
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 13 September 2021
Sec. Thoracic Oncology
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.724692
This article is part of the Research Topic New Trends in Early-Stage Lung Cancer Presenting as Ground-Glass Opacities: Clinical, Pathological and Molecular Aspects View all 14 articles
 Zihan Wei1,2†
Zihan Wei1,2† Ziyang Wang1,2†
Ziyang Wang1,2† Yuntao Nie1
Yuntao Nie1 Kai Zhang1
Kai Zhang1 Haifeng Shen1
Haifeng Shen1 Xin Wang1,2
Xin Wang1,2 Manqi Wu1,2
Manqi Wu1,2 Fan Yang1
Fan Yang1 Kezhong Chen1*
Kezhong Chen1*Background and Aims: Nodular ground-glass lesions have become increasingly common with the increased use of computed tomography (CT), while the genomic features of ground-glass opacities (GGOs) remain unclear. This study aims to comprehensively investigate the molecular alterations of GGOs and their correlation with radiological progression.
Methods: Studies from PubMed, Embase, Cochrane Library, and Web of Science, using PCR, targeted panel sequencing, whole exosome sequencing, and immunohistochemistry, and reporting genomic alterations or PD-L1 expressions in lung nodules presenting as GGOs until January 21, 2021 were included in this study. Chi-square test, random-effects model, and Z-test analysis were adopted to analyze the data.
Results: A total of 22 studies describing mutations in lung adenocarcinoma (LUAD) with GGOs were analyzed. EGFR was the most frequently mutative gene (51%, 95%CI 47%–56%), followed by TP53 (18%, 95%CI 6%–31%), HER2 (10%, 95%CI 0%–21%), ROS1 (6%, 95%CI 0%–18%), and KRAS (6%, 95%CI 3%–9%). The correlation between the frequency of EGFR mutation and radiological was observed and the differences were found to be not statistically significant in the subgroups, which are listed as below: radiological: gGGO 47.40%, 95%CI [38.48%; 56.40%]; sGGO 51.94%, 95%CI [45.15%; 58.69%]. The differences of the frequency of KRAS mutation in the different subgroups were also consistent with this conclusion, which are listed as: radiological gGGO 3.42, 95%CI [1.35%; 6.13%]; sGGO 12.27%, 95%CI [3.89%; 23.96%]. The pooled estimated rate of PD-L1 was 8.82%, 95%CI [5.20%–13.23%]. A total of 11.54% (3/26) of the SMGGNs were confirmed to be intrapulmonary spread by WES.
Conclusions: Somatic genetic alterations are considered in early-stage GGO patients without distinct changes of the frequency following the progress of the tumor. This review sheds insight on molecular alterations in LUAD with GGOs.
Ground-glass opacities (GGOs), defined as hazy increased density of the lungs with bronchial and vascular margins on computed tomography (CT) (1, 2), often associate with lung cancers, especially lung adenocarcinomas (LUADs), and are commonly detected in East-Asia patients. GGOs, being radiologically distinct clinical entities, which were known to have an indolent clinical course, present a superior survival after resection, especially pure GGOs with a nearly 100% long-term disease-free survival (DFS), shown in many previous studies (3, 4), indicating the unique biology of GGOs. However, the molecular characteristics of GGO-associated lung cancers have not been systematically reviewed due to the limitation of sample size and different criteria used while reporting, and, therefore, the tumor evolutionary mechanism behind the slow-growing appearance in GGOs is not clear. In addition, there are many patients with synchronous multiple ground-glass nodules (SMGGNs) on their initial CT. And some of them are found to have an intrapulmonary spread, even if the initial lesions seem to be in a fairly early-stage.
Therefore, we meta-analyzed the extracted data under certain criteria to demonstrate the dynamic genomic alterations in the diversity of GGO patients. This review can provide a novel insight into the molecular alterations in LUAD patients with GGOs and new views for the biology behavior of GGOs.
Three distinctive keywords were identified as follows: “ground-glass opacity”, “gene alterations”, and “PD-L1”. MeSH term database from the National Center for Biotechnology Information (NCBI) was searched to find all the possible expressions for these keywords which were defined as free words. The final search strategy was combined with both the MeSH terms and free words, which is listed as follows: #1: “GGO” OR “GGN” OR “ground glass opacity” OR “ground glass nodule” OR “ground glass nodules” OR “ground-glass opacity” OR “ground-glass nodule” OR “ground-glass nodules” OR “subsolid nodule” OR “subsolid nodules” OR “subsolid pulmonary nodules”, #2: “Gene” OR “Cistron” OR “Cistrons” OR “Genetic Materials” OR “Genetic Material” OR “genetic feature” OR “genetic characteristics” OR “genetic characteristic” OR “genetic features” OR “Genomic alteration” OR “Genomic alterations” OR “EGFR” OR “epidermal growth factor receptor” OR “TTF-1” OR “thyroid transcription factor 1” OR “ALK” OR “anaplastic lymphoma kinase” OR “KRAS” OR “Kirsten rat sarcoma” OR “HER2” OR “human epidermal growth factor receptor type 2” OR “oncogenic driver”, and #3: “PD-L1” OR “programmed cell death 1 ligand 1 protein” OR “PDL1” OR “CD274” OR “B7-H1” OR “B7H1”. “#1 AND #2” and “#1 AND #3” were searched in the four databases up to January 21, 2021, without language limitations, respectively.
Firstly, all the papers retrieved from the search were screened by reviewing the titles and abstracts, during which period, reviews, case studies, editorials, meeting abstracts, and papers not meeting any of our search criteria were excluded. Then, the full contents of the rest papers were evaluated carefully to distinguish the ones that perfectly fit our inclusion criteria, analyzing the molecular alterations in a consecutive cohort of patients with GGOs, during which period, some papers were excluded for the following reasons (1): the cohort was developed to analyze the characters of the nodules with specific molecular alterations (2); insufficient data for analyses; and (3) papers not written in English. Two authors (ZWe and ZWa) conducted the procedure independently to evaluate the study eligibility for our review. This analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (5).
The following basic data were extracted from the selected papers: author(s), year of publication, size and region of the cohort, characteristics of the patients in the study, radiological and pathological details of the nodules, the methodological details, and relevant statistical findings for the entire cohort and/or by population subgroups. Two authors (ZWe and ZWa) collected these data independently, and any discrepancies between the two authors were resolved by discussions with a third author (KC).
We firstly performed a descriptive analysis summarizing all the rates of gene alterations reported in the eligible works. Then, the rates of the gene alterations which had been reported in more than three studies were pooled using random-effects meta-analysis models allowing for the inherent heterogeneity of observational studies (6), after a data-transformation and normality-check using the variance-stabilizing double-arcsine transformation method (7). Q and I2 statistics were calculated to assess the heterogeneity between study-specific estimates (8). Forest plots were adopted to show a graphical presentation of the meta-analysis results, whereas Z-test was applied to check the level of significance of the differences of the pooled estimated rates from different groups, where the values of p less than 0.05 were considered to be significant. The publication biases were assessed by Egger test, which is based on a weighted linear regression of the effect on its standard error. All the analyses were implemented with R (version 4.0.3).
In this systematic review, the 25 included studies were all cross-sectional studies. All the patients had been diagnosed with lung cancer before or during their treatment. The authors finalized the list of included articles through discussion and agreement. Data from the articles were independently extracted by two authors (ZWe and ZWa) who were not involved in any of the reviewed studies. As recommended by the Agency for Healthcare Research and Quality, the assessment of the methodological quality of the included studies was made from 11 perspectives with the Cross-Sectional/Prevalence Study Quality, a scoring system specific for a cross-sectional study (Supplementary Table 1).
After removing the duplicated records, 680 records related to gene alterations and 25 records related to PD-L1 expression were selected for further assessment with the titles and abstracts. From the remaining records, 27 records related to gene alterations and 6 records related to PD-L1 expression were carefully selected through the evaluation of the full contents as the second round of selection. Finally, 22 gene-alteration-reported articles and 4 PD-L1-expression-reported articles were included in the following analysis, as shown in a PRISMA diagram (Figure 1).
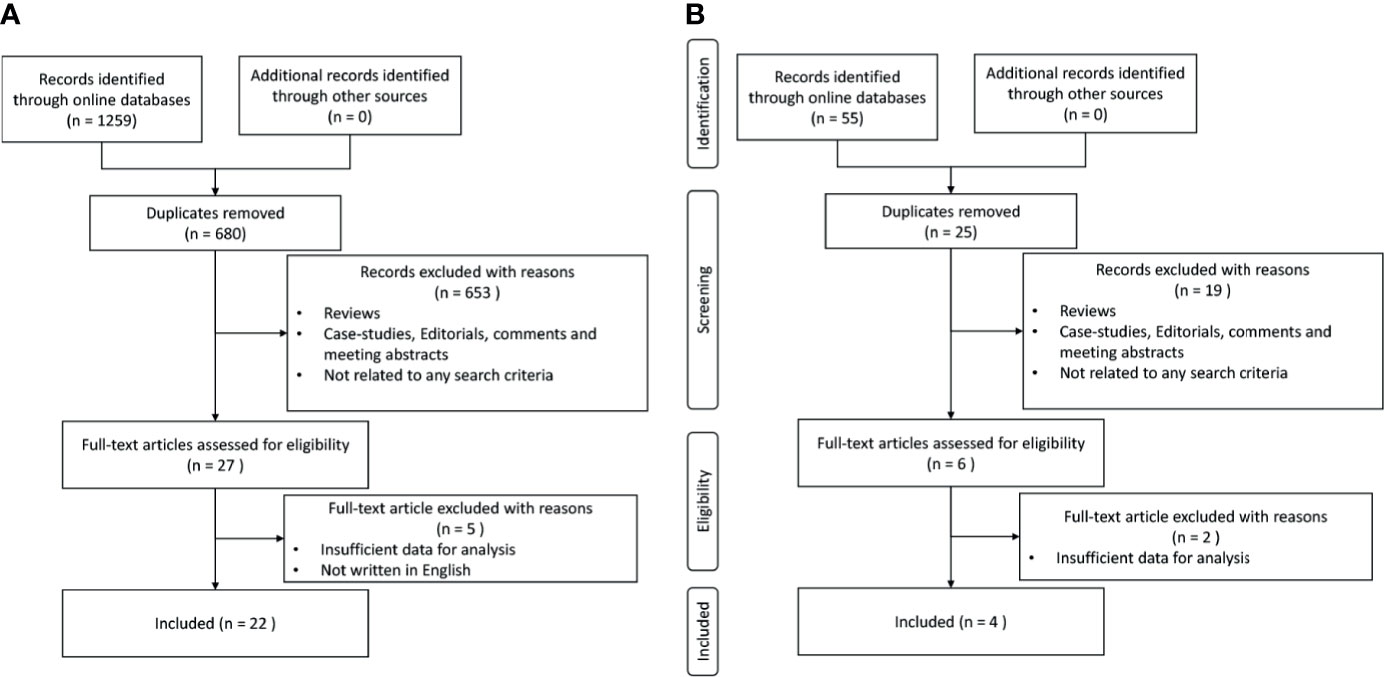
Figure 1 Process of study selection. (A) Study selection with genetic alterations. (B) Study selection with PD-L1 expressions.
All the cohorts from the 25 studies included were composed of Asians (Table 1), except that one cohort (29) also included Caucasians. In the 22 cohorts reporting gene alterations, the median cohort size was 135 [interquartile range (IQR): 25–210], of which, 16 cohorts included patients with solitary pulmonary nodules, while 6 cohorts put their attention on multiple pulmonary nodules, and the pathological subtypes of all these nodules were adenocarcinoma. While nearly half of the studies (11/22) focused on early-stage LUAD, 8 of the 22 studies included some stage III/IV cases (3 articles did not mention the clinical stage of the nodules). No subgroup analysis was performed due to the lack of data. Only two studies used whole exosome sequencing in their analysis, other than PCR or targeted gene sequencing. Among the four articles reporting PD-L1 expression included (Table 1) in our review, only one article reported gene alterations at the same time. All four cohorts were formed with Asians, two with Chinese, and two with Japanese. No significant publication bias were seen in the analysis (EGFR, p = 0.9419; KRAS, p = 0.7106; ALK, p = 0.0918; PD-L1, p = 0.89).
Being the most validated genetic mutation, EGFR was the most prominent variation as well [51%, 95%CI (47%, 56%)], followed by TP53 [18%, 95%CI (6%, 31%)], HER2 [10%, 95%CI (0%, 21%)], ROS1 [6%, 95%CI (0%, 18%)], KRAS [6%, 95%CI (3%, 9%)] (Figure 2). Meanwhile, we summarized the rates of the top two validated gene alterations, EGFR mutation and KRAS mutation, to conduct a subgroup analysis.
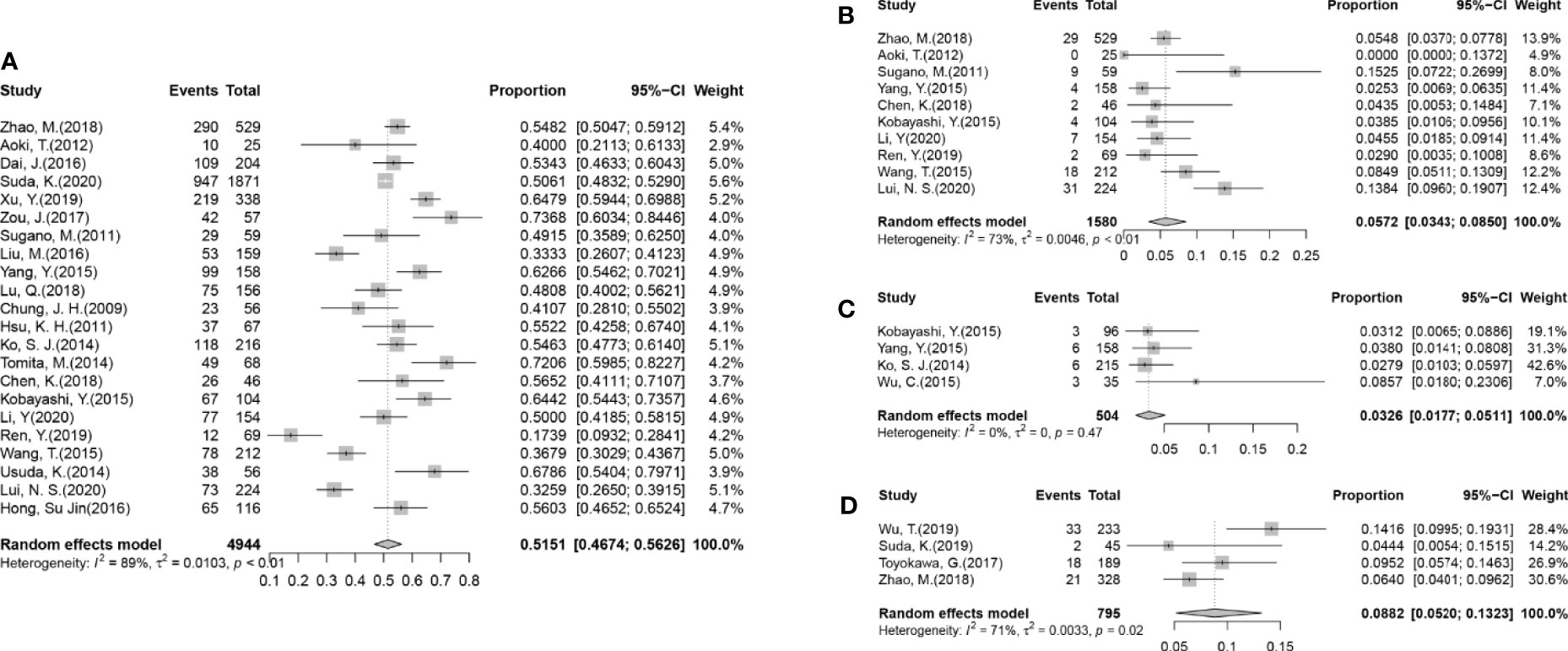
Figure 2 Forest plots from the meta-analysis of published gene alterations in GGOs. (A) The pooled estimated rate of the EGFR mutation in 22 articles. (B) The pooled estimated rate of the KRAS mutation in 10 articles. (C) The pooled estimated rate of the ALK rearrangement in four articles. (D) The pooled estimated rate of the PD-L1 expression in four articles.
All the 25 articles that were included reported the rates of EGFR mutations in their cohorts (Table 1), in which 2,536/4,944 cases (51.29%) were found to harbor EGFR mutations. After performing a meta-analysis with the random-effects model (Figure 2A), the pooled estimated rate of EGFR mutations was found to be 51.51% [95%CI (46.74%, 56.26%)].
Further analyses were conducted according to the radiological subgroups with the random-effects model (Figure 3). A G/T ratio, defined as the ratio of the ground-glass opacity (GGO) component to the tumor size at CT, ≥50% is suggested to be a sign of pathologically noninvasiveness. Additionally, the rates of lymph node metastasis range from 21% to 26% in lesions ≤3 cm with a G/T ratio ≤ 50% (34–36). Therefore, G/T ratio was used to divide the nodules into two groups in our review: gGGO (ground-glass dominant GGO) 50%<G/T ratio ≤ 100%; sGGO (solid dominant GGO) 0<G/T ratio ≤ 50%. The data of each subgroup were extracted from 10 articles that reported the necessary details according to the division criteria, and the EGFR mutation rate of each subgroup after analyzing with the random-effects model was listed in Table 2. It was found that the EGFR mutation rate has a marginal increment with the radiological progression of GGOs, but the difference was not statistically significant (p = 0.4828). Our results showed that with the radiological progression of GGOs, the frequency of EGFR mutation was stable.
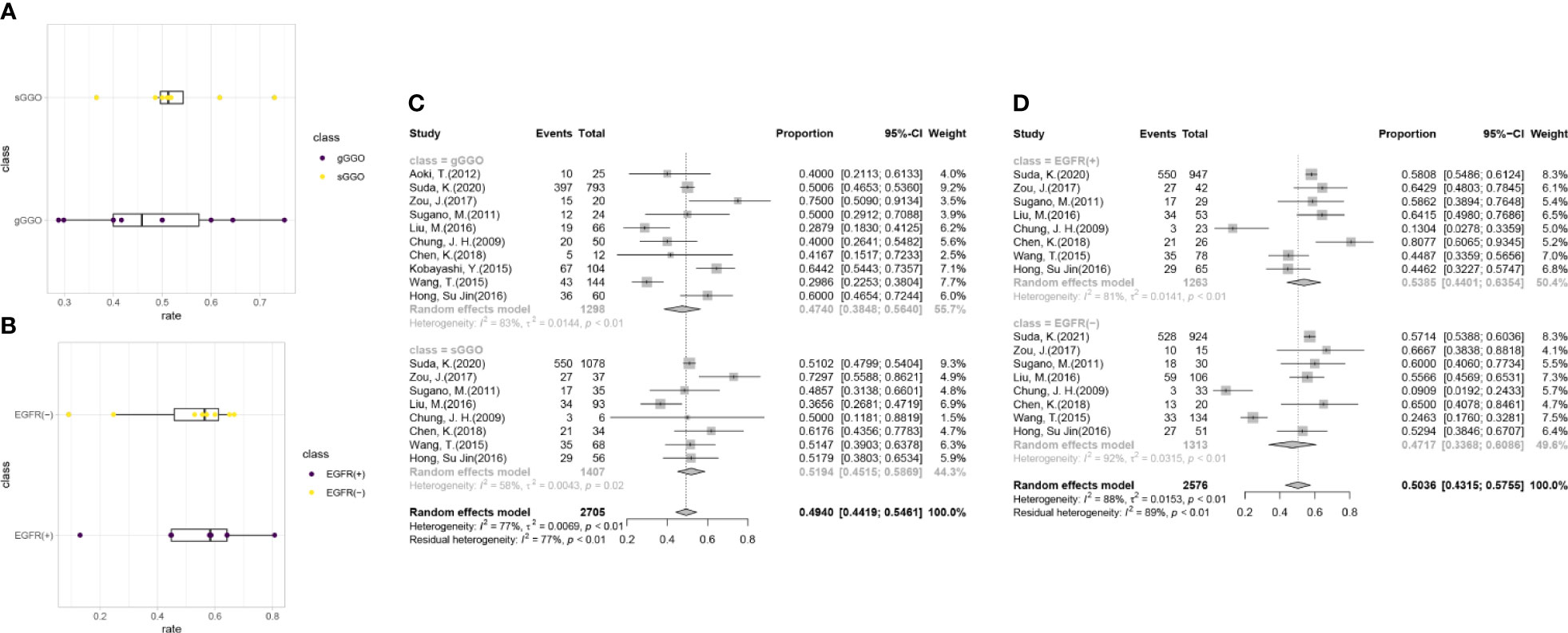
Figure 3 Subgroup analysis for the EGFR mutations. (A) The distribution of the EGFR gene mutation rates in the subgroups with a different radiological density. (B) The distribution of sGGOs in the subgroups with a different status of EGFR mutation. (C) Forest plots from the subgroup meta-analysis of EGFR alterations in GGOs with different radiological subtypes. (D) Forest plots from the subgroup meta-analysis of the percentage of sGGOs in GGOs with a different status of EGFR mutation. gGGO, ground-glass dominant GGO; 50%<G/T ratio ≤ 100%; sGGO, solid dominant GGO; 0<G/T ratio ≤ 50%.
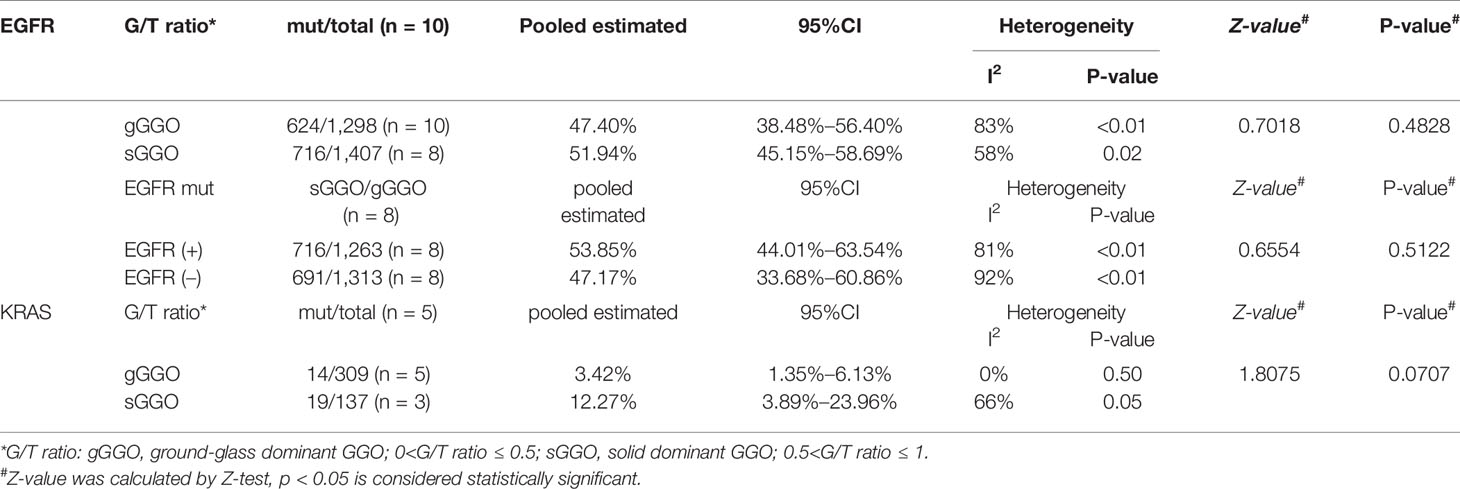
Table 2 Details of the radiological subgroup analysis for the EGFR mutation and KRAS mutation by the random effect model.
Moreover, the mutation rates of EGFR subtypes were collected and analyzed (Supplementary Figure 2), and we found that the rate of L858R mutation was approximately equal to that of 19del mutation, composed over half of all EGFR mutations together. Though the rate of T790M mutation was relatively low (0.58%), there were still early-stage GGOs harboring T790M mutations.
In order to uncover whether EGFR mutation would have an influence on tumor progression, we divided the researches collected in our analysis into two groups by the mutation status of EGFR, among which, two studies included only cases with gGGOs and were excluded in the following analysis. The result shows that the proportion of the sGGOs was fairly the same in the groups with a different EGFR-mutation status, which is 53.85% [95%CI (44.01%, 63.54%)] in the EGFR(+) group and 47.17% [95%CI (33.68%, 60.86%)] in the EGFR (–) group, with a p-value at 0.5122 (Table 2). Though the heterogeneity between studies was still high, the result in each study can still confirm our results—sGGOs compose about 50% in whether EGFR(+) or EGFR (–) groups in each study.
A total of 10 articles had reported the rates of KRAS mutation (Figure 2B and Supplementary Figure 1), in which 106/1,580 (6.71%) cases were reported to harbor the KRAS mutation, which was far less than the EGFR mutation. The pooled estimated rate of the KRAS mutation was 5.72% [95%CI (3.43%, 8.50%)]. Subgroups were also divided according to the criteria mentioned before. In the radiological subgroups, it was clear to demonstrate that the rate of the KRAS mutation increased with the decrease of G/T ratio numerically (gGGO 3.42%, sGGO 12.27%), but the difference was not statistically significant (p = 0.07), due to the large 95% confidence intervals and high heterogeneities (Table 2).
The rate of ALK rearrangement in GGOs, reported in 4 of the 22 studies, was 18/504 (3.57%) after enumeration (Figure 2C), and the pooled estimated rate was 3.26% [95%CI (1.17%, 5.11%)]. The heterogeneity of the studies reporting an ALK arrangement was fairly low (I2 = 0%, p = 0.47).
Among all 795 cases, 74 (9.31%) were found with the PD-L1 expression (Figure 2D). Though with a significant heterogeneity in the method and cut-off values assessing the PD-L1 expression, it can be confirmed that the rate of the PD-L1 expression in GGOs is fairly low, as the pooled estimated rate of the PD-L1 expression of the nodules was 8.82% (95% CI 5.20%–13.23%). There was only one article (33) that provided details in the subgroups, so a meta-analysis could not be performed on the subgroups. Still, it was shown in the articles that the rate of PD-L1 expression was significantly lower in GGOs (4.44%–14.16%) than solid nodules (18.36%–35.04%) in the same cohorts (9, 31–33).
A total of 6/22 articles included synchronous multiple ground-glass nodules (SMGGNs) in their cohorts, while only the data from 4 articles could be extracted for analysis. In the 123 cases with SMGGNs included, 8 cases (6.50%) possessed identical mutations in their resected nodules which were doubted to be an intrapulmonary spread. Only one article used whole-exosome sequencing (WES) and confirmed 3/26 (11.54%) cases to have an intrapulmonary spread. The genetic alterations of the cases are listed below (Table 3). The distribution of genetic alterations appeared to have no significant differences between genetic alteration rates in whether multicentric origin nodules (Table 3A) or intrapulmonary spread nodules (Table 3B).
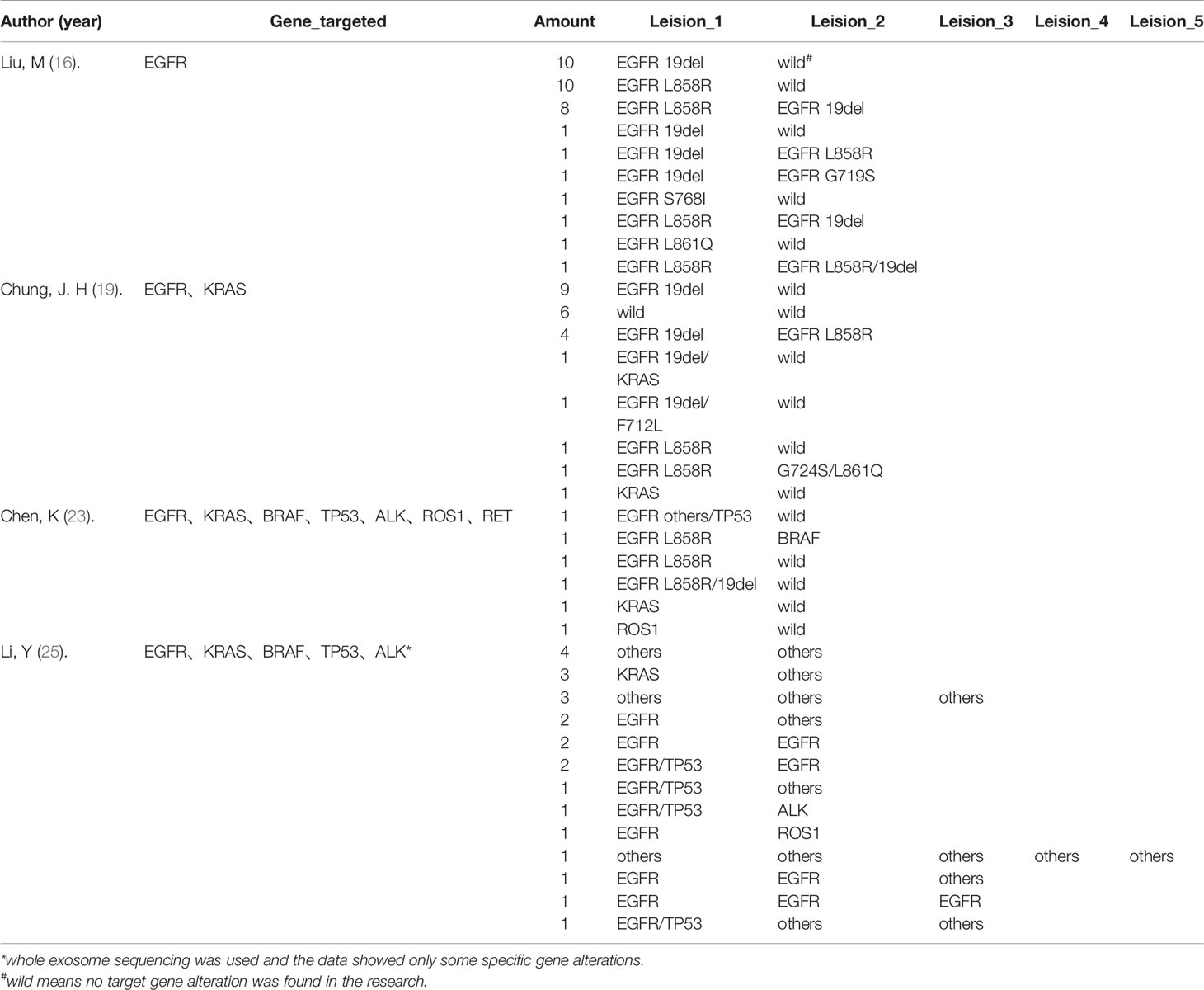
Table 3 Gene mutations in synchronous multiple ground-glass nodules (SMGGNs).TABLE 3A Data extracted from four articles which showed the gene alterations among multicentric SMGGNs.

Table 3B Three cases confirmed by Li, Y (25). which applied WES to analyze gene alterations in their research.
This systematic review investigated the molecular alterations in lung nodules presenting as ground-glass opacities and analyzed the trend of tumor genetic alterations along with the radiological progress.
As it is known to us that EGFR mutation was first reported in 2004 (37), and associate with non-smokers, female, LUAD tightly (38, 39). EGFR mutations were present in 10% of cases in Caucasians, while 30% in East Asians (40, 41), which may explain why the cohorts included in this review were mostly Asian. Among all the reported genetic alterations analyzed in this review, EGFR mutation was clearly the most validated and highest incidence genetic alteration, which is similar to previous studies (41). Some researchers had mentioned in their study (42) that EGFR functioned in tumor genesis, and also played an important role in tumor processing (41, 43). To the best of our best knowledge, this review is the first meta-analysis for the EGFR mutation rate especially in GGOs, which shows that with the progression in radiological, there is no significant difference in the rate of EGFR mutation (p ≥ 0.05), suggesting that EGFR mutation, as a driver mutation for lung cancer, count for tumorigenesis in a relatively early stage, and maintain consistency in the progression of tumors. In addition, though relatively low, there were still early-stage GGOs harboring T790M mutations, indicating that T790M might play a role in tumorigenesis, which is known to associate with chemotherapy resistance.
Transforming from gGGOs to sGGOs have always been considered as a sign of tumor progression and is widely used in daily clinical work. So, we defined such transformation as tumor progression in our meta-analysis. Researchers have a heated discussion about the factors distinguishing between the easy-to-progress GGOs and indolent GGOs for a long time with no consensus, which mainly lie in a large size, the presence of a solid portion, old age, gene alterations, and so on (44–46). Unfortunately, our findings indicated that EGFR mutation has a little impact on tumor progression. Statistically, there is no difference between the distribution of gGGOs and sGGOs whether in the EGFR mutation group or in the wild type group. Whether some other signaling pathways can and under what conditions they will regulate the progression of GGOs, and whether there were any signs besides genetic alterations such as genetic heterogeneity or chromosome instability require more studies to confirm, which may help us get a deeper understanding of the biological behavior of GGOs.
When taking KRAS mutation as one of the earliest discoveries of genetic alterations in lung cancers (41, 47) and reported as very important for tumor progression (48) into account, there seemed to bear discrepancy at different stages of tumor progression. Though not statistically different, the frequency of KRAS mutation seems to increase with the increasing G/T ratio, suggesting a relationship between KRAS mutation and tumor progression, resulting in a higher frequency in more progressed lung nodules presenting with GGOs.
Using antibodies targeting the PD-1 pathway is a promising and effective option of immunotherapy, a newly developed treatment of NSCLC (49, 50), where PD-L1 is used as a biomarker to predict the immunotherapy response (51). We clearly showed that the incidence of PD-L1 expression was much lower in GGOs than pure-solid tumors in several articles, which was verified by Suda et al. in a clinical experiment including 124 qualified patients (4% vs. 25%, p < 0.01) (32). Wu et al. also found in a small-size cohort that even for the same patient, the volume of synchronous GGOs showed no significant change before and after treatment (4,160.2 vs 4,185.5 mm3, p = 0.6050) than solid nodules (52). Therefore, it is predictable that PD-1 treatment is less effective for patients with GGOs.
When compared to lung cancer presenting as solid nodule(s), Zhao et al (9) reported that the EGFR mutation rate was higher in solid nodules than in GGOs, especially the subtype mutation of 19 del, agreeing with previous studies (14). They also reported that in patients with GGOs, there are significantly more frequent HER2 mutations (p = 0.033), while less frequent ALK translocations (p = 0.014) and PIK3CA mutations (p = 0.012), compared to patients with solid nodules (GGOs/Solid nodules = 529/718). However, contrary to other studies in our analysis, Hong et al. (30) find that EGFR mutations were significantly more frequent in tumors with GGOs than in solid tumors, which may be caused by the different cohort sizes, regions of the cohorts, and the methods used to detect the mutations, suggesting that more researches are needed to elucidate the difference in the rates of mutations between GGOs and solid nodules.
Despite PCR, WES, and WGS which are used in the studies included in our study, with the development of molecular diagnostic technology, the field of liquid biopsy has gradually developed a lot. Analyzing circulating tumor DNA (ctDNA) at the genomic level, a newly-emerged non-invasive approach, is proposed to have the ability to distinguish between malignant and benign disease (53, 54) and at the same time detect the molecular alterations carried by the nodules, and then guide targeted therapy and immunotherapy. However, it has been shown that indolent GGO-predominant lung cancers shed lower-level ctDNA, which is less detectable to help identify cancer in patients (55). Therefore, for very early lesions, such as GGOs, it is difficult to achieve an early diagnosis by ctDNA SNV testing under the current technology limitations with a low sensitivity.
Two studies reported independently that different pathological lesions could share identical mutations even in pre-invasive LUADs, such as AAH and AIS lesions (56, 57). This review also points out that different GGOs could share the same mutation in patients with SMGGNs, shows that SMGGNs might have an intrapulmonary spread, despite the multicentric regions. Detecting the mutation status of a specific gene by PCR is only a small fragment of the whole genome and does not represent the expression status of the whole genome, so using whole exon sequencing (WES) or whole-genome sequencing (WGS) to determine an intrapulmonary metastasis of GGO sounds more convincing. In the 26 cases with SMGGNs reported by Li et al. (25) using WES, 3 cases (11.54%) were confirmed to have an intrapulmonary spread. Despite the articles analyzed in our review, we noted that Li et al. (58) reported another two cases to be an intrapulmonary spread by WES in a case report. Though EGFR mutations were found in four of the five confirmed cases, it is still too early to come to a conclusion that specific molecular alterations are associated with the intrapulmonary spreading of GGOs. We need to be concerned that though GGO is usually considered an early-stage lesion, it has a certain probability of metastasis. However, the exact mechanism of metastasis in GGOs, non-invasive cancer, is still unclear. We noted that these GGOs were all in a close proximity which might result in dissemination along the airway. Furthermore, whether these multiple GGOs sharing the same mutation affects the prognosis needs to be explored by an in-depth longer follow-up clinical and mechanistic analysis.
With the development of an immune checkpoint inhibitor treatment, especially the inspiring results of neoadjuvant immune therapy, a series of studies have focused on the immune-environment of early-stage lung cancer patients. The TRACEx cohort reported that sparsely infiltrated tumors exhibited a waning of neoantigen editing during tumor evolution, compared with immune-infiltrated tumor regions exhibiting an ongoing immunoediting, with either loss of heterozygosity in human leukocyte antigens or depletion of expressed neoantigens (59).Recently, some researchers have used single-cell tumor sequencing to map the tumor microenvironment and have found that GGO has less endothelial cell angiogenesis, downregulated EGR1 expression, upregulated KLF6 expression, a significantly higher proportion of NK cells, and showing a marked metabolic disorder and immune response stress, compared to an advanced lung cancer (60, 61). These studies, although with a limited sample size, initially revealed distinct immune mechanisms in GGOs from non-GGO lung adenocarcinomas, helping us to further understand the essence of the inert progression of GGO and to identify the nodules with a poorer prognosis at an early stage.
Our research revealed that EGFR mutation is not associated with the radiological progression of GGOs, which means EGFR mutation was a driver mutation for lung cancer in a fairly early stage, and maintains consistency in the progression of tumors. On the contrary, the frequency of KRAS mutation was higher in progressed lung nodules, indicating a position for KRAS mutation in tumor progression. Immunotherapy, as one of the recently discovered effective therapies for advanced lung cancer, is less effective against GGOs, which may be due to the low expression level of PD-L1 in early-stage lung cancer, found by our research. Though GGOs are usually considered early-stage lesions, there does have a possibility for SMGGNs to have an intrapulmonary spread, the mechanism behind which is still unclear. The limitation of our meta-analysis lies in its retrospective design; postsurgical follow-up or treatment plans at recurrence would differ among attending surgeons. Also, the high heterogeneity between the methodologies and results of researches is another limitation of our research. Overall, this review summarizes the published estimates of the rates of molecular alterations in lung nodules presenting as GGOs, which may help clinical treatment decisions for GGOs and provide a novel insight in revealing the molecular alterations behind GGOs.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
ZWe and ZWa conceived and planned the experiments. ZWe and ZWa carried out the experiments. ZWe, ZWa, HS, XW, and MW contributed to the data preparation. ZWe, ZWa, YN, and KZ verified the analytical methods. ZWe, ZWa, YN, KZ, HS, XW, and MW contributed to the interpretation of the results. ZWe and ZWa took the lead in writing the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the National Natural Science Funds (grant No. 82072566) and Peking University People’s Hospital Research and Development Funds (grant No. RS2019-01).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank our colleagues from the Department of Thoracic Surgery, Peking University People’s Hospital, Beijing, China who provided their insight and expertise that greatly assisted the research, although they may not be informed of all the interpretations/conclusions of this paper.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.724692/full#supplementary-material
1. Austin JH, Müller NL, Friedman PJ, Hansell DM, Naidich DP, Remy-Jardin M, et al. Glossary of Terms for CT of the Lungs: Recommendations of the Nomenclature Committee of the Fleischner Society. Radiology (1996) 200(2):327–31. doi: 10.1148/radiology.200.2.8685321
2. Henschke CI, Yankelevitz DF, Mirtcheva R, McGuinness G, McCauley D, Miettinen OS. CT Screening for Lung Cancer: Frequency and Significance of Part-Solid and Nonsolid Nodules. AJR Am J Roentgenol (2002) 178(5):1053–7. doi: 10.2214/ajr.178.5.1781053
3. Fu F, Zhang Y, Wen Z, Zheng D, Gao Z, Han H, et al. Distinct Prognostic Factors in Patients With Stage I Non-Small Cell Lung Cancer With Radiologic Part-Solid or Solid Lesions. J Thorac Oncol (2019) 14(12):2133–42. doi: 10.1016/j.jtho.2019.08.002
4. Hattori A, Hirayama S, Matsunaga T, Hayashi T, Takamochi K, Oh S, et al. Distinct Clinicopathologic Characteristics and Prognosis Based on the Presence of Ground Glass Opacity Component in Clinical Stage IA Lung Adenocarcinoma. J Thorac Oncol (2019) 14(2):265–75. doi: 10.1016/j.jtho.2018.09.026
5. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann Intern Med (2009) 151(4):264–9. doi: 10.7326/0003-4819-151-4-200908180-00136
6. DerSimonian R, Laird N. Meta-Analysis in Clinical Trials Revisited. Contemp Clin Trials (2015) 45(Pt A):139–45. doi: 10.1016/j.cct.2015.09.002
7. Freeman MF, Tukey JW. Transformations Related to the Angular and the Square Root. Ann Math Stat (1950) 21(4):607–11. doi: 10.1214/aoms/1177729756
8. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring Inconsistency in Meta-Analyses. BMJ (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
9. Zhao M, Zhan C, Li M, Yang X, Yang X, Zhang Y, et al. Aberrant Status and Clinicopathologic Characteristic Associations of 11 Target Genes in 1,321 Chinese Patients With Lung Adenocarcinoma. J Thorac Dis (2018) 10(1):398–407. doi: 10.21037/jtd.2017.12.68
10. Aoki T, Hanamiya M, Uramoto H, Hisaoka M, Yamashita Y, Korogi Y. Adenocarcinomas With Predominant Ground-Glass Opacity: Correlation of Morphology and Molecular Biomarkers. Radiology (2012) 264(2):590–6. doi: 10.1148/radiol.12111337
11. Dai J, Shi J, Soodeen-Lalloo AK, Zhang P, Yang Y, Wu C, et al. Air Bronchogram: A Potential Indicator of Epidermal Growth Factor Receptor Mutation in Pulmonary Subsolid Nodules. Lung Cancer (2016) 98:22–8. doi: 10.1016/j.lungcan.2016.05.009
12. Suda K, Mitsudomi T, Shintani Y, Okami J, Ito H, Ohtsuka T, et al. Clinical Impacts of EGFR Mutation Status: Analysis of 5780 Surgically Resected Lung Cancer Cases. Ann Thorac Surg (2021) 111(1):269–76. doi: 10.1016/j.athoracsur.2020.05.041
13. Zheng M, Wang N, Wang R. Comprehensive Study of Surgical Treated Lung Adenocarcinoma With Ground Glass Nodule Component. Med Sci monitor Int Med J Exp Clin Res (2019) 25:8492–8. doi: 10.12659/msm.919532
14. Zou J, Lv T, Zhu S, Lu Z, Shen Q, Xia L, et al. Computed Tomography and Clinical Features Associated With Epidermal Growth Factor Receptor Mutation Status in Stage I/II Lung Adenocarcinoma. Thorac Cancer (2017) 8(3):260–70. doi: 10.1111/1759-7714.12436
15. Sugano M, Shimizu K, Nakano T, Kakegawa S, Miyamae Y, Kaira K, et al. Correlation Between Computed Tomography Findings and Epidermal Growth Factor Receptor and KRAS Gene Mutations in Patients With Pulmonary Adenocarcinoma. Oncol Rep (2011) 26(5):1205–11. doi: 10.3892/or.2011.1412
16. Liu M, He W-X, Song N, Yang Y, Zhang P, Jiang G-N. Discrepancy of Epidermal Growth Factor Receptor Mutation in Lung Adenocarcinoma Presenting as Multiple Ground-Glass Opacities. Eur J Cardiothorac Surg (2016) 50(5):909–13. doi: 10.1093/ejcts/ezw113
17. Yang Y, Yang Y, Zhou X, Song X, Liu M, He W, et al. EGFR L858R Mutation is Associated With Lung Adenocarcinoma Patients With Dominant Ground-Glass Opacity. Lung Cancer (2015) 87(3):272–7. doi: 10.1016/j.lungcan.2014.12.016
18. Lu Q, Ma Y, An Z, Zhao T, Xu Z, Chen H. Epidermal Growth Factor Receptor Mutation Accelerates Radiographic Progression in Lung Adenocarcinoma Presented as a Solitary Ground-Glass Opacity. J Thorac Dis (2018) 10(11):6030–9. doi: 10.21037/jtd.2018.10.19
19. Chung J-H, Choe G, Jheon S, Sung S-W, Kim TJ, Lee KW, et al. Epidermal Growth Factor Receptor Mutation and Pathologic-Radiologic Correlation Between Multiple Lung Nodules With Ground-Glass Opacity Differentiates Multicentric Origin From Intrapulmonary Spread. J Thorac Oncol (2009) 4(12):1490–5. doi: 10.1097/JTO.0b013e3181bc9731
20. Hsu K-H, Chen K-C, Yang T-Y, Yeh Y-C, Chou T-Y, Chen H-Y, et al. Epidermal Growth Factor Receptor Mutation Status in Stage I Lung Adenocarcinoma With Different Image Patterns. J Thorac Oncol (2011) 6(6):1066–72. doi: 10.1097/JTO.0b013e31821667b0
21. Ko S-J, Lee YJ, Park JS, Cho Y-J, Yoon HI, Chung J-H, et al. Epidermal Growth Factor Receptor Mutations and Anaplastic Lymphoma Kinase Rearrangements in Lung Cancer With Nodular Ground-Glass Opacity. BMC Cancer (2014) 14:312. doi: 10.1186/1471-2407-14-312
22. Tomita M, Ayabe T, Chosa E, Kawagoe K, Nakamura K. Epidermal Growth Factor Receptor Mutations in Japanese Men With Lung Adenocarcinomas. Asian Pac J Cancer Prev (2014) 15(24):10627–30. doi: dx.doi.org/10.7314//APJCP.2014.15.24.10627
23. Chen K, Chen W, Cai J, Yang F, Lou F, Wang X, et al. Favorable Prognosis and High Discrepancy of Genetic Features in Surgical Patients With Multiple Primary Lung Cancers. J Thorac Cardiovasc Surg (2018) 155(1):371–9.e1. doi: 10.1016/j.jtcvs.2017.08.141
24. Kobayashi Y, Mitsudomi T, Sakao Y, Yatabe Y. Genetic Features of Pulmonary Adenocarcinoma Presenting With Ground-Glass Nodules: The Differences Between Nodules With and Without Growth. Ann Oncol (2015) 26(1):156–61. doi: 10.1093/annonc/mdu505
25. Li Y, Li X, Li H, Zhao Y, Liu Z, Sun K, et al. Genomic Characterisation of Pulmonary Subsolid Nodules: Mutational Landscape and Radiological Features. Eur Respir J (2020) 55(2):1901409. doi: 10.1183/13993003.01409-2019
26. Ren Y, Huang S, Dai C, Xie D, Zheng L, Xie H, et al. Germline Predisposition and Copy Number Alteration in Pre-Stage Lung Adenocarcinomas Presenting as Ground-Glass Nodules. Front Oncol (2019) 9:288. doi: 10.3389/fonc.2019.00288
27. Wang T, Zhang T, Han X, Liu XI, Zhou N, Liu Y. Impact of the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society Classification of Stage IA Adenocarcinoma of the Lung: Correlation Between Computed Tomography Images and EGFR and KRAS Gene Mutations. Exp Ther Med (2015) 9(6):2095–103. doi: 10.3892/etm.2015.2422
28. Usuda K, Sagawa M, Motono N, Ueno M, Tanaka M, Machida Y, et al. Relationships Between EGFR Mutation Status of Lung Cancer and Preoperative Factors - are They Predictive? Asian Pac J Cancer Prev (2014) 15(2):657–62. doi: 10.7314/APJCP.2014.15.2.657
29. Lui NS, Benson J, He H, Imielski BR, Kunder CA, Liou DZ, et al. Sub-Solid Lung Adenocarcinoma in Asian Versus Caucasian Patients: Different Biology But Similar Outcomes. J Thorac Dis (2020) 12(5):2161–71. doi: 10.21037/jtd.2020.04.37
30. Hong SJ, Kim TJ, Choi YW, Park J-S, Chung J-H, Lee KW. Radiogenomic Correlation in Lung Adenocarcinoma With Epidermal Growth Factor Receptor Mutations: Imaging Features and Histological Subtypes. Eur Radiol (2016) 26(10):3660–8. doi: 10.1007/s00330-015-4196-z
31. Wu T, Zhou F, Soodeen-Lalloo AK, Yang X, Shen Y, Ding X, et al. The Association Between Imaging Features of TSCT and the Expression of PD-L1 in Patients With Surgical Resection of Lung Adenocarcinoma. Clin Lung Cancer (2019) 20(2):e195–207. doi: 10.1016/j.cllc.2018.10.012
32. Suda K, Shimoji M, Shimizu S, Sato K, Chiba M, Tomizawa K, et al. Comparison of PD-L1 Expression Status Between Pure-Solid Versus Part-Solid Lung Adenocarcinomas. Biomolecules (2019) 9(9):456. doi: 10.3390/biom9090456
33. Toyokawa G, Takada K, Okamoto T, Shimokawa M, Kozuma Y, Matsubara T, et al. Computed Tomography Features of Lung Adenocarcinomas With Programmed Death Ligand 1 Expression. Clin Lung Cancer (2017) 18(6):e375–e83. doi: 10.1016/j.cllc.2017.03.008
34. Aoki T, Tomoda Y, Watanabe H, Nakata H, Kasai T, Hashimoto H, et al. Peripheral Lung Adenocarcinoma: Correlation of Thin-Section CT Findings With Histologic Prognostic Factors and Survival. Radiology (2001) 220(3):803–9. doi: 10.1148/radiol.2203001701
35. Matsuguma H, Yokoi K, Anraku M, Kondo T, Kamiyama Y, Mori K, et al. Proportion of Ground-Glass Opacity on High-Resolution Computed Tomography in Clinical T1 N0 M0 Adenocarcinoma of the Lung: A Predictor of Lymph Node Metastasis. J Thorac Cardiovasc Surg (2002) 124(2):278–84. doi: 10.1067/mtc.2002.122298
36. Nakata M, Sawada S, Yamashita M, Saeki H, Kurita A, Takashima S, et al. Objective Radiologic Analysis of Ground-Glass Opacity Aimed at Curative Limited Resection for Small Peripheral Non-Small Cell Lung Cancer. J Thorac Cardiovasc Surg (2005) 129(6):1226–31. doi: 10.1016/j.jtcvs.2004.10.032
37. Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating Mutations in the Epidermal Growth Factor Receptor Underlying Responsiveness of non-Small-Cell Lung Cancer to Gefitinib. N Engl J Med (2004) 350(21):2129–39. doi: 10.1056/NEJMoa040938
38. Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, et al. EGF Receptor Gene Mutations are Common in Lung Cancers From "Never Smokers" and are Associated With Sensitivity of Tumors to Gefitinib and Erlotinib. Proc Natl Acad Sci USA (2004) 101(36):13306–11. doi: 10.1073/pnas.0405220101
39. Tsao M-S, Sakurada A, Cutz J-C, Zhu C-Q, Kamel-Reid S, Squire J, et al. Erlotinib in Lung Cancer - Molecular and Clinical Predictors of Outcome. N Engl J Med (2005) 353(2):133–44. doi: 10.1056/NEJMoa050736
40. Shigematsu H, Takahashi T, Nomura M, Majmudar K, Suzuki M, Lee H, et al. Somatic Mutations of the HER2 Kinase Domain in Lung Adenocarcinomas. Cancer Res (2005) 65(5):1642–6. doi: 10.1158/0008-5472.CAN-04-4235
41. Kosaka T, Yatabe Y, Onozato R, Kuwano H, Mitsudomi T. Prognostic Implication of EGFR, KRAS, and TP53 Gene Mutations in a Large Cohort of Japanese Patients With Surgically Treated Lung Adenocarcinoma. J Thorac Oncol (2009) 4(1):22–9. doi: 10.1097/JTO.0b013e3181914111
42. Chou Y-T, Lin H-H, Lien Y-C, Wang Y-H, Hong C-F, Kao Y-R, et al. EGFR Promotes Lung Tumorigenesis by Activating miR-7 Through a Ras/ERK/Myc Pathway That Targets the Ets2 Transcriptional Repressor ERF. Cancer Res (2010) 70(21):8822–31. doi: 10.1158/0008-5472.CAN-10-0638
43. Sun F, Xi J, Zhan C, Yang X, Wang L, Shi Y, et al. Ground Glass Opacities: Imaging, Pathology, and Gene Mutations. J Thorac Cardiovasc Surg (2018) 156(2):808–13. doi: 10.1016/j.jtcvs.2018.02.110
44. Kobayashi Y, Mitsudomi T. Management of Ground-Glass Opacities: Should All Pulmonary Lesions With Ground-Glass Opacity be Surgically Resected? Trans Lung Cancer Res (2013) 2(5):354–63. doi: 10.3978/j.issn.2218-6751.2013.09.03
45. Lee SW, Leem C-S, Kim TJ, Lee KW, Chung J-H, Jheon S, et al. The Long-Term Course of Ground-Glass Opacities Detected on Thin-Section Computed Tomography. Respir Med (2013) 107(6):904–10. doi: 10.1016/j.rmed.2013.02.014
46. Huang C, Wang C, Wang Y, Liu J, Bie F, Wang Y, et al. The Prognostic Significance of Pure Ground Glass Opacities in Lung Cancer Computed Tomographic Images. J Cancer (2019) 10(27):6888–95. doi: 10.7150/jca.33132
47. Slebos RJ, Kibbelaar RE, Dalesio O, Kooistra A, Stam J, Meijer CJ, et al. K-Ras Oncogene Activation as a Prognostic Marker in Adenocarcinoma of the Lung. N Engl J Med (1990) 323(9):561–5. doi: 10.1056/NEJM199008303230902
48. Uras IZ, Moll HP, Casanova E. Targeting KRAS Mutant Non-Small-Cell Lung Cancer: Past, Present and Future. Int J Mol Sci (2020) 21(12):4325. doi: 10.3390/ijms21124325
49. Herbst RS, Baas P, Kim D-W, Felip E, Pérez-Gracia JL, Han J-Y, et al. Pembrolizumab Versus Docetaxel for Previously Treated, PD-L1-Positive, Advanced Non-Small-Cell Lung Cancer (KEYNOTE-010): A Randomised Controlled Trial. Lancet (2016) 387(10027):1540–50. doi: 10.1016/S0140-6736(15)01281-7
50. Qu J, Mei Q, Liu L, Cheng T, Wang P, Chen L, et al. The Progress and Challenge of Anti-PD-1/PD-L1 Immunotherapy in Treating non-Small Cell Lung Cancer. Ther Adv Med Oncol (2021) 13:1758835921992968–. doi: 10.1177/1758835921992968
51. Emancipator K. Keytruda and PD-L1: A Real-World Example of Co-Development of a Drug With a Predictive Biomarker. AAPS J (2020) 23(1):5. doi: 10.1208/s12248-020-00525-1
52. Wu F, Li W, Zhao W, Zhou F, Xie H, Shi J, et al. Synchronous Ground-Glass Nodules Showed Limited Response to Anti-PD-1/PD-L1 Therapy in Patients With Advanced Lung Adenocarcinoma. Clin Trans Med (2020) 10(3):e149. doi: 10.1002/ctm2.149
53. Jiang N, Zhou J, Zhang W, Li P, Liu Y, Shi H, et al. RNF213 Gene Mutation in Circulating Tumor DNA Detected by Targeted Next-Generation Sequencing in the Assisted Discrimination of Early-Stage Lung Cancer From Pulmonary Nodules. Thorac Cancer (2021) 12(2):181–93. doi: 10.1111/1759-7714.13741
54. Abbosh C, Birkbak NJ, Wilson GA, Jamal-Hanjani M, Constantin T, Salari R, et al. Phylogenetic ctDNA Analysis Depicts Early-Stage Lung Cancer Evolution. Nature (2017) 545(7655):446–51. doi: 10.1038/nature22364
55. Chabon JJ, Hamilton EG, Kurtz DM, Esfahani MS, Moding EJ, Stehr H, et al. Integrating Genomic Features for Non-Invasive Early Lung Cancer Detection. Nature (2020) 580(7802):245–51. doi: 10.1038/s41586-020-2140-0
56. Hu X, Fujimoto J, Ying L, Fukuoka J, Ashizawa K, Sun W, et al. Multi-Region Exome Sequencing Reveals Genomic Evolution From Preneoplasia to Lung Adenocarcinoma. Nat Commun (2019) 10(1):2978. doi: 10.1038/s41467-019-10877-8
57. Krysan K, Tran LM, Grimes BS, Fishbein GA, Seki A, Gardner BK, et al. The Immune Contexture Associates With the Genomic Landscape in Lung Adenomatous Premalignancy. Cancer Res (2019) 79(19):5022. doi: 10.1158/0008-5472.CAN-19-0153
58. Li R, Li X, Xue R, Yang F, Wang S, Li Y, et al. Early Metastasis Detected in Patients With Multifocal Pulmonary Ground-Glass Opacities (GGOs). Thorax (2018) 73(3):290–2. doi: 10.1136/thoraxjnl-2017-210169
59. Rosenthal R, Cadieux EL, Salgado R, Bakir MA, Moore DA, Hiley CT, et al. Neoantigen-Directed Immune Escape in Lung Cancer Evolution. Nature (2019) 567(7749):479–85. doi: 10.1038/s41586-019-1032-7
60. Xing X, Yang F, Huang Q, Guo H, Wang JJSA. Decoding the Multicellular Ecosystem of Lung Adenocarcinoma Manifested as Pulmonary Subsolid Nodules by Single-Cell RNA Sequencing. (2021) 7(5):eabd9738. doi: 10.1126/sciadv.abd9738
Keywords: ground-glass-opacity, lung cancer, systematic review, molecular alteration, EGFR, PD-L1
Citation: Wei Z, Wang Z, Nie Y, Zhang K, Shen H, Wang X, Wu M, Yang F and Chen K (2021) Molecular Alterations in Lung Adenocarcinoma With Ground-Glass Nodules: A Systematic Review and Meta-Analysis. Front. Oncol. 11:724692. doi: 10.3389/fonc.2021.724692
Received: 14 June 2021; Accepted: 18 August 2021;
Published: 13 September 2021.
Edited by:
Cheng Zhan, Fudan University, ChinaReviewed by:
Song Xu, Tianjin Medical University General Hospital, ChinaCopyright © 2021 Wei, Wang, Nie, Zhang, Shen, Wang, Wu, Yang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kezhong Chen, Y2hlbmtlemhvbmdAcGt1cGguZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.