2/p53 pathway, and the loss of PTEN activates the PI3K/AKT pathway and causes DNA damage repair, which is further augmented by adding anti-androgen therapy. Therefore, the combination has some synergistic or additive benefits. While the underlying basic mechanism of our patient’s anti-tumor response remains uncertain, our case highlights the possible benefit and safety of combination carboplatin/abiraterone acetate/radiation in treated mCRPC and suggests that further prospective studies are warranted to evaluate whether this combination therapy is effective in this population.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
ZM and CE: drafting of the article, acquisition of data, and final approval of the manuscript. DA: providing histopathology pictures and final approval of the manuscript. All authors contributed to the article and approved the submitted version.
This funding is supporting the University of Kentucky Markey Cancer Center’s Research Communications Office. They assisted with preparation of this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank the patient for allowing us to share his medical history and clinical course. The content is solely the responsibility of the authors. The University of Kentucky Markey Cancer Center’s Research Communications Office assisted with preparation of this manuscript. This research was supported by the Biospecimen Procurement & Translational Pathology Shared Resource Facility of the University of Kentucky Markey Cancer Center, NCI Cancer Center Support Grant (P30 CA177558).
1. Pezaro C, Omlin A, Lorente D, Rodrigues DN, Ferraldeschi R, Bianchini D, et al. Visceral Disease in Castration-Resistant Prostate Cancer. Eur Urol (2014) 65(2):270–3. doi: 10.1016/j.eururo.2013.10.055
2. Halabi S, Kelly WK, Ma H, Zhou H, Solomon NC, Fizazi K, et al. Meta-Analysis Evaluating the Impact of Site of Metastasis on Overall Survival in Men With Castration-Resistant Prostate Cancer. J Clin Oncol (2016) 34(14):1652–9. doi: 10.1200/JCO.2015.65.7270
3. de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, CHu L, et al. Abiraterone and Increased Survival in Metastatic Prostate Cancer. New Engl J Med (2011) 364(21):1995–2005. doi: 10.1056/NEJMoa1014618
4. Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. Increased Survival With Enzalutamide in Prostate Cancer After Chemotherapy. N Engl J Med (2012) 367(13):1187–97. doi: 10.1056/NEJMoa1207506
5. Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel Plus Prednisone or Mitoxantrone Plus Prednisone for Advanced Prostate Cancer. New Engl J Med (2004) 351(15):1502–12. doi: 10.1056/NEJMoa040720
6. Ghashghaei M, Kucharczyk M, Elakshar S, Muanza T, Niazi T. Combining Prostate Cancer Radiotherapy With Therapies Targeting the Androgen Receptor Axis. Curr Oncol (2019) 26(5):e640–50. doi: 10.3747/co.26.5005
7. Cho E, Mostaghel EA, Russell KJ, Liao JJ, Konodi MA, Kurland BF, et al. External Beam Radiation Therapy and Abiraterone in Men With Localized Prostate Cancer: Safety and Effect on Tissue Androgens. Int J Radiat Oncol Biol Phys (2015) 92(2):236–43. doi: 10.1016/j.ijrobp.2015.01.020
8. Myers MP, Stolarov JP, Eng C, Li J, Wang SI, Wigler MH, et al. P-TEN, the Tumor Suppressor From Human Chromosome 10q23, is a Dual-Specificity Phosphatase. Proc Natl Acad Sci USA (1997) 94(17):9052–7. doi: 10.1073/pnas.94.17.9052
9. de Bono JS, Oudard S, Ozguroglu M, Ozguroglu M, Hansen S, Machiels JP, et al. Prednisone Plus Cabazitaxel or Mitoxantrone for Metastatic Castration-Resistant Prostate Cancer Progressing After Docetaxel Treatment: A Randomised Open-Label Trial. Lancet (2010) 376(9747):1147–54. doi: 10.1016/S0140-6736(10)61389-X
10. Trump DL, Marsh JC, Kvols LK, Citrin D, Davis TE, Hahn RG, et al. A Phase II Trial of Carboplatin (NSC 241240) in Advanced Prostate Cancer, Refractory to Hormonal Therapy. An Eastern Cooperative Oncology Group Pilot Study. Invest New Drugs (1990) 8(Suppl 1):S91–4. doi: 10.1007/BF00171992
11. Corn PG, Heath EI, Zurita A, Ramesh N, Xiao L, Sei E, et al. Cabazitaxel Plus Carboplatin for the Treatment of Men With Metastatic Castration-Resistant Prostate Cancers: A Randomised, Open-Label, Phase 1-2 Trial. Lancet Oncol (2019) 20(10):1432–43. doi: 10.1016/S1470-2045(19)30408-5
12. de Bono J, Mateo J, Fizazi K, Saad F, Shore N, Sandhu S, et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. New Engl J Med (2020) 382(22):2091–102. doi: 10.1056/NEJMoa1911440
13. Abida W, Cheng ML, Armenia J, Middha S, Autio KA, Vargas HA, et al. Analysis of the Prevalence of Microsatellite Instability in Prostate Cancer and Response to Immune Checkpoint Blockade. JAMA Oncol (2019) 5(4):471–8. doi: 10.1001/jamaoncol.2018.5801
14. Hofman MS, Emmett L, Sandhu S, Iravani A, AM J, JC G, et al. [(177)Lu]Lu-PSMA-617 Versus Cabazitaxel in Patients With Metastatic Castration-Resistant Prostate Cancer (TheraP): A Randomised, Open-Label, Phase 2 Trial. Lancet (2021) 397(10276):797–804. doi: 10.1016/S0140-6736(21)00237-3
15. Jamaspishvili T, Berman DM, Ross AE, Scher HI, De Marzo AM, Squire JA, et al. Clinical Implications of PTEN Loss in Prostate Cancer. Nat Rev Urol (2018) 15(4):222–34. doi: 10.1038/nrurol.2018.9
16. Armstrong AJ, Netto GJ, Rudek MA, Halabi S, Wood D, Creel P, et al. A Pharmacodynamic Study of Rapamycin in Men With Intermediate- to High-Risk Localized Prostate Cancer. Clin Cancer Res (2010) 16(11):3057–66. doi: 10.1158/1078-0432.CCR-10-0124
17. Kruczek K, Ratterman M, Tolzien K, Sulo S, Lestingi TM, Nabhan C. A Phase II Study Evaluating the Toxicity and Efficacy of Single-Agent Temsirolimus in Chemotherapy-Naive Castration-Resistant Prostate Cancer. Br J Cancer (2013) 109(7):1711–6. doi: 10.1038/bjc.2013.530
18. George DJ, Halabi S, Healy P, Jonasch D, Anand M, Rasmussen J, et al. Phase 2 Clinical Trial of TORC1 Inhibition With Everolimus in Men With Metastatic Castration-Resistant Prostate Cancer. Urol Oncol (2020) 38(3):79 e15–22. doi: 10.1016/j.urolonc.2019.08.015
19. Massard C, Chi KN, Castellano D, de Bono J, Gravis G, Dirix L, et al. Phase Ib Dose-Finding Study of Abiraterone Acetate Plus Buparlisib (BKM120) or Dactolisib (BEZ235) in Patients With Castration-Resistant Prostate Cancer. Eur J Cancer (2017) 76:36–44. doi: 10.1016/j.ejca.2017.01.024
20. Wei XX, Hsieh AC, Kim W, Friedlander T, AM L, Louttit M, et al. A Phase I Study of Abiraterone Acetate Combined With BEZ235, a Dual PI3K/mTOR Inhibitor, in Metastatic Castration Resistant Prostate Cancer. Oncologist (2017) 22(5):503–e543. doi: 10.1634/theoncologist.2016-0432
21. Graham L, Banda K, Torres A, Carver BS, Chen Y, Pisano K, et al. A Phase II Study of the Dual mTOR Inhibitor MLN0128 in Patients With Metastatic Castration Resistant Prostate Cancer. Invest New Drugs (2018) 36(3):458–67. doi: 10.1007/s10637-018-0578-9
22. Hotte SJ, Chi KN, Joshua AM, Tu D, Macfarlane RJ, Gregg RW, et al. A Phase II Study of PX-866 in Patients With Recurrent or Metastatic Castration-Resistant Prostate Cancer: Canadian Cancer Trials Group Study Ind205. Clin Genitourin Cancer (2019) 17(3):201–208 e201. doi: 10.1016/j.clgc.2019.03.005
23. de Bono JS, De Giorgi U, Rodrigues DN, Massard C, Bracarda S, Font A, et al. Randomized Phase II Study Evaluating Akt Blockade With Ipatasertib, in Combination With Abiraterone, in Patients With Metastatic Prostate Cancer With and Without PTEN Loss. Clin Cancer Res (2019) 25(3):928–36. doi: 10.1158/1078-0432.CCR-18-0981
24. Bono JSD, Sweeney C, Bracarda S, Sternberg CN, Chi KN, Olmos D, et al. PI3K/AKT Pathway Biomarkers Analysis From the Phase III IPATential150 Trial of Ipatasertib Plus Abiraterone in Metastatic Castration-Resistant Prostate Cancer. J Clin Oncol (2021) 39(6_suppl):13–3. doi: 10.1200/JCO.2021.39.6_suppl.13
25. Aparicio AM, Shen L, Tapia EL, Lu JF, Chen HC, Zhang J, et al. Combined Tumor Suppressor Defects Characterize Clinically Defined Aggressive Variant Prostate Cancers. Clin Cancer Res (2016) 22(6):1520–30. doi: 10.1158/1078-0432.CCR-15-1259
26. Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, BS C, et al. Integrative Genomic Profiling of Human Prostate Cancer. Cancer Cell (2010) 18(1):11–22. doi: 10.1016/j.ccr.2010.05.026
27. Brandmaier A, Hou SQ, Shen WH. Cell Cycle Control by PTEN. J Mol Biol (2017) 429(15):2265–77. doi: 10.1016/j.jmb.2017.06.004
28. Papa A, Pandolfi PP. The PTEN(-)PI3K Axis in Cancer. Biomolecules (2019) 9(4). doi: 10.3390/biom9040153
29. Maehama T, Dixon JE. The Tumor Suppressor, PTEN/MMAC1, Dephosphorylates the Lipid Second Messenger, Phosphatidylinositol 3,4,5-Trisphosphate. J Biol Chem (1998) 273(22):13375–8. doi: 10.1074/jbc.273.22.13375
30. Ming M, He YY. PTEN in DNA Damage Repair. Cancer Lett (2012) 319(2):125–9. doi: 10.1016/j.canlet.2012.01.003
31. Puc J, Keniry M, Li HS, Pandita TK, Choudhury AD, Memeo L, et al. Lack of PTEN Sequesters CHK1 and Initiates Genetic Instability. Cancer Cell (2005) 7(2):193–204. doi: 10.1016/j.ccr.2005.01.009
32. Dasari S, Tchounwou PB. Cisplatin in Cancer Therapy: Molecular Mechanisms of Action. Eur J Pharmacol (2014) 740:364–78. doi: 10.1016/j.ejphar.2014.07.025
33. Polkinghorn WR, Parker JS, Lee MX, Kass EM, Spratt DE, Iaquinta PJ, et al. Androgen Receptor Signaling Regulates DNA Repair in Prostate Cancers. Cancer Discovery (2013) 3(11):1245–53. doi: 10.1158/2159-8290.CD-13-0172
34. Sekhar KR, Wang J, Freeman ML, Kirschner AN. Radiosensitization by Enzalutamide for Human Prostate Cancer is Mediated Through the DNA Damage Repair Pathway. PloS One (2019) 14(4):e0214670. doi: 10.1371/journal.pone.0214670
35. Goto H, Izawa I, Li P, Inagaki M. Novel Regulation of Checkpoint Kinase 1: Is Checkpoint Kinase 1 a Good Candidate for Anti-Cancer Therapy? Cancer Sci (2012) 103(7):1195–200. doi: 10.1111/j.1349-7006.2012.02280.x
36. Paul BT, Blanchard Z, Ridgway M, ElShamy WM. BRCA1-IRIS Inactivation Sensitizes Ovarian Tumors to Cisplatin. Oncogene (2015) 34(23):3036–52. doi: 10.1038/onc.2014.237
37. Goodwin JF, Schiewer MJ, Dean JL, Schrecengost RS, de Leeuw R, Han S, et al. A Hormone-DNA Repair Circuit Governs the Response to Genotoxic Insult. Cancer Discovery (2013) 3(11):1254–71. doi: 10.1158/2159-8290.CD-13-0108
38. Koontz BF, Hoffman KE, Halabi S, Healy P, Anand M, George DJ, et al. Combination of Radiation Therapy and Short-Term Androgen Blockade With Abiraterone Acetate Plus Prednisone for Men With High- and Intermediate-Risk Localized Prostate Cancer. Int J Radiat Oncol Biol Phys (2021) 109(5):1271–8. doi: 10.1016/j.ijrobp.2020.11.059
Keywords: metastatic castration refractory prostate cancer, PI3K/AKT pathway, carboplatin, abiraterone acetate, ATM/Chk2/p53 signal pathway
Citation: Myint ZW, Allison DB and Ellis CS (2021) A Case Report of Metastatic Castration-Resistant Prostate Cancer Harboring a PTEN Loss. Front. Oncol. 11:731002. doi: 10.3389/fonc.2021.731002
Received: 25 June 2021; Accepted: 27 August 2021;
Published: 23 September 2021.
Edited by:
Sakthivel Muniyan, University of Nebraska Medical Center, United StatesReviewed by:
Dharmesh Gopalakrishnan, University at Buffalo, United StatesCopyright © 2021 Myint, Allison and Ellis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zin W. Myint, zin.myint@uky.edu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.